Abstract
Background
Musculoskeletal disorders negatively affect millions of patients worldwide, placing significant demand on health care systems. Digital technologies that improve clinical outcomes and efficiency across the care pathway are development priorities. We developed the musculoskeletal Digital Assessment Routing Tool (DART) to enable self-assessment and immediate direction to the right care.
Objective
We aimed to assess and resolve all serious DART usability issues to create a positive user experience and enhance system adoption before conducting randomized controlled trials for the integration of DART into musculoskeletal management pathways.
Methods
An iterative, convergent mixed methods design was used, with 22 adult participants assessing 50 different clinical presentations over 5 testing rounds across 4 DART iterations. Participants were recruited using purposive sampling, with quotas for age, habitual internet use, and English-language ability. Quantitative data collection was defined by the constructs within the International Organization for Standardization 9241-210-2019 standard, with user satisfaction measured by the System Usability Scale. Study end points were resolution of all grade 1 and 2 usability problems and a mean System Usability Scale score of ≥80 across a minimum of 3 user group sessions.
Results
All participants (mean age 48.6, SD 15.2; range 20-77 years) completed the study. Every assessment resulted in a recommendation with no DART system errors and a mean completion time of 5.2 (SD 4.44, range 1-18) minutes. Usability problems were reduced from 12 to 0, with trust and intention to act improving during the study. The relationship between eHealth literacy and age, as explored with a scatter plot and calculation of the Pearson correlation coefficient, was performed for all participants (r=–0.2; 20/22, 91%) and repeated with a potential outlier removed (r=–0.23), with no meaningful relationships observed or found for either. The mean satisfaction for daily internet users was highest (19/22, 86%; mean 86.5, SD 4.48; 90% confidence level [CL] 1.78 or –1.78), with nonnative English speakers (6/22, 27%; mean 78.1, SD 4.60; 90% CL 3.79 or –3.79) and infrequent internet users scoring the lowest (3/22, 14%; mean 70.8, SD 5.44; 90% CL 9.17 or –9.17), although the CIs overlap. The mean score across all groups was 84.3 (SD 4.67), corresponding to an excellent system, with qualitative data from all participants confirming that DART was simple to use.
Conclusions
All serious DART usability issues were resolved, and a good level of satisfaction, trust, and willingness to act on the DART recommendation was demonstrated, thus allowing progression to randomized controlled trials that assess safety and effectiveness against usual care comparators. The iterative, convergent mixed methods design proved highly effective in fully evaluating DART from a user perspective and could provide a blueprint for other researchers of mobile health systems.
International Registered Report Identifier (IRRID)
RR2-10.2196/27205
Keywords: mobile health, mHealth, eHealth, digital health, digital technology, musculoskeletal, triage, physiotherapy triage, usability, acceptability, mobile phone
Introduction
Background
Musculoskeletal disorders (MSDs) are prevalent across all ages, have shown an increase in the global disease burden over the past decade [1-3], and are associated with increased life expectancy and reduced activity [4,5]. MSDs are leading contributors to years lived with disability, early work retirement, and reduced ability to participate socially [5]. In many countries, they present the most significant proportional reason for lost productivity in the workplace, leading to significant impacts on the Gross Domestic Product and health care costs [6,7].
In the United Kingdom, the MSD burden of care poses a significant financial challenge to the National Health Service (NHS), costing £4.76 billion (US $3.84 billion) of resources and using up to 30% of primary care physician visits annually [8,9]. A freedom of information request has revealed that the average waiting times for NHS musculoskeletal outpatient physiotherapy services exceeded 6 weeks in the year to April 2019, with some patients waiting 4 months for routine physiotherapy appointments [10]. Longer waiting times can result in delays to physiotherapy services, with detrimental effects on pain, disability, and quality of life for waiting patients [11,12], highlighting the need for a targeted policy response [3,13].
Reducing inconsistency in clinical pathway delivery, including unwarranted secondary care consultations and investigations, forms part of the “Getting It Right First Time (GIRFT)” national program implemented within the UK NHS and has demonstrated cost reduction across the musculoskeletal pathway, particularly relevant in overburdened health care systems [14] Musculoskeletal triage as a single point of entry is effective in improving user satisfaction, diagnostic agreement, appropriateness of referrals, and reduction in patient waiting times [15], where it has been demonstrated to be effective using several methods by a range of clinicians [16-18]. However, using clinicians to provide MSD triage carries its own challenges [19].
Mobile health (mHealth), defined by the World Health Organization as a medical or public health practice that is supported by mobile devices [20], has seen rapid evolution and adoption, and currently, smartphone apps have the potential to make the treatment and prevention of diseases cost-efficient and widely accessible [21,22]. Optima Health has developed the mHealth Digital Assessment Routing Tool (DART) specifically for triaging MSDs, delivering a narrower but deeper assessment than that found with more generic symptom checkers. A digital alternative to clinician-led triage, which is able to replicate the same stratification of care and reduction in costs, is a desirable objective, although some mHealth tools have not demonstrated cost-effectiveness or have merely shown a shift in spending to another part of the health system [23]. It is also recognized that many mHealth apps fail to scale up from a prototype to successful implementation, with inattention to usability during the design and testing phases being identified as a potential cause of the high abandonment rate [24-27]. Although acknowledging usability is crucial in the design, development, testing, and implementation of mHealth apps [28-32], a consistent approach to testing has not yet been established, with researchers using a combination of different study methodologies [33].
An iterative, convergent mixed methods design was used to assess the usability of DART, using cyclical evaluation and improvement plus mixed methods to provide richness while quantifying use, maximizing usability, and therefore supporting system adoption [34]. The testing protocol for this study has been described in detail in a previous publication [35].
DART Overview
DART is a first contact mHealth system comprising an algorithm distinguished by 9 body areas, providing the patient with a recommendation for the most appropriate level of intervention based on their responses (Figure 1). Screening for serious pathologies is completed at the start of the assessment, with less urgent medical referrals being identified as the patient passes through the questioning. The referrals recommended by the algorithm are configured to match the service provider’s local MSD pathways. DART typically signposts emergency or routine medical assessments, specific condition specialists, physiotherapy, self-management programs, and psychological support services.
Figure 1.
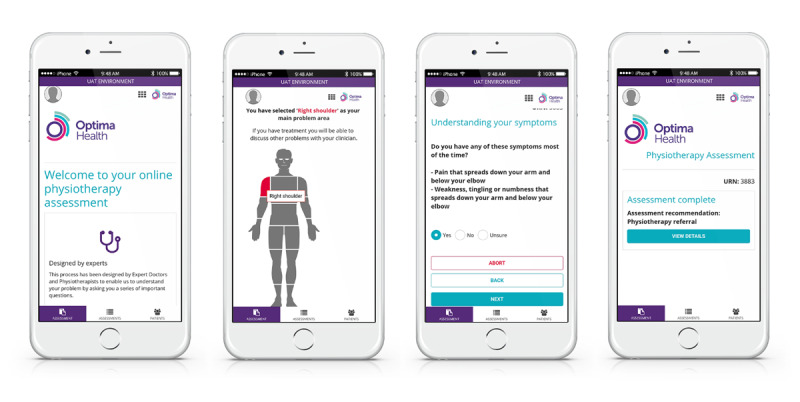
The Digital Assessment Routing Tool mobile health system.
