Highlights
-
•
Lifetime use of psilocybin, tryptamine, and ketamine has increased over time.
-
•
Past twelve-month ketamine use increased consistently since 2006.
-
•
Lifetime use of mescaline and salvia have decreased in recent years.
-
•
Generally, age groups 12–17 and 18–25 show decreases for most hallucinogen types.
-
•
Age group 26+ generally showed increases in use.
Keywords: Hallucinogens, Psychedelics, Ketamine, Psilocybin, Tryptamine psychedelics, Drug use trends
Abstract
Aims
Information on time trends in use of different plant-based hallucinogens is lacking. The current study used nationally representative U.S. data to assess overall and age-specific time trends in the prevalence of lifetime and 12-month use of plant-based hallucinogens and dissociative agents.
Methods
Participants were respondents aged ≥ 12 years (N = 1,006,051) from the National Survey on Drug Use and Health, 2002–2019. Predictors were continuous years. Outcomes included illicit use of peyote, mescaline, psilocybin, ketamine, salvia, and tryptamine. Sociodemographic variables (gender; age; race/ethnicity; educational level; family income) were modeled as covariates. Trends were estimated overall and by age (12–17, 18–25, 26+). Prevalence differences [PDs] were obtained for each category, along with 95 % confidence intervals [CI].
Results
Increases in lifetime use were observed for psilocybin (2002–2019 PD=+1.61), tryptamine (2006–2014 PD=+0.55; 2015–2019 PD=+0.44), and ketamine (2006–2014 PD=+0.27; 2015–2019 PD=+0.21). Mescaline use decreased (PD = −0.89). While overall lifetime salvia use increased between 2006 and 2014 (PD=+1.81), prevalence did not change between 2015 and 2019. Twelve-month use of tryptamine and ketamine increased between 2006 and 2014 (PD=+0.14; +0.03, respectively). Twelve-month ketamine use also increased from 2015 to 2019 (PD=+0.03). By age, participants aged 12–17 and 18–25 showed decreases in use of most types of hallucinogens, but those age 26+ generally showed increases.
Conclusions
While use of plant-based hallucinogens and dissociative agents remains rare, lifetime use of ketamine, tryptamine, and psilocybin is increasing in adults. Considering these increases alongside concerns about unsupervised use of illicit products whose dose and composition is uncertain, clinicians and policymakers should remain mindful of the rising rates of illicit use in the general population.
1. Introduction
Hallucinogens are a diverse group of psychoactive substances that can alter perception, mood, and cognition (Nichols, 2004). While most hallucinogens do not cause acute harm and carry low risk for dependence (Hendricks et al., 2015, Krebs and Johansen, 2013, Nesvag et al., 2015, Nichols, 2004), they are considered a Schedule I substance by the US Drug Enforcement Administration (DEA) (defined as drugs with no currently accepted medical use and a high potential of abuse) and produce powerful mind-altering effects that may pose risks, such as feelings of overwhelming distress or a state of fear or dread (a’bad trip’) (DiSclafani et al., 1981, Ungerleider et al., 1968). Further, use of hallucinogens has been associated with the exacerbation of psychosis (Dos Santos et al., 2017, Malhotra et al., 1997) and Hallucinogen-Persisting Perception Disorder, characterized by lingering perceptual symptoms that cause distress long after an episode of use (Halpern and Pope, 2003, Orsolini et al., 2017). At the same time, recent research suggests that hallucinogens may be associated with benefits such as enhanced creative thinking (Mason et al., 2021) or improvements in mental health (Johnson, Hendricks, Barrett, & Griffiths, 2019), which may reflect changes in their illicit use in the general population.
Plant-based hallucinogens and dissociative agents have been used within various cultures’ medicinal and religious practices for thousands of years (Carod-Artal, 2015, Nichols, 2004). Although hallucinogens were classified as a Schedule I substance in the 1970 U.S. Controlled Substances Act (Belouin & Henningfield, 2018), empirical studies have brought a resurgence of attention to potential risks and benefits related to their use, which have recently resulted in multiple state initiatives to reevaluate their legal status (Ballotpedia, 2020, California Legislative Information, 2021). Multiple risks associated with recreational illicit use of these hallucinogens, including memory impairment (Morgan et al., 2014), dysphoria or anxiety (Bienemann et al., 2020, Carbonaro et al., 2016), delusional thinking among frequent (>4x/week) ketamine users (Morgan, Muetzelfeldt, & Curran, 2009), and effects that mimic psychosis (Kapur & Seeman, 2002), which can lead to significant distress (Ceban et al., 2021). When hallucinogenic drugs are purchased and used illicitly, individuals may lack information about safe use, including dosing, and therefore may be more at risk for these adverse effects. At the same time, the past decade has seen increasing efforts to fund large-scale clinical trials to determine potential therapeutic benefit (Rubin, 2019). Early findings suggest promise of psychedelic-assisted treatment for psychopathology in supervised settings (Reiff et al., 2020), and in 2020, Oregon became the first state to legalize psilocybin treatment from licensed providers (Ballotpedia, 2020). In addition to treatment potential, state legalization of substances such as psilocybin for medical use may be partially motivated by reductions in costs for drug-related offenses, and to generate state and local tax revenue (Legislative Analyst's Office, 2021). Additionally, emerging clinical research is paired with increasing private investor interest in the potential of psychedelic treatments, and some market reports forecast that the psychedelic drug market could reach nearly eleven billion dollars by 2027 (PR Newswire, 2021). Although clinical research remains preliminary, recent studies suggest beneficial effects of plant-based hallucinogens and ketamine in treating depression (Berman et al., 2000, Carhart-Harris et al., 2016, Galvez et al., 2014, Griffiths et al., 2016), substance use disorders (Reiff et al., 2020), and post-traumatic stress disorder (Ivan Ezquerra-Romano et al., 2018, Reiff et al., 2020), notably in those resistant to other forms of pharmacological intervention (Cantor, 2021, Carhart-Harris et al., 2016, Serafini et al., 2014). However, if such findings and encourage individuals to use these substances recreationally, increases in use and ensuing adverse consequences may occur in the general population. Consistent with this, findings from the DEA’s Drug Abuse Warning Network (DAWN) show that although ED visits involving hallucinogens were relatively rare compared to other drugs, they increased between 2004 and 2011 (Substance Abuse and Mental Health Services Administration & Center for Behavioral Health Statistics and Quality, 2011). However, ED data are not necessarily generalizable to the larger population.
To provide generalizable information about non-medical (i.e., illicit) hallucinogen use prevalences and time trends in the U.S. population necessitates data from nationally representative samples. Previous epidemiological studies and reports of hallucinogen use in the US population are primarily derived from three sources of information: (1) the National Survey on Drug Use and Health (NSDUH), (2) the National Epidemiologic Survey on Alcohol and Related Conditions-III (NESARC-III), and (3) the Monitoring the Future (MTF) survey of US adolescents. The NSDUH, collected annually, is a critical source for identifying trends over time in substance use. While recent NSDUH findings show increases in both lifetime and 12-month adult use of overall hallucinogen use since 2002 (Livne, Shmulewitz, Walsh, & Hasin, In press), this study did not evaluate the use of plant-based hallucinogens and dissociative agents specifically. Other NSDUH studies report relatively stable lifetime use trends for peyote, mescaline, and psilocybin throughout the 2000 s (Johnson et al., 2019), and increases in adult salvia (Wu, Woody, Yang, Li, & Blazer, 2011) use from 2006 to 2008 and tryptamine (Palamar & Le, 2018) use from 2008 to 2014. However, these studies did not test for trends by age, or are out of date. A study using NESARC-III data identified 2012–2013 lifetime and twelve-month prevalence of any hallucinogen use as 9.32 % and 0.62 %, respectively (Shalit, Rehm, & Lev-Ran, 2019). However, because the NESARC is not an annual survey, it does not provide trend information, and does not include specific categories of hallucinogens. Third, MTF annual reports are a useful tool for identifying adolescent use prevalences. Data from the annual MTF survey show a steady lifetime prevalence of hallucinogen use other than LSD (including mescaline, peyote, psilocybin, salvia, ketamine) of around 3 % in the pooled sample of adolescents since 2015 (Miech, Johnston, O’Malley, Bachman, Schulenberg, & Me., 2017). These findings, paired with NSDUH results showing a decline in any adolescent hallucinogen since 2002, suggest that patterns of hallucinogen use are not consistent across the lifespan. This is further supported by a study showing that illicit microdosing (a self-administered, sub-hallucinogenic dose) is more popular among adults than other age groups (Cameron, Nazarian, & Olson, 2020). However, no study has compared age-specific trends for use of plant-based hallucinogen categories.
