Abstract
We have characterized an open reading frame of 2,454 bp on chromosome I of Schizosaccharomyces pombe as the gene encoding trehalose-6P phosphatase (tpp1+). Disruption of tpp1+ caused in vivo accumulation of trehalose-6P upon heat shock and prevented cell growth at 37 to 40°C. Accumulation of trehalose-6P in cells bearing a chromosomal disruption of the tpp1+ gene and containing a plasmid with tpp1+ under the control of the thiamine-repressible promotor correlated with tpp1+ repression. The level of tpp1+ mRNA rose upon heat shock, osmostress, or oxidative stress and was negatively controlled by cyclic AMP-dependent protein kinase activity. Expression of tpp1+ during oxidative or osmotic stress, but not during heat shock, was under positive control by the wis1-sty1 (equivalent to phh1 and spc1) mitogen-activated protein kinase pathway. Analysis of Tpp1 protein levels suggests that the synthesis of trehalose-6P phosphatase may also be subjected to translational or posttranslational control.
In yeast cells, trehalose functions as a carbohydrate reserve and also as a stress metabolite (16, 32, 34). Biosynthesis and mobilization of this nonreducing disaccharide are under multiple controls and are exquisitely regulated (13, 18, 29, 30). Trehalose accumulation requires only two enzymes. In a first step, trehalose-6P synthase (TPS1) transfers a glucosyl residue from UDP-glucose to glucose-6P to yield trehalose-6P, which is subsequently dephosphorylated by trehalose-6P phosphatase (TPS2) to trehalose (5). In Saccharomyces cerevisiae, the genes involved in trehalose synthesis have been cloned and sequenced (1, 9, 33). Purification of the corresponding enzymes has revealed that trehalose is synthesized by a large bifunctional complex which includes two catalytic activities, trehalose-6P synthase and trehalose-6P phosphatase, closely associated with the regulatory subunits TSL1 and TPS3 (2, 9, 19). Disruption of TPS1 (also called CIF1, FDP1, TSS1, and GGS1), which codes for the smallest (56-kDa) polypeptide of the trehalose synthase-phosphatase complex, causes not only absence of trehalose accumulation but also inability of the yeast cells to grow on glucose and other rapidly fermentable sugars. Hence, TPS1 seems to catalyze the formation of trehalose-6P and to play an important role in the regulation of sugar metabolism in S. cerevisiae (1, 14). Disruption of TPS2, encoding a larger (100-kDa) component of the trehalose synthase complex, causes accumulation of the phosphorylated intermediate trehalose-6P and renders cells permanently unable to grow at 40°C (9).
While much work has been done on trehalose synthesis in S. cerevisiae, less is known about the process of trehalose accumulation in other yeast species (22). In this context, evidence indicates that disruption of tpp1+ in the fission yeast Schizosaccharomyces pombe, which is homologous to the S. cerevisiae TPS1 gene, does not affect growth in glucose but prevents spore germination (4). These findings suggest that the trehalose pathway may have different roles in the two species. In contrast to the situation in S. cerevisiae (31), there is no information on the enzyme which splits trehalose-6P into trehalose and Pi in the fission yeast. In this work we have identified a gene (tpp1+) encoding a trehalose-6P phosphatase which is involved in trehalose biosynthesis in S. pombe. Also, we have characterized the phenotype associated with tpp1+ disruption and determined the main regulatory pathways that control the expression of this gene at the mRNA and protein levels under various stress conditions.
MATERIALS AND METHODS
Strains and culture media.
The S. pombe strains employed in this study are listed in Table 1. They were routinely grown with shaking at 28°C in YES (21) or EMM2 minimal medium with or without thiamine (5 mg/liter) (7). Culture media were supplemented with adenine, leucine, histidine, or uracil (100 mg/liter; Sigma Chemical Co.), depending on the requirements of each strain. Solid media were made by the addition of 2% (wt/vol) Bacto Agar (Difco Laboratories, Detroit, Mich.). Transformation of strains was performed by the lithium acetate method as described elsewhere (7). Escherichia coli DH5αF′ was employed as a host to propagate plasmids. It was grown at 37°C in Luria-Bertani medium plus 50 μg of ampicillin/ml.
TABLE 1.
