Abstract
Purposeof Review
The pathogenesis of eosinophilic granulomatosis with polyangiitis (eGPA) is driven largely by CD4 + type 2 helper T cells (Th2), B cells, and eosinophils. Interleukin (IL)-4 and IL-13 are critical cytokines in Th2 cell–mediated inflammation; however, inhibition of IL-4 and IL-13 does not reduce serum eosinophil counts and has even been associated with hypereosinophilia. This review explores the role of IL-4, IL-5, and IL-13 in Th2-mediated inflammation to consider the potential clinical consequences of inhibiting these individual cytokines in eGPA.
Recent Findings
Treatments for eosinophilic granulomatosis with polyangiitis (eGPA) are rapidly evolving through using biologic therapies to modulate the Th2 inflammatory response via eosinophil inhibition. While IL-4, IL-5, IL-13, and IL-25 can all affect eosinophils, only IL-5 inhibition has demonstrated therapeutic benefit to-date. In this review, we report a clinical vignette of a patient with adult-onset asthma who developed severe manifestations of eGPA after switching from mepolizumab (an IL-5 inhibitor) to dupilumab (an inhibitor of IL-4 and IL-13).
Summary
By understanding the role of IL-4, IL-5, and IL-13 in Th2-mediated vasculitis, we can start to understand how eGPA might respond differently to focused cytokine inhibition.
Keywords: Eosinophilic granulomatosis with polyangiitis, IL-4, IL-5, IL-13, Th2 inflammatory response
Introduction
Formerly known as Churg-Strauss syndrome, eosinophilic granulomatosis with polyangiitis (eGPA) is a systemic autoimmune small-to-medium vessel necrotizing vasculitis characterized by granulomatous and eosinophilic inflammation in the setting of peripheral and tissue eosinophilia. Patients with eGPA often progress through three sequential stages of disease [1]. The first stage is characterized by allergic symptoms: allergic rhinitis, sinusitis, and adult-onset asthma. This is followed by the second stage of eosinophilic organ infiltration with involvement of the lungs, heart, and gastrointestinal system. Finally, patients enter the third, vasculitic stage, with palpable purpura, mononeuritis multiplex, and constitutional symptoms.
Many patients with systemic eGPA have positive antineutrophil cytoplasmic antibody (ANCA) serologies, and thus eGPA is considered an ANCA-associated vasculitis (AAV) along with granulomatosis with polyangiitis (GPA) and microscopic polyangiitis (MPA). However, it is estimated that only 40–60% of patients with eGPA are ANCA-positive [1, 2]. Patients who are ANCA-positive may develop more of the vasculitic features of the disease (purpura, neuropathy, pulmonary-renal syndrome) while ANCA-negative patients may experience more eosinophil-driven symptoms (pulmonary infiltrates or cardiomyopathy) [2]. Genome-wide association studies (GWAS) suggest that ANCA-positive and ANCA-negative eGPA are genetically distinct entities. For example, there is a strong association between ANCA-positive eGPA and HLA-DQ alleles, whereas ANCA-negative eGPA is associated with variations at the GPA33 and IL5/IRF1 genetic loci [3•]. In addition, cases of ANCA-negative eGPA with extremely high eosinophil counts often share many features with idiopathic hypereosinophilic syndrome (HES) and are considered by some experts to be a distinct category of “overlap HES” [4]. Taken together, eGPA appears to be an autoimmune syndrome with distinct disease subsets delineated by ANCA status.
The primary driver of disease in eGPA is the CD4 + T helper cell, in particular the type 2 (Th2) subtype. Th2 cells primarily produce type 2 cytokines, specifically interleukin (IL)-4, IL-5, and IL-13. These cytokines influence the behavior of B cells, eosinophils, and mast cells, which are all key components of the inflammatory response in tissues affected by eGPA [5]. Recently, IL-5 has received substantial attention as a therapeutic target in eGPA. This interest stems from the critical roles IL-5 and the IL-5 receptor (IL-5R) play in maintaining eosinophil survival. IL-5 inhibition with mepolizumab has proven effective as a treatment for eGPA [6••]. While dupilumab, an inhibitor of both IL-4 and IL-13, is effective for a variety of severe allergic diseases, its utility for eGPA is unknown and can even cause peripheral eosinophilia in some patients [7–9]. In this article, we review the role of IL-4, IL-5, and IL-13 in Th2-mediated inflammation and eosinophil biology to better understand the potential consequences of inhibiting these cytokines in eGPA.
