Abstract
Microplastics (MPs), small pieces of plastic (∼5 mm), are released into the environment not only as a result of the decomposition of large-sized plastics but also from day-to-day use of plastic products. Chronic exposure to MPs has been attributed to harmful effects on aquatic organisms and rodents. Effects include gastrointestinal toxicity, hepatotoxicity, neurotoxicity, and reproductive and developmental toxicities. Exposure to MPs may also potentially affect human health. Herein, we reviewed the impact of MPs on male and female reproductive systems and the associated mechanisms involved in the reproductive and developmental toxicities of MPs. We performed a literature search in Google Scholar and PubMed using the following keywords: MPs and reproductive toxicity; MPs and developmental studies; MPs and infertility; MPs and aquatics; and MPs and rodents. Evidence of MPs accumulation has been reported in many organs of humans and experimental models. The harmful effects of MPs have been manifested in male and female reproductive systems of mammalian and aquatic animals, including developmental effects on gametes, embryos, and their offspring. This review describes various signaling pathways involved in MPs-associated male and female reproductive and developmental toxicities.
Keywords: BTB-blood–testis barrier, MPs-microplastics, developmental toxicity, signaling, reproductive toxicity
Introduction
Plastics are frequently used in day-to-day life due to their low cost, ease of handling, transportation, production process, and widespread applications. The production of plastics has been increasing continuously for the last 60 years. Many of these are broken down into small plastics called microplastics (MPs) (Avio et al., 2017). Mechanical stress, sunlight, and an oxidizing atmosphere decompose large plastics into MPs, typically 5 mm in diameter. Numerous samples from the environment, including rivers, Antarctic snow, and biogas plants, have been collected to identify various types of MPs such as polypropylene (PP), polyethylene (PE), polystyrene (PS), and polyethylene terephthalate (PT) (Dumichen et al., 2017; Aves et al., 2022). The generation of MPs is not only limited to their degradative by-products but also emerged from clothing microfibres (Hernandez et al., 2017; Galvao et al., 2020), indoor dust (Zhang et al., 2020), cosmetics (facial scrubs) (Napper et al., 2015), tap water (Tong et al., 2020), and seafood (MPs accumulation) (Leung et al., 2021).
MPs affect the normal functioning of the organisms and may cause several organ-specific toxicities such as neuronal, digestive, reproductive, and developmental toxicity (Yin et al., 2021). Compromised sperm quality in men and infertility problems in women have been reported among plastic industry workers. (Jelnes, 1988; Hougaard et al., 2009). Indeed, micro- and nano-particles of plastics may pose more risk to the reproductive system. Various studies have been conducted on animals in order to understand the effect of MPs on male and female fertility (Hou B. et al., 2021; Haddadi et al., 2022; Wei et al., 2022). Moreover, MPs may also affect the growth of offspring when the mother is exposed for a longer duration, suggesting the detrimental effects of MPs on development and growth (Luo et al., 2019; Wang et al., 2019; Hu et al., 2021). Therefore, further research studies are required to understand the in-depth biological effects of MPs on the reproductive and development process, as they can affect future generations.
Reproductive toxic effects of MPs exposure
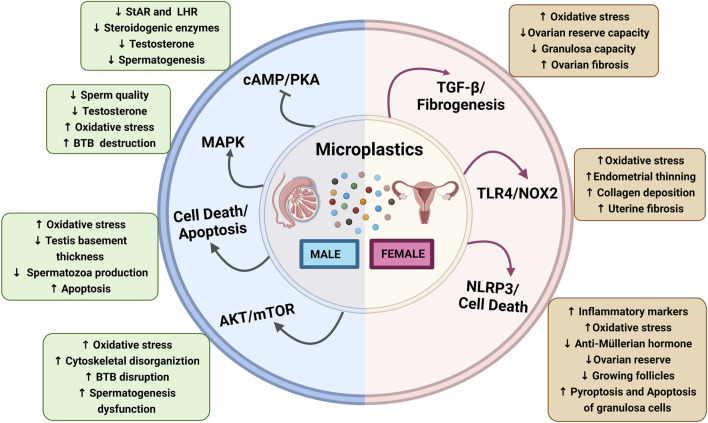
Reproductive toxicity is defined as exposure to any substance that interferes with the normal functioning of male and female reproductive organs, causing the loss of fertility (United Nations Economic Commission for Europe (UNECE), 2011). Continuous exposure to environmental toxicants and pollutants such as MPs can compromise the fertility of males and females (Wei et al., 2022). It has been reported that MPs induce reproductive toxicity in various organisms, including rodents (An et al., 2021; Hou B. et al., 2021) and aquatic species such as oysters (Sussarellu et al., 2016), cladocerans (Jaikumar et al., 2019), Caenorhabditis elegans (Chen et al., 2022), and zebrafish (Danio rerio) (Qiang and Cheng, 2021). In this section, we have covered the effect of MPs on structural, functional, and hormonal changes in male and female reproductive organs (Figure 1).
FIGURE 1.
Schematic diagram showing microplastics affecting male and female reproductive functions: Microplastics affecting male reproductive functions via the activation of different signaling pathways, Akt/mTOR, apoptosis, MAPK, and inhibition of cAMP/PK3 that results in increased oxidative stress, BTB disruption, and spermatogenesis dysfunction and decrease in steroidogenic enzymes and LHR downregulation. Microplastics affecting the female reproductive functions altered by the activation of NLRP3, TLR4/NOX2, and TGF-β/fibrogenesis that results in increased oxidative stress, endometrial thinning, collagen deposition, inflammatory markers, and pyroptosis and apoptosis of granulosa cells while ovarian reserve, growing follicles, granulosa cells capacity, and anti-müllerian hormone levels are decreased. Abbreviations: cAMP, cyclic adenosine monophosphate; AKT, Ak strain transforming; BTB, blood–testes barrier; LHR, luteinizing hormone receptor; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; NOX-2, NADPH oxidase-2; NLRP3, nod-like receptor family pyrin domain containing 3; PKA, protein kinase A; StAR, steroidogenic acute regulatory protein; TGF-β, transforming growth factor-β; TLR4, toll-like receptor-4.
