Abstract
Escherichia coli mukF, mukE, and mukB null mutants have common phenotypes such as temperature-dependent colony formation, anucleate cell production, chromosome cutting by septum closure, and abnormal localization of SeqA-DNA clusters. We show here that the associated muk null mutations cause hypersensitivity to novobiocin. Null mutation of either dam or seqA suppressed partially the temperature-sensitive lethality but failed to suppress the anucleate cell production and the hypersensitivity to novobiocin caused by muk null mutations.
The mukF, mukE, and mukB genes are essential for faithful partitioning of sister chromosomes into both daughter cells in Escherichia coli (15, 17, 28). Null mutation of each muk gene causes the medium-dependent, temperature-sensitive, lethal phenotype and produces a significant number of anucleate cells of normal size during growth at permissive low temperature (16, 28). The three Muk proteins form a complex in vitro (29). Purified MukB protein has a DNA binding activity, an ATP and GTP binding activity (15), and a Mg2+-dependent ATPase activity (12, 29). The N-terminal globular domain of MukB binds to filaments of the FtsZ protein polymer (12) and to eukaryotic microtubules in vitro (13). To analyze the in vivo function of MukB, various suppressor mutations and synthetic-lethal mutations have been identified (10, 25–27). Mutations of the topA gene, encoding topoisomerase I, suppress the temperature-sensitive growth and anucleate cell production caused by null mutation of each muk gene. The suppression correlates with excess negative supercoiling by DNA gyrase, because the gyrase inhibitor coumermycin reverses the suppression caused by the topA mutations, suggesting that muk mutations cause a defect in chromosome folding and DNA condensation (20).
DNA is fully methylated by DNA adenine methyltransferase (Dam methylase) in E. coli wild-type cells (1, 2, 5). Following initiation from the chromosomal origin (oriC), newly synthesized nascent DNA strands acquire a hemimethylated state at Dam methylation sites. The seqA gene is essential for control of synchronous initiation of chromosome replication (3, 14, 23). The purified SeqA protein preferentially binds GATC sequences in hemimethylated DNA (4, 22). SeqA is localized as discrete foci in exponentially growing wild-type cells of E. coli (7, 18). Formation of the visible SeqA foci depends on Dam methylation (7, 18) and ongoing replication (8), suggesting clusters of SeqA molecules which bind to hemimethylated nascent DNA strands. A single SeqA focus localized at midcell seems to separate into two foci, and these foci subsequently migrate rapidly in opposite directions to 1/4 and 3/4 positions of the cell (7, 8, 18). In the mukB null mutant, SeqA clusters are abnormal in size and subcellular localization (7, 18), suggesting that MukB may participate in separation or migration of SeqA-DNA clusters. Interestingly, E. coli and related bacteria possess MukF, MukE, and MukB together with SeqA, MutH, and Dam methylase (8).
Weitao et al. (24) showed that, when a seqA null mutation was introduced into a mukB null mutant by P1 transduction, a resulting transductant recovered from the temperature-sensitive growth, the anucleate cell production, and the hypersensitivity to novobiocin caused by the mukB null mutation. However, we report here that either a dam or seqA single mutation or a seqA dam double mutation suppresses partially only the temperature-sensitive growth, not the hypersensitivity to novobiocin and the anucleate cell production, of mukF, mukE, and mukB null mutants.
Partial suppression of temperature-sensitive growth of muk null mutants by a dam or seqA null mutation. To examine the effect of the dam null mutation on the phenotypes of muk null mutants, we introduced a dam::cat mutation into mukB, mukE, mukF, and mukFEB null mutants (Table 1) and also into the isogenic muk+ strain YK1100 by transduction with phage P1vir (21), which was grown in dam-deficient KA468 cells. Chloramphenicol-resistant transductants were isolated after incubation for 5 days at 22°C on L agar medium (9) containing chloramphenicol (7 μg/ml) and sodium citrate (20 mM). After single-colony isolation of 10 transductants at 22°C, these transductants were confirmed for the dam mutation by observing the localization of SeqA with immunofluorescence microscopy (7). In all these transductants, SeqA was distributed throughout the whole nucleoid instead of displaying discrete foci, indicating the absence of Dam methylase (7).
