Abstract
The current COVID-19 pandemic raises several clinical challenges. Cases of COVID-19-associated arthritis have been reported, and inconsistently described as either COVID-19 viral arthritis or COVID-19 reactive arthritis. We aimed to review all the reported cases of ‘COVID-19-associated arthritis’, which we propose, is a better term to define the entire spectrum of new-onset arthritis believed to be associated with SARS-CoV-2 infection. We performed a systematic literature review using MEDLINE, EMBASE and the Cochrane Database of Systematic Reviews to search for articles published up to 13 December 2021. We included cohort studies, case series and case reports describing patients diagnosed with COVID-19 reactive or viral arthritis by a physician, irrespective of fulfilment of classification criteria. To identify relevant studies, medical subject headings and keywords related to ‘COVID-19/SARS-CoV-2 infection’ and ‘reactive arthritis’ were used. Our search retrieved 419 articles, of which 31 were included in the review. A total of 33 cases were reported in these 31 articles, the majority being adults (28/33=85%) with peripheral joint involvement (26/33=79%). Most of the patients responded well to treatment and the disease was self-limiting. These 33 case reports describe a possible causal relationship between exposure to SARS-CoV-2 and the onset of arthritis. However, since these cases were reported during a pandemic, other aetiologies cannot be fully excluded. The exact mechanism through which SARS-CoV-2 might trigger arthritis is not fully understood and robust epidemiological data to support a causal relationship are still lacking.
Keywords: COVID-19; arthritis, reactive; arthritis, infectious
Key messages.
WHAT IS ALREADY KNOWN ON THIS TOPIC
Cases of SARS-CoV-2-associated arthritis have been reported, and inconsistently described as cases of either viral arthritis or reactive arthritis.
WHAT THIS STUDY ADDS
We performed a systematic literature review of COVID-19-associated arthritis and comprehensively describe 33 case reports, gathering all the information about this potential new entity in one single article.
We propose a unified designation: ‘COVID-19-associated arthritis’—in which SARS-CoV-2 infection has been proven, there is a plausible temporal relation between COVID-19 and the onset of arthritis, and the arthritis cannot be justified by other aetiologies.
Nonetheless, causality between SARS-CoV-2 infection and the onset of arthritis still needs to be proven, as robust mechanistic and epidemiological data are still lacking.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE AND, OR POLICY
COVID-19-associated arthritis is a potential new disease entity, in which varied joint involvement has been reported, generally with a self-limiting course and good response to treatment (non-steroid anti-inflammatory drugs and glucocorticoids).
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a novel coronavirus that was first identified in Wuhan, central China, and is responsible for the 2019–2022 pandemic. Globally, there have been 512 million confirmed cases of Coronavirus disease 2019 (COVID-19), the disease caused by SARS-CoV-2, including >6 million deaths, reported to World Health Organization (WHO) up to 4 May 2022.1
Coronaviruses belong to the family of ribonucleic acid (RNA) viruses, and they are characterised by the presence of crown-like spikes on their surface. Hundreds of coronaviruses circulate among animals (eg, cats, pigs, camels and bats) but cross-species virus transmission (‘spill over events’) can occur when the exposure to other species increases and the natural barriers to infection of new hosts are overcome.2 Seven coronaviruses are known to infect humans, including SARS-CoV-2 (table 1).3
Table 1.
Coronaviruses known to infect humans
| Severity of the disease | HCoV | Epidemiological history |
| Mild-to-moderate upper respiratory tract illnesses, like the common cold | HCoV-229E | Most common causes of respiratory tract infection throughout the world. |
| HCoV-HKU1 | ||
| HCoV-NL63 | ||
| HCoV-OC43 | ||
| Highly pathogenic and deadly HCoV | SARS-CoV-1 | First emerged in November 2002, in Foshan, China, and no human cases have been reported since May 2004. |
| MERS-CoV | First emerged in April 2012, in Zarqa, Jordan, and has been causing periodical endemics mainly in the Middle East regions. | |
| SARS-CoV-2 | First emerged in December 2019, in Wuhan, China, and is an ongoing pandemic. |
HCoV, human coronavirus; MERS-CoV, Middle East respiratory syndrome coronavirus; SARS-CoV-1, Severe Acute Respiratory Syndrome coronavirus 1; SARS-CoV-2, Severe Acute Respiratory Syndrome coronavirus 2.
Organ dysfunction caused by SARS-CoV-2 can be a consequence of cytotoxic damage (direct injury) and immunological insult (indirect damage) to host cells. Pneumonia and acute respiratory distress syndrome are the major complications of COVID-19. Other COVID-19 complications may include acute liver, cardiac and kidney injury, as well as secondary infection, immunothrombosis and hyperinflammatory response.4
As with other respiratory tract infections, musculoskeletal symptoms can develop with coronavirus infections. Arthralgia is reported in 15% of patients with COVID-19, and myalgia is even more common (44%). However, musculoskeletal symptoms do not seem to be related to COVID-19 severity.5
The current COVID-19 pandemic raises several intriguing questions, as well as clinical challenges in the context of rheumatic and musculoskeletal diseases. Cases of ‘SARS-CoV-2-associated arthritis’ have been reported, and inconsistently described as cases of either viral arthritis or reactive arthritis. This distinction is sometimes difficult, and classically the term reactive arthritis has been used to describe an immune-mediated sterile arthritis (typically monoarticular or oligoarticular, often involving the lower extremities, and sometimes associated with dactylitis and enthesitis) occurring several days to weeks after bacterial infection of the gastrointestinal or genitourinary tract, in genetically predisposed individuals; while the term viral arthritis has been used to describe the (often symmetric and polyarticular) articular manifestations of viral infections, potentially triggered by a variety of mechanisms, including direct invasion of the joint cells or tissues, immune complex formation and direct or indirect immune dysregulation due to persistent/latent viral infection, sometimes in the context of an upper respiratory tract infection. Microorganisms that have been associated with reactive and viral arthritis are listed in table 2.6
Table 2.
Microorganisms associated with reactive arthritis and viral arthritis
| Reactive arthritis | Viral arthritis |
| A. Definite causes of classical reactive arthritis | A. Enterovirus infections |
| A1. Gastrointestinal pathogens |
|
|
|
|
B. Hepatitis viruses |
|
|
|
|
|
|
| A2. Genitourinary pathogens | C. Parvovirus B19 |
|
D. Rubella and rubella vaccine virus |
|
E. Alphaviruses |
| A3. Respiratory pathogen |
|
|
|
| B. Probable and possible causes of reactive arthritis |
|
|
|
|
|
|
|
|
F. Flavivirus |
|
|
|
|
|
G. Mumps virus |
|
H. Adenovirus |
|
I. Herpesvirus |
|
|
|
|
|
|
|
|
| J. HIV infection |
To date, there is no agreement regarding viral or reactive arthritis diagnostic or classification criteria. The diagnosis is essentially clinical, based on a careful history and physical examination.7 In this article, we aimed to review all the reported cases of ‘COVID-19-associated arthritis’, which we propose, is a better term to define the entire spectrum of new-onset arthritis believed to be associated with SARS-CoV-2 infection.