MPs-induced male reproductive toxicity
MPs-induced reproductive dysfunctions have been observed in the aquatic species, which is attributed to the MPs accumulation in reproductive tissues (Issac and Kandasubramanian, 2021; Qiang and Cheng, 2021; Cormier et al., 2022; Liu Y. et al., 2022). Toxicity evaluation of PE-MPs (<400 µm) in freshwater hydra at concentrations of 0.01, 0.02, 0.04, and 0.08 g/ml for 3–96 h have reported morphological (clubbed and disintegrated tentacles) and reproductive (hydranth numbers) changes; however, these changes are non-lethal (Murphy and Quinn, 2018). Furthermore, Qiang et al. have investigated the testicular toxicity of PS-MPs in zebrafish, and exposure to 100 and 1,000 μg/L of MPs has shown increased testicular apoptosis (Qiang and Cheng, 2021). Moreover, the effect of MPs on testis has not been limited to zebrafish but has also gained attention in other organisms. Earthworms (Eisenia andrei) exposed to nano-plastics (>100 nm) for 21 days have been shown to reduce male reproductive functions and deformities in sperm cells, such as reduced mature bundle, damaged plasma membranes, and reduced density of sperm and viability of coelomocytes (Kwak and An, 2021). In addition, chronic exposure to PS-MPs (10 µm) for 28 days exhibits a significant decrease in testosterone, spermatogenic cells, and disrupted blood–testis barrier (BTB) integrity in Balb/c mice (Jin et al., 2021). Some dose-response studies have been performed to evaluate the effects of MPs on the male reproductive organs (Park et al., 2020; Ijaz et al., 2021). PS-MPs have been assessed at the concentration of 2, 20, 200, and 2,000 μg/L in SD rats, and the lowest observed adverse effect level (LOAEL) showed at a dose of 20 μg/L, while the highest concentration (2,000 μg/L) has been reported maximum toxicity (Ijaz et al., 2021). Furthermore, at the highest concentration, PS-MPs have been reported to decrease sperm counts, motility, and viability and also reduce the follicle-stimulating hormone (FSH), luteinizing hormone (LH), and testicular and plasma testosterone levels (Ijaz et al., 2021). MPs (4 and 10 µm) exposure to male Balb/c mice for 28 days have disrupted the BTB and caused testicular inflammation via the downregulation of BTB-linked proteins (tight junction protein zonula occludens-1, occludin, basal ectoplasmic specializations (ES) protein, N-cadherin and β-catenin, and gap junction protein CX43) in the testis (Wei et al., 2021).
The steroidogenic enzymes P450scc, P450c17, 3β-HSD, and 17β-HSD are involved in testosterone synthesis in Leydig’s cells (Sun et al., 2019). Jin et al. reported that administration of 100 and 1,000 μg/L of PS-MPs (0.5, 4, and 10 μm) in mice with drinking water for 180 days had shown a significant decrease in steroidogenic enzymes and steroidogenic acute regulatory protein (StAR) levels (Jin et al., 2022). MPs may also cause morphological changes in sperm, such as absent and small head and acrosome loss (Jin et al., 2022). A repeated oral toxicity study for 28 days in SD rats has shown that PP-MPs induce lesions in the testis and epididymis at 25 mg/kg/day (Jin et al., 2022). A recent study has reported that exposure to PS-MPs (1%–10% crushed PS disposable plates for 90 days) showed a remarkable decrease in epididymal sperm count, motility, and serum testosterone level in male albino Wistar rats (Ilechukwu et al., 2022). Based on the above mentioned findings from diverse model systems, it is pertinent that MPs exposure adversely affects the male reproductive system (Figure 1). However, more evidence is required to validate the mechanism of MPs toxicity on male fertility and reproductive health. Hence, it is suggested that future mechanistic investigations are urgently needed to understand the MPs-associated reproductive toxicities.
MPs-induced female reproductive toxicity
The harmful effects of MPs have not been limited to the male reproductive system (D'Angelo and Meccariello, 2021) but have also been shown to adversely affect the female reproductive system (Wei et al., 2022). Although, as compared to male reproductive studies, a limited number of studies are conducted to evaluate the harmful effects on the female reproductive system. MPs-associated female reproductive toxicity has been studied in several species, including zebrafish (Qiang and Cheng, 2021), oysters (Sussarellu et al., 2016), zooplankton (copepods) (Cole et al., 2015), medaka fishes (Yan et al., 2020; Li et al., 2022), mice (Liu Z. et al., 2022; Wei et al., 2022), and rats (An et al., 2021). Here, we have summarized the available studies on MPs exposure in female reproductive cells/organs.
Copepods, a zooplankton species, were subjected to PS-beads (20 μm, 75 MPs/ml) and cultured algae (250 µgCL−1) for 24 h and have shown a significant reduction in ingestion, fecundity, and survival rate, though no change in laying out eggs were observed (Cole et al., 2015). PS-MPs (2 and 6 µm) exposure (0.023 mg/L) to oysters for 2 months has reported a decrease in diameter and number of oocytes (Sussarellu et al., 2016). The combined effect of MPs with heavy metals in medaka fishes (Oryzias melastigma) has been shown to perturb similar changes such as irregular oocytes, partly adhesion, and empty follicle in the ovaries of female medaka (Yan et al., 2020). Moreover, PS-MPs (5 µm) exposure (0.1 mg/day) for 24–26 days by oral gavage has perturbs folliculogenesis such as disrupted follicles maturation, differentiation, and increased number of atretic and cyst follicles in Wistar rats (Haddadi et al., 2022). In addition, estrous cycle disruption due to exposure to MPs has also been reported in female rats (Haddadi et al., 2022). PS-MPs (5 µm) led to a significant decrease in the duration of the fourth estrus cycle and reduced the duration of the metestrus phase compared to control rats (Haddadi et al., 2022). Further, the continuous exposure to PS-MPs (30 mg/kg) for 35 days impaired the follicles development, quality, and maturation of oocytes in the ovaries of mice (Liu Z. et al., 2022). Furthermore, the accumulation of MPs (0.5 µm) in granulosa cells of rats has been reported to interfere with the normal functioning, growth, and differentiation of oocytes and lead to female reproductive toxicity via apoptosis, pyroptosis, and fibrosis (An et al., 2021; Hou J. et al., 2021). Therefore, extensive exposure to MPs aggravates various toxicities such as decreased diameter and number of oocytes, decreased or empty follicles, inflamed ovaries, and reduced ovarian reserve (Yan et al., 2020; Hou J. et al., 2021; Liu Z. et al., 2022).
Overall, these studies suggest that MPs are associated with negative effects on the female reproductive system (Figure 1).
MPs-induced developmental toxicity
Developmental toxicity is defined as any reversible or irreversible functional or structural alteration caused by environmental insult, diet, and toxic chemicals or physical factors that affect organisms’ normal growth, differentiation, development, or behaviour (Hougaard, 2021). In this section, we have discussed the development and growth effects such as fetal growth, deformities, and death in offspring whose parents are exposed to MPs for an extended period. A recent study investigated four generations (F0, F1, F2, and F3) of developmental effects in Daphnia magna with 21 days of exposure to MPs and reported a significantly reduced population growth rate and reproduction (Martins and Guilhermino, 2018). Furthermore, there is a slow recovery up to F3 generation, which accounts for the developmental toxicity in the Daphnia magna population (Martins and Guilhermino, 2018). Moreover, a transgenerational study of PS-MPs in the marine medaka (Oryzias melastigma) has reported a delayed incubation time and gonads maturation, hatching rate, and body length of offspring at the dose of 20 and 200 mg/L (Wang et al., 2019). Findings of aquatic studies have also been translated into rodents. The results of 90 days of repeated exposure (0.125, 0.5, 2 mg/day) to PE-MPs (40–48 µm) have shown a significant reduction in the number of live births/dam, sex ratio, and pups’ body weight in the Institute of Cancer Research (ICR) mice (Park et al., 2020). In addition, MPs have disrupted maternal-fetal connection in allogenic pregnant mice, as evident by increased embryo resorption rate and decreased number and diameter of uterine arterioles (Hu et al., 2021). This finding indicates that MPs may pose a threat to fetus development. Several studies have reported the developmental and reproductive toxicities of MPs in different species, summarized in Table 1. However, more studies are required to decipher the mechanisms of MP-associated developmental toxicity and how MPs could cross the placental barrier and impact growth in utero and postnatal stages.