IL-5 in the Th2 Response
IL-5 is produced by both Th2 cells and group 2 innate lymphoid cells (also known as ILC2 cells) and is further upregulated by IL-25 [10]. IL-5 regulates nearly every aspect of an eosinophil’s life, from priming and chemotaxis to the very survival of the cell itself [11]. Combined with inflammatory signaling, IL-5 will trigger extracellular trap cell death in eosinophils, also known as EETosis [11]. This is a “pro-inflammatory” cellular suicide, in which the eosinophils extrude all cellular contents including at least 200 granules of cytotoxic proteins and cytokines [11]. While the individual eosinophil dies, its cellular contents continue to drive inflammatory changes, which lead to tissue damage [11]. Not only is IL-5 a potent signal in eGPA pathophysiology, it is also very selective. Its receptor is located on eosinophil precursors, eosinophils themselves, mast cells, and basophils, making it an ideal target for biologic therapies in the treatment of eGPA [12].
The Role of Anti-IL-5 Therapies in eGPA
Three monoclonal antibodies, mepolizumab, reslizumab, and benralizumab, work through different mechanisms to prevent IL-5 and IL-5R from binding [13, 14]. Mepolizumab is approved by the United States Food and Drug Administration (FDA) for use in eGPA at a dose of 300 mg every 28 days, higher than the 100-mg dose used for severe asthma. This dosing is based on a randomized controlled trial in which mepolizumab or placebo was added to 136 patients’ treatment regimen for refractory or relapsing eGPA [6••]. The patients who received mepolizumab had more weeks in remission and a higher proportion of patients in remission compared to the placebo group [6••].
Clinical trials of reslizumab and benralizumab for eGPA are ongoing (ClinicalTrials.gov ID NCT02947945 and NCT04157348, respectively). These agents are not yet approved for use in this condition. Though these treatments are promising, it is important to note that monotherapy with anti-IL-5 biologic agents is not recommended for active, severe eGPA [15]. Rather, cyclophosphamide or rituximab is favored for induction therapy in severe cases [15]. The efficacy of these agents highlights that the pathophysiology of eGPA involves numerous interconnected pathways.
IL-4 and IL-13 in the Th2 Response
The roles of IL-4 and IL-13 are closely linked in the Th2 response. Both are produced by multiple cells, specifically CD4 + T cells, basophils, eosinophils, mast cells, and natural killer (NK) T cells, and ILC2 cells. Once expressed, IL-4 and IL-13 bind to multiple sites on either IL-4 receptors (IL-4R) or IL-13 receptors (IL-13R) to activate intracellular signaling [16••].
IL-4 is a critical cytokine in Th2 cell–mediated responses and drives B cell growth and B cell survival (Table 1) [17]. IL-4 binds to the IL-4Rα receptor binding chain to form the IL-4/IL-4Rα complex. Once this primary complex is formed, IL-4/IL-4Rα can then bind the IL-2Rγc (γc) or the IL-13Rα1 receptor binding chain, forming either a type I or type II functional IL-4/receptor complex respectively [16••]. The type I complex (IL-4/IL-4Rα/γc) is found in lymphocytes and myeloid cells while the type II complex (IL-4/IL-4Rα/IL-13Rα1) is present in myeloid cells and all non-hematopoietic cells. Once formed, the type I and type II complexes induce intracellular signaling through the JAK/STAT pathway, specifically through Janus Kinase (JAK) 1 and JAK3 [16••]. Activation of JAK1 and JAK3 leads to phosphorylation of the Signal Transducer and Activator of Transcription 6 (STAT6) [15], which in turn upregulates transcription of GATA-binding protein 3 (GATA3), major histocompatibility complex class II (MHCII), and IgE class switching [10, 18]. IL-4 is also a key regulator of several mast cell functions, including upregulation of the high-affinity receptor for IgE, or FceRI, expression [6••, 9]. By increasing expression of FceRI, IL-4 indirectly promotes the binding of IgE to mast cells, leading to IgE-induced degranulation [6••]. Not only does IL-4 drive key immunologic pathways, but it also stimulates its own positive feedback promoting the differentiation of Th cells specifically Th2 cells while blocking their differentiation into other Th subsets [6••].
Table 1.
