Abstract
Background
We previously demonstrated that flurbiprofen increased arterial oxygen partial pressure and reduced intrapulmonary shunts. The present study aims to investigate whether flurbiprofen improves intraoperative regional cerebral oxygen saturation (rScO2) and reduces the incidence of postoperative delirium (POD) in elderly patients undergoing one-lung ventilation (OLV).
Methods
One hundred and twenty patients undergoing thoracoscopic lobectomy were randomly assigned to the flurbiprofen-treated group (n = 60) and the control-treated group (n = 60). Flurbiprofen was intravenously administered 20 minutes before skin incision. The rScO2 and partial pressure of arterial oxygen (PaO2) were recorded during the surgery, and POD was measured by the Confusion Assessment Method (CAM) within 5 days after surgery. The study was registered in the Chinese Clinical Trial Registry with the number ChiCTR1800020032.
Results
Compared with the control group, treatment with flurbiprofen significantly improved the mean value of intraoperative rScO2 as well as the PaO2 value (P < 0.05, both) and significantly reduced the baseline values of the rScO2 area under threshold (AUT) (P < 0.01) at 15, 30, and 60 min after OLV in the flurbiprofen-treated group. After surgery, the POD incidence in the flurbiprofen-treated group was significantly decreased compared with that in the control group (P < 0.05).
Conclusion
Treatment with flurbiprofen may improve rScO2 and reduce the incidence of POD in elderly patients undergoing thoracoscopic one-lung ventilation surgery for lung cancer.
Clinical trial registration
http://www.chictr.org/cn/, identifier ChiCTR1800020032.
Keywords: flurbiprofen, one-lung ventilation, regional cerebral oxygen saturation, postoperative delirium, thoracic surgery
Introduction
One-lung ventilation (OLV) refers to the mechanical separation of the two lungs to allow ventilation of only one lung, while the other lung is compressed by the surgeon or allowed to passively deflate (1). This non-physiological ventilation approach is a standard approach to facilitate surgical exposure for pulmonary and other thoracic surgeries by using either a double-lumen tube or bronchial blocker (2). However, hypoxemia is one of the most common complications associated with OLV, occurring in 7–28% of individuals subjected to OLV (3, 4). It is thought that hypoxemia during OLV is related to ventilation/perfusion disturbance due to intrapulmonary shunts (5, 6). Although hypoxic pulmonary vasoconstriction (HPV) allows redirection of blood flow into the ventilated lung, approximately 4–10% of patients still experience an oxygen saturation of <90% (1, 7, 8).
Brain cellular function may be impaired or damaged by the reduction in oxygen delivery under this level of peripheral oxygen desaturation (9). Brain oximetry based on cerebral near-infrared spectroscopy (NIRS) enables continuous and noninvasive measurement of cerebral tissue oxygen saturation (SctO2) or regional cerebral oxygen saturation (rScO2) (10, 11). The incidence of cerebral oxygen desaturation, depicted as a decrease in SctO2 of more than 15% from the baseline level, has been reported to be as high as 70–100% in thoracic surgical patients undergoing OLV (7, 12). Cerebral hypoxia is a well-established risk factor for postoperative delirium (POD), which is an acute fluctuating brain dysfunction characterized by inattention, disorganized thinking, and altered levels of consciousness (13, 14). The reported incidence of POD ranges from 7 to 23% in patients who receive OLV (15, 16). It has been reported that cerebral desaturation, defined by 90% baseline for left SctO2, may be associated with an increased risk of POD in thoracotomy with OLV (17).
We have demonstrated that treatment with flurbiprofen reduced the Qs/Qt ratio and further increased the PaO2 level during OLV, possibly due to the upregulation of the vasoactive agent thromboxane B2 (TXB2)/6-keto-prostaglandin F1α (6-K-PGF1α) ratio (18). However, whether flurbiprofen can further alleviate cerebral desaturation and reduce POD incidence is largely unclear. In the present study, we aimed to determine whether flurbiprofen alleviates the reduction in the intraoperative rScO2 value and decreases the incidence of POD.
Patients and methods
Trial design
This is a prospective, randomized, double-blind and controlled trial implemented in First Affiliated Hospital, University of Science and Technology of China (USTC). This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee. All the patients were informed of the full details of the study protocol and signed the informed consent from. The study was registered in the Chinese Clinical Trial Registry with the number ChiCTR1800020032.
Randomization and blinding
The patients were randomized to either the flurbiprofen treatment group (Group F) or the control group (Group C) before entering the operation room. An allocation sequence was created by a computer-generated list. Allocation concealment was implemented by using sequentially numbered, opaque, sealed envelopes. Randomization and drug preparation were performed by an independent investigator who was not involved in the administration of anesthesia. Flurbiprofen and fat emulsions of the same appearance were sent to the anesthesiologist in an unmarked syringe before intravenous injection. Data collection was performed by another independent researcher involved in the administration of anesthesia. Data were collected in a blinded manner by the study patients, anesthesiologists, other researchers, and statisticians.
Patients
From June 2020 to April 2021, patients who were scheduled for elective pulmonary lobectomy and underwent video-assisted thoracoscopic surgery (VATS) were assessed before the study. Figure 1 shows the flow diagram of participant recruitment. The inclusion criteria were as follows: (1) age between 65 and 75 years; (2) American Society of Anesthesiologists (ASA) status II-III; and (3) anticipated duration of one-lung ventilation >60 min.