TABLE 1.
Bacterial strains used
| Strain | Genotype | Source or reference |
|---|---|---|
| YK1100 | W3110 except trpC9941 | 28 |
| AZ5372 | YK1100 except ΔmukB::kan | 28 |
| AZ5450 | YK1100 except mukE::kan | 28 |
| AZ5381 | YK1100 except mukF::kan | 28 |
| OT7 | PB 103 except ΔmukFEB::kan | 29 |
| KK347 | YK1100 except ΔmukFEB::kan | This worka |
| NK7253 | NK7254 except seqA::tet | 14 |
| KA468 | NH5402-1 except dam-13::Tn9 | T. Katayama |
P1vir[OT7]→YK1100.
These muk dam double-null mutants were exponentially grown at 22°C in M9 minimal medium (19) supplemented with 0.2 mM MgSO4, 0.1 mM CaCl2, glucose (0.5%), Casamino Acids (0.4%; Difco), and l-tryptophan (50 μg/ml) (MCAT medium) or L medium (1% tryptone-peptone [Difco], 0.5% yeast extract (Difco), 0.5% NaCl, pH 7.4). The cultures were diluted with 0.84% NaCl and spread onto MCAT-agar and L-agar plates, respectively. These plates were incubated at 22, 30, 37, and 42°C for 1 to 4 days to allow formation of visible colonies. The muk dam double-null mutants were able to form colonies at 22, 30, and 37°C (Table 2), while the parental muk null mutants were unable to form colonies at 37°C. These double mutants also failed to grow at 42°C (Table 2). Thus the dam mutation partially suppressed the temperature-sensitive colony formation of these muk null mutants.
TABLE 2.
Effect of dam and seqA mutations on temperature-sensitive colony formation and hypersensitivity to novobiocin in mukB, mukE, mukF, and mukFEB null mutants
| Strain | Relevant genotype | Colony-forming abilitya in:
|
Novobiocin resistanceb (μg/ml) at 22°C in:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MCAT medium at:
|
L medium at:
|
L medium | MCAT medium | ||||||||
| 22°C | 30°C | 37°C | 42°C | 22°C | 30°C | 37°C | 42°C | ||||
| YK1100 | Wild type | 1 | 0.8 | 1.0 | 0.9 | 1 | 0.7 | 3.1 | 1.9 | 200 | 200 |
| KK266 | dam | 1 | 1.0 | 0.8 | 1.1 | 1 | 0.9 | 1.0 | 0.7 | 100 | |
| KK259 | seqA | 1 | 1.0 | 0.9 | 1.1 | 1 | 1.2 | 0.9 | 0.6 | 100 | |
| KK278 | dam seqA | 1 | 0.9 | 1.2 | 1.2 | 1 | 2.2 | 1.5 | 1.7 | 100 | |
| AZ5372 | mukB | 1 | 0.8 | 2 × 10−4 | <1 × 10−5 | 1 | 0.3 | 8 × 10−4 | <1 × 10−5 | 20 | 20 |
| KK267 | mukB dam | 1 | 1.3 | 0.7 | <1 × 10−5 | 1 | 1.0 | 0.4 | <1 × 10−5 | 20 | 20 |
| KK248 | mukB seqA | 1 | 1.0 | 0.8 | <1 × 10−5 | 1 | 1.1 | 1.0 | <1 × 10−5 | 20 | 20 |
| KK279 | mukB dam seqA | 1 | 1.1 | 0.2 | <1 × 10−5 | 1 | 1.0 | 1.4 | <1 × 10−5 | 20 | 10 |
| AZ5450 | mukE | 1 | 0.8 | 5 × 10−4 | <1 × 10−5 | 1 | 1.0 | 2 × 10−4 | <1 ×10−5 | 20 | |
