Abstract
Fertility and hatchability are 2 major parameters that highly influence the reproductive performance of chicken breeds. The objective of this study is to investigate how the genetic background of chickens affects the aspects of fertility, hatchability, and embryonic mortality pattern. Six different native chicken genotypes (black, black-barred, brown, gray, naked neck, and frizzle) kept under similar conditions were evaluated. A total of 1,645 fertile pedigreed eggs from all genetic groups were collected and incubated in forced draft setter. Fertility, hatchability, embryonic mortality, and hatched chick weight were determined. The data were subjected to a one-way analysis of variance with breed (genotype) as a fixed effect. Sire component of variance were used to compute heritability estimates for hatchability traits. The results showed that the fertility and hatchability of the eggs produced from the naked neck or frizzle genotypes exhibited higher values compared to the other genetic groups. An increase in the relative weight of hatched chicks was detected in hatching eggs weighing 44 g or higher. Therefore, attention should be given to the egg size produced by native chicken populations to achieve maximum hatchability performance. Multiple regression analysis revealed that the settable egg weight and the egg weight loss during incubation are the main factors affecting the relative weight of hatched chicks of all genotypes.
Key words: naked neck, frizzle, genotype, genetic group, Saudi chicken
INTRODUCTION
Native chicken breeds represent great value to the majority of consumers, particularly in the rural sector of the most developing and underdeveloped countries. Their meat and eggs are preferred by the majority of rural communities and often urban people (Pym et al., 2006; Ajayi and Agaviezor, 2016; Fathi et al., 2017a). However, poor productive performance of the native chicken breeds is considered an important factor affecting their spread on a large scale. Recently, great attention has been focused on native chicken populations as significant genetic resources. They may contain genes that are important for adaptation to harsh weather and disease resistance. Many strategies are adopted to improve their productive performance through environmental factors (husbandry, nutrition, and health) and/or breeding protocols (selection and crossbreeding). Fertility and hatchability are significant criteria of profitability in the hatchery enterprise for small and medium-sized stakeholders (Peters et al. 2008; Adeleke et al., 2012). Unfortunately, poor fertility and hatchability rates of native chicken breeds are a major threat to preserving these valuable constituents for poultry production (Adebambo, 2005; Allanah et al., 2014; Adedeji et al., 2015). Hatchability is a complex quantitative trait that depends on genetic make-up, incubational conditions, and nutritional factors. Embryonic mortality that occurs during the incubation period, in most cases, is related to inadequate incubation conditions rather than to poor egg quality and an imbalanced diet of the breeder flock (Kumar et al., 2013).There are significant relationships between settable egg weight and hatching results. Egg weight directly effects hatchability, embryonic mortality, hatching weights, and subsequent performance of chicks (Alkan et al., 2008; Çağlayan et al., 2009; Duman and Şekeroğlu, 2017).
In Saudi Arabia, people traditionally raise native chickens for their preferred eggs and ornamented appearance, especially those segregating for major genes (Fathi et al., 2017a). As a result of a national project funded by King Abdulaziz City for Science and Technology in Saudi Arabia and launched in 2011, six distinct chicken breeds based on their plumage color, productive performance, and genetic evaluation were characterized and established (Fathi et al., 2017a,b and Fathi et al., 2018). To present, there is no comprehensive study on fertility and hatchability performance that has been conducted on well-characterized Saudi native chicken breeds. However, an attempt was found on eggs of indigenous chickens collected from free-range local farms in Saudi Arabia's eastern region (Abudabos et al., 2017). The current study aimed at evaluating the features of fertility, hatchability, and embryonic mortality pattern as affected by genotype and egg weight of native chicken genotypes that are conserved at Qassim University research farm. Besides, stepwise multiple regression analysis was applied to determine the factors affecting chick weight at hatch as influenced by genotype and/or settable egg weight.
MATERIALS AND METHODS
Birds, Management and Experimental Design
Six genotypes of Saudi native chickens (black, black-barred, brown, gray, naked neck, and frizzle) were utilized to evaluate the fertility, hatchability, and embryonic mortality pattern. The morphological description, productive performance, and genetic origin of these breeds are fully explained in the previous review accomplished by Fathi et al. (2017a). Each genotype was represented by 6 to 8 pens. Each family was assigned to one sire for eight dams in parental half-sib pedigreed pens. The families were housed in littered floor pens measuring 150 cm (L) × 150 cm (W). The birds were fed on a breeder diet that contained 16.6% crude protein, 2,875 ME kcal/kg, 3.7% calcium, and 0.45% available phosphorous. The feed and drinking water were supplied ad libitum throughout the entire experimental period. All birds were kept under similar environmental, nutritional, and health conditions. The care and handling of the birds were in accordance with the regulations of the committee of research ethics for basic and applied sciences at Qassim University.