Integration of DART with the provider’s clinical record system means that assessment data and recommendations can be made instantly available to the receiving clinician. Using a link on the clinical provider’s website, DART can be accessed 24/7 using a mobile device or computer, directing users to care at an earlier stage of their injury than would be possible via a traditional clinician-led triage process (Figure 2). Alternatively, DART can be delivered over the telephone by a nonclinician. Reduction in treatment waiting times and reallocation of triage clinical resources to more complex assessments and management could hold significant benefits for the user and health care system.
Figure 2.
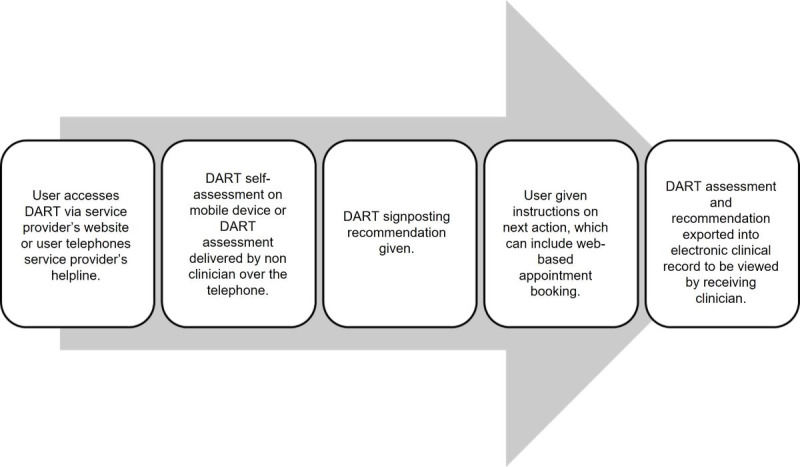
Integration of the DART mobile health system within an existing musculoskeletal disorder pathway. DART: Digital Assessment Routing Tool.
Previous Work
This usability study is part of a larger project, bringing DART from concept to implementation through a series of clinical and academic research work packages. Clinical algorithm validity was assessed by a panel of clinical experts using vignettes incorporating common MSD presentations, as well as red flags and complex presentations, with the panel deeming the validity to be sufficient to allow DART to proceed to further research studies. The protocol devised for this usability study went through a series of iterations within an internal review process, comprising the research project team and DART system developers to arrive at the final version [35]. The objective of this study was to optimize usability before evaluating the safety and effectiveness of DART through a randomized controlled trial, the pilot protocol for which has been published [36].
Methods
Study Design
This study used an iterative, convergent mixed methods design, the protocol for which has been published elsewhere [35]. Step 1 involved in-depth interviews with 5 participants to identify key usability issues, followed by step 2, where group sessions captured greater diversity of data from a potential DART user population (Figure 3). Quantitative data collection was defined by the constructs of effectiveness, efficiency, and satisfaction within the International Organization for Standardization (ISO) 9241-210-2019 standard [30] and provided researchers with a focus for qualitative data collection during both steps. Accessibility was monitored throughout the testing process following the principles described in ISO 30071-1-2019 for embedding inclusion within the design process [31]. Mixed methods data collection and analysis continued cyclically through all rounds of testing until the fourth DART mHealth system iteration was found to perform according to the agreed criteria and the study end points of all grade 1 and 2 usability problems being resolved, as well as a mean System Usability Scale (SUS) score of ≥80, were achieved. The relationship between the likelihood to recommend a system and the mean SUS score has been found to be strongly correlated, and a score of ≥80 was chosen as a study end point as achievement of this threshold is considered to increase the probability of users recommending the system to a friend, therefore positively affecting adoption [37].
Figure 3.
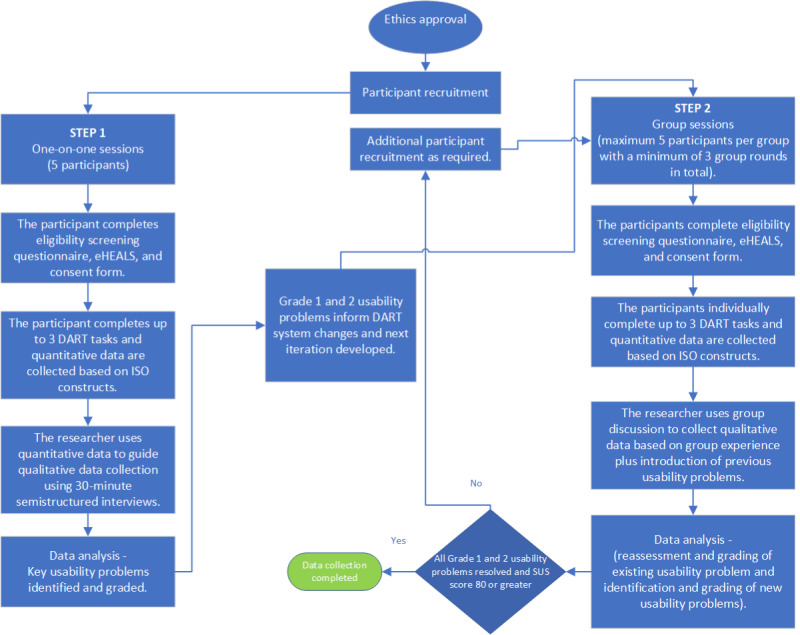
DART usability study iterative, convergent mixed methods design. New participants were recruited for each testing round. Participants raising specific issues in previous rounds were invited individually to review and provide feedback on changes. DART: Digital Assessment Routing Tool; eHEALS: eHealth Literacy Scale; ISO: International Organization for Standardization.
Participant Recruitment
A stratified purposive sampling method was used to gather information from participants by using a sampling matrix and quotas [38], categorized by participant age, internet use, sex, and English for speakers of other languages (ESOL) groups—all of which are subgroups that have shown to contribute small differences in internet use [39]. For this study, “daily internet users” were defined as individuals who access the internet every day or almost every day, and “infrequent internet users” were those who were not daily users but had accessed the internet within the past 3 months [39]. Recruitment was conducted via flyers and emails to local community groups, Optima Health’s existing client base of employers and staff, and Queen Mary University of London students, as well as via social media. In the latter stages, snowballing yielded participants with characteristics of interest; study recruitment continued throughout the study process until the study end points were reached. Potential participants expressing an interest were sent a patient information sheet and consent form and had the opportunity to review this material before consenting to join the study. A total of 33 individuals expressed an interest in participating, of whom 22 (67%) enrolled in the study after meeting the screening criteria.
Inclusion and Exclusion Criteria
The study participant inclusion criteria were as follows: (1) adults aged >18 years; (2) able to speak and read English; (3) living in the United Kingdom; (4) accessed the internet at least once every 3 months; (5) access to a smartphone, tablet, or laptop; and (6) current or previous experience of a musculoskeletal condition.
The study participant exclusion criteria were as follows: (1) significant visual or memory impairment sufficient to affect the ability to answer questions and recall information in an individual or group discussion setting; (2) medically trained musculoskeletal health care professional, such as a physician or physiotherapist; (3) relatives or friends of the researchers; and (4) Optima Health employees.