TABLE 1.
Summary of experimental studies showing developmental and harmful effects of MPs in different species and their offspring.
| S.N | Type of toxicity | Model systems | MPs types | MPs sizes and concentrations used | Reported effects and inferences | Reference |
|---|---|---|---|---|---|---|
| 1 | Developmental toxicity (postnatal) | Daphnia magna | Pristine polymer microspheres | 1–5 μm (0.1 mg/L) | Decreased growth, reproduction, and population growth rate led to the extinction of F1 generation | Martins and Guilhermino, (2018) |
| MPs deposition was seen until F3 generation | ||||||
| 2 | Developmental toxicity (postnatal and prenatal) | Marine medaka (Oryzias melastigma) | PS- MPs | 10 µm (20 and 200 mg/L) | Delayed incubation time reduced the heart and hatching rate and length of body of the offspring | Wang et al. (2019) |
| 3 | Developmental toxicity (postnatal) | Marine medaka (Oryzias melastigma) | PS-MPs (Phenanthrene) | 10 μm (2–200 μg/L) | Higher dose deposited on the chorion reduced the growth and hatching rate and delayed hatching time. MPs at low dose do not accumulate phenanthrene | Li et al. (2020) |
| 4 | Developmental toxicity (prenatal and postnatal), and reproductive toxicity | Marine medaka (Oryzias melastigma) | MPs + Phenanthrene | 13 μm (200 μg/L) | Exacerbated bradycardia in embryos, causing transgenerational toxicity from mother to offspring | Li et al. (2022) |
| 5 | Developmental toxicity (postnatal) | Zebrafish (Danio rerio) | Polyamide (PA) MPs | 6.37–8.13 μm 200 mg/L | Reduced hatching rate and inhibited musculoskeletal development in zebrafish larvae | Zou et al. (2020) |
| Macrophages induced proinflammation, apoptosis, and multi-xenobiotics resistance | ||||||
| 6 | Developmental toxicity (postnatal) | Zebrafish (Danio rerio) | Pristine PE-MPs (Medium density) | 20–60 μm (6.2, 12.5, 25.0, 50.0 and 100 mg/L) | Harmful effects such as bigger swim bladder, increased yolk sac, and reduced hatching rate of larvae | Malafaia et al. (2020) |
| Larvae at concentrations of 50 and 100 mg/L MPs showed more significant external morphological changes and higher teratogenic abnormality rates | ||||||
| 7 | Developmental toxicity (prenatal and postnatal) | Zebrafish (Danio rerio) | Pristine PS- MPs + Butylated hydroxyanisole (BHA) | 65 nm to 20 μm, (2 mg/L) and (BHA, 1 mg/L) | MPs aggravate the accumulation of BHA in zebrafish larvae viz. reduced hatching rates, increased malformation rates, and decreased calcified vertebrae | Zhao et al. (2020) |
| 8 | Developmental toxicity (prenatal and postnatal) | Zebrafish (Danio rerio) | Pristine PE-MPs and spiked with benzo α pyrene (MP-BaP) | 20–27 µm (1% w/w in the fish diet) | MPs and MP-BaP 30 and 90 dpf (day post-fertilization) lead to altered growth parameters such as reduced fecundity, egg morphology, and yolk area | Tarasco et al. (2022) |
| Impairment in the development of caudal fins and bone quality | ||||||
| 9 | Developmental toxicity (prenatal and postnatal) | Zebrafish (Danio rerio) | PS- MPs | 10 μm (200 particles/mL) | Larvae development deformities, moderate hatching rate, and altered antioxidant and cellular function | De Marco et al. (2022) |
| 10 | Developmental (prenatal) and reproductive toxicity | Prawn | PS-MPs | (2 and 20 mg/L) | The quality of testicular germ cells and sex hormones are altered, causing decreased hatching success and survival of F1 larvae. PS-MPs bioaccumulated in different tissues of larvae and decreased immunity due to paternal exposure | Sun et al. (2022) |
| 11 | Developmental and (prenatal and postnatal) reproductive toxicity | Mice | PS nanoplastics | 100 nm (0.1, 1 and 10 mg/L) | Prenatal and postnatal PS-NPs exposure declines birth and postnatal body weight in offspring | Huang et al. (2022) |
| Transgenerational testicular toxicities in offspring (reduced testis weight and sperm counts) | ||||||
| 12 | Developmental toxicity (postnatal) | ICR Mice | PS- MPs | 0.5 and 5 µm (100 and 1,000 μg/L) | Risk of metabolic disorders in offspring | Luo et al. (2019) |
| Intergenerational effects on the F1 offspring | ||||||
| 13 | Developmental (prenatal and postnatal) reproductive toxicity | Male and female ICR mice | PE-MPs | 40–48 μm (0.125, 0.5, and 2 mg/mouse) | Reduced number of live births/dam, sex ratio, and body weight of pups | Park et al. (2020) |
| Immune disruption in the offspring of PE-treated maternal or paternal mice | ||||||
| 14 | Developmental toxicity (prenatal) | C57BL/6-mated Balb/c mice (Allogenic mice) | PS-MPs | 10 μm (250 μg/mouse) | Increased resorption rate and reduced number and diameter of uterine arterioles | Hu et al. (2021) |
| Immunological barrier homeostasis disruption in the peripheral blood, placenta, and spleen |
Potential signaling pathways involved in MPs-induced male and female reproductive toxicity
So far, MPs and their effects on reproductive and developmental organs have been reported in different model systems, primarily in laboratory experiments. MPs-induced reproductive toxicity, dysfunctions, and impairments in fertility are associated with many signaling pathways (Figure 1).
