Abstract
Polycystic ovary syndrome (PCOS) is one of the most prevalent gynecological endocrine conditions affecting reproductive women. It can feature a variety of symptoms, such as obesity, insulin resistance, skin conditions, and infertility. Women with PCOS are susceptible to illnesses including mood disorders, diabetes, hypertension, and dyslipidemia. Among them, depression is the most common in PCOS and has a detrimental effect on quality of life. Depression may occasionally develop due to the pathological traits of PCOS, but its exact pathogenesis in PCOS have eluded researchers to date. Therefore, there is an urgent need to explore the pathogenesis and treatments of depression in PCOS. The present review discusses the epidemiology of depression in PCOS, potential pathogenic mechanisms underlying PCOS and depression, as well as some potential factors causing depression in PCOS, including obesity, insulin resistance, hyperandrogenism, inflammation, and infertility. Meanwhile, some common treatment strategies for depression in PCOS, such as lifestyle intervention, acupuncture, oral contraceptive pills, psychological intervention, and insulin-sensitizer, are also reviewed. To fully understand the pathogenesis and treatment of depression in PCOS, a need remains for future large-scale multi-center randomized controlled trials and in-depth mechanism studies.
Keywords: polycystic ovary syndrome, depression, pathogenesis, mechanism, treatments
Introduction
Polycystic ovary syndrome (PCOS) is a common disease in women and is characterized by hyperandrogenemia, chronic anovulation, and polycystic ovary morphology (1). Endocrine and energy metabolism disorders lead to a variety of clinical symptoms of PCOS, including anovulation and amenorrhea (75–80%), infertility (75%), excessive hair growth (70%), and obesity (50%) (2). The prevalence of PCOS is rising and can range from 5 to 20% depending on the demographic studied and the diagnostic criteria applied (3). Patients with PCOS experience significant social and financial pressure, which increases the prevalence of mental illnesses such as anxiety and sadness. According to a recent study, anxiety and sadness are prevalent in women with PCOS with rates of 38.6 and 25.7%, respectively (4). The most prevalent cause of disability globally is depression, which is three to eight times higher in women with PCOS than in control groups (5, 6). Global standards indicate that all PCOS patients should be screened for depression at diagnosis, reflecting growing concern about this aspect of the condition (7). Obesity, insulin resistance, hyperandrogenism, inflammation, and infertility—all pathogenic aspects of PCOS—have been linked in studies to the emergence of depression. This review discusses the epidemiology of depression in PCOS, potential pathogenic mechanisms underlying the association between depression and PCOS, and some common treatment strategies for depression in this condition.
Depression in PCOS
Epidemiology
Using the PHQ-9 (patient health questionnaire-9), 64.1 percent of women with PCOS have been found to have depressive disorders, a significantly higher proportion than in a non-PCOS group (P < 0.01). In PCOS patients, the odds ratio for depressive disorders was 5.7, and univariate analysis showed significantly higher body mass index (BMI) in women with than without PCOS. Age, marital status, educational attainment, and place of employment did not significantly influence the onset of depression (8). Other research has shown that depression is more likely to occur in black than white women with PCOS (P < 0.001) (9). In comparison to non-PCOS counterparts, the prevalence of depression in PCOS was considerably higher in the overweight and obese categories (PCOS 33.2% vs. non-PCOS 16.2%; P < 0.001) (10). Women with PCOS also have a higher incidence of depression-related hospitalizations than those without PCOS (11). The prevalence of PCOS-related depression in various geographic areas is displayed in Table 1.
Table 1.
Summary of studies indicating prevalence by geographic region and examining the impact of PCOS-related treatments on depression in randomized controlled trials.
| Type | Country and case inclusion period | Prevalence (%) | Groups (number of subjects) | Treatment | Treatment length | Assessment scales | Results | References |
|---|---|---|---|---|---|---|---|---|
| Summary of studies indicating prevalence of depression in PCOS by geographic region | United States, 2005–2008 | 64.1% | PCOS with depression (n = 75), PCOS without depression (n = 42) |
/ | 35 months | PHQ-9 | The prevalence of depressive disorders among women with PCOS was 64.1% | (8) |
| United States, 1985–1986 | 36% | No PCOS (n = 1,044), PCOS (n =83) |
/ | 12 months | CESD | CES-D scores were higher among women with PCOS, and black women experienced higher depression burden than white women | (9) | |
| Australia, 1973–1978 | 27.3% | PCOS (n = 478), non-PCOS (n = 8,134) |
/ | 60 months | CESD-10 | Women with PCOS, reported higher prevalence of depression than women without PCOS (27.3 vs. 18.8%) | (10) | |
| Korean, 2007–2010 | 15.35% | PCOS (n = 26,251), Non PCOS (n = 131,480) |
/ | 36 months | / | The risk of developing depression in women with PCOS was higher compared to women without PCOS | (11) | |
| Syria and Jordan, 2017 | 83% in Syria and 65% in Jordan | Syria (active, n = 30 vs. control, n = 30), Jordan (active, n = 30 vs. control, n = 28) |
/ | 5 months | Beck depression inventory | Syria and Jordan highlighted a high prevalence of depression (Syria = 83% vs. Jordan = 65%) | (12) | |
| Randomize-d controlled trials assessing the effect of PCOS-related treatments on depression in women with PCOS | Netherlands, 2010–2016 | / | CAU (n = 60). CBTLS (n = 63) CBTLS+SMS (n = 60) |