To address these knowledge gaps, using NSDUH data from 2002 to 2019, the current study examined trends in the prevalence of illicit use of peyote, mescaline, psilocybin, ketamine, salvia, and tryptamine psychedelics in a US nationally representative sample, overall and by age group (12–17; 18–25; 26 + ).
2. Methods
2.1. Sample
Data were drawn from the 2002–2019 NSDUH public use data files, which provide annual national statistics on substance use and related psychiatric and health correlates (National Survey on Drug Use and Health). The NSDUH includes non-institutionalized civilians aged ≥ 12 years in all 50 US states and the District of Columbia, covering residents of households and select group quarters (National Survey on Drug Use and Health). To participate, households were selected at random, and participants were notified via mail. An interviewer visited each household and completed a screener survey with one resident ≥ 18 years on a handheld computer, after which the survey algorithm randomly selected zero, one, or two household members for participation (Substance Abuse and Mental Health Services Administration, 2015). After the screener and selection process, the interviewer completed the NSDUH survey with participants in a private area of their home, which took around one hour to complete. Written informed consent was obtained from adults or from parents or guardians for adolescent participants. Computer-assisted interviewing was conducted by an interviewer, as well as audio computer-assisted self-interviewing. All NSDUH procedures are approved by the Institutional Review Board at RTI international. Respondents received $30 upon completion of the interview (Substance Abuse and Mental Health Services Administration, October 2019). Sampling weights were computed to adjust for non-response and oversampling, to ensure consistency with population estimates from the US Census Bureau.
The combined total sample size from 2002 to 2019 was 1,006,051, with survey response rates during these years ranging from 64.9 % to 79 % (Substance Abuse and Mental Health Services Administration, September 2020). When combining data over years, new sample weights were created by dividing the original weight by the number of data sets combined, as recommended by SAMSHA (Substance Abuse and Mental Health Services Administration, 2014) and as previously performed (Hasin, Shmulewitz, & Keyes, 2019).
2.2. Measures
2.2.1. Outcomes: Hallucinogen use
Lifetime use was assessed for peyote, mescaline, psilocybin, ketamine, salvia, and tryptamine psychedelics using the following question: “Have you ever, even once, used [substance]?”. To ascertain whether the respondent had ever used tryptamine psychedelics, the instrument queried if the respondent had used dimethyltryptamine (DMT), alpha-methyltryptamine (AMT), or 5-MeO-DIPT (“Foxy”). Responses were coded as binary (“yes”/”no”). Twelve-month use of hallucinogens was assessed for ketamine, salvia, and tryptamine psychedelics, using the following question: “How long has it been since you last used [substance]?” Responses of “within the past 30 days” and “more than 30 days ago, but within the past 12 months” were coded as past 12-month users of that substance.
2.2.2. Predictor: Year
Peyote, mescaline, and psilocybin use were each assessed from 2002 to 2019. Data regarding ketamine, salvia, and tryptamine psychedelic use first became available in 2006, and due to questionnaire changes in 2015, data for these categories could not be combined over the entire period but were analyzed in separate time intervals (2006–2014 and 2015–2019).
2.2.3. Covariates: Sociodemographic characteristics
Sociodemographic covariates included gender (male, female), age in three categories (12–17 years, 18–25 years, 26 + years); educational attainment (<high school, high school graduate or above); total annual family income (≤$49,999, ≥$50,000); and race/ethnicity (non-Hispanic white, Black, Hispanic, Native American/Alaska Native, Native Hawaiian/Other Pacific Islander, Asian, more than one race).
2.2.4. Analysis
Weighted prevalence of hallucinogen use and sociodemographic variables were estimated, pooled for all available years. For each type of hallucinogen, time trends were estimated using logistic regression, with use as the outcome, continuous year as the predictor and sociodemographic variables (gender, age, race/ethnicity, educational attainment, and family income) as covariates. Log-odds were back-transformed to the prevalence scale and change over time was indicated by the difference in the model-predicted prevalence between the last year and the first year (prevalence difference, PD) (Brown et al., 2017, Hasin et al., 2019, Mauro et al., 2018). Prevalence differences significantly different from 0.0 (i.e., 95 % confidence interval [CI] not including 0.0) indicate change over time, with a negative difference indicating a decrease over time, and a positive difference indicating an increase over time.
To determine whether trends differed by age group (12–17; 18–25; 26 + ), an interaction term of time*age was included in the regression model. This allows trends over time (PD) to be estimated separately within each age group. Contrasts between each age group (12–17, 18–25) and ≥ 26 years (reference group), i.e. PD for ≥ 26 years minus PD for 12–17 or 18–25 indicated whether trends differed by age, with estimates significantly different from 0 indicating trend differences. Interaction terms of age with each of the control variables were also included, to allow covariate effects to differ within each age group and ensure that differential trends were not primarily driven by sociodemographic effects differing in the age groups. Contrasts between the age groups with 26 + years as the reference group (difference-in-prevalence difference) indicated whether trends differed by age, with estimates significantly different from 0 indicating trend differences. The 26 + age group was selected as the reference group because it was the most prevalent age category in the sample.
Additional models were run for each outcome, treating year as a categorical variable, to estimate year-by-year adjusted prevalence for graphing. Analyses were conducted using SAS 9.4 and SUDAAN 11.0.4 software (Research Triangle Institute., 2012, SAS Institute Inc, 2013). Collinearity diagnostics (Alin, 2010) were performed using Proc Reg and results showed little evidence for multicollinearity among independent variables, with tolerance ranging between 0.51 and 0.99 and Variance inflation Factor (VIF) ranging between 1.01 and 1.96 (SAS Institute Inc, 2013). Assumption of linearity of independent variables and log-odds were validated by plotting the scatter plot of year vs the log odds of the predicted outcome. For each outcome the plot showed a linear pattern, indicating that the assumption is satisfied.
2.2.4.1. Sensitivity analysis
Because mescaline is extracted from peyote and the two substances share similar nicknames (Drug Enforcement Administration, 2020, Drug Enforcement Administration, 2020), a combined variable encompassing peyote or mescaline use was created and analyzed to address potential double-reporting between the two substances.
2.2.4.2. Missing data
The NSDUH datasets provide pre-coded statistically imputed or recoded demographic and drug use variables which are designed to replace missing or non-informative values (Substance Abuse and Mental Health Services Administration, 2021). The computer-assisted interviewing (CAI) format of the survey allows for two possibilities for missing data: (1) if a respondent entered a response of “don’t know” or “refused” when answering a given item, or (2) a respondent broke off the interview prior to reaching the end of the survey. For all variables used in this analysis, two strategies were implemented to address missing data: imputation and recoding. Imputed variables have statistically estimated values in place of missing data, i.e., non-informative response options such as “don’t know” or “refused” (Substance Abuse and Mental Health Services Administration, 2020). Imputed variables included the sociodemographic variables and ketamine, salvia, and tryptamine psychedelic use variables for 2015–2019. Recoded variables with missing data due to non-informative responses are coded as “no”. Recoded variables included peyote, mescaline, and psilocybin lifetime use variables, and ketamine, salvia, and tryptamine psychedelic use variables for 2006–2014. Among recoded variables, the prevalence of recoded missing information was very low (range, 0.12 % for peyote use to 0.22 % for salvia use, 2006–2014).
2.2.4.3. Influential outliers
The presence of potential influential outliers was addressed by calculating the leverage estimate from the diagonal element of the Hat matrix for each model, identifying the observations that were potential influential outliers, calculating the percentage they comprised from the overall sample of that model, and rerunning the model after excluding these observations. Observations whose leverage statistic exceeded the cutoff value 3 times larger than the mean leverage value (3*(p/n)) were considered 'influential outliers'. This statistic was calculated for each outcome (and in each relevant time period). Influential outliers ranged between 2.13 % and 5.16 % across all models.
3. Results
3.1. Descriptives
In the overall study sample, pooled over all years, 52 % were female, 62 % were non-Hispanic whites, 40 % were aged 26+, 41 % had less than high school education (including all participants aged 12–17 years), and 55 % had a total annual family income of ≤$49,999 (Supplementary Table 1).