Egg Collection and Incubation Procedure
A total of 1,645 pedigreed eggs from the half-sib families were collected and properly identified according to each genotype. Cracked, dirty, and misshapen eggs were excluded. The fertile eggs were preserved before being set in a holding room with a temperature of 14° to 16°C and 75% relative humidity. Prior to setting, the weight of eggs was individually recorded to the nearest 0.01 g. The eggs were incubated in an automatically forced draft machine maintained at a temperature of 37.8°C and 30°C of wet bulb thermometer. The incubated eggs were turned on an hourly basis at a 90° angle. The eggs were candled on the 7th day of incubation to recognize the infertile (clear) eggs that did not show any embryonic development. Fertility was defined as the proportion of the fertile eggs to the total number of settable eggs for each genotype. The eggs containing blood rings were classified as early embryonic death. After candling and elimination of clear eggs, they were reset in the setter. On d 18 of incubation, the candling inspection was performed, and the eggs containing dead embryos were broken out to identify mid-embryonic mortality according to Tesarova et al. (2021). The eggs containing survival embryos were transferred to hatching baskets after weighing to determine the egg weight loss. On hatching day, the unhatched eggs were opened and examined to verify the embryonic mortality pattern, including pipped and dead-in-shell deaths. The intact shell with a dead chick inside during the last 3 d of incubation was considered a dead-in-shell case. The chick failed to hatch with an emerged beak via the shell was recorded as a pipped chick. Upon hatching, the baby chicks were sorted for each family within the breed and weighed to the nearest 0.01 g. The hatchability rate was calculated by dividing the number of hatched chicks by the number of fertile settable eggs. The remaining shell for each egg was collected and weighed to the nearest 0.01 g. To measure the shell thickness of hatching eggs, pieces from three different regions were measured with a dial gauge micrometer (Ames, Massachusetts, USA) to the nearest 0.01 mm.
Statistical Analysis
The data of fertility, hatchability and embryonic mortality were subjected to a one-way analysis of variance with breed as the fixed effect using JMP Ver. 11 (SAS Institute, 2013). The model applied is as follows:
Where:
Yij is the observation taken on the Jth egg, μ is the overall mean, Bi is the a fixed effect of the ith breed, eij is the random error assumed to be independent normally distributed with mean = 0 and variance = σ2.
The settable eggs were classified into three different egg weight classes including, small (˂40 g), medium (≥40 g to ≤44 g) and large (˃ 44 g). All results are presented as mean and the pooled SEM. The significance of difference between means was assessed using Tukey's test. Significance was set as P < 0.05. Correlation procedure (PROC CORR) was used to compute the relationship between egg properties and chick weight at hatch. To estimate variance components Restricted Maximum Likelihood (Reml) method was applied using VARCOMP procedure. Heritability estimates for hatchability traits computed from sire component of variance and from parental half-sibs analysis were calculated as follows:
Stepwise regression analysis was applied using PROC REG to determine factors affecting chick weight at hatch according to breed and/or egg weight using the following model:
Where:
Yi = the dependent variable, a = the intercept, βn = the regression coefficients, Xn = independent variables, ei = error.
RESULTS
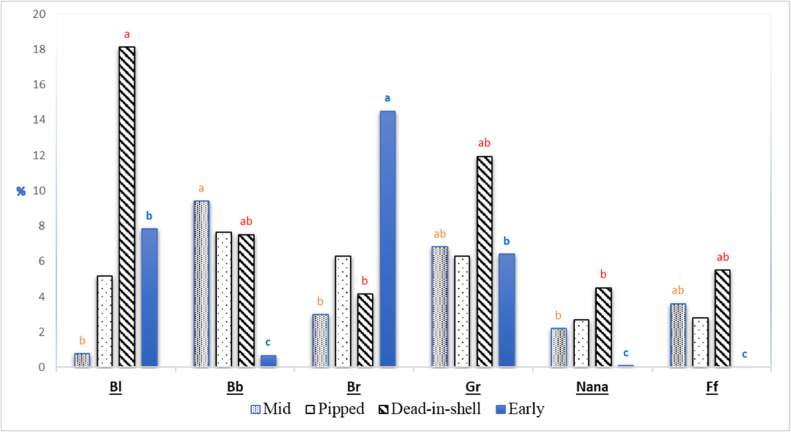
The results of the fertility, hatchability, and embryonic mortality patterns according to genotype effect are presented in Table 1. Both fertility and hatchability of fertile eggs were significantly affected (P ˂ 0.05) by genotype. The brown breed recorded a significantly lower fertility (P ˂ 0.01) compared with that of hens carrying Na and F genes or the black breed. Moreover, the highest fertility percentage was recorded for the naked neck genotype (95.6). On the other hand, the black and gray breeds recorded significantly lower hatchability (68.2 and 68.6%, respectively) compared to the naked neck and frizzle genotypes. The black-barred and brown breeds were intermediate. With respect to the embryonic mortality pattern, a significant difference in all mortality types was noticed among genotypes, except for pipped chicks (Figure 1).
