Abstract
A group of genes regulated by arginine was found clustered in the order argF-ORF1-argC-argJ-ORF4 between other, as yet uncharacterized, open reading frames (ORFs). Transcription starts were identified immediately upstream from argF and ORF4. Arginine repressed transcription that was initiated at argF but induced transcription of ORF4. The functions of ORF1 and ORF4 are unknown, but analysis of the sequence of ORF4 suggests that it is a membrane protein, possibly involved in transport of arginine or a related metabolite. Mobility shift and DNase I footprinting have revealed specific binding of pure Escherichia coli ArgR to the promoter region of Thermus thermophilus argF. These results suggest that argF transcription is controlled by a repressor homologous to those characterized in enteric bacteria and bacilli. Thermus argF mRNA is devoid of Shine-Dalgarno (SD) sequences. However, downstream from the ATG start codon of argF and many other Thermus genes (with or without an SD box), sequences were found to be complementary to nucleotides 1392 to 1409 of Thermus 16S rRNA, suggesting that an mRNA-rRNA base pairing in this region is important for correct translation initiation.
In arginine biosynthesis two alternative pathways have evolved to split off the acetyl group of N-acetylornithine. Enterobacteriaceae, Vibrionaceae, Myxococcus xanthus, and possibly also the archaeon Sulfolobus acidocaldarius use a linear pathway in which the formation of ornithine is mediated by acetyl ornithinase (encoded by argE) (14, 12, 31, 36). Other bacteria, archaea, and eukaryotic microbes recycle the acetyl group by transacetylation of N-acetylornithine and glutamate (26). The transacetylation is catalyzed by ornithine acetyltransferase (encoded by argJ), an enzyme which in some organisms is also able to use acetyl coenzyme A to acetylate glutamate and in this way bypasses the first step of the linear pathway (11, 26). Ornithine carbamoyltransferase (encoded by argF) converts ornithine and carbamoyl phosphate (CP) into citrulline. CP is extremely thermolabile, and in Pyrococcus furiosus (17), Pyrococcus abyssi (25), and Thermus thermophilus ZO5 (32), it appears to be protected from thermal decomposition into the indiscriminate carbamoylating agent cyanate, by channeling towards citrulline and carbamoyl aspartate.
Arginine genes may be either scattered (as in several proteobacteria, Aquifex aeolicus, cyanobacteria, and archaea) or clustered in two different patterns (2, 36): divergently transcribed clusters (enteric bacteria and Vibrionaceae) or clusters of variable extension where argC and argJ are found together, as in the gram-positive organisms Thermotoga maratima and Thermus (see below). Nevertheless, regulation appears to be similar in most of these organisms: an ArgR repressor interacts with specific operator sequences (called Arg boxes) overlapping the promoter region (5, 6, 12, 18, 28). Homologous proteins were also reported to activate genes involved in arginine degradation in bacilli (19, 20). However, a nonhomologous arginine regulatory protein has been identified in Pseudomonas aeruginosa (22). Except for proteobacteria and gram-positive organisms, little is known about the regulation of arginine biosynthesis. T. thermophilus (24) is an interesting bacterium in two respects, as a paradigm for investigations on extreme thermophily and as a representative of a deep-branching division which also contains Deinococcus radiodurans (its closest relative), Chloroflexus, and Thermomicrobium (34). In this study we describe the organization and expression of an unusual type of arginine gene clustering in T. thermophilus HB27.