| KK269 | mukE dam | 1 | 0.9 | 0.6 | <1 × 10−5 | 1 | 2.6 | 1.8 | <1 × 10−5 | 20 | |
| KK250 | mukE seqA | 1 | 1.0 | 1.0 | <1 × 10−5 | 1 | 0.8 | 0.1 | <1 × 10−5 | 20 | |
| KK281 | mukE dam seqA | 1 | 0.9 | 0.1 | <1 × 10−5 | 1 | 1.0 | 4 × 10−3 | <1 × 10−5 | 20 | |
| AZ5381 | mukF | 1 | 1.0 | 1 × 10−4 | <1 × 10−5 | 1 | 0.9 | 4 × 10−4 | <1 × 10−5 | 20 | |
| KK268 | mukF dam | 1 | 0.9 | 0.7 | <1 × 10−5 | 1 | 1.1 | 1.0 | <1 × 10−5 | 20 | |
| KK251 | mukF seqA | 1 | 1.5 | 0.9 | <1 × 10−5 | 1 | 1.0 | 0.3 | <1 × 10−5 | 20 | |
| KK280 | mukF dam seqA | 1 | 0.9 | 0.1 | <1 × 10−5 | 1 | 0.9 | 3 × 10−3 | <1 × 10−5 | 20 | |
| KK347 | mukFEB | 1 | 0.7 | 5 × 10−5 | <1 × 10−5 | 1 | 0.9 | 7 × 10−5 | <1 × 10−5 | 20 | |
| KK351 | mukFEB dam | 1 | 1.0 | 0.7 | <1 × 10−5 | 1 | 1.1 | 0.5 | <1 × 10−5 | 20 | |
| KK354 | mukFEB seqA | 1 | 1.4 | 0.4 | <1 × 10−5 | 1 | 0.8 | 0.1 | <1 × 10−5 | 20 | |
| KK282 | mukFEB dam seqA | 1 | 1.3 | 0.1 | <1 × 10−5 | 1 | 1.2 | 0.1 | <1 × 10−5 | 20 | |
Colony-forming ability at 22°C was defined as 1.
The maximum concentration of novobiocin allowing survival of more than 50% of cells.
To test the effect of a seqA null mutation, the seqA::tet mutation from strain NK7253 was introduced into the muk null mutants by P1 transduction. After 5 days of incubation at 22°C in L-agar plates containing tetracycline (7 μg/ml) and sodium citrate (20 mM), five tetracycline-resistant transductants from each transduction experiment were isolated and the seqA null mutation was verified by immunofluorescence microscopy. None of the transductants were stained by the anti-SeqA antibody, indicating the seqA::tet mutation. These muk seqA double mutants formed colonies at 22 and 30°C as efficiently as the parental muk null mutants having the wild-type seqA gene and also as efficiently the seqA transductants obtained from the isogenic muk+ strain (Table 2). In contrast to the parental muk null mutants, these muk seqA double mutants were able to form colonies at 37°C but failed to grow at 42°C (Table 2). Thus, the seqA null mutation also partially suppressed the temperature-sensitive growth of these muk null mutants. The above results were inconsistent with the phenotypes of a seqA mukB double-mutant strain described by Weitao et al. (24). Their double mutant was unable to grow at 25°C but was able to grow at 37°C in M9 glucose medium supplemented with Casamino Acids (24), suggesting a cold-sensitive phenotype for growth.