Table 1.
Fertility, hatchability, and embryonic mortality pattern of varied genotypes of chickens.
| Trait, % | Genotype |
|||||||
|---|---|---|---|---|---|---|---|---|
| Bl | Bb | Br | Gr | Na | F | SEM | Prob. | |
| Fertility, % | 94.0a | 90.2 ab | 77.3b | 89.2 ab | 95.6a | 92.0a | 1.98 | <0.01 |
| Hatchability, % | 68.2b | 74.8ab | 72.0ab | 68.6b | 90.5a | 88.1a | 3.20 | <0.05 |
| Early | 7.8ab | 0.7b | 14.5a | 6.4ab | 0.1b | 0.0b | 1.32 | <0.01 |
| Mid | 0.8b | 9.4a | 3.0ab | 6.8ab | 2.2ab | 3.6ab | 0.76 | <0.01 |
| Pipped | 5.2 | 7.7 | 6.3 | 6.3 | 2.7 | 2.8 | 0.79 | 0.44 |
| Dead-in-shell | 18.1a | 7.5ab | 4.1b | 11.9ab | 4.5b | 5.5b | 1.38 | <0.01 |
Abbreviations: Bl, black breed; Bb, black-barred breed; Br, brown breed; F, frizzle breedand; Gr, gray breed; Na, naked neck breed.
Means within row with different superscript litters are significantly different from each other.
Figure 1.
Embryonic mortality pattern as affected by chicken genotype. a..cSignificant letters stand/provide while P ≤ .01. Bl = black breed, Bb = black-barred breed, Br = brown breed, and Gr = gray breed, Nana = naked neck breed, Ff = frizzle breed.
The brown breed recorded the highest percentage of early embryonic mortality among all the different genetic groups. This difference was significant when compared with the black-barred, naked neck, and frizzle genetic groups. The highest percentage of mid embryonic mortality was recorded for the black-barred breed (9.4%) among all the different genetic groups, while the black one recorded the lowest percentage (0.8%). The black breed had significantly higher dead-in-shell deaths (18.1%) when compared with the naked neck and frizzle genetic groups (4.5 and 5.5%, respectively) and the brown breed (4.1%).
Hatchability aspects as affected by chicken genotype are presented in Table 2. Generally, it was seen that the genotype had a significant effect (P ˂ 0.001) on all studied traits. The naked neck and frizzle genotypes recorded the highest chick weight compared to the other genotypes carrying no marker genes. Accordingly, there was a significant increase in eggshell weight for the naked neck and frizzle genotypes compared to individuals who do not carry these marker genes. Shell thickness was significantly increased (P 0.001) in the naked neck, black, and gray breeds when compared to the frizzle and black-barred genotypes. The egg weight loss during the incubation period was significantly lower (P ˂ 0.001) in the fertile eggs produced from black-barred, brown and gray breeds compared with those of the other genotypes. Upon hatch, the remaining eggshell percentage and the relative weight of the newly hatched chick were significantly higher in the frizzle genotype compared to the other genotypes.
Table 2.
Some hatchability aspects of varied genotypes of chicken populations.
| Trait | Genotype |
|||||||
|---|---|---|---|---|---|---|---|---|
| Bl | Bb | Br | Gr | Na | F | SEM | Prob. | |
| Settable egg weight, g | 39.9c | 40.2c | 39.6c | 40.6c | 45.9a | 44.2b | 0.16 | <0.001 |
| Chick weight, g | 25.0c | 25.9c | 26.2c | 25.9c | 31.6a | 31.2a | 0.15 | <0.001 |
| Eggshell weight, g | 3.4c | 3.3c | 3.3c | 3.6b | 4.1a | 4.1a | 0.02 | <0.001 |
| Shell Thickness, µ | 313.2abc | 293.0c | 298.6bc | 325.9a | 315.3ab | 299.1c | 1.33 | <0.001 |
| Egg weight loss, % | 11.8a | 8.5b | 9.1b | 9.8b | 12.1a | 11.3a | 0.13 | <0.001 |
| Chick, % | 62.8c | 64.5c | 66.3bc | 64.2c | 69.0b | 70.6a | 0.28 | <0.001 |
| Shell, % | 8.5bc | 8.2c | 8.4bc | 8.9b | 8.9b | 9.3a | 0.04 | <0.001 |
Abbreviations: Bl, black breed; Bb, black-barred breed; Br, brown breed; F, frizzle breedand; Gr, gray breed; Na, naked neck breed.