Data Collection
Following the attainment of consent, participants completed a short questionnaire, including the eHealth Literacy Scale (eHEALS) [40], to provide demographic data and were given instructions by the researcher on how to log into the DART system test site. The first 5 participants in step 1 attended one-on-one video call interviews lasting up to 60 minutes where they could choose up to 3 existing or previous musculoskeletal conditions to complete assessments while being encouraged to give feedback using the concurrent think-aloud method [41]. Participant choice was not limited to specific body sites as usability features were synonymous across all 9 body sites. The participants in step 2 tested DART individually and then attended 30-minute video call group discussion sessions facilitated by the researcher.
Assessing DART performance using satisfaction scales alone was not considered adequate; thus, data collection parameters were defined using the ISO constructs (Figure 4). Following their DART assessments, all participants completed a questionnaire and the SUS [42-44]. The researcher (physiotherapist with postgraduate MSD qualifications) assessed the clinical accuracy of the DART recommendation based on the diagnosis the participant had been given by their treating clinician. Quantitative data were also taken from the DART system itself.
Figure 4.
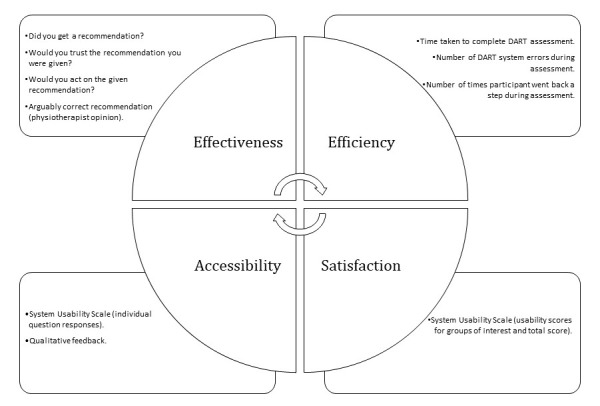
Data collection methods used to assess DART performance against the International Organization for Standardization 9241-210-2019 and International Organization for Standardization 30071-1-2019 standards constructs of effectiveness, efficiency, and satisfaction [30] and accessibility [31]. DART: Digital Assessment Routing Tool.
Quantitative data aligned to the ISO 9241-210-2019 standard constructs were generated from participant questionnaires and DART system data, as shown in Figure 4. These informed the researcher’s qualitative data collection, aided by the use of a visual joint display, merging both types of data to illuminate not only usability problem themes but also potential system improvements (Multimedia Appendix 1). Qualitative data recorded during the interviews and group sessions were transcribed verbatim using the Otter transcription software (Otter.ai; automated video and audio transcription software) and checked for accuracy against the original recording. During group sessions, previous usability problems were introduced to participants to assess the impact of changes made to the previous iteration. In addition, users who raised specific issues in previous rounds were invited individually to review and feedback on changes. In addition to usability problems, any participant feedback on accessibility or positive aspects of DART was recorded.
Data analysis occurred after each round of testing and leveraged the strengths of the convergent mixed methods design to identify usability issues and inform the changes required for subsequent DART iterations (Figure 5). Of particular importance was the thematic analysis of qualitative data provided by real-world users, which ensured their views were included in the DART system development to improve usability.
Figure 5.
The convergent mixed methods design, where both data types are collected simultaneously to allow the analysis and grading of usability problems, thus informing the next system iteration.
Data analysis was performed to identify the overall satisfaction score and differences between groups (mean score, SD, and confidence level [CL]). Statistical analyses examined the relationship between participant age and eHealth literacy using Pearson correlations.
Restrictions imposed during the COVID-19 pandemic led to all data collection sessions being conducted remotely using Microsoft Teams videoconferencing software and web-based questionnaires.
Ethics Approval
This study received approval from the Queen Mary University of London Ethics of Research Committee (QMREC2018/48/048) in June 2020.
Data Analysis
Extending the convergent mixed methods design from data collection to analysis, the reporting used a weaving approach where usability problems were brought together on a theme-by-theme basis and presented through joint displays [45].
Quantitative data from web-based questionnaires and measures of efficiency from the DART system were analyzed and reported to identify key usability issues. Participant SUS raw scores were converted and analyzed by groups of specific interest (daily internet users, infrequent internet users, and ESOL), and the amalgamated mean score across all participants was converted into a percentile score to provide benchmarking against other web-based systems [46].
To minimize bias, quantitative data were collected by an independent researcher during the initial 5 semistructured interviews, and web-based questionnaires were used for the group sessions. Using a thematic analysis approach, qualitative data derived from transcripts of interviews and group sessions were reviewed and analyzed systematically by the 2 researchers independently. Patterns and clusters of meaning within the data were identified and labeled according to the area of system functionality. Data not directly related to the overall research question were excluded. The 2 researchers then worked together to agree and create a thematic framework with higher-order key usability themes able to address the research objective [47,48]. Data were indexed into usability problems of key importance to the study and quotes extracted for each subtheme, thus providing the details required to make the system changes needed to remove or mitigate grade 1 and 2 usability issues. The researchers, working independently initially and then together, arrived at a consensus and allocated a problem severity grade to each usability problem. This was obtained by considering the impact and frequency of the problem, leading to a decision on the risk of not addressing the problem versus the reward of correcting it [49] (Table 1). Once problems had been graded, matched system developments were passed to the DART system developers to guide the next iteration. Actions to address all grade 1 and 2 usability problems were completed for the next iteration, together with closely associated grade 3 and 4 problems if they fell within the scope of the development work. All usability problems remained on record and were reassessed after each round and, if necessary, regraded. Positive feedback about the system was also reported.
Table 1.
Usability problem grading criteria, adapted from guidance issued by The Food and Drug Administration [49].
| Grade | Impact | Frequency | Implications | Action |
| 1 | High | High, moderate, or low | Prevents effective use of the system | Address in next study iteration |
| 2 | Moderate or low | High or moderate | Affects the quality of system delivery | Address in next study iteration |
| 3 | Moderate or low | Low or moderate | Minor issues for several users or a small number of users highlighting concerns important to them | Document and address in later development |
| 4 | Low | Low | Small issues that, if resolved, could improve user satisfaction | Document and address in later development |
Statistical Analysis
The relationship between participant age and eHealth literacy was analyzed using Pearson correlations in Microsoft Excel (a spreadsheet with statistical analysis functionality) to identify user groups less likely to use DART successfully.
Differences in satisfaction scores were present between groups, with expert internet users having the highest mean score (mean 86.5, SD 4.48; 90% CL 1.78).
Results
Overview
A total of 22 participants were enrolled and completed the study (Table 2). The first testing round comprised 23% (5/22) of participants who completed qualitative “think-aloud” data collection led by a researcher familiar with the system and with training in the use of the method. It has been suggested that this relatively small number of participants is sufficient to expose 75% of usability issues, including all catastrophic problems, with further testing of subsequent iterations using new participants to identify less serious problems [50]. This proved to be the case, and data sufficiency was achieved. This was supported by a narrow study aim and the quality of dialog with the first 5 participants. The final sample size was not predefined and was re-evaluated after each round of results [51].
Table 2.