| Cytokines | Cellular targets | ||||
|---|---|---|---|---|---|
| ILC2 | Th2 | B cells | Mast cells (MC) | Eosinophils | |
| IL-4 | -Appropriately stimulated ILC2 can produce IL-4 |
-Produced by Th2 cells to drive Th2 inflammatory response -Promote differentiation of Th to Th2 cells -Cellular effects driven through STAT6 signaling |
-Promotes B cell growth and survival -Upregulates MHCII expression -Promotes IgE class switching |
- MC produce IL-4 to further stimulate inflammation -IL-4 promotes expression of FceRI on MC. IgE binding to FceRI leads to degranulation -Promotes proliferation, adhesion, and chemotaxis of mature MC -Promotes apoptosis of immature MC |
-Eosinophils produce IL-4, promoting positive feedback to the Th2 response |
| IL-5 | -ILC2 produce IL-5 in defense against helminths | -Produced by Th2 cells to drive Th2 inflammatory response |
-Maintains B cell survival and proliferation -Promotes IgG and IgM production |
-Subsets of mast cells can produce IL-5 |
Potentially controls eosinophil function and development: -Priming -Chemotaxis -Cell survival -Cell death -EETosis -Degranulation |
| IL-13 | - Appropriately stimulated ILC2 can produce IL-13 | -Produced by Th2 cells to drive Th2 inflammatory response as an effector cytokine (regulates smooth muscle contraction and mucous production) |
-B cells can express IL-13 after binding of CD40 and IL-4 or IL-2 -Potentially acts as a growth factor for IgE-producing B cells |
-Mast cells produce IL-13 if stimulated by DAMPS, IgE, and cytokines |
-Eosinophils produce IL-13, promoting positive feedback to the Th2 response -Upregulates eotaxin production to recruit eosinophils to sites of inflammation -Promotes production of chemokines that can activate eosinophils via CCR3 receptor binding |
| IL-25 | -High levels can stimulate ILC2 to produce IL-4, 5, and 13, which then promote Th differentiation to Th2 cells and Th2-mediated inflammation |
-Produced by Th2 cells to drive Th2 inflammatory response -Stimulates Th2 cells to produce more IL-4, 5, and 13. This drives further differentiation of Th cells to Th2 cells |
-Promotes production of IgG1, IgE, and IgA | -Can promote eosinophil activity in allergic responses |
-Can activate eosinophils in allergic responses -Can recruit eosinophils in allergic asthma through promoting eotaxin production |
IL-13 is capable of binding either the IL-13Rα1 or IL-13Rα2 receptor binding chains. If IL-13 binds to IL-13Rα1, the IL-13/IL-13Rα1 complex will then bind IL-4Rα to form a functional complex that, similar to IL-4, activates STAT6 signaling [16••]. Unlike IL-4, however, IL-13 is an effector cytokine. The down-stream consequences of JAK/STATs signaling through IL-13 cause prominent clinical features of the Th2 response, such as airway smooth muscle contraction and airway mucous production [19]. These are key components of airway hyperresponsiveness (AHR) seen Th2-mediated diseases such as asthma. In patients with asthma, pulmonary expression of IL-13 has been correlated with asthma severity [19]. Murine models also highlight the importance IL-13 plays in AHR as IL-13-deficient (-/-) mice do not develop AHR while IL-4-deficient (-/-) mice still mounted AHR after sensitization and challenge with methacholine [20].
Impact of Dupilumab on Th2-mediated Inflammation and Eosinophil Biology
Dupilumab is a monoclonal antibody that targets the IL-4 receptor alpha chain (IL-4Rα), which is used by the cytokine receptors for both IL-4 and IL-13 [7]. Blocking IL-4 and IL-13 signaling decreases CCL17 and eotaxins, important chemokines for eosinophils, in addition to IgE levels [12]. Thus, dupilumab reduces Th2-mediated inflammation and is approved by the FDA to treat asthma, nasal polyposis, and atopic dermatitis [21].
Despite the utility of dupilumab in multiple diseases with prominent eosinophilia, this treatment does not decrease serum eosinophil counts and has even been associated with hypereosinophilia in rare cases [7–9]. Although the mechanisms underlying this association between dupilumab and elevated eosinophilia are not fully known, studies have shown that the recruitment of eosinophils is not abolished in IL-4- or IL-13-deficient mice [22, 23]. CD4 + T cells from IL-4 -/- mice still produce IL-5 after in vitro stimulation with antigen, suggesting these cytokines are not required for eosinophilic inflammation [22, 23]. Guntur et al. also studied the relationship between IL-4, IL-5, and IL-13 in murine models using IL-13 -/- mice [14]. When IL-13 -/- mice underwent IL-4 neutralization (which would in theory simulate duplimuab’s effect), the mice developed a peripheral eosinophilia [14].
Rarely, patients treated with dupilumab can develop severe complications related to persistent eosinophilic inflammation. In the SINUS-24 and SINUS-52 trials for dupilumab in patients with chronic rhinosinusitis phenotype with nasal polyps (CRSwNP), each trial reported one patient who developed eGPA while on dupilumab [24, 25]. Additionally, there has also been a case report of a patient who developed eosinophilic pneumonia shortly after receiving a dupilumab injection, initially prescribed for severe eosinophilic and allergic asthma [26]. It is not clear whether dupilumab was directly responsible for these cases. No direct causality between dupilumab and the development of eGPA or other eosinophilic diseases has been established. The degree of overlap between mild eGPA, mild asthma, and CRSwNP is such that patients are often diagnosed with asthma up to 9 years before receiving an eGPA diagnosis [27]. It may be possible that the patients who were diagnosed with eGPA during SINUS-24 and SINUS-52 trials may have been initially misclassified prior to the trial.