Methods
Protocol and search strategy
The systematic literature review (SLR) was conducted and reported according to the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines.8
The literature search was performed in MEDLINE, EMBASE and the Cochrane Database of Systematic Reviews (CENTRAL) for articles published up to 13 December 2021. We selected published articles in English, Portuguese, Turkish, Spanish or French. To identify the relevant studies, medical subject headings and keywords related to ‘COVID-19/SARS-CoV-2 infection’ and ‘reactive arthritis’ were used. The search strategies used to identify relevant studies are provided in online supplemental table 1.
rmdopen-2021-002026supp001.pdf (74.4KB, pdf)
The American College of Rheumatology and European Alliance of Associations for Rheumatology (EULAR) meeting abstracts from the last 2 years (2020–2021) were searched and only included if they had not already been published as original studies. Reference lists of all relevant studies retrieved from the electronic search were manually searched to identify additional potentially eligible studies.
The following study designs were allowed: cohort studies, case series and case reports; to gather information about the clinical, biological and demographic features of these patients.
EndNote V.20 was used to manage the references obtained from the search results of each of the databases. The protocol was written and defined before starting the search and no deviations were performed during the process.
Study selection
Two reviewers (ASP and BF) independently screened the titles and abstracts of the retrieved articles applying predefined inclusion criterion: patients diagnosed clinically with COVID-19 reactive or viral arthritis by a physician, irrespective of fulfilment of published classification criteria for these conditions. We excluded articles with incomplete information, non-human studies, study population/individuals with pre-existing chronic inflammatory rheumatic diseases or in which another rheumatic disease was subsequently diagnosed and could be the cause of the new-onset arthritis, cases of arthritis associated with vaccination against SARS-CoV-2, and other types of studies not specified above (eg, reviews without original data and editorials).
Titles, abstracts and full texts were screened for inclusion by two independent authors (ASP and BF). Any discrepancies were discussed until a consensus was reached or with the involvement of a third reviewer (PMM) whenever necessary.
Data extraction
Data were collected from each article in a data collection sheet and included: author(s), country(ies) of origin of the data, year of publication, study design, number of patients, demographic features (age, gender), full details about SARS-CoV-2 infection diagnosis and symptoms, tests used to confirm the diagnosis, imaging investigations and medications. Other data included: time of presentation of arthritis, time between SARS-CoV-2 infection/COVID-19 onset and articular symptoms, site and number of joints involved and joint aspiration results. Serological tests were included if they were performed, as well as immunological tests including rheumatoid factor and anticitrullinated protein antibodies, and other laboratory tests such as acute phase reactants and full blood count results. The chronicity of the manifestations was recorded.
Data synthesis
Data were summarised by stratifying the results into two groups: COVID-19-associated arthritis with onset up to 1 week after the initial COVID-19 manifestations, and COVID-19-associated arthritis with onset >1 week after the initial COVID-19 manifestations. The 1-week cut-off was arbitrarily defined, in order to distinguish early onset cases from medium-onset/late-onset cases, as they may have different underlying pathophysiological mechanisms.
Results
General results
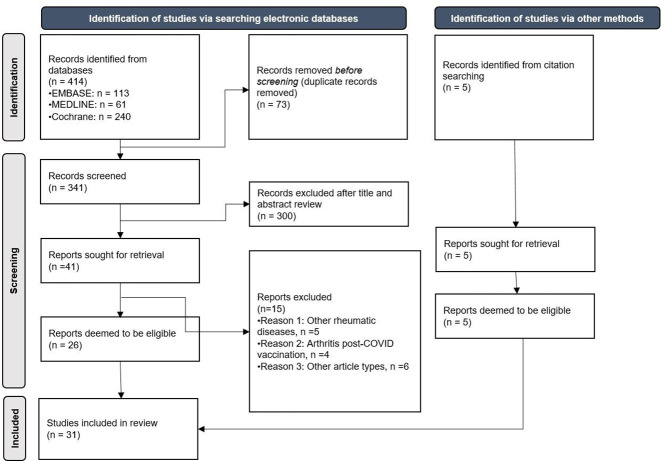
Our search retrieved 419 articles (414 identified via searching electronic databases and 5 identified from citation searching). Of these, we excluded duplicates within and across databases, and a total of 346 articles were assessed (341 from electronic databases and 5 from hand search). After review of the titles and abstracts, 46 articles were retrieved for full-text evaluation, of which 31 were included in the review (26 from electronic databases and 5 from hand search). A total of 33 cases were reported in these 31 articles. Figure 1 depicts the flow of information through the different phases of the SLR. The 15 articles excluded and the reasons for exclusion are shown in online supplemental table 2.
Figure 1.
Flow of information through the different phases of the systematic literature review.
COVID-19-associated arthritis with onset up to 1 week after COVID-19
A total of six articles reported seven cases of onset of arthritis up to 1 week after COVID-19 diagnosis (table 3). Of these, three cases occurred in children9 10 and four in adults.11–14 Onset of arthritis was 1 week before COVID-19 symptoms in one case,14 concomitant in one case11 and within the first week of onset of COVID-19 symptoms in the remaining five cases.9 10 12 13 All the cases reported had a confirmation of the infection by nasopharyngeal and/or oropharyngeal swab by real-time-polymerase chain reaction (RT-PCR), except one, in which infection was confirmed by a positive IgM against SARS-CoV-2 detected by enzyme linked immunosorbent assay (ELISA).10 Only one case was Human Leukocyte Antigen (HLA)-B27 positive.12
Table 3.