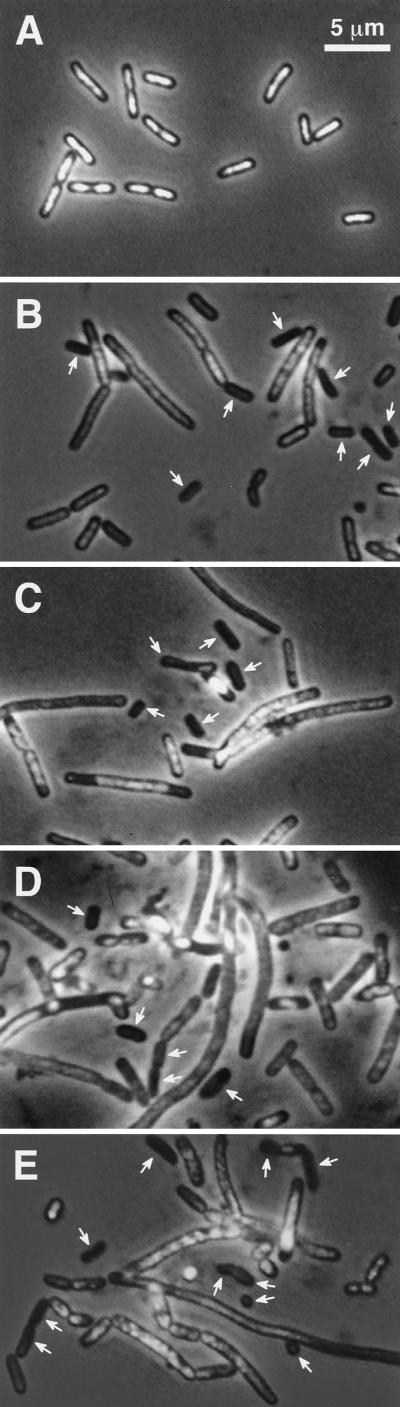
No suppression of anucleate cell formation in muk null mutants by dam and seqA null mutations. We analyzed by fluorescence and phase-contrast microscopy (9) the number of anucleate cells in exponentially growing cultures in MCAT medium at the permissive temperature of 22°C. The percentages of anucleate cells were 0.03, 4.4, 5.0, 4.2, and 5.5% for YK1100, AZ5372, KK267, KK248, and KK279, respectively, indicating that dam and seqA null mutations were unable to suppress anucleate cell formation. These bacterial cells grown exponentially at 22°C were further incubated at 37°C for 4 h. The parental mukB mutant showed heterogeneous lengths of elongated cells having abnormally localized nucleoids and nonseparated large nucleoids (Fig. 1B). In contrast, the mukB dam and mukB seqA double mutants and mukB dam seqA triple mutants showed elongated cells less frequently, indicating that the mutants had partially recovered from defects in cell division. However, anucleate cells were still frequently (10 to 15%) produced at 37°C in these genetic backgrounds (Fig. 1C to E). Thus, dam and seqA mutations were unable to suppress anucleate cell production in the mukB mutant. Similar results were obtained with the other muk mutants having the mukF or mukE null mutation (data not shown).
FIG. 1.
Production of anucleate cells. Cells were exponentially grown at 22°C in MCAT medium and then incubated at 37°C for 4 h. Cells were fixed and stained with DAPI (4′,6′-diamidino-2-phenylindole) (9). (A) YK1100 (wild type). (B) AZ5372 (mukB). (C) KK267 (mukB dam). (D) KK248 (mukB seqA). (E) KK279 (mukB dam seqA). Arrows, anucleate cells or cells with a small amount of chromosomal DNA.