3.2. Peyote use
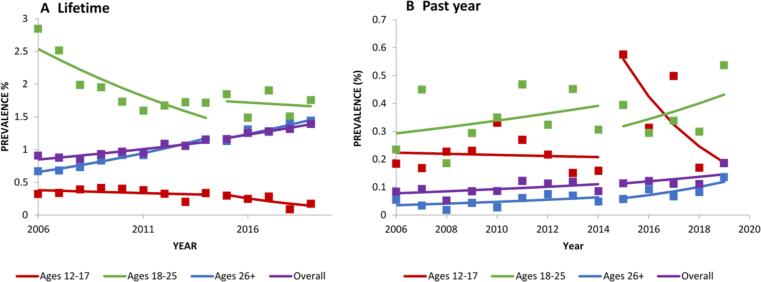
3.2.1. Lifetime
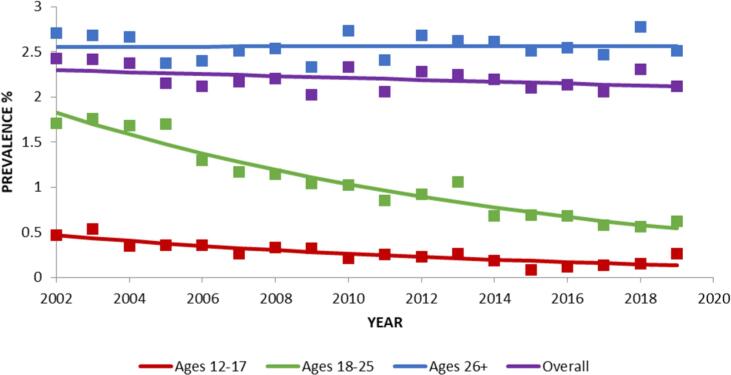
Overall, there was no significant change in the prevalence of lifetime peyote use between 2002 and 2019 (Table 1; Fig. 1). However, within specific age groups, lifetime peyote use decreased significantly in age groups 12–17 from 2002 to 2019, (PD = -0.33), and 18–25 (PD = -1.29), which was significantly different from the lack of change in participants ages 26+ (Table 1; Fig. 1).
Table 1.
Change over time in lifetime prevalence of peyote, mescaline, and psilocybin use, NSDUH, 2002–2019, (N = 1,006,051).
| Lifetime |
||||
|---|---|---|---|---|
| Prevalence % (SE)a | Prevalence difference % (95 % CI)a,b | Trend differences by age group % (95 % CI)c | ||
| Start year | End year | |||
| Peyote | 2002 | 2019 | ||
| Whole sample | 2.30 (0.06) | 2.12 (0.06) | −0.18 (−0.38, 0.02) | |
| Ages 12–17 | 0.47 (0.04) | 0.14 (0.08) | −0.33 (−0.57, −0.09) | −0.34 (−0.58, −0.10) |
| Ages 18–25 | 1.83 (0.07) | 0.54 (0.03) | −1.29 (−1.58, −1.00) | −1.28 (−1.57, −0.99) |
| Ages ≥ 26 | 2.55 (0.07) | 2.56 (0.07) | 0.01 (−0.23, 0.25) | Reference |
| Mescaline | ||||
| Whole sample | 3.69 (0.07) | 2.80 (0.06) | −0.89 (−1.13, −0.65) | |
| Ages 12–17 | 0.38 (0.04) | 0.08 (0.01) | −0.30 (−0.40, −0.20) | 0.51 (0.22, 0.80) |
| Ages 18–25 | 2.40 (0.09) | 0.53 (0.03) | −1.87(−2.09, −1.65) | −1.06 (−1.41, −0.71) |
| Ages ≥ 26 | 4.23 (0.09) | 3.42 (0.08) | −0.81(−1.10, −0.52) | Reference |
| Psilocybin | ||||
| Whole sample | 7.57 (0.10) | 9.18 (0.11) | 1.61 (1.26, 1.96) | |
| Ages 12–17 | 3.30 (0.13) | 1.40 (0.08) | −1.90 (−2.21, −1.59) | −4.80 (−5.33, −4.27) |
| Ages 18–25 | 13.59 (0.16) | 8.79 (0.13) | −4.80 (−5.27, −4.33) | −7.70 (−8.31, −7.09) |
| Ages ≥ 26 | 7.04 (0.11) | 9.93 (0.14) | 2.89 (2.48, 3.30) | Reference |
Adjusted for gender, age, race/ethnicity, educational level, and family income.
Calculated as difference between the predicted prevalence in the end and start year.
Estimated as the difference between the prevalence difference for each age group (12–17 and 18–25 versus reference group).
Fig. 1.
Change over time in lifetime prevalence in peyote use, overall and by age. Trends were analyzed from 2002 to 2019. Square markers indicate adjusted prevalences.
3.3. Mescaline use
3.3.1. Lifetime
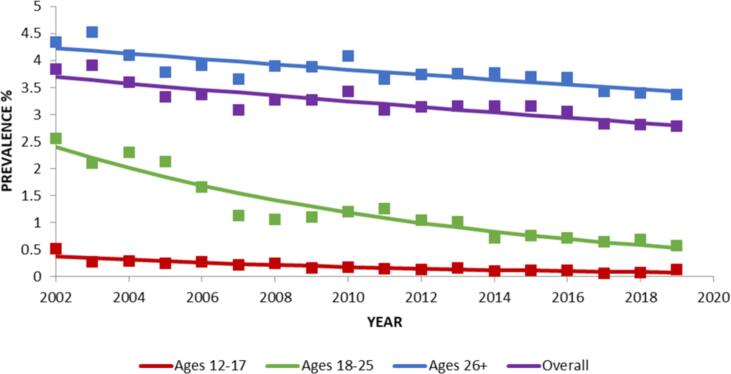
Between 2002 and 2019, overall lifetime mescaline use decreased significantly (PD = -0.89; Table 2; Fig. 2). Lifetime mescaline use also decreased significantly in age groups 12–17 (PD = -0.30), 18–25 (PD = -1.87), and 26+ (PD = -0.81; Table 1; Fig. 2).
Table 2.
Change over time in lifetime and 12-month prevalence of salvia use, NSDUH.
|
Lifetime |
12-month |
|||||||
|---|---|---|---|---|---|---|---|---|
| Prevalence % (SE)a |
Prevalence difference % (95 % CI)a,b | Trend differences by age group % (95 % CI)c | Prevalence % (SE)a |
Prevalence difference % (95 % CI)a,b | Trend differences by age group % (95 % CI)c | |||
| Start year | End year | Start year | End year | |||||
| 2006 | 2014 | 2006 | 2014 | |||||
| Whole sample | 0.67 (0.03) | 2.48 (0.08) | 1.81 (1.69, 1.93) | 0.66 (0.05) | 0.24 (0.01) | −0.42 (−0.54, −0.30) | ||
| Ages 12–17 | 1.66 (0.15) | 1.23 (0.13) | −0.43 (−0.68, −0.18) | −1.77 (−2.08, −1.46) | 0.70 (0.11) | 0.38 (0.06) | −0.32 (−0.48, −0.16) | −0.33 (−0.49, −0.17) |
| Ages 18–25 | 5.53 (0.13) | 9.02 (0.21) | 3.50 (2.95, 4.05) | 2.15 (1.58, 2.72) | 2.51 (0.10) | 0.94 (0.05) | −1.56 (−1.80, −1.32) | −1.57 (−1.81, −1.33) |
| Ages ≥ 26 | 0.31 (0.02) | 1.65 (0.07) | 1.34 (1.18, 1.50) | Reference | 0.09 (0.01) | 0.10 (0.02) | +0.01 (−0.05, 0.07) | Reference |
| 2015 | 2019 | 2015 | 2019 | |||||
| Whole sample | 1.95 (0.07) | 1.85 (0.06) | −0.10 (−0.32, 0.12) | 0.08 (0.01) | 0.05 (0.01) | −0.03 (−0.07, 0.01) | ||
| Ages 12–17 | 0.46 (0.08) | 0.34 (0.09) | −0.12 (−0.36, 0.12) | −0.52 (−0.83, −0.21) | 0.08 (0.03) | 0.08 (0.03) | 0.00 (−0.06, 0.06) | 0.02 (−0.06, 0.10) |
| Ages 18–25 | 5.58 (0.23) | 2.17 (0.12) | −3.41 (−4.02, −2.80) | −3.80 (−4.43, −3.17) | 0.35 (0.07) | 0.20 (0.05) | −0.15 (−0.35, 0.05) | −0.13 (−0.33, 0.07) |
| Ages ≥ 26 | 1.55 (0.07) | 1.94 (0.07) | 0.39 (0.15, 0.63) | Reference | 0.04 (0.01) | 0.02 (0.01) | −0.02 (−0.06, 0.02) | Reference |
Note: Significant trends (p-values < 0.05) are shown in bold. 2002–2019 (N = 1,006,051); 2002–2014 (N = 723,283); 2006–2014 (N = 502,467); 2015–2019 (N = 241,708).