COVID-19-associated arthritis with onset up to 1 week after COVID-19 initial manifestations
| Study, country | N, gender, age (years) | COVID-19 symptoms | Time between onset of COVID-19 and onset of articular symptoms (SARS-CoV-2 diagnostic test) | Type of arthritis (and EA involvement) | Blood tests (included immunology) | Serologies | Arthrocentesis | Treatment for COVID-19 | Treatment for arthritis | Last assessment and chronicity |
| Alivernini et al11, Italy | 1, male, 61 | Cough, mild dyspnoea, severe asthenia and anorexia | Concomitant (RT-PCR) | Polyarthritis | RF and ACPA negative | NA | Absence of calcium pyrophosphate or monosodium urate crystals | Lopinavir-ritonavir and HCQ 400 mg/day | Etoricoxib 200 mg/day, baricitinib 4 mg/day, PDN 10 mg/day | Symptoms resolved (DAS28-CRP: 2.8 after 8 days) |
| Apaydin et al, Turkey | 1, male, 37 | Watery diarrhoea and dry cough | 1 week (nasopharyngeal and oropharyngeal swab RT-PCR) | Polyarthritis (knees, wrists, ankles, elbows and MTP joints) | Normal UA; RF, ASO, ANCA, ACPA, ANA and ENA negative; HLA-B27 positive |
HBV, HCV, HIV, EBV, HSV type 1 and 2, parvovirus B19, rubella, CMV, toxoplasma, brucellosis, syphilis and gonorrhoea negative | 617 leucocytes/mm3, with negative Gram staining and bacterial cultures | NA | HCQ 400 mg/day and MPD 16 mg/day; SSZ 2 g/day | Chronic symptoms (arthralgia) |
| Houshmand et al9, Iran | 1, male, 10 | Fever, urticaria | 2 days (nasopharyngeal and oropharyngeal swab RT-PCR) | Knees and right elbow | RF, ANA negative | Urine analysis was normal; stool examinations for ova and parasites and occult blood were normal | Dry tap without any joint fluid | Acetaminophen, cetirizine 10 mg/day, desloratadine 5 mg/day, hydroxyzine 10 mg/day | Not used | No chronicity |
| Liew et al13, Singapore | 1, male, 47 | Low-grade fever | 3 days (nasopharyngeal and oropharyngeal swab RT-PCR) | Knee and balanitis | NA | HIV, syphilis, chlamydia and gonorrhoea testing were negative | Turbid yellow fluid, without crystals; synovial Gram stain, gonococcal, bacteria cultures and gonococcal and chlamydia PCR were negative; synovial fluid PCR and viral cultures for SARS CoV-2 were also negative | NA | Etoricoxib and intra-articular triamcinolone (knee joint) | Remission |
| Sinaei et al10, Iran | 1, male, 8 | Low-grade fever and cough | 1 week (COVID-19 IgM indirect ELISA) | Hip | NA | UA normal, ANA negative; RF positive | NA | NA | Skin traction and naproxen 500 mg/day | No chronicity |
| 1, female, 6 | High-grade fever | 1 week (nasopharyngeal and oropharyngeal swab RT-PCR) | Hips | NA | UA normal, RF and ANA negative | NA | NA | Ibuprofen 40 mg/kg/day in three doses/day | Complete recovery after 4 days | |
| Talarico et al14, Italy | 1, male, 45 | Anosmia, dysgeusia and myalgia without any respiratory symptoms | 1 week before other COVID-19 symptoms (nasopharyngeal and oropharyngeal swab RT-PCR) | Symmetric polyarthritis (MCP, PIP) | ACPA positive; RF negative | NA | NA | No specific treatment | Medium doses of MPD | Complete remission |
ACPA, anticitrullinated protein antibodies; ANA, antinuclear antibodies; ANCA, antineutrophil cytoplasmic antibodies; ASO, antistreptolysin O; CMV, cytomegalovirus; CRP, C reactive protein; DAS28, Disease Activity Score 28 joints; EA, extra-articular; EBV, Epstein-Barr virus; ENA, extractable nuclear antigen; HBV, hepatitis B virus; HCQ, hydroxychloroquine; HCV, hepatitis C virus; HLA, human leucocyte antigen; HSV, herpes simplex virus; MCP, metacarpophalangeal; MPD, methylprednisolone; MTP, metatarsophalangeal; NA, not available; PDN, prednisolone; PIP, proximal interphalangeal; RF, rheumatoid factor; RT-PCR, real-time PCR; SSZ, sulfasalazine; UA, uric acid.
Polyarthritis was observed in three cases,11 12 14 oligoarthritis in two cases9 10 and monarthritis in two cases.10 13 In the report by Liew et al,13 balanitis (a typical extra-articular manifestation of classical reactive arthritis) was observed in association with knee monarthritis.
While non-steroid anti-inflammatory drugs (NSAIDs) were used in two paediatric patients,10 no specific treatment was used in the third paediatric patient9 and none of them progressed to chronicity of the arthritis. Alivernini et al11 reported that NSAIDs, baricitinib and glucocorticoids were started, Apaydın et al12 reported that hydroxychloroquine (HCQ), glucocorticoids and sulfasalazine were started, Liew et al13 reported that NSAIDs were started and intra-articular triamcinolone was administered in the knee joint and Talarico et al14 reported that medium doses of glucocorticoids were started. There was one case in which the patient did not achieve remission before publication of the article12 and the remaining where chronicity was not reported.
COVID-19-associated arthritis with onset >1 week after COVID-19
A total of 25 articles reported 26 cases of onset of arthritis >1 week after COVID-19 (table 4). Except for two patients aged 14 and 16 years,15 16 all were adult patients, and the ages ranged from 27 years17 18 to 73 years.19 Half of the patients (13/26) were male.15 17 19–29 The time of occurrence of arthritis after COVID-19 varied between cases. One report did not specify the period of time between onset of COVID-19 and onset of arthritis, describing it as a ‘short period’,20 while for the remaining cases, the shortest period of time was 10 days,30 31 and the longest period of time was 48 days (development of polyarthritis 3 days after a 45-day hospitalisation for COVID-19 pneumonia).28 SARS-CoV-2 infection was confirmed by RT-PCR or antigen test in all the patients with exception of one,20 in which a subsequent SARS-CoV-2 antibody test was positive.
Table 4.