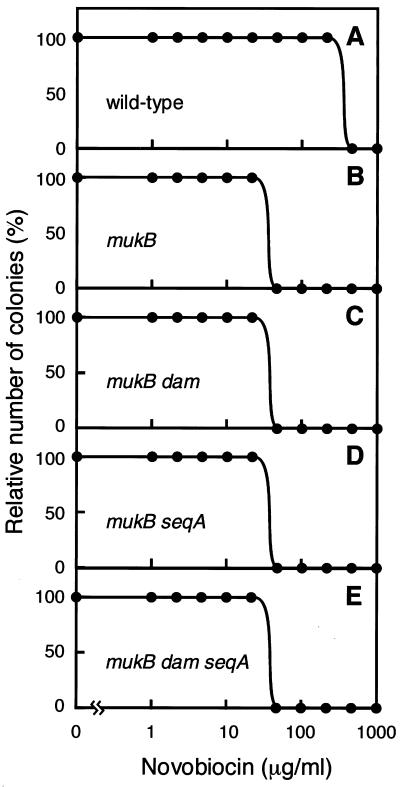
No suppression of the novobiocin hypersensitivity of muk null mutants by dam and seqA mutations. Novobiocin inhibits DNA supercoiling reactions by blocking the B subunit of DNA gyrase (for reviews, see references 6 and 11). We anticipated that novobiocin might have an additive lethal effect in such muk mutants. Bacterial cultures grown exponentially at 22°C in MCAT or L medium were diluted and spread onto MCAT-agar or L-agar plates, respectively, containing various concentrations of novobiocin (0 to 1,000 μg/ml). The plates were incubated at 22°C for 2 days, and the colonies were counted. The maximal concentration of novobiocin allowing growth of the wild-type strain was 200 μg/ml; the corresponding concentration was 20 μg/ml for all muk null mutants (Fig. 2 and Table 2). Thus, muk null mutants are hypersensitive to novobiocin as expected. Introduction of either the dam or seqA null mutation or both the dam and seqA null mutations into muk null mutants failed to suppress the novobiocin hypersensitivity of these muk null mutants (Fig. 2, Table 2). dam or seqA single mutants with the muk+ genetic background were slightly more sensitive to novobiocin (maximum concentration of novobiocin allowing survival of more than 50% of cells, 100 μg/ml) than the wild-type strain (Table 2). The absence of SeqA and Dam is thus not sufficient to suppress the novobiocin hypersensitivity of muk null mutants. It is therefore unlikely that the lack of SeqA restores novobiocin hypersensitivity in the mukB null mutant to the level of the muk+ strain as described by Weitao et al. (24).
FIG. 2.
Colony formation of various strains in the absence or presence of various concentrations of novobiocin. The numbers of colonies that appeared after 3 days of incubation at 22°C were scored. (A) YK1100 (wild type). (B) AZ5372 (mukB). (C) KK267 (mukB dam). (D) KK248 (mukB seqA). (E) KK279 (mukB dam seqA).
Discrepancy between our results and the previously reported results on the effect of the seqA null mutation. Weitao et al. (24) previously described a seqA mukB double-null mutant that showed cold-sensitive growth in minimum glucose medium supplemented with Casamino Acids, no production of anucleate cells, and resistance to novobiocin similar to that of the wild-type strain. By contrast, our present results revealed that all the isolated seqA mukB double mutants were able to grow at 22, 30, and 37°C in MCAT medium; however, these double mutants produced anucleate cells and were still as hypersensitive to novobiocin as the mukB single mutant on the seqA+ genetic background. Probably, the seqA mukB double mutant isolated and analyzed by Weitao et al. (24) had a spontaneous third mutation that was able to suppress the production of anucleate cells and novobiocin hypersensitivity.
Role of the MukFEB complex in dynamic localization of SeqA-DNA clusters. The medium-dependent lethality of muk null mutants may be primarily due to cutting chromosomal DNA by septum closure, the so-called “guillotine effect,” resulting from abnormal localization and structure of nucleoids (16, 28). In the absence of the MukFEB complex, the SeqA-DNA clusters are distributed irregularly and sometimes they seem to fuse to each other due to entanglement of DNA strands in the clusters (7, 18). The MukFEB complex appears to participate in the reorganization of replicated sister chromosomal strands to form two separated, folded sister chromosomes localized at the 1/4 and 3/4 positions. A high degree of entanglement between sister chromosomes likely occurs frequently in muk null mutants. The absence of SeqA-DNA clusters in the dam or seqA null mutants may get rid of the worst condition and rescue partially the viability of muk null mutants. Alternatively, dam and seqA null mutations may cause abnormal expression of various genes and restore indirectly the temperature-sensitive lethality of muk null mutants.
Acknowledgments
We thank Y. Kawata and N. Fukuda for assistance in this laboratory. We thank Barry Holland for critical reading of the manuscript.
