Abstract
Mutation and genetic complementation studies suggested that two chromosomal loci, agr and sar, are involved in the upregulation of several exotoxin genes and the downregulation of a number of surface protein genes in a growth phase-dependent manner in Staphylococcus aureus. We purified recombinant T7-tagged SarA from Escherichia coli and determined its effect on transcription from several S. aureus promoters by using purified RNA polymerase reconstituted with either ςA or ςB from S. aureus. Of the seven ςA-dependent promoters that we tested, SarA repressed transcription from agrP2, agrP3, cna, sarP1, and sea promoters and did not affect sec and znt promoters. Furthermore, SarA had no effect on transcription from the ςB-dependent sarP3 promoter. In vitro experimental data presented in this report suggest that SarA expression is autoregulated.
The pathogenicity of Staphylococcus aureus is attributed to the production of numerous extracellular toxins (e.g., hemolysins, enterotoxins, toxic shock syndrome toxins, proteases, and leukocidins) and surface proteins (e.g., protein A, fibronectin binding proteins, and collagen adhesion protein). Mutation and genetic complementation studies have identified two genetic loci, agr (accessory gene regulator) and sar (staphylococcal accessory regulator), that are involved in the regulation of expression of many of the toxin genes at the transcription level (for agr, references 20, 26, and 31, and for sar, references 1, 9, 11, and 17). The agr locus is divergently transcribed into two RNA molecules, called RNAII and RNAIII, from the promoters P2 and P3, respectively. Of the four open reading frames in the RNAII region, AgrA and AgrC have similarity with two-component signal transduction systems (18, 19, 22). Recently, AgrC was located in S. aureus membranes, and it was shown to be autophosphorylated on a histidine residue (22). An octapeptide, which is posttranslationally processed from AgrD presumably by AgrB, is necessary for the phosphorylation of AgrC. RNAIII, as an RNA molecule (517 nucleotides [nt]), upregulates the expression of several extracellular toxin genes at postexponential phase of growth (27–29). However, the agr system downregulates expression of coagulase and some surface proteins, e.g., protein A and fibronectin binding protein, at exponential phase (26, 34, 36). Interestingly, RNAIII also regulates expression of alpha-toxin posttranscriptionally (27).
The sar locus is at least partly involved in the upregulation of transcription from the agrP2 and agrP3 promoters (6, 10). Three overlapping RNAs, terminating at the same site, are transcribed from three distinct promoters (∼580-nt sarA from promoter P1, ∼840-nt sarC from P3, and ∼1,150-nt sarB from P2) in a growth phase-dependent fashion (1). The largest gene product, SarA, is encoded at the 3′ region of all the transcripts. SarA has been shown to bind to different promoters, including agrP2, agrP3 (9, 28, 33), the collagen adhesion gene promoter cna (3), sec, spa, hla, and fnb (11). The level of cna transcript in agr+ or agr S. aureus cells was elevated in a sarA background, implying that cna expression is downregulated by SarA in an agr-independent pathway (3).
SarH1, a homolog of SarA, was recently identified on a separate global regulatory locus on the S. aureus chromosome (38). Like sarA, sarH1 is transcribed from both a ςA-dependent promoter and a ςB-dependent promoter. Both SarA and RNAIII repress sarH1 expression, and some of the previously reported effects of sarA and agr on target gene expression (hla, spa, and ssp) appear to be mediated by sarH1. The relative concentrations of RNAIII, SarA, SarH1, and possibly other regulatory factors most likely dictate target gene expression. A sequence similarity search (using The Institute for Genomic Research and the University of Oklahoma databases) resulted in the identification of three more homologs of SarA. The calculated molecular masses of SarH2 and SarH4 are each 14 kDa, and the amino acid sequences are 35 and 24% identical to SarA, respectively. SarH3 has the same molecular mass as SarH1. The amino- and carboxy-terminal halves of both SarH1 and SarH3 have a high degree of sequence identity with SarA (33 and 30% at the amino and carboxyl termini, respectively). The sar homologs are located on separate loci.