Participant characteristics (N=22).
| Characteristic | Daily internet users | Infrequent internet users | ESOLa,b | All groups | |
| Total sample, n (%) | 19 (86) | 3 (14) | 6 (27) | 22 (100) | |
| Age (years) | |||||
|
|
Values, mean (SD) | 47.6 (15.7) | 55 (11.4) | 41 (8.5) | 48.6 (15.2) |
|
|
Values, range | 20-77 | 47-68 | 31-55 | 20-77 |
| Sex (male), n (%) | 9 (41) | 1 (5) | 3 (14) | 10 (46) | |
| eHEALSc score | |||||
|
|
Values, mean (SD) | 29 (8) | 25 (4) | 26 (12.3) | 28.8 (7.8) |
|
|
Values, range | 8-38 | 21-29 | 8-37 | (8-38) |
aESOL: English for speakers of other languages.
bAll ESOL participants were also daily internet users.
ceHEALS: eHealth Literacy Scale.
There was representation from all the groups of interest; however, not all quotas were met, and small sample sizes, especially infrequent internet users, resulted in a skew of data in favor of daily internet users. This compromised detailed statistical analyses across groups (Table 3).
Table 3.
Recruitment matrix showing minimum quotas and number of participants recruited by characteristics of interest (N=22)a.
| Characteristic | Daily internet user (n=19) | Infrequent internet user (n=3) | |||
|
|
Quota | Enrolled, n (%) | Quota | Enrolled, n (%) | |
| Age (years) | |||||
|
|
18-54 | 2-4 | 7 (37) | 1-3 | 2 (67) |
|
|
55-74 | 2-4 | 10 (53) | 1-3 | 1 (33) |
|
|
≥75 | 1-3 | 1 (5) | 2-4 | 0 (0) |
| Sex | |||||
|
|
Male | Minimum 6 | 7 (37) | Minimum 4 | 1 (33) |
|
|
Female | Minimum 6 | 10 (53) | Minimum 4 | 2 (67) |
| ESOLb | |||||
|
|
Non-ESOL | Minimum 6 | 15 (79) | Minimum 6 | 3 (100) |
|
|
ESOL | Minimum 2 | 6 (32) | Minimum 2 | 0 (0) |
aTotal study participants quota was 20.
bESOL: English for speakers of other languages.
We were interested to know whether the frequency of internet use, age, eHealth literacy, or being a speaker of English as a second language would affect DART usability, as these factors have been highlighted as potential variables in mHealth adoption [39]. There was a wide range of eHEALS scores across participants (mean 28.8, SD 7.8; 95% CI 25.1-32.3), with the highest score of 38/40 achieved by a daily internet user aged 27 years and the lowest score of 8/40 achieved by an ESOL daily internet user aged 31 years. The oldest participant (aged 77 years) achieved a score of 37/40, and the youngest participant (aged 20 years) scored 30/40.
The relationship between eHealth literacy and age, as explored with a scatterplot and calculation of Pearson correlation coefficients, was performed for all participants (20/22, 91%; r=–0.2) and repeated with the potential outlier removed, as indicated in Figure 6 in red (19/22, 86%; r=–0.23), with no meaningful relationship observed or found for either.
Figure 6.
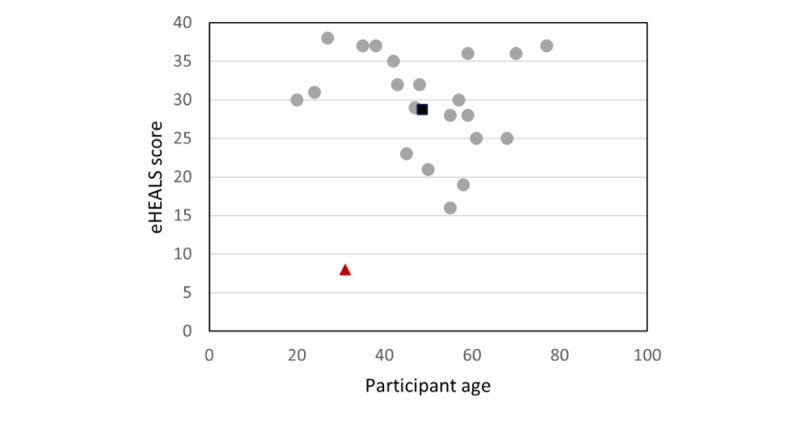
Relationship between age and eHealth literacy scores across all participants. (eHEALS scores range from 0 to 40 scale, with higher scores being better). eHEALS: eHealth Literacy Scale.
A total of 50 assessments were completed by the 22 participants across a possible 9 body sites (Figure 7). The most frequently chosen body site was the low back and pelvis (13/22, 26%), followed by shoulder and knee (both 9/22, 18%). Two body sites were not selected by participants for testing: chest and upper back and elbow. Within a typical MSD triage service, these are often the least occurring body sites. However, the usability features are consistent with those of the other body regions; thus, it is unlikely that any new problems would have been identified through the selection of these pathways.
Figure 7.
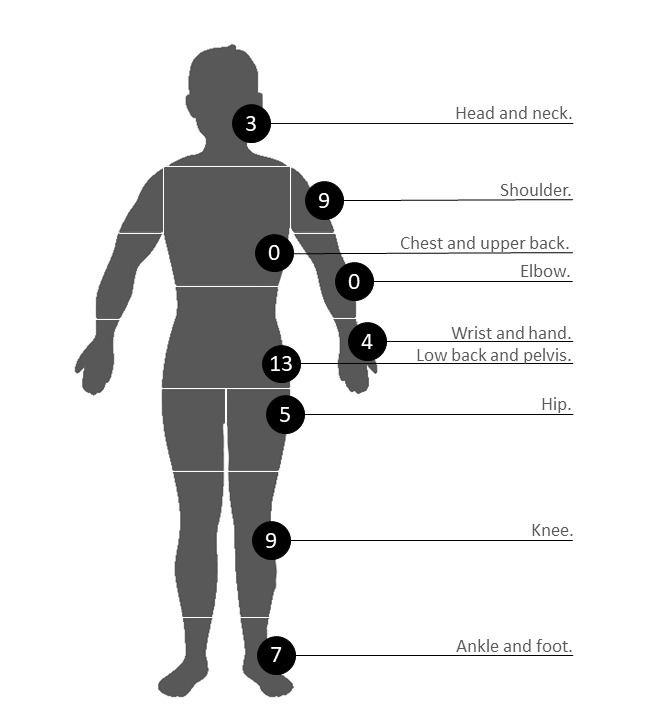
Body sites selected by participants. The number of selections represents the total of the front, back, and either side of a given body site. The Digital Assessment Routing Tool algorithms are designed to assess for musculoskeletal disorder conditions that occur or refer to pain in the selected body site.
Usability Problems
A total of 19 individual usability problems were identified across all 5 rounds of testing, of which 12 (63%) were initially classified as grade 1 or 2. These grades were either reduced or resolved over the iterations. DART iteration 4 was reviewed by participants during testing round 4, and no grade 1 or 2 usability problems were found. This was validated during testing round 5, and the study end points were achieved (Figure 8).
Figure 8.