Cognitive behavioral lifestyle sessions combined with a healthy diet and physical therapy | 12 months | BDI-II, RSES, FNAES |
A three-component lifestyle intervention based on CBT could improve depression in women with PCOS | (13) |
| United States, 2013–2015 | / | CBT+LS (n = 20), LS (n = 13) |
Cognitive-behavioral therapy (CBT) and lifestyle modification (LS) | 16 weeks | CESD, STAI | CBT+LS significantly improved depressive symptoms in women with PCOS compared with LS alone | (14) | |
| China, 2018–2019 | / | Intervention group (n = 61), control group (n = 61) |
Transtheoretical model-based mobile health application intervention program | 12 months | SAS, SDS | TTM-based mobile health application program can decrease depression in patients with PCOS | (15) | |
| Australia, not mentioned | / | HPLC: (n = 14); LPHC: (n = 14) |
High-protein, low-carbohydrate diet (HPLC) | 16 weeks | HADS and the Rosenberg Self Esteem Scale | The HPLC diet was associated with significant reduction in depression | (16) | |
| Brazil, 2014–2016 | / | CAT (n = 23), IAT (n = 22), CG (n = 24) |
Continuous and intermittent aerobic physical training | 16 weeks | HADS | Both CAT and IAT groups had significant reductions in depression scores | (17) | |
| China, 2016–2019 | / | A (n = 20). LS (n = 20). |
Acupuncture | 4 months | SAS, SDS | Acupuncture can effectively relieve depression in patients with PCOS, and the mechanism may be related to the regulation of serum β-endorphin and androgen | (18) | |
| Swedish, 2005–2008 | / | Acupuncture (n = 28); exercise (n = 29); control (n = 15) |
Acupuncture | 16 weeks | MADRS-S, BSA-S | Acupuncture can lead to a modest improvement in depression scores in women with PCOS | (19) | |
| China, 2012–2016 | / | Acupuncture group (n = 27), sham acupuncture group (n = 27) |
Acupuncture | 16 weeks | Zung-SAS and Zung-SDS | Acupuncture can influence serum levels of NE and 5-HT, improving symptoms of depression in PCOS patients | (20) | |
| United States, 2008–2014 | / | OCP group (n = 45), LS group (n = 44), combined group (n = 43) |
Oral contraceptive pills (OCPs; ethinyl estradiol/norethindrone acetate) | 16 weeks | Positive screens on the Prime-MD | OCPs result in significant improvements in depressive symptoms | (21) | |
| Athens, 2012–2013 | / | Intervention group (n = 23), control group (n = 15) |
Mindfulness stress management program | 8 weeks | DASS 21 | Mindfulness techniques ameliorate stress, anxiety, depression and the quality of life in women with PCOS | (22) | |
| Danish, 2014–2016 | / | MI+ SA (n = 19), SA (n = 18) |
Motivational interviewing | 6 weeks | WHO-5 and MDI | Motivational interviewing can significantly improve depression scores | (23) | |
| Germany, 2011–2012 | / | Pioglitazone (n = 20), metformin (n = 20) |
Pioglitazone | 6 weeks | HDR-17 | Pioglitazone improves depression with mechanisms largely unrelated to its insulin-sensitizing action | (24) | |
| China, 2016–2018 | / | PM (n = 28), M (n = 26), placebo (n = 21) |
Pioglitazone metformin complex preparation (PM) | 12 weeks | SCL-90-R | Pioglitazone metformin alleviates depression via inhibiting NLRP3 inflammasome | (25) |
Pathogenesis of PCOS
Hypothalamic-pituitary-gonad dysfunction is thought to be the primary cause of PCOS. Gonadotropin-releasing hormone (GnRH) is pulse-released in the hypothalamus under typical circumstances. Increased frequency of release encourages luteinizing hormone (LH) to be released from the anterior pituitary gland, while reduced frequency encourages the production of follicle stimulating hormone (FSH) (26). The amounts of GnRH, LH, and FSH secreted by the pituitary and hypothalamus are controlled by the levels of estrogen, progesterone, androgen, and other steroid hormones generated by the ovary. In PCOS patients, dysfunction in the GnRH neuronal network in the brain resulted in decreased responsiveness to gonadal steroid hormone negative feedback, destroying the afore-mentioned feedback loop and causing rises in GnRH pulse frequency, increasing LH pulse frequency, decreasing FSH pulse frequency, and abnormally increasing the LH/FSH ratio (27). Ovarian granulosa cells' reaction to reduced FSH release frequency leads to a distortion in the selection of dominant follicles, with follicle stagnation, a polycystic state detected on ultrasound examination, and infertility (28). However, elevated LH causes theca cells in the ovary to produce excessive androgen, which causes hyperandrogenemia (29). In addition, peripheral estrogen synthesis occurs as a result of high serum androgen levels in PCOS (30). The pro-inflammatory nature of PCOS is caused by excess estrogen, which increases the synthesis of a variety of inflammatory cytokines (31). Inflammatory markers IL-6 and tumor necrosis factor alpha (TNF-α) are linked to insulin resistance, another metabolic abnormality which may be induced in PCOS (32).
Pathogenesis of depression
The pathogenesis of depression is associated with alterations in the hypothalamic-pituitary-adrenal (HPA) axis (hypothalamus, pituitary, adrenal and downstream target organs) and a decrease in monoamine neurotransmitter level (33). Corticotropin releasing hormone, adreno-cortico-tropic-hormone and cortisol secretion increase when the body is under psychological stress (such as competition for a job) (34). Excessive cortisol inhibits HPA axis activity, maintaining body hormone homeostasis. However, if stress persists, cortisol will remain high, leading to desensitization of cortisol receptors and further stimulation of the HPA axis, which eventually destroy the negative feedback regulation between cortisol and its receptors, causing continuous hyperactivity of the HPA axis, and forming a vicious cycle leading to depression (35). Decreased secretion of serotonin, acetylcholine and other neurotransmitters also negatively affects function of the HPA axis and similarly leads to depression (33).