Means within row with different superscript litters are significantly different from each other.
According to the egg weight classification as shown in Table 3, it could be noticed, as expected, that the chick weight and shell weight significantly increased (P ˂ 0.001) as egg weight increased in a linear manner. It seems that the hatching egg weight does not have a significant effect on both shell thickness and the relative weight of hatched chicks. The relative weight of the remaining shell upon hatch significantly decreased (P ˂ 0.001) in large eggs compared with those of small and medium categories. In terms of egg weight loss during the incubation period, the large category recorded a significantly higher percentage (P ˂ 0.001) compared to small and medium eggs.
Table 3.
Some hatchability traits as affected by settable egg weight.
| Trait | Egg weight |
||||
|---|---|---|---|---|---|
| Small1 | Medium2 | Large3 | SEM | Prob. | |
| Settable egg weight, g | 37.5c | 42.2b | 47.9a | 0.16 | <0.001 |
| Chick weight, g | 25.3c | 28.4b | 32.8a | 0.15 | <0.001 |
| Eggshell weight, g | 3.5c | 3.9b | 4.1a | 0.02 | <0.001 |
| Shell thickness, µ | 311.9 | 310.1 | 311.8 | 1.33 | 0.83 |
| Egg weight loss, % | 10.8b | 10.9b | 11.7a | 0.13 | <0.001 |
| Chick weight, % | 67.4 | 67.3 | 68.4 | 0.28 | 0.06 |
| Shell, % | 9.4a | 9.2a | 8.5b | 0.04 | <0.001 |
Means within row with different superscript litters are significantly different from each other.
Egg weight ˂40 g.
Egg weight ≥40 g to ≤44 g.
Egg weight ˃44 g.
Table 4 gives the estimated heritability values and phenotypic correlations for the hatchability traits. It is of interest to note that the heritability values were apparently high (0.75, 0.47, and 0.46) for chick weight, its relative weight, and eggshell weight, respectively. Low heritability values (0.36 and 0.11) were recoded for shell thickness and egg weight loss, respectively. However, shell percentage trait is considered a non-inheritable character (h2 approaches zero). As expected, chick weight at hatch and eggshell was highly significant and positively correlated with incubated egg weight (0.78 and 0.47, respectively). It is clear that there was no relationship between chick weight at hatch and both shell thickness and egg weight loss. However, chick weight was negatively correlated with shell percentage (−0.19). A significantly negative correlation coefficient (−0.16) was found between relative chick weight and egg weight loss. No relationship (r = 0.0) was found between the shell thickness and the relative weight of the hatched chick.
Table 4.
Estimates of heritability (on the diagonal) and phenotypic correlations for the hatchability properties of settable eggs.
| Trait | Egg weight |
|||||
|---|---|---|---|---|---|---|
| (CWT) | (SWT) | (STH) | (EL) | (CW%) | (S%) | |
| Settable egg weight (EWT) | 0.78*** | 0.47**** | 0.02 | 0.11 | 0.07 | −0.42*** |
| Chick weight (CWT) | 0.75 | 0.49*** | −0.01 | −0.07 | 0.67*** | −0.19*** |
| Eggshell weight (SWT) | 0.46 | 0.25*** | 0.07 | 0.25*** | 0.60*** | |
| Shell Thickness (STH) | 0.36 | 0.03 | 0.00 | 0.25*** | ||
| Egg weight loss (EL) | 0.11 | −0.16** | −0.03 | |||
| Chick weight (CW%) | 0.47 | 0.28*** | ||||
| Shell (S%) | 0.01 | |||||
p < 0.01.
p < 0.001.
p < 0.0001.
The results of the regression analysis for chick weight at hatch as a dependent variable are given in Table 5. Overall, irrespective of genetic group or egg weight classification, it was found that the settable egg weight was the best predictor of hatched chick weight, followed by shell weight and egg weight loss during incubation. It could be noticed that the R2 increased from 0.61 to 0.65 in the third step. With respect to genetic group effect, it could be observed that the settable egg weight was the unique factor affecting chick weight at hatch in black, black-barred, and brown genotypes. In chickens carrying the major genes (Na and F), water loss during the incubation period was the most significant factor following egg weight (R2 = 0.71 and 0.64 for Nana and Ff, respectively). However, according to egg weight classification, shell properties and water loss represent the main factors affecting chick weight at hatch in medium-sized eggs, while the other categories (small and large) exhibited the same trend as found in all studied genotypes.
Table 5.