IL-4 and IL-13 clearly hold numerous important roles in Th2-mediated diseases. Though IL-4 and IL-13 inhibition decreases Th2-mediated inflammation, eosinophils seem to elude this blockade’s control. Below, we present a clinical vignette of a patient with asthma and CRSwNP polyposis but no prior symptoms of vasculitis who developed life-threatening features of ANCA-negative eGPA after switching from mepolizumab to dupilumab.
Clinical Vignette
A 63-year-old man presented in for management of severe persistent asthma and nasal polyposis. Despite treatment with beclomethasone inhaler (80 mcg 2 puffs twice daily), albuterol (90 mcg per actuation as needed) and mepolizumab (100 mg subcutaneously every 28 days), he was unable to decrease his prednisone dose below 6 mg per day. He recently suffered an asthma exacerbation on this regimen, his first since initiating mepolizumab four years ago. Given his active disease and presence of nasal polyposis, he was advised to switch mepolizumab to dupilumab with a loading dose of 400 mg followed by 200 mg subcutaneously every 15 days.
Seven months later, the patient presented to an urgent care with 10 days of worsening sinus congestion, sinus drainage, and a cough. He was SARS-CoV-2 negative; his physical exam was unremarkable; and a chest X-ray did not reveal an acute cardiopulmonary process. Lab testing showed a WBC of 9.6 × 109/L and an elevated eosinophil count of 0.7 × 109/L (7.3% of his total WBC). Review of prior records demonstrated normal eosinophil counts (0.18 × 109/L) prior to initiation of mepolizumab several years ago, as well as normal eosinophil counts after 3 years on mepolizumab (0.05 × 109/L).
Over the next several months, the patient was treated with multiple courses of antibiotics and prednisone tapers for recurrent sinus and upper respiratory symptoms. In addition, his dupilumab was increased to 300-mg subcutaneous injections every 15 days. Despite these treatments, his symptoms continued to recur whenever his prednisone was tapered back to 6 mg daily.
He then developed body aches, weakness, severe fatigue, and a feeling like “death warmed over.” He also noted a dark rash that began on both his shins and subsequently spread up to the thighs. He presented to an urgent care with hypotension of 65/41 mmHg, heart rate of 99 beats per minute, respiratory rate of 22 breaths per minute, oxygen saturation of 96% on room air, and a temperature of 36.9 °C. He was subsequently admitted to a tertiary medical center for emergent evaluation.
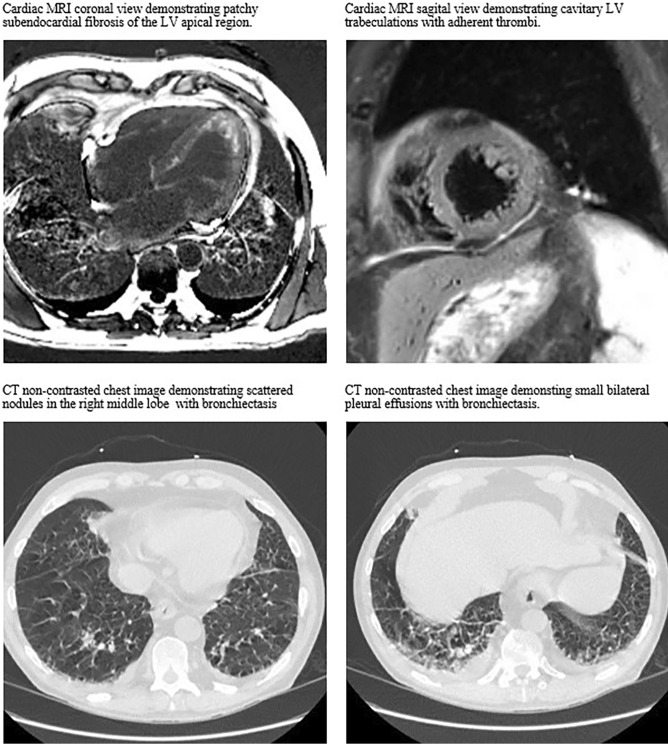
On admission, his physical exam was remarkable for purpura on his feet, knees, and thighs bilaterally. Lab work revealed an elevated troponin, inflammatory markers, leukocytosis with eosinophilia, and mild creatinine elevation with pyuria (Table 2). CT chest showed clustered pulmonary nodules in the right middle lobe and small bilateral pleural effusions (Fig. 1). An electrocardiogram showed lateral ST segment depressions, and echocardiography revealed a hyper-trabeculated left ventricular (LV) apex, raising concern for eosinophilic myocarditis. Cardiac MRI confirmed the presence of patchy subendocardial fibrosis of the LV apical region, including the wall itself and cavitary trabeculation in the apex, consistent with eosinophilic endomyocardial fibrosis (Fig. 1). The presence of LV apical thrombi and thrombi adherent to the trabeculations were also identified, as well as subendocardial scarring in multiple coronary artery territories. This finding was concerning for coronary embolic phenomenon from the LV thrombus, though his ejection fraction remained normal at 66%.