For all experiments, Thermus was grown at 75°C in arginine- and uracil-free liquid medium (4) with 20 mM pyruvate as the carbon source and 10 mM ammonium sulfate as the nitrogen source. Chromosomal DNA partially digested with Sau3A was used to construct a λ-ZAP genomic library (Stratagene), which was screened as described by Sanchez et al. (27). Primer extension experiments were performed according to the method described by Kholti et al. (15) except that hybridization experiments were performed overnight at 45°C. Primers were oligonucleotides complementary to the sequences 60 to 80 nucleotides downstream from the ATG codon of each open reading frame (ORF). S1 nuclease mapping was also done according to the method described by Kholti et al. (15). For mobility shift assays, pure Escherichia coli and Bacillus stearothermophilus arginine repressors (final concentration, 45 μg/ml) were incubated for 30 min at 37°C with a 32P-end-labeled DNA fragment in binding buffer (1 mM Tris-HCl [pH 7.4], 5 mM MgCl2, 250 mM KCl, 2.5 mM CaCl2, 0.5 mM dithiothreitol, and 2.5% glycerol) with a 100-fold excess of sonicated herring sperm DNA in the presence or absence of 10 mM arginine. DNA-protein complexes were then immediately loaded on a 6% polyacrylamide gel. For DNase I footprinting experiments (10) a single 32P-end-labeled DNA fragment (100 ng/ml) and a 100-fold excess of nonspecific competitor DNA were incubated for 30 min at 37°C in binding buffer (as for the mobility shift assay described above) containing pure repressor (9 μg/ml). DNase I was added at a final concentration of 0.7 mg/ml, and the digestion was terminated after 30 s by the addition of stop buffer (0.6 M ammonium acetate and 0.05 M EDTA [final concentration]) and 10 μg of yeast tRNA. After DNA precipitation the reaction products were analyzed on a 6% denaturating polyacrylamide gel.
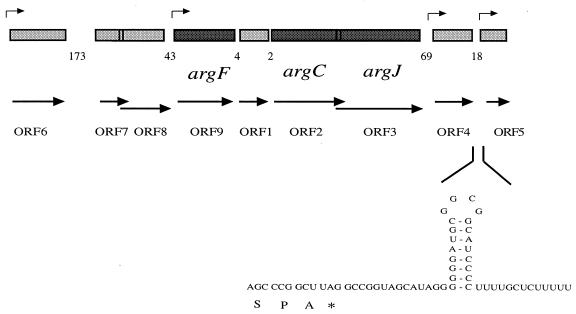
Clustering of arginine biosynthetic genes and unidentified ORFs. By screening the λ-ZAP library we identified in order of transcription ORF6-ORF7-ORF8-argF (i.e., ORF9)-ORF1-argC (i.e., ORF2)-argJ (i.e., ORF3)-ORF4-ORF5 (Fig. 1). The start codon of argJ overlaps the stop codon of argC. This is also the case for ORF7 and ORF8. A putative rho-independent terminator was found between ORF4 and ORF5. A BLAST search showed that ORF6-like proteins belong to the UPF0078 family (National Center for Biotechnology Information database). They occur in several bacteria, such as E. coli (YgiH), Bacillus subtilis (YneS), and Mycoplasma pneumoniae (YgiH), as potential integral membrane proteins containing several putative transmembrane regions. At least five such regions occur in ORF6 (data not shown). Alignment studies showed highly conserved regions in the N-terminal (GATN) and central parts (FKGGKAVAT) of the protein. ORF7 encodes a polypeptide of 113 amino acids; it is homologous to five genes scattered throughout the genome of Methanococcus jannaschii, one of which was found next to carB, which encodes a subunit of CP synthetase. Two exemplars of this gene were found in Synechocystis. These ORF7 homologues all overlap a neighboring gene. In Thermus, ORF7 overlaps ORF8. ORF8-like genes occur in E. coli (yaiS), B. subtilis (ypjG), Mycobacterium leprae (lmbE), and Streptomyces lincolnensis (lmbE). Few genes homologous to ORF1 or ORF4 were found. An ORF1 homologue occurs in Synechocystis with 32% identity at the amino acid level. For ORF4, a similar gene was encountered in D. radiodurans (31% identical amino acids). Interestingly, the N-terminal part of ORF4 is similar to the signal sequence of outer membrane proteins of the OmpA family (Fig. 2). No similarity was found beyond the signal sequence. ORF4 thus probably encodes a membrane protein; in this respect it is reminiscent of the P. aeruginosa OpcD porin (23), which facilitates the diffusion of basic amino acids. There is no obvious similarity between the two sequences, but expression of both OpcD and Thermus ORF4 (see below) is strongly induced by arginine.