We report here that SarA represses transcription from agrP2, agrP3, cna, sarP1, and sea promoters in vitro. This negative regulation by SarA is observed on several selective primary ς factor (ςA)-dependent promoters, but not on the alternative ς factor (ςB)-dependent promoter sarP3.
The oligonucleotide primers, plasmids, and bacterial strains used in this study are listed in Table 1. The restriction enzyme sites at the termini of each primer are underlined. PCR amplification was carried out using recombinant Pfu DNA polymerase (Stratagene, La Jolla, Calif.). Plasmid DNA from pALC561 and S. aureus RN6650 and chromosomal DNA from S. aureus COL were used as templates for the amplification of sarA, the agrP2-P3 promoter region, and the cna promoter region, respectively. Reaction conditions were as follows: melting temperature, 96°C (2 min); annealing temperature, 45°C (2 min); and elongation temperature, 72°C (1 min).
TABLE 1.
DNA oligomers, plasmids, and bacterial strains used in this study
| Oligonucleotide primer, plasmid, or strain | DNA sequence or descriptiona | Source or reference |
|---|---|---|
| Primers | ||
| SarA (forward) | CGGGATCCATGG CAATTACAAAAATCAATG (BamHI) | |
| SarA (reverse) | CCCAAGCTTTCATTTATTTACTCGAC (HindIII) | |
| agr (forward) | GGGGTACCCCACTCCTTCCTTAATTAAG (KpnI) | |
| agr (reverse) | GCTCTAGAGCCGTGGCAAACTGGTC (XbaI) | |
| cna (forward) | GGGGTACCGCACTTGTATTCGTTATACTG (KpnI) | |
| cna (reverse) | GCTCTAGACTCGTGCTGCAAATGCTTC (XbaI) | |
| Plasmids | ||
| pALC561 | Contains the entire sar operon | 1 |
| pET24a(+) | Kmr, expression vector | Novagen |
| pET24a(+)-sarA | sarA gene cloned into pET24(a+) for overexpression of SarA | This study |
| pMP7 | Apr, transcriptional terminator on either site of the multicloning site | 12 |
| pMP7-agrP2-P3 | Apr, agrP2-P3 promoter region containing part of the agrB and hld genes | This study |
| pMP7-cna | cna promoter region and a part of cna gene | This study |
| pMP7-zntPR | Apr, contains znt promoter and the zntR | 37 |
| pMJB1167 | Contains sec promoter and a part of sec gene | 32 |
| pMJB1168 | Contains sea promoter and a part of sea gene | 32 |
| Strains | ||
| E. coli DH5α | F−supE44 ΔlacU169 (Φ80 lacZ Δm15) hsdR17 recA1 endA1 gyrA96 thi-1 recA1 | Life Technologies |
| E. coli BL21(DE3) | F−ompT hsdSB(rB− mB−) gal dcm (DE3) T7 RNA polymerase under lacUV5 control | Novagen |
| S. aureus COL | Highly Mcr clinical isolate | 16 |
Sequences are 5′ to 3′; restriction enzyme (as indicated in parentheses) sites are underlined. Abbreviations: Apr, ampicillin resistant; Kmr, kanamycin resistant; Mcr, methicillin resistant.
pET24a(+)-sarA (Table 1) was created by introducing a DNA fragment containing the sarA gene into the BamHI and HindIII sites of pET24a(+). Escherichia coli strain BL21(DE3) (Novagen, Madison, Wis.), harboring pET24a(+)-sarA, was used for overproduction of T7-tagged SarA. The recombinant SarA was purified using a monoclonal T7-tagged affinity column (Novagen).