COVID-19-associated arthritis with onset >1 week after COVID-19 initial manifestations
| Study, country | N, gender, age (years) | COVID-19 symptoms | Time between onset of COVID-19 and onset of articular symptoms (SARS-CoV-2 diagnostic test) | Type of arthritis (and EA involvement) | Blood tests (included immunology) | Serologies | Arthrocentesis | Treatment for COVID-19 | Treatment for arthritis | Last assessment and chronicity |
| Cincinelli et al,17 Italy | 1, male, 27 | Mild body temperature elevation (up to 37.3°C), influenza-like symptoms and mild, bilateral conjunctival injection | 2 weeks (nasopharyngeal swab RT-PCR) | Monarthritis (first MCP) | NA | NA | Not done | NA | NSAIDs and paracetamol without resolution and then PDN 10 mg/day | Absence of pain or range of motion limitation and minimal residual swelling of the affected joint |
| Coath, et al,20 UK | 1, male, 53 | Fever, night sweats, malaise, 2 kg weight loss and loss of sense of smell | Short period but not specified (no swab was obtained; symptoms were highly suggestive of COVID-19 and subsequent SARS-CoV-2 antibody test was positive) |
Bilateral sacroiliitis and arthritis of left first costovertebral and costotransverse joints | Positive HLA-B27 | NA | Not done | NA | NSAIDs and MPD 120 mg intramuscular | Asymptomatic after 4–5 months |
| Danssaert et al, USA 38 |
1, female, 37 | Cough, congestion, fevers, chills and myalgia | 12 days after testing positive for SARS-CoV-2 | Extensor tendinitis (second, third and fourth compartments of right hand) | RF negative and positive ANA (speckled pattern); UA normal |
Lyme serology negative | Not done | NA | Initially, hydromorphone intramuscular, oxycodone and lidocaine patch; subsequently, NSAID gel, gabapentin and oral hydromorphone; wrist splint, tramadol and occupational therapy for wrist tendinitis | Tenderness of the dorsal aspect of the wrist and hand |
| De Stefano L et al21, Italy | 1, male, 30s | Arthromyalgia, fatigue, diarrhoea and anosmia | 40 days after COVID-19 (nasopharyngeal swab RT-PCR) | Monarthritis (elbow) and psoriasis lesions | ANA, ENA, RF, ACPA, HLA-B27 negative | NA | Negative for SARS- CoV-2 RNA on RT-PCR and no crystals detected | Symptomatic treatment | NSAIDs | Arthritis completely resolved |
| Di Carlo et al22, Italy | 1, male, 55 | Fever | 37 days (nasopharyngeal swab RT-PCR) | Monarthritis (ankle) | HLA-B27 negative | U. urealyticum, M. hominis and C. trachomatis in the genitourinary system and enterobacteriaceae (C. jejuni, S. flexneri, Y. enterocolitica, C. difficile, Salmonella spp) were negative | It was not possible to aspirate the synovial fluid | NA | MPD 4 mg/day | Asymptomatic on follow-up |
| Dutta et al15, India | 1, male, 14 | Mild symptoms | 3 weeks after COVID-19 (nasopharyngeal swab RT-PCR) | Polyarthritis (right elbow, bilateral knees and ankles) | ACPA, HLA-B27, ASO, ANA negative | HIV and mycoplasma negative | NA | Conservative treatment | NSAIDs, intravenous MPD at 2 mg/kg/day, PDN | Asymptomatic on follow-up |
| Fragata et al,34 Portugal | 1, female, 41 | Myalgias, fatigue, coryza and loss of smell and taste and low fever (38°C) | 4 weeks after COVID-19 symptoms (nasopharyngeal and oropharyngeal swab RT-PCR) | Polyarthritis (PIP, DIP and MCP joints) | ANA, anti-dsDNA, RF ACPA, ENA negative; normal UA | Echoviruses, parvovirus B19, HIV 1 and 2, HBV and HCV were negative | Not done | Symptomatic treatment | NSAIDs (ibuprofen 1200 mg/day), 5 days of PDN 5 mg/day | No joint complaints or new inflammatory signs at 3-month follow-up |
| Gasparotto et al,23 Italy | 1, male, 60 | Hyperpyrexia, headache, asthenia and dyspnoea; interstitial pneumonia | 32 days after diagnosis of COVID-19 (nasopharyngeal swab RT-PCR) | Oligoarthritis (right ankle and knee) | RF, ACPA, ANA, ENA and HLA-B27 were negative | Urine and blood cultures were negative; urethral swab and stool culture did not show evidence of bacterial infection | 20 cc of a cloudy, yellow and highly inflammatory synovial fluid with 20 000/mm3 WBCs of which 90% polymorphonucleates and 10% monocytes; no crystals were detected; negative cultures | Azithromycin, ceftriaxone, HCQ 400 mg/day, anticoagulation, low-flow oxygen; underwent nasotracheal intubation and received broad-spectrum antibiotics, antimycotic prophylaxis, continuous diuretic infusion, anticoagulants, noradrenalin | Ibuprofen 600 mg | Asymptomatic on follow-up (up to 6 months after discontinuation of therapy) |
| Mukarram et al 46 Pakistan | 1, male, 34 | Low-grade fever, dry cough, severe fatigue/lethargy, loss of appetite and ageusia | 14 days (nasopharyngeal swab RT-PCR) | Monarthritis (knee) | NA | NA | NA | Azithromycin (for 5 days), zinc and multivitamins | NSAIDs and intra-articular steroid injection | Arthritis resolved completely within 10 days |
| Gibson et al,25 UK | 1, male, 37 | Fever, non-productive cough and fatigue | 5 weeks after COVID-19 (nasopharyngeal swab RT-PCR) | Symmetrical polyarthritis (wrists, PIP) and Achilles tendinitis | RF, ACPA, ANA/ENA and HLA-B27 negative | NA | NA | NA | PDN 20 mg daily and NSAIDs | In remission with normal inflammatory markers at follow-up |
| Hønge et al,26 Denmark |
1, male, 53 | Fatigue, shortness of breath and fever up to 40°C, hypoxia <90% despite oxygen therapy | 16 days after infection (oropharyngeal swab RT-PCR) | Oligoarthritis (knee and ankles) | RF, ACPA, HLA-B27, ANAs were negative | HIV negative; blood cultures negative | Polynuclear cells and a smaller number of mononuclear cells detected; no crystals detected; negative cultures | 200 mg intravenous remdesivir followed by 100 mg/day; 6 mg intravenous dexamethasone | NSAIDs (ibuprofen 400 mg orally three times per day) and PDN 25 mg/day | Arthritis resolved completely after 4 months of follow-up |
| Jali, Saudi Arabia35 | 1, female, 39 | Fever, sore throat, fatigue, generalised body pain and headache | 3 weeks (nasopharyngeal swab RT-PCR) | Polyarthritis (PIPs and DIPs) | RF, ACPA, ANA negative | Hepatitis and HIV negative | NA | NA | NSAID | Remission |
| Kocyigit et al,32 Turkey | 1, female, 53 | Headache and ageusia, cough, sputum and dyspnoea | 41 days after infection (nasopharyngeal swab RT-PCR) | Monarthritis (knee) | RF, ACPA, HLA-B27, ANA were negative; normal UA |
NA | 12 mL cloudy-yellow synovial fluid with 18 000/mm3 WBC of which 80% polymorphonucleates; negative cultures and no crystals detected | Favipiravir, HCQ, azithromycin, anticoagulantion and oxygen therapy | Diclofenac 150 mg/day | Arthritis not observed in follow-up examinations |