Figure 1.
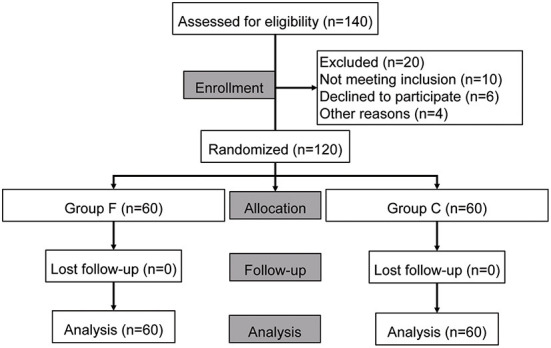
Flow chart of study.
Exclusion criteria were as follows: (1) severe impairment of respiratory function (forced expiratory volume in 1 s of <50% of the predicted values); (2) presence of contraindications for using flurbiprofen or intralipid; (3) treatment with NSAIDs drugs within 1 month before surgery; (4) scored<24 on the Mini-mental State Examination (MMSE) or who were unable to complete baseline cognitive assessment; (5) history of smoking, alcohol or drug abuse; (6) difficulty to maintain oxygenation with one-lung ventilation intraoperatively; (7) duration of one-lung ventilation was smaller than 60 min; (8) unable to collect data due to the cerebral oximeter machine malfunction; (9) researchers believe that other situations do not meet the conditions of this study. For example, VATS was intraoperatively converted to an open thoracotomy procedure.
Treatment
The participants were randomized to either the flurbiprofen treatment group (Group F) or the control group (Group C). Flurbiprofen 100 mg (50 mg/5 ml, Beijing Tide Pharmaceutical, China) was in Group F, and the placebo (Intralipid, Chengdu Huarui Pharmaceutical, China) was in Group C. The trial drug was dissolved in 100 ml of normal saline and intravenous drip within 15 min. All subjects received the drug (flurbiprofen or placebo) 20 min before incision. The length of the trial drug to OLV start was approximately 30 min.
General anesthesia
No premedication was used prior to surgery. General anesthesia was initiated with intravenous 0.05 mg/kg midazolam, 2 mg/kg propofol, 0.4 μg/kg sufentanil, and 1.0 mg/kg rocuronium. After the induction of anesthesia, an endobronchial tube was inserted and confirmed by bronchoscopy. Intubation was performed using a double-lumen tube. An additional 0.15 μg/kg sufentanil was given before the incision. Subsequently, intraoperative anesthesia was maintained with a continuous infusion of propofol (4–8 mg/kg/h) and remifentanil (0.05–0.2 μg/kg/min) to achieve a target bispectral index (BIS) value between 40 and 50. Cisatracurium was administered intermittently as required during the surgery. All patients were mechanically ventilated to maintain 35–45 mmHg ETCO2. Patients were mechanically ventilated in constant-flow volume-controlled mode following the protocol with a tidal volume of 6 to 8 mL/kg (two-lung ventilation) or 4–6 mL/kg (one-lung ventilation), inspiratory-to-expiratory time ratio of 1:2, and positive end-expiratory pressure 5 cm H2O. Pulse oxygen saturation was maintained above 92%. All surgical operations were performed by the same surgeon team, without any additional administration of local anesthetic by surgeons. Postoperative analgesia was regularly conducted using patient-controlled analgesia (PCA): Sufentanil was delivered at a rate of 2 μg/hr, with a 1.5 μg bolus and lockout interval of 15 min for breakthrough pain. Tramadol (50 mg) was provided for rescue analgesia if the visual analog scale (VAS) score was ≥4.
Monitoring indicators
Throughout the perioperative period, electrocardiography (ECG), heart rate (HR), SpO2, blood pressure, and CVP were continuously monitored. A bispectral index (BIS) sensor (Aspect Medical Systems, Inc., USA) applied to the forehead was used to monitor the depth of anesthesia, and the BIS value was kept at 40–50. The nasopharyngeal temperature was maintained at 36.3–37.2°C. Phenylephrine was given at a bolus of 25 μg if mean blood pressure (MBP) decreased to <80% of the preoperative baseline or systolic blood pressure decreased to <90 mmHg; atropine (0.3 mg) was given if HR decreased to <50 beats/min. If MBP or HR increased by >20% of the preoperative baseline, patients received fentanyl (0.05 mg) followed by perdipine (0.2 mg) or esmolol (10 mg). These treatments were repeated if necessary.
rScO2 monitoring
All patients were monitored with the INVOS 5100C cerebral oximeter before anesthesia induction until extubation. A fiberoptic sensor was positioned on each side on the forehead of the patients and covered by an opaque plastic patch to prevent ambient light. Baseline absolute rScO2 values were taken in the awake patient after 2 min of breathing 100% oxygen through a face mask. The screen of the cerebral oximeter was covered with an opaque bag to blind the rScO2 data to anesthesia providers and surgeons for monitoring. The rScO2 data were recorded at 30-s intervals on the device's accessory disk drive for later analysis. With the whole rScO2 data of each subject, the baseline, mean and minimum absolute rScO2 values of both sides during surgery were recorded, calculated and sifted. According to reference, cerebral desaturation was defined as either of the following two situations: (1) a baseline absolute value over 50%, rScO2 reduced to <75 percent of baseline; (2) or a baseline absolute value <50%, rScO2 reduced to <80 percent of baseline (19). Thus, in this trial, the rScO2 threshold was defined as 75% of the baseline absolute value if the baseline value was ≥50%, and if the baseline value was <50%, the rScO2 threshold was defined as 75% of the baseline value. The area under the threshold (AUT-rScO2) was also calculated. AUT was based on the following formula: AUT (present) = AUT (past) + (rScO2 threshold- rScO2 value) × sample rate. The AUT was 0 if the rScO2 value was above the defined rScO2 threshold (20).