Stepwise regression analysis for hatched chick weight as a dependent variable.
| Partial regression coefficient |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model1 | Step | Intercept | Settable egg wt | Swt | STH | SP | Water loss | R2 | Prob. |
| Overall | 1 | −1.8 | 0.72 | - | - | - | - | 0.61 | P < 0.0001 |
| 2 | −4.9 | 0.65 | 1.61 | - | - | - | 0.63 | P < 0.0001 | |
| 3 | −3.7 | 0.66 | 1.65 | - | - | −0.18 | 0.65 | P < 0.0001 | |
| Bl | 1 | 13.7 | 0.28 | 0.21 | P < 0.001 | ||||
| Bb | 1 | 10.3 | 0.39 | 0.26 | P < 0.001 | ||||
| Br | 1 | 11.1 | 0.38 | 0.20 | P < 0.01 | ||||
| Gr | 1 | 11.4 | 0.36 | 0.21 | P < 0.0001 | ||||
| 2 | 8.63 | 0.25 | 1.97 | 0.29 | P < 0.0001 | ||||
| 3 | 45.3 | −0.62 | 11.88 | −4.16 | 0.33 | P < 0.05 | |||
| Na | 1 | 1.54 | 0.66 | 0.58 | P < 0.0001 | ||||
| 2 | 6.37 | 0.65 | −0.37 | 0.71 | P < 0.0001 | ||||
| F | 1 | 0.08 | 0.71 | 0.58 | P <0.0001 | ||||
| 2 | 2.73 | 0.71 | −0.23 | 0.64 | P < 0.0001 | ||||
| S | 1 | 3.6 | 0.58 | 0.20 | P < 0.0001 | ||||
| M | 1 | 19.2 | 2.37 | 0.15 | P < 0.0001 | ||||
| 2 | 19.9 | 8.3 | −2.6 | 0.21 | P < 0.0001 | ||||
| 3 | 21.4 | 8.9 | −2.8 | −0.19 | 0.27 | P < 0.0001 | |||
| 4 | 23.9 | 8.9 | −10.1 | −2.7 | −0.19 | 0.29 | P < 0.05 | ||
| L | 1 | −1.3 | 0.71 | 0.31 | P < 0.0001 | ||||
| 2 | −19.2 | 0.88 | 1.2 | 0.36 | P < 0.0001 | ||||
| 3 | −16.3 | 0.87 | 1.2 | −0.24 | 0.41 | P < 0.0001 | |||
n = 1,645.
Models are computed based on the eggs collected from; overall, black (Bl), black-barred (Bb), brown (Br), gray (Gr), naked neck (Na), frizzle (F), small (S), medium (M) and large (L) egg weight, respectively.
DISCUSSION
It is well recognized that the hatchability percentage plays a significant role in the poultry industry. Accordingly, improving the hatchability of eggs, particularly chick weight, is of utmost importance in poultry breeding strategies. There is a lack of research evaluating the hatching performance of Saudi native chicken genotypes. Fertility was significantly affected by genotype, and the brown breed recorded the lowest percentage. Similar results were reported by several investigators (Alsobayel et al., 2013; Allanah et al., 2014; Grochowska et al., 2019). The most important finding of this study was that the naked neck and frizzle genotypes had a higher hatchability percentage compared with other native breeds. In agreement with our findings, Sharifi et al. (2010a) reported that the naked neck genotype (NaNa) had higher fertility, hatchability, and number of live chicks in comparison with the normally feathered hens (nana) under thermal stress. Additionally, the genotype combining the 2 major genes (FFdw-) proved to be superior in fertility to the normally feathered dwarf type (ffdw-) (Sharifi et al., 2010b). Moreover, the chickens carrying the naked neck and frizzle genes had significantly higher packed cell volume concentration than the normal plumage genotype (Asumah et al., 2022). On the other hand, low hatchability and high embryonic mortality were observed in genotypes having frizzle or naked genes in Nigerian indigenous chickens (Ajayi and Agaviezor, 2016). Adeleke et al. (2012) found that the fertile eggs produced from naked neck sires had the highest dead-in-shell compared with the others produced from frizzle and normal plumage. The results of the embryonic mortality pattern revealed that the black breed had the highest percentage of dead in shell type, followed by the gray breed. The survivability of an embryo can be considered as a function of its genotype, which depends on genes received from the sire and dam, and the egg environment, which generally depends entirely on the dam (Wolc et al., 2009). In the current study, the native breeds carrying the naked neck and frizzle genes exhibited lower shell death compared with the other normally feathered genotypes. Also, the naked neck and frizzle hens recorded nearly zero early embryonic mortality compared with normally feathered genotypes. Early embryonic mortality could be attributed to chromosomal aberrations and lethal genes, which suggests that embryo survival is a trait of both sire and dam (Liptói and Hidas, 2006). Accordingly, chickens having Na or F genes are preferred for raising under Saudi environmental conditions.