Table 2.
Admission laboratory testing and hypereosinophilia evaluation patient results
| Admission laboratory testing | Patient test result | Test reference range | |
|---|---|---|---|
| High-sensitivity troponin (ng/L) | 597 | 24–36 | |
| Pro-brain natriuretic peptide, N-terminal (NT-Pro-BNP) (pg/mL) | 3885 | < 175 | |
| Total creatine kinase (CK) (U/L) | 140 | 30–220 | |
| Inflammatory markers | |||
| C-reactive protein (mg/dL) | 7.95 | < 0.6 | |
| Erythrocyte sedimentation rate (mm/h) | 38 | < 20 | |
| Complete blood count with differential | |||
| White blood cell count (× 109/L) | 21.6 | 3.2–9.8 | |
| Hemoglobin (g/dL) | 13.3 | 3.7–17.3 | |
| Hematocrit (%) | 42.1 | 39–49 | |
| Platelet (× 109/L) | 189 | 150–450 | |
| Neutrophil count (× 109/L) | 7.6 | 2.0–8.6 | |
| Lymphocyte count (× 109/L | 1.2 | 0.6–4.2 | |
| Monocyte count (× 109/L) | 0.9 | 0–0.9 | |
| Eosinophil count (× 109/L) | 11.76 | 0–0.70 | |
| Basophil count (× 109/L) | 0.12 | 0–0.20 | |
| Urinalysis with microscopy | |||
| Protein | Negative | Negative | |
| Blood | 2 + | Negative | |
| Red blood cell count [per high powered field (hpf)] | 21 | < 3 | |
| White blood cell count (per hpf) | 9 | < 5 | |
| RBC Casts (per hpf) | 0 | 0 | |
| Blood chemistries | |||
| BUN (mg/dL) | 14 | 7–20 | |
| Creatinine (mg/dL) | 1.1 | 0.6–1.3 | |
| GFR (mL/min/1.73 m2) | 71 | > 65 | |
| AST (U/L) | 42 | 15–41 | |
| ALT (U/L) | 19 | 17–63 | |
| Lactate (mmol/L) | 1.4 | 0.6–2.2 | |
| Thyroid function panel | |||
| TSH (µL/mL) | 6.64 | 0.34–5.66 | |
| Free T4 (ng/dL) | 0.66 | 0.52–1.21 | |
| Viral PCR | |||
| COVID-19 | Negative | Negative | |
| Flu A/B | Negative | Negative | |
| Hypereosinophilia evaluation | Patient result | ||
| Flow cytometry |
Eosinophilia with normal immunophenotype (44%) Negative for increased blasts Negative for monoclonal B cells No phenotypically abnormal T cell population |
||
| Chromosome analysis |
46 X, Y Male karyotype. No clonal abnormality detected |
||
| Antineutrophil cytoplasmic antibodies | Negative | ||
| Rheumatoid factor | Negative | ||
| Antinuclear antibodies | Negative | ||
| Cyclic citrullinated peptide antibodies | Negative | ||
| Cryoglobulin | Negative | ||
| Complement levels | |||
| C3 | 131 mg/dL (reference range: 81–157 mg/dL) | ||
| C4 | 25 mg/dL (reference range: 13–39 mg/dL) | ||
| IgE level | 9 (reference range: 4–269 IU/mL) | ||
| BCR/ABL1 PCR | Negative | ||
| JAK2 V617F | Negative | ||
| T cell receptor gene rearrangement panel | Negative | ||
| Myeloid NGS panel | Negative | ||
| FIP1L1-PDGFRA | Negative | ||
| Tryptase | 7.2 ng/mL (reference range: < 11.5 ng/mL) | ||
| Vitamin B-12 | 267 pg/mL (reference range: 123–730 pg/mL) | ||
| Stool cultures | Negative | ||
| Giardia antigen | Negative | ||
| Cryptosporidia antigen | Negative | ||
| HIV antibody screen | Negative | ||
Fig. 1.
Cardiopulmonary radiographic findings
The serologic workup for a secondary eosinophilia and vasculitis was unrevealing: ANCA and cryoglobulin screen were all negative (Table 2). Complement and IgE levels were normal. Infectious workup with stool cultures, HIV antibody, giardia antigen, and cryptosporidia antigen was also negative. Peripheral blood smear was unremarkable, but bone marrow biopsy revealed markedly increased eosinophils in the bone marrow (41%) without increased blasts or significant dysplasia. Flow cytometry and chromosome analysis were normal. Oncogenic mutations such as BCR/ABL1 PCR, JAK2 V617F, T cell receptor gene rearrangement panel, myeloid NGS panel, and FIP1L1-PDGFRA were negative. Tryptase and vitamin B-12 levels were normal.
