Abstract
Ralstonia eutropha H16 degraded (mobilized) previously accumulated poly(3-hydroxybutyrate) (PHB) in the absence of an exogenous carbon source and used the degradation products for growth and survival. Isolated native PHB granules of mobilized R. eutropha cells released 3-hydroxybutyrate (3HB) at a threefold higher rate than did control granules of nonmobilized bacteria. No 3HB was released by native PHB granules of recombinant Escherichia coli expressing the PHB biosynthetic genes. Native PHB granules isolated from chromosomal knockout mutants of an intracellular PHB (i-PHB) depolymerase gene of R. eutropha H16 and HF210 showed a reduced but not completely eliminated activity of 3HB release and indicated the presence of i-PHB depolymerase isoenzymes.
The mechanism of degradation of denatured, exogenous, crystalline poly(3-hydroxybutyrate) (PHB) by extracellular PHB depolymerases has been extensively studied during the last decade (for a recent review, see reference 6). However, intracellular PHB (i-PHB) degradation, i. e., the mobilization of previously accumulated amorphous PHB, is poorly understood. The beneficial effect of accumulated PHB on survival in the absence of a carbon source has been described for several species, including Ralstonia eutropha (4), Legionella pneumophila (5), and Hydrogenophaga pseudoflava (15). Recently, a DNA sequence of a putative i-PHB depolymerase of R. eutropha H16 was determined (GenBank accession no. AB017612). However, the physiological relevance of this gene during mobilization of PHB has not been investigated.
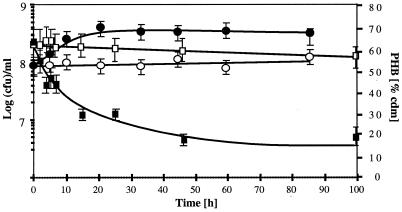
PHB-rich cells of R. eutropha H16 (DSM428) were resuspended and incubated in carbon-free mineral-salt medium (12) with (mobilization) or without (control) NH4Cl for 6 days. The number of viable cells, in CFU per milliliter, increased about fourfold in the presence of NH4Cl within 30 h and remained constantly high for 6 days (Fig. 1). In contrast, the CFU/ml remained almost unchanged in the absence of a nitrogen source. The PHB content of strain H16 (assayed through gas chromatography [1]) rapidly decreased from 70 to ≈30% during the first day of mobilization and decreased further, below 20%, after 6 days (Fig. 1). The PHB content of the control decreased only very little. The CFU/ml of the PHB-free mutant PHB−4 (DSM541) decreased rapidly by several orders of magnitude after 3 days regardless of the absence or presence of a nitrogen source. Similar results were obtained with other bacteria such as Acidovorax delafieldii (DSM50403), Alcaligenes faecalis (14), a Comamonas sp. (DSM6781) and two Paracoccus denitrificans strains (DSM1404 and DSM413); however, the amount of PHB accumulation and the increase of the CFU/ml during mobilization of PHB were not as pronounced for these bacteria as for R. eutropha (data not shown). We conclude that R. eutropha H16 and other bacteria are able to mobilize previously accumulated PHB and to use the degradation products for one or two cell divisions even in the absence of an exogenous carbon source. Very little PHB is mobilized if protein synthesis is not possible due to the absence of a nitrogen source, indicating a coupling of PHB mobilization to the cellular consumption of energy. This conclusion is supported by the observation that decoupling agents, e. g., inhibitors of the proton motive force, such as carbonyl cyanide m-chlorophenylhydrazone, increase the rate of PHB mobilization (4). In the absence of PHB (strain PHB−4), the bacteria die quickly from carbon starvation (data not shown).
FIG. 1.
Growth of R. eutropha H16 on endogeneous PHB. PHB-rich bacteria were resuspended in carbon-free (with NH4Cl; black symbols) or carbon- and nitrogen-free (white symbols) mineral-salt medium. The numbers of CFU on nutrient broth agar (circles) and the PHB content (squares) were determined. Error bars were calculated from four determinations for CFU per milliliter and two determinations for PHB, respectively, and represent standard deviations. cdm, cellular dry matter.
Effect of PHB mobilization on enzyme activities.