It was seen that both genotype and incubated egg weight significantly affected hatchability performance. The highest chick weight was recorded for naked neck and frizzle genotypes compared with the other genetic groups, resulting from the higher settable egg weight associated with naked neck or frizzle chickens. Inconsistent with our findings, Alsobayel et al. (2012) indicated that the breed had a significant effect on most studied traits of fertility and hatchability in commercial broiler breeders. Egg weight was also found to be significantly related to chick weight at hatch and egg weight loss during incubation (Caglayan et al., 2008; Alsobayel et al., 2013; Abudabos et al., 2017). Similarly, the weight of settable eggs produced from the naked neck genotype was higher than that of the Nigerian local breeds (Adedeji et al., 2015). This may be attributed to the greater adaptation of naked neck hens to harsh environmental conditions, particularly hot ambient temperatures, than the other native chicken breeds. Chick quality has been found to be affected by settable egg weight (Ramaphala and Mbajiorgu, 2013; Iqbal et al., 2016; Abudabos et al., 2017). Therefore, sorting fertile eggs by weight prior to incubation might be advantageous to improve chicken uniformity and productive efficiency (Wilson, 1991). Also, genotype represents the most important determinant of the percentage of egg weight loss rather than breeder flock age and egg storage time (Grochowska et al., 2019). In the current study, egg weight loss during incubation was shown to be significantly influenced by genotype as well as the egg weight. The naked neck and frizzle chickens had a significantly (P ˂ 0.001) lower egg weight loss when compared to the normally feathered breeds. This result is in accordance with the findings of Abudabos (2010) and Alsobayel et al. (2013), who indicated that broiler breeder strain significantly affected the percentage of egg weight loss during incubation and storage period, respectively. It seems that egg weight classification did not significantly (P ≥ 0.06) influence the relative weight of hatched chicks. However, an increase (almost 1%) in chick percentage was recorded in large eggs as compared to small and medium egg weight categories. A reduction has been noticed in the remaining shell percentage of large eggs compared to those of small and medium eggs. This may be due to the fact that the chick with the large weight needs more calcium for the formation of bone, nails, and beak.
As expected, chick weight at hatch was positively correlated (P ˂ 0.001) with the settable egg weight. This finding generally agrees with that of several studies. Narushin et al. (2002) reported that the egg weight alone had the biggest correlation with chick weight (r = 0·56) in comparison with the other egg characteristics. In selected Japanese quail hens for egg production, Alkan et al. (2008) found a significant positive phenotypic correlation (r = 0.72) between egg weight and newly hatched chick weight. Embryo weight is not correlated with egg weight during the first half of the incubation period, while the correlation increases thereafter and reaches a maximum (0.5–0.95) at the time of hatching (Wilson, 1991). A highly significant positive correlation (0.25) was found between the weight and thickness of the eggshell. This result is in agreement with those of Hristakieva et al. (2017), who found a positive correlation coefficient (r = 0.52 and r = 0.68) in eggs produced by 34-wk-old and 46-wk-old turkeys. It is important to note that the egg weight loss during incubation was negatively correlated (r = −0.16) with the relative hatched weight, that is, the lower the egg weight loss, the higher the newly hatched chick weight. Similarly, Hristakieva et al. (2017) reported a negative correlation (up to r = −0.80) between the egg weight loss during incubation and both absolute and relative hatchling weights of turkeys. There was no relationship between chick weight at hatch and eggshell thickness (r = −0.01). This result is fully in agreement with the findings of El-Safty (2011), who found a correlation coefficient (−0.02) between chick weight and eggshell thickness in ostrich. According to a high heritability estimate of the relative weight of the hatched chick, this trait could be improved by selection within each genotype. The low heritability value recoded for egg weight loss means that the external factors surrounded by settable eggs must be taken into consideration during incubation. However, the heritability estimates of chick weight at hatch are widely varied in indigenous chickens in the tropics, resulting from the method of estimation, location at which the data was collected (Ndung'u et al., 2020), or due to the small sample size used for each genotype (Singh et al., 2009). An insubstantial overestimation of heritability may be obtained for hatchability traits resulting from a small sire effect. Based on stepwise multiple regression for chick weight at hatch as a predictor, it could be concluded that the egg weight and the egg weight loss during incubation are significant factors affecting the relative weight of hatched chicks, whether the analysis is performed based on genotype or egg weight classification.