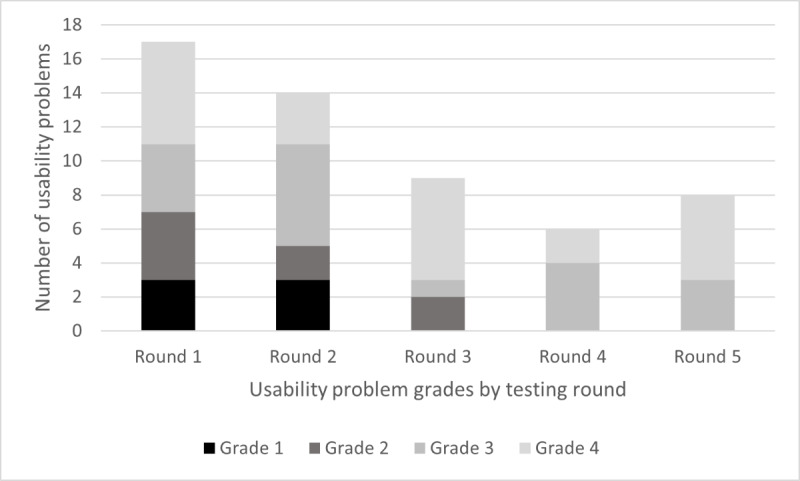
Number of usability problems across testing rounds by grade. The incidence and problem grading changed over the 5 rounds of testing, with grade 1 and 2 problems being negated or reduced to a lower grade. All grade 3 and 4 issues were documented, reviewed, and prioritized for future Digital Assessment Routing Tool development.
Within the grade 1 and 2 usability problems, 3 main themes and 7 contributory subthemes were identified (Figure 9).
Figure 9.
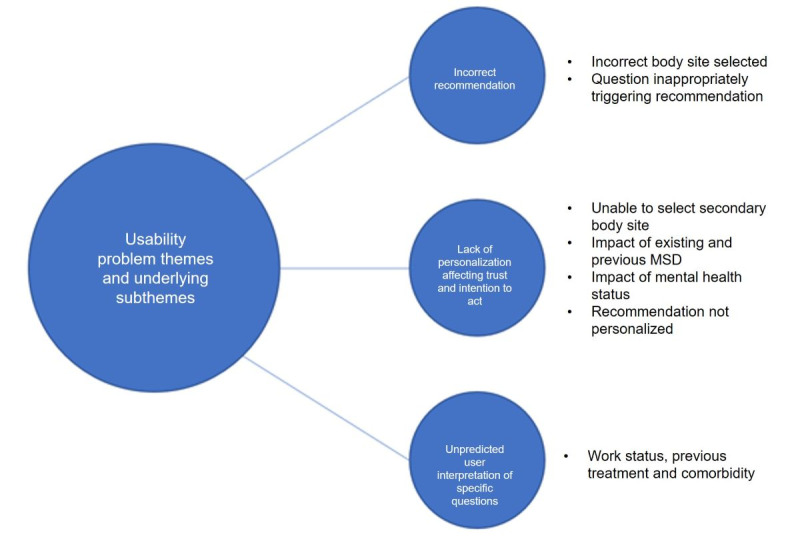
Grade 1 and 2 usability problem themes and underlying subthemes. MSD: musculoskeletal disorder.
Over each of the 5 testing rounds, grade 1 and 2 usability problems were discussed with the participants and regraded (Table 4).
Table 4.
Grade 1 and 2 usability problem themes, subthemes, and participant quotes over 5 rounds of testinga.
| Underlying theme and subthemes | Usability problem grade of subtheme (testing round) | Participant quotes | |||
| Theme 1: incorrect DARTb recommendation compared with expert opinion | |||||
|
|
Incorrect body site selected |
|
|
||
|
|
Question inappropriately triggering recommendation—systemic inflammatory disease and central nervous system condition |
|
|
||
| Theme 2: lack of personalization affecting participants’ trust and intention to act | |||||
|
|
Unable to select secondary body site |
|
|
||
|
|
Impact of existing and previous MSDc |
|
|
||
|
|
Impact of mental health status |
|
|
||
|
|
Recommendations not sufficiently personalized |
|
|
||
| Theme 3: participant difficulty in interpreting questions | |||||
|
|
Specific questions (work status, previous treatment, and comorbidities) |
|
|
||
aUsability problems were clustered into subthemes based on specific areas of DART functionality. Problem grades were reduced in severity over testing rounds as problems were negated or reduced during DART iterations (grade 1 is the most severe, and grade 4 is the least severe).
bDART: Digital Assessment Routing Tool.
cMSD: musculoskeletal disorder.
Construction of a joint display showed how different types of data were combined to assess performance against the ISO 9241-210-2019 constructs (Tables 5, 6, and 7).
Table 6.
Display of quantitative data by International Organization for Standardization 9241-210-2019 standard constructs (effectiveness, efficiency, and satisfaction) over 5 testing roundsa.
| Construct, goal, and testing round | Result | ||||
| Construct 1: effectivenessa | |||||
|
|
Assessment results for a recommendation being given; participants in testing round achieving construct theme (%) | ||||
|
|
|
Round 1 | 13 (100) | ||
|
|
|
Round 2 | 11 (100) | ||
|
|
|
Round 3 | 11 (100) | ||
|
|
|
Round 4 | 10 (100) | ||
|
|
|
Round 5 | 5 (100) | ||
|
|
Assessment results for a correct clinical recommendation; participants in testing round achieving construct theme (%) | ||||
|
|
|
Round 1 | 11 (85) | ||
|
|
|
Round 2 | 5 (45) | ||
|
|
|
Round 3 | 11 (100) | ||
|
|
|
Round 4 | 10 (100) | ||
|
|
|
Round 5 | 10 (100) | ||
|
|
Assessment of whether the participant would trust; participants in testing round achieving construct theme (%) | ||||
|
|
|
Round 1 | 13 (100) | ||
|
|
|
Round 2 | 9 (82) | ||
|
|
|
Round 3 | 11 (100) | ||
|
|
|
Round 4 | 8 (80) | ||
|
|
|
Round 5 | 5 (100) | ||
|
|
Assessment of whether the participant would act upon, n (%) | ||||
|
|
|
Round 1 | 12 (92) | ||
|
|
|
Round 2 | 8 (73) | ||
|
|
|
Round 3 | 11 (100) | ||
|
|
|
Round 4 | 8 (80) | ||
|
|
|
Round 5 | 4 (80) | ||
| Construct 2: efficiencyb | |||||
|
|
Time taken to reach recommendation (minutes) | ||||
|
|
|
Round 1 | |||
|
|
|
|
Values, mean (SD) | Not recorded | |
|
|
|
|
Values, range | Not recorded | |
|
|
|
Round 2 | |||
|
|
|
|
Values, mean (SD) | 5.7 (5.35) | |
|
|
|
|
Values, range | 1-18 | |
|
|
|
Round 3 | |||
|
|
|
|
Values, mean (SD) | 5.4 (4.54) | |
|
|
|
|
Values, range | 1-15 | |
|
|
|
Round 4 | |||
|
|
|
|
Values, mean (SD) | 3.5 (1.5) | |
|
|
|
|
Values, range | 1-5 | |
|
|
|
Round 5 | |||
|
|
|
|
Values, mean (SD) | 7.4 (2.13) | |
|
|
|
|
Values, range | 3-17 | |
|
|
|
All groups | |||
|
|
|
|
Values, mean (SD) | 5.2 (4.44) | |
|
|
|
|
Values, range | 1-18 | |
|
|
DARTc system errors | ||||
|
|
|
Round 1 | 0 | ||
|
|
|
Round 2 | 0 | ||
|
|
|
Round 3 | 0 | ||
|
|
|
Round 4 | 0 | ||
|
|
|
Round 5 | 0 | ||
|
|
DART system backsteps | ||||
|
|
|
Round 1 | 1 | ||
|
|
|
Round 2 | 2 | ||
|
|
|
Round 3 | 2 | ||
|
|
|
Round 4 | 2 | ||
|
|
|
Round 5 | 6 | ||
| Construct 3: satisfactiond | |||||
|
|
System Usability Scale score per rounde | ||||
|
|
|
Round 1 | |||
|
|
|
|
Values, n (%) | 5 (23) | |
|
|
|
|
Values, mean (SD) | 91.6 (4.23) | |
|
|
|
|
Margin of error | 4.46 or –4.46 | |
|
|
|
Round 2 | |||
|
|
|
|
Values, n (%) | 6 (27) | |
|
|
|
|
Values, mean (SD) | 87 (10.23) | |
|
|
|
|
Margin of error | 12.72 or –12.72 | |
|
|
|
Round 3 | |||
|
|
|
|
Values, n (%) | 5 (23) | |
|
|
|
|
Values, mean (SD) | 79.5 (16.91) | |
|
|
|
|
Margin of error | 21.02 or –21.02 | |
|
|
|
Round 4 | |||
|
|
|
|
Values, n (%) | 2 (9) | |
|
|
|
|
Values, mean (SD) | 78.8 (18.75) | |
|
|
|
|
Margin of error | N/Af | |
|
|
|
Round 5 | |||
|
|
|
|
Values, n (%) | 4 (18) | |
|
|
|
|
Values, mean (SD) | 78.8 (5.73) | |
|
|
|
|
Margin of error | 9.11 or –9.11 | |
aQuantitative data show the number of participants in each round and the percentage that achieved the construct theme.