Oxidative stress and MAPK signaling pathway
Oxidative stress is a primary mediator in male and female reproductive dysfunctions (Khan et al., 2011; Agarwal et al., 2014; Ahmad et al., 2017). Excessive reactive oxygen species (ROS) generation creates an imbalance between oxidant and antioxidant status, leading to lipid peroxidation, DNA damage, and protein breakdown (Ahmad et al., 2017; Yu et al., 2018). Several experimental studies have confirmed that microplastics cause ROS production, which increases oxidative stress in gonads (An et al., 2021; Kim et al., 2021; Wei et al., 2021). In addition, mitogen-activated protein kinases (MAPK) pathways are activated through different stimuli, viz. chemical agents and UV-induced damage, cytokines, and oxidative stress (Stramucci et al., 2018). Xie et al. reported that MPs exposure activated MAPK signaling via oxidative stress in the mouse testis (Xie et al., 2020). Moreover, PS-MPs exposure for 6 weeks induces ROS generation and increases the phosphorylation of p38 and JNK MAPK in the testis of Balb/c mice (Xie et al., 2020).
Nuclear factor erythroid 2-related factor 2(Nrf2) is a critical transcription factor and acts as an antioxidant which is negatively regulated by Kelch-like ECH-associated protein 1 (Keap-1) (Kovac et al., 2015). Li et al. have shown that PS-MPs increase oxidative stress, activate the p38 MAPK, and deplete the nuclear Nrf2 pathway, which leads to poor quantity and quality of sperms and compromised BTB integrity (Li et al., 2021). The integrity of the BTB junction is regulated via N-clathrin during internalization, while selective infiltration is regulated by occludin through tight connections of BTB (Mruk and Cheng, 2010; Lie et al., 2013). Interestingly, PS-MPs have been reported to damage BTB and significantly decrease the expressions of connexin-43, claudin, and N-cadherin in rats (Li et al., 2021). In a nutshell, the MAPK-Nrf2 pathway and oxidative stress-associated mechanism seem to be involved in MPs-induced reproductive dysfunctions (Figure 1). However, more investigations are needed to identify the precise role of this signaling in MP-induced reproductive adverse effects.
Akt and mTOR signaling pathway
Mammalian target of rapamycin (mTOR) plays a vital role in the cellular processes by providing energy and cytoskeletal structure. mTOR forms two complexes, mTORC1 and mTORC2, and exerts opposite physiological functions by binding with raptor and rictor (Jesus et al., 2017; Wang and Zhang, 2019). Moreover, it plays a crucial role in the maintenance and process of spermatogenesis (Jesus et al., 2017). The ribosomal protein S6 (rpS6), a downstream gene of mTORC1, has been reported to disorganize the F-actin, resulting in leaky BTB via the rpS6-Akt-MMP-9 signaling pathway (Mok et al., 2014; Mok et al., 2015). Also, rictor helps to develop F-actin organization and maintains the BTB integrity via protein kinase C alpha (PKC-α) and gap junctions (Mok et al., 2013). A recent study has shown that PS-MPs trigger ROS-mediated imbalance of mTORC1 and mTORC2 signaling, which alter the expression of actin-related protein 3 (Arp3) and epidermal growth factor receptor pathway substrate 8 (Eps8) actin-binding proteins, eventually disrupting BTB integrity and spermatogenesis (Wei et al., 2021). The current research is still in the infancy phase and requires more studies to validate the role of Akt and mTOR signaling pathways in MPs-associated reproductive toxicity.
Inflammasome (NLRP3) and fibrotic signaling pathways
NLRP3, NOD-like receptor protein 3, is a multi-protein that acts as the defense mechanism against microorganisms, endogenous damage, and toxic stimuli but is also involved in male and female infertility (de Rivero Vaccari, 2020; Sano et al., 2022). The activation of NLRP3 triggers an apoptotic and inflammatory response by converting pro-caspase-1 to caspase-1 and pro-interleukin-1 (pro-IL-1β) and pro-interleukin-18 (pro-IL-18) into interleukin-1β (IL-1β) and interleukin-18 (IL-18), respectively (Charan et al., 2022). A recent study has ascertained that PS-MPs trigger the NLRP3/caspase-1 signaling pathway by oxidative stress, leading to decreased ovarian reserve in rats (Hou J. et al., 2021). However, limited findings are reported on NLRP3-mediated biological effects of MPs.
Fibrosis is a process of development of connective tissue as a repairing response to injury and affects organ structure and function, including the reproductive organs (Amargant et al., 2020). A recent study has reported that PS-MPs exposure causes fibrosis in ovaries via activation of toll-like receptor-4/NADPH oxidase-2 (TLR4/NOX2) signaling (Wu et al., 2022). This study has also reported an increase in oxidative stress, which consequently leads to activation of NOTCH and transforming growth factor-β (TGF-β)-mediated fibrosis in the endometrial epithelial cells and uterus (Wu et al., 2022). Furthermore, PS-MPs have elevated the expression of Wnt/β-catenin, alpha-smooth muscle actin (α-SMA), TGF-β, and fibronectin in ovarian granulosa cells, thereby leading to ovarian fibrosis (An et al., 2021). Therefore, inflammatory and fibrotic signaling might be involved in the MPs-induced reproductive toxicity, particularly in females (Figure 1).
Cell death and apoptotic signaling pathways
Apoptosis pathways are the most explored in MPs-induced male and female reproductive toxicity (Qiang and Cheng, 2021; Liu Z. et al., 2022). Qiang et al. have reported that MPs induced the caspase-dependent apoptosis in zebrafish testis, which is mediated via the upregulation of several caspases and p53 (Qiang and Cheng, 2021). Furthermore, PS-MP exposure for 35 days leads to perturbed mitochondrial membrane potential and increased inflammatory and apoptotic markers (caspase-3 and Bax) that result in ovarian inflammation and poor quality of oocytes in mice (Liu Z. et al., 2022). Hence, these studies suggest that both (intrinsic and extrinsic) apoptotic pathways are involved in MPs-induced cell death and apoptosis in reproductive organs (Figure 1).
Steroidogenic and endocrine signaling pathways
Testosterone is crucial in spermatogenesis and is secreted by Leydig cells and regulated by LH signaling (Ramaswamy and Weinbauer, 2014). LH binds to its receptor (LHR) at the Leydig cell membrane, which, in turn, increases cAMP and other downstream pathways such as protein kinase A (PKA), StAR, and steroid synthases (Tremblay, 2015). Of note, Jin et al. have reported that chronic exposure to PS-MPs reduce testosterone, LH, and FSH contents in rat serum and downregulate the expression of StAR via inhibiting the AC/cAMP/PKA pathway in vitro (Jin et al., 2022). However, the effects of MPs on steroidogenic and endocrine signaling pathways are meager.
Conclusion
MPs have been shown to accumulate not only in various organs in experimental models but also in human organs such as blood, lymph, placenta, meconium, and lungs (Segovia-Mendoza et al., 2020; Amato-Lourenco et al., 2021; Braun et al., 2021; Cobanoglu et al., 2021; Ragusa et al., 2021; Jenner et al., 2022). However, MPs accumulation is not identified in the reproductive organs of humans. Assessment of MPs-associated adverse effects in humans is challenging. The concentration and size of MPs and the duration of exposure used in experimental models might be quite low and/or high compared to human exposure to MPs. However, marine organisms can be one of the indirect sources of MPs accumulation in humans from seafood and other packaged food materials. Thus, more regulations and awareness are required to curb the generation of MPs in industries and landfill sites.