FIG. 1.
Schematic drawing of the organization of arginine biosynthetic genes in T. thermophilus. The genes involved in arginine biosynthesis are: argF (ornithine carbamoyltransferase), argC (N-acetyl-gamma-glutamyl-phosphate reductase), and argJ (glutamate N-acetyltransferase). Other ORFs encode proteins with unknown functions. Arrows show the direction and starting points of transcription. The number of nucleotides present between each pair of ORFs is indicated. A putative rho-independent terminator is present between ORF4 and ORF5. The deduced C-terminal amino acid sequence of ORF4 is shown; the stop codon is indicated by an asterisk.
FIG. 2.
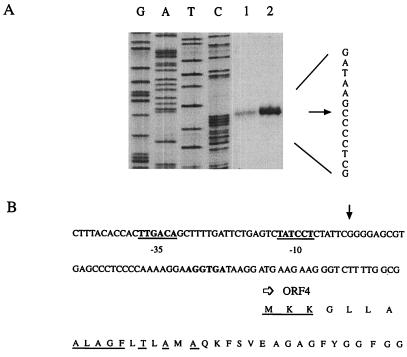
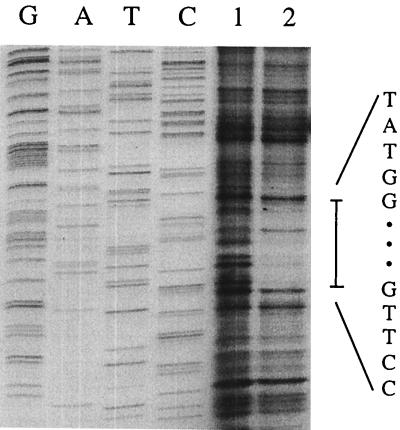
(A) Primer extension mapping of the transcriptional starting point of the T. thermophilus ORF4 region. Equal amounts (about 50,000 cpm) of 5′-end-labeled 20-mer primer were mixed with 100 μg of RNA extracted from cultures grown in minimal medium (1) or supplemented with arginine (2). After precipitation and hybridization the extension reaction was performed. The position of the transcript is indicated by the arrow; the sequence shown is of the noncoding strand. Lanes G, A, T, and C represent chain-terminating DNA-sequencing reactions of the noncoding strand with the oligonucleotide also used as a primer in the primer extension experiment. (B) Nucleotide and deduced amino acid sequences of a portion of the T. thermophilus ORF4 gene and its promoter region. The arrow pointing downward indicates the transcriptional start site. Also, −35 and −10 promoter sequences are underlined and in bold type. A putative ribosome binding site is indicated by bold letters. The underlined amino acids of ORF4 are identical to the signal sequence of seven proteins of the OmpA family (E coli, Salmonella enterica serovar Typhimurium, B. subtilis, Pseudomonas luteolum, Serratia marcescens, Enterobacter aerogenes, and Shigella dysenteriae).
Starting points of transcription. Primer extension experiments performed for each of the genes present in the cluster showed that transcription was initiated just before ORF4 (Fig. 2), ORF5, and ORF6 (data not shown), whereas no transcription starts were observed immediately upstream of ORF7, ORF8, ORF1, argC, or argJ. Transcription from the ORF6 promoter was very weak and not influenced by arginine, whereas transcription of ORF4 was strongly activated in the presence of arginine. In primer extension experiments, argF cDNA synthesis stopped within the coding region, presumably because of a secondary structure forming in the transcript. S1 nuclease mapping showed two major bands (Fig. 3) corresponding to a T residue and a C residue, 2 and 4 nucleotides upstream from the AUG codon, respectively. The intensity was strongly reduced with RNA extracted from cells grown in the presence of arginine. Previous experiments already indicated that arginine represses OTCase synthesis in Thermus (27, 31). The region thus appears to contain four consecutive transcription units: ORF6, ORF7, ORF8, argF; ORF1, argC, argJ; ORF4; and ORF5.
FIG. 3.