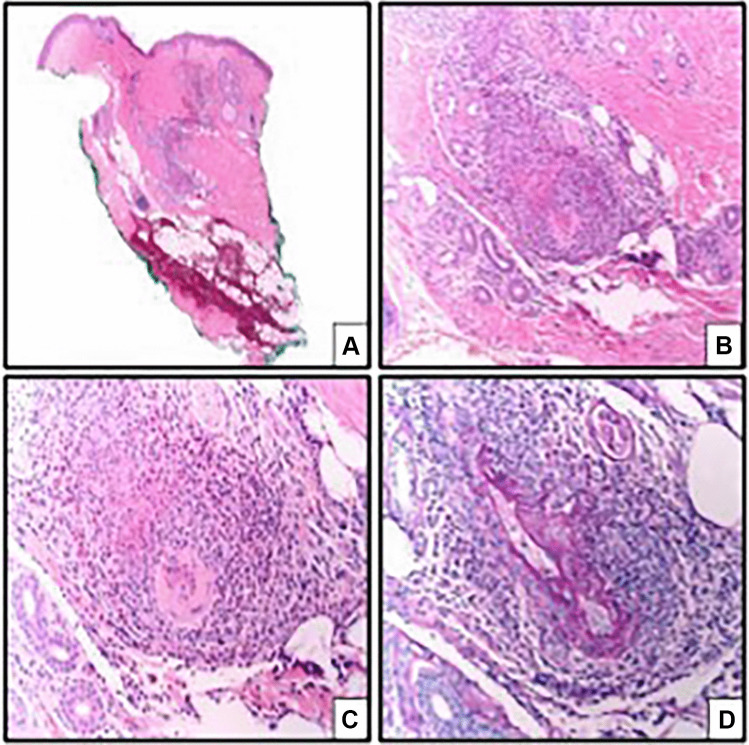
A skin biopsy of his lower extremity lesions demonstrated a small-vessel vasculitis with an eosinophilic infiltrate (Fig. 2). Thus, the patient was diagnosed with ANCA-negative eosinophilic granulomatosis with polyangiitis (eGPA) with overlapping features of idiopathic hypereosinophilic syndrome (HES). He was treated with 3 days of intravenous methylprednisolone 1000 mg daily, followed by a gradual oral prednisone taper. His eosinophil count normalized within 24 h of beginning steroids. Cyclophosphamide 0.75 g/m2 monthly infusions were initiated during this admission, and he was transitioned to daily azathioprine (2 mg/kg) and monthly subcutaneous mepolizumab 300 mg for maintenance therapy without relapse. Both this patient’s asthma and vasculitic manifestations of eGPA remain in remission on his current therapies of azathioprine, mepolizumab, and a gradual steroid taper scheduled to end in early 2022.
Fig. 2.
Cutaneous lesion biopsy result
Conclusions
Despite inhibiting two major drivers of the Th2 response, IL-4 and IL-13, the patient in the vignette still developed severe manifestations of a Th2-mediated disease. EGPA is a complicated disease process involving complex interactions within the Th2 inflammatory response. Targeting cytokines such as IL-5 are leading to new therapies used in refractory and relapsing disease. However, it may be possible that certain cytokines should not be inhibited in eGPA as eosinophilia levels can be increased with combined IL-4 and IL-13 blockade. For this reason, it is important to recognize cases that are highly suspicious for eGPA as the treatments for refractory asthma alone or in the setting of eGPA can be vastly different, as evidenced by the clinical vignette presented in this review. Further investigation is needed to determine whether IL-4 and IL-13 blockade is involved in triggering eosinophil activity or merely permits uncontrolled disease in eGPA.
Author Contribution
All authors contributed significantly to the manuscript text, figures, tables, and/or editing process.
Availability of Data and Material
Availability of data is not applicable. All figures and tables are original to this manuscript and have not been previously published.
Code Availability
Not applicable.
Compliance with Ethical Standards
Consent for Publication
The patient described in the case report provided informed consent for publication. All identifying information has been removed.