The toxic effects of MPs are mainly studied in rodents and aquatic experimental models, but the implication of these findings to the human population is still debatable. MPs might trigger their adverse effects via oxidative stress, apoptosis, inflammatory and fibrotic response, and altering hormonal balance. Other than these findings, the mechanism(s) of MPs toxicities remain largely unknown. The current understanding of MPs-associated reproductive toxicity is limited and is a nascent area of research, which needs future mechanistically focused investigation to understand the harmful effects of MPs on male and female reproductive organs, including the risk of developmental effects.
Author contributions
SpK and SbK conceptualized the idea of review, critically checked the manuscript, and did all the necessary corrections. ID collected all the works of literature and wrote and formatted the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- AC
Adenylyl cyclase
- Akt
Ak strain transforming
- Arp3
Actin-related protein 3
- αSMA
Alpha-smooth muscle actin
- Bax
B-cell lymphoma 2–associated X protein
- BTB
Blood–testes barrier
- 3β-HSD
3-beta hydroxysteroid dehydrogenase
- 17β-HSD
17β-hydroxysteroid dehydrogenases
- cAMP
Cyclic adenosine monophosphate
- DNA
Deoxyribonucleic acid
- FSH
Follicle-stimulating hormone
- ICR
Institute of Cancer Research
- JNK
c-Jun N-terminal kinase
- LH
Luteinizing hormone
- LHR
Luteinizing hormone receptor
- MPs
Microplastics
- p38 MAPK
p38 mitogen-activated protein kinase
- MMP 9
Matrix metallopeptidase 9
- mTOR
Mammalian target of rapamycin
- mTORC1
mTOR complex 1
- mTORC2
mTOR complex 2
- NOX
2 NADPH oxidase-2
- Nrf2
Nuclear factor erythroid 2-related factor 2
- NLRP3
Nod-like receptor family pyrin domain containing 3
- P450scc
Cholesterol side-chain cleavage enzyme
- PE
Polyethylene
- PET
Polyethylene terephthalate
- PP
Polypropylene
- PS
Polystyrene
- PKA
Protein kinase A
- ROS
Reactive oxygen species
- SD
Sprague Dawley
- rpS6
Ribosomal protein S6
- StAR
Steroidogenic acute regulatory protein
- TGF β
Transforming growth factor-β
- TLR4
Toll-like receptor 4
- UV
Ultraviolet
- Wnt
Wingless-related integration site.
References
- Agarwal A., Virk G., Ong C., Du Plessis S. S. (2014). Effect of oxidative stress on male reproduction. World J. Mens. Health 32 (1), 1–17. 10.5534/wjmh.2014.32.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad G., Almasry M., Dhillon A. S., Abuayyash M. M., Kothandaraman N., Cakar Z. (2017). Overview and sources of reactive oxygen species (ROS) in the reproductive system, in Oxidative stress in human reproduction (Cham: Springer; ), 1–16. [Google Scholar]
- Amargant F., Manuel S. L., Tu Q., Parkes W. S., Rivas F., Zhou L. T., et al. (2020). Ovarian stiffness increases with age in the mammalian ovary and depends on collagen and hyaluronan matrices. Aging Cell 19 (11), e13259. 10.1111/acel.13259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato-Lourenco L. F., Carvalho-Oliveira R., Junior G. R., Dos Santos Galvao L., Ando R. A., Mauad T. (2021). Presence of airborne microplastics in human lung tissue. J. Hazard. Mat. 416, 126124. 10.1016/j.jhazmat.2021.126124 [DOI] [PubMed] [Google Scholar]
- An R., Wang X., Yang L., Zhang J., Wang N., Xu F., et al. (2021). Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology 449, 152665. 10.1016/j.tox.2020.152665 [DOI] [PubMed] [Google Scholar]
- Aves A., Revell L., Gaw S., Ruffell H., Schuddeboom A., Wotherspoon N., et al. (2022). First evidence of microplastics in Antarctic snow. Cryosphere 16, 2127–2145. 10.5194/tc-16-2127-2022 [DOI] [Google Scholar]
- Avio C. G., Gorbi S., Regoli F. (2017). Plastics and microplastics in the oceans: from emerging pollutants to emerged threat. Mar. Environ. Res. 128, 2–11. 10.1016/j.marenvres.2016.05.012 [DOI] [PubMed] [Google Scholar]
- Braun T., Ehrlich L., Henrich W., Koeppel S., Lomako I., Schwabl P., et al. (2021). Detection of microplastic in human placenta and meconium in a clinical setting. Pharmaceutics 13 (7), 921. 10.3390/pharmaceutics13070921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charan H. V., Dwivedi D. K., Khan S., Jena G. (2022). Mechanisms of NLRP3 inflammasome-mediated hepatic stellate cell activation: therapeutic potential for liver fibrosis. Genes Dis. 10.1016/j.gendis.2021.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Yang Y., Wang C., Hua X., Li H., Xie D., et al. (2022). Reproductive toxicity of UV-photodegraded polystyrene microplastics induced by DNA damage-dependent cell apoptosis in Caenorhabditis elegans . Sci. Total Environ. 811, 152350. 10.1016/j.scitotenv.2021.152350 [DOI] [PubMed] [Google Scholar]
- Cobanoglu H., Belivermis M., Sikdokur E., Kilic O., Cayir A. (2021). Genotoxic and cytotoxic effects of polyethylene microplastics on human peripheral blood lymphocytes. Chemosphere 272, 129805. 10.1016/j.chemosphere.2021.129805 [DOI] [PubMed] [Google Scholar]
- Cole M., Lindeque P., Fileman E., Halsband C., Galloway T. S. (2015). The impact of polystyrene microplastics on feeding, function and fecundity in the marine copepod Calanus helgolandicus . Environ. Sci. Technol. 49 (2), 1130–1137. 10.1021/es504525u [DOI] [PubMed] [Google Scholar]
- Cormier B., Cachot J., Blanc M., Cabar M., Clérandeau C., Dubocq F., et al. (2022). Environmental microplastics disrupt swimming activity in acute exposure in Danio rerio larvae and reduce growth and reproduction success in chronic exposure in D. rerio and Oryzias melastigma. Environ. Pollut. 308, 119721. 10.1016/j.envpol.2022.119721 [DOI] [PubMed] [Google Scholar]
- D'Angelo S., Meccariello R. (2021). Microplastics: a threat for male fertility. Int. J. Environ. Res. Public Health 18 (5), 2392. 10.3390/ijerph18052392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco G., Conti G. O., Giannetto A., Cappello T., Galati M., Iaria C., et al. (2022). Embryotoxicity of polystyrene microplastics in zebrafish Danio rerio . Environ. Res. 208, 112552. 10.1016/j.envres.2021.112552 [DOI] [PubMed] [Google Scholar]