S. pombe strains used in this study
| Strain | Genotype | Reference or source |
|---|---|---|
| JY742 | h+ade6-M216 leu1-32 ura4-D18 | 7 |
| P698 | h90ura4-D18 | A. Duran |
| MMP-3 | h+ade6-M216 leu1-32 ura4-D18 Δtpp1::ura4+ | This study |
| MMP-5 | h90ura4-D18 Δtpp1::ura4+ | This study |
| MMPI-3 | h+ade6-M216 leu1-32 ura4-D18 tpp1HA (ura4+) | This study |
| TK107 | h−ura4D-18 leu1-32 Δphh1::ura4+ | T. Kato |
| CHP429 | h−ura4D-18 leu1-32 ade6-M216 his7-366 | C. S. Hoffman |
| CHP433 | h−ura4D-18 leu1-32 his7-366 Δpka1::ura4+ | 17 |
| CHP458 | h−ura4D-18 leu1-32 his7-366 Δpka1::ura4+ Δsck1::his7+ | 17 |
Cloning and disruption of tpp1+.
The S. pombe sequence that codes for a putative trehalose-6P phosphatase was amplified by PCR using DNA from cosmid clone CRFc60GT219 as a template (obtained from the Resource Center/Primary Database of the German Human Genome Project, Max-Planck Institut für Molekulare Genetik, Berlin-Charlottenburg, Germany) and the 5′ oligonucleotide TPP5 (CAGTGTCGACGAAGAAGTTGCCAATAG), incorporating a SalI site, and the 3′ oligonucleotide TPP3 (CCACCCGGGTCGGGCATCTTCGTTGAA), incorporating a SmaI site (both restriction sites are italicized). The resulting 2.5-kbp product was cloned into the pREP3X expression vector (20) as a SalI-SmaI fragment and sequenced. The tpp1+ gene was interrupted by the ura4+ gene as follows. Plasmid pREP3X-tpp1 was digested with NotI and BglII, releasing an internal fragment of 1.4 kbp from the cloned sequence, which was then replaced with an S. pombe 1.8-kbp NotI-BglII fragment containing the ura4+ gene (15), giving rise to plasmid pMTM-10. This plasmid was digested with SalI and SmaI, releasing a 2.9-kbp fragment that was gel purified and transformed into the haploid strain JY742 and the homothallic strain P698. Stable ura+ transformants were screened for tpp1+ disruption by Southern blot hybridization.
Determination of neutral trehalose activity, trehalose, and trehalose-6P.
Neutral trehalose activity was assayed after cell breakage as described previously (7). Trehalose and trehalose-6P were extracted from the cells as indicated previously (3), and the extract was heated at 100°C for 10 min in the presence of 0.1 M NaOH and neutralized with HCl. The resulting preparation was deproteinized by phenol treatment and resolved by thin-layer chromatography (TLC) analysis on silica gel 60 plates (Merck, Darmstadt, Germany) using butanol-ethanol-water (5:3:2 [vol/vol/vol]) as a solvent (35). Authentic trehalose-6P, trehalose, and glucose were run in parallel as a control. Sugar spots were visualized by charring them at 95°C after spraying them with 25% H2SO4. For quantification, the areas matching trehalose-6P spots in duplicate plates were scraped, dissolved in methanol-water (1:1 [vol/vol]), and identified by mass spectrometry in the electronic-impact mode in a Finnigan (Manchester, United Kingdom) Trace MS 2000 by comparison with authentic trehalose-6P (Sigma Chemical Co.). The samples were directly inserted, a 70-eV potential of ionization was applied, and the temperature range for sample evaporation was 50 to 350°C at 50°C/min. The source temperature was 200°C. In both cases, the appearance of fragments at m/zs of 73, 85, 97, 127, 145, 163, 235, and 325 was observed, the last being characteristic of trehalose-6P and absent in the mass spectrum of nonphosphorylated trehalose. A quantitative estimation was performed of the amounts of trehalose-6P in cell extracts of mutant and wild-type cells after heat shock based on the relative abundance of the m/z 325 fragment.
Northern and Southern blot hybridization.
Wild-type and disruption mutant strains were grown in YES medium and subjected to different stresses. Total mRNAs were then extracted, transferred to nylon membranes, and hybridized to tpp1+ and leu1+ (internal control) 32P-labeled probes as previously described (7, 26). Southern blot hybridization was performed under high-strigency conditions, employing the tpp1+ open reading frame (ORF) as a digoxigenin-labeled probe (7).
Construction of a Tpp1-HA-tagged strain.