Binding of SarA to the cna promoter was studied in an electrophoretic mobility shift assay (EMSA) following a published protocol (3, 33), with a buffer solution containing 10 mM Tris-HCl (pH 7.6), 50 mM KCl, 2 mM dithiothreitol, 2 mM EDTA, 0.05% Triton X-100, and 3.5 nM probe DNA. A 260-bp DNA fragment flanking 80 bp upstream of the cna promoter was PCR amplified using the cna oligomers listed in Table 1, under the reaction conditions described above. The DNA fragment was end labeled with [γ-32P]ATP (specific activity, 3,000 Ci mmol−1; ICN Pharmaceuticals), purified in a Qiagen spin column, and used as the probe for the EMSA.
In vitro transcription reactions were carried out following our published protocol (15, 37). RNA polymerase purified from S. aureus was reconstituted with either ςA or ςB and was used in transcription reactions. Purified plasmid DNA (uncut) was used as a template in all the transcription assays described below.
Effect of SarA on binding to and transcription from the cna promoter.
We first studied the binding of our recombinant SarA preparation to cna promoter DNA. As shown in Fig. 1, SarA bound with the cna promoter region, and this binding was similar to that observed with a recombinant SarA (33). At SarA concentrations between 3.4 and 17 nM (Fig. 1, lanes 2 to 4), the probe shifted as a major band on the top and some smearing occurred. However, at higher SarA concentrations, 34 and 68 nM, the probe shifted as a single major band (lanes 5 and 6). The smearing of the shifted probe could arise from unsaturated binding of SarA to multiple sites on the probe (3). The binding of SarA to the cna promoter was reversed by adding a 25-fold excess of unlabeled probe, compared to binding in the presence of the labeled probe DNA (lane 7).
FIG. 1.
Binding of SarA to the cna promoter. EMSA was done as described in the text.
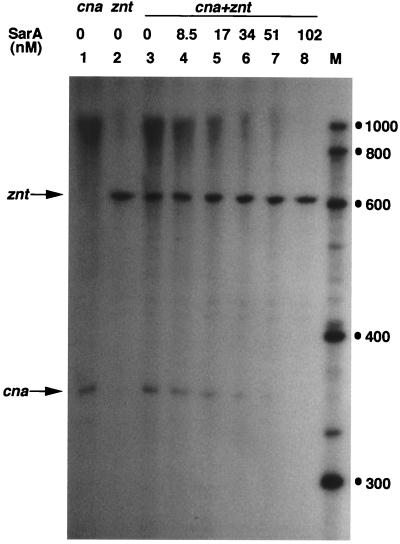
To study the specific effect of SarA on transcription from the cna promoter, the cna promoter region and the zinc resistance znt promoter region (37) were separately cloned into the plasmid pMP7, which contains two divergent transcription terminator sequences flanking the multiple cloning site (12). Thus, detection of precise transcripts originating from the cloned sequence was straightforward. pMP7-cna (Table 1) contains about 80 bp upstream of the cna promoter sequence. Fusion transcripts arising from the cloned cna sequence and the nucleotide sequence between the cloned cna sequence and the transcription termination site in vector pMP7 were obtained. Since SarA has no effect on transcription from the znt promoter, this plasmid served as a control for the quantitative determination of the cna transcript in different assays. pznt-PR, used for the transcription template of znt, contains 160 nt upstream of the znt transcription start site (37). As shown in Fig. 2, SarA inhibited transcription from the cna promoter in a concentration-dependent fashion, and >50% inhibition of transcription was achieved at a concentration of 17 nM SarA (data were quantitated by scanning using the Sigma gel software [not shown]). These results are consistent with the in vivo observation that the sar locus represses transcription of the cna gene in an agr-independent manner (3). This correlation of in vivo and in vitro results suggests that T7-tagged SarA and native SarA are biochemically similar. The above results unambiguously confirm the genetic model which predicts that SarA negatively regulates transcription from the cna promoter independent of any other cellular factor. Negative regulation by SarA has also been proposed for the expression of V8 serine protease and of a likely metalloprotease, based on mutation and genetic complementation studies (5, 23).
FIG. 2.