| Ono et al, 27 Japan | 1, male, 50s | Fever with chills and severe fatigue; mildly hypoxic | 21 days (nasopharyngeal swab RT-PCR) | Oligoarthritis (ankles) and Achilles enthesitis | RF, ACPA, ANA and HLA-B27 were negative | Syphilis, HIV, ASO, Mycoplasma, C. pneumoniae negative; gonococcal and C. trachomatis urine PCR negative | Mild inflammatory fluid without crystals; cultures of synovial fluid was also negative | Favipiravir, supportive care with empiric cefepime and vancomycin; intubation | NSAIDs and intra-articular corticosteroid injection | Moderate improvement |
| Ouedraogo et al,28 2USA | 1, male, 45 | Productive cough and fever, multiorgan failure | 48 days (nasopharyngeal swab RT-PCR) | Monarthritis (left knee) | RF, ACPA were negative | Gonorrhoea, Chlamydia, HBV, C. difficile and HIV, CMV, EBV, enterovirus, parvovirus B19, and T. pallidum were negative | 11 000/mm3 WBC with 94% polymorphonuclears, no crystals and negative cultures | Azithromycin, ceftriaxone, HCQ and tocilizumab; intubation, extracorporeal membrane oxygenation and dyalisis | Oral corticosteroids | Significant improvement |
| Parisi et al33, Italy | 1, female, 58 | Arthralgia, fever, cough, nausea, diarrhoea and dysgeusia | 25 days (nasopharyngeal swab RT-PCR) | Monarthritis (ankle) and Achilles tendinitis | RF, ACPA, ANA, ENA and dsDNA negative | NA | NA | Paracetamol | Ibuprofen 600 mg twice a day | Synovitis still present 30 days after treatment initiation |
| Santacruz et al,39 Colombia | 1, female, 30 | Odynophagia, anosmia, dysgeusia, bilateral conjunctivitis, fever of 38.5°C and dyspnoea | 1 month (positive antigen test) | Dactylitis of left fourth toe and extra-articular findings (conjunctivitis, oral lesions, palatal erosion, blennorrhagic keratoderma, subungual hyperkeratosis and onycholysis, circinate vulvitis) | HLA-B27 and HLA-B15 positive | M. pneumoniae, C. trachomatis negative; VDRL and HIV negative | NA | Dexamethasone | PDN 15 mg/day | Remission |
| Saricaoglu et al,19 Turkey | 1, male, 73 | Fever, weakness and dry cough | 22 days (nasopharyngeal and oropharyngeal swab RT-PCR) | Polyarthritis (MTP, PIPs and DIPs) | ACPA and RF negative; normal UA | NA | NA | HCQ, azithromycin and ceftriaxone | NSAIDs | Completely resolved with NSAID therapy |
| Schenker et al,30 Germany | 1, female, 65 | Respiratory symptoms, fever and shortness of breath; generalised myalgia and back pain | 10 days (tested positive but test not specified) | Symmetric polyarthritis of ankles, wrists and knee joints | HLA-B27 positive; all auto-antibody tests were negative | Negative (not specified which ones) | NA | NA | Prednisolone | Regressed after starting prednisolone |
| Shokraee et al,36 Iran | 1, female, 58 | Unproductive cough, shortness of breath and extreme fatigue | 15 days (nasopharyngeal swab RT-PCR) | Sacroiliitis and right hip arthritis | NA | Brucellosis and tuberculosis were negative | NA | Interferon-β1, dexamethasone, ceftriaxone, enoxaparin and nortriptyline | 100 mg indomethacin twice a day and 80 mg intramuscular depot steroid | In remission after 14 days |
| Sidhu et al31, UK | 1, female, 31 | Fever, cough, malaise, weight loss, acute swelling of lips, dysphagia and widespread urticarial rash | 10 days (nasopharyngeal swab RT-PCR) | Polyarthritis (wrists, elbows, knees and hands) | ANA, ANCA, IgM-RF, ACPA and HLA-B27 were all negative | Urine and blood cultures were negative | NA | Not reported | PDN 30 mg/day | In remission after 2 months |
| Sureja et al18, India | 1, female, 27 | Fever and body aches | Two weeks after COVID-19 (nasopharyngeal swab RT-PCR) | Polyarthritis (bilateral knee, ankle and midfoot joints and mild arthritis of the wrist, MCP and PIP joints) | RF positive (low titter); ACPA, ANA and HLA-B27 negative | NA | NA | 1 mg/kg CS in the form of oral MPD and favipiravir | CS 0.25 mg/kg tapered and stopped over 3 weeks, NSAIDs and oral opioids | Improved significantly (at 4-week follow-up) |
| Yokogawa N et al,29 Japan | 1, male, 57 | Cough, fever and malaise | 15 days after COVID-19 (nasopharyngeal swab RT-PCR) | Monarthritis (right knee) | RF and ACPA negative | Negative HBV surface antigen, HCV and HIV | Negative for SARS- CoV-2 RNA on RT- PCR and free from crystals | Symptomatic treatment | Without treatment | Resolved |
| Colatutto et al,37 Italy | 1, female, 58 | Cough | Within 1 month (nasopharyngeal swab RT-PCR) | Sacroiliitis | ANA, RF and HLA-B27 negative | NA | NA | HCQ and azithromycin | NSAIDs | Mild low back pain |
| 1, female, 53 | Cough | Within 1 month (nasopharyngeal swab RT-PCR) | Sacroiliitis | ANA and RF negative | NA | NA | HCQ and azithromycin | NSAIDs | Mechanical low back pain | |
| Salvatierra et al16, Spain | 1, female, 16 | Anosmia, ageusia, odynophagia and fever | 11 days (SARS-CoV-2 serology positive for IgG and IgM) | Dactylitis (left second, fourth and fifth toes) | ANA, RF and HLA-B27 negative | NA | NA | NA | Naproxen 500 mg twice daily for 5 days | Resolved |
ACPA, anticitrullinated protein antibodies; ANA, antinuclear antibodies; ASO, antistreptolysin O; C. difficile, Clostridium difficile; C. jejuni, Campylobacter jejuni; CMV, citomegalovirus; C. pneumoniae, Chlamydia pneumoniae; CS, corticosteroids; C. trachomatis, Chlamydia trachomatis; DIP, distal interphalangeal; ds-DNA, double-stranded DNA; EA, extra-articular; EBV, Epstein-Barr virus; ENA, extractable nuclear antigen; HBV, hepatitis B virus; HCQ, hydroxychloroquine; HCV, hepatitis C virus; HLA, human leucocyte antigen; MCP, metacarpophalangeal; M. hominis, Mycoplasma hominis; MPD, methylprednisolone; M. pneumoniae, Mycoplasma pneumoniae; MTP, metatarsophalangeal; N, number of patients; NA, not available; NSAID, non-steroid anti-inflammatory drug; PDN, prednisolone; PIP, proximal interphalangeal; RF, rheumatoid factor; RT-PCR, real-time PCR ; S. flexneri, Shighella flexneri; UA, uric acid; U. urealyticum, Ureaplasma urealyticum; VDRL, venereal disease research laboratory; WBC, white blood cell; Y. enterocolitica, Yersinia enterocolitica.
The number and location of joints affected were variable. Eight cases were reported as monarthritis17 21 22 24 28 29 32 33; of these, four affecting the knee,24 28 29 32 two the ankle,22 33 one the elbow21 and one affecting the MCP joint.17 In addition to monarthritis, psoriasis was reported in one case21 and Achilles tendinitis in another case.33 Oligoarthritis was reported in three cases; knee and ankle involvement was reported in two cases,23 26 and bilateral ankle arthritis and Achilles enthesitis in one case.27 Polyarticular involvement was reported in eight cases; one of the cases was juvenile15 and seven were adult cases.18 19 25 30 31 34 35 Although the joint involvement pattern differed between cases, proximal and distal interphalangeal joints of the hands and feet, knees, wrists, ankles and elbows were the most frequently affected joints.