Conflict of Interest
The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Immunotherapy and Immunomodulators
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Gioffredi A, Maritati F, Oliva E, Buzio C. Eosinophilic granulomatosis with polyangiitis: an overview. Front Immuno Published Online. 2014;5:549. doi: 10.3389/fimmu.2014.00549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fagni F, Bello F, Emmi G. Eosinophilic granulomatosis: dissecting the pathophysiology. Front Med. 2021 doi: 10.3389/fmed.2021.627776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.• Lyons PA, Peters JE, Alberici F, Liley J, Coulson RMR, et al. Genome-wide association study of eosinophilic granulomatosis with polyangiitis reveals genomic loci stratified by ANCA status. Nat Commun. 2019;10(1):5120. 10.1038/s41467-019-12515-9. COMMENT: Study showing genetic differences in ANCA-positive and ANCA-negative eGPA. [DOI] [PMC free article] [PubMed]
- 4.Klion AD. How I treat hypereosinophilic syndromes. Blood. 2015;126(9):1069–1077. doi: 10.1182/blood-2014-11-551614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaglio A, Buzio C, Zwerina J. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): state of the art. Allergy. 2013;68:261–273. doi: 10.1111/all.12088. [DOI] [PubMed] [Google Scholar]
- 6.•• Wechsler ME, Akuthota P, Jayne D, Khoury P, Kilon CA, et al. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017;376(20):1921–1932. COMMENT: Study that led to FDA approval for mepolizumab use in eGPA. [DOI] [PMC free article] [PubMed]
- 7.Wenzel S, Ford L, Pearlman D, Spector S, Sher L, Skobieranda F, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med. 2013;368:2455–2466. doi: 10.1056/nejmoa1304048. [DOI] [PubMed] [Google Scholar]
- 8.Eger K, Pet L, Weersink EJ, Bel EH. Complications of switching from anti-IL-5 or anti-IL-5R to dupilumab in corticosteroid-dependent severe asthma. J Allergy Clin Immunol Pract. 2021;9(7):2913–2915. doi: 10.1016/j.jaip.2021.02.042. [DOI] [PubMed] [Google Scholar]
- 9.Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496. doi: 10.1056/NEJMoa1804092. [DOI] [PubMed] [Google Scholar]
- 10.Isozaki T, Homma T, Sagara H, Kasama T. Role of cytokines in eGPA and the possibility of treatment with an anti-IL-5 antibody. J Clin Med. 2020;9:3890. doi: 10.3390/jcm9123890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagase H, Ueki S, Fujieda S. The roles of IL-5 and anti-IL-5 treatment in eosinophilic diseases: asthma, eosinophilic granulomatosis with polyangiitis, and eosinophilic chronic rhinosinusitis. Allergol Int. 2020;69:178–186. doi: 10.1016/j.alit.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Radonjic-Hoseli S, Valent P, Kilon AD, Wechsler ME, Simon H. Novel targeted therapies for eosinophilic-associated diseases and allergy. Annu Rev Pharmacol Toxicol. 2015;55:633–656. doi: 10.1146/annurev-pharmtox-010814-124407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faverio P, Bonaiti G, Bini F, Vaghi A, Pesci A. Mepolizumab as the first targeted treatment for eosinophilic granulomatosis with polyangiitis: a review of current evidence and potential place in therapy. Ther Clin Risk Manag. 2018;14:2385–2396. doi: 10.2147/tcrm.s159949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guntur VP, Manka LA, Denson JL, Dunn RM, Dollin YT, Gill M, Kolakowski C, Strand MJ, Wechsler ME. Benralizumab as a steroid-sparing treatment option in eosinophilic granulomatosis with polyangiitis. J Allergy Clin Immunol Pract. 2021;9(3):1186–1193.e1. doi: 10.1016/j.medcli.2021.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Chung SA, Langford CA, Maz M, Abril A, Gorelik M, Guyatt G, et al. American college of rheumatology/vasculitis foundation guideline for the management of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 2021;-:1–18. doi: 10.1002/art.41773. [DOI] [PubMed] [Google Scholar]
- 16.Junttila IS. Tuning the cytokine responses: an update on interleukin (IL)-4 and IL-13 receptor complexes. Front Immunol. 2018 doi: 10.3389/fimmu.2018.00888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiaojing M. (ed). Regulation of cytokine gene expression in immunity and diseases. In: Advances in experimental medicine and biology. 1st ed. Springer Dordrecht; 2016; p. 941. ISBN: 978–94–024–0919–2. 10.1007/978-94-024-0921-5
- 18.Toru H, Ra C, Nonoyama S, Suzuki K, Yata J, Nakahata T. Induction of the high-affinity IgE receptor (FceRI) on human mast cells by IL-4. Int Immunol. 1996;8(9):1367–1373. doi: 10.1093/intimm/8.9.1367. [DOI] [PubMed] [Google Scholar]
- 19.May RD, Fung M. Strategies targeting the IL-4/IL-13 axes in disease. Cytokine. 2015;75(1):89–116. doi: 10.1016/j.cyto.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 20.Walter DM, McIntire JJ, Berry G, McKenzie ANJ, Donaldson DD, et al. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol. 2001;167(8):4668–4675. doi: 10.4049/jimmunol.167.8.4668. [DOI] [PubMed] [Google Scholar]
- 21.Highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761055s020lbl.pdf. Accessed 10 July 2021.