bTime taken to complete an assessment (time taken to reach a disposition was not measured during round 1, as the “think-aloud” method of data capture was prioritized at this stage); number of system errors where the participant was unable to navigate to the end of the assessment because of a system technical error; backsteps where the participant moved back to the previous question.
cDART: Digital Assessment Routing Tool.
dSystem Usability Scale scores by round, group of interest, and across all groups.
eResponses were scored on a 5-point Likert scale (1=strongly disagree and 5=strongly agree) and converted to a score of between 0 and 4, with 4 being the most positive usability rating. Converted scores for all participants are multiplied by 2.5 to give a range of possible total values from 0 to 100. We used 90% CI to allow the benchmarking of the overall DART System Usability Scale score with other studies using this value [46].
fN/A: not applicable.
Table 7.
System Usability Scale score per group for construct 3 (satisfaction) of the International Organization for Standardization 9241-210-2019 standard.
| System Usability Scalea score per group | Daily internet users (n=19) | Infrequent internet users (n=3) | ESOLb internet users (n=6) | All participants (n=22) |
| Values, mean (SD) | 86.5 (4.48) | 70.8 (5.44) | 78.1 (4.60) | 84.3 (12.73) |
| Margin of error | 1.78 or –1.78 | 9.17 or –9.17 | 3.79 or –3.79 | 4.67 or –3.79 |
aResponses were scored on a 5-point Likert scale (1=strongly disagree and 5=strongly agree) and converted to a score of between 0 and 4, with 4 being the most positive usability rating. Converted scores for all participants are multiplied by 2.5 to give a range of possible total values from 0 to 100. We used 90% CI to allow the benchmarking of the overall Digital Assessment Routing Tool System Usability Scale score with other studies using this value [46].
bESOL: English for speakers of other languages.
Table 5.
Display of qualitative data by International Organization for Standardization 9241-210-2019 standard constructs (effectiveness, efficiency, and satisfaction)a.
| Construct and goal | Participant quotes | ||
| Construct 1: effectiveness | |||
|
|
Assessment results for a recommendation being given |
|
|
|
|
Assessment results for a correct clinical recommendation; |
|
|
|
|
Assessment of whether the participant would trust |
|
|
|
|
Assessment of whether the participant would act upon |
|
|
| Construct 2: efficiency | |||
|
|
Time taken to reach recommendation (minutes) |
|
|
|
|
DARTb system errors |
|
|
|
|
DART system backsteps |
|
|
| Construct 3: satisfaction | |||
|
|
System Usability Scale score per round |
|
|
aParticipant quotes provide a deeper understanding of system performance and usability problems.
bDART: Digital Assessment Routing Tool.
Effectiveness
All assessments resulted in a recommendation. Other measures of effectiveness improved over the DART iterations, culminating in a high degree of efficiency being reached (Figure 10).
Figure 10.
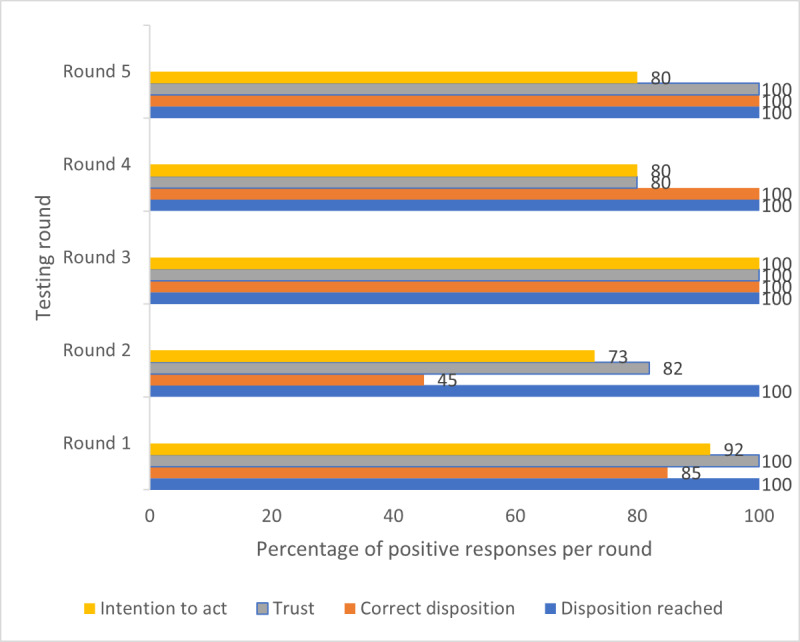
Combined measures of effectiveness by testing round. Results are displayed as the percentage of total assessments to allow comparison, as there were different numbers of participants and assessments in each round. The percentage of assessments in each round resulted in a positive response to the following queries: (1) whether a disposition was achieved, (2) whether it was a clinically correct disposition, (3) whether the participant would trust the disposition, and (4) whether the participant would act on the disposition.
Of the 50 assessments, 8 (16%) resulted in an incorrect recommendation being given, equating to a grade 1 usability issue. Qualitative data revealed that the selection of the incorrect body site at the start of the assessment was responsible for one of these errors. A total of 7 inappropriate clinical escalations were triggered by 2 specific screening questions for systemic inflammatory disease (SID) and central nervous system conditions. Both were reviewed against the evidence base, rewritten, and incorporated into iteration 3. Subsequent testing rounds, including inviting the participants who revealed this problem to retest, confirmed that this usability issue was solved. Participants said their trust and willingness to act would increase if all their symptoms are considered, and this could be achieved by adding a text box on the body site page where they could enter information about problems in other body areas. A related theme was participants wanting to personalize their assessment by adding additional information, and DART iteration 3 included the addition of a free text box at the end of each page. This improved both trust and intention to act, with all participants during testing round 5 arriving at a correct recommendation that they would trust, with just one assessment where the participant said they would not act on the recommendation related to their previous experience of their MSD resolving spontaneously. A small number of participants felt that the lack of personalization of the DART recommendation page made them less likely to act on the advice. This was a result of a test version being used for the study, containing a simple generic recommendation rather than the detailed advice and next actions that would be found on a production version. However, the importance of this feedback was noted and will guide the final DART version to be deployed.