In addition to peripheral joint arthritis, sacroiliitis was reported in four cases (one patient with concomitant arthritis of the left first costovertebral and costotransverse joints, one with concomitant monarthritis of the hip and two with isolated sacroiliitis).20 36 37 Extensor tendinitis of the hand was reported in one case,38 and dactylitis with extra-articular involvement (conjunctivitis, oral lesions, palatal erosion, blennorrhagic keratoderma, subungual hyperkeratosis and onycholysis, circinate vulvitis)39 and without other symptoms16 in two cases. Three cases were HLA-B27 positive.20 30 39
NSAIDs and glucocorticoids were the most prescribed drugs for the treatment of the arthritis. NSAID monotherapy was used in nine patients,16 19 21 23 32 33 35 37 glucocorticoid monotherapy in five patients,22 28 30 31 39 NSAIDs plus glucocorticoids in eight patients,15 17 18 20 25 26 34 36 NSAIDs plus intra-articular steroid injection in two patients24 27 and NSAID gel plus opioid drugs in one patient.38 The arthritis resolved spontaneously (without any specific treatment for the arthritis) in one patient.29 '‘Significant improvement’, ‘remission’, ‘regression’ or ‘resolution’ of arthritis was observed in 20 patients,15 16 19–26 28–32 34–36 39 ‘moderate improvement’ in 1 patient,27 ‘residual swelling’ in 1 patient,17 ‘tenderness’ in 1 patient,38 low back pain in 2 patients37 and persistent synovitis (‘synovitis still present’) was reported in 1 patient,33 at the last evaluation of the cases. Table 5 presents a summary of patients’ characteristics and their frequency among reported cases.
Table 5.
Summary of patients’ characteristics and their frequency among reported cases
| Onset up to 1 week after COVID-19 | Onset at least 1 week after COVID-19 | |
| Adult patients | 4 | 24 |
| Juvenile patients | 3 | 2 |
| Male | 6 | 13 |
| Female | 1 | 13 |
| Peripheral joint involvement | 7 | 24 |
|
2 | 8 |
|
2 | 3 |
|
3 | 8 |
|
0 | 2 |
|
0 | 3 |
| Axial involvement | 0 | 4 |
| Positive ANAs | 0 | 1 |
| Positive RF | 0 | 1 |
| Positive ACPA | 1 | 0 |
| Positive HLA B27+ | 1 | 3 |
| No treatment | 1 | 1 |
| NSAIDs/Analgesics | 4 | 19 |
| Glucocorticoids | 3 | 13 |
| csDMARDs | 0 | 0 |
| Remission | 6 | 20 |
| Minor residual symptoms | 1 | 6 |
ACPA, anticitrullinated protein antibodies; ANA, antinuclear antibodies; csDMARD, conventional synthetic disease-modifying antirheumatic drug; HLA, human leucocyte antigen; NSAID, non-steroid anti-inflammatory drug; RF, rheumatoid factor.
Discussion
In this systematic review, we gathered all published data on patients with COVID-19-associated arthritis. We summarised a total of 33 cases in two categories: onset up to 1 week after COVID-19 (7 cases), and onset >1 week after COVID-19 (26 cases).
We propose a new classification for cases of arthritis possibly associated with SARS-CoV-2 infection: ‘COVID-19-associated arthritis’. The distinction between reactive and viral arthritis in the context of SARS-CoV-2 infection is artificial and there is no consensual definition for either of them, both in terms of clinical presentation as well as in terms of the required period of time between the onset of COVID-19 and the onset of arthritis. Some of the cases described are outside of the interval of time described as typical of reactive arthritis (1–4 weeks); these are reports in which the articular symptoms started before or at the same time as COVID-19 symptoms or in which the articular symptoms started at least 1 month after of the diagnosis of COVID-19.
Since important differential diagnoses of viral and reactive arthritis include crystal arthropathy, septic arthritis and other chronic inflammatory joint diseases,40 serological evaluation and synovial fluid analysis are critical investigations. Arthrocentesis results were reported in 10 cases,11–13 21 23 26–29 32 and no crystal and/or microorganisms were detected in the synovial fluid of these patients.
Our study has limitations. It is based on a small number of reports and these being descriptive and voluntary observations they are prone to ascertainment and publication bias, and findings may not be generalisable to other populations and settings. Moreover, some cases were better documented than others, and for example, important investigations such as autoantibody and serological testing and synovial fluid analysis were not systematically performed. Furthermore, the SLR did not find epidemiological data about the incidence or prevalence of cases of viral and/or reactive arthritis during specific periods of the pandemic, which could be compared with the incidence or prevalence of cases of COVID-19 infection during the same period of the pandemic, and with the incidence or prevalence of cases of viral and/or reactive arthritis before the pandemic, in the same geographical area—this type of data could allow to better infer regarding a potential causal association between COVID-19 and arthritis, as studied in Guillain-Barré syndrome.41
Despite these limitations, taken together, these case reports describe a possible causal association between exposure to SARS-CoV-2 and the onset of arthritis. All the cases reported had a confirmed diagnosis of COVID-19, defined by either a positive nasal or throat swab RT-PCR for viral RNA or a subsequent positive serological test for anti-SARS-CoV-2 IgM or IgG. There was only one case in which a nasal or oropharyngeal swab was not obtained during the symptomatic period,20 but the patient symptoms were highly suggestive of COVID-19, and a subsequent SARS-CoV-2 antibody test (IgG) was positive. However, since these cases were reported during a pandemic period, we cannot exclude that they could simply be the reflection of the background incident cases of viral and reactive arthritis, for which the list of potential culprits is extensive (table 2) and often undetermined in clinical practice. Since we do not have a full picture of the incidence of viral and reactive arthritis during the pandemic period that could be compared with prepandemic figures (in the same geographical area), in which SARS-CoV-2 infection was not an existing cause for arthritis, we cannot be sure that these cases are truly associated with SARS-CoV-2 infection and exclude the possibility of coincidental cases (caused by other virus or bacteria). Nevertheless, in most cases, the authors concluded that the most likely diagnosis was viral or reactive arthritis associated with COVID-19, as other causes to explain the arthritis were excluded by autoantibody testing/serology, arthrocentesis and patient history/symptoms. Conversely, it could also be argued that the reported diagnostic workup was not sufficiently extensive and was not done consistently and in a standardised way across reports.