Neuropsychological tests
The Confusion Assessment Method (CAM) is a widely used and well-validated screening tool for delirium with a sensitivity of 94% (95% confidence interval [CI] = 91–97%) and specificity of 89% (95% CI = 85–94%) and has been successfully adapted for use in the intensive care unit (ICU) setting (the CAM-ICU was used where appropriate) (21, 22). POD was defined as any episode of delirious symptoms within five postoperative days. Delirium was assessed twice daily, between 06:00–08:00 and 18:00–20:00, using the Chinese version of the Confusion Assessment Method (CAM) in non-intubated patients and the CAM-ICU in intubated patients. (17). The research personnel who were responsible for delirium assessment participated in a 4-h training session with the following agenda: (1) an introduction to the symptoms, diagnosis, and treatment of delirium, (2) a lecture on how to use CAM and CAM-ICU for delirium assessments, and (3) a simulation training course with a quiz at the end of training. All trainees were required to answer all quiz questions correctly. Research personnel who were responsible for out-come assessment were not allowed to access patient data collected during surgery (17).
Sample collection
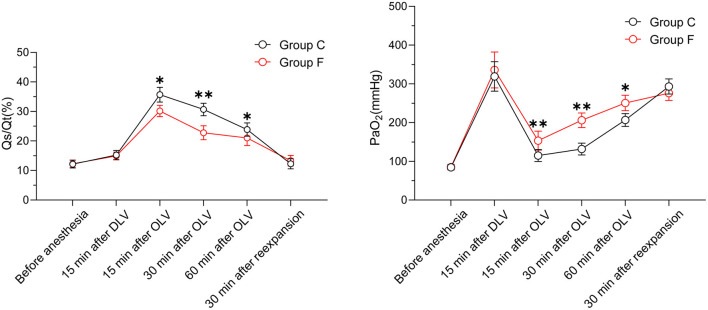
Blood samples were collected from arterial catheters before anesthesia, 15 min after double-lung ventilation (supine position), 15, 30, and 60 min after OLV, and 30 min after re-expansion of the collapsed lung. The samples were tested by blood gas analyses. The Qs/Qt ratio was determined from the following formula: (CCO2 – arterial oxygen content)/ (CCO2 – mixed venous oxygen content), where CCO2 = end-pulmonary capillary oxygen content.
Other outcomes
Baseline blood pressure, baseline heart rate, operation time, anesthesia time, OLV time, intraoperative blood loss, urine volume, infusion volume, length of hospital stay, length of stay in the ICU, pulmonary infection rate, chest tube removal time, visual analog score, and death rate 1 month after surgery were recorded for every patient.
Sample capacity
Few studies have tested the effect of flurbiprofen on cerebral oxygen saturation in one-lung ventilation. However, based on our pre-experiment outcome, we considered a difference of 3% for the mean rScO2 between both groups and a pooled SD of 7% of the means. With a power (β) of 0.95 and a 5% significance level, a minimum sample size of 59 patients for each group was estimated. Considering a drop-out rate of 20%, 70 subjects whose elective pulmonary lobectomy was undergoing VATS with an ASA status of II-III were registered in each group (140 patients in total).
Statistical analysis
Data were analyzed using SPSS 16.0 software. Values are expressed as the mean ± standard deviation (SD) or n (%). The comparison of the two groups of normal data was performed using one-way analysis, the comparison of two groups of nonnormal data was performed using the independent sample t-test and the Mann–Whitney U test, and the comparison of count data was performed by the chi-square test. Multiple reading data were analyzed by repeated-measures analysis of variance. A P < 0.05 was considered statistically significant, and a P < 0.01 was considered highly statistically significant.
Results
Patient demographics
One hundred and twenty patients aged between 65 and 75 years underwent elective pulmonary lobectomy for this study. There were 60 patients in each group. The characteristics of the 120 patients are summarized in Table 1. There were no significant differences in age, sex, ASA grade, year of education, MMSE score, hemoglobin before surgery, clinical characteristics, or surgery side between the two groups (P >0.05).
Table 1.
Patient characteristics.
| Characteristic | Group C (n = 60) | Group F (n = 60) | p Valuea |
|---|---|---|---|
| Age (years) | 68.38 ± 3.08 | 68.63 ± 2.91 | 0.9310 |
| Gender | 0.6011 | ||
| Male | 38 (63.3%) | 40 (66.7%) | |
| Female | 22 (36.7%) | 20 (33.3%) | |
| ASA physical status | 0.7158 | ||
| II | 11 (18.3%) | 13 (21.7%) | |
| III | 49 (81.7%) | 47 (78.3%) | |
| BMI (kg/m2) | 24.78 ± 2.48 | 25.28 ± 2.29 | 0.4299 |
| Education (years) | 0.4324 | ||
| ≤9 years | 52 (86.7) | 52 (83.3) | |
| >9 years | 8 (13.3) | 10 (16.7) | |
| MMSE before surgery | 27.52 ± 2.018 | 27.19 ± 1.966 | 0.8032 |
| Clinical characteristics | |||
| History of stroke | 8 (13.3%) | 10 (16.7%) | 0.6291 |
| Hypertension | 26 (43.3%) | 24 (40.0%) | 0.6932 |
| Diabetes | 15 (25.0%) | 18 (30.0%) | 0.5294 |
| Surgery side | 0.5104 | ||
| Left | 25 (41.7%) | 28 (46.7%) | |
| Right | 35 (58.3%) | 32 (53.3%) | |
| Hemoglobin (g/dl) | 12.64 ± 1.42 | 12.62 ± 1.74 | 0.9742 |
Values are n (%) or mean ± SD.