Adjusted for gender, age, race/ethnicity, educational level, and family income.
Calculated as difference between the predicted prevalence in the end and start year.
Estimated as the difference between the prevalence difference for each group (12–17 and 18–25 versus the ≥ 26 reference group).
Fig. 2.
Change over time in lifetime prevalence in mescaline use, overall and by age. Trends were analyzed from 2002 to 2019. Square markers indicate adjusted prevalences.
3.4. Psilocybin use
3.4.1. Lifetime
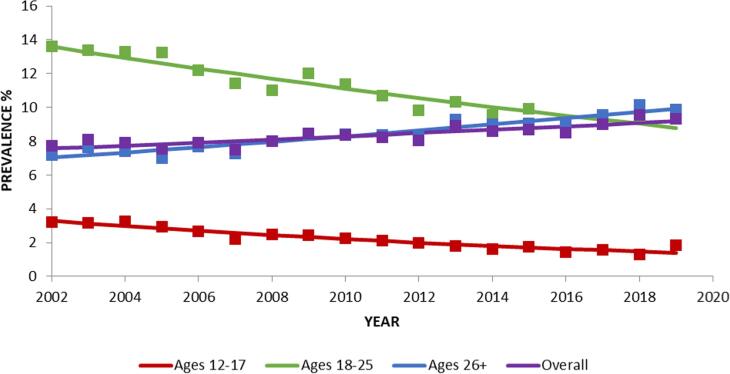
Overall lifetime psilocybin use increased significantly from 2002 to 2019 (PD=+1.61; Table 1; Fig. 3). Lifetime psilocybin use decreased significantly in age groups 12–17 (PD = −1.90) and 18–25 (PD = −4.80) but increased significantly in respondents aged 26+ (PD=+2.89).
Fig. 3.
Change over time in lifetime prevalence in psilocybin use, overall and by age. Trends were analyzed from 2002 to 2019. Square markers indicate adjusted prevalences.
3.5. Salvia use
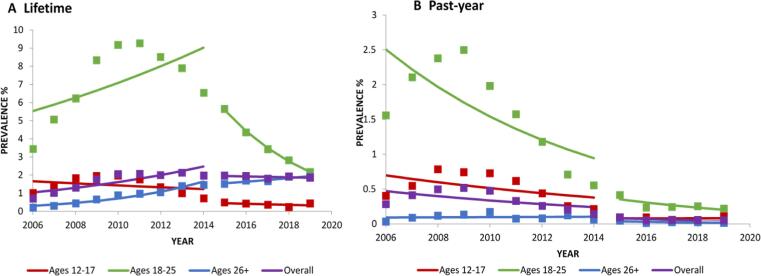
3.5.1. Lifetime
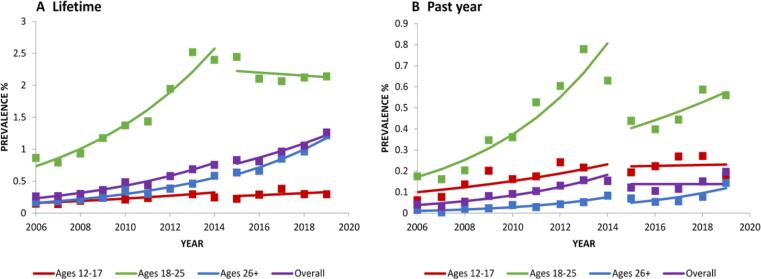
There was an overall significant increase in lifetime salvia use between 2006 and 2014 (PD=+1.81; Table 2; Fig. 4a), with no significant change from 2015 to 2019 (PD = −0.10), although descriptively, the increase appears to peak around 2011, followed by a decreasing trend (Fig. 4a). From 2006 to 2014, lifetime salvia use decreased significantly in respondents aged 12–17 (PD = −0.43) but increased significantly in age groups 18–25 (PD=+3.50) and 26+ (PD=+1.34; Table 4). From 2015 to 2019, similar trends were observed in ages 26+ (PD=+0.39), but a decrease was observed in ages 18–25 (PD = −3.41; Table 2).
Fig. 4.
Change over time in lifetime, past-year prevalence in salvia use, overall and by age. Trends were analyzed from 2006 to 2014 and 2015 to 2019. Square markers indicate adjusted prevalences.
Table 4.
Change over time in lifetime and 12-month prevalence of ketamine use, NSDUH.
|
Lifetime |
12-month |
|||||||
|---|---|---|---|---|---|---|---|---|
| Prevalence % (SE)a |
Prevalence difference % (95 % CI)a,b | Trend differences by age group % (95 % CI)c | Prevalence % (SE)a |
Prevalence difference % (95 % CI)a,b | Trend differences by age group % (95 % CI)c | |||
| Start year | End year | Start year | End year | |||||
| 2006 | 2014 | 2006 | 2014 | |||||
| Whole sample | 0.85 (0.04) | 1.12 (0.04) | 0.27 (0.15, 0.39) | 0.08 (0.01) | 0.11 (0.09) | 0.03 (0.01, 0.07) | ||
| Ages 12–17 | 0.38 (0.05) | 0.31 (0.04) | −0.07 (−0.19, 0.05) | −0.58 (−0.80, −0.36) | 0.22 (0.08) | 0.21 (0.07) | −0.01 (−0.13, 0.11) | −0.04 (−0.16, 0.06) |
| Ages 18–25 | 2.54 (0.12) | 1.49 (0.07) | −1.05 (−1.36, −0.74) | −1.57 (−1.92, −1.22) | 0.29 (0.03) | 0.39 (0.04) | 0.10 (−0.02, 0.22) | 0.07 (−0.05, 0.19) |
| Ages ≥ 26 | 0.66 (0.04) | 1.17 (0.05) | 0.52 (0.36, 0.68) | Reference | 0.04 (0.04) | 0.06 (0.01) | 0.02 (−0.02, 0.06) | Reference |
| 2015 | 2019 | 2015 | 2019 | |||||
| Whole sample | 1.18 (0.05) | 1.39 (0.05) | 0.21 (0.05, 0.37) | 0.11 (0.01) | 0.15 (0.01) | 0.033 (0.029, 0.037) | ||
| Ages 12–17 | 0.31 (0.07) | 0.14 (0.04) | −0.17 (−0.37, 0.03) | −0.45 (−0.69, −0.21) | 0.56 (0.25) | 0.19 (0.01) | −0.37 (−0.80, 0.06) | −0.43 (−0.86, 0.00) |
| Ages 18–25 | 1.74 (0.08) | 1.66 (0.08) | −0.08 (−0.28, 0.12) | −0.36 (−0.42, −0.30) | 0.32 (0.06) | 0.43 (0.05) | 0.11 (−0.11, 0.33) | 0.05 (−0.19, 0.29) |
| Ages ≥ 26 | 1.17 (0.05) | 1.46 (0.05) | 0.29 (0.13, 0.45) | Reference | 0.06 (0.01) | 0.12 (0.02) | 0.06 (0.00, 0.12) | Reference |
Note: Significant trends (p-values < 0.05) are shown in bold. 2002–2019 (N = 1,006,051); 2002–2014 (N = 723,283); 2006–2014 (N = 502,467); 2015–2019 (N = 241,708).
Adjusted for gender, age, race/ethnicity, educational level, and family income.
Calculated as difference between the predicted prevalence in the end and start year.
Estimated as the difference between the prevalence difference for each group (12–17 and 18–25 versus the ≥ 26 reference group).
3.5.2. Twelve-months
From 2006 to 2014, there was an overall significant decrease in 12-month salvia use (PD = −0.42; Table 2; Fig. 4b), and among age groups 12–17 (PD = −0.32) and 18–25 (PD = −1.56; Table 4). From 2015 to 2019, there was no significant change in 12-month salvia use overall or by age group.