Effect of SarA protein on transcription from the cna promoter. pMP7-cna and pMP7-zntPR DNA (Table 1) were used as templates for the determination of transcriptional activities from the cna and znt promoters (indicated at the top), respectively. The specific transcripts are indicated by arrows. Lane M, RNA markers (sizes are in nucleotides).
Effect of SarA on several ςA-dependent promoters.
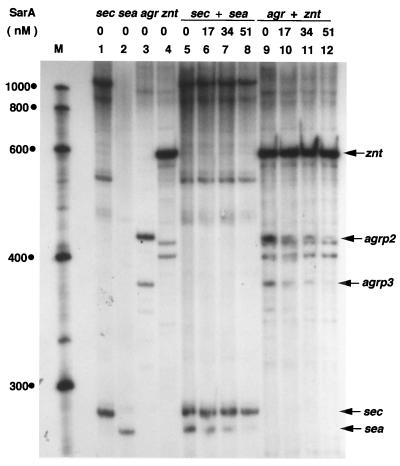
Since SarA binds to the agrP2-P3 promoter region and the genes transcribed from the agrP2 promoters play a pivotal role in the autoregulation of the agr operon, and since RNAIII transcribed from agrP3 regulates a number of toxin genes, we cloned the agrP2-P3 promoter region into the plasmid pMP7. The DNA from the recombinant plasmid pMP7-agrP2-P3 (Table 1) was used as a template to determine the effect of SarA on transcription from the agrP2 and agrP3 promoters (Fig. 3, lanes 9 to 12). pMP7-agrP2-P3 includes up to the +45 sequence of RNAIII and the +90 sequence of RNAII start site. All the SarA binding sites in the agrP2-P3 promoter region, as reported by different laboratories (9, 28, 33), are present within the cloned sequence. Fusion transcripts arising from the cloned agr sequence and the sequence between the cloned agr DNA and the transcription termination sites in the vector were obtained. In the transcription assays, transcript from the znt promoter served as a control for quantification (data not shown). Note that two unrelated RNA bands migrated just below the transcript derived from the agrP2 promoter (Fig. 3, compare lanes 3 and 4). Contrary to the current hypothesis that SarA activates transcription from the agr operon, these results demonstrate that SarA directly inhibits transcription from both the agrP2 and agrP3 promoters. It can be suggested that SarA, together with some yet-uncharacterized cellular factor(s), activates transcription of the agr operon. Alternatively, SarA may regulate expression of one or more factors which activate agr operon expression in the cell.
FIG. 3.
SarA protein affects transcription of several specific ςA-dependent promoters in a dose-dependent manner. Plasmid DNA containing the specific promoter along with a partial or complete gene adjacent to the promoter, as indicated in Table 1, was used as the template. The different promoter DNAs used in each lane are noted at the top. The specific transcripts are indicated by arrows. Lane M, RNA markers (sizes are in nucleotides).
Genetic studies of five different staphylococcal enterotoxins (SEs) (SEA through SEE) have been reported. The corresponding genes show 50 to 85% nucleotide sequence identity (2). SEA and SED levels in culture supernatants are highest during exponential growth, whereas maximal levels of SEB and SEC are attained during postexponential growth (13, 30). SEC expression is regulated by agr, but the expression of SEA is not dependent on agr (39). The agr effect on SEC is on the sec mRNA level (35). A systematic analysis using deletion mutants revealed that inclusion of the region extending 7 bp upstream of the promoter sequence (−42) is enough to maintain a 100% level of SEA compared to that in wild-type S. aureus (4). Since SarA binds to the promoter region (upstream of the −35 sequence) of sec (11) and deletion of the sequence 7 bp upstream of the sea promoter region has no effect on sea transcription in vivo, we tested the effect of SarA on transcription from the sea and sec promoters. Plasmid pMJB1167 (Table 1), used as a template for sec transcription, contains about 300 bp upstream of the promoter sequence, and pMJB1168, used as the template for sea transcription, includes sequence up to position −80 of the sea promoter region. As shown in Fig. 3 (lanes 5 to 8), expression from the sec promoter is unaffected or marginally affected by SarA, while expression from the sea promoter is severely repressed. Thus, it can be concluded that the binding of SarA upstream of the sec promoter does not affect its transcription. Recently, SarH1, a homolog of SarA, was shown to bind with the agrP3 promoter region and the promoter regions of the spa and ssp genes in vitro but did not affect transcription from these promoters in vivo (38). Apparently, promoter binding and transcriptional regulation of SarA may not always be correlated. The relative binding affinities of RNA polymerase and SarA to the promoter DNA may be a determining factor in the ultimate role of SarA on transcription from the different promoters. How SarA represses transcription cannot be explained with currently available data, and further investigation of this aspect should prove interesting.