The comparison among the two groups of normal data using one-way analysis, the comparison of count data by chi-square test.
ASA, American Society of Anesthesiologists; BMI, body mass index; MMSE, Minimum Mental State Examination.
Cerebral oxygen saturation values
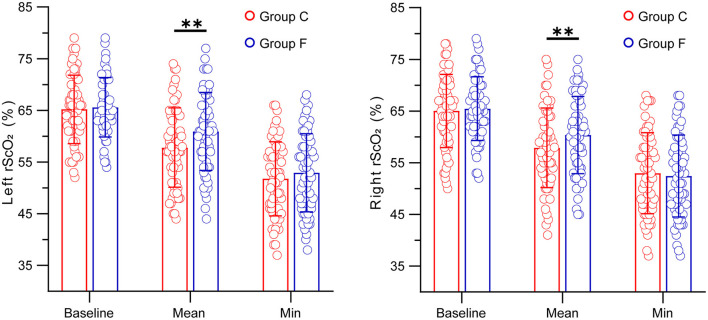
In terms of the baseline values of rScO2 measured before anesthesia induction and the minimum values of rScO2 during a surgical operation, our findings showed that there were no significant differences between the flurbiprofen-treated group and the control group in either the right or left hemisphere (P > 0.05) (Figure 2). The mean value of rScO2 on both sides in the flurbiprofen-treated group was significantly higher than that in the control group (right hemisphere, P < 0.05; left hemisphere, P < 0.01) (Figure 2). In the flurbiprofen-treated group, the area under the threshold (AUT) of baseline rScO2, either in the right hemisphere or left hemisphere, was less than that in the control group, with significant differences (P < 0.01 both) (Figure 3). PaO2 levels throughout the intraoperative period in the flurbiprofen-treated group were higher than those in the control group (P < 0.05) (Figure 4).
Figure 2.
Baseline, mean and minimum of the left and right rScO2 values in Group F and Group C. All data are presented as mean ± SD, **P < 0.01 vs Group C.
Figure 3.
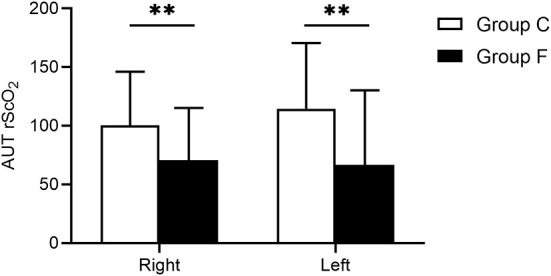
The left and right AUT rScO2 of baseline rScO2 values in Group F and Group C. All data are presented as mean ± SD, **P < 0.01 vs. Group C. AUT rScO2, the area under the threshold of rScO2 readings.
Figure 4.
PaO2 and Qs/Qt values in Group F and Group C. All data are presented as mean ± SD, *P < 0.05 and **P < 0.01 vs. Group C.
**P < 0.01 vs Group C.
POD incidence and other outcomes
Throughout the follow-up period after surgery, there were significant differences in POD development between the flurbiprofen-treated group and the control group at postoperative day 1 (7 vs. 14, P < 0.05), postoperative day 2 (5 vs. 12, P < 0.05), postoperative day 3 (4 vs. 8, P < 0.05), postoperative day 4 (1 vs. 5, P < 0.05), and postoperative day 5 (1 vs. 2, P < 0.05). The overall incidence of POD in the flurbiprofen-treated group was significantly lower than that in the control group (7 vs. 15, P < 0.05) (Figure 5). There were no significant differences in the baseline mean blood pressure, baseline heart rate, duration of anesthesia, duration of surgery, duration of OLV, phenylephrine and blood loss, urine output, fluid infusion during the surgery, chest tube removal time, lung infection rate, visual analog scale score, or ICU stay. However, the hospital stay of Group F was significantly shorter than that of Group C. No patient died at the one-month postoperative follow-up (Table 2). There were no significant differences in the intraoperative arterial blood oxygen saturation (SaO2) and arterial blood partial pressure of carbon dioxide (pCO2), as shown in Table 3.
Figure 5.
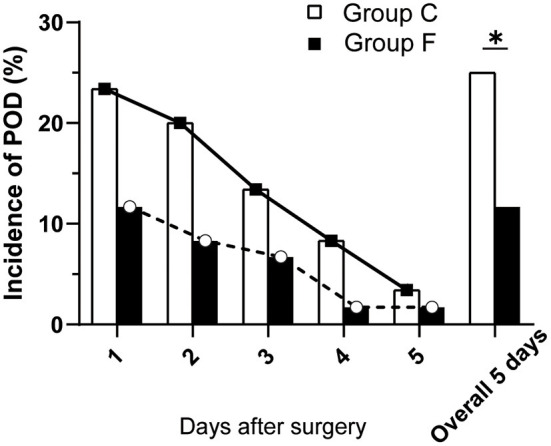
Incidence of POD in the two groups on postoperative days 1, 2, 3, 4, 5 and overall. All data are presented as n (%), *P < 0.05 vs. Group C. POD, postoperative delirium.