Pathogenesis of depression in PCOS
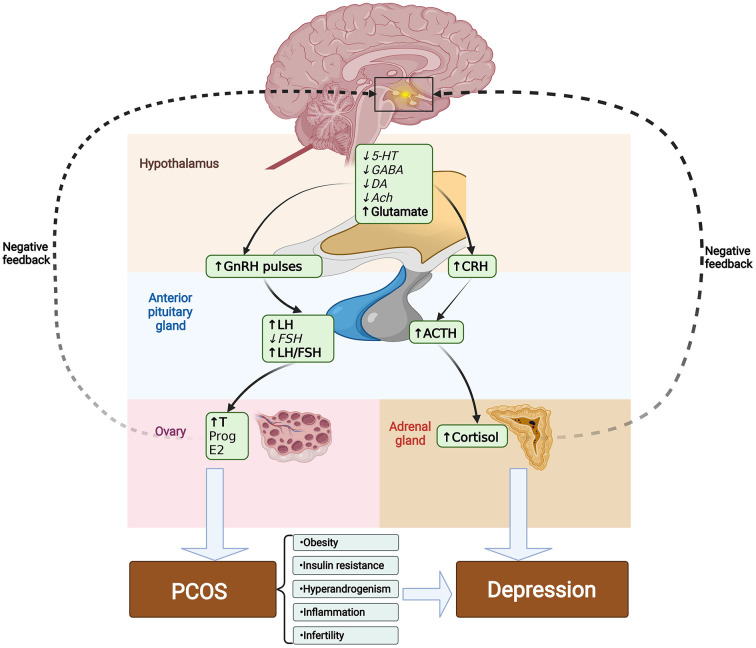
Inhibitory neurotransmitters such as serotonin (5-HT), dopamine (DA), gamma-aminobutyric acid (GABA), and acetylcholine (Ach) are diminished in PCOS. In contrast, glutamate levels, which are the primary stimulants of GnRH and LH, are raised in PCOS-related disorders, and these neurotransmitter alterations could play a part in the pathophysiology of depression in PCOS (36). The specific mechanisms driving the higher prevalence of depressive symptoms in PCOS-positive women are not yet established, however there are many possible contributing factors: obesity, insulin resistance, hyperandrogenism, inflammation, and infertility (see Figure 1).
Figure 1.
Changes in neurotransmitters may be involved in the pathogenesis of PCOS-induced depression. In PCOS, GnRH and LH inhibitory neurotransmitters such as 5-HT, DA, GABA and Ach are decreased. While the major stimulants of GnRH and LH such as glutamate are increased. Elevated frequency of release in GnRH encourages LH to be released from the anterior pituitary gland, while reduced frequency encourages the production of FSH, which abnormally increases the LH/FSH ratio. Elevated LH causes theca cells in the ovary to produce excessive androgen and eventually exacerbates the progression of PCOS. Decreased secretion of serotonin, acetylcholine and other neurotransmitters also negatively affects function of the HPA axis, which increases the levels of CRH, ACTH, and cortisol, causing continuous hyperactivity of the HPA axis, and leading to depression. Meanwhile, the pathological traits of PCOS, including obesity, insulin resistance, hyperandrogenism, inflammation, and infertility can exacerbate the onset of depression. Italic font indicates lower levels compared to normal, whereas bold font indicates higher levels. 5-HT, serotonin; DA, dopamine; GABA, gamma-aminobutyric acid; Ach, acetylcholine; GnRH, gonadotropin-releasing hormone; LH, luteinizing hormone; FSH, follicle stimulating hormone; T, Testosterone; E2, estradiol; CRH, corticotropin releasing hormone; ACTH, adreno-cortico-tropic-hormone.
Obesity
Up to 80% of women with PCOS have morbid obesity (37). An increased depression score and a higher likelihood of depression were significantly correlated in obese women with PCOS (38); The HPA axis is dysregulated in obesity, with extensive over-production of cortisol (39). In PCOS, abnormally high levels of systemic cortisol lead to the overexpression of mineralocorticoid and glucocorticoid receptors, which in turn reduces negative feedback and ultimately results in a state of sustained hypercortisolism (40). Excessive secretion of cortisol and impaired glucocorticoid-mediated feedback mechanism result in the development of depression in PCOS (41). Additionally, by activating the highly expressed glucocorticoid receptors in the visceral and intra-abdominal areas, cortisol stimulates the deposition of central fat (42) and promotes lipid accumulation, which leads to visceral and central obesity and, ultimately, metabolic disorders (43) in a vicious cycle. Additionally, the obese patient's perception that they are physically unattractive may exacerbate depressive symptoms and their perception of their own femininity (44). In conclusion, obesity, as a result of abnormal metabolism of PCOS, is closely related to the dysfunction of HPA axis, leading to the development of depression.
Insulin resistance
About 75% of women with PCOS meet the World Health Organization (WHO) criteria for insulin resistance (45). According to one study, insulin resistance may act as a physiological mediator and has an independent relationship with depression in PCOS, with Homeostatic Model Assessment of Insulin Resistance score linked to a 2.3-fold higher risk of depression (46). One proposed molecular reason for the emergence of depression through insulin resistance is elevated cortisol, accompanied by elevated sympathetic nerve activity and decreased levels of 5-HT in the central nervous system (47). 5-HTs are potent neurotransmitters with a broad range of effects (48). Depression can result from diminished central 5-HT function (49). Decreased level of 5-HT in patients with depression can antagonize the direct inhibition of insulin secretion, leading to insulin dysfunction and insulin resistance (50), thus forming a vicious cycle between insulin resistance and depression.
Hyperandrogenism
A large majority of PCOS-affected women has clinical and/or biochemical hyperandrogenism (47). Clinically, hyperandrogenism can manifest as hirsutism, acne, and alopecia (51). When compared to women without depression in PCOS, those with depression in PCOS had higher levels of free testosterone, according to a meta-regression analysis (52). Hyperandrogenemia associated with obesity, hirsutism, acne and hair loss may alter self-image, which can lead to depression (53). It is reported that the serum concentrations of 5-HT, 5-hydroxyindoleacetic acid (5-HIAA), and the DA metabolite homovanillic acid were significantly lower while the testosterone and the depressive subscales were significantly higher in women with PCOS compared to those without PCOS, suggesting a relationship among hyperandrogenism, depression and altered neurotransmitter contents in PCOS (54). Animal studies have also demonstrated that dehydroepiandrosterone caused depression-like behavior in mice with PCOS, potentially via down-regulation of brain monoamines and associated metabolites, indicating a role of hyperandrogenism in the mental health conditions found in PCOS (55). Above all, the hyperandrogenism associated with PCOS that results in depression may be based on the monoamine hypothesis of depression.