Autoregulation of sar operon expression.
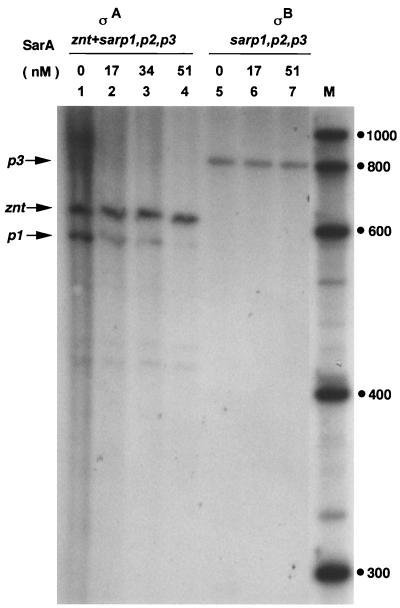
From in vitro binding experiments with an uncharacterized 12-kDa protein and the sarP1 promoter region, Manna et al. (24) suggested that the expression of sarA is autoregulated. This is in contrast with the conclusion drawn by Blevins et al. (3) from their mutation and genetic complementation experiments that SarA is produced constitutively and the DNA upstream of the sarA gene promoter makes no contribution to the regulation of SarA production. We previously observed that of the three sar transcripts, the shortest one, derived from the promoter P1, is ςA dependent while that from the P3 promoter is dependent on an alternative ς factor, ςB (15). It was of interest to determine the effect of SarA on transcription from the sar promoters. The S. aureus core RNA polymerase was reconstituted with either ςA or ςB (14, 15). Plasmid DNA containing the entire sar operon was used as the template in the transcription assays. As shown in Fig. 4, transcription from the sarP1 promoter was specifically inhibited by SarA (lanes 2 to 4), while SarA had no effect on transcription from the sarP3 promoter (lanes 5 to 7). We could not detect any transcript from the sarP2 promoter (Fig. 4; also previously reported in reference 15), implying that transcription from this promoter is positively regulated by one or more unknown factors in the cell.
FIG. 4.
Effect of SarA on transcription from the sar promoters. pALC561 DNA containing the entire sar operon was used as the template. pMP7-zntPR DNA was used as a control in lanes 1 to 4. RNA polymerase reconstituted with either ςA (lanes 1 to 4) or ςB (lanes 5 to 7) from S. aureus, in the presence of different concentrations of SarA, was used for the transcription reactions. Lane M, RNA markers (sizes are in nucleotides).
Our results presented in Fig. 4 show that SarA repressed transcription from the ςA-dependent sarP1, but it did not affect transcription from the ςB-dependent sarP3. Most of the genetic studies on the regulation of toxin gene expression have been carried out using S. aureus strain 8325-4 or its derivatives (RN6390 and RN6390B) as the parent strain. Recently, a naturally occurring 11-bp deletion within the rsbU gene, encoding anti-anti-ςB factor, in S. aureus strain 8325-4 was reported (21). Most likely, this mutation severely affects cellular levels of active ςB (25). Note that the synthesis of hla mRNA in derivatives of S. aureus 8325-4 and in strain V8, which lacks RNAIII, was significantly repressed (27). The hla mRNA level was undetectable in the S. aureus 8325-4 derivative with a deletion in the RNAIII region, while S. aureus V8, with a similar deletion in the RNAIII region, still produced hla mRNA, albeit at a 10-fold-lower level than in the parent strain. It can be suggested that in addition to RNAIII, ςB also regulates hla transcription indirectly. Whether SarA in combination with another protein whose expression is ςB dependent is involved in toxin gene regulation in S. aureus remains an open question.