A 5′-truncated version of tpp1+ was amplified by PCR using DNA from the pREP3X-tpp1+ plasmid as a template and the 5′ oligonucleotide TPP5F (TCAGCTGCTGTTCTCGAGTCCTTG, which hybridizes at positions 846 to 869 in the tpp1+ ORF and shows an internal XhoI site) together with the 3′ oligonucleotide TPP3F (GATGCGGCCGCGGTTAGTAAAATTTGCCA, which hybridizes at positions 2436 to 2456 in the tpp1+ ORF and incorporates a NotI site placed immediately upstream of the stop codon). The restriction sites in both oligonucleotides are italicized. The resulting 1.6-kbp product was then double digested with XhoI and NotI and cloned into plasmid pSFL172 (12), creating plasmid pSFL172-F, which expresses a truncated version of Tpp1p fused to a triple hemagglutinin (HA) tag at its C terminus (Tpp1pHA) under the regulation of the strong thiamine promoter (Pnmt1). A version of this plasmid without the promoter (pSFL172-I) was constructed and digested at the unique BglII site within tpp1+, and the linear fragment was transformed into the haploid strain JY742 to target integration at the tpp1+ locus. Uracil prototrophs were selected, and identification of strains with one copy of tpp1-HA expressed from the genomic tpp1+ promoter was performed by immunoblotting whole-cell extracts with anti-HA antibody and by Southern blot analysis.
SDS-PAGE and Western immunoblotting.
Total-cell homogenates were prepared under native conditions, and 20 μg of protein was resolved in each case by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as previously described (21). The gels were transferred to nitrocellulose filters (Amersham-Pharmacia) and incubated with anti-HA (clone 12CA5; Roche Molecular Biochemicals) or anti-α-tubulin (loading control; Sigma Chemical Co.) antibodies. The immunoreactive bands were revealed with a mouse horseradish peroxidase-conjugated secondary antibody (Sigma Chemical Co.) and the ECL system (Amersham-Pharmacia).
Nucleotide sequence accession number.
The nucleotide sequence of the tpp1+ gene has been submitted to the EMBL database under accession no. AJ242743.
RESULTS AND DISCUSSION
Isolation and disruption of the trehalose-6P phosphatase gene in S. pombe.
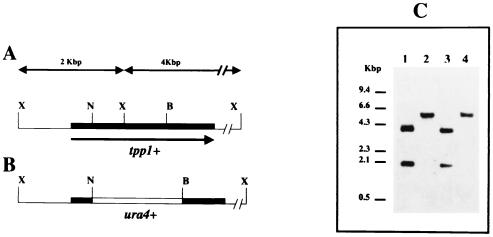
In an attempt to identify new genes related to trehalose metabolism in S. pombe, a putative trehalose-6P phosphatase gene was identified in clone ICRFc60G1219 from the S. pombe sequencing project. The ORF was 2,454 bp long, mapped in chromosome I, and presumably coded for a protein containing 813 amino acids, showing 36% identity with the trehalose-6P phosphatase protein from S. cerevisiae (TPS2). This sequence was amplified by PCR using DNA from the corresponding cosmid clone as a template and the oligonucleotides TPP5 and TPP3 (see Materials and Methods), cloned into the pREP3X expression vector (20), and sequenced to confirm identity with the sequence of the cosmid clone. To verify that the cloned sequence represented the coding region of a trehalose-6P phosphatase gene, it was interrupted by the ura4+ gene and integrated via homologous recombination into h+ and h90 ura4-D18 strains JY742 and P698, respectively (Fig. 1A and B). Disruption of the phosphatase gene was not expected to be lethal, since disruption of other members of the trehalose biosynthetic pathway (tpp1+) does not have a noticeable effect on the growth of vegetative cells of the fission yeast (4). Congruent with this, prototrophs for uracil were recovered at high frequency from each recipient strain. Effective disruption of the putative trehalose-6P phosphatase gene was verified by PCR and Southern blot hybridization using the analyzed ORF as a probe (Fig. 1C).
FIG. 1.
Disruption of the tpp1+ gene from S. pombe. (A) Restriction map of the genomic region containing the tpp1+ gene. The solid bar and the arrow below indicate the tpp1+ ORF. (B) Construct used for the disruption of the tpp1+ gene. An internal NotI-BamHI fragment of the tpp1+ gene was replaced by the ura4+ cassette. The restriction sites X, N, and B correspond to XhoI, NotI, and BamHI sites, respectively. (C) Southern hybridization analysis of XhoI-digested genomic DNA from strains JY742 (h+ tpp1+; lane 1) and P698 (h90 tpp1+; lane 3) and transformants MMP-3 (h+ tpp1::ura4+; lane 2) and MMP-5 (h90 Δtpp1::ura4+; lane 4), with the complete tpp1+ ORF as a digoxigenin-labeled probe.