Samples of R. eutropha taken during mobilization of PHB were used for morphological and biochemical characterization of native PHB granules and for determination of i-PHB depolymerase, NAD+-dependent 3-hydroxybutyrate dehydrogenase (3HB DH), p-nitrophenylbutyrate esterase (p-NPB esterase), and p-nitrophenylacetate esterase activity. Native PHB granules were purified by two subsequent glycerol density gradient centrifugations of crude extracts (all values show the amount of glycerol in 100 mM Tris-HCl buffer [vol/vol], pH 8.0 [first gradient: 5 ml, 87%; 10 ml, 50%; and about 10 ml of crude extract] [second gradient: 5 ml [each] 90, 80, 60, and 40%, and 5 to 10 ml of 1:3 diluted fraction of the first gradient]) (SW28 rotor at 20,000 rpm for 40 min at 4°C). PHB granules isolated from cells that were incubated under mobilization conditions (with NH4Cl) or under control conditions (without NH4Cl) are referred to as mobilized granules or control granules, respectively. The mean size of the granules was determined through electron microscopy, after staining with 3% phosphotungstic acid, by measuring the diameters of about 300 individual granules.
The diameters of native granules isolated from R. eutropha had an almost Gaussian distribution, and the mean values were higher for the control culture (without NH4Cl) (454 ± 15 nm) than for mobilized cells (387 ± 15 nm). This indicated that a significant portion of the PHB molecules had been degraded during the 8-h mobilization. The color of the granule preparations was different: native control granules were white, but mobilized PHB granules were white-beige. Both types of native PHB granules were subjected to an autohydrolysis assay in 100 mM sodium phosphate buffer, pH 7. The rates of 3HB release and optical-density decrease were about three times higher for mobilized granules than for control granules. The mean size of the mobilized granules decreased from 402 ± 15 nm to 352 ± 15 nm within 12 h of incubation at 30°C. Apparently, mobilized granules have significantly higher surface-bound i-PHB depolymerase activity than control granules. The release of 3HB by native PHB granules was not influenced by the addition of soluble cell extracts, indicating that soluble i-PHB depolymerase is below the detection limit or absent. When a soluble crude extract of Pseudomonas lemoignei cells containing 3HB dimer hydrolase activity was added to the supernatant of an autohydrolysis assay, the amount of released 3HB was increased and indicated that oligomeric esters of 3HB were released from native PHB granules in addition to monomeric 3HB. Native PHB granules isolated from recombinant Escherichia coli, which expressed the R. eutropha PHB biosynthetic genes, showed no detectable i-PHB depolymerase activity. However, the granules were rapidly hydrolyzed by i-PHB depolymerase purified from Rhodospirillum rubrum (3), confirming that the E. coli granules were in the same amorphous state as the R. eutropha granules and that the absence of autohydrolysis activity was caused by the lack of i-PHB depolymerase activity in E. coli.
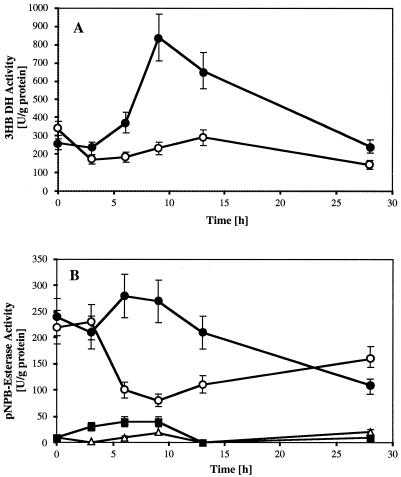
Soluble 3HB DH activity increased about threefold from ≈300 U/g of protein to more than 800 U/g during the first 9 h of mobilization and subsequently decreased to the original value after 28 h (Fig. 2A). The 3HB DH activity of the control remained almost unchanged. A similar time course of activity was measured for soluble p-NPB esterase, and a transient 3.5-fold-higher activity (270 U/g of protein) was determined than for the control (75 U/g; Fig. 2B). Interestingly, low levels of p-NPB esterase activity were also present in purified native granules (Fig. 2B), and the specific activities were higher in mobilized granules than in control granules. No significant p-nitrophenylacetate esterase activity was measured for any of the samples. These results reveal that the bacterial PHB metabolism quickly responds to starvation conditions by activation of i-PHB mobilization, resulting in a release of free 3HB and subsequent (transient) induction of 3HB DH activity.