Table 2.
Perioperative data and postoperative outcomes.
| Information | Group C (n = 60) | Group F (n = 60) | p Valuea |
|---|---|---|---|
| Baseline MBP (mmHg) | 92.25 ± 6.428 | 93.18 ± 5.792 | 0.4425 |
| Baseline HR (beats/min) | 69.37 ± 4.215 | 67.12 ± 6.14 | 0.5914 |
| Duration of anesthesia (min) | 247.73 ± 48.12 | 250.14 ± 45.73 | 0.9048 |
| Duration of surgery (min) | 186.17 ± 40.23 | 187.20 ± 46.56 | 0.9209 |
| Duration of OLV (min) | 129.33 ± 17.62 | 135.60 ± 21.20 | 0.5055 |
| Phenylephrine (μg) | 267.47 ± 129.77 | 283.23 ± 79.45 | 0.6831 |
| Blood loss (ml) | 287.83 ± 84.45 | 284.50 ± 58.34 | 0.8448 |
| Urine output (ml) | 480.33 ± 139.12 | 467.67 ± 114.64 | 0.7503 |
| Fluid infusion (ml) | 1,953.33 ± 257.95 | 2,015.17 ± 277.71 | 0.6897 |
| Hospital-stay (days) | 7.38 ± 2.27 | 6.52 ± 2.43 | 0.043 |
| Chest tube removal (days) | 4.11 ± 1.17 | 3.98 ± 1.30 | 0.574 |
| visual analog scale | 2.76 ± 0.87 | 2.45 ± 1.01 | 0.597 |
| Lung infectionb | 10(16.66) | 7(11.66) | 0.198 |
| 1st month postoperative mortality rate | 0 | 0 | - |
| ICU-stay (days) | 0.88 ± 2.37 | 0.87 ± 2.42 | 0.820 |
Values are n (%) or mean±SD.
The comparison among the two groups of normal data using one-way analysis, the comparison of count data by chi-square test.
Lung infection was defined through imaging and clinical manifestations 1 week after surgery.
MBP, mean blood pressure; HR, heart rate; OLV, one-lung ventilation.
Table 3.
Intraoperative data.
| Information | Group C (n = 60) | Group F (n = 60) | p Valuea |
|---|---|---|---|
| Oxygen saturation (%) | |||
| Before anesthesia | 98.3 ± 1.4 | 98.2 ± 0.8 | 0.9957 |
| 15 min after DLV | 99.2 ± 0.5 | 99.3 ± 0.7 | 0.9957 |
| 15 min after OLV | 97.1 ± 1.8 | 96.9 ± 1.5 | 0.8714 |
| 30 min after OLV | 97.4 ± 1.1 | 97.3 ± 0.9 | 0.9957 |
| 60 min after OLV | 97.8 ± 0.9 | 97.6 ± 0.6 | 0.8714 |
| 30 min after re-expansion | 99.3 ± 0.4 | 98.9 ± 0.8 | 0.1894 |
| Partial pressure of carbon dioxide (mmHg) | |||
| Before anesthesia | 40.1 ± 3.5 | 41.4 ± 2.4 | 0.1888 |
| 15 min after DLV | 37.7 ± 4.7 | 37.8 ± 4.1 | 0.9899 |
| 15 min after OLV | 43.5 ± 3.1 | 44.0 ± 3.7 | 0.9599 |
| 30 min after OLV | 44.4 ± 2.3 | 44.1 ± 2.8 | 0.9972 |
| 60 min after OLV | 45.3 ± 3.5 | 45.6 ± 2.6 | 0.9972 |
| 30 min after re-expansion | 41.1 ± 2.1 | 42.0 ± 4.3 | 0.6023 |
Values are the mean±SD.
The comparison among the two groups of normal data using One-way analysis, the comparison of count data by chi-square test.
DLV, double-lung ventilation; OLV, one-lung ventilation.
Discussion
Hypoxemia remains challenging for intraoperative management, despite intervention including an increase in the inspired fraction of oxygen followed by a recruitment maneuvered and escalation of positive end-expiratory pressure (PEEP) to the ventilated lung (3, 6). Our study previously reported that intraoperative use of flurbiprofen increased arterial oxygen partial pressure and reduced intrapulmonary shunt (18). In the present study, our findings indicate that flurbiprofen may improve intraoperative rScO2 and further reduce the POD incidence in patients undergoing OLV. To the best of our knowledge, this is the first report to demonstrate the effects of flurbiprofen on intraoperative rScO2 and POD in elderly patients undergoing OLV.