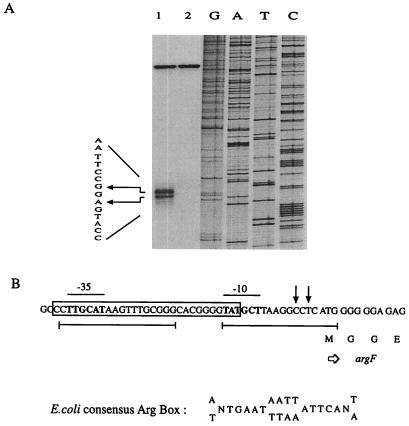
(A) S1 nuclease mapping of the transcriptional starting point of the argF region. Equal amounts (50,000 cpm) of the 5′-end-labeled 150-bp PCR fragment containing the promoter region of argF were hybridized with RNA extracted from cultures grown in minimal medium (lane 1) and minimal medium supplemented with arginine (lane 2). After precipitation and hybridization, S1 nuclease activity proceeded. The position of the transcript is indicated by the arrows; the sequence shown is of the noncoding strand. Lanes G, A, T, and C represent chain-terminating DNA-sequencing reactions of the noncoding strand. (B) Nucleotide and deduced amino acid sequences of a portion of the T. thermophilus argF gene and its promoter region. The arrows indicate the transcriptional start sites. Also, −35 and −10 promoter sequences are indicated and in bold type. Sequences showing similarity to the E. coli consensus Arg box are underlined. Boxed sequences were protected from DNase I treatment.
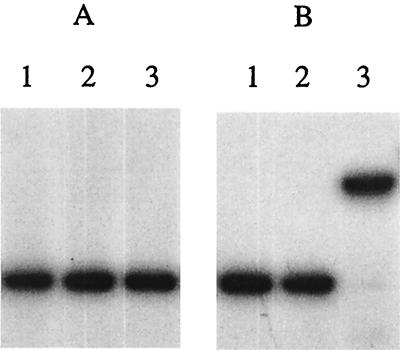
In vitro binding of the E. coli arginine repressor to the Thermus argF promoter region. Thermus argF promoter sequences overlapping the −35 and −10 elements exhibit similarity to the palindromic sequence of the E. coli consensus Arg box; the sequence overlapping the −35 region shows up to 62% identity (Fig. 4). We studied the interaction of pure E. coli ArgR and B. stearothermophilus AhrC with the promoter region of Thermus argF and ORF4 by mobility shift electrophoresis and footprinting. In the presence of arginine, E. coli ArgR binds to the promoter region of Thermus argF (Fig. 4), where it protects a 29-nucleotide region from DNase I digestion (Fig. 5). This region contains the first putative Arg box covering the −35 region and part of the second Arg box (Fig. 4). The latter is not completely protected by E. coli ArgR, probably because of the 7-bp spacing of the two boxes (3). The B. stearothermophilus Arg repressor did not interact with the argF promoter. No arginine repressor was reported so far in Thermus, but in the genome of D. radiodurans there is an ORF encoding a protein clearly homologous to the Arg repressor characterized in enteric bacteria and bacilli. Thus, it appears that Thermus argF is controlled by such a repressor. No specific interaction between the E. coli and B. stearothermophilus arginine repressors could be demonstrated with the Thermus ORF4 promoter region (data not shown). Further studies should show whether repression of argF and induction of ORF4 involve the same Thermus protein.
FIG. 4.
Mobility shift experiments for the Thermus argF promoter region with E. coli ArgR and B. stearothermophilus AhrC in the absence (A) and presence (B) of arginine. Lanes l, no added protein; lanes 2, argF promoter fragment incubated with B. stearothermophilus AhrC; lanes 3, argF promoter fragment incubated with E. coli ArgR.
FIG. 5.
DNase I footprinting of the 210-bp fragment of the Thermus argF promoter region protected by the E. coli arginine repressor. Lanes: G, A, T, and C, sequencing ladders; 1, DNase I reference ladder (without E. coli ArgR); 2, DNase I treatment in the presence of E. coli ArgR. The approximately 29-bp-long protected stretch is indicated by a bar.
Translation initiation signals.