Acknowledgments
We are grateful to W. Hendrickson and V. Kapatral for critical reading of the manuscript and constructive criticisms. The RNA polymerase used in this study was prepared by R. Deora during his graduate studies in this laboratory, and R. Fleming assisted in the construction of the pMP7-cna plasmid.
This work was supported by the Stephen W. and Alice A. Benedict Medical Research Fund administered by the University of Illinois at Chicago.
REFERENCES
- 1.Bayer M G, Heinrichs J H, Cheung A L. The molecular architecture of the sar locus in Staphylococcus aureus. J Bacteriol. 1996;178:4563–4570. doi: 10.1128/jb.178.15.4563-4570.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betley M J, Soltis T M, Couch J L. Molecular biological analysis of staphylococcal toxins. In: Novick R P, editor. Molecular biology of the staphylococci. New York, N.Y: VCH Publishers; 1990. pp. 327–342. [Google Scholar]
- 3.Blevins J S, Gillaspy A F, Rechtin T M, Hulburt B K, Smeltzer M S. The staphylococcal accessory regulator (sar) represses transcription of the Staphylococcus aureus collagen adhesion gene (cna) in an agr-independent manner. Mol Microbiol. 1999;33:317–326. doi: 10.1046/j.1365-2958.1999.01475.x. [DOI] [PubMed] [Google Scholar]
- 4.Borst D W, Betley M J. Promoter analysis of the staphylococcal enterotoxin A gene. J Biol Chem. 1994;269:1883–1888. [PubMed] [Google Scholar]
- 5.Chan P F, Foster S J. Role of SarA in virulence determinant production and environmental signal transduction in Staphylococcus aureus. J Bacteriol. 1998;180:6232–6241. doi: 10.1128/jb.180.23.6232-6241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung A L, Bayer M G, Heinrichs J H. sar genetic determinants necessary for transcription of RNAII and RNAIII in the agr locus of Staphylococcus aureus. J Bacteriol. 1997;179:3963–3971. doi: 10.1128/jb.179.12.3963-3971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung A L, Eberhardt K, Heinrichs J H. Regulation of protein A synthesis by the sar and agr loci of Staphylococcus aureus. Infect Immun. 1997;65:2243–2249. doi: 10.1128/iai.65.6.2243-2249.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung A L, Koomey M J, Butler C A, Projan S J, Fischetti V A. Regulation of exoprotein gene expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc Natl Acad Sci USA. 1992;89:6462–6466. doi: 10.1073/pnas.89.14.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chien Y-T, Cheung A L. Molecular interactions between two global regulators: sar and agr, in Staphylococcus aureus. J Biol Chem. 1998;273:2645–2652. doi: 10.1074/jbc.273.5.2645. [DOI] [PubMed] [Google Scholar]
- 10.Chien Y-T, Manna A, Cheung A L. SarA level is a determinant of agr activation in Staphylococcus aureus. Mol Microbiol. 1998;30:991–1001. doi: 10.1046/j.1365-2958.1998.01126.x. [DOI] [PubMed] [Google Scholar]
- 11.Chien Y-T, Manna A, Projan S, Cheung A L. SarA, a global regulator of virulence determinants in Staphylococcus aureus, binds to a conserved motif essential for sar-dependent gene regulation. J Biol Chem. 1999;274:37169–37176. doi: 10.1074/jbc.274.52.37169. [DOI] [PubMed] [Google Scholar]