FIG. 2.
Time course of 3HB DH activity (A) and of p-NPB esterase activity (B) in the soluble fraction (circles) and in the granule fraction (triangles and squares) in R. eutropha. Values measured during mobilization (with NH4Cl) of accumulated PHB on carbon-free mineral-salt medium are indicated with black symbols; those for nonmobilized (without NH4Cl) control cells or granules are indicated with white symbols (A and B). Error bars represent standard deviations.
Characterization of the i-PHB depolymerase.
The pH optima of autohydrolysis of native mobilized and control granules were around pH 7. No significant activity was found at pH values below 5 and above 8, and no second pH optimum near pH 9 (11) was detectable in our granule preparation. However, the rate of 3HB release generally was about three times higher for mobilized granules than for control granules. Monovalent cations, such as Na+ and K+, did not affect the release of 3HB significantly in the range up to 100 mM. Divalent cations, such as Mg2+ or Ca2+ (1 mM), had a marginal stimulating effect, but the presence of low concentrations of EDTA (0 to 5 mM) did not reduce activity. High concentrations of EDTA reduced the rate of 3HB release significantly (30% inhibition at 15 mM and 55% inhibition at 60 mM). When 5 mM dl-3HB (corresponding to about 2 mM d-3HB as determined enzymatically with 3HB DH) was added to the assay mixture, no measurable effect on the release of 3HB was found. Apparently, the release of 3HB is not subject to product inhibition by 3HB. Detergents (sodium dodecyl sulfate or Triton X-100; each, 0.2%) inhibited the release of 3HB completely. Reducing agents, such as 4 mM dithiothreitol, inhibited autohydrolysis almost completely (88%). The serine esterase inhibitor dodecylsulfonyl chloride (1 mM) effectively inhibited the release of 3HB (95%), whereas another inhibitor, phenylmethylsulfonyl fluoride (1 mM), hardly affected autodigestion of the PHB granules (6% inhibition). Solvents, such as 2-propanol (0.5%), inhibited the reaction significantly (40%).
Construction of knockout mutants in the putative i-PHB depolymerase gene of R. eutropha.
A putative i-PHB depolymerase gene (i-phaZ) (see GenBank accession number above) was PCR amplified (1,510 bp) from chromosomal DNA of R. eutropha H16 using the oligonucleotides i-PHB depolymerase uni (5′-CGTCTCTAGAGCAAGATCGTTAGCACGGA) and i-PHB depolymerase rev (5′-CGAATC-TAGAGCATGAGCGCTTGCCG). The XbaI-digested PCR product (1,496 nucleotides) was cloned into pUC18. A 826-bp deletion (833 bp − 7 bp derived from the primer, including two stop codons and a frameshift) was generated by PCR (Δi-phaZ-left, 5′-CTGAATTCTCAGCGC-ACCGACTTGATGTCG; and Δi-phaZ-right, 5′-GCGAATTCTGATGACCGTCGAGGG-AGAAC [Vent DNA polymerase]). The truncated gene was sequenced, cloned into the XbaI site of pLO3 (9) (yielding pSKLO3-1660), and transformed into E. coli S17-1. The plasmid pSKLO3-1660 was transferred to R. eutropha strain H16 and megaplasmid-free strain HF210 (7) by conjugation (spot mating) and selection for Tc resistance and prototrophy (heterogenotes). Homogenotes were obtained by selection for sucrose-resistant colonies on Luria-Bertani sucrose (15%) agar as described elsewhere (10). The correctness of the constructions was verified by Southern and PCR analysis of selected Tc-sensitive colonies of H16 (H16-SK1544) and HF210 (HF210-SK1542).
Characterization of i-PHB depolymerase gene knockout mutants.