- 22.Hogan SP, Mould A, Kikutani H, Ramsay AJ, Foster PS. Aeroallergen-induced eosinophilic inflammation, lung damage, and airways hyperreactivity in mice can occur independently of IL-4 and allergen-specific immunoglobulins. J Clin Invest. 1997;99(6):1329–1339. doi: 10.1172/jci119292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Webb DC, McKenzie AN, Koskinen AM, Yang M, Mattes J, Foster PS. Integrated signals between IL-13, IL-4, and IL-5 regulate airways hyperreactivity. J Immunol. 2000;165(1):108–113. doi: 10.4049/jimmunol.165.1.108. [DOI] [PubMed] [Google Scholar]
- 24.Kariyawasam HH, James LK, Gane SB. Dupilumab: clinical efficacy of blocking IL-4/IL-13 signalling in chronic rhinosinusitis with nasal polyps. Drug Des Devel Ther. 2020;14:1757–1769. doi: 10.2147/DDDT.S243053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bachert C, Han JK, Desrosiers M, Hellings PW, Amin N, Lee SE, Mullol J, Greos LS, Bosso JV, Laidlaw TM, Cervin AU, Maspero JF, Hopkins C, Olze H, Canonica GW, Paggiaro P, Cho SH, Fokkens WJ, Fujieda S, Zhang M, Lu X, Fan C, Draikiwicz S, Kamat SA, Khan A, Pirozzi G, Patel N, Graham NMH, Ruddy M, Staudinger H, Weinreich D, Stahl N, Yancopoulos GD, Mannent LP. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet. 2019;394(10209):1638–1650. doi: 10.1016/s0140-6736(19)31881-1. [DOI] [PubMed] [Google Scholar]
- 26.Menzella F, Montanari G, Patricelli G, Cavazza A, Galeone C, Ruggiero P, Bagnasco D, Facciolongo N. A case of chronic eosinophilic pneumonia in a patient treated with dupilumab. Ther Clin Risk Manag. 2019;15:869–875. doi: 10.2147/tcrm.s207402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furuta S, Iwamoto T, Nakajima H. Update on eosinophilic granulomatosis with polyangiitis. Allergol Int. 2019;68(4):430–436. doi: 10.1016/j.alit.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Moon BG, Takaki S, Miyake K, Takatsu K. The role of IL-5 for mature B-1 cells in homeostatic proliferation, cell survival, and Ig production. J Immunol. 2004;172(10):6020–6029. doi: 10.4049/jimmunol.172.10.6020. [DOI] [PubMed] [Google Scholar]
- 29.Roufosse F. Targeting the interleukin-5 pathway for treatment of eosinophilic conditions other than asthma. Front Med (Lausanne) 2018;5:49. doi: 10.3389/fmed.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hajoui O, Janani R, Tulic M, Joubert P, Ronis T, et al. Synthesis of IL-13 by human B lymphocytes: regulation and role in IgE production. J Allergy Clin Immunol. 2004;114(3):657–663. doi: 10.1016/j.jaci.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 31.McLeod JJ, Baker B, Ryan JJ. Mast cell production and response to IL-4 and IL-13. Cytokine. 2015;75(1):57–61. doi: 10.1016/j.cyto.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Angkasekwinai P, Sodthawon W, Jeerawattanawart S, Hansakon A, Pattanapanyasat K, et al. ILC2s activated by IL-25 promote antigen-specific Th2 and Th9 functions that contribute to the control of Trichinella spiralis infection. PLoS ONE. 2017;12(9):e0184684. doi: 10.1371/journal.pone.0184684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu D, Cao T, Wang N, Liu C, Ma N, et al. IL-25 attenuates rheumatoid arthritis through suppression of Th17 immune responses in an IL-13-dependent manner. Sci Rep. 2016;6:36002. doi: 10.1038/srep36002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Y, Shao Z, Shangguan G, Bie Q, Zhang B. Biological properties and the role of IL-25 in disease pathogenesis. J Immunol Res. 2018;2018:6519465. doi: 10.1155/2018/6519465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valizadeh A, Khosravi A, Zadeh LJ, Parizad EG. Role of IL-25 in immunity. J Clin Diagn Res. 2015;9(4):OE01–OE4. doi: 10.7860/jcdr/2015/12235.5814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doran E, Cai F, Holweg CTJ, Wong K, Brumm J, et al. Interleukin-13 in asthma and other eosinophilic disorders. Front Med (Lausanne) 2017;4:139. doi: 10.3389/fmed.2017.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Availability of data is not applicable. All figures and tables are original to this manuscript and have not been previously published.
Not applicable.