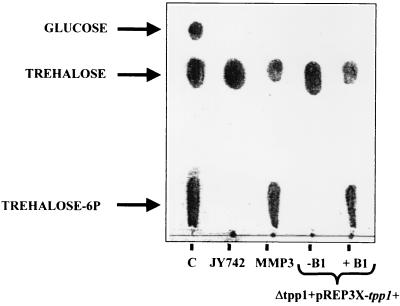
The intracellular concentration of the intermediate trehalose-6P in heat-shocked exponential cells of S. pombe is about 2 orders of magnitude lower than for the trehalose pool (4). Thus, if the isolated sequence coded for an enzyme able to dephosphorylate trehalose-6P, a strain disrupted in such a gene would then hyperaccumulate trehalose-6P instead of (or in addition to) trehalose after mild heat stress. To investigate this, cultures from the wild-type and disruption strains were heat shocked to trigger trehalose-6P synthase activity as previously described (28), and intracellular disaccharides were then extracted and resolved by TLC on silica gel 60 plates (35) (Fig. 2). Indeed, following heat shock, wild-type cells showed negligible content of trehalose-6P and accumulated significant amounts of trehalose. However, all the disruption strains tested showed high yields of trehalose-6P in addition to trehalose (Fig. 2). Hence, the gene disrupted in S. pombe appears to code for a main trehalose-6P phosphatase, although additional nonspecific phosphatase activities are likely able to dephosphorylate part of the increased trehalose-6P pool, apparently with lower affinity. We have called this gene tpp1+ (for trehalose-6P phosphatase of pombe). A quantitative estimation of the amount of trehalose-6P in extracts of mutant and wild-type cells after heat shock was performed by mass spectrometry in the electronic-impact mode. The results showed a 10-fold increase in trehalose-6P in tpp1-disrupted cells compared to that in wild-type cells (10.5 versus 1.1 mg/g [wet weight] [mean of two independent determinations]).
FIG. 2.
Analysis of disaccharides present in wild-type and trehalose-6P phosphatase disruption mutants of S. pombe. Cells were grown to mid-log phase in YES medium (control strain JY742 and Δtpp1 strain MMP3) and in EMM2 minimal medium strain Δtpp1+pREP3X-tpp1+ with or without thiamine (±B1). After heat shock (40°C for 90 min), trehalose and trehalose-6P were extracted and resolved by TLC analysis on silica gel 60 plates. Authentic trehalose-6P, trehalose, and glucose were run in parallel as a control (lane C). Corresponding areas in duplicate plates were analyzed by mass spectrometry, as described in the text.
To demonstrate further that the accumulation of trehalose-6P in Δtpp1 strains was due to loss of tpp1+ function, a leu1-32 Δtpp1 disrupted strain was transformed with plasmid pREP3X-tpp1+, which expresses the gene under the control of the strong thiamine-repressible promoter (20). Leucine prototrophs were isolated, and the trehalose and trehalose-6P contents in either thiamine-repressed (i.e., no Tpp1 activity) or thiamine-derepressed (i.e., full Tpp1 activity) strains were analyzed by TLC. As indicated in Fig. 2, trehalose and trehalose-6P accumulated in the presence of thiamine, while only trehalose was detected chromatographically in cells growing in the absence of thiamine. Therefore, tpp1+ codes for a protein whose activity is able to dephosphorylate trehalose-6P in vivo.
The predicted amino acid sequence of Tpp1p is 813 amino acids long (97.3 kDa) and exhibited the highest identities with S. cerevisiae trehalose-6P phosphatase (TPS2; 36%), an S. pombe putative trehalose-6P synthase (35%), and an Arabidopsis thaliana putative trehalose-6P synthase (31%). A search for potential phosphorylation sites in the Tpp1 sequence showed the presence of a cyclic AMP phosphorylation site at position 819 (-KKLS-), although there is at present no evidence that Tpp1p activation and deactivation are mediated by phosphorylation in yeasts (31).
Properties of tpp1+-disrupted cells.