- 12.Chugani S A, Parsek M R, Hershberger C D, Murakami K, Ishihama A, Chakrabarty A M. Activation of the catBCA promoter: probing the interaction of CatR and RNA polymerase through in vitro transcription. J Bacteriol. 1997;179:2221–2227. doi: 10.1128/jb.179.7.2221-2227.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czop J K, Bergdoll M S. Staphylococcal enterotoxin synthesis during the exponential, transitional, and stationary growth phases. Infect Immun. 1974;9:229–235. doi: 10.1128/iai.9.2.229-235.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deora R, Misra T K. Characterization of the primary ς factor of Staphylococcus aureus. J Biol Chem. 1996;271:21828–21834. doi: 10.1074/jbc.271.36.21828. [DOI] [PubMed] [Google Scholar]
- 15.Deora R, Tseng T, Misra T K. Alternative transcription factor ςSB of Staphylococcus aureus: characterization and role in transcription of the global regulatory locus sar. J Bacteriol. 1997;179:6355–6359. doi: 10.1128/jb.179.20.6355-6359.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duthie E S, Lorenz L L. Staphylococcal coagulase: mode of action and antigenicity. J Gen Microbiol. 1952;6:95–107. doi: 10.1099/00221287-6-1-2-95. [DOI] [PubMed] [Google Scholar]
- 17.Heinrichs J H, Bayer M G, Cheung A L. Characterization of the sar locus and its interaction with agr in Staphylococcus aureus. J Bacteriol. 1996;178:418–423. doi: 10.1128/jb.178.2.418-423.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji G, Beavis R C, Novick R P. Cell density control of staphylococcal virulence mediated by an octapeptide pheromone. Proc Natl Acad Sci USA. 1995;92:12055–12059. doi: 10.1073/pnas.92.26.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji G, Beavis R, Novick R P. A novel type of bacterial interference caused by a hypervariable autoinducing peptide. Science. 1997;276:2027–2030. doi: 10.1126/science.276.5321.2027. [DOI] [PubMed] [Google Scholar]
- 20.Kornblum J B, Kreiswirth B N, Projan S J, Ross H, Novick R P. agr: a polycistronic locus regulating exoprotein synthesis in Staphylococcus aureus. In: Novick R P, editor. Molecular biology of the staphylococci. New York, N.Y: VCH Publishers; 1990. pp. 373–402. [Google Scholar]
- 21.Kullik I, Giachino P, Fuchs T. Deletion of the alternative sigma factor ςB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J Bacteriol. 1998;180:4814–4820. doi: 10.1128/jb.180.18.4814-4820.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lina G, Jarrud S, Ji G, Greenland T, Pedraza A, Etienne J, Novick R P, Vandenesch F. Transmembrane topology and histidine protein kinase activity of AgrC, the agr signal receptor in Staphylococcus aureus. Mol Microbiol. 1998;28:655–662. doi: 10.1046/j.1365-2958.1998.00830.x. [DOI] [PubMed] [Google Scholar]
- 23.Lindsay J A, Foster S J. Interactive regulatory pathways control virulence determinant production and stability in response to environmental conditions in Staphylococcus aureus. Mol Gen Genet. 1999;262:323–331. doi: 10.1007/s004380051090. [DOI] [PubMed] [Google Scholar]
- 24.Manna A C, Bayer M G, Cheung A L. Transcriptional analysis of different promoters in the sar locus in Staphylococcus aureus. J Bacteriol. 1998;180:3828–3836. doi: 10.1128/jb.180.15.3828-3836.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miyazaki E, Chen J-M, Ko C, Bishai W R. The Staphylococcus aureus rsbW (orf159) gene encodes an anti-sigma factor of SigB. J Bacteriol. 1999;181:2846–2851. doi: 10.1128/jb.181.9.2846-2851.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morfeldt E, Janzon A, von Gabin A, Arvidson S, Lofdahl S. Cloning of a chromosomal locus (exp) which regulates the expression of several exoprotein genes in Staphylococcus aureus. Mol Gen Genet. 1988;211:435–440. doi: 10.1007/BF00425697. [DOI] [PubMed] [Google Scholar]