Inflammation
Previous research has shown that PCOS is an inflammatory condition (56). Various studies have shown elevated serum levels of TNF-α in PCOS (32, 52). In addition, high estrogen release during PCOS increases interleukin 4 (IL-4), IL-1, IL-6, and interferon γ (IFN-γ) production (31) and these increases are associated with depression-like symptoms in human and animal studies (57). When this cytokine signal enters the brain, depression may result, having a noticeable impact on brain monoamines like 5-HT and DA. Tryptophan, the major precursor for 5-HT production, is depleted when cytokines activate the enzyme indoleamine 2,3-dioxygenase, which metabolizes the conversion of tryptophan into kynurenine. This results in lower numbers of 5-HT receptors in the brain (56). Cytokines may potentially affect the availability of 5-HT by interfering with synaptic reuptake through use of presynaptic transporters such as the high-affinity 5-HT transporter (58). For instance, it has been observed that IL-1 and TNF activate p38 mitogen-activated protein kinase, which results in the phosphorylation and increased expression of 5-HT reuptake pumps, increasing the intake of 5-HT and, consequently, the manifestation of depressive symptoms (59). Depression also increases inflammation. Stress promotes the expression of cytokines in the brain and peripheral areas of the body, activates microglia, and causes an inflammatory response through the sympathetic nervous system (60). Furthermore, major depressive disorder intensifies the body's inflammatory reaction to stress. Proinflammatory cytokines (such IL-1 and IL-6) are linked to both acute and chronic stress, and affect the severity and speed of development of depression (61). To sum up, excessive inflammation caused by PCOS is an important inducer of depression.
Infertility
Up to 72% of patients with PCOS experienced infertility (2), of which the clinical pregnancy rate using in vitro fertilization and embryo transfer (IVF-ET) technology is only 29% (62). The treatment of infertility may impact emotions through the interaction of estrogen and/or progesterone and serotonin and worsen the incidence of depression in women with PCOS due to the high social, family, and economic pressures they confront (63). According to one study, 40.8% of infertile women suffer from depression, which is associated with the duration of infertility and is most prevalent 4–6 years after diagnosis (26). Another study reported that, the fertility problem inventory subscale scores and the overall stress scale score in the infertility patients were negatively correlated with clinical pregnancy outcome, indicating that the higher the pressure, the lower the pregnancy success rate of IVF-ET (64). Two common immunoendocrine mechanisms that underpin infertility may act as mediators between psychopathology/stress and poor reproduction. Infertility has been linked to immunological imbalance, which can lead to parasecretion of hormones, cytokines, and neuropeptides. These offer a typical molecular pathogenesis for depression in PCOS (65).
Treatment
No specific drug treatment has been identified for PCOS depression to date, and most studies in this field have assessed the effectiveness of PCOS-specific treatment regimens. Randomized controlled trials on effectiveness are summarized in Table 1.
Lifestyle intervention
For patients with PCOS, healthy lifestyle is the primary means of treatment, such as the combined application of diet and exercise. One study reported that diet, exercise, and cognitive behavioral therapy (CBT) combined with a lifestyle intervention program significantly improved depression and self-esteem in patients with PCOS, and weight loss was positively associated with self-esteem (13). Another randomized controlled study including 33 overweight/obese women with PCOS and depressive symptoms found that weekly CBT and lifestyle change for 8 weeks significantly reduced weight and enhanced quality of life compared to lifestyle change alone. Both groups experienced a decrease in heart rate at 8 weeks, which could be interpreted as an improvement in sympathetic responses to stress or habituation triggered by repeated measurements. This finding raises the possibility of a connection between CBT, weight loss, and regulation of the stress response (14). Dietary modifications have a substantial impact on PCOS, and studies have shown that adopting a high-protein, low-carbohydrate (HPLC) diet is linked to a considerable decline in depressive symptoms and an increase in self-esteem. It is likely that HPLC diets are associated with better compliance and, therefore, more effectiveness in the long-term treatment of obesity due to a greater sense of wellbeing (16). Exercise can regulate PCOS patients' body weight, endocrine function, depression, and other factors. Anxiety and sadness ratings dropped significantly in women with PCOS undertaking continuous and intermittent aerobic physical exercise, according to a randomized controlled trial assessing the effects on sexual function and mood in these patients. This finding may be related to the decline in testosterone level (17).
Acupuncture
Acupuncture therapy is widely used in the treatment of depression (66), and has good efficacy in alleviating PCOS symptoms (67). One study reported reduced BMI, Ferriman-Gallway score, self-rating anxiety scale (SAS) and self-rating depression scale (SDS) scores, while PCOS health-related quality of life questionnaire scores, serum sex hormone binding globulin (SHBG) levels and β -endorphin levels were elevated after acupuncture intervention in PCOS patients. These results indicated that acupuncture therapy can effectively relieve depression in PCOS patients, and the mechanism may be related to the regulation of serum β-endorphin and androgen levels (18). Physiology, energy/vitality, general health impression, and mental component scores in the medical outcomes study short-form 36 (SF-36) domain of PCOS patients in the acupuncture group improved after intervention and during follow-up, according to a secondary analysis from a randomized controlled trial. And the effects of acupuncture persisted for at least 4 months after treatment ended (19). Another secondary investigation to assess how electroacupuncture affected anxiety and sadness in unmarried women with PCOS found that acupuncture could alter serum norepinephrine (NE) and 5-HT levels and thereby lessen PCOS depression symptoms (20).
Oral contraceptive pills
For women with PCOS, hormonal contraceptives are the primary line of treatment to control menstruation. OCP use was linked to a significant improvement in the psychological component of health-related quality of life in a survey of more than 1,000 women in the general population (68). Studies have shown that low-dose hormonal contraceptive therapy combined with lifestyle changes can improve the psychosocial functioning of PCOS in overweight/obese women and significantly reduce depressive symptoms through a mechanism associated with reduced androgen levels, in addition to well-established benefits like improved menstrual cycle and reduced hirsutism (21). However, few studies on the effects or mechanisms of oral contraceptives on depression in PCOS, and larger and longer-term studies are required to investigate these.