Furthermore, the exact mechanism through which SARS-CoV-2 might cause arthritis is not fully understood, and mechanistic data are still lacking. The most common hypothesis is the existence of molecular mimicry between SARS-CoV-2 viral epitopes and the synovial membrane causing local inflammation, but other theories have proposed a role for the presence of circulating immune complexes or localisation of the virus directly on joint tissue.42 Molecular mimicry triggers humoral and cellular autoreactivity in the host.43 Primary SARS-CoV-2 infection induces systemic inflammation that can impact the musculoskeletal system allowing direct viral infection.42 Recent studies have found an association between the microbiome and reactive arthritis. Any disruption of the equilibrium between gut and microbiome is called dysbiosis, which sometimes can activate a T helper 17-mediated immune response in the host’s intestinal lamina propria, thereby promoting local and systemic inflammation,44 which could be another pathogenic link between SARS-CoV-2 and arthritis. The dysbiotic gut microbiota that persists after disease resolution could be a factor predisposing to the development of persistent symptoms and/or multisystem inflammation syndromes that occur in some patients after clearing the virus.45
In conclusion, this study summarised 33 cases of (possible) COVID-19-associated arthritis. The pattern of joint involvement described in these cases was diverse, but most patients had peripheral involvement with either polyarthritis or monarthritis. Most of the patients responded well to treatment (NSAIDs and glucocorticoids).
Mild disease symptoms resembling viral or reactive arthritis, a plausible temporal relationship between SARS-CoV-2 infection and the onset of arthritis, good response to treatment and low propensity to chronicity of the arthritis are preliminary characteristic features of COVID-19-associated arthritis. However, extensively excluding other possible causes of arthritis remains critical and challenging. Therefore, data should be interpreted with caution and bearing in mind the lack of robust mechanistic and epidemiological data, and that cases were reported with a short follow-up time after the onset of arthritis.
Moreover, we feel that the term ‘COVID-19-associated arthritis’ is more appropriate because it acknowledges the unknown mechanistic links, the lack of epidemiological data, the heterogeneity of clinical presentation and the lack of uniformity in the literature, with these cases ‘randomly’ being named as either reactive arthritis or viral arthritis. Homogenising the nomenclature will hopefully contribute to more consistent data collection and reporting, and further investigation of this new potential condition. As knowledge evolves, refinement of the nomenclature and/or establishment of robust classification/diagnostic criteria is expected to occur.
The definitions of viral and reactive arthritis (in general) are still evolving and the inconsistency in nomenclature found in this review suggests that the use of the term ‘COVID-19-associated arthritis’ is more appropriate and inclusive, when describing arthritis in the context of SARS-CoV-2 infection, at least until more mechanistic data regarding the potential link between SARS-CoV-2 and arthritis are available. Our review informs regarding the interpretation of future case reports of COVID-19-associated arthritis and the refinement of the definition and characteristics of this potential emerging new entity.
Footnotes
Twitter: @pedrommcmachado
BF and ASP contributed equally.
Contributors: BF and ASP performed the literature search, performed the data extraction and analysis and wrote the first draft of the manuscript. PMM designed the study, supervised the work and acted as systematic literature review methodologist and third reviewer. All the authors contributed to writing the manuscript, read and approved the final manuscript.
Funding: BF is supported by the Turkish Society for Rheumatology. ASP is supported by a scientific training bursary for young fellows from EULAR. PMM is supported by the National Institute for Health Research (NIHR), University College London Hospitals (UCLH), Biomedical Research Centre (BRC).
Disclaimer: None of the institutions had a role in the study, including study design, collection, analysis and interpretation of data; writing of the report and decision to submit the paper for publication.
Competing interests: PMM has received consulting/speaker’s fees from AbbVie, BMS, Celgene, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Orphazyme, Pfizer, Roche and UCB, all unrelated to this manuscript. ASP and BF: no conflicts of interest.
Provenance and peer review: Commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.WHO coronavirus (COVID-19) Dashboard, 2022. Available: https://covid19.who.int/ [Accessed 26 Feb 2022].
- 2.Grainger R, Machado PM, Robinson PC. Novel coronavirus disease-2019 (COVID-19) in people with rheumatic disease: epidemiology and outcomes. Best Pract Res Clin Rheumatol 2021;35:101657. 10.1016/j.berh.2020.101657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Z, Lian X, Su X, et al. From SARS and MERS to COVID-19: a brief summary and comparison of severe acute respiratory infections caused by three highly pathogenic human coronaviruses. Respir Res 2020;21:224. 10.1186/s12931-020-01479-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anka AU, Tahir MI, Abubakar SD, et al. Coronavirus disease 2019 (COVID-19): an overview of the immunopathology, serological diagnosis and management. Scand J Immunol 2021;93:e12998. 10.1111/sji.12998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schett G, Manger B, Simon D, et al. COVID-19 revisiting inflammatory pathways of arthritis. Nat Rev Rheumatol 2020;16:465–70. 10.1038/s41584-020-0451-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taniguchi Y, Nishikawa H, Yoshida T, et al. Expanding the spectrum of reactive arthritis (REA): classic REA and infection-related arthritis including poststreptococcal REA, Poncet's disease, and iBCG-induced REA. Rheumatol Int 2021;41:1387–98. 10.1007/s00296-021-04879-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitt SK. Reactive arthritis. Infect Dis Clin North Am 2017;31:265–77. 10.1016/j.idc.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 8.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–34. 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 9.Houshmand H, Abounoori M, Ghaemi R, et al. Ten-year-old boy with atypical COVID-19 symptom presentation: a case report. Clin Case Rep 2021;9:304–8. 10.1002/ccr3.3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sinaei R, Pezeshki S, Parvaresh S, et al. Post SARS-CoV-2 infection reactive arthritis: a brief report of two pediatric cases. Pediatr Rheumatol Online J 2021;19:89. 10.1186/s12969-021-00555-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alivernini S, Cingolani A, Gessi M, et al. Comparative analysis of synovial inflammation after SARS-CoV-2 infection. Ann Rheum Dis 2021;80:e91. 10.1136/annrheumdis-2020-218315 [DOI] [PubMed] [Google Scholar]