Several studies have shown that the loss of trehalose-6P synthase activity in S. pombe greatly reduces tolerance of heat stress and severely compromises the ability of spores to germinate (4, 7, 23). We reasoned that a limited trehalose availability in Δtpp1 cells might make these cells functionally similar to Δtps1 cells in some respects. Disruption of tpp1+ did not have any noticeable effect on the growth rates of strains growing at 25 to 30°C in either rich (YES) or minimal (EMM-2) medium, which were found to be close to those shown by control strains. Under these growth conditions, the level of trehalose-6P is almost undetectable in normal and tpp1+-disrupted cells (reference 4 and our results). However, after a shift to 37°C (YES agar medium) or 40°C (YES liquid medium), which enhances trehalose synthesis in wild-type cells and triggers trehalose-6P accumulation in tpp1+-disrupted cells, the former were able to resume growth, while the latter ceased cell proliferation (data not shown). This finding indicates that tpp1+ function is indispensable for growth at high temperatures. Interestingly, disruption of TPS2 in S. cerevisiae also results in temperature-sensitive growth, suggesting that accumulation of high levels of the metabolic intermediate trehalose-6P may be inhibitory to growth or promote a shortage of free intracellular Pi (9).
Consistent with the presence in Δtpp1 strains of at least one additional phosphatase activity able to partially dephosphorylate the trehalose-6P pool, purified spores from strain MMP-5 (h90 Δtpp1::ura4 ura4D-18) were able to germinate with the same efficiency as spores from the control strain P698 (h90 ura4D-18) (data not shown). In the absence of such “extra” phosphatase activity, Δtpp1 spores should be phenotypically similar to Δtps1 spores (4), i.e., unable to mobilize trehalose and therefore unable to germinate, since neutral trehalase of S. pombe does not recognize trehalose-6P as a substrate (data not shown). Besides, all the known routes for neutral trehalase activation previously described in S. pombe (8, 10, 24, 25, 27) were shown to be fully functional in Δtpp1 strains, which is clearly different from Δtps1 strains, where neutral trehalase is activated only by osmostress (6).
Different pathways regulate transcription of tpp1+ and the corresponding protein levels under stress.
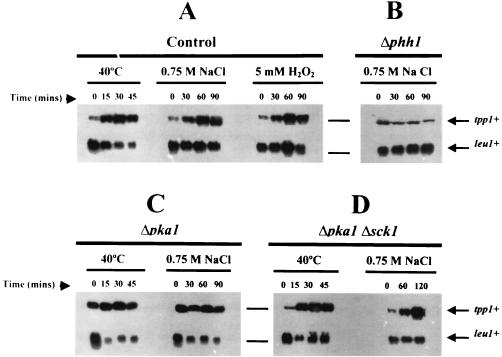
The expression of tpp1+ under various adverse environmental conditions was analyzed by Northern blot hybridization experiments. The corresponding mRNA migrates as a single band of about 2.5 kb, which coincides with the size of the tpp1+ ORF. Similar to other enzymes involved in the synthesis and breakdown of trehalose, the level of tpp1+ mRNA increases rapidly during heat shock and more slowly during osmotic and oxidative stress (Fig. 3A), suggesting the existence of at least two different and conserved regulatory pathways (26). In this context, the absence of increase in tpp1+ expression in Δphh1 cells during osmostress (and also during oxidative stress but not during heat shock [Fig. 3B]) demonstrates that the wis1-sty1 (equivalent to phh1 and spc1) stress-activated mitogen-activated protein kinase cascade regulates this pathway.
FIG. 3.
Analysis of tpp1+ expression in S. pombe. (A) Increase in the level of expression of tpp1+ mRNA following heat, osmotic, or oxidative stress in control strain CHP429. (B) Evidence for regulation of the expression of tpp1+ by the wis1-sty1 (equivalent to phh1 and spc1) mitogen-activated protein kinase pathway during osmotic stress using strain TK107 (Δphh1::ura4+). (C) Analysis of Δpka1 strain CHP433 indicates that Pka1 activity negatively modulates the basal level and the expression of tpp1+ during heat and osmotic shock. (D) Expression of tpp1+ in a Δpka1 Δsck1 strain suggests that derepression of tpp1+ in the absence of Pka1 is dependent on the cellular level of Sck1 activity.
In addition, basal expression (zero time) of tpp1+ mRNA in cells growing in rich medium appears to be negatively controlled by cyclic AMP-dependent protein kinase (Pka1) function (Fig. 3C). Interestingly, this result is similar to those shown previously for tps1+, which codes for trehalose-6P synthase, and for ntp1+, which codes for neutral trehalase (reference 11 and unpublished results). Thus, basal expression of the genes involved in either synthesis or degradation of trehalose appears to be repressed by Pka1 activity. However, in a double disruptant for Pka1 and Sck1 (a protein kinase that supresses some defects of Δpka1 mutants and regulates neutral-trehalase activation under different conditions) (17, 25), tpp1+ expression recovered the wild-type basal levels (Fig. 3D). This suggests that Sck1 function is somewhat responsible for the derepressing effect observed in Pka1-deficient cells. In this context, it is worth mentioning that in the double-mutant ntp1+, expression follows the same pattern as in tpp1+ (not shown), which might be consistent with a model in which these protein kinases regulate transcription of enzymes involved in trehalose metabolism.