- de Rivero Vaccari J. P. (2020). The inflammasome in reproductive biology: a promising target for novel therapies. Front. Endocrinol. 11, 8. 10.3389/fendo.2020.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumichen E., Eisentraut P., Bannick C. G., Barthel A. K., Senz R., Braun U. (2017). Fast identification of microplastics in complex environmental samples by a thermal degradation method. Chemosphere 174, 572–584. 10.1016/j.chemosphere.2017.02.010 [DOI] [PubMed] [Google Scholar]
- Galvao A., Aleixo M., De Pablo H., Lopes C., Raimundo J. (2020). Microplastics in wastewater: microfiber emissions from common household laundry. Environ. Sci. Pollut. Res. Int. 27 (21), 26643–26649. 10.1007/s11356-020-08765-6 [DOI] [PubMed] [Google Scholar]
- Haddadi A., Kessabi K., Boughammoura S., Rhouma M. B., Mlouka R., Banni M., et al. (2022). Exposure to microplastics leads to a defective ovarian function and change in cytoskeleton protein expression in rat. Environ. Sci. Pollut. Res. Int. 29, 34594–34606. 10.1007/s11356-021-18218-3 [DOI] [PubMed] [Google Scholar]
- Hernandez E., Nowack B., Mitrano D. M. (2017). Polyester textiles as a source of microplastics from households: a mechanistic study to understand microfiber release during washing. Environ. Sci. Technol. 51 (12), 7036–7046. 10.1021/acs.est.7b01750 [DOI] [PubMed] [Google Scholar]
- Hou B., Wang F., Liu T., Wang Z. (2021). Reproductive toxicity of polystyrene microplastics: In vivo experimental study on testicular toxicity in mice. J. Hazard. Mat. 405, 124028. 10.1016/j.jhazmat.2020.124028 [DOI] [PubMed] [Google Scholar]
- Hou J., Lei Z., Cui L., Hou Y., Yang L., An R., et al. (2021). Polystyrene microplastics lead to pyroptosis and apoptosis of ovarian granulosa cells via NLRP3/Caspase-1 signaling pathway in rats. Ecotoxicol. Environ. Saf. 212, 112012. 10.1016/j.ecoenv.2021.112012 [DOI] [PubMed] [Google Scholar]
- Hougaard K. S., Hannerz H., Feveile H., Bonde J. P. (2009). Increased incidence of infertility treatment among women working in the plastics industry. Reprod. Toxicol. 27 (2), 186–189. 10.1016/j.reprotox.2009.01.003 [DOI] [PubMed] [Google Scholar]
- Hougaard K. S. (2021). Next generation reproductive and developmental Toxicology: crosstalk into the future. Front. Toxicol. 3, 652571. 10.3389/ftox.2021.652571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Qin X., Zhang J., Zhu Y., Zeng W., Lin Y., et al. (2021). Polystyrene microplastics disturb maternal-fetal immune balance and cause reproductive toxicity in pregnant mice. Reprod. Toxicol. 106, 42–50. 10.1016/j.reprotox.2021.10.002 [DOI] [PubMed] [Google Scholar]
- Huang T., Zhang W., Lin T., Liu S., Sun Z., Liu F., et al. (2022). Maternal exposure to polystyrene nanoplastics during gestation and lactation induces hepatic and testicular toxicity in male mouse offspring. Food Chem. Toxicol. 160, 112803. 10.1016/j.fct.2021.112803 [DOI] [PubMed] [Google Scholar]
- Ijaz M. U., Shahzadi S., Samad A., Ehsan N., Ahmed H., Tahir A., et al. (2021). Dose-dependent effect of polystyrene microplastics on the testicular tissues of the male sprague dawley rats. Dose. Response. 19 (2), 15593258211019882. 10.1177/15593258211019882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilechukwu I., Ehigiator B., Ben I., Okonkwo C., Olorunfemi O., Modo E. U., et al. (2022). Chronic toxic effects of polystyrene microplastics on reproductive parameters of male rats. Environ. Anal. Health Toxicol. 37 (2), e2022015–e2022010. 10.5620/eaht.2022015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issac M. N., Kandasubramanian B. (2021). Effect of microplastics in water and aquatic systems. Environ. Sci. Pollut. Res. Int. 28 (16), 19544–19562. 10.1007/s11356-021-13184-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaikumar G., Brun N. R., Vijver M. G., Bosker T. (2019). Reproductive toxicity of primary and secondary microplastics to three cladocerans during chronic exposure. Environ. Pollut. 249, 638–646. 10.1016/j.envpol.2019.03.085 [DOI] [PubMed] [Google Scholar]
- Jelnes J. E. (1988). Semen quality in workers producing reinforced plastic. Reprod. Toxicol. 2 (3-4), 209–212. 10.1016/0890-6238(88)90024-x [DOI] [PubMed] [Google Scholar]
- Jenner L. C., Rotchell J. M., Bennett R. T., Cowen M., Tentzeris V., Sadofsky L. R. (2022). Detection of microplastics in human lung tissue using μFTIR spectroscopy. Sci. Total Environ. 831, 154907. 10.1016/j.scitotenv.2022.154907 [DOI] [PubMed] [Google Scholar]
- Jesus T. T., Oliveira P. F., Sousa M., Cheng C. Y., Alves M. G. (2017). Mammalian target of rapamycin (mTOR): a central regulator of male fertility? Crit. Rev. Biochem. Mol. Biol. 52 (3), 235–253. 10.1080/10409238.2017.1279120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H., Ma T., Sha X., Liu Z., Zhou Y., Meng X., et al. (2021). Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mat. 401, 123430. 10.1016/j.jhazmat.2020.123430 [DOI] [PubMed] [Google Scholar]
- Jin H., Yan M., Pan C., Liu Z., Sha X., Jiang C., et al. (2022). Chronic exposure to polystyrene microplastics induced male reproductive toxicity and decreased testosterone levels via the LH-mediated LHR/cAMP/PKA/StAR pathway. Part. Fibre Toxicol. 19 (1), 13–17. 10.1186/s12989-022-00453-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S., Ahmad T., Parekh C. V., Trivedi P. P., Kushwaha S., Jena G. (2011). Investigation on sodium valproate induced germ cell damage, oxidative stress and genotoxicity in male Swiss mice. Reprod. Toxicol. 32 (4), 385–394. 10.1016/j.reprotox.2011.09.007 [DOI] [PubMed] [Google Scholar]
- Kim J. H., Yu Y. B., Choi J. H. (2021). Toxic effects on bioaccumulation, hematological parameters, oxidative stress, immune responses and neurotoxicity in fish exposed to microplastics: A review. J. Hazard. Mat. 413, 125423. 10.1016/j.jhazmat.2021.125423 [DOI] [PubMed] [Google Scholar]