This work was supported by grants from the Ministry of Education, Science, Sports, and Culture of Japan and by a grant from the Human Frontier Science Program (RG-386/95M).
REFERENCES
- 1.Bakker A, Smith D W. Methylation of GATC sites is required for precise timing between rounds of DNA replication in Escherichia coli. J Bacteriol. 1989;171:5738–5742. doi: 10.1128/jb.171.10.5738-5742.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boye E, Løbner-Olesen A. The role of dam methyltransferase in the control of DNA replication in E. coli. Cell. 1990;62:981–989. doi: 10.1016/0092-8674(90)90272-g. [DOI] [PubMed] [Google Scholar]
- 3.Boye E, Stokke T, Kleckner N, Skarstad K. Coordinating DNA replication initiation with cell growth: differential roles for DnaA and SeqA proteins. Proc Natl Acad Sci USA. 1996;93:12206–12211. doi: 10.1073/pnas.93.22.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brendler T, Austin S. Binding of SeqA protein to DNA requires interaction between two or more complexes bound to separate hemimethylated GATC sequences. EMBO J. 1999;18:2304–2310. doi: 10.1093/emboj/18.8.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell J L, Kleckner N. E. coli oriC and the dnaA gene promoter are sequestered from dam methyltransferase following the passage of the chromosomal replication fork. Cell. 1990;62:967–979. doi: 10.1016/0092-8674(90)90271-f. [DOI] [PubMed] [Google Scholar]
- 6.Drlica K, Zhao X. DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev. 1997;61:377–392. doi: 10.1128/mmbr.61.3.377-392.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hiraga S, Ichinose C, Niki H, Yamazoe M. Cell cycle-dependent duplication and bidirectional migration of SeqA-associated DNA-protein complexes in E. coli. Mol Cell. 1998;1:381–387. doi: 10.1016/s1097-2765(00)80038-6. [DOI] [PubMed] [Google Scholar]
- 8.Hiraga S, Ichinose C, Onogi T, Niki H, Yamazoe M. Bidirectional migration of SeqA-bound hemimethylated DNA clusters and pairing of oriC copies in Escherichia coli. Genes Cells. 2000;5:327–341. doi: 10.1046/j.1365-2443.2000.00334.x. [DOI] [PubMed] [Google Scholar]
- 9.Hiraga S, Niki H, Ogura T, Ichinose C, Mori H, Ezaki B, Jaffé A. Chromosome partitioning in Escherichia coli: novel mutants producing anucleate cells. J Bacteriol. 1989;171:1496–1505. doi: 10.1128/jb.171.3.1496-1505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jaffé A, Vinella D, D'Ari R. The Escherichia coli histone-like protein HU affects DNA initiation, chromosome partitioning via MukB, and cell division via MinCDE. J Bacteriol. 1997;179:3494–3499. doi: 10.1128/jb.179.11.3494-3499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine C, Hiasa H, Marians K J. DNA gyrase and topoisomerase IV: biochemical activities, physiological roles during chromosome replication, and drug sensitivities. Biochim Biophys Acta. 1998;1400:29–43. doi: 10.1016/s0167-4781(98)00126-2. [DOI] [PubMed] [Google Scholar]
- 12.Lockhart A, Kendrick-Jones J. Interaction of the N-terminal domain of MukB with the bacterial tubulin homologue FtsZ. FEBS Lett. 1998;430:278–282. doi: 10.1016/s0014-5793(98)00677-2. [DOI] [PubMed] [Google Scholar]
- 13.Lockhart A, Kendrick-Jones J. Nucleotide-dependent interaction of the N-terminal domain of MukB with microtubules. J Struct Biol. 1998;124:303–310. doi: 10.1006/jsbi.1998.4056. [DOI] [PubMed] [Google Scholar]