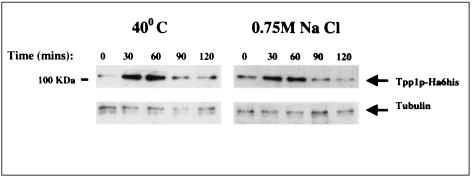
Finally, to test whether the above-described increase in mRNA expression of tpp1+ upon thermal, osmotic, or oxidative stress correlated with a similar increase in Tpp1p protein levels, we constructed a strain carrying a chromosomal HA-tagged version of tpp1+. One of these strains, MMPI-3, was subjected to either thermal shock or osmostress, and the level of Tpp1p-HA fusion was estimated by Western blot analysis employing anti-HA antibody. As shown in Fig. 4, Tpp1HA protein migrates as a single 100-kDa band, almost identical to the 97.3-kDa mass calculated on the basis of its amino acid sequence (see above). Moreover, a clear rise in Tpp1pHA synthesis was observed when the Tpp1pHA-tagged strain was subjected to thermal or osmotic stress. However, unlike the results obtained from Northern blot experiments concerning tpp1+ mRNA (Fig. 3), Tpp1pHA synthesis reached a maximum 30 to 60 min after the treatment, decreased rapidly, and was independent of the type of stress applied. This implies the existence of a control of Tpp1p expression at the translational or posttranslational level during heat and osmotic stresses. How the transcriptional and translational machinery coordinates Tpp1p synthesis with Tpp1p activity during the S. pombe life cycle and what the interaction of Tpp1p with other members of the trehalose synthesis and breakdown pathways are unresolved questions which deserve further investigation.
FIG. 4.
Tpp1pHA protein levels following thermal or osmotic stress. The strain MMPI-3 was grown in YES medium to mid-log phase and harvested before or after heat (40°C) or osmotic (0.75 M NaCl) stress for the indicated times. The SDS-PAGE gels were transferred to nitrocellulose filters and incubated with anti-HA or anti-α-tubulin (loading control) antibodies.
ACKNOWLEDGMENTS
A. F. and T. S. contributed equally to this work.
We thank F. Garro for expert technical assistance. We are indebted to C. S. Hoffman, S. L. Forsburg, T. Kato, and A. Duran for kind provision of plasmids and strains.
A. F. is a predoctoral fellow of PFPI from the University of Murcia. This work was supported in part by grant PB97-1049 from DGES, Spain.
REFERENCES
- 1.Bell W, Klaassen P, Ohnacker M, Boller T, Herweiger M, Schoppink P, Van der Zee P, Wiemken A. Characterization of the 56-kDa subunit of yeast trehalose-6-phosphate synthase and cloning of its gene reveal its identity with the product of CIF1, a regulator of carbon catabolite inactivation. Eur J Biochem. 1992;209:951–959. doi: 10.1111/j.1432-1033.1992.tb17368.x. [DOI] [PubMed] [Google Scholar]
- 2.Bell W, Sun W, Hohmann S, Wera S, Reinders A, De Virgilio C, Wiemken A, Thevelein J M. Composition and functional analysis of the Saccharomyces cerevisiae trehalose synthase complex. J Biol Chem. 1998;273:33311–33319. doi: 10.1074/jbc.273.50.33311. [DOI] [PubMed] [Google Scholar]
- 3.Blazquez M A, Lagunas R, Gancedo C, Gancedo J M. Trehalose-6-phosphate, a new regulator of yeast glycolysis that inhibits hexokinases. FEBS Lett. 1993;329:51–54. doi: 10.1016/0014-5793(93)80191-v. [DOI] [PubMed] [Google Scholar]
- 4.Blazquez M A, Stucka R, Feldmann H, Gancedo C. Trehalose-6-P synthase is dispensable for growth on glucose but not for spore germination in Schizosaccharomyces pombe. J Bacteriol. 1994;176:3895–3902. doi: 10.1128/jb.176.13.3895-3902.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabib E, Leloir F. The biosynthesis of trehalose phosphate. J Biol Chem. 1958;231:259–275. [PubMed] [Google Scholar]
- 6.Cansado J, Vicente-Soler J, Soto T, Fernandez J, Gacto M. Trehalose-6P synthase is essential for trehalase activation triggered by glucose, nitrogen source or heat shock, but not by osmostress, in Schizosaccharomyces pombe. Biochim Biophys Acta. 1998;1381:271–278. doi: 10.1016/s0304-4165(98)00039-7. [DOI] [PubMed] [Google Scholar]