Psychological intervention
Official guidelines stated that medical staff should evaluate psychological status and provide constructive criticism and adjustments to remove the psychological barriers for patients with PCOS. When necessary, they should also consult with support or guidance groups for reasonable psychological support and intervention in patients with obesity (69). After practicing mindfulness-based stress reduction, blood pressure, blood glucose, emotional distress, and quality of life all improve in women with PCOS (70). Motivational interviewing as a tool for addiction therapy, is now being used to treat psychological disorders and obesity (71, 72). One study revealed that the World Health Organization 5 Wellbeing Index (P = 0.028) and Major Depression Inventory (P = 0.008) scores were considerably raised when motivational interviewing was supplemented with conventional guidance (23). Large trials are required to augment the evidence on psychological intervention for depression in women with PCOS due to the limited sample sizes and trial periods in the existing published studies.
Insulin-sensitizer
Insulin sensitizers are used to treat insulin resistance associated with PCOS. Pioglitazone is a thiazolidinedione (TZD), which is an insulin sensitizer. TZDs have been found in numerous animal and human studies to be effective in treating neurological and psychiatric conditions like depression and to have strong antidepressant properties (73, 74). Pioglitazone outperformed metformin in terms of reduced depression [38.3 vs. 8.3% reduction from baseline scores, F(1,37) = 73.513, P < 0.001] (24). According to one study, pioglitazone metformin can successfully suppress the activation of NLRP3 inflammasome, lessen the release of pro-inflammatory cytokines, and enhance many indicators, including total testosterone, leading to a reduction in psychological distress in PCOS (25).
Summary and outlook
The mechanism underlying the rising prevalence of depression in PCOS may be related to the pathological traits of PCOS, including obesity, insulin resistance, hyperandrogenism, inflammation, and infertility. However, rather than focusing on the biological mechanisms of depression in PCOS, contemporary research has largely concentrated on the similarities and correlations between the pathological features of PCOS and depression. Meanwhile, there are few randomized controlled trials that deeply study the effective treatment strategy and explore the mechanism. According to previous studies, PCOS-induced depression can be effectively treated with acupuncture, oral contraceptives, psychological therapy, and insulin sensitization agents. Nevertheless, effective data supporting the use of these medications to specifically target depression in PCOS are yet lacking due to varying methodologies and a dearth of randomized controlled trials with a large sample size. To fully understand the pathogenesis and treatment of depression in PCOS, a need remains for future large-scale multi-center randomized controlled trials and in-depth mechanism studies.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was supported by the National Natural Science Foundation of China (31960178, 82160923, and 82060895), Applied Basic Research Programs of Science and Technology Commission Foundation of Yunnan Province (2019FA007), Key Laboratory of Traditional Chinese Medicine for Prevention and Treatment of Neuropsychiatric Diseases, Yunnan Provincial Department of Education, Scientific Research Projects for High-level Talents of Yunnan University of Chinese Medicine (2019YZG01), Young Top-Notch Talent in 10,000 Talent Program of Yunnan Province (YNWR-QNBJ-2019-235), the Yunnan University of Chinese Medicine Joint Special Project of Applied Basic Research (2019FF002-004), and Yunnan Science and Technology Department Project (202103AC100005).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.American College of Obstetricians and Gynecologists' Committee on Practice Bulletins—Gynecology . ACOG practice bulletin no. 194: polycystic ovary syndrome. Obstet Gynecol. (2018) 131:e157–e71. 10.1097/AOG.0000000000002656 [DOI] [PubMed] [Google Scholar]
- 2.Skiba MA, Islam RM, Bell RJ, Davis SR. Understanding variation in prevalence estimates of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. (2018) 24:694–709. 10.1093/humupd/dmy022 [DOI] [PubMed] [Google Scholar]
- 3.Azziz R, Carmina E, Chen Z, Dunaif A, Laven JS, Legro RS, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. (2016) 2:16057. 10.1038/nrdp.2016.57 [DOI] [PubMed] [Google Scholar]
- 4.Chaudhari AP, Mazumdar K, Mehta PD. Anxiety, depression, and quality of life in women with polycystic ovarian syndrome. Indian J Psychol Med. (2018) 40:239–46. 10.4103/IJPSYM.IJPSYM_561_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCarron RM, Shapiro B, Rawles J, Luo J. Depression. Ann Intern Med. (2021) 174:Itc65–80. 10.7326/AITC202105180 [DOI] [PubMed] [Google Scholar]
- 6.Cooney LG, Lee I, Sammel MD, Dokras A. High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. (2017) 32:1075–91. 10.1093/humrep/dex044 [DOI] [PubMed] [Google Scholar]
- 7.Teede HJ, Misso ML, Costello MF, Dokras A, Laven J, Moran L, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil Steril. (2018) 110:364–79. 10.1016/j.fertnstert.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhattacharya SM, Jha A. Prevalence and risk of depressive disorders in women with polycystic ovary syndrome (PCOS). Fertil Steril. (2010) 94:357–9. 10.1016/j.fertnstert.2009.09.025 [DOI] [PubMed] [Google Scholar]