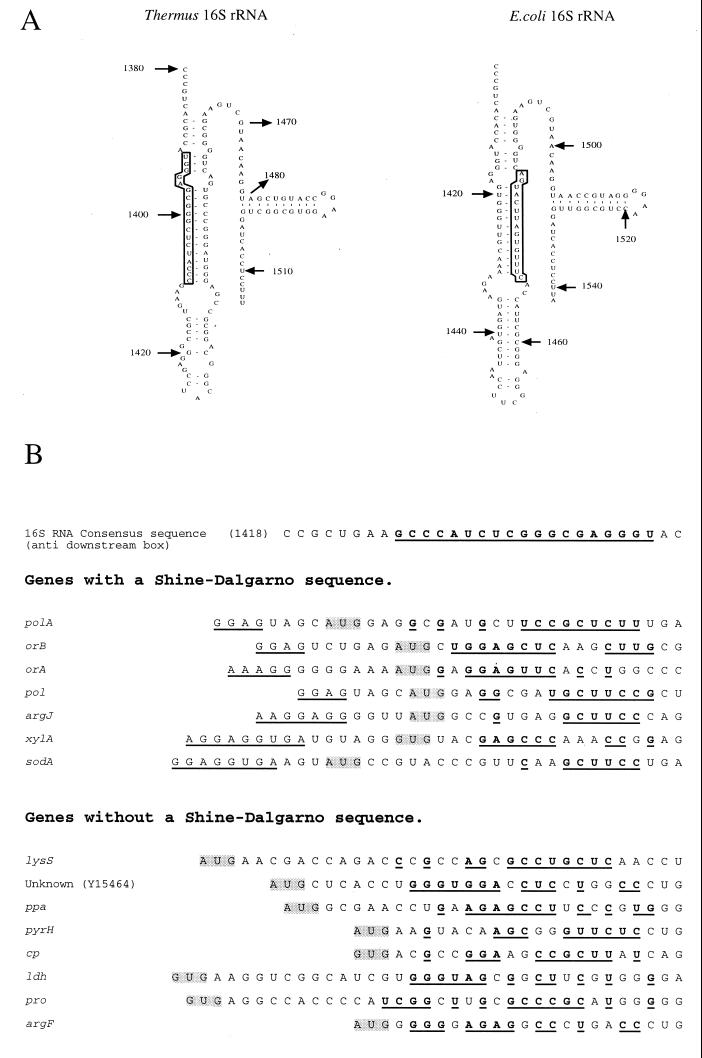
The argF mRNA is devoid of a Shine-Dalgarno (SD) sequence. Some mRNAs lacking SD sequences were found in bacteria, archaea, eukarya (35), and eukaryotic organelles (21), suggesting that information within the coding sequence may be sufficient to signal the translational start in diverse biological systems. In several genes of E. coli and of E. coli bacteriophages, Sprengart et al. (29) identified sequences complementary to nucleotides 1469 to 1483 of 16S rRNA. This region is exposed on the ribosome surface and expected to come into contact with the 3′ end of 16S RNA. Mutations were found in the E. coli gln gene which increase the complementarity of downstream sequences to 16S rRNA and result in higher translational activity (8). We found that 64% of 130 analyzed genes from Thermus (including argF) showed such a downstream box, where at least 6 consecutive nucleotides or at least 8 nucleotides out of 12 complemented the nucleotide-1392-to-1409 region of Thermus 16S rRNA (Fig. 6). This sequence differs from the one mentioned by Sprengart et al. (29, 30) but is situated in a similarly exposed stem-loop structure (Fig. 6). This consensus sequence was found in Thermus mRNAs containing an SD sequence as well as in mRNAs lacking one. These observations suggest that an mRNA-rRNA interaction at the level of these downstream sequences could in some cases compensate for absent SD boxes or reinforce interactions with existing ones.
FIG. 6.
(A) Sequences at the 3′ ends of Thermus and E. coli 16S rRNAs. The region of the 16S RNA molecule complementary to sequences downstream of the AUG start codon of several Thermus and E. coli genes is indicated by boxed nucleotides. (B) Examples of “downstream boxes” in genes from Thermus. Sequences complementary to the region of 16S rRNA shown above are in bold type and underlined. The SD sequence is underlined, and the start codon is indicated by a grey box throughout.