- 7.Cansado J, Soto T, Fernandez J, Vicente-Soler J, Gacto M. Characterization of mutants devoid of neutral trehalase activity in the fission yeast Schizosaccharomyces pombe: partial protection from heat shock and high-salt stress. J Bacteriol. 1998;180:1342–1345. doi: 10.1128/jb.180.5.1342-1345.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrillo D, Vicente-Soler J, Gacto M. Cyclic AMP signalling pathway and trehalase activation in the fission yeast Schizosaccharomyces pombe. Microbiology. 1994;140:1467–1472. doi: 10.1099/00221287-140-6-1467. [DOI] [PubMed] [Google Scholar]
- 9.De Virgilio C, Burckert N, Bell W, Jena P, Boller T, Wiemken A. Disruption of TPS2, the gene encoding the 100-kDa subunit of the trehalose-6-phosphate synthase/phosphatase complex in Saccharomyces cerevisiae, causes accumulation of trehalose-6-phosphate and loss of trehalose-6-phosphate phosphatase activity. Eur J Biochem. 1993;212:315–323. doi: 10.1111/j.1432-1033.1993.tb17664.x. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez J, Soto T, Vicente-Soler J, Cansado J, Gacto M. Osmo-stress-induced changes in neutral trehalase activity of the fission yeast Schizosaccharomyces pombe. Biochim Biophys Acta. 1997;1357:41–48. doi: 10.1016/s0167-4889(97)00010-4. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez J, Soto T, Vicente-Soler J, Cansado J, Gacto M. Heat-shock response in Schizosaccharomyces pombe cells lacking cyclic AMP-dependent phosphorylation. Curr Genet. 1997;31:112–118. doi: 10.1007/s002940050183. [DOI] [PubMed] [Google Scholar]
- 12.Forsburg S L, Sherman D A. General purpose tagging vectors for fission yeast. Gene. 1997;191:191–195. doi: 10.1016/s0378-1119(97)00058-9. [DOI] [PubMed] [Google Scholar]
- 13.François J, Neves M J, Hers H G. The control of trehalose biosynthesis in Saccharomyces cerevisiae: evidence for a catabolite inactivation and repression of trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase. Yeast. 1991;7:575–587. doi: 10.1002/yea.320070605. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez M I, Stucka R, Blazquez M A, Feldmann H, Gancedo C. Molecular cloning of CIF1, a yeast gene necessary for growth on glucose. Yeast. 1992;8:183–192. doi: 10.1002/yea.320080304. [DOI] [PubMed] [Google Scholar]
- 15.Grimm C, Kohli J. Observations on integrative transformation in Schizosaccharomyces pombe. Mol Gen Genet. 1988;215:87–93. doi: 10.1007/BF00331308. [DOI] [PubMed] [Google Scholar]
- 16.Hottiger T, De Virgilio C, Hall M N, Boller T, Wiemken A. The role of trehalose synthesis for the acquisition of thermotolerance in yeast. II. Physiological concentrations of trehalose increase the thermal stability of protein in vitro. Eur J Biochem. 1994;219:187–193. doi: 10.1111/j.1432-1033.1994.tb19929.x. [DOI] [PubMed] [Google Scholar]
- 17.Jin M, Fujita M, Culley B M, Apolinario E, Yamamoto M, Maundrell K, Hoffman C S. Sck1, a high copy number suppressor of defects in the cAMP-dependent protein kinase pathway in fission yeast, encodes a protein homologous to the Saccharomyces cerevisiae SCH9 kinase. Genetics. 1995;140:457–467. doi: 10.1093/genetics/140.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jorge J A, Polizeli M L T M, Thevelein J M, Terenzi H F. Trehalases and trehalose hydrolysis in fungi. FEMS Microbiol Lett. 1999;154:165–171. doi: 10.1111/j.1574-6968.1997.tb12639.x. [DOI] [PubMed] [Google Scholar]
- 19.Londesborough J, Vuorio O. Trehalose-6-phosphate synthase/phosphatase complex from baker's yeast: purification of a proteolytically activated form. J Gen Microbiol. 1991;137:323–330. doi: 10.1099/00221287-137-2-323. [DOI] [PubMed] [Google Scholar]