- 14.Lu M, Campbell J L, Boye E, Kleckner N. SeqA: a negative modulator of replication initiation in E. coli. Cell. 1994;77:413–426. doi: 10.1016/0092-8674(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 15.Niki H, Imamura R, Kitaoka M, Yamanaka K, Ogura T, Hiraga S. E. coli MukB protein involved in chromosome partition forms a homodimer with a rod-and-hinge structure having DNA binding and ATP/GTP binding activities. EMBO J. 1992;11:5101–5109. doi: 10.1002/j.1460-2075.1992.tb05617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niki H, Jaffé A, Imamura R, Ogura T, Hiraga S. The new gene mukB codes for a 177 kd protein with coiled-coil domains involved in chromosome partitioning of E. coli. EMBO J. 1991;10:183–193. doi: 10.1002/j.1460-2075.1991.tb07935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Niki H, Yamaichi Y, Hiraga S. Dynamic organization of chromosomal DNA in Escherichia coli. Genes Dev. 2000;14:212–223. [PMC free article] [PubMed] [Google Scholar]
- 18.Onogi T, Niki H, Yamazoe M, Hiraga S. The assembly and migration of SeqA-Gfp fusion in living cells of Escherichia coli. Mol Microbiol. 1999;31:1775–1782. doi: 10.1046/j.1365-2958.1999.01313.x. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. p. A. 3.. [Google Scholar]
- 20.Sawitzke J A, Austin S. Suppression of chromosome segregation defects of Escherichia coli muk mutants by mutations in topoisomerase I. Proc Natl Acad Sci USA. 2000;97:1671–1676. doi: 10.1073/pnas.030528397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
- 22.Slater S, Wold S, Lu M, Boye E, Skarstad K, Kleckner N. E. coli SeqA protein binds oriC in two different methyl-modulated reactions appropriate to its roles in DNA replication initiation and origin sequestration. Cell. 1995;82:927–936. doi: 10.1016/0092-8674(95)90272-4. [DOI] [PubMed] [Google Scholar]
- 23.von Freiesleben U, Rasmussen K V, Schaechter M. SeqA limits DnaA activity in replication from oriC in Escherichia coli. Mol Microbiol. 1994;14:763–772. doi: 10.1111/j.1365-2958.1994.tb01313.x. [DOI] [PubMed] [Google Scholar]
- 24.Weitao T, Nordström K, Dasgupta S. Mutual suppression of mukB and seqA phenotypes might arise from their opposing influences on the Escherichia coli nucleoid structure. Mol Microbiol. 1999;34:157–168. doi: 10.1046/j.1365-2958.1999.01589.x. [DOI] [PubMed] [Google Scholar]
- 25.Yamanaka K, Mitani T, Ogura T, Niki H, Hiraga S. Cloning, sequencing, and characterization of multicopy suppressors of a mukB mutation in Escherichia coli. Mol Microbiol. 1994;13:301–312. doi: 10.1111/j.1365-2958.1994.tb00424.x. [DOI] [PubMed] [Google Scholar]
- 26.Yamanaka K, Ogura T, Koonin E V, Niki H, Hiraga S. Multicopy suppressors, mssA and mssB, of an smbA mutation of Escherichia coli. Mol Gen Genet. 1994;243:9–16. doi: 10.1007/BF00283870. [DOI] [PubMed] [Google Scholar]
- 27.Yamanaka K, Ogura T, Niki H, Hiraga S. Identification and characterization of the smbA gene, a suppressor of the mukB null mutant in Escherichia coli. J Bacteriol. 1992;174:7517–7526. doi: 10.1128/jb.174.23.7517-7526.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamanaka K, Ogura T, Niki H, Hiraga S. Identification of two new genes, mukE and mukF, involved in chromosome partitioning in Escherichia coli. Mol Gen Genet. 1996;250:241–251. doi: 10.1007/BF02174381. [DOI] [PubMed] [Google Scholar]
- 29.Yamazoe M, Onogi T, Sunako Y, Niki H, Yamanaka K, Ichimura T, Hiraga S. Complex formation of MukB, MukE and MukF proteins involved in chromosome partitioning in Escherichia coli. EMBO J. 1999;18:5873–5884. doi: 10.1093/emboj/18.21.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]