- Kovac S., Angelova P. R., Holmstrom K. M., Zhang Y., Dinkova-Kostova A. T., Abramov A. Y. (2015). Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim. Biophys. Acta 1850 (4), 794–801. 10.1016/j.bbagen.2014.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak J. I., An Y. J. (2021). Microplastic digestion generates fragmented nanoplastics in soils and damages earthworm spermatogenesis and coelomocyte viability. J. Hazard. Mat. 402, 124034. 10.1016/j.jhazmat.2020.124034 [DOI] [PubMed] [Google Scholar]
- Leung M. M., Ho Y. W., Lee C. H., Wang Y., Hu M., Kwok K. W. H., et al. (2021). Improved Raman spectroscopy-based approach to assess microplastics in seafood. Environ. Pollut. 289, 117648. 10.1016/j.envpol.2021.117648 [DOI] [PubMed] [Google Scholar]
- Li S., Wang Q., Yu H., Yang L., Sun Y., Xu N., et al. (2021). Polystyrene microplastics induce blood-testis barrier disruption regulated by the MAPK-Nrf2 signaling pathway in rats. Environ. Sci. Pollut. Res. Int. 28 (35), 47921–47931. 10.1007/s11356-021-13911-9 [DOI] [PubMed] [Google Scholar]
- Li Y., Wang J., Yang G., Lu L., Zheng Y., Zhang Q., et al. (2020). Low level of polystyrene microplastics decreases early developmental toxicity of phenanthrene on marine medaka (Oryzias melastigma). J. Hazard. Mat. 385, 121586. 10.1016/j.jhazmat.2019.121586 [DOI] [PubMed] [Google Scholar]
- Li Y., Yang G., Wang J., Lu L., Li X., Zheng Y., et al. (2022). Microplastics increase the accumulation of phenanthrene in the ovaries of marine medaka (Oryzias melastigma) and its transgenerational toxicity. J. Hazard. Mat. 424, 127754. 10.1016/j.jhazmat.2021.127754 [DOI] [PubMed] [Google Scholar]
- Lie P. P., Cheng C. Y., Mruk D. D. (2013). Signalling pathways regulating the blood-testis barrier. Int. J. Biochem. Cell Biol. 45 (3), 621–625. 10.1016/j.biocel.2012.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Zhang J., Zhao H., Cai J., Sultan Y., Fang H., et al. (2022). Effects of polyvinyl chloride microplastics on reproduction, oxidative stress and reproduction and detoxification-related genes in Daphnia magna. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 254, 109269. 10.1016/j.cbpc.2022.109269 [DOI] [PubMed] [Google Scholar]
- Liu Z., Zhuan Q., Zhang L., Meng L., Fu X., Hou Y. (2022). Polystyrene microplastics induced female reproductive toxicity in mice. J. Hazard. Mat. 424, 127629. 10.1016/j.jhazmat.2021.127629 [DOI] [PubMed] [Google Scholar]
- Luo T., Zhang Y., Wang C. Y., Wang X. Y., Zhou J. J., Shen M. L., et al. (2019). Maternal exposure to different sizes of polystyrene microplastics during gestation causes metabolic disorders in their offspring. Environ. Pollut. 255, 113122. 10.1016/j.envpol.2019.113122 [DOI] [PubMed] [Google Scholar]
- Malafaia G., de Souza A. M., Pereira A. C., Goncalves S., da Costa Araujo A. P., Ribeiro R. X., et al. (2020). Developmental toxicity in zebrafish exposed to polyethylene microplastics under static and semi-static aquatic systems. Sci. Total Environ. 700, 134867. 10.1016/j.scitotenv.2019.134867 [DOI] [PubMed] [Google Scholar]
- Martins A., Guilhermino L. (2018). Transgenerational effects and recovery of microplastics exposure in model populations of the freshwater cladoceran Daphnia magna Straus. Sci. Total Environ. 631, 421–428. 10.1016/j.scitotenv.2018.03.054 [DOI] [PubMed] [Google Scholar]
- Mok K. W., Chen H. Q., Lee W. M., Cheng C. Y. (2015). rpS6 regulates blood-testis barrier dynamics through arp3-mediated actin microfilament organization in rat sertoli cells. An in vitro study. Endocrinology 156 (5), 1900–1913. 10.1210/en.2014-1791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok K. W., Mruk D. D., Cheng C. Y. (2014). rpS6 regulates blood-testis barrier dynamics through Akt-mediated effects on MMP-9. J. Cell Sci. 127 (22), 4870–4882. 10.1242/jcs.152231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok K. W., Mruk D. D., Lee W. M., Cheng C. Y. (2013). Rictor/mTORC2 regulates blood-testis barrier dynamics via its effects on gap junction communications and actin filament network. FASEB J. 27 (3), 1137–1152. 10.1096/fj.12-212977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk D. D., Cheng C. Y. (2010). Tight junctions in the testis: new perspectives. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365 (1546), 1621–1635. 10.1098/rstb.2010.0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy F., Quinn B. (2018). The effects of microplastic on freshwater Hydra attenuata feeding, morphology & reproduction. Environ. Pollut. 234, 487–494. 10.1016/j.envpol.2017.11.029 [DOI] [PubMed] [Google Scholar]
- Napper I. E., Bakir A., Rowland S. J., Thompson R. C. (2015). Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics. Mar. Pollut. Bull. 99 (1-2), 178–185. 10.1016/j.marpolbul.2015.07.029 [DOI] [PubMed] [Google Scholar]
- Park E. J., Han J. S., Park E. J., Seong E., Lee G. H., Kim D. W., et al. (2020). Repeated-oral dose toxicity of polyethylene microplastics and the possible implications on reproduction and development of the next generation. Toxicol. Lett. 324, 75–85. 10.1016/j.toxlet.2020.01.008 [DOI] [PubMed] [Google Scholar]
- Qiang L., Cheng J. (2021). Exposure to polystyrene microplastics impairs gonads of zebrafish (Danio rerio). Chemosphere 263, 128161. 10.1016/j.chemosphere.2020.128161 [DOI] [PubMed] [Google Scholar]
- Ragusa A., Svelato A., Santacroce C., Catalano P., Notarstefano V., Carnevali O., et al. (2021). Plasticenta: first evidence of microplastics in human placenta. Environ. Int. 146, 106274. 10.1016/j.envint.2020.106274 [DOI] [PubMed] [Google Scholar]