- 9.Greenwood EA, Yaffe K, Wellons MF, Cedars MI, Huddleston HG. Depression over the lifespan in a population-based cohort of women with polycystic ovary syndrome: longitudinal analysis. J Clin Endocrinol Metab. (2019) 104:2809–19. 10.1210/jc.2019-00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damone AL, Joham AE, Loxton D, Earnest A, Teede HJ, Moran LJ. Depression, anxiety and perceived stress in women with and without PCOS: a community-based study. Psychol Med. (2019) 49:1510–20. 10.1017/S0033291718002076 [DOI] [PubMed] [Google Scholar]
- 11.Lee IO, Kim JC, Seo JW, Pak HY, Chung JE. Risk of developing major depressive disorder in polycystic ovary syndrome: a retrospective cohort study. J Obstet Gynaecol. (2021) 41:1157–61. 10.1080/01443615.2020.1849071 [DOI] [PubMed] [Google Scholar]
- 12.Alkoudsi KT, Basheti IA. Prevalence of anxiety and depression among women with Polycystic Ovary Syndrome living in war versus non war zone countries: a randomized controlled trial assessing a pharmacist intervention. Res Social Adm Pharm. (2020) 16:689 98. 10.1016/j.sapharm.2019.08.027 [DOI] [PubMed] [Google Scholar]
- 13.Jiskoot G, Dietz de Loos A, Beerthuizen A, Timman R, Busschbach J, Laven J. Long-term effects of a three-component lifestyle intervention on emotional well-being in women with Polycystic Ovary Syndrome (PCOS): a secondary analysis of a randomized controlled trial. PLoS ONE. (2020) 15:e0233876. 10.1371/journal.pone.0233876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooney LG, Milman LW, Hantsoo L, Kornfield S, Sammel MD, Allison KC, et al. Cognitive-behavioral therapy improves weight loss and quality of life in women with polycystic ovary syndrome: a pilot randomized clinical trial. Fertil Steril. (2018) 110:161–71.e1. 10.1016/j.fertnstert.2018.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L, Liu Y, Tan H, Huang S. Transtheoretical model based mobile health application for PCOS. Reprod Health. (2022) 19:117. 10.1186/s12978-022-01422-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galletly C, Moran L, Noakes M, Clifton P, Tomlinson L, Norman R. Psychological benefits of a high-protein, low-carbohydrate diet in obese women with polycystic ovary syndrome–a pilot study. Appetite. (2007) 49:590–3. 10.1016/j.appet.2007.03.222 [DOI] [PubMed] [Google Scholar]
- 17.Lopes IP, Ribeiro VB, Reis RM, Silva RC, Dutra de Souza HC, Kogure GS, et al. Comparison of the effect of intermittent and continuous aerobic physical training on sexual function of women with polycystic ovary syndrome: randomized controlled trial. J Sex Med. (2018) 15:1609–19. 10.1016/j.jsxm.2018.09.002 [DOI] [PubMed] [Google Scholar]
- 18.Zhang HL, Huo ZJ, Wang HN, Wang W, Chang CQ, Shi L, et al. Acupuncture ameliorates negative emotion in PCOS patients: a randomized controlled trial. Zhongguo Zhen Jiu. (2020) 40:385–90. 10.13703/j.0255-2930.20191231-k0005 [DOI] [PubMed] [Google Scholar]
- 19.Stener-Victorin E, Holm G, Janson PO, Gustafson D, Waern M. Acupuncture and physical exercise for affective symptoms and health-related quality of life in polycystic ovary syndrome: secondary analysis from a randomized controlled trial. BMC Complement Altern Med. (2013) 13:131. 10.1186/1472-6882-13-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Z, Dong H, Wang Q, Zhang L, Wu X, Zhou Z, et al. Effects of electroacupuncture on anxiety and depression in unmarried patients with polycystic ovarian syndrome: secondary analysis of a pilot randomised controlled trial. Acupunct Med. (2019) 37:40–6. 10.1136/acupmed-2017-011615 [DOI] [PubMed] [Google Scholar]
- 21.Dokras A, Sarwer DB, Allison KC, Milman L, Kris-Etherton PM, Kunselman AR, et al. Weight loss and lowering androgens predict improvements in health-related quality of life in women with PCOS. J Clin Endocrinol Metab. (2016) 101:2966–74. 10.1210/jc.2016-1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stefanaki C, Bacopoulou F, Livadas S, K andaraki A, Karachalios A, Chrousos GP, et al. Impact of a mindfulness stress management program on stress, anxiety, depression and quality of life in women with polycystic ovary syndrome: a randomized controlled trial. Stress. (2015) 18:57–66. 10.3109/10253890.2014.974030 [DOI] [PubMed] [Google Scholar]
- 23.Moeller LV, Lindhardt CL, Andersen MS, Glintborg D, Ravn P. Motivational interviewing in obese women with polycystic ovary syndrome - a pilot study. Gynecol Endocrinol. (2019) 35:76–80. 10.1080/09513590.2018.1498832 [DOI] [PubMed] [Google Scholar]
- 24.Kashani L, Omidvar T, Farazmand B, Modabbernia A, Ramzanzadeh F, Tehraninejad ES, et al. Does pioglitazone improve depression through insulin-sensitization? Results of a randomized double-blind metformin-controlled trial in patients with polycystic ovarian syndrome and comorbid depression. Psychoneuroendocrinology. (2013) 38:767–76. 10.1016/j.psyneuen.2012.08.010 [DOI] [PubMed] [Google Scholar]
- 25.Guo QJ, Shan J, Xu YF, Hu YY, Huo CL, Song JY, et al. Pioglitazone metformin complex improves polycystic ovary syndrome comorbid psychological distress via inhibiting NLRP3 inflammasome activation: a prospective clinical study. Mediators Inflamm. (2020) 2020:3050487. 10.1155/2020/3050487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stern E, Ruf-Zamojski F, Zalepa-King L, Pincas H, Choi SG, Peskin CS, et al. Modeling and high-throughput experimental data uncover the mechanisms underlying Fshb gene sensitivity to gonadotropin-releasing hormone pulse frequency. J Biol Chem. (2017) 292:9815–29. 10.1074/jbc.M117.783886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coyle C, Campbell RE. Pathological pulses in PCOS. Mol Cell Endocrinol. (2019) 498:110561. 10.1016/j.mce.2019.110561 [DOI] [PubMed] [Google Scholar]
