Abstract
Long non-coding RNA WAC antisense RNA 1 (lncRNA WAC-AS1) is involved in the replication of the hepatitis B virus (HBV). The purpose of this study was to determine its functions and specific mechanism. The levels of lncRNA WAC-AS1, RNA (miR)-192-5p and were examined in serum of HBV-infected patients and in HepG2.2.15 cells using quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) and Western blotting. Using the database starBase, the target binding sites of lncRNA WAC-AS1 and miR-192-5p were predicted and confirmed by dual-luciferase reporter assay and RNA pull-down assay. The expression of pgRNA and HBV DNA was determined by qRT-PCR, while the levels of HBeAg and HBsAg were measured by enzymelinked immunosorbent assay (ELISA). Using laser scanning confocal microscopy, the light chain 3 (LC3) expression was analyzed. qRT-PCR and Western blotting were used to assess the expression of beclin-1, p62, and LC3I/II. Overexpression of lncRNA WAC-AS1, upregulation of ATG7. and downregulation of miR-192-5p were observed in the serum of HBV-infected patients and the in vitro model. miR-192-5p directly targets lncRNA WAC-AS1. LncRNA WAC-AS1 was downregulated in lncRNA WAC-AS1-shRNA‒transfected cells. miR-192-5p was upregulated in lncRNA WAC-AS1-shRNA-transfected cells and downregulated in cells transfected with a miR-192-5p inhibitor. In HepG2 2.15 cells, the downregulation of lncRNA WAC-AS1 inhibited HBV replication and autophagy. In contrast, the miR-192-5p inhibitor-transfected group exhibited the opposite results, and ATG7 overexpression reversed the effects of miR-192-5p mimic or lncRNA WAC-AS1-shRNA on HBV replication and cell autophagy. Our findings indicate that lncRNA WAC-AS1 regulates HBV replication by reinforcing the autophagy induced by miR-192-5p/ATG7. Consequently, lncRNA WAC-AS1 may serve as a therapeutically- promising target in HBV patients.
Key words: Hepatitis B virus, lncRNA WAC-AS1, miR-192-5p/ATG7 axis, autophagy
Introduction
Hepatitis B virus (HBV), the etiological agent of hepatitis B, belongs to the hepatophilic DNA virus family. The main clinical manifestations of HBV infection are fatigue, lack of appetite, and abnormal liver function and inflammation, as well as necrosis and fibrosis of liver tissue.1 Patients with chronic HBV infections are more likely to develop severe liver disease, hepatocellular carcinoma, and liver cirrhosis.2 Nevertheless, the existing therapies have certain limitations, such as an unsatisfactory sustained response rate, significant side effects, and drug resistance.3 Therefore, it is important to develop effective and sustainable methods or markers for the treatment of HBV infection.
Long non-coding RNA (lncRNA), a type of non-coding RNA that is more than 200 nucleotides in length, plays vital roles in various potentially carcinogenic processes, including those related to cell proliferation, HBV replication, and autophagy. Feng et al. have previously suggested that the lncRNA proliferating cell nuclear antigen pseudogene 1 (PCNAP1) modulates HBV replication and enhances tumor growth in liver cancer.4 LncRNA WAC-AS1, a novel lncRNA, has been shown to be associated with glioma prognosis and ferroptosis.5 Besides, lncRNA WAC-AS1 can promote glycolysis and tumor progression in hepatocellular carcinoma.6 However, the latent mechanisms underlying the effect of lncRNA WACAS1 on HBV replication have not been fully elucidated.
Recent evidence suggests that host miRNAs may be involved in host–virus interactions and that they may participate in mediating the life cycle of the infecting virus. Li et al. have demonstrated the clinical significance of serum miR-487b in HBV-related hepatocellular carcinoma and its potential mechanism.7 MiR-192-5p has been shown to be a tumor suppressor in many cancers, including cervical cancer,8 colon cancer,9 and non-alcoholic fatty liver disease.10 However, whether miR- 192-5p is related to the pathological progression of HBV replication is still unclear.
Thus, this study aimed to shed light on the relevance of the lncRNA WAC-AS1/miR-192-5p/ATG7 axis in HBV infection and uncover the latent mechanisms of this axis in HBV replication and hepatic cell autophagy. Furthermore, to explore whether pregnancy affects the expression of lncRNA WAC-AS1/miR-192-5p in serum of female patients with HBV infection, the expression of lncRNA WACAS1/ miR-192-5p in the serum of healthy pregnant women, pregnant women with chronic hepatitis B (CHB), non-pregnant women carrying HBV, and non-pregnant women with CHB were determined in this study. The findings would form a basis for discovery of promising therapeutic targets that can be used for HBV treatment.
Materials and Methods
Serum collection
To explore the expression of lncRNA WAC-AS1/miR-192-5p in healthy pregnant women, pregnant women with CHB, non-pregnant women carrying HBV, and non-pregnant women with CHB, serum samples were acquired from 10 participants from each of these groups and promptly stored at -80°C. This experimental protocol was approved by the Ethics Committee of Nanjing First People’s Hospital, and all the participants provided written informed consent.
Cell culture
HepG2 and HepG2.2.15 cells were obtained from the American Type Culture Collection and cultivated in Dulbecco’s modified Eagle’s medium (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% fetal bovine serum (Invitrogen, Waltham, MA, USA) with 1% penicillin-streptomycin and maintained at 37˚C in a 5% CO2 incubator. Stable expression and replication of HBV in culture are the characteristic features of HepG2.2.15 cells.
Cell transfection
Negative control of lncRNA WAC-AS1-shRNA (sh-NC), lncRNA WAC-AS1-shRNA; miR-192-5p inhibitor, negative control of miR-192-5p inhibitor (inhibitor-NC); miR-192-5p mimics, negative control of miR-192-5p mimics (mimics-NC); and negative control of ATG7-over expression plasmid (OE-NC) and ATG7-over expression plasmid (ATG7-OE) were synthesized by Gene Create (Wuhan, China). These synthesized sequences and vectors were then transfected into HepG2.2.15 cells using Lipofectamine 2000 Transfection Regent (Invitrogen; 11668019) according to the manufacturer’s protocol. The cells were collected 24 h after transfection, and qRT-PCR analysis and Western blotting were performed to evaluate gene expression and protein expression, respectively.
qRT-PCR analysis
Following transfection, the gene expression of lncRNA WACAS1, miR-192-5p, beclin-1 and p62 mRNA, ATG7, and GAPDH was measured using qRT-PCR. In brief, total RNA was extracted from HepG2 and HepG2.2.15 cells using the TRlpure Total RNA Extraction Reagent (ELK Biotechnology, Wuhan, China) according to the manufacturer’s instructions. Next, the total RNA was reverse-transcribed to cDNA using M-MLV Reverse Transcriptase (ELK Biotechnology) and qRT-PCR analysis was conducted using QuFast SYBR Green PCR Master Mix (ELK Biotechnology) using the StepOneTM Real-Time PCR kit (Life Technologies, Carlsbad, CA, USA) according to the manufacturers’ instructions. The expression of specific genes was evaluated using the 2−ΔΔCt method.11 Sequences of the primers used in this study are listed in Table 1.
Detection of HBV DNA, HBsAg, and HBeAg
HBV DNA was isolated from the supernatant using Blood/Cell/Tissue Genomic DNA Extraction Kit (EP007; ELK Biotechnology) and its concentration was determined using qPCR analysis (QuantStudio 6 Flex System; Life Technologies). The levels of HBsAg and HBeAg in the cell culture supernatant were detected using human HBeAg and HbsAg ELISA kits, according to the manufacturer’s protocol.
Table 1.
Primer sequences for PCR.
| Gene name | Sequences: 5’-3’ | |
|---|---|---|
| LncWAC-AS1 | Forward, 5'-GAGATTGAAATTGTAGAAAAGAACC-3'; | Reverse, 5'-CTCCCCTCATGCATATACTGGTAT-3'; |
| β-actin | Forward, 5 -GTCCACCGCAAATGCTTCTA-3 ; | Reverse,5'-TGCTGTCACCTTCACCGTTC-3'; |
| ATG7 | Forward, 5'-TTCCTCCTCTTGACATTTGCAG-3'; | Reverse, 5'-TATCTTCGTCCTTTGACCTTGG-3'; |
| pgRNA | Forward, 5'-TAATTCCAGGATCCTCAACAAC-3'; | Reverse, 5'-CAAATGGCACTAGTAAACTGAGC-3'; |
| Beclin1 | Forward, 5'-GACAGAGCGATGGTAGTTCTGG-3'; | Reverse, 5'-TGGGCTGTGGTAAGTAATGGAG-3'; |
| P62 | Forward, 5'-GAACAGATGGAGTCGGATAACTG-3'; | Reverse, 5'-CTGGGAGAGGGACTCAATCAG-3'; |
| miR-192-5p | Forward, 5'-CCTGACCTATGAATTGACAGCC-3'; | Reverse, 5'-CTCAACTGGTGTCGTGGAGTC-3'; |
| U6 | Forward, 5'-CTCGCTTCGGCAGCACAT-3'; | Reverse, 5'-AACGCTTCACGAATTTGCGT-3'. |
Dual-luciferase reporter assay
The data base known as starBase was used to identify the relationship between miR-192-5p and lncRNA WAC-AS1. The 3’UTR of lncRNA WAC-AS1, containing the miR-192-5p binding site or mutated target site, was synthesized by carrying out a genomic PCR (ELK Biotechnology) and was cloned into pMIR vectors (Ambion, Austin, TX, USA) to construct the reporter vector lncRNA WAC-AS1 wild-type (lncRNA WAC-AS1-WT) or lncRNA WAC-AS1 mutant-type vector (lncRNA WAC-AS1- MUT). Next, 293T cells were transfected with lncRNA WACAS1- WT or lncRNA WAC-AS1-MUT combined with miR-192-5p mimic or mimic control using Lipofectamine 2000 transfection regent (Invitrogen). The transfected cells were incubated for 48 h and dual-luciferase reporter assay (Beyotime Technology, Jiangsu, China) was performed. Firefly luciferase activity of the plasmids was normalized to Renilla luciferase activity.
RNA pull-down assay
The cells were collected and resuspended in RIPA lysis buffer (AS1004; ASPEN, Wuhan, China). A biotinylated probe was added to the cells, which were then mixed with magnetic beads. Next, the cells were washed several times and the supernatant was discarded. Finally, the re-suspended magnetic beads were collected. The complex was mixed with 200 μL Trizol reagent and RNA was extracted. Finally, miR-192-5p enrichment was evaluated using qRT-PCR.
Western blot analysis
Following transfection for 48 h, total proteins from HepG2 cells and HepG2.2.15 cells were extracted using RIPA buffer (AS1004; ASPEN) and evaluated using the BCA protein assay kit (AS1086; ASPEN). Next, the proteins were separated using SDSPAGE and transferred onto PVDF membranes (IPVH00010; Millipore, Burlington, MA, USA). After blocking with 5% skim milk in PBST for 1.5 h, the membranes were incubated with primary antibodies against beclin-1 (cat. no. #3738; 1:2000 dilution; Cell Signaling Technology, Danvers, MA, USA), p62 (cat. no. ab109012; 1:2000 dilution; Abcam), and LC3I/II (cat. no. #4108; 1: 1000 dilution; Cell Signaling Technology), ATG7 (cat. no. ab133528; 1:1000 dilution; Abcam), or β-actin (cat. No. TDY051; 1:10000 dilution; Beijing TDY Biotech Co., Ltd., Beijing, China) overnight at 4˚C. The membranes were then washed and incubated with a secondary antibody for 1 h. Finally, the target protein bands were examined using an ECL detection system reagent (ASPEN, AS1059) according to the manufacturer’s protocol.
Figure 1.
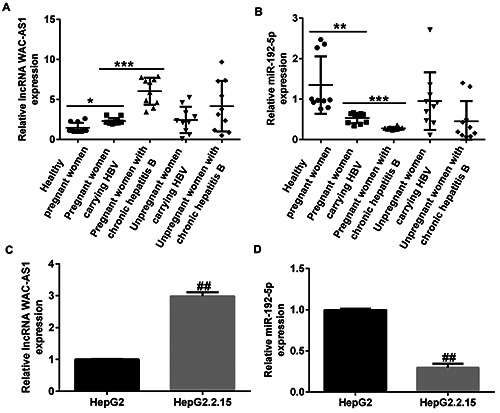
Expression of lncRNA WAC-AS1 and miR-192-5p in sera of HBV-infected patients and in hepatic cell lines. Levels of (A) lncRNA WAC-AS1 and (B) miR-192-5p in serum samples of healthy pregnant women, pregnant women carrying HBV, pregnant women with chronic hepatitis B, non-pregnant women carrying HBV, and non-pregnant women with chronic hepatitis B were estimated by qRT-PCR. (C) Determination of lncRNA WAC-AS1 expression in HepG2.2.15 and HepG2 cells. (D) qRT-PCR analysis of miR- 192-5p levels in HepG2.2.15 and HepG2 cells. *p<0.05; **p<0.01; ***p<0.001; ##p<0.01 vs HepG2.
Confocal microscopy
The HepG2.2.15 cells were transfected with GFP-LC3 (Beyotime) for 24 h before being spread and cultured overnight on a cover glass. Next, the cells were fixed using freshly prepared 4% paraformaldehyde, and confocal microscopy (OLYMPUS, IX51) was performed to visualize and quantify the cells.
Statistical analysis
Results are expressed as mean ± standard deviation of three independent measurements. Differences in means among groups were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test or Student’s t-test. All statistical analyses were carried out using the GraphPad Prism 6.0 statistical software; p<0.05 and p<0.01 were considered to be statistically significant.
Results
High expression of lncRNA WAC-AS1 and low expression of miR-192-5p were observed in sera of HBV-infected patients and in HepG2.2.15 cells
Firstly, the expression of lncRNA WAC-AS1 in healthy pregnant women, pregnant women carrying HBV, pregnant women with CHB, non-pregnant women carrying HBV, and non-pregnant women with CHB was evaluated using qRT-PCR. As presented in Figure 1 A,B, the expression of lncRNA WAC-AS1 was significantly upregulated and that of miR-192-5p was significantly downregulated in the sera of HBV-infected patients than in sera of healthy pregnant women. Figure 1 C,D indicate that the expression of lncRNA WAC-AS1 was up-regulated and that of miR-192-5p was down-regulated to a greater extent in HepG2.2.15 cells than in HepG2 cells (p<0.01). We also found that the expression of lncRNA WAC-AS1 was significantly down-regulated in lncRNA WAC-AS1-shRNA-transfected HepG2.2.15 cells than in control and shRNA-NC groups (Supplementary Figure 1A; p<0.01). Furthermore, the levels of pgRNA, HBV DNA, HBeAg, and HBsAg had remarkably decreased in the lncRNA WAC-AS1- shRNA group than in the shRNA-NC group (Supplementary Figure1 B-E; all p<0.01).
To clarify the function of lncRNA WAC-AS1 in HBV infection, putative targets of lncRNA WAC-AS1 were investigated using starBase. We found that miR-192-5p was a latent target of lncRNA WAC-AS1 (Figure 2A). Subsequently, the correlation between lncRNA WAC-AS1 and miR-192-5p was confirmed by dual luciferase reporter assay (Figure 2B) and RNA pull down assay (Figure 2C). These results suggest that miR-192-5p directly targets lncRNA WAC-AS1.
Downregulation of lncRNA WAC-AS1 suppressed the replication of HBV and inhibited cell autophagy in HepG2.2.15 cells by regulating miR-192-5p
We also explored the related mechanism between miR-192-5p and lncRNA WAC-AS1 in HBV infection. Results of qRT-PCR analysis revealed that lncRNA WAC-AS1 was down-regulated in lncRNA WAC-AS1-shRNA‒transfected HepG2.2.15 cells (Figure 3A; p<0.01). A significant reduction of miR-192-5p expression was observed in miR-192-5p inhibitor-treated HepG2.2.15 cells than in inhibitor-NC‒treated cells (Figure 3B; p<0.01). Moreover, miR-192-5p expression was up-regulated in lncRNAWAC-AS1- shRNA‒transfected HepG2.2.15 cells than in sh-NC‒transfected cells. However, opposite results were observed in lncRNA WACAS1- shRNA+ miR-192-5p inhibitor‒transfected cells, as verified by suppressed miR-192-5p levels. Besides, the levels of pgRNA, HBV DNA, HBeAg and HBsAg were significantly suppressed in lncRNA WAC-AS1-shRNA‒transfected group (all p<0.01), whereas the decrease in these levels were reversed by miR-192-5p inhibitor (Figure 3 C-F).
Figure 2.
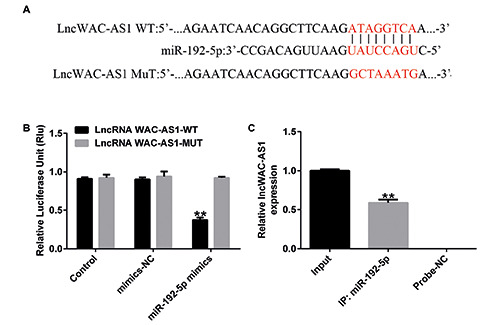
LncRNA WAC-AS1 directly targets miR-192-5p. A) starBase was used to predict the binding sites of miR-192-5p in the 3′- UTR of lncRNA WAC-AS1. Association between miR-192-5p and lncRNA WAC-AS1 was verified using dual-luciferase reporter assay (B) and RNA pull down assay (C). **p<0.01.
Figure 3.
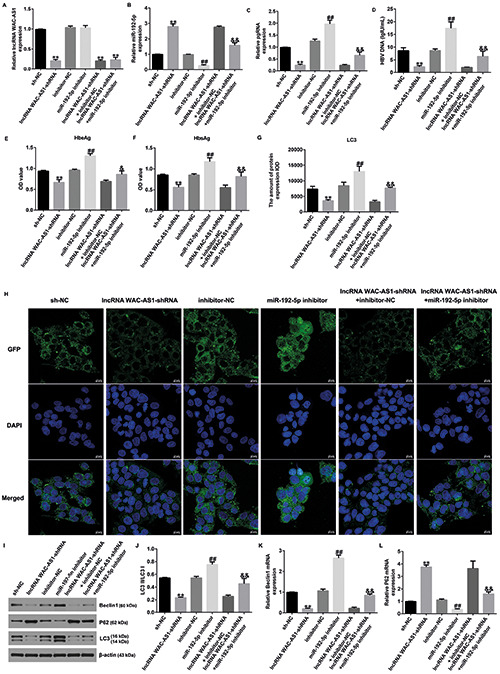
A) MiR-192-5p inhibitor reversed the effects of lncRNA WAC-AS1-shRNA on replication of HBV and autophagy in HepG2.2.15 cells. B) qRT-PCR analysis of lncRNA WAC-AS1 or miR-192-5p in different groups. Expression of pgRNA (C), HBV DNA (D), HbeAg (E), and HbsAg (F) was evaluated. G) Statistical analysis of LC3 expression. H) Representative confocal images of LC3 (green) expression in HepG2.2.15 cells are shown. I) Western blot analysis of beclin-1, p62, and LC3 expression. J) Ratio of LC3II/I. K-L) mRNA levels of beclin-1 and p62 in different groups. **p<0.01 vs sh-NC; ##p<0.01 vs inhibitor-NC; &&p<0.01 vs lncRNA WACAS- shRNA+inhibitor-NC.
Laser scanning confocal microscopy revealed a significant reduction of LC3 expression in lncRNA WAC-AS1-shRNA‒transfected cells, while this reduction was reversed by miR-192-5p inhibitor. Meanwhile, we observed increased LC3 expression in miR-192-5p inhibitor‒transfected HepG2.2.15 cells than in inhibitor-NC‒transfected cells (Figure 3 G-H; p<0.01). Furthermore, we determined the levels of autophagy-associated proteins such as beclin-1, p62, and LC3I/II in HepG2.2.15 cells. As depicted in Figure 2I-L, beclin-1 and LC3 showed low expression, while p62 was over-expressed in lncRNA WAC-AS1- shRNA‒transfected cells (all p<0.01). However, we observed opposite results in miR-192-5p inhibitor‒transfected group compared to inhibitor-NC‒transfected group or lncRNA WAC-AS1- shRNA+inhibitor-NC‒transfected group. Overall, these results suggest that lncRNA WAC-AS1 reduces the replication of HBV and inhibits cell autophagy in HepG2.2.15 cells by regulating miR- 192-5p.
Upregulation of miR-192-5p suppressed HBV replication and inhibited cell autophagy in HepG2.2.15 cells by regulating ATG7
Previous reports have shown that a low expression of ATG7 can inhibit HBV replication. Accordingly, we investigated the levels of ATG7 in HepG2.2.15 cells using western blotting and qRT-PCR. As shown in Supplementary Figure 2, ATG7 was upregulated in HepG2.2.15 cells (p<0.01), revealing that ATG7 is involved in the progression of HBV replication. To investigate the functions of ATG7 in HepG2.2.15 cells, the miR-192-5p mimic, mimic-NC, OENC, or ATG7-OE were transfected into HepG2.2.15 cells for 24 h. qRT-PCR analysis results suggested that transfection with the ATG7- OE prominently increased ATG7 expression in HepG2.2.15 cells. We also found that miR-192-5p was upregulated in miR-192-5p mimic‒transfected cells (Figure 4 A,B; p<0.01). Additionally, we observed that the levels of pgRNA (Figure 4C; p<0.01), HBV DNA (Figure 4D; p<0.01), HBeAg (Figure 4E; p<0.01), and HBsAg (Figure 4F; p<0.01) were increased in the ATG7-OE group than in NC group; however, these levels were reduced in the miR-192-5p mimic‒transfected group than in mimic-NC‒transfected group.
Results of laser scanning confocal microscopy revealed that ATG7-plasmid transfection promoted cellular autophagy, which was evidenced by decreased LC3 expression (p<0.01). However, opposite results were obtained for the miR-192-5p mimic‒transfected cells (Figure 4G-H). We also investigated the expression of autophagy-related proteins (ATG7, beclin-1, p62, and LC3I/II) using qRT-PCR and Western blotting. Results of these analyses, illustrated in Figure 3I-J, suggested that ATG7-plasmid transfection increased ATG7, beclin-1, and LC3 expression, and reduced p62 levels (all p<0.01); however, these findings were reversed for cells transfected with the miR-192-5p mimic when compared with cells transfected with mimic-NC or miR-192-5p mimic+mimic- NC. Similar results were obtained for the mRNA expression of these proteins (Figure 4 K-M; all p<0.01). Taken together, these data indicate that upregulation of miR-192-5p suppresses HBV replication and inhibits cell autophagy in HepG2.2.15 cells by down-regulating ATG7.
LncRNA WAC-AS1 mediates HBV replication and cell autophagy in HepG2.2.15 cells by regulating ATG7 expression
To better explain the correlation and functions of lncRNA WAC-AS1, miR-192-5p, and ATG7 in HBV replication and cellular autophagy, qRT-PCR was performed. The results suggested that lncRNA WAC-AS1 was down-regulated in lncRNA WAC-AS1- shRNA‒transfected cells, while its expression showed no marked change following transfection with ATG7-OE (Figure 5A). We also observed that pgRNA (Figure 5B; p<0.01), HBV DNA (Figure 5C; p<0.01), HBeAg (Figure 5D; p<0.01), and HBsAg (Figure 5E; p<0.01) levels were dramatically reduced in the lncRNA WAC-AS1-shRNA‒transfected group than in sh- NC‒transfected group; however, the decrease in these levels was reversed in ATG7-plasmid‒transfected cells when compared with OE-NC‒transfected cells.
Results of laser scanning confocal microscopy revealed that lncRNA WAC-AS1-shRNA reduced the expression of LC3 in HepG2.2.15 cells, and this suppression was abolished by transfection with ATG7-OE (Figure 5 F,G). Moreover, Western blot analysis demonstrated that transfection with lncRNA WAC-AS1- shRNA decreased ATG7, beclin-1, and LC3 expression but increased p62 levels; however, these findings were reversed for cells transfected with ATG7-OE (Figure 5 H,I). In addition, we observed similar findings at the mRNA level, wherein we noted an increase in ATG7 and beclin-1 mRNA levels and a decrease in p62 mRNA levels (Figure 5 J-L; all p<0.01). These findings reveal that lncRNA WAC-AS1 mediates HBV replication and cell autophagy in HepG2.2.15 cells through the miR-192-5p/ATG7 axis.
Discussion
It is estimated that nearly 250 million individuals are infected with HBV worldwide, and the incidence of HBV infection is relatively high in China.12 It is reported that HBV infection can result in liver cirrhosis and hepatocellular carcinoma, which is related to a high mortality rate.13 Therefore, it is necessary to discover promising biomarkers for early diagnosis and treatment of HBV infection. Blocking HBV replication is vital to prevent CHB liver injury.14 For pregnant women with CHB, antiviral therapy during pregnancy can not only normalize ALT levels in pregnant women with abnormal liver function, prevent progression of the disease, and reduce maternal and infant complications, but also suppress HBV viral load and effectively block mother-to-child transmission of HBV.15,16
LncRNAs are associated with multiple diseases, including HBV infection.17 LncRNA PCNAP1 has been reported to modulate hepatitis B virus replication.4 LncRNA HOTAIR can modulate hepatitis B virus transcription and replication by enhancing the SP1 transcription factor.18 However, the role of lncRNA WAC-AS1 in HBV replication is unclear. Thus, our study focused on revealing the functions of lncRNA WAC-AS1 in the pathogenesis of HBV infection. First, we evaluated the levels of lncRNA WACAS1 present in sera of 10 hepatitis B-carrying pregnant women, 10 hepatitis B-positive pregnant women, and 10 healthy pregnant women using HepG2 and HepG2.2.15 cells by qRT-PCR. Interestingly, our data suggested that lncRNA WAC-AS1 expression was remarkably upregulated in sera of HBV-infected patients than in sera of healthy pregnant women. These observations were further confirmed in HepG2.2.15 cells using qRT-PCR. Our findings are similar to other previous reports, which suggest that lncRNA WAC-AS1 is dysregulated in various diseases.5,6 For instance, Xia et al. demonstrated that lncRNA WAC-AS1 promotes glycolysis and tumor progression in hepatocellular carcinoma.6 Thus, it can be speculated that the downregulation of lncRNA WAC-AS1 might be able to regulate HBV replication. To further determine the role of lncRNA WAC-AS1 in HBV replication, HepG2.2.15 cells were co-transfected with control-shRNA and lncRNA WAC-AS1-shRNA. We found that the levels of lncRNA WAC-AS1 were lower in the lncRNA WAC-AS1-shRNA group than in control and shRNA-NC groups. Furthermore, we observed that pgRNA, HBV DNA, HBeAg and HBsAg levels were remarkably decreased in the lncRNA WAC-AS1-shRNA group than in shRNA-NC group, suggesting that lncRNA WAC-AS1 participates in the progression of HBV replication.
Previous research has indicated that abnormal expression patterns of miRNA molecules play a role in mediating HBV replication and disease development.19 It is also known that HBV induces autophagy to promote its replication via the miR-192-3p-XIAP axis through NF kappa B signaling.20 Therefore, we hypothesized that lncRNA WAC-AS1 might affect HBV replication by regulating miRNA expression. Accordingly, we investigated the underlying targets of lncRNA WAC-AS1 in the regulation of HBV replication. The results of dual-luciferase reporter assay and RNA pull down assay demonstrated that miR-192-5p directly interacts with lncRNA WAC-AS1, indicating a latent mechanism of miR-192-5p in HBV replication. MiR-192-5p is found to be regulated in cholangiocarcinoma cells through the activation of the MEK/ERK pathway.21 Furthermore, Fu et al. have indicated that miR-192-5p inhibits proliferation, migration, and invasion of papillary thyroid carcinoma cells via the regulation of SH3RF3.22 We further assessed miR-192-5p expression in the sera of HBV-infected patients and healthy pregnant women, as well as in HepG2 and HepG2.2.15 cells. qRT-PCR analysis revealed that a low expression miR-192-5p was observed in the sera of HBV-infected patients and in HepG2.2.15 cells. HBV is a virus that possesses the ability to induce autophagy to promote its replication. In addition, several reports have demonstrated that HBV is controlled through metabolic processes, including autophagy.23,24 miR-192-5p has also been revealed to play critical roles in the regulation cell autophagy.25,26 Thus, in our study, we also evaluated the roles of miR-192-5p in HBV replication and cell autophagy. Recently, serum miRNA-125b was verified to be related to serum HBV DNA and HBsAg levels in CHB patients.27 Our results, which suggest that the downregulation of lncRNA WAC-AS1 reduces replication of HBV and inhibits HepG2 2.15 cell autophagy, are in line with this report. Our conclusion is supported by the observation of decreased pgRNA, HBV DNA, HBeAg and HBsAg levels; reduced beclin-1 and LC3 expression; increased p62 levels; and decreased autophagosome numbers in lncRNA WAC-AS1- shRNA-transfected cells, along with the observation of opposite results seen in miR-192-5p inhibitor-transfected cells. Taken together, these findings reveal that lncRNA WAC-AS1 reduces the replication of HBV in HepG2.2.15 cells by regulating miR-192-5p.
Figure 4.
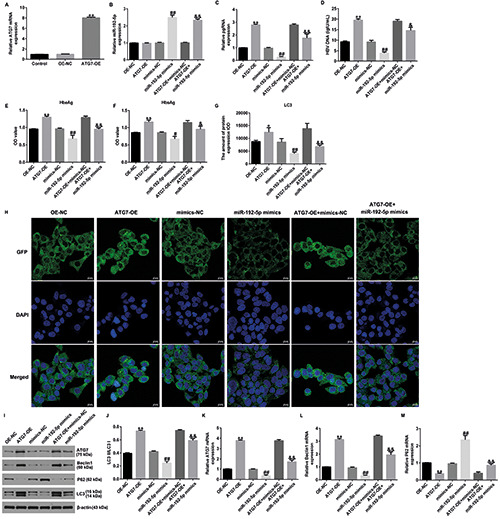
ATG7-plasmid reversed the effects of miR-192-5p mimic on HBV replication and cell autophagy in HepG2.2.15 cells. A) qRTPCR analysis of ATG7 in ATG7-plasmid-transfected cells. B) miR-192-5p expression was determined by qRT-PCR analysis. Expression of pgRNA (C), HBV DNA (D), HbeAg (E), and HbsAg (F) in different groups was measured. G) Quantification of LC3 expression. H) LC3 (green) expression in HepG2.2.15 cells was determined by laser scanning confocal microscopy. I) Western blot analysis of beclin- 1, p62, and LC3 expression. J) Ratio of LC3II/I. K-M) mRNA levels of ATG7, beclin-1, and p62 in different groups. *p<0.05 vs OENC; **p<0.01 vs OE-NC; #p<0.05 vs mimics-NC; ##p<0.01 vs mimics-NC; &p<0.05 vs ATG7-OE+mimics-NC; &&p<0.01 vs ATG7- OE+mimics-NC.
Figure 5.
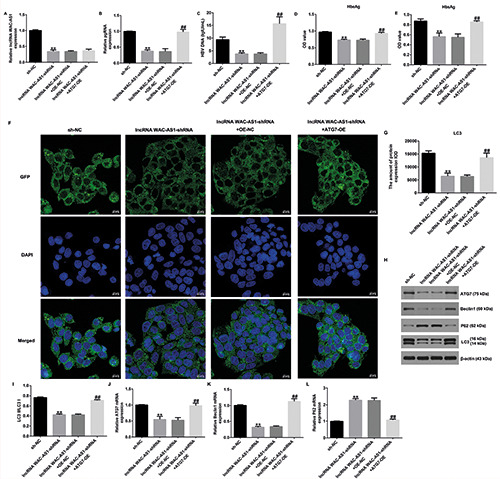
ATG7-plasmid reversed the effects of lncRNA WAC-AS1-shRNA on HBV replication and autophagy in HepG2.2.15 cells. A) qRT-PCR analysis of lncRNA WAC-AS1 in different groups. Expression of pgRNA (B), HBV DNA (C), HbeAg (D), and HbsAg (E) in different groups was determined. F) Representative confocal images of LC3 (green) expression in HepG2.2.15 cells are shown. G) Quantification of LC3 expression. H) Detection of beclin-1, p62, and LC3 expression. I) Ratio of LC3II/I. J-L) mRNA levels of ATG7, beclin-1, and p62 were analyzed by qRT-PCR. **p<0.01 vs sh-NC; ##p<0.01 vs lncRNA WAC-AS-shRNA+OE-NC.
Studies have shown that ATG7 is the target gene of miR-192-5p.25,26,28 Wang et al. have suggested that ASPP2 inhibits HBV replication by preventing nuclear translocation of HSF1 and attenuating transactivation of ATG7.29 ATG7 is an essential autophagy effector enzyme, which, in concert with other ATG proteins, also regulates immunity, protein secretion, and cell death, as well as independently regulates the cell apoptosis and cell cycle.30 Next, we explored whether miR-192-5p affects HBV replication by regulating ATG7 expression in HepG2.2.15 cells. Western blotting and qRT-PCR analysis revealed that ATG7 was upregulated in HepG2.2.15 cells, thus revealing that ATG7 is involved in the progression of HBV replication. To better elucidate the regulatory relationship between miR-192-5p and ATG7 in HepG2.2.15 cells, rescue experiments were performed. We found that miR-192-5p was upregulated in the miR-192-5p mimic-transfected cells. The levels of pgRNA, HBV DNA, HBeAg, and HBsAg were remarkably increased in the ATG7-plasmid group than in control-plasmid group. Moreover, ATG7-plasmid transfection decreased LC3 expression; increased ATG7, beclin-1, and LC3 expression; and reduced p62 levels. However, these findings were reversed following miR-192-5p mimic transfection, demonstrating that miR-192-5p mimic suppressed HepG2.2.15 cell autophagy by targeting ATG7. To better explain the mechanism of the correlating role of lncRNA WAC-AS1, miR-192-5p, and ATG7 in HBV replication, HepG2.2.15 cells were transfected with lncRNA WAC-AS1- shRNA, control-shRNA, control-plasmid, or ATG7-plasmid for 24 h. ATG7-plasmid transfection reversed the effects of lncRNA WAC-AS1-shRNA on HBV replication, cell autophagy, and autophagosome numbers in HepG2.2.15 cells.
However, there are some limitations of this study. For example, this study was not performed in vivo. Furthermore, only one cell line (HepG2.2.15 cells) was used to study the effects of lncRNA WAC-AS1/miR-192-5p/ATG7 on HBV replication. Besides, miR-192-5p has more than one target genes, and only ATG7 was analyzed in this study. Moreover, we did not analyze whether the levels of lncRNA WAC-AS1 are correlated with the severity of HBV infections. We will further investigate these issues in the future.
Taken together, our findings suggest that lncRNA WAC-AS1 regulates HBV replication by reinforcing miR-192-5p/ATG7-stimulated autophagy, thereby indicating that lncRNA WAC-AS1 may serve as an innovative therapeutic target to treat HBV patients.
Funding Statement
Funding: This research was supported by the Chinese National Research Grant of the Thirteenth Five-Year Plan for the Key Projects in Infectious Diseases (Grant No.2017ZX10201201-001-002) and Development Project of The Affiliated Wuxi Maternity and Child Health Care Hospital of Nanjing Medical University (HB2020005).
References
- 1.Li Q, Lomonosova E, Donlin MJ, Cao F, O'Dea A, Milleson B, et al. Amide-containing alpha-hydroxytropolones as inhibitors of hepatitis B virus replication. Antiviral Res 2020;177: 104777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y, Veeraraghavan V, Pinkerton M, Fu J, Douglas MW, George J, et al. Viral biomarkers for hepatitis B virus-related hepatocellular carcinoma occurrence and recurrence. Front Microbiol 2021;12:665201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao LH, Zhao PL, Liu ZM, Sun SC, Xu DB, Zhang JD, et al. Efficacy and safety of nucleoside analogues in preventing vertical transmission of the hepatitis B virus from father to infant. Genet Mol Res 2015;14:15539-46. [DOI] [PubMed] [Google Scholar]
- 4.Feng J, Yang G, Liu Y, Gao Y, Zhao M, Bu Y, et al. LncRNA PCNAP1 modulates hepatitis B virus replication and enhances tumor growth of liver cancer. Theranostics 2019;9:5227-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng J, Zhou Z, Qiu Y, Wang M, Yu H, Wu Z, et al. A prognostic ferroptosis-related lncRNAs signature associated with immune landscape and radiotherapy response in glioma. Front Cell Dev Biol 2021;9:675555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xia X, Zhang H, Xia P, Zhu Y, Liu J, Xu K, et al. Identification of Glycolysis-related lncRNAs and the novel lncRNA WACAS1 promotes glycolysis and tumor progression in hepatocellular carcinoma. Front Oncol 2021;11:733595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Guo Y, Wang X, Ge A, Wang H, Fan K, et al. Clinical significance of serum miR-487b in HBV-related hepatocellular carcinoma and its potential mechanism. Infect Dis (Lond) 2021;53:546-54. [DOI] [PubMed] [Google Scholar]
- 8.Dong RF, Zhuang YJ, Wang Y, Zhang ZY, Xu XZ, Mao YR, et al. Tumor suppressor miR-192-5p targets TRPM7 and inhibits proliferation and invasion in cervical cancer. Kaohsiung J Med Sci 2021;37:699-708. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Su X, Lin M, Fu B, Zhou C, Ling C, et al. Expression of miR-192-5p in colon cancer serum and its relationship with clinicopathologic features. Am J Transl Res 2021;13:9371-6. [PMC free article] [PubMed] [Google Scholar]
- 10.Liu XL, Cao HX, Wang BC, Xin FZ, Zhang RN, Zhou D, et al. miR-192-5p regulates lipid synthesis in non-alcoholic fatty liver disease through SCD-1. World J Gastroenterol 2017;23:8140-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livak and Schmittgen. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 2001;25:402-8. [DOI] [PubMed] [Google Scholar]
- 12.Xie M, Yang Z, Liu Y, Zheng M. The role of HBV-induced autophagy in HBV replication and HBV related-HCC. Life Sci 2018;205:107-12. [DOI] [PubMed] [Google Scholar]
- 13.Wang D, Yan X, Zhang M, Ren C, Wang L, Ma J, et al. Association between liver cirrhosis and estimated glomerular filtration rates in patients with chronic HBV infection. Medicine (Baltimore) 2020;99:e21387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Yan L, Shi Y, Lv D, Shang J, Bai L, et al. Hepatitis B virus infection: Overview. Adv Exp Med Biol 2020;1179:1-16. [DOI] [PubMed] [Google Scholar]
- 15.Han GR, Jiang HX, Wang CM, Ding Y, Wang GJ, Yue X, et al. Long-term safety and efficacy of telbivudine in infants born to mothers treated during the second or third trimesters of pregnancy. J Viral Hepat 2017;24:514-21. [DOI] [PubMed] [Google Scholar]
- 16.Pan CQ, Duan Z, Dai E, Zhang S, Han G, Wang Y, et al. Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med 2016;374:2324-34. [DOI] [PubMed] [Google Scholar]
- 17.Shahen SM, Elshenawy SZ, Mohamed SE, Talaat RM. Genetic polymorphisms in the miR-372 (rs12983273) and LncRNA HULC (rs7763881) genes and susceptibility to hepatitis B virus (HBV) infection. Mol Biol Rep 2021;48:7901-6. [DOI] [PubMed] [Google Scholar]
- 18.Ren F, Ren JH, Song CL, Tan M, Yu HB, Zhou YJ, et al. LncRNA HOTAIR modulates hepatitis B virus transcription and replication by enhancing SP1 transcription factor. Clin Sci (Lond. 2020;134:3007-22. [DOI] [PubMed] [Google Scholar]
- 19.Li F, Deng Y, Zhang S, Zhu B, Wang J, Wang J, et al. Human hepatocyte-enriched miRNA-192-3p promotes HBV replication through inhibiting Akt/mTOR signalling by targeting ZNF143 in hepatic cell lines. Emerg Microbes Infect 2022;11:616-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J, Chen J, Liu Y, Zeng X, Wei M, Wu S, et al. Hepatitis B virus induces autophagy to promote its replication by the axis of miR-192-3p-XIAP through NF kappa B signaling. Hepatology 2019;69:974-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang C, Yuan P, Wang J, Zhang Y, Chang X, Jin D, et al. MiR- 192-5p regulates the proliferation and apoptosis of cholangiocarcinoma cells by activating MEK/ERK pathway. 3 Biotech 2021;11:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu S, Ma C, Tang X, Ma X, Jing G, Zhao N, et al. MiR-192-5p inhibits proliferation, migration, and invasion in papillary thyroid carcinoma cells by regulation of SH3RF3. Biosci Rep 2021;41:BSR20210342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L, Ming X, Li W, Bi M, Yan B, Wang X, et al. The microRNA-155 mediates hepatitis B virus replication by reinforcing SOCS1 signalling-induced autophagy. Cell Biochem Funct 2020;38:436-42. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Lin Y, Liu S, Zhu Y, Lu K, Broering R, Lu M. OGlcNAcylation modulates HBV replication through regulating cellular autophagy at multiple levels. FASEB J 2020;34: 14473-89. [DOI] [PubMed] [Google Scholar]
- 25.Lou L, Tian M, Chang J, Li F, Zhang G. MiRNA-192-5p attenuates airway remodeling and autophagy in asthma by targeting MMP-16 and ATG7. Biomed Pharmacother 2020;122: 109692. [DOI] [PubMed] [Google Scholar]
- 26.Zhou W, Yu Q, Ma J, Xu C, Wu D, Li C. Triamcinolone acetonide combined with 5-fluorouracil suppresses urethral scar fibroblasts autophagy and fibrosis by increasing miR-192-5p expression. Am J Transl Res 2021;13:5956-68. [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou P, Dong M, Wang J, Li F, Zhang J, Gu J. Baseline serum miR-125b levels predict virologic response to nucleos(t)ide analogue treatment in patients with HBeAg-positive chronic hepatitis B. Exp Ther Med 2018;16:3805-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao L, Wang B, Sun L, Sun B, Li Y. Association of miR-192-5p with Atherosclerosis and its Effect on Proliferation and Migration of Vascular Smooth Muscle Cells. Mol Biotechnol 2021;63:1244-51. [DOI] [PubMed] [Google Scholar]
- 29.Wang S, Sun Y, Wang Y, Wang A, Kou B, Che Y, et al. ASPP2 inhibits hepatitis B virus replication by preventing nucleus translocation of HSF1 and attenuating the transactivation of ATG7. J Cell Mol Med 2021;25:6899-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Collier JJ, Suomi F, Oláhová M, McWilliams TG, Taylor RW. Emerging roles of ATG7 in human health and disease. EMBO Mol Med 2021;13:e14824. [DOI] [PMC free article] [PubMed] [Google Scholar]