Concluding remarks. Clusters of functionally related genes containing apparently unrelated ORFs have been reported in other organisms (7, 11). It was claimed that the inclusion of genes in unrelated operons may be selectively neutral if the latter are constitutive (16). This is clearly not the case for ORF1, which is coregulated with argF, argC, and argJ. One may argue that the product of coregulated ORFs could stabilize enzymes encoded by the same polycystronic mRNA (13). However, in Thermus, the argF and argJ gene products appear intrinsically stable (1, 27). Another possibility would be that the unknown ORF product is involved in the formation of multienzyme complexes channeling arginine metabolic precursors (17, 32). The situation in D. radiodurans, the closest known relative of Thermus, is also intriguing. Deinococcus argF is followed at 8 bp by a homologue of mutT (encoding an enzyme degrading 8-oxo-dGTP). This mutT gene overlaps argC, itself followed at 4 nucleotides by the gene for glycerol-3-phosphate dehydrogenase (33). In Deinococcus, however, the regulation of those genes has not yet been studied. We feel that the inclusion of unknown or already identified “foreign” genes into defined operons is a neglected area of molecular physiology in need of further investigation.
Nucleotide sequence accession number.
The nucleotide sequence data reported in this paper are available in the EMBL Nucleotide Database under accession number Y18353.
Acknowledgments
This work was supported by a grant from the Flanders Scientific Foundation for Scientific Research (FWO).
We thank D. Charlier for the gift of pure arginine repressor and are grateful for the excellent help of Nadine Huysveld.
REFERENCES
- 1.Baetens M, Legrain C, Boyen A, Glansdorff N. Genes and enzymes of the acetyl cycle of arginine biosynthesis in the extreme thermophilic bacterium Thermus thermophilus HB27. Microbiology. 1998;144:479–492. doi: 10.1099/00221287-144-2-479. [DOI] [PubMed] [Google Scholar]
- 2.Bringel F, Frey L, Boivin S, Hubert J C. Arginine biosynthesis and regulation in Lactobacillus plantarum: the carA gene and the argCJBDF cluster are divergently transcribed. J Bacteriol. 1997;179:2697–2706. doi: 10.1128/jb.179.8.2697-2706.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charlier D, Roovers M, Van Vliet F, Boyen A, Cunin R, Nakamura Y, Glansdorff N, Pierard A. Arginine regulon of Escherichia coli K12. A study of repressor-operator interactions and of in vitro binding affinities versus in vivo repression. J Mol Biol. 1992;226:367–386. doi: 10.1016/0022-2836(92)90953-h. [DOI] [PubMed] [Google Scholar]
- 4.Degryse E, Glansdorff N, Piérard A. A comparative analysis of extreme thermophilic bacteria belonging to the genus Thermus. Arch Microbiol. 1978;177:189–196. doi: 10.1007/BF00402307. [DOI] [PubMed] [Google Scholar]
- 5.Dimova D, Weigel P, Takahashi M, Marc F, Van Duyne G D, Sakanyan V. Thermostability, oligomerisation and DNA-binding properties of the regulatory protein ArgR from the hyperthermophilic bacterium Thermotoga neapolitana. Mol Gen Genet. 2000;263:119–130. doi: 10.1007/pl00008670. [DOI] [PubMed] [Google Scholar]
- 6.Dion M, Charlier D, Wang H, Gigot D, Savchenko A, Hallert J N, Glansdorff N, Sakanyan V. The highly thermostable arginine repressor of Bacillus stearothermophilus: gene cloning and repressor-operator interactions. Mol Microbiol. 1997;25:385–398. doi: 10.1046/j.1365-2958.1997.4781845.x. [DOI] [PubMed] [Google Scholar]
- 7.Fani R, Lio P, Lazeano A. Molecular evolution of the histidine biosynthetic pathway. J Mol Evol. 1995;41:760–774. doi: 10.1007/BF00173156. [DOI] [PubMed] [Google Scholar]