- 20.Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993;123:127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- 21.Moreno S, Klar A, Nurse P. Molecular genetic analysis of the fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 22.Reinders A, Romano I, Wiemken A, De Virgilio C. The thermophilic yeast Hansenula polymorpha does not require trehalose synthesis for growth at high temperatures but does for normal acquisition of thermotolerance. J Bacteriol. 1999;181:4665–4668. doi: 10.1128/jb.181.15.4665-4668.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribeiro M J S, Reinders A, Boller T, Wiemken A, De Virgilio C. Trehalose synthesis is important for the acquisition of thermotolerance in Schizosaccharomyces pombe. Mol Microbiol. 1997;25:571–581. doi: 10.1046/j.1365-2958.1997.4961856.x. [DOI] [PubMed] [Google Scholar]
- 24.Soto T, Fernandez J, Cansado J, Vicente-Soler J, Gacto M. Glucose-induced, cyclic-AMP-independent signalling pathway for activation of neutral trehalase in the fission yeast Schizosaccharomyces pombe. Microbiology. 1995;141:2665–2671. [Google Scholar]
- 25.Soto T, Fernandez J, Cansado J, Vicente-Soler J, Gacto M. Protein kinase Sck1 is involved in trehalase activation by glucose and nitrogen source in the fission yeast Schizosaccharomyces pombe. Microbiology. 1997;143:2457–2463. doi: 10.1099/00221287-143-7-2457. [DOI] [PubMed] [Google Scholar]
- 26.Soto T, Fernandez J, Dominguez A, Vicente-Soler J, Cansado J, Gacto M. Analysis of the ntp1+ gene, encoding neutral trehalase in the fission yeast Schizosaccharomyces pombe. Biochim Biophys Acta. 1998;1443:225–229. doi: 10.1016/s0167-4781(98)00214-0. [DOI] [PubMed] [Google Scholar]
- 27.Soto T, Fernandez J, Vicente-Soler J, Cansado J, Gacto M. Nitrogen-source-induced activation of neutral trehalase in Schizosaccharomyces pombe and Pachysolen tannophilus: role of cAMP as second messenger. FEMS Microbiol Lett. 1995;132:229–232. doi: 10.1016/0378-1097(95)00269-b. [DOI] [PubMed] [Google Scholar]
- 28.Soto T, Fernandez J, Vicente-Soler J, Cansado J, Gacto M. Accumulation of trehalose by overexpression of tps1, coding for trehalose-6-phosphate synthase, causes increased resistance to multiple stresses in the fission yeast Schizosaccharomyces pombe. Appl Environ Microbiol. 1999;65:2020–2024. doi: 10.1128/aem.65.5.2020-2024.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thevelein J M. Regulation of trehalose mobilization in fungi. Microbiol Rev. 1984;48:42–59. doi: 10.1128/mr.48.1.42-59.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thevelein J M, Hohmann S. Trehalose synthase: guard to the gate of glycolysis in yeast? Trends Biochem Sci. 1995;20:3–10. doi: 10.1016/s0968-0004(00)88938-0. [DOI] [PubMed] [Google Scholar]
- 31.Vandercammen A, François J, Hers H G. Characterization of trehalose-6-phosphate synthase and trehalose-6-phosphate phosphatase of Saccharomyces cerevisiae. Eur J Biochem. 1989;182:613–620. doi: 10.1111/j.1432-1033.1989.tb14870.x. [DOI] [PubMed] [Google Scholar]
- 32.Van Laere A. Trehalose, reserve and/or stress metabolite? FEMS Microbiol Rev. 1989;63:201–210. [Google Scholar]
- 33.Vuorio O E, Kalkkinen N, Londesborough J. Cloning of two related genes encoding the 56-kDa and 123-kDa subunits of trehalose synthase from the yeast Saccharomyces cerevisiae. Eur J Biochem. 1993;216:849–861. doi: 10.1111/j.1432-1033.1993.tb18207.x. [DOI] [PubMed] [Google Scholar]
- 34.Wiemken A. Trehalose in yeast, stress protectant rather than reserve carbohydrate. Antonie Leeuwenhoek. 1990;58:209–217. doi: 10.1007/BF00548935. [DOI] [PubMed] [Google Scholar]
- 35.Zähringer H M, Burgert M, Holzer H, Nwaka S. Neutral trehalase Nth1p of Saccharomyces cerevisiae encoded by the NTH1 gene is a multiple stress responsive protein. FEBS Lett. 1997;412:615–620. doi: 10.1016/s0014-5793(97)00868-5. [DOI] [PubMed] [Google Scholar]