- 12.Apaydin H, Guven SC, Kucuksahin O, et al. A case of human leukocyte antigen B27 positive reactive arthritis associated with severe acute respiratory syndrome coronavirus 2 infection. North Clin Istanb 2021;8:423–4. 10.14744/nci.2020.88965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liew IY, Mak TM, Cui L, et al. A case of reactive arthritis secondary to coronavirus disease 2019 infection. J Clin Rheumatol 2020;26:233. 10.1097/RHU.0000000000001560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talarico R, Stagnaro C, Ferro F, et al. Symmetric peripheral polyarthritis developed during SARS-CoV-2 infection. Lancet Rheumatol 2020;2:e518–9. 10.1016/S2665-9913(20)30216-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dutta S, Dey S, Poddar A, et al. Post-COVID reactive arthritis. Indian J Pediatr 2022;89:103. 10.1007/s12098-021-03992-2 [DOI] [PubMed] [Google Scholar]
- 16.Salvatierra J, Martínez-Peñalver D, Salvatierra-Velasco L. CoVid-19 related dactyitis. Joint Bone Spine 2020;87:660. 10.1016/j.jbspin.2020.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cincinelli G, Di Taranto R, Orsini F, et al. A case report of monoarthritis in a COVID-19 patient and literature review: simple actions for complex times. Medicine 2021;100:e26089. 10.1097/MD.0000000000026089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sureja NP, Nandamuri D. Reactive arthritis after SARS-CoV-2 infection. Rheumatol Adv Pract 2021;5:rkab001. 10.1093/rap/rkab001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saricaoglu EM, Hasanoglu I, Guner R. The first reactive arthritis case associated with COVID-19. J Med Virol 2021;93:192–3. 10.1002/jmv.26296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coath FL, Mackay J, Gaffney JK. Axial presentation of reactive arthritis secondary to COVID-19 infection. Rheumatology 2021;60:e232–3. 10.1093/rheumatology/keab009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Stefano L, Rossi S, Montecucco C, et al. Transient monoarthritis and psoriatic skin lesions following COVID-19. Ann Rheum Dis 2020. 10.1136/annrheumdis-2020-218520. [Epub ahead of print: 04 Aug 2020]. [DOI] [PubMed] [Google Scholar]
- 22.Di Carlo M, Tardella M, Salaffi F. Can SARS-CoV-2 induce reactive arthritis? Clinical and Experimental Rheumatology 2021;39:S25–6. [PubMed] [Google Scholar]
- 23.Gasparotto M, Framba V, Piovella C, et al. Post-COVID-19 arthritis: a case report and literature review. Clin Rheumatol 2021;40:3357–62. 10.1007/s10067-020-05550-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. MukarramI, MukarramM, IshaqK, et al. Post COVID-19 reactive arthritis: an emerging existence in the spectrum of musculoskeletal complications of SARS-CoV-2 infection. CSMC 2020;7:1–4. 10.24966/CSMC-8801/100101 [DOI] [Google Scholar]
- 25.Gibson M, Sampat K, Coakley G. EP15 A self-limiting symmetrical polyarthritis following COVID-19 infection. Rheumatol Adv Pract 2020;4:24. 10.1093/rap/rkaa052.014 [DOI] [Google Scholar]
- 26.Hønge BL, Hermansen M-LF, Storgaard M. Reactive arthritis after COVID-19. BMJ Case Rep 2021;14. 10.1136/bcr-2020-241375. [Epub ahead of print: 02 Mar 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ono K, Kishimoto M, Shimasaki T, et al. Reactive arthritis after COVID-19 infection. RMD Open 2020;6:e001350. 10.1136/rmdopen-2020-001350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ouedraogo F, Navara R, Thapa R, et al. Reactive arthritis Post-SARS-CoV-2. Cureus 2021;13:e18139. 10.7759/cureus.18139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yokogawa N, Minematsu N, Katano H, et al. Case of acute arthritis following SARS-CoV-2 infection. Ann Rheum Dis 2021;80:e101. 10.1136/annrheumdis-2020-218281 [DOI] [PubMed] [Google Scholar]
- 30.Schenker HM, Hagen M, Simon D, et al. Reactive arthritis and cutaneous vasculitis after SARS-CoV-2 infection. Rheumatology 2021;60:479–80. 10.1093/rheumatology/keaa689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sidhu A, Selvan S, Alkutobi Z. EP02 a rare case of reactive arthritis secondary to COVID-19. Rheumatol Adv Pract 2020;4i:17. 10.1093/rap/rkaa052.001 [DOI] [Google Scholar]
- 32.Kocyigit BF, Akyol A. Reactive arthritis after COVID-19: a case-based review. Rheumatol Int 2021;41:2031–9. 10.1007/s00296-021-04998-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parisi S, Borrelli R, Bianchi S, et al. Viral arthritis and COVID-19. Lancet Rheumatol 2020;2:e655–7. 10.1016/S2665-9913(20)30348-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fragata I, Mourão AF. Coronavirus disease 19 (COVID-19) complicated with post-viral arthritis. Acta Reumatol Port 2020;45:278–80. [PubMed] [Google Scholar]
- 35.Jali I. Reactive arthritis after COVID-19 infection. Cureus 2020;12:e11761. 10.7759/cureus.11761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shokraee K, Moradi S, Eftekhari T, et al. Reactive arthritis in the right hip following COVID-19 infection: a case report. Trop Dis Travel Med Vaccines 2021;7:18. 10.1186/s40794-021-00142-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Colatutto D, Sonaglia A, Zabotti A, et al. Post-COVID-19 arthritis and sacroiliitis: natural history with longitudinal magnetic resonance imaging study in two cases and review of the literature. Viruses 2021;13. 10.3390/v13081558. [Epub ahead of print: 06 08 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Danssaert Z, Raum G, Hemtasilpa S. Reactive arthritis in a 37-year-old female with SARS-CoV2 infection. Cureus 2020;12:e9698. 10.7759/cureus.9698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santacruz JC, Londoño J, Santos AM, et al. Extra-Articular manifestations in reactive arthritis due to COVID-19. Cureus 2021;13:e18620. 10.7759/cureus.18620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toivanen A, Toivanen P. Reactive arthritis. Best Pract Res Clin Rheumatol 2004;18:689–703. 10.1016/j.berh.2004.05.008 [DOI] [PubMed] [Google Scholar]
- 41.Keddie S, Pakpoor J, Mousele C, et al. Epidemiological and cohort study finds no association between COVID-19 and Guillain-Barré syndrome. Brain 2021;144:682–93. 10.1093/brain/awaa433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Silva Andrade B, Siqueira S, de Assis Soares WR, et al. Long-COVID and Post-COVID health complications: an up-to-date review on clinical conditions and their possible molecular mechanisms. Viruses 2021;13. 10.3390/v13040700. [Epub ahead of print: 18 04 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim B, Kaistha SD, Rouse BT. Viruses and autoimmunity. Autoimmunity 2006;39:71–7. 10.1080/08916930500484708 [DOI] [PubMed] [Google Scholar]
- 44.Manasson J, Shen N, Garcia Ferrer HR, et al. Gut microbiota perturbations in reactive arthritis and postinfectious spondyloarthritis. Arthritis Rheumatol 2018;70:242–54. 10.1002/art.40359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeoh YK, Zuo T, Lui GC-Y, et al. Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 2021;70:698–706. 10.1136/gutjnl-2020-323020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mukarram MS. Post COVID-19 reactive arthritis: an emerging existence in the spectrum of musculoskeletal complications of SARS-CoV-2 infection. CSMC 2020;7:1–4. 10.24966/CSMC-8801/100101 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2021-002026supp001.pdf (74.4KB, pdf)