- 8.Faxén M, Plumbridge J, Isaksson L A. Codon choice and potential complementarity between mRNA downstream of the initiation codon and bases 1471–1480 in 16S ribosomal RNA affects expression of glnS. Nucleic Acid Res. 1991;19:5247–5251. doi: 10.1093/nar/19.19.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried M, Crothers D. CAP and RNA polymerase interaction with the lac promoter: binding stoichiometry and long range effects. Nucleic Acids Res. 1983;11:141–158. doi: 10.1093/nar/11.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galas D J, Schmitz A. DNase footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978;5:3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gifford C M, Wallace S S. The genes encoding endonuclease VIII and endonuclease III in Escherichia coli are transcribed as the terminal genes in operons. Nucleic Acids Res. 2000;28:762–769. doi: 10.1093/nar/28.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glansdorff N. Biosynthesis of arginine and polyamines. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: American Society for Microbiology; 1996. pp. 408–433. [Google Scholar]
- 13.Glansdorff N. On the origin of operons and their possible role in evolution towards thermophily. J Mol Evol. 1999;49:432–438. doi: 10.1007/pl00006566. [DOI] [PubMed] [Google Scholar]
- 14.Harris B Z, Singer M. Identification and characterization of the Myxococcus xanthus argE gene. J Bacteriol. 1998;180:6412–6414. doi: 10.1128/jb.180.23.6412-6414.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kholti A, Charlier D, Gigot D, Huysveld N, Roovers M, Glansdorff N. pyrH-encoded UMP-kinase directly participates in pyrimidine-specific modulation of promoter activity in Escherichia coli. J Mol Biol. 1998;280:571–582. doi: 10.1006/jmbi.1998.1910. [DOI] [PubMed] [Google Scholar]
- 16.Lawrence J G, Roth J R. Selfish operons: horizontal transfer may drive the evolution of gene clusters. Genetics. 1996;143:1843–1860. doi: 10.1093/genetics/143.4.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Legrain C, Demarez M, Glansdorff N, Piérard A. Ammonia-dependent synthesis and metabolic channeling of carbamoyl phosphate in the hyperthermophilic archaeon Pyrococcus furiosus. Microbiology. 1995;141:1093–1099. doi: 10.1099/13500872-141-5-1093. [DOI] [PubMed] [Google Scholar]
- 18.Maas W K. The arginine repressor of Escherichia coli. Microbiol Rev. 1994;58:631–640. doi: 10.1128/mr.58.4.631-640.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maghnouj A, Franco de Sousa Cabral T, Stalon V, Vander Wauven C. The arcABDC gene cluster, encoding the arginine deiminase pathway of Bacillus licheniformis, and its activation by the arginine repressor ArgR. J Bacteriol. 1998;180:6468–6475. doi: 10.1128/jb.180.24.6468-6475.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller C M, Baumberg S, Stockley P G. Operator interactions by the Bacillus subtilis arginine repressor/activator, AhrC: novel positioning and DNA-mediated assembly of a transcriptional activator at catabolic sites. Mol Microbiol. 1997;26:37–48. doi: 10.1046/j.1365-2958.1997.5441907.x. [DOI] [PubMed] [Google Scholar]
- 21.Montoya J, Ojala D, Attardi G. Distinctive features of the 5′-terminal sequences of the human mitochondrial mRNAs. Nature. 1981;290:465–470. doi: 10.1038/290465a0. [DOI] [PubMed] [Google Scholar]