3.6. Tryptamine psychedelic use
3.6.1. Lifetime
Overall lifetime tryptamine psychedelic use increased between 2006 and 2014 (PD=+0.55), and between 2015 and 2019 (PD=+0.44; Table 3; Fig. 5a). Lifetime tryptamine psychedelic use increased significantly from 2006 to 2014 in age groups 12–17 (PD=+0.17), 18–25 (PD=+1.84), and 26+ (PD=+0.39), and from 2015 to 2019 in age group 26+ (PD=+0.56; Table 3; Fig. 5a).
Table 3.
Change over time in lifetime and 12-month prevalence of tryptamine use, NSDUH.
|
Lifetime |
12-month |
|||||||
|---|---|---|---|---|---|---|---|---|
| Prevalence % (SE)a |
Prevalence difference % (95 % CI)a,b | Trend differences by age group % (95 % CI)c | Prevalence % (SE)a |
Prevalence difference % (95 % CI)a,b | Trend differences by age group % (95 % CI)c | |||
| Start year | End year | Start year | End year | |||||
| 2006 | 2014 | 2006 | 2014 | |||||
| Whole sample | 0.23 (0.01) | 0.79 (0.04) | 0.55 (0.45, 0.65) | 0.04 (0.00) | 0.18 (0.02) | 0.14 (0.10, 0.18) | ||
| Ages 12–17 | 0.16 (0.03) | 0.33 (0.05) | 0.17 (0.07, 0.27) | −0.23 (−0.37, −0.09) | 0.10 (0.03) | 0.23 (0.07) | 0.13 (0.03, 0.23) | 0.07 (−0.05, 0.19) |
| Ages 18–25 | 0.73 (0.04) | 2.58 (0.12) | 1.84 (1.55, 2.13) | 1.45 (1.14, 1.76) | 0.17 (0.02) | 0.81 (0.07) | 0.64 (0.48, 0.80) | 0.57 (0.41, 0.73) |
| Ages ≥ 26 | 0.16 (0.02) | 0.56 (0.05) | 0.39 (0.29, 0.49) | Reference | 0.01 (0.00) | 0.08 (0.02) | 0.07 (0.03, 0.11) | Reference |
| 2015 | 2019 | 2015 | 2019 | |||||
| Whole sample | 0.78 (0.04) | 1.22 (0.05) | 0.44 (0.28, 0.60) | 0.14 (0.01) | 0.14 (0.01) | 0.00 (0.00, 0.00) | ||
| Ages 12–17 | 0.27 (0.06) | 0.33 (0.08) | 0.06 (−0.16, 0.28) | −0.51 (−0.75, −0.27) | 0.22 (0.06) | 0.23 (0.12) | 0.01 (−0.19, 0.21) | −0.06 (−0.26, 0.14) |
| Ages 18–25 | 2.22 (0.10) | 2.13 (0.11) | −0.09 (−0.42, 0.24) | −0.66 (−0.86, −0.46) | 0.40 (0.05) | 0.58 (0.08) | 0.18 (−0.04, 0.40) | 0.10 (−0.14, 0.34) |
| Ages ≥ 26 | 0.61 (0.03) | 1.17 (0.06) | 0.56 (0.42, 0.70) | Reference | 0.05 (0.01) | 0.12 (0.02) | 0.07 (0.01, 0.13) | Reference |
Note: Significant trends (p-values < 0.05) are shown in bold. 2002–2019 (N = 1,006,051); 2002–2014 (N = 723,283); 2006–2014 (N = 502,467); 2015–2019 (N = 241,708).
Adjusted for gender, age, race/ethnicity, educational level, and family income.
Calculated as difference between the predicted prevalence in the end and start year.
Estimated as the difference between the prevalence difference for each group (12–17 and 18–25 versus the ≥ 26 reference group).
Fig. 5.
Change over time in lifetime, past-year prevalence in Tryptamine Psychedelics use, overall and by age. Trends were analyzed from 2006 to 2014 and 2015 to 2019. Square markers indicate adjusted prevalences.
3.6.2. Twelve-months
From 2006 to 2014, there was an overall significant increase in 12-month tryptamine psychedelic use (PD=+0.14), with no significant change from 2015 to 2019 (Table 3; Fig. 5b). 12-month tryptamine psychedelic use significantly increased from 2006 to 2014 across all age groups (12–17 (PD=+0.13); 18–25 (PD=+0.64); 26+ (PD=+0.07)), and from 2015 to 2019 in ages + 26 (PD=+0.07; Table 3; Fig. 5b).
3.7. Ketamine use
3.7.1. Lifetime
There was an overall significant increase in lifetime ketamine use between 2006 and 2014 (PD=+0.27; Table 4; Fig. 6a), and between 2015 and 2019 (PD=+0.21; Table 4; Fig. 6a). Lifetime ketamine use, from 2006 to 2014, decreased significantly in age group 18–25 (PD = -1.05) and increased significantly in respondents aged 26+ (PD=+0.52), and increased significantly in the 26 + age group (PD=+0.29) from 2015 to 2019 (Table 4; Fig. 6a).
Fig. 6.
Change over time in lifetime, past-year prevalence in ketamine use, overall and by age a,b. Trends were analyzed from 2006 to 2014 and 2015 to 2019. Square markers indicate adjusted prevalences.
3.7.2. Twelve-months
Overall past 12-month ketamine use increased significantly between 2006 and 2014 (PD=+0.03), and from 2015 to 2019 (PD=+0.03; Table 4; Fig. 6b). There was no significant change in past 12-month ketamine use by age between 2006 and 2014. However, past 12-month ketamine use increased in respondents aged + 26 between 2015 and 2019 (PD=+0.06; Table 4; Fig. 6b).
3.8. Sensitivity analysis
Overall lifetime combined mescaline or peyote use decreased significantly from 2002 to 2019 (PD = −0.88; Supplementary Table 3; Supplementary Fig. 1). Lifetime mescaline or peyote use also decreased across all age groups (12–17 (PD = −0.53); 18–25 (PD = −2.60); 26+ (PD = −0.66).
3.9. Removal of influential outliers
After running all models excluding the observations identified as potential influential outliers, the direction of the trend in lifetime tryptamine use among those aged 18–25 changed to an increase and became significant between 2015 and 2019 (PD=+0.47). All other results of trends did not change, and all significant values remained significant.
4. Discussion
Using data from the U.S. nationally representative NSDUH surveys, this study examined temporal trends in the illicit use of plant-based hallucinogens and dissociative agents between 2002 and 2019. Our findings indicate that the direction and magnitude of changes over time varied by type of hallucinogen, age group, time intervals, and timeframe of use (lifetime or past 12 months). For example, overall lifetime use of psilocybin, tryptamine, salvia, and ketamine use increased, while mescaline use decreased, while past 12 month use increased for ketamine and tryptamines and decreased for salvia. Age groups 12–17 and 18–25 generally showed decreases in use across most types of hallucinogens, while adults age 26 + generally showed increases.
This is the first study to report trends in lifetime use of mescaline, peyote and psilocybin using data from a large-scale, nationally representative survey of the US population. The decrease in overall mescaline use is consistent with past popular media reports (Oberhaus, December 14, 2018), and occurred across all age groups. While overall lifetime peyote use remained unchanged across survey years, respondents aged 12–17 and 18–25 showed a significant decrease in peyote use, consistent with MTF reports of adolescent use (Miech, Johnston, O’Malley, Bachman, Schulenberg, & Patrick, 2017). Given that mescaline is extracted from peyote, it should be noted that pure mescaline is rare and is more commonly used in the form of peyote buttons or other mescaline-producing plants. Mescaline use may therefore be overreported in NSDUH, while peyote may be underreported, as respondents may have confused the two. Combined mescaline or peyote use followed the same trends as mescaline use alone, and future studies that assess both mescaline and peyote use may benefit from clear descriptions of each substance to eliminate potential confusion between the two. In any case, a possible cause of the decreases in mescaline and peyote use could be the rapid decline in the supply of peyote to the US due to habitat destruction, illegal poaching, and unsustainable harvesting practices (Oberhaus, December 14, 2018).