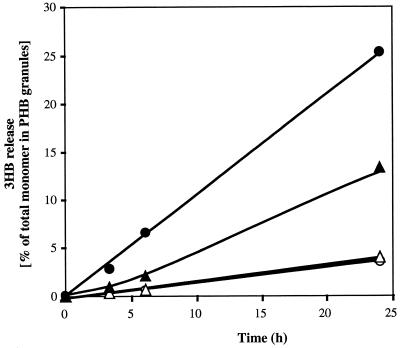
The deletion of the putative i-PHB depolymerase gene had no measurable effect on growth or accumulation of PHB. The PHB content decreased by 49 and 41% for the wild-type strain H16 and the megaplasmid-free strain HF 210 within 12 h of applying mobilization conditions. The PHB content of the i-phaZ deletion mutants also decreased but only to a minor extent (25 and 28% degradation for the H16 mutant and the HF210 mutant, respectively). Apparently, the PHB-mobilizing activity is reduced but not completely eliminated in the mutants. These results were confirmed by analyzing the rates of 3HB release of isolated native PHB granules (Fig. 3). The rate for mobilized mutant granules was only half of that for mobilized wild-type granules. No significant differences were found between the i-PHB depolymerase deletion mutants of H16 and of the megaplasmid-free strain HF210. Apparently, there is no additional copy of an i-PHB depolymerase gene on the megaplasmid as has been described for the cfx genes (8). Interestingly, control granules of strains H16 and HF210 and of the deletion mutants showed the same low rates of 3HB release and indicated the presence of i-PHB depolymerase isoenzymes on the chromosome. This assumption is supported by observations of Saito et al. (11), who found evidence for i-PHB depolymerase isoenzymes in the same strain. The presence of isoenzymes allows R. eutropha to adapt the carbon flux of 3HB quickly to the cellular demand: one enzyme is synthesized constitutively at a low level of activity; the other can be induced during carbon starvation. Depending on the intracellular concentrations of the specific substrates (3HB-coenzyme A [3HB-CoA] for the PHB synthase and PHB for the depolymerase), the synthase and the depolymerases might be responsible for balancing transient changes in the carbon flux coming from gluconate to acetyl-CoA and in the consumption of acetyl-CoA by the energy metabolism and anabolism. If the supply of a suitable carbon source (e. g., gluconate or fructose) is high enough that the generation of acetyl-CoA (and 3HB-CoA) is higher than its consumption, a high PHB synthase activity level and a low i-PHB depolymerase activity level would result in net accumulation of PHB. In contrast, if the supply of the carbon source suddenly ceases, depletion of acetyl-CoA would reduce the concentration of PHB precursors and lead to a halt of PHB synthesis. A constitutively expressed i-PHB depolymerase can immediately supply the metabolism with 3HB, which can be converted to acetyl-CoA easily and used for energy generation via energy metabolism. If carbon is the only growth-limiting factor, a transient induction of a second i-PHB depolymerase can enable the cells to increase the rate of PHB mobilization and to perform one or two cell divisions. This assumption might explain an apparent contradiction between the results of Doi et al. (2) and Taidi et al. (13), who found evidence for the simultaneous synthesis and degradation of PHB. It will be necessary to study the expression of both i-PHB depolymerases separately under PHB-accumulating and PHB-mobilizing conditions in the future.
FIG. 3.
Time course of 3HB release in vitro by native PHB granules isolated from R. eutropha wild-type H16 (circles) and from the i-PHB depolymerase deletion mutant H16-SK1544 (triangles). The PHB granules were isolated after 12 h of mobilization (with NH4Cl; black symbols) or from control cells (without NH4Cl; white symbols) and were diluted with 100 mM potassium phosphate buffer, pH 7.0, to an optical density at 600 nm of about 1. The amount of 3HB released from granules was determined periodically from granule-free supernatant by the 3HB DH assay. The 100% values were determined after alkaline hydrolysis of the granules to 3HB.
Acknowledgments
This work was supported by the Deutsche Forschungsgemeineschaft, the Graduiertenkolleg “Chemische Aktivitäten von Mikroorganismen” and a scholarship of the Studienstiftung des Deutschen Volkes to R.H. We thank O. Lenz for providing pLO3.