- 22.Nishijyo T, Park S-M, Lu C-D, Itoh Y, Abdelal A T. Molecular characterization and regulation of an operon encoding a system for transport of arginine and ornithine and the ArgR regulatory protein in Pseudomonas aeruginosa. J Bacteriol. 1998;180:5559–5566. doi: 10.1128/jb.180.21.5559-5566.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochs M M, Lu C D, Hancock R E, Abdelal A T. Amino acid-mediated induction of the basic amino acid-specific outer membrane porin OprD from Pseudomonas aeruginosa. J Bacteriol. 1999;181:5426–5432. doi: 10.1128/jb.181.17.5426-5432.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oshima T, Imahori K. Description of Thermus thermophilus (Yoshima and Oshima) comb. nov., a nonsporulating thermophilic bacterium from a Japanese thermal spa. Int J Syst Bacteriol. 1974;24:102–112. [Google Scholar]
- 25.Purcarea C, Evans D R, Herve G. Channeling of carbamoyl phosphate to the pyrimidine and arginine biosynthetic pathways in the deep sea hyperthermophilic archaeon Pyrococcus abyssi. J Biol Chem. 1999;274:6122–6129. doi: 10.1074/jbc.274.10.6122. [DOI] [PubMed] [Google Scholar]
- 26.Sakanyan V, Petrosyan P, Lecocq M, Boyen A, Legrain C, Demarez M, Hallet J N, Glansdorff N. Genes and enzymes of the acetyl cycle of arginine biosynthesis in Corynebacterium glutamicum: enzyme evolution in the early steps of the arginine pathway. Microbiology. 1996;142:99–108. doi: 10.1099/13500872-142-1-99. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez R A, Baetens M, van de Casteele M, Roovers M, Legrain C, Glansdorff N. Ornithine carbamoyltransferase from the extreme thermophile Thermus thermophilus. Analysis of the gene and characterisation of the protein. Eur J Biochem. 1997;248:466–477. doi: 10.1111/j.1432-1033.1997.00466.x. [DOI] [PubMed] [Google Scholar]
- 28.Soutar A, Baumberg S. Implication of a repression system, homologous to those of other bacteria, in the control of arginine biosynthesis genes in Streptomyces coelicolor. Mol Gen Genet. 1996;251:245–251. doi: 10.1007/BF02172924. [DOI] [PubMed] [Google Scholar]
- 29.Sprengart M L, Fatscher H P, Fuchs E. The initiation of translation in E. coli: apparent base pairing between the 16S RNA and downstream sequences of the mRNA. Nucleic Acids Res. 1990;18:1719–1723. doi: 10.1093/nar/18.7.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sprengart M L, Porter A G. Functional importance of RNA interactions in selection of translation initiation codons. Mol Microbiol. 1997;24:19–28. doi: 10.1046/j.1365-2958.1997.3161684.x. [DOI] [PubMed] [Google Scholar]
- 31.Van de Casteele M, Demarez M, Legrain C, Glansdorff N, Piérard A. Pathways of arginine biosynthesis in extreme thermophilic archaea and eubacteria. J Gen Microbiol. 1990;136:1177–1183. [Google Scholar]
- 32.Van de Casteele M, Legrain C, Demarez L, Chen P G, Piérard A, Glansdorff N. Molecular physiology of carbamoylation under extreme conditions: what can we learn from extreme thermophilic microorganisms? Comp Biochem Physiol. 1997;188A:463–473. doi: 10.1016/s0300-9629(97)00007-8. [DOI] [PubMed] [Google Scholar]
- 33.White O, Eisen J A, Heidelberg J F, Hickey E K, Peterson J D, et al. Genome sequence of the radioresistant bacterium Deinococcus radiodurans R1. Science. 1999;286:1571–1577. doi: 10.1126/science.286.5444.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woese C R. Bacterial evolution. Microbiol Rev. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu C J, Janssen G R. Translation of vph mRNA in Streptomyces lividans and Escherichia coli after removal of the 5′ untranslated leader. Mol Microbiol. 1996;22:339–355. doi: 10.1046/j.1365-2958.1996.00119.x. [DOI] [PubMed] [Google Scholar]
- 36.Xu Y, Liang Z, Legrain C, Rüger H J, Glansdorff N. Evolution of arginine biosynthesis in the bacterial domain: novel gene-enzyme relationships from psychrophilic Moritella strains (Vibrionaceae) and the evolutionary significance of N-α-acetyl ornithinase. J Bacteriol. 2000;182:1609–1615. doi: 10.1128/jb.182.6.1609-1615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]