In contrast, lifetime psilocybin use increased overall across survey years, and this category remains the most common plant-based hallucinogen used in the US. This increase was driven by respondents aged 26 + and may reflect changing norms related to substance use, reflected in legalization (Ballotpedia, 2020), microdosing practices (Polito & Stevenson, 2019), or therapeutic uses. Psilocybin is increasingly studied for its potential therapeutic benefits (Carhart-Harris et al., 2016, Griffiths et al., 2016, Hendricks et al., 2015). Notably, a recent study found high doses of psilocybin to be associated with a decrease in both self and clinician-reported measures of depression and anxiety, along with increases in quality of life and optimism among cancer patients (Griffiths et al., 2016). If therapeutic effects are replicated and evident on a larger scale, adults in the general population may increasingly perceive psilocybin as non-risky substance and use it regardless of therapeutic indication, continuing an upward trend in use. Although psilocybin carries low risk for dependence, individuals may be at a higher risk for adverse experiences such as dysphoria (Bienemann et al., 2020) when using illicitly. However, we show a decrease in use among adolescents, indicating that trends vary across the lifespan. Our findings are consistent with data from MTF showing declines in use of hallucinogens other than LSD in adolescents since 2001. Of note, MTF data do not show declining adolescent rates in use of all substances, since vaping of nicotine and cannabis significantly increased in MTF participants between 2017 and 2019, potentially reflecting a shift in use towards substances with high perceived availability among this age group (Johnston et al., 2020). However, given our findings on the increases in adult use of psilocybin, further research into reasons for psilocybin use among adults is needed to provide additional insight into the increases in this age group.
Our findings show that lifetime and 12-month ketamine use increased overall between 2006 and 2014 and 2015 to 2019. Similar to psilocybin use, significant increases in lifetime or past year use were exclusively observed in adults 26+, perhaps also due to reports suggesting therapeutic effects of ketamine for mood disorders, paired with a surge of attention in popular media to potential benefits of ketamine (Dodge, November 4, 2021). While ketamine treatment for psychiatric disorders is shown to be safe under supervised use (Serafini et al., 2014), illicit use may be more prone to adverse effects (Ceban et al., 2021) or dissociative symptoms (Morgan et al., 2009). Increases in use up to 2019 occurred simultaneously with FDA approval of esketamine, an antidepressant administered as a nasal spray derived from ketamine (Food and Drug Administration, 2019). Additionally, news reporting showing outcomes of clinical trials assessing the efficacy of hallucinogenic drugs such as psilocybin and ketamine (Eschner, 2022, LaMotte, 2022) as well as anecdotal reports of practices such as “microdosing” (Batty, 2021) increases their exposure to the general population, which could encourage increases in their use. As ketamine becomes more widely accepted for treatment, providers should also monitor potential recreational use and self-medication behaviors among adults. However, ketamine use decreased or remained stable among younger adults and adolescents. As with psilocybin, future research regarding motivations for ketamine use may provide insight into trends, including whether ketamine is used as a club drug or for self-medication purposes.
Overall and age-specific lifetime salvia trends reported in this study add to findings from a previous NSDUH study (Wu et al., 2011). The differences in lifetime and 12-month trends (overall and age-specific) may indicate a cohort effect, since with each year, fewer young people (12–17 years) and more older people (26 + years) used salvia. This may be partially attributed to the changing legal status of salvia, which became illegal to possess or sell in many US states between 2008 and 2011 (Drug Enforcement Administration, 2020, Drug Enforcement Administration, 2020) and is currently banned in 29 states. Considering that salvia carries a low risk for dependence, and as other substances including cannabis become more available for recreational use, young adults may instead be opting for use of more easily accessible cannabis.
Our finding of an overall upward trend in both lifetime and 12-month tryptamine use is consistent with results from a recent study (Palamar & Le, 2018), and affirms a growing concern about the increase in popularity of synthetic tryptamines (Araujo, Carvalho, Bastos Mde, Guedes de Pinho, & Carvalho, 2015), novel psychoactive substances that have broad negative health outcomes (Boland et al., 2005, Jovel et al., 2014). Further, our finding that this trend occurred across all age groups is alarming, considering that use of these synthetic substances was only found among the young adult age group in the past (Palamar & Le, 2018). One prior study found that the proportion of users trying the tryptamine dimethyltryptamine (DMT) for the first time is higher than new users of LSD, psilocybin, and ketamine (Winstock, Kaar, & Borschmann, 2014), and the prevalence of tryptamine use was higher among those who also used other hallucinogens (Palamar & Le, 2018). These findings suggest that tryptamines may be used as an alternative to other substances, possibly due to availability and lower cost. Considering the growing popularity of tryptamines, further research on the health risks and the sociodemographic and clinical correlates of these substances is warranted.
This study has limitations. First, due to the nature of NSDUH’s self-report methods, results may be subject to bias and potential underreporting of substance use. Self-reports may also be inaccurate about the substance used if respondents could not differentiate between plant-based hallucinogens with similar characteristics. Second, data for salvia, tryptamines, and ketamine became available in 2006, and analysis of these hallucinogen categories could not be performed across the entire timeframe due to questionnaire changes in 2015, and therefore were analyzed over two separate time intervals. Third, past 12-month use data were not available for peyote, mescaline, and psilocybin. Because of this limitation, future study of past 12-month use of these hallucinogens is warranted. Fourth, the current study evaluated temporal trends in a combined category for adults ages 26 +. Trends in use may differ among phases of older adulthood, and future research to delineate patterns in use among more nuanced categories of adults is warranted. Fifth, analysis of hallucinogen use prevalences would benefit from additional evaluation using other demographic variables, for example in trend patterns by gender or by race/ethnicity. Finally, while findings can be considered in the context of increased interest of potential therapeutic benefit of hallucinogens, specific information on patterns of use is lacking. As such, further research is warranted to determine settings and motivations related to hallucinogen use, including whether use was supervised, for religious or spiritual purposes, or for self-medication purposes and the positive or negative experiences and/or consequences associated with such use.
5. Conclusions
Our findings provide a large-scale overview of changes in use of plant-based hallucinogens and dissociative agents over time, and a succinct picture of current use rates. Lifetime and 12-month use of ketamine increased consistently overall and continued to increase in those aged 26 + years in more recent years. Lifetime use of psilocybin also increased overall and in adults 26+, while lifetime tryptamine use increased overall and in those aged 18–25 and 26 + more recently. These findings, paired with a growing body of work suggesting that hallucinogens such as ketamine and psilocybin may offer therapeutic benefits, indicate that hallucinogen use may become more widely accepted in the general population. As researcher and investor interest in the potential of hallucinogen use for treatment purposes increases, clinicians and policymakers must also remain mindful of the already rising rates of use of these substances in the general population and of the potential risks related to their use. Although use in the general population remains rare, recent increases in prevalence justify monitoring if these increases are associated with an increase in harm related to use. Such monitoring of recreational use motivations and potential harm related to use would provide information for appropriate risk prevention.
Funding
This work was supported by the National Institute on Drug Abuse [T32DA031099 (Hasin)], and the New York State Psychiatric Institute.
CRediT authorship contribution statement
Claire A. Walsh: Conceptualization, Writing – original draft, Writing – review & editing. Ofir Livne: Conceptualization, Software, Writing – review & editing. Dvora Shmulewitz: Methodology, Formal analysis, Writing – review & editing. Malki Stohl: Methodology, Formal analysis. Deborah S. Hasin: Conceptualization, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.abrep.2022.100454.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Alin. (2010). Multicollinearity. Wiley Interdisciplinary Reviews, Computational Statistics 2(3), 370–374. Retrieved from https://doi.org/10.1002/wics.84.
- Araujo A.M., Carvalho F., Bastos Mde L., Guedes de Pinho P., Carvalho M. The hallucinogenic world of tryptamines: An updated review. Archives of Toxicology. 2015;89(8):1151–1173. doi: 10.1007/s00204-015-1513-x. [DOI] [PubMed] [Google Scholar]
- Ballotpedia. (2020). Oregon Measure 109, Psilocybin Mushroom Services Program Initiative (2020). Retrieved from https://ballotpedia.org/Oregon_Measure_109,_Psilocybin_Mushroom_Services_Program_Initiative_(2020).
- Batty, D. (2021). People ‘microdosing’ on psychedelics to improve wellbeing during pandemic. Retrieved from The Guardian: https://www.theguardian.com/society/2021/dec/02/people-microdosing-on-psychedelics-to-improve-wellbeing-during-pandemic.