- 28.Medeiros SF, Barbosa BB, Medeiros MAS, Yamamoto MMW. Morphology and biochemistry of ovulation. Rev Bras Ginecol Obstet. (2021) 43:480–6. 10.1055/s-0041-1731379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witchel SF, Oberfield SE, Peña AS. Polycystic ovary syndrome: pathophysiology, presentation, and treatment with emphasis on adolescent girls. J Endocr Soc. (2019) 3:1545–73. 10.1210/js.2019-00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meier RK. Polycystic ovary syndrome. Nurs Clin North Am. (2018) 53:407–20. 10.1016/j.cnur.2018.04.008 [DOI] [PubMed] [Google Scholar]
- 31.Mobeen H, Afzal N, Kashif M. Polycystic ovary syndrome may be an autoimmune disorder. Scientifica. (2016) 2016:4071735. 10.1155/2016/4071735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong P, Guan B, Lin Y, Zhang S. Changes in inflammatory factors, oxidative stress, glucose and lipid metabolism, and insulin resistance in patients with polycystic ovary syndrome. Cell Mol Biol. (2022) 67:45–50. 10.14715/cmb/2021.67.5.6 [DOI] [PubMed] [Google Scholar]
- 33.Joseph DN, Whirledge S. Stress and the HPA axis: balancing homeostasis and fertility. Int J Mol Sci. (2017) 18:2224. 10.3390/ijms18102224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shapero BG, Curley EE, Black CL, Alloy LB. The interactive association of proximal life stress and cumulative HPA axis functioning with depressive symptoms. Depress Anxiety. (2019) 36:1089–101. 10.1002/da.22957 [DOI] [PubMed] [Google Scholar]
- 35.Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM. Glucocorticoids, cytokines and brain abnormalities in depression. Prog Neuropsychopharmacol Biol Psychiatry. (2011) 35:722–9. 10.1016/j.pnpbp.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaudhari N, Dawalbhakta M, Nampoothiri L. GnRH dysregulation in polycystic ovarian syndrome (PCOS) is a manifestation of an altered neurotransmitter profile. Reprod Biol Endocrinol. (2018) 16:37. 10.1186/s12958-018-0354-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cinar N, Kizilarslanoglu MC, Harmanci A, Aksoy DY, Bozdag G, Demir B, et al. Depression, anxiety and cardiometabolic risk in polycystic ovary syndrome. Hum Reprod. (2011) 26:3339–45. 10.1093/humrep/der338 [DOI] [PubMed] [Google Scholar]
- 38.Maya J, Siegel J, Cheng TQ, Rousseau-Pierre T. Prevalence and risk factors of polycystic ovarian syndrome among an ethnically diverse overweight/obese adolescent population. Int J Adolesc Med Health. (2020) 34:1–6. 10.1515/ijamh-2019-0109 [DOI] [PubMed] [Google Scholar]
- 39.Incollingo Rodriguez AC, Epel ES, White ML, Standen EC, et al. Hypothalamic-pituitary-adrenal axis dysregulation and cortisol activity in obesity: a systematic review. Psychoneuroendocrinology. (2015) 62:301–18. 10.1016/j.psyneuen.2015.08.014 [DOI] [PubMed] [Google Scholar]
- 40.Lee SH, Paz-Filho G, Mastronardi C, Licinio J, Wong ML. Is increased antidepressant exposure a contributory factor to the obesity pandemic? Transl Psychiatry. (2016) 6:e759. 10.1038/tp.2016.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Butler T, Harvey P, Cardozo L, Zhu YS, Mosa A, Tanzi E, et al. Epilepsy, depression, and growth hormone. Epilepsy Behav. (2019) 94:297–300. 10.1016/j.yebeh.2019.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pavlatou MG, Vickers KC, Varma S, Malek R, Sampson M, Remaley AT, et al. Circulating cortisol-associated signature of glucocorticoid-related gene expression in subcutaneous fat of obese subjects. Obesity. (2013) 21:960–7. 10.1002/oby.20073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paredes S, Ribeiro L. Cortisol: the villain in metabolic syndrome? Rev Assoc Med Bras. (2014) 60:84–92. 10.1590/1806-9282.60.01.017 [DOI] [PubMed] [Google Scholar]
- 44.Mack LR, Tomich PG. Gestational diabetes: diagnosis, classification, and clinical care. Obstet Gynecol Clin North Am. (2017) 44:207–17. 10.1016/j.ogc.2017.02.002 [DOI] [PubMed] [Google Scholar]
- 45.Tosi F, Bonora E, Moghetti P. Insulin resistance in a large cohort of women with polycystic ovary syndrome: a comparison between euglycaemic-hyperinsulinaemic clamp and surrogate indexes. Hum Reprod. (2017) 32:2515–21. 10.1093/humrep/dex308 [DOI] [PubMed] [Google Scholar]
- 46.Greenwood EA, Pasch LA, Cedars MI, Legro RS, Eisenberg E, Huddleston HG. Insulin resistance is associated with depression risk in polycystic ovary syndrome. Fertil Steril. (2018) 110:27–34. 10.1016/j.fertnstert.2018.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pomytkin IA, Cline BH, Anthony DC, Steinbusch HW, Lesch KP, Strekalova T. Endotoxaemia resulting from decreased serotonin tranporter (5-HTT) function: a reciprocal risk factor for depression and insulin resistance? Behav Brain Res. (2015) 276:111–7. 10.1016/j.bbr.2014.04.049 [DOI] [PubMed] [Google Scholar]
- 48.De Deurwaerdere P, Di Giovanni G. 5-HT interaction with other neurotransmitters: an overview. Prog Brain Res. (2021) 259:1-5. 10.1016/bs.pbr.2021.01.001 [DOI] [PubMed] [Google Scholar]
- 49.Daut RA, Fonken LK. Circadian regulation of depression: a role for serotonin. Front Neuroendocrinol. (2019) 54:100746. 10.1016/j.yfrne.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gluvic Z, Zaric B, Resanovic I, Obradovic M, Mitrovic A, Radak D, et al. Link between metabolic syndrome and insulin resistance. Curr Vasc Pharmacol. (2017) 15:30–9. 10.2174/1570161114666161007164510 [DOI] [PubMed] [Google Scholar]
- 51.Amiri M, Ramezani Tehrani F, Nahidi F, Bidhendi Yarandi R, Behboudi-Gandevani S, Azizi F. Association between biochemical hyperandrogenism parameters and Ferriman-Gallwey score in patients with polycystic ovary syndrome: a systematic review and meta-regression analysis. Clin Endocrinol. (2017) 87:217–30. 10.1111/cen.13389 [DOI] [PubMed] [Google Scholar]