CONCLUSIONS
In conclusion, our study indicates that the naked neck and frizzle chicken breeds recorded the higher figures of fertility, hatchability, and chick weight at hatch among all native breeds. Sorting settable egg weights of native chicken genotypes prior to incubation might be essential for producing a good hatching performance. The category of large egg weight (over 44 g) recorded an increase (almost 1%) in the relative weight of hatched chicks. It was concluded that heavier settable eggs produced a higher relative weight of newly hatched chicks, particularly in the naked neck and frizzle genotypes. A negative correlation coefficient between the relative weight of the hatched chick and the egg weight loss during incubation was found. The results of multiple regression analysis suggest that the settable egg weight and egg weight loss during incubation are the main factors affecting the relative weight of hatched chicks.
DISCLOSURES
The authors declare no conflicts of interest.
REFERENCES
- Abudabos A. The effect of broiler breeder strain and parent flock age on hatchability and fertile hatchability. Int. J. Poult. Sci. 2010;9:231–235. [Google Scholar]
- Abudabos A.M., Aljumaah R.S., Algawaan A.S., Al-Sornokh H., Al-Atiyat R.M. Effects of hen age and egg weight class on the hatchability of free-range indigenous chicken eggs. Braz. J. Poult. Sci. 2017;19:33–40. [Google Scholar]
- Adebambo A.O. Physiological response of poultry to the environmental condition. Poult. Sci. 2005;8:17–19. [Google Scholar]
- Adedeji T.A., Amao S.R., Popoola A.D., Ogundipe R.I. Fertility, hatchability and eggs quality traits of Nigerian locally adapted chickens in the derived savanna environment of Nigeria. J. Biol. 2015;5:36–42. [Google Scholar]
- Adeleke M.A., Peters S.O., Ozoje M.O., Ikeobi C.O., Bamgbose A.M., Adebambo O.A. Effect of crossbreeding on fertility, hatchability and embryonic mortality of Nigerian local chickens. Trop. Anim. Health Prod. 2012;44:505–510. doi: 10.1007/s11250-011-9926-x. [DOI] [PubMed] [Google Scholar]
- Ajayi F.O., Agaviezor B.O. Fertility and hatchability performance of pure and crossbred indigenous chicken strains in the high rainforest zone of Nigeria. Int. J. Livest. Prod. 2016;7:141–144. [Google Scholar]
- Alkan S., Karabag K., Galic A., Balcioglu M.S. Effects of genotype and egg weight on hatchability traits and hatching weight in Japanese quail. S. Afr. J. Anim. Sci. 2008;38:231–237. [Google Scholar]
- Allanah T.O., Okonkwo J.C., Omeje S.I. Fertility and hatchability characterization of three strains of egg type chickens. Sci. J. Biol. Sci. 2014;3:59–68. [Google Scholar]
- Alsobayel A.A., Almarshade M.A., Albadry M.A. Effect of breed, age and storage period on fertility and hatchability of hatching eggs of commercial broilers breeders. Arab. Gulf J. Sci. Res. 2012;30:1–6. [Google Scholar]
- Alsobayel A.A., Almarshade M.A., Albadry M.A. Effect of breed, age and storage period on egg weight, egg weight loss and chick weight of commercial broiler breeders raised in Saudi Arabia. J. Saudi Soc. Agri. Sci. 2013;12:53–57. [Google Scholar]
- Asumah C., Adomako K., Olympio O.S., Hagan B.A., Yeboah E.D. Influence of thermoregulatory (Na & F) genes on performance and blood parameters of F2 and F3 generations of crosses of local and commercial chickens. Trop. Anim. Health Prod. 2022;54:207. doi: 10.1007/s11250-022-03207-6. [DOI] [PubMed] [Google Scholar]
- Caglayan T., Garip M., Kirikci K., Gunlu A. Effect of egg weight on chick weight, egg weight loss and hatchability in rock partridges (A. graeca) Ital. J. Anim. Sci. 2008;8:567–574. [Google Scholar]
- Çağlayan T., Garip M., Kırıkçı K., Günlü A. Effect of egg weight on chick weight, egg weight loss and hatchability in rock partridges (A. graeca) Ital. J. Anim. Sci. 2009;8:567–574. [Google Scholar]
- Duman M.I., Şekeroğlu A. Effect of egg weights on hatching results, broiler performance and some stress parameters. Braz. J. Poult. Sci. 2017;19:255–262. [Google Scholar]
- El-Safty S.A. Using stepwise regression analysis to determine the factors affecting chick weight at hatch in ostrich (struthiocamelus) Egypt. Poult. Sci. 2011;31:695–704. [Google Scholar]
- Fathi M.M., Al-Homidan I., Abou-Emera O.K., Al-Moshawah A. Characterisation of Saudi native chicken breeds: a case study of morphological and productive traits. Worlds Poult. Sci. J. 2017;73:916–927. [Google Scholar]