Recently, an increasing number of published works have suggested NSAIDs (such as parecoxib, and flurbiprofen) that are commonly used for postoperative analgesia and can reduce POD after major noncardiac surgery in elderly patients (23, 24). However, the pharmacological mechanism underlying this beneficial action of NSAIDs on neuropsychiatric function is not entirely understood. It may involve the NSAID-elicited reduction of proinflammatory cytokines (TNF-α, IL-1β, and IL-6), which is thought to be primarily triggered by surgical operation and damage synapses and neurons and ultimately lead to POD via vagal afferents and by crossing the blood-brain barrier (25, 26). In addition to neuroinflammation, there are many other risk factors contributing to POD, such as age, sex, hypothermia, category of surgery, surgical position, duration of surgery, and mechanical ventilation. Given the complex etiology of POD, it is largely unknown whether flurbiprofen can in particular impact cerebral homeostasis, which has been shown to be closely related to the high risk of developing POD.
A variety of studies have indicated that the incidence of cerebral desaturation, defined as a decrease in SctO2 of more than 15% from the baseline level, can be as high as 70–100% in thoracic surgical patients (7). The cerebral desaturation during thoracic surgery might be due to a reduction in cerebral oxygen delivery that could be caused by an impairment either in arterial oxygen content or in cerebral blood flow that might also be related to a decrease in cardiac output. OLV together with the lateral decubitus position which is required for most cases of thoracic surgery, is accompanied by substantial physiological disturbances, including increases in pulmonary vascular resistance and pulmonary arteriovenous shunt, hypoxic pulmonary vasoconstriction, and reduction in alveolar-arterial oxygen tension. With the increase in pulmonary resistance during the collapse of the operated lung, the right ventricular cardiac output would be expected to decrease, especially under anesthesia where compensatory reflex mechanisms may be blunted (17). Furthermore, with impaired right ventricular performance, an increase in right-sided filling pressures could increase cerebral venous blood volume and affect cerebral saturation in this manner. Thus, these mechanisms could contribute to the decreases seen in cerebral oxygen saturation. It has been well-documented that flurbiprofen alleviates inflammation and pain by inhibiting cyclooxygenase (COX) activity and the synthesis of thromboxane A2 (TXA2)/prostaglandin I2 (PGI2), which are vasoactive agents that affect pulmonary arterial pressure, the Qs/Qt ratio, and PaO2. In the present study, flurbiprofen improved intraoperative rScO2 by increasing PaO2, probably owing to flurbiprofen-elicited adequate oxygen delivery.
Many studies have shown that the alteration of cerebral oxygen saturation is also of great clinical significance. For example, a study implied that once the maximum percentage decrease in rScO2 was more than 11%, the sensitivity and specificity of postoperative cognitive dysfunction occurrence were 86.5 and 77.8%, respectively. This suggests that if the maximum percentage decrease in rScO2 exceeds 11%, appropriate measures should be taken to increase rScO2 to prevent the risk of developing cerebral ischemia (27). Our study found that the maximum percentage decrease in rScO2 in single-lung ventilation was more than 11%. Furthermore, among patients undergoing coronary artery bypass grafting, maintenance of intraoperative monitored rScO2 values above a “safety threshold” was associated with a lower incidence of major organ dysfunction and a shorter hospital stay (28). Another study by Plachky et al. showed a positive relationship between decreased rScO2 during the anhepatic phase and a hypoxia/ischemia-induced increase in neuron-specific enolase, which is used as an index of cerebral damage, during orthotopic liver transplantation (29). Collectively, even a small improvement in cerebral oxygen saturation is clinically significant, and near-infrared spectroscopy determination has strong potential with clinical utility in differing settings. Therefore, we also cautiously compared the differences in hemoglobin, phenylephrine use, and intraoperative partial pressure of carbon dioxide, which may affect cerebral oxygen saturation, in the two groups, and the results were not significantly different. We consider that flurbiprofen may have improved the decrease in cerebral oxygen saturation during one-lung ventilation and this may contribute to the improvement of postoperative cognitive dysfunction.
Hypoxemia during one-lung ventilation triggers a concern that organ cellular function may be impaired or injured by the reduction in oxygen delivery (9, 12). Initially, perioperative cerebral oximetry monitoring primarily focused on cardiac surgery patients as this cohort had a known significant incidence of postoperative neurocognitive disorder and cerebral vascular incidents (22). As there was a growing recognition of the potential for intraoperative monitoring with cerebral oximetry, it was applied to non-cardiac surgery scenarios such as thoracic surgery, which often has an increased risk of intraoperative hypoxemia, and was one potential application of great interest. Although the validity needs to be further tested, multiple studies have shown a relationship between cerebral oxygen desaturations and neurocognitive deficits in patients undergoing thoracic surgical procedures (17, 30, 31). For example, Monique Roberts and colleagues indicated that intraoperative cerebral oxygen desaturations, frequent during one-lung ventilation, are significantly associated with worse early cognitive recovery, and a high risk of POD (31). Furthermore, it is important to understand how the severity of cerebral desaturation and POD is related and if there is a SctO2 threshold below which the risk of delirium is increased. In another study performed by Fan Cui and colleagues, cerebral desaturation defined by <90% baseline for left SctO2 and <85% baseline for right SctO2, may be associated with an increased risk of post-thoracotomy delirium (17). Our findings may support an additional mechanism by which flurbiprofen-elicited cerebral oxygen saturation might, at least in part, contribute to a reduction in POD.