Efficiency
Quantitative indicators of efficiency remained high throughout testing, reinforced by qualitative data.
Round 5 had a larger number of ESOL participants, and it was noted the mean time for this group was slightly longer (6 minutes). The longest time (18 minutes) taken to complete an assessment was by an ESOL participant (Figure 11). All participants without exception said that the time taken to complete an assessment was acceptable and that the format of the questions was clear and supported their ability to make decisions easily.
Figure 11.
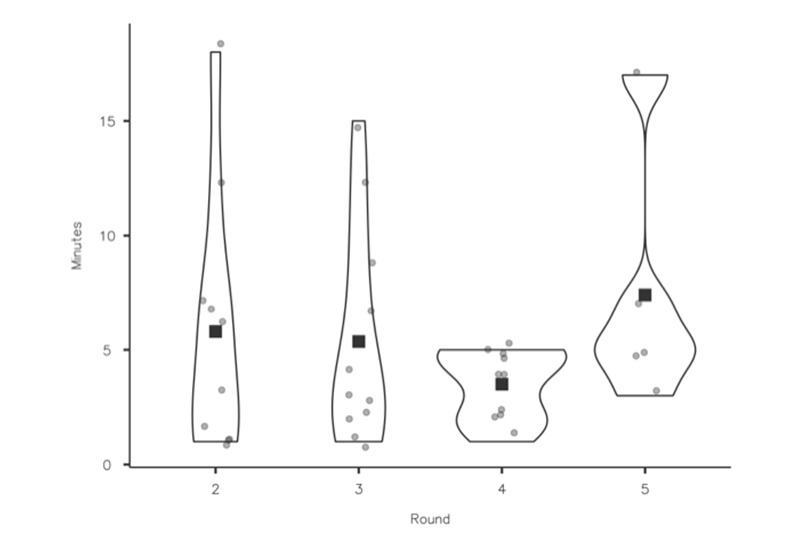
Efficiency (time to complete an assessment). Time taken for participants to complete assessments. A total of 16 participants completed 34 assessments in total across rounds 2 to 5. Time was not recorded in round 1, as participants were encouraged to use the “think-aloud” technique.
System errors were defined as DART technical errors, such as presenting the user with an error message or the system timing out. No system errors were encountered during any testing rounds.
The number of times a participant moved back in the pathway to review the previous question remained consistent across the first 4 testing rounds, even with the introduction of participants who used the internet less frequently and ESOL users. Backsteps increased to 6 in round 5 and were linked to one of the ESOL participants, who had the lowest recorded eHEALS score (Figure 12). He told us some of the questions were more challenging to answer and required him to reread them.
Figure 12.
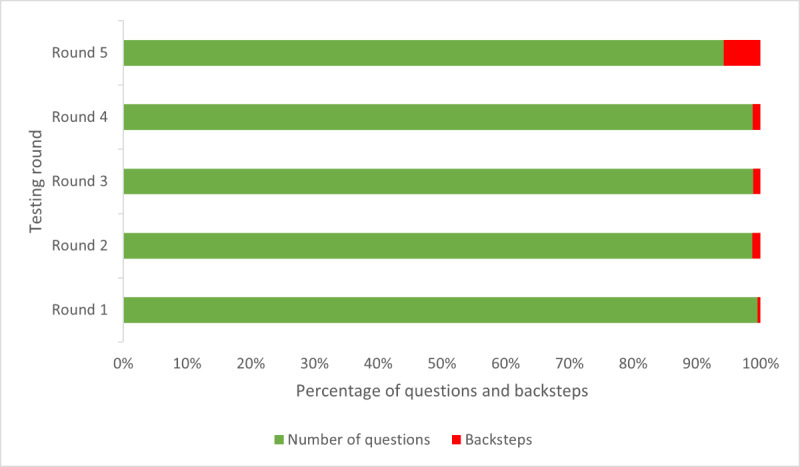
Measures of efficiency (backsteps) by testing round. Number of times a participant moved back a step in the question set to review their previous question and response, with backsteps shown as a percentage of the total number of questions in the assessment.
Satisfaction
Satisfaction was measured quantitively across all groups using the SUS, with qualitative data providing deeper insights into specific question responses. Although high levels of system satisfaction were prevalent throughout testing, cumulative satisfaction scores reduced with each round as infrequent internet users and ESOL participants were recruited (Figure 13). However, the final mean SUS score of 84.3 (SD 12.73; 90% CL 4.67) across all groups achieved the predefined study end point of a score of ≥80, representing a “good” or better system and associated with an increase in the probability that users would recommend DART to a friend [37].
Figure 13.
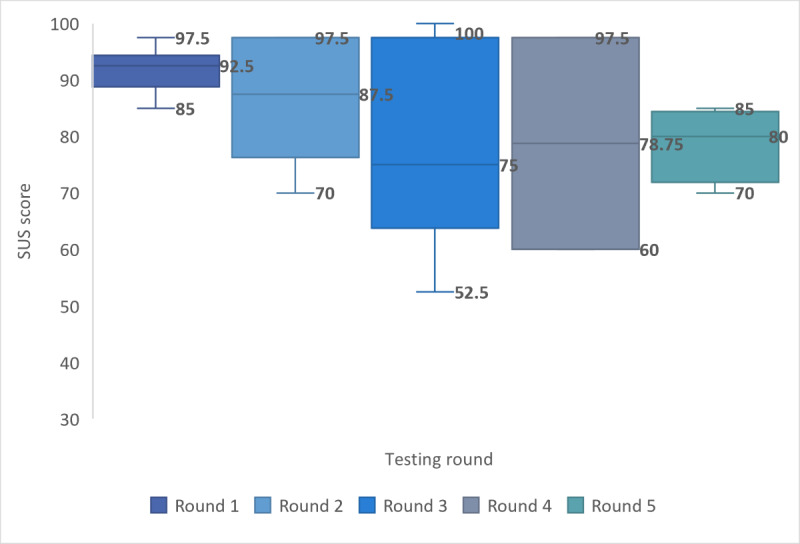
Measure of satisfaction by testing round. SUS: System Usability Scale.
Differences in mean satisfaction scores were present between groups, with daily internet users scoring highest (19/22, 86%; mean 86.5, SD 4.48; 90% CL 1.78 or –1.78), and nonnative English speakers (6/22, 27%; mean 78.1, SD 4.60; 90% CL 3.79 or –3.79) and infrequent internet users scoring the lowest (3/22, 14%; mean 70.8, SD 5.44; 90% CL 9.17 or –9.17), although the CIs overlap.
Although care should be taken in examining individual SUS item responses as external validity only exists on aggregated scores [51], analysis of the highest scoring questions did reveal some useful insights. All participant groups scored highest when saying that they would not need to learn many things before they could use DART. Both daily and infrequent internet users agreed they would not need technical support to use DART, with ESOL participants agreeing to a slightly lesser extent.