- Ramaswamy S., Weinbauer G. F. (2014). Endocrine control of spermatogenesis: role of FSH and LH/testosterone. Spermatogenesis 4 (2), e996025. 10.1080/21565562.2014.996025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano M., Komiyama H., Shinoda R., Ozawa R., Watanabe H., Karasawa T., et al. (2022). NLRP3 inflammasome is involved in testicular inflammation induced by lipopolysaccharide in mice. Am. J. Reprod. Immunol. 87 (4), e13527. 10.1111/aji.13527 [DOI] [PubMed] [Google Scholar]
- Segovia-Mendoza M., Nava-Castro K. E., Palacios-Arreola M. I., Garay-Canales C., Morales-Montor J. (2020). How microplastic components influence the immune system and impact on children health: focus on cancer. Birth Defects Res. 112 (17), 1341–1361. 10.1002/bdr2.1779 [DOI] [PubMed] [Google Scholar]
- Stramucci L., Pranteda A., Bossi G. (2018). Insights of crosstalk between p53 protein and the MKK3/MKK6/p38 MAPK signaling pathway in cancer. Cancers 10 (5), 131. 10.3390/cancers10050131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Wang D., Lin J., Liu Y., Xu L., Lv R., et al. (2019). Icariin protects mouse Leydig cell testosterone synthesis from the adverse effects of di (2-ethylhexyl) phthalate. Toxicol. Appl. Pharmacol. 378, 114612. 10.1016/j.taap.2019.114612 [DOI] [PubMed] [Google Scholar]
- Sun S., Jin Y., Luo P., Shi X. (2022). Polystyrene microplastics induced male reproductive toxicity and transgenerational effects in freshwater prawn. Sci. Total Environ. 842, 156820. 10.1016/j.scitotenv.2022.156820 [DOI] [PubMed] [Google Scholar]
- Sussarellu R., Suquet M., Thomas Y., Lambert C., Fabioux C., Pernet M. E. J., et al. (2016). Oyster reproduction is affected by exposure to polystyrene microplastics. Proc. Natl. Acad. Sci. U. S. A. 113 (9), 2430–2435. 10.1073/pnas.1519019113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasco M., Gavaia P. J., Bensimon-Brito A., Cordelières F. P., Santos T., Martins G., et al. (2022). Effects of pristine or contaminated polyethylene microplastics on zebrafish development. Chemosphere 303, 135198. 10.1016/j.chemosphere.2022.135198 [DOI] [PubMed] [Google Scholar]
- Tong H., Jiang Q., Hu X., Zhong X. (2020). Occurrence and identification of microplastics in tap water from China. Chemosphere 252, 126493. 10.1016/j.chemosphere.2020.126493 [DOI] [PubMed] [Google Scholar]
- Tremblay J. J. (2015). Molecular regulation of steroidogenesis in endocrine Leydig cells. Steroids 103, 3–10. 10.1016/j.steroids.2015.08.001 [DOI] [PubMed] [Google Scholar]
- United Nations Economic Commission for Europe (UNECE) (2011). “Globally harmonized system of classification and labelling ofChemicals (GHS),” in Part 3. Health and environmental hazards (United Nations, New York/Geneva: Reproductive Toxicity; ). Chapter 3.7. [Google Scholar]
- Wang J., Li Y., Lu L., Zheng M., Zhang X., Tian H., et al. (2019). Polystyrene microplastics cause tissue damages, sex-specific reproductive disruption and transgenerational effects in marine medaka (Oryzias melastigma). Environ. Pollut. 254, 113024. 10.1016/j.envpol.2019.113024 [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhang H. (2019). Regulation of autophagy by mTOR signaling pathway. Adv. Exp. Med. Biol. 1206, 67–83. 10.1007/978-981-15-0602-4_3 [DOI] [PubMed] [Google Scholar]
- Wei Y., Zhou Y., Long C., Wu H., Hong Y., Fu Y., et al. (2021). Polystyrene microplastics disrupt the blood-testis barrier integrity through ROS-Mediated imbalance of mTORC1 and mTORC2. Environ. Pollut. 289, 117904. 10.1016/j.envpol.2021.117904 [DOI] [PubMed] [Google Scholar]
- Wei Z., Wang Y., Wang S., Xie J., Han Q., Chen M. (2022). Comparing the effects of polystyrene microplastics exposure on reproduction and fertility in male and female mice. Toxicology 465, 153059. 10.1016/j.tox.2021.153059 [DOI] [PubMed] [Google Scholar]
- Wu H., Xu T., Chen T., Liu J., Xu S. (2022). Oxidative stress mediated by the TLR4/NOX2 signalling axis is involved in polystyrene microplastic-induced uterine fibrosis in mice. Sci. Total Environ. 838 (2), 155825. 10.1016/j.scitotenv.2022.155825 [DOI] [PubMed] [Google Scholar]
- Xie X. M., Deng T., Duan J. F., Xie J., Yuan J. L., Chen M. Q. (2020). Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway. Ecotoxicol. Environ. Saf. 190, 110133. 10.1016/j.ecoenv.2019.110133 [DOI] [PubMed] [Google Scholar]
- Yan W., Hamid N., Deng S., Jia P. P., Pei D. S. (2020). Individual and combined toxicogenetic effects of microplastics and heavy metals (Cd, Pb, and Zn) perturb gut microbiota homeostasis and gonadal development in marine medaka (Oryzias melastigma). J. Hazard. Mat. 397, 122795. 10.1016/j.jhazmat.2020.122795 [DOI] [PubMed] [Google Scholar]
- Yin K., Wang Y., Zhao H. J., Wang D. X., Guo M. H., Mu M. Y., et al. (2021). A comparative review of microplastics and nanoplastics: toxicity hazards on digestive, reproductive and nervous system. Sci. Total Environ. 774, 145758. 10.1016/j.scitotenv.2021.145758 [DOI] [Google Scholar]
- Yu P., Liu Z., Wu D., Chen M., Lv W., Zhao Y. (2018). Accumulation of polystyrene microplastics in juvenile Eriocheir sinensis and oxidative stress effects in the liver. Aquat. Toxicol. 200, 28–36. 10.1016/j.aquatox.2018.04.015 [DOI] [PubMed] [Google Scholar]
- Zhang J., Wang L., Kannan K. (2020). Microplastics in house dust from 12 countries and associated human exposure. Environ. Int. 134, 105314. 10.1016/j.envint.2019.105314 [DOI] [PubMed] [Google Scholar]
- Zhao H-J., Xu J-K., Yan Z-H., Ren H-Q., Zhang Y. (2020). Microplastics enhance the developmental toxicity of synthetic phenolic antioxidants by disturbing the thyroid function and metabolism in developing zebrafish. Environ. Int. 140, 105750. 10.1016/j.envint.2020.105750 [DOI] [PubMed] [Google Scholar]
- Zou W., Xia M., Jiang K., Cao Z., Zhang X., Hu X. (2020). Photo-oxidative degradation mitigated the developmental toxicity of polyamide microplastics to zebrafish larvae by modulating macrophage-triggered proinflammatory responses and apoptosis. Environ. Sci. Technol. 54 (21), 13888–13898. 10.1021/acs.est.0c05399 [DOI] [PubMed] [Google Scholar]