There are several limitations. First, oxygen delivery is not solely dependent on saturation but rather must be considered in concert with hemoglobin level and, more importantly, cardiac output (8). Thus, whether hypoxemia reflected through peripheral oxygen saturation results in hypoxia is patient-dependent. Additionally, due to a lack of direct evidence, we do not know whether the participation of neuroinflammation underlies the beneficial actions of flurbiprofen (25, 26). Finally, this was a single-center study. The same team of performing surgeons and anesthetists ensured the standardization and consistency of the work. To add more evidence, multicenter studies are warranted to test the results and conclusions of our trial.
Conclusions
Collectively, premedication with flurbiprofen may improve intraoperative rScO2 and reduce the incidence of POD in elderly patients undergoing thoracoscopic surgery with one-lung ventilation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The Ethics Committee at the First Affiliated Hospital of USTC approved this prospective trial. Written informed consent was obtained from all patients recruited to the study, in accordance with the code of the Declaration of Helsinki.
Author contributions
LS and J-qC: data curation and writing-original draft preparation. X-lY: writing-review and editing. J-cH and WG: analyze or synthesize study data. X-qC and DW: development or design of methodology. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant from Anhui Provincial Key Research and Development Project Foundation (No. 1804h08020286).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
Abbreviations
- rScO2
regional cerebral oxygen saturation
- POD
postoperative delirium
- OLV
one-lung ventilation
- PaO2
partial pressure of arterial oxygen
- CAM
Confusion Assessment Method
- AUT
the area under threshold
- HPV
hypoxic pulmonary vasoconstriction
- NIRS
near-infrared spectroscopy
- SctO2
cerebral tissue oxygen saturation
- VATS
video-assisted thoracoscopic surgery
- ASA
American Society of Anesthesiologists
- MMSE
Mini-mental State Examination
- BIS
bispectral index
- PCA
patient-controlled analgesia
- HR
heart rate
- MBP
mean blood pressure
- PEEP
positive end-expiratory pressure.
References
- 1.Bernasconi F, Piccioni F. One-lung ventilation for thoracic surgery: current perspectives. Tumori. (2017) 103:495–503. 10.5301/tj.5000638 [DOI] [PubMed] [Google Scholar]
- 2.Mcgrath B, Tennuci C, Lee G. The history of one-lung anesthesia and the double-lumen tube. J Anesth Hist. (2017) 3:76–86. 10.1016/j.janh.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 3.Ishikawa S, Lohser J. One-lung ventilation and arterial oxygenation. Curr Opin Anaesthesiol. (2011) 24:24–31. 10.1097/ACO.0b013e3283415659 [DOI] [PubMed] [Google Scholar]
- 4.Yoon S, Kim BR, Min SH, Lee J, Bahk JH, Seo JH. Repeated intermittent hypoxic stimuli to operative lung reduce hypoxemia during subsequent one-lung ventilation for thoracoscopic surgery: A randomized controlled trial. PLoS ONE. (2021) 16:e0249880. 10.1371/journal.pone.0249880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lohser J, Slinger P. Lung injury after one-lung ventilation: a review of the pathophysiologic mechanisms affecting the ventilated and the collapsed lung. Anesth Analg. (2015) 121:302–18. 10.1213/ANE.0000000000000808 [DOI] [PubMed] [Google Scholar]
- 6.Campos JH, Feider A. Hypoxia during one-lung ventilation-a review and update. J Cardiothorac Vasc Anesth. (2018) 32:2330–8. 10.1053/j.jvca.2017.12.026 [DOI] [PubMed] [Google Scholar]
- 7.Hemmerling TM, Bluteau MC, Kazan R, Bracco D. Significant decrease of cerebral oxygen saturation during single-lung ventilation measured using absolute oximetry. Br J Anaesth. (2008) 101:870–5. 10.1093/bja/aen275 [DOI] [PubMed] [Google Scholar]
- 8.Durkin C, Romano K, Egan S, Lohser J. Hypoxemia during one-lung ventilation: does it really matter? Curr Anesthesiol Rep. (2021) 1–7. 10.1007/s40140-021-00470-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watts ME, Pocock R, Claudianos C. Brain energy and oxygen metabolism: emerging role in normal function and disease. Front Mol Neurosci. (2018) 11:216. 10.3389/fnmol.2018.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benni PB, Macleod D, Ikeda K, Lin HM. A validation method for near-infrared spectroscopy based tissue oximeters for cerebral and somatic tissue oxygen saturation measurements. J Clin Monit Comput. (2018) 32:269–84. 10.1007/s10877-017-0015-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu Y, Zhang K, Zhang L, Zong H, Meng L, Han R. Cerebral near-infrared spectroscopy (NIRS) for perioperative monitoring of brain oxygenation in children and adults. Cochrane Database Syst Rev. (2018) 1:CD010947. 10.1002/14651858.CD010947.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazan R, Bracco D, Hemmerling TM. Reduced cerebral oxygen saturation measured by absolute cerebral oximetry during thoracic surgery correlates with postoperative complications. Br J Anaesth. (2009) 103:811–6. 10.1093/bja/aep309 [DOI] [PubMed] [Google Scholar]