REFERENCES
- 1.Brandl H, Gross R A, Lenz R W, Fuller R C. Pseudomonas oleovorans as a source of poly(β-hydroxyalkanoates) for potential applications as biodegradable polyesters. Appl Environ Microbiol. 1988;54:1977–1982. doi: 10.1128/aem.54.8.1977-1982.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doi Y, Segawa A, Kawaguchi Y, Kunioka M. Cyclic nature of poly(3-hydroxyalkanoate) metabolism in Alcaligenes eutrophus. FEMS Microbiol Lett. 1990;67:165–170. doi: 10.1016/0378-1097(90)90188-v. [DOI] [PubMed] [Google Scholar]
- 3.Handrick R, Jendrossek D. Extracellular and intracellular polyhydroxy-alkanoate depolymerases: homologies and differences. In: Steinbüchel A, editor. Biochemical principles and mechanisms of biosynthesis and biodegradation of polymers. Weinheim, Germany: Wiley-VCH; 1998. pp. 57–67. [Google Scholar]
- 4.Hippe H. Abbau und Wiederverwertung von Poly-β-hydroxybuttersäure durch Hydrogenomonas H16. Arch Mikrobiol. 1967;56:248–277. [PubMed] [Google Scholar]
- 5.James B W, Mauchline W S, Dennis P J, Keevil C W, Wait R. Poly-3-hydroxybutyrate in Legionella pneumophila, an energy source for survival in low-nutrient environments. Appl Environ Microbiol. 1999;65:822–827. doi: 10.1128/aem.65.2.822-827.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jendrossek, D. Microbial degradation of polyesters. In T. Scheper (ed.), Biopolyesters. Advances in biochemical engineering/biotechnology, in press. [DOI] [PubMed]
- 7.Kortlücke C, Friedrich B. Maturation of membrane-bound hydrogenase of Alcaligenes eutrophus H16. J Bacteriol. 1992;174:6290–6293. doi: 10.1128/jb.174.19.6290-6293.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kusian B, Bowien B. Organization and regulation of cbb CO2 assimilation genes in autotrophic bacteria. FEMS Microbiol Rev. 1997;21:135–155. doi: 10.1111/j.1574-6976.1997.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 9.Lenz O, Friedrich B. A novel multicomponent regulatory system mediates H2 sensing in Alcaligenes eutrophus. Proc Natl Acad Sci USA. 1998;95:12474–12479. doi: 10.1073/pnas.95.21.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenz O, Schwartz E, Dernedde J, Eitinger M, Friedrich B. The Alcaligenes eutrophus H16 hoxX gene participates in hydrogenase regulation. J Bacteriol. 1994;176:4385–4393. doi: 10.1128/jb.176.14.4385-4393.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saito T, Takizawa K, Saegusa H. Intracellular poly(3-hydroxybutyrate) depolymerase in Alcaligenes eutrophus. Can J Microbiol. 1995;41(Suppl. 1):187–191. [Google Scholar]
- 12.Schlegel H G, Kaltwasser H, Gottschalk G. Ein Submersverfahren zur Kultur wasserstoffoxidierender Bakterien: Wachstumsphysiologische Untersuchungen. Arch Mikrobiol. 1961;38:209–222. [PubMed] [Google Scholar]
- 13.Taidi B, Mansfield D A, Anderson A. Turnover of poly(3-hydroxybutyrate) (PHB) and its influence on the molecular mass of the polymer accumulated by Alcaligenes eutrophus during batch culture. FEMS Microbiol Lett. 1995;129:201–206. [Google Scholar]
- 14.Tanio T, Fukui T, Shirakura Y, Saito T, Tomita K, Kaiho T, Masamune S. An extracellular poly(3-hydroxybutyrate) depolymerase from Alcaligenes faecalis. Eur J Biochem. 1982;124:71–77. doi: 10.1111/j.1432-1033.1982.tb05907.x. [DOI] [PubMed] [Google Scholar]
- 15.Yoon S C, Choi M H. Local sequence dependence of polyhydroxyalkanoic acid degradation in Hydrogenophaga pseudoflava. J Biol Chem. 1999;274:37800–37808. doi: 10.1074/jbc.274.53.37800. [DOI] [PubMed] [Google Scholar]