All groups did not feel they would use DART frequently or that the functions in the system were well integrated. This was not an unexpected finding as DART is intended for single-time use to determine the correct level of intervention for the user’s MSD and, therefore, would not be used frequently. In contrast to most other mHealth systems, DART is not designed to provide an MSD intervention, with no requirement for the user to navigate to additional features within the system.
Satisfaction adjectives were associated with each participant’s individual total score to aid in explaining the results to non–human factor professionals [52], with 91% (20/22) of participant scores equating DART as a “good,” “excellent,” or “best imaginable system.” The remaining 2 (8%) participants rated DART as “fair,” with none rating it as “poor” or “worst imaginable.”
Using the normalizing process described by Sauro [46], DART ranks within the 96th to 100th percentile (SUS score 84.1-100) of systems tested using the SUS, with an associated adjective rating of “Excellent” [53]. Benchmarking of the DART SUS score against the mean score of 67 (SD 13.4) from 174 studies assessing the usability of public-facing websites utilizing the SUS revealed that DART was among the highest scoring systems assessed in this way [46].
Accessibility
Accessibility has been central to the design of the DART user interface, and the Appian platform on which DART is constructed includes features supporting accessibility for a wide range of users, including those with disabilities who use assistive technologies such as screen readers.
DART’s simplicity of use was recognized in the qualitative data by all participants from all groups, who felt that it was sufficiently simple to use and liked the fact that all assessments resulted in a disposition being given. This supports the theory of a low barrier to entry for DART, provided users have internet access:
It is so simple, its one most simple of the things, websites, I've engaged with.
DART016
It was very quick. And I quite like that it has one thing for one page, which is a very short question, it gives you a few options, and then you answer so you don't have to go through long text questions, one after the other. So, it just takes you very quickly step by step. And it's quite, I don't know, for me, it was super easy and clear to answer questions.
DART017
We asked participants whether they could think of anyone who may not be able to use DART:
I wonder how somebody like my mom's age would cope with it and I actually thought there wouldn’t be too many who wouldn’t.
One of the ESOL participants tested DART with the help of her daughter, which she told us was her normal practice when she needed to use the internet and common practice within her community:
No it’s easy to do. My daughter is helping me. Little bit, I understand most of the things, but little bit some questions, what can I say? So, my daughter guides me and help me.
DART012
When asked how other ESOL users would use DART, she said the following:
I think they need somebody's help, their partner or their children, somebody, or some of their friends, some can help them and then they can do it.
Overall, participants felt that DART would be easily accessible to people who are familiar with using the internet but that a telephone-delivered alternative would be required for some users. Additional benefits of reducing the time to receiving a diagnosis or treatment and financial savings were also mentioned:
If I had this actual system, I would have saved £150 in cash and probably three months of pain had I been able to access it when I had my problems with my back.
DART013
Discussion
Principal Findings
The use of the iterative, convergent mixed methods design proved effective; rich data provided objective measures of system performance together with identification of serious usability problems and solutions by real-world users. The results from this study indicate that through a series of iterations, DART usability reached a sufficiently high standard to proceed to further safety and effectiveness trials.
Theme 1: Factors Leading to an Incorrect Recommendation Being Given
Selection of the appropriate body site is crucial to driving matched clinical algorithms within DART, and failure to do so accounted for 8 incorrect recommendations. In addition, participant confidence in DART being able to recognize their body site selection was considered important to most participants, being related to their wider trust in the system and associated intention to act on the recommendation they received. As a result, the body site diagram was refined across all iterations.
A specific DART question designed to screen for SID triggered false-positive recommendations for participants describing mechanical pain. Correct identification of SID can be problematic for primary care clinicians because of varied symptom presentation and overlap with more common osteoarthritic joint conditions [53-55]. A study using patient-entered responses showed that osteoarthritis was diagnosed in 38% of SID cases [56], a result that is clinically significant, given the interdependency of early recognition of SID, minimizing a poor patient outcome [57,58]. During DART testing, these participants prioritized the presence of pain characteristics of osteoarthritis over the hot and swollen multiple joint symptoms presented in SID. This problem was addressed by a detailed review of the literature on the differential diagnosis of SID, rewriting, and inclusion of new questions within all algorithms with associated linked age logic. Subsequent testing rounds, including participants who revealed this problem, confirmed success in negating this usability issue. The implications of creating false positives are often underestimated, with most symptom checker development taking a conservative approach, resulting in systems typically being more risk averse than health care practitioners [59-61]. However, the failure to provide an accurate routing decision can affect user trust and system adoption [62], as well as the creation of unnecessary referrals to urgent or emergency services.
Theme 2: Impact of Assessment Personalization on Participant Trust and Intention to Act on the Given Recommendation
Personalization of assessments was perceived by some participants as a key advantage of a patient-clinician triage interaction over an mHealth system. It has been shown that lack of personalization can affect trust and intention to act, with implications for system adoption [63]. Our participants wanted to “tell their story,” not feeling involved in the assessment process unless they could provide information personal to them, including entering details of secondary body site areas. It has been estimated that 8% of patients with MSD presenting to a primary care physician have a problem in >1 body site [64]; thus, an additional comment box was added to the body site diagram page, inviting users to enter other problem areas, something our participants said would address this problem.
In all but 1 assessment, participants who said they would trust DART also said that they would act on the recommendation they were given. A direct correlate of eHealth user trust is information quality, defined by knowledgeable, impartial, and expert sources. These are important factors that lead users to believe the system is acting in their best interest, as they feel a clinician would do [65].
Trust factors have a significant direct effect on user intention to act [66], a key requirement for successful DART introduction into an MSD digital pathway and system adoption. However, we found other factors may be at play, such as previous experience in MSD management. One of the participants told us that they would trust the DART recommendation for physiotherapy but would have waited to see whether their problem improved without treatment, as it had before. It has been recognized that when users of eHealth make decisions on system trust and intention to act, especially those with high eHealth literacy, they will often corroborate information using other web-based content to “triangulate” advice, particularly if the primary source is not familiar to them [63,66]. Interestingly, it has been shown that eHealth users in the United Kingdom with access to free NHS health care are less likely to use health corroboration than users in other countries with private health systems [66]. The NHS website is considered a trusted source of information for many citizens in the United Kingdom, and deploying DART within an NHS pathway may enhance a user’s trust in the given recommendations.
Qualitative data revealed a usability problem that was not considered during development—that serious condition-screening questions on DART had the potential to cause user anxiety in some individuals, who otherwise would not have considered the potential for their problem to be serious. On the basis of this feedback, we placed these less frequently occurring conditions in the context of their incidence to allay unnecessary user anxiety. This is an important consideration for developers of mHealth triage systems, as although the creation of false positives is well recognized and largely accepted as a prudent conservative approach to medical risk management [62], it is also suggested that a significant proportion of potential users would reject using a symptom checker for fear of receiving a wrong diagnosis or an assessment that could cause them anxiety [60].
Theme 3: Impact of User Interpretation When Answering Specific DART Questions
A small number of questions provoked some unexpected participant responses attributable to individual interpretation, likely influenced by their personal experiences. This theme was not identified during previous validation work completed by the panel of expert clinicians. It was only possible to reveal and understand this important usability factor by using a convergent mixed methods design with real-world users, reinforcing the advantage of this methodology over the common practice of using vignettes constructed or delivered by clinicians. An expert clinician recognizes conditions by virtue of pattern recognition