- 13.Tang L, Kazan R, Taddei R, Zaouter C, Cyr S, Hemmerling TM. Reduced cerebral oxygen saturation during thoracic surgery predicts early postoperative cognitive dysfunction. Br J Anaesth. (2012) 108:623–9. 10.1093/bja/aer501 [DOI] [PubMed] [Google Scholar]
- 14.Li XM, Li F, Liu ZK, Shao MT. Investigation of one-lung ventilation postoperative cognitive dysfunction and regional cerebral oxygen saturation relations. J Zhejiang Univ Sci B. (2015) 16:1042–8. 10.1631/jzus.B1500030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murakawa K, Kitamura Y, Watanabe S, Hongo S, Shinomiya K, Sendo T. Clinical risk factors associated with postoperative delirium and evaluation of delirium management and assessment team in lung and esophageal cancer patients. J. Pharm Health Care Sci. (2015) 1. 10.1186/s40780-014-0002-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee H-S, Hayashi K, Motoishi M, Sawai S, Horimoto K, Hanaoka J. Postoperative delirium after lung resection for primary lung cancer: Risk factors, risk scoring system, and prognosis. PLoS ONE. (2019) 14. 10.1371/journal.pone.0223917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cui F, Zhao W, Mu DL, Zhao X, Li XY, Wang DX, et al. Association between cerebral desaturation and postoperative delirium in thoracotomy with one-lung ventilation: a prospective cohort study. Anesth Analg. (2021) 133:176–86. 10.1213/ANE.0000000000005489 [DOI] [PubMed] [Google Scholar]
- 18.Chai XQ, Ma J, Xie YH, Wang D, Chen KZ. Flurbiprofen axetil increases arterial oxygen partial pressure by decreasing intrapulmonary shunt in patients undergoing one-lung ventilation. J Anesth. (2015) 29:881–6. 10.1007/s00540-015-2060-6 [DOI] [PubMed] [Google Scholar]
- 19.Casati A, Fanelli G, Pietropaoli P, Proietti R, Tufano R, Montanini S, et al. Monitoring cerebral oxygen saturation in elderly patients undergoing general abdominal surgery: a prospective cohort study. Eur J Anaesthesiol. (2007) 24:59–65. 10.1017/S0265021506001025 [DOI] [PubMed] [Google Scholar]
- 20.Fischer GW, Lin HM, Krol M, Galati MF, Di Luozzo G, Griepp RB, et al. Noninvasive cerebral oxygenation may predict outcome in patients undergoing aortic arch surgery. J Thorac Cardiovasc Surg. (2011) 141:815–21. 10.1016/j.jtcvs.2010.05.017 [DOI] [PubMed] [Google Scholar]
- 21.Rolfson Db M.J., Jhangri Gs, Rockwood K. (1999). Validity of the confusion assessment method in detecting postoperative delirium in the elderly. Int Psychogeriatr 11, 431-438. 10.1017/S1041610299006043 [DOI] [PubMed] [Google Scholar]
- 22.Sanders RD, Pandharipande PP, Davidson AJ, Ma D, Maze M. Anticipating and managing postoperative delirium and cognitive decline in adults. BMJ. (2011) 343:d4331. 10.1136/bmj.d4331 [DOI] [PubMed] [Google Scholar]
- 23.Ma XD, Li BP, Wang DL, Yang WS. Postoperative benefits of dexmedetomidine combined with flurbiprofen axetil after thyroid surgery. Exp Ther Med. (2017) 14:2148–52. 10.3892/etm.2017.4717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X, Wang Y, Hu Y, Wang L, Zhao W, Wei L, et al. Effect of flurbiprofen axetil on postoperative delirium for elderly patients. Brain Behav. (2019) 9:e01290. 10.1002/brb3.1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cunningham C, Skelly DT. Non-steroidal anti-inflammatory drugs and cognitive function: are prostaglandins at the heart of cognitive impairment in dementia and delirium? J Neuroimmune Pharmacol. (2012) 7:60–73. 10.1007/s11481-011-9312-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subramaniyan S, Terrando N. Neuroinflammation and perioperative neurocognitive disorders. Anesth Analg. (2019) 128:781–8. 10.1213/ANE.0000000000004053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li XM, Shao MT, Wang JJ, Wang YL. Relationship between post-operative cognitive dysfunction and regional cerebral oxygen saturation and beta-amyloid protein. J Zhejiang Univ Sci B. (2014) 15:870–8. 10.1631/jzus.B1400130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murkin JM, Adams SJ, Novick RJ, Quantz M, Bainbridge D, Iglesias I, et al. Monitoring brain oxygen saturation during coronary bypass surgery: a randomized, prospective study. Anesth Analg. (2007) 104:51–8. 10.1213/01.ane.0000246814.29362.f4 [DOI] [PubMed] [Google Scholar]
- 29.Plachky J, Hofer S, Volkmann M, Martin E, Bardenheuer HJ, Weigand MA. Regional cerebral oxygen saturation is a sensitive marker of cerebral hypoperfusion during orthotopic liver transplantation. Anesth Analg. (2004) 99:344–9. 10.1213/01.ANE.0000124032.31843.61 [DOI] [PubMed] [Google Scholar]
- 30.Brinkman R, Amadeo RJ, Funk DJ, Girling LG, Grocott HP, Mutch WA. Cerebral oxygen desaturation during one-lung ventilation: correlation with hemodynamic variables. Can J Anaesth. (2013) 60:660–6. 10.1007/s12630-013-9954-2 [DOI] [PubMed] [Google Scholar]
- 31.Roberts ML, Lin HM, Tinuoye E, Cohen E, Flores RM, Fischer GW, et al. The association of cerebral desaturation during one-lung ventilation and postoperative recovery: a prospective observational cohort study. J Cardiothorac Vasc Anesth. (2021) 35:542–50. 10.1053/j.jvca.2020.07.065 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.