- Belouin S.J., Henningfield J.E. Psychedelics: Where we are now, why we got here, what we must do. Neuropharmacology. 2018;142:7–19. doi: 10.1016/j.neuropharm.2018.02.018. [DOI] [PubMed] [Google Scholar]
- Berman R.M., Cappiello A., Anand A., Oren D.A., Heninger G.R., Charney D.S., Krystal J.H. Antidepressant effects of ketamine in depressed patients. Biological Psychiatry. 2000;47(4):351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Bienemann B., Ruschel N.S., Campos M.L., Negreiros M.A., Mograbi D.C. Self-reported negative outcomes of psilocybin users: A quantitative textual analysis. PLoS ONE. 2020;15(2):e0229067. doi: 10.1371/journal.pone.0229067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland D.M., Andollo W., Hime G.W., Hearn W.L. Fatality due to acute alpha-methyltryptamine intoxication. Journal of Analytical Toxicology. 2005;29(5):394–397. doi: 10.1093/jat/29.5.394. [DOI] [PubMed] [Google Scholar]
- Brown Q.L., Sarvet A.L., Shmulewitz D., Martins S.S., Wall M.M., Hasin D.S. Trends in Marijuana Use Among Pregnant and Nonpregnant Reproductive-Aged Women, 2002–2014. JAMA. 2017;317(2):207–209. doi: 10.1001/jama.2016.17383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- California Legislative Information. (February 17 2021). SB-519 Controlled substances: decriminalization of certain hallucinogenic substances. Retrieved from https://leginfo.legislature.ca.gov/faces/billNavClient.xhtml?bill_id=202120220SB519.
- Cameron L.P., Nazarian A., Olson D.E. Psychedelic Microdosing: Prevalence and Subjective Effects. Journal of Psychoactive Drugs. 2020;52(2):113–122. doi: 10.1080/02791072.2020.1718250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantor, C. (2021). Psilocybin Found to Rapidly Improve Depressive Symptoms in Clinical Trial. Retrieved from https://www.columbiapsychiatry.org/news/psilocybin-found-rapidly-improve-depressive-symptoms-clinical-trial.
- Carbonaro T.M., Bradstreet M.P., Barrett F.S., MacLean K.A., Jesse R., Johnson M.W., Griffiths R.R. Survey study of challenging experiences after ingesting psilocybin mushrooms: Acute and enduring positive and negative consequences. Journal of Psychopharmacology. 2016;30(12):1268–1278. doi: 10.1177/0269881116662634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carhart-Harris R.L., Bolstridge M., Rucker J., Day C.M., Erritzoe D., Kaelen M.…Nutt D.J. Psilocybin with psychological support for treatment-resistant depression: An open-label feasibility study. Lancet Psychiatry. 2016;3(7):619–627. doi: 10.1016/S2215-0366(16)30065-7. [DOI] [PubMed] [Google Scholar]
- Carod-Artal F.J. Hallucinogenic drugs in pre-Columbian Mesoamerican cultures. Neurologia. 2015;30(1):42–49. doi: 10.1016/j.nrl.2011.07.003. [DOI] [PubMed] [Google Scholar]
- Ceban F., Rosenblat J.D., Kratiuk K., Lee Y., Rodrigues N.B., Gill H.…McIntyre R.S. Prevention and Management of Common Adverse Effects of Ketamine and Esketamine in Patients with Mood Disorders. CNS Drugs. 2021;35(9):925–934. doi: 10.1007/s40263-021-00846-5. [DOI] [PubMed] [Google Scholar]
- DiSclafani A., 2nd, Hall R.C., Gardner E.R. Drug-induced psychosis: Emergency diagnosis and management. Psychosomatics. 1981;22(10):845–850. doi: 10.1016/S0033-3182(81)73092-5. 855. [DOI] [PubMed] [Google Scholar]
- Dodge, D. (November 4 2021). The Ketamine Cure. Retrieved from The New York Times: https://www.nytimes.com/2021/11/04/well/ketamine-therapy-depression.html.
- Dos Santos R.G., Bouso J.C., Hallak J.E.C. Ayahuasca, dimethyltryptamine, and psychosis: A systematic review of human studies. Therapeutic Advances in Psychopharmacology. 2017;7(4):141–157. doi: 10.1177/2045125316689030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration. (2020). SALVIA DIVINORUM AND SALVINORIN A. Retrieved from https://www.deadiversion.usdoj.gov/drug_chem_info/salvia_d.pdf.
- Drug Enforcement Administration. (April 2020). Drug Fact Sheet: Peyote and Mescaline. Retrieved from https://www.dea.gov/sites/default/files/2020-06/Peyote%20and%20Mescaline-2020_0.pdf.
- Eschner, K. (2022). The Promises and Perils of Psychedelic Health Care. Retrieved from The New York Times: https://www.nytimes.com/2022/01/05/well/psychedelic-drugs-mental-health-therapy.html.
- Food and Drug Administration. (2019). FDA approves new nasal spray medication for treatment-resistant depression; available only at a certified doctor’s office or clinic. Retrieved from https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified.
- Galvez V., O'Keefe E., Cotiga L., Leyden J., Harper S., Glue P.…Loo C.K. Long-lasting effects of a single subcutaneous dose of ketamine for treating melancholic depression: A case report. Biological Psychiatry. 2014;76(3):e1–e2. doi: 10.1016/j.biopsych.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Griffiths R.R., Johnson M.W., Carducci M.A., Umbricht A., Richards W.A., Richards B.D.…Klinedinst M.A. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. Journal of Psychopharmacology. 2016;30(12):1181–1197. doi: 10.1177/0269881116675513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern J.H., Pope H.G., Jr. Hallucinogen persisting perception disorder: What do we know after 50 years? Drug and Alcohol Dependence. 2003;69(2):109–119. doi: 10.1016/s0376-8716(02)00306-x. [DOI] [PubMed] [Google Scholar]
- Hasin, D. S., Shmulewitz, D., & Keyes, K. (2019). Alcohol use and binge drinking among U.S. men, pregnant and non-pregnant women ages 18-44: 2002-2017. Drug Alcohol Depend, 205, 107590. doi:10.1016/j.drugalcdep.2019.107590. [DOI] [PMC free article] [PubMed]
- Hendricks P.S., Thorne C.B., Clark C.B., Coombs D.W., Johnson M.W. Classic psychedelic use is associated with reduced psychological distress and suicidality in the United States adult population. Journal of Psychopharmacology. 2015;29(3):280–288. doi: 10.1177/0269881114565653. [DOI] [PubMed] [Google Scholar]
- Ivan Ezquerra-Romano I., Lawn W., Krupitsky E., Morgan C.J.A. Ketamine for the treatment of addiction: Evidence and potential mechanisms. Neuropharmacology. 2018;142:72–82. doi: 10.1016/j.neuropharm.2018.01.017. [DOI] [PubMed] [Google Scholar]
- Johnson M.W., Hendricks P.S., Barrett F.S., Griffiths R.R. Classic psychedelics: An integrative review of epidemiology, therapeutics, mystical experience, and brain network function. Pharmacology & Therapeutics. 2019;197:83–102. doi: 10.1016/j.pharmthera.2018.11.010. [DOI] [PubMed] [Google Scholar]
- Johnston, L., Miech, R., O'Malley, P., Bachman, J., Schulenberg, J., & Patrick, M. (2020). Monitoring the Future 2019 Overview: Key Findings on Adolescent Drug Use. Retrieved from The University of Michigan: https://cdn.ymaws.com/www.fadaa.org/resource/resmgr/files/resource_center/mtf-overview2019.pdf.
- Jovel A., Felthous A., Bhattacharyya A. Delirium due to intoxication from the novel synthetic tryptamine 5-MeO-DALT. Journal of Forensic Science. 2014;59(3):844–846. doi: 10.1111/1556-4029.12367. [DOI] [PubMed] [Google Scholar]
- Kapur S., Seeman P. NMDA receptor antagonists ketamine and PCP have direct effects on the dopamine D(2) and serotonin 5-HT(2)receptors-implications for models of schizophrenia. Molecular Psychiatry. 2002;7(8):837–844. doi: 10.1038/sj.mp.4001093. [DOI] [PubMed] [Google Scholar]
- Krebs T.S., Johansen P.O. Psychedelics and mental health: A population study. PLoS ONE. 2013;8(8):e63972. doi: 10.1371/journal.pone.0063972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMotte, S. (2022). How psilocybin, the psychedelic in mushrooms, may rewire the brain to ease depression, anxiety and more. Retrieved from CNN: https://www.cnn.com/2022/06/11/health/psilocybin-brain-changes-life-itself-wellness-scn/index.html.
- Legislative Analyst's Office. (2021). Psilocybin Legalization. Retrieved from https://lao.ca.gov/BallotAnalysis/BallotDetail?id=1361.