- Fathi M.M., Al-Homidan I., Motawei M.I., Abou-Emera O.K., El-Zarei M.F. Evaluation of genetic diversity of Saudi native chicken populations using microsatellite markers. Poult Sci. 2017;96:530–536. doi: 10.3382/ps/pew357. [DOI] [PubMed] [Google Scholar]
- Fathi M.M., El-Zarei M.F., Al-Homidan I., Abou-Emera O.K. Genetic diversity of Saudi native chicken breeds segregating for naked neck and frizzle genes using microsatellite markers. Asian-Australas J. Anim. Sci. 2018;31:1871–1880. doi: 10.5713/ajas.18.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grochowska E., Kinal A., Sobek Z., Siatkowski I., Bednarczyk M. Field study on the factors affecting egg weight loss, early embryonic mortality, hatchability, and chick mortality with the use of classification tree technique. Poult. Sci. 2019;98:3626–3636. doi: 10.3382/ps/pez180. [DOI] [PubMed] [Google Scholar]
- Hristakieva P., Oblakova M., Mincheva N., Lalev M., Kaliasheva K. Phenotypic correlations between the egg weight, shape of egg, shell thickness, weight loss and hatchling weight of turkeys. Slovak J. Anim. Sci. 2017;50:90–94. [Google Scholar]
- Iqbal J., Khan S.H., Mukhtar N., Ahmed T., Pasha R.A. Effects of egg size (weight) and age on hatching performance and chick quality of broiler breeder. J. Appl. Anim. Res. 2016;44:54–64. [Google Scholar]
- Kumar A., Das K., Bharti A., Kumar R., Singh A.K. Embryonic morality pattern in black rock, gramapriya and vanaraja breeds of chicken. Progress. Res. 2013;8:98–100. [Google Scholar]
- Liptói K., Hidas A. Investigation of possible genetic background of early embryonic mortality in poultry. Worlds Poult. Sci. J. 2006;62:326–337. [Google Scholar]
- Narushin V.G., Romanov M.N., Bogatyr V.P. Ap–animal production technology: relationship between pre-incubation egg parameters and chick weight after hatching in layer breeds. Biosyst. Eng. 2002;83:373–381. [Google Scholar]
- Ndung'u C.W., Okeno T.O., Muasya T.K. Pooled parameter estimates for traits of economic importance in indigenous chicken in the tropics. Livest. Sci. 2020;239 [Google Scholar]
- Peters S.O., Ilori B.M., Ozoje M.O., Ikeobi C.O.N., Adebambo O.A. Gene segregation effects on fertility and hatchability of pure and crossbred chicken genotypes in the humid tropics. Int. J. Poult. Sci. 2008;7:954–958. [Google Scholar]
- Pym R.A.E., GuerneBleich E., Hoffmann I. The relative contribution of indigenous chicken breeds to poultry meat and egg production and consumption in the developing countries of Africa and Asia. Proceedings of the XII European Poultry Conference. 2006; Vol. 1014. [Google Scholar]
- Ramaphala N.O., Mbajiorgu C.A. Effect of egg weight on hatchability and chick hatch-weight of Cobb 500 broiler chickens. Asian J. Anim. Vet. Adv. 2013;8:885–892. [Google Scholar]
- SAS Institute . SAS Institute Inc.; Cary, NC: 2013. JMP Version 11. User's Guide. [Google Scholar]
- Sharifi A.R., Horst P., Simianer H. The effect of naked neck gene and ambient temperature and their interaction on reproductive traits of heavy broiler dams. Poult. Sci. 2010;89:1360–1371. doi: 10.3382/ps.2009-00593. [DOI] [PubMed] [Google Scholar]
- Sharifi A.R., Horst P., Simianer H. The effect of frizzle gene and dwarf gene on reproductive performance of broiler breeder dams under high and normal ambient temperatures. Poult. Sci. 2010;89:2356–2369. doi: 10.3382/ps.2010-00921. [DOI] [PubMed] [Google Scholar]
- Singh G., Singh S.K., Kumar B., Kumar H. Genetic study of egg and hatch weight in different genetic groups of chicken. Indian J. Anim. Res. 2009;43:200–202. [Google Scholar]
- Tesarova M.P., Skoupa M., Foltyn M., Tvrdon Z., Lichovnikova M. Research note: Effects of preincubation and higher initiating incubation temperature of long-term stored hatching eggs on hatchability and day-old chick and yolk sac weight. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson H.R. Interrelationships of egg size, chick size, posthatching growth and hatchability. Worlds Poult. Sci. J. 1991;47:5–20. [Google Scholar]
- Wolc A., White I.M.S., Olori V.E., Hill W.G. Inheritance of fertility in broiler chickens. Genet. Sel. Evol. 2009;41:47. doi: 10.1186/1297-9686-41-47. [DOI] [PMC free article] [PubMed] [Google Scholar]