- 27.Morfeldt E, Taylor D, von Gabin A, Arvidson S. Activation of alpha toxin in Staphylococcus aureus by the trans-encoded antisense RNA, RNAIII. EMBO J. 1995;14:4569–4577. doi: 10.1002/j.1460-2075.1995.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morfeldt E, Tegmark K, Arvidson S. Transcriptional control of the agr-dependent virulence gene regulator, RNAIII, in Staphylococcus aureus. Mol Microbiol. 1996;21:1227–1237. doi: 10.1046/j.1365-2958.1996.751447.x. [DOI] [PubMed] [Google Scholar]
- 29.Novick R P, Ross H F, Projan S J, Kornblum J, Kreiswirth B, Moghazeh S. Synthesis of staphylococcal virulence factors is controlled by a regulatory RNA molecule. EMBO J. 1993;12:3967–3975. doi: 10.1002/j.1460-2075.1993.tb06074.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otero A, García M L, García M C, Moreno B, Bergdoll M S. Production of staphylococcal enterotoxins C1 and C2 and thermonuclease throughout the growth cycle. Appl Environ Microbiol. 1990;56:555–559. doi: 10.1128/aem.56.2.555-559.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng H-L, Novick R P, Kreiswirth B, Kornblum J, Schlievert P. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol. 1988;170:4365–4372. doi: 10.1128/jb.170.9.4365-4372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao L, Karls R K, Betley M J. In vitro transcription of pathogenesis-related genes by purified RNA polymerase from Staphylococcus aureus. J Bacteriol. 1995;177:2609–2614. doi: 10.1128/jb.177.10.2609-2614.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rechtin T M, Gillaspy A F, Schumacher M A, Brennan R G, Smeltzer M S, Hulburt B K. Characterization of the SarA virulence gene regulator of Staphylococcus aureus. Mol Microbiol. 1999;33:307–316. doi: 10.1046/j.1365-2958.1999.01474.x. [DOI] [PubMed] [Google Scholar]
- 34.Recsei P R, Kreiswirth B, O'Reilly M, Schlievert P, Gruss A, Novick R P. Regulation of exoprotein gene expression in Staphylococcus aureus by agr. Mol Gen Genet. 1986;202:58–61. doi: 10.1007/BF00330517. [DOI] [PubMed] [Google Scholar]
- 35.Regassa L B, Couch J L, Betley M J. Steady-state staphylococcal enterotoxin type C mRNA is affected by a product of the accessory gene regulator (agr) and by glucose. Infect Immun. 1991;59:955–962. doi: 10.1128/iai.59.3.955-962.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saravia-Otten P, Müller H-P, Arvidson S. Transcription of Staphylococcus aureus fibronectin binding protein genes is negatively regulated by agr and an agr-independent mechanism. J Bacteriol. 1997;179:5259–5263. doi: 10.1128/jb.179.17.5259-5263.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh V K, Xiong A, Usgaard T R, Chakrabarti S, Deora R, Misra T K, Jayaswal R K. ZntR is an autoregulatory protein and negatively regulates the chromosomal zinc resistance operon znt of Staphylococcus aureus. Mol Microbiol. 1999;33:200–207. doi: 10.1046/j.1365-2958.1999.01466.x. [DOI] [PubMed] [Google Scholar]
- 38.Tegmark K, Karlson A, Arvidson S. Identification and characterisation of SarH1, a new global regulator of virulence gene expression in Staphylococcus aureus. Mol Microbiol. 2000;37:398–409. doi: 10.1046/j.1365-2958.2000.02003.x. [DOI] [PubMed] [Google Scholar]
- 39.Tremaine M T, Brockman D K, Betley M J. Staphylococcal enterotoxin A gene (sea) expression is not affected by the accessory gene regulator (agr) Infect Immun. 1993;61:356–359. doi: 10.1128/iai.61.1.356-359.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]