- 52.Sayin NC, Gücer F, Balkanli-Kaplan P, Yüce MA, Ciftci S, Kücük M, et al. Elevated serum TNF-alpha levels in normal-weight women with polycystic ovaries or the polycystic ovary syndrome. J Reprod Med. (2003) 48:165–70. [PubMed] [Google Scholar]
- 53.Elsenbruch S, Benson S, Hahn S, Tan S, Mann K, Pleger K, et al. Determinants of emotional distress in women with polycystic ovary syndrome. Hum Reprod. (2006) 21:1092–9. 10.1093/humrep/dei409 [DOI] [PubMed] [Google Scholar]
- 54.Shi X, Zhang L, Fu S, Li N. Co-involvement of psychological and neurological abnormalities in infertility with polycystic ovarian syndrome. Arch Gynecol Obstet. (2011) 284:773–8. 10.1007/s00404-011-1947-1 [DOI] [PubMed] [Google Scholar]
- 55.Yu Q, Hao S, Wang H, Song X, Shen Q, Kang J. Depression-like behavior in a dehydroepiandrosterone-induced mouse model of polycystic ovary syndrome. Biol Reprod. (2016) 95:79. 10.1095/biolreprod.116.142117 [DOI] [PubMed] [Google Scholar]
- 56.Shelton RC, Miller AH. Inflammation in depression: is adiposity a cause? Dialogues Clin Neurosci. (2011) 13:41–53. 10.31887/DCNS.2011.13.1/rshelton [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haapakoski R, Ebmeier KP, Alenius H, Kivimäki M. Innate and adaptive immunity in the development of depression: an update on current knowledge and technological advances. Prog Neuropsychopharmacol Biol Psychiatry. (2016) 66:63–72. 10.1016/j.pnpbp.2015.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quan N, Banks WA. Brain-immune communication pathways. Brain Behav Immun. (2007) 21:727–35. 10.1016/j.bbi.2007.05.005 [DOI] [PubMed] [Google Scholar]
- 59.Zhu CB, Lindler KM, Owens AW, Daws LC, Blakely RD, Hewlett WA. Interleukin-1 receptor activation by systemic lipopolysaccharide induces behavioral despair linked to MAPK regulation of CNS serotonin transporters. Neuropsychopharmacology. (2010) 35:2510–20. 10.1038/npp.2010.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. (2012) 37:137–62. 10.1038/npp.2011.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Young JJ, Bruno D, Pomara N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J Affect Disord. (2014) 169:15–20. 10.1016/j.jad.2014.07.032 [DOI] [PubMed] [Google Scholar]
- 62.Feng JG, Guo Y, Ma LA, Xing J, Sun RF, Zhu W. Prevalence of dermatologic manifestations and metabolic biomarkers in women with polycystic ovary syndrome in north China. J Cosmet Dermatol. (2018) 17:511–7. 10.1111/jocd.12387 [DOI] [PubMed] [Google Scholar]
- 63.Williams KE, Marsh WK, Rasgon NL. Mood disorders and fertility in women: a critical review of the literature and implications for future research. Hum Reprod Update. (2007) 13:607–16. 10.1093/humupd/dmm019 [DOI] [PubMed] [Google Scholar]
- 64.Aimagambetova G, Issanov A, Terzic S, Bapayeva G, Ukybassova T, Baikoshkarova S, et al. The effect of psychological distress on IVF outcomes: reality or speculations? PLoS ONE. (2020) 15:e0242024. 10.1371/journal.pone.0242024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haimovici F, Anderson JL, Bates GW, Racowsky C, Ginsburg ES, Simovici D, et al. Stress, anxiety, and depression of both partners in infertile couples are associated with cytokine levels and adverse IVF outcome. Am J Reprod Immunol. (2018) 79:e12832. 10.1111/aji.12832 [DOI] [PubMed] [Google Scholar]
- 66.Smith CA, Armour M, Lee MS, Wang LQ, Hay PJ. Acupuncture for depression. Cochrane Database Syst Rev. (2018) 3:Cd004046. 10.1002/14651858.CD004046.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lim DC, Chen W, Cheng LN, Xue CC, Wong FW, O'Sullivan AJ, et al. Acupuncture for polycystic ovarian syndrome. Cochrane Database Syst Rev. (2011) 2011:Cd007689. 10.1002/14651858.CD007689.pub2 [DOI] [PubMed] [Google Scholar]
- 68.Borenstein J, Yu HT, Wade S, Chiou CF, Rapkin A. Effect of an oral contraceptive containing ethinyl estradiol and drospirenone on premenstrual symptomatology and health-related quality of life. J Reprod Med. (2003) 48:79–85. [PubMed] [Google Scholar]
- 69.Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. American association of clinical endocrinologists and American college of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. (2016) 22 (Suppl. 3):1–203. 10.4158/EP161365.GL [DOI] [PubMed] [Google Scholar]
- 70.Raja-Khan N, Agito K, Shah J, Stetter CM, Gustafson TS, Socolow H, et al. Mindfulness-based stress reduction for overweight/obese women with and without polycystic ovary syndrome: design and methods of a pilot randomized controlled trial. Contemp Clin Trials. (2015) 41:287–97. 10.1016/j.cct.2015.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hettema J, Steele J, Miller WR. Motivational interviewing. Annu Rev Clin Psychol. (2005) 1:91–111. 10.1146/annurev.clinpsy.1.102803.143833 [DOI] [PubMed] [Google Scholar]
- 72.Armstrong MJ, Mottershead TA, Ronksley PE, Sigal RJ, Campbell TS, Hemmelgarn BR. Motivational interviewing to improve weight loss in overweight and/or obese patients: a systematic review and meta-analysis of randomized controlled trials. Obes Rev. (2011) 12:709–23. 10.1111/j.1467-789X.2011.00892.x [DOI] [PubMed] [Google Scholar]
- 73.Rosa AO, Kaster MP, Binfaré RW, Morales S, Martín-Aparicio E, Navarro-Rico ML, et al. Antidepressant-like effect of the novel thiadiazolidinone NP031115 in mice. Prog Neuropsychopharmacol Biol Psychiatry. (2008) 32:1549–56. 10.1016/j.pnpbp.2008.05.020 [DOI] [PubMed] [Google Scholar]
- 74.Sepanjnia K, Modabbernia A, Ashrafi M, Modabbernia MJ, Akhondzadeh S. Pioglitazone adjunctive therapy for moderate-to-severe major depressive disorder: randomized double-blind placebo-controlled trial. Neuropsychopharmacology. (2012) 37:2093–100. 10.1038/npp.2012.58 [DOI] [PMC free article] [PubMed] [Google Scholar]