- Livne, O., Shmulewitz, D., Walsh, C., & Hasin, D. (In press). Adolescent and adult time trends in U.S. hallucinogen use, 2002–2019: any use, and use of ecstasy, LSD, and PCP. Addiction. [DOI] [PMC free article] [PubMed]
- Malhotra A.K., Pinals D.A., Adler C.M., Elman I., Clifton A., Pickar D., Breier A. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17(3):141–150. doi: 10.1016/S0893-133X(97)00036-5. [DOI] [PubMed] [Google Scholar]
- Mason N.L., Kuypers K.P.C., Reckweg J.T., Muller F., Tse D.H.Y., Da Rios B.…Ramaekers J.G. Spontaneous and deliberate creative cognition during and after psilocybin exposure. Translational Psychiatry. 2021;11(1):209. doi: 10.1038/s41398-021-01335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro P.M., Carliner H., Brown Q.L., Hasin D.S., Shmulewitz D., Rahim-Juwel R.…Martins S.S. Age Differences in Daily and Nondaily Cannabis Use in the United States, 2002–2014. Journal of Studies on Alcohol and Drugs. 2018;79(3):423–431. doi: 10.15288/jsad.2018.79.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech, R. A., Johnston, L. D., O’Malley, P. M., Bachman, J. G., Schulenberg, J. E., & Me, P. (2017). Monitoring the future study national survey results on drug use, 1975-2016: Volume 1, secondary school students. University of Michigan, Institute for Social Research.
- Miech, R. A., Johnston, L. D., O’Malley, P. M., Bachman, J. G., Schulenberg, J. E., & Patrick, M. (2017). Monitoring the future study national survey results on drug use, 1975-2016: Volume 1, secondary school students. Retrieved from University of Michigan, Institute for Social Research.
- Morgan C., Dodds C., Furby H., Pepper F., Fam J., Freeman T.…Stone J. Long-Term Heavy Ketamine Use is Associated with Spatial Memory Impairment and Altered Hippocampal Activation. Frontiers in Psychiatry. 2014;5 doi: 10.3389/fpsyt.2014.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C.J., Muetzelfeldt L., Curran H.V. Ketamine use, cognition and psychological wellbeing: A comparison of frequent, infrequent and ex-users with polydrug and non-using controls. Addiction. 2009;104(1):77–87. doi: 10.1111/j.1360-0443.2008.02394.x. [DOI] [PubMed] [Google Scholar]
- National Survey on Drug Use and Health. Retrieved from https://nsduhweb.rti.org/respweb/homepage.cfm.
- Nesvag R., Bramness J.G., Ystrom E., Suzanne Krebs T., Johansen P.O. The link between use of psychedelic drugs and mental health problems. Journal of Psychopharmacology. 2015;29(9):1035–1036. doi: 10.1177/0269881115596156. [DOI] [PubMed] [Google Scholar]
- Nichols D.E. Hallucinogens. Pharmacology & Therapeutics. 2004;101(2):131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Oberhaus, D. (December 14, 2018). The Decline of American Peyote. Retrieved from VICE Magazine: https://www.vice.com/en/article/zmdzbw/the-decline-of-american-peyote-v24n5.
- Orsolini L., Papanti G.D., De Berardis D., Guirguis A., Corkery J.M., Schifano F. The “Endless Trip” among the NPS Users: Psychopathology and Psychopharmacology in the Hallucinogen-Persisting Perception Disorder. A Systematic Review. Frontiers Psychiatry. 2017;8:240. doi: 10.3389/fpsyt.2017.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palamar J.J., Le A. Trends in DMT and other tryptamine use among young adults in the United States. American Journal on Addictions. 2018;27(7):578–585. doi: 10.1111/ajad.12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polito V., Stevenson R.J. A systematic study of microdosing psychedelics. PLoS ONE. 2019;14(2):e0211023. doi: 10.1371/journal.pone.0211023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PR Newswire. (2021). Psychedelic Drugs Market Size Is Projected To Reach $10.75 Billion By 2027. Retrieved from https://www.prnewswire.com/news-releases/psychedelic-drugs-market-size-is-projected-to-reach-10-75-billion-by-2027--301273405.html.
- Reiff, C. M., Richman, E. E., Nemeroff, C. B., Carpenter, L. L., Widge, A. S., Rodriguez, C. I., … Novel Treatments, a. D. o. t. A. P. A. C. o. R. (2020). Psychedelics and Psychedelic-Assisted Psychotherapy. The American Journal of Psychiatry, 177(5), 391-410. doi:10.1176/appi.ajp.2019.19010035. [DOI] [PubMed]
- Research Triangle Institute. (2012). SUDAAN® Statistical Software for Analyzing Correlated Data. Release 11. Vol 1 and 2. . Retrieved from https://www.rti.org/impact/sudaan-statistical-software-analyzing-correlated-data.
- Rubin R. Philanthropists Fund Johns Hopkins Center for Study of Psychedelics. JAMA. 2019;322(19):1849–1851. doi: 10.1001/jama.2019.17126. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. (2013). SAS® 9.4 Software. Retrieved from Cary, NC.
- Serafini G., Howland R.H., Rovedi F., Girardi P., Amore M. The role of ketamine in treatment-resistant depression: A systematic review. Current Neuropharmacology. 2014;12(5):444–461. doi: 10.2174/1570159X12666140619204251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalit N., Rehm J., Lev-Ran S. Epidemiology of hallucinogen use in the U.S. results from the National epidemiologic survey on alcohol and related conditions III. Addictive Behaviors. 2019;89:35–43. doi: 10.1016/j.addbeh.2018.09.020. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2014). Results from the 2013 National Survey on Drug Use and Health. Retrieved from https://scholar.google.com/scholar_lookup?title=Summary+of+National+Findings&publication_year=2014. [PubMed]
- Substance Abuse and Mental Health Services Administration. (2015). Overview of NSDUH Sampling and Data Collection Procedures. In National Survey on Drug Use and Health: 2014 and 2015 Redesign Changes. Rockville, MD. [PubMed]
- Substance Abuse and Mental Health Services Administration. (2020). NATIONAL SURVEY ON DRUG USE AND HEALTH: 2002-2019 PUBLIC USE FILE CODEBOOK. Retrieved from https://www.datafiles.samhsa.gov/dataset/nsduh-2002-2019-ds0001-nsduh-2002-2019-ds0001. [PubMed]
- Substance Abuse and Mental Health Services Administration. (2021). 2019 National Survey on Drug Use and Health (NSDUH) Methodological Resource Book. Retrieved from Rockville, Maryland: https://www.samhsa.gov/data/sites/default/files/reports/rpt34660/NSDUHmrbEditImputation2019.pdf.
- Substance Abuse and Mental Health Services Administration. (October 2019). 2018 National Survey on Drug Use and Health Public Use File Codebook. Retrieved from https://www.datafiles.samhsa.gov/sites/default/files/field-uploads-protected/studies/NSDUH-2018/NSDUH-2018-datasets/NSDUH-2018-DS0001/NSDUH-2018-DS0001-info/NSDUH-2018-DS0001-info-codebook.pdf.
- Substance Abuse and Mental Health Services Administration. (September 2020). 2019 NSDUH Detailed Tables. Retrieved from https://www.samhsa.gov/data/report/2019-nsduh-detailed-tables.
- Substance Abuse and Mental Health Services Administration, & Center for Behavioral Health Statistics and Quality. (2011). Drug Abuse Warning Network, 2011: National Estimates of Drug-Related Emergency Department Visits. Retrieved from https://www.samhsa.gov/data/sites/default/files/DAWN2k11ED/DAWN2k11ED/DAWN2k11ED.pdf.
- Ungerleider J.T., Fisher D.D., Fuller M., Caldwell A. The “bad trip”–the etiology of the adverse LSD reaction. American Journal of Psychiatry. 1968;124(11):1483–1490. doi: 10.1176/ajp.124.11.1483. [DOI] [PubMed] [Google Scholar]
- Winstock A.R., Kaar S., Borschmann R. Dimethyltryptamine (DMT): Prevalence, user characteristics and abuse liability in a large global sample. Journal of Psychopharmacology. 2014;28(1):49–54. doi: 10.1177/0269881113513852. [DOI] [PubMed] [Google Scholar]
- Wu L.T., Woody G.E., Yang C., Li J.H., Blazer D.G. Recent national trends in Salvia divinorum use and substance-use disorders among recent and former Salvia divinorum users compared with nonusers. Subst Abuse Rehabil. 2011;2011(2):53–68. doi: 10.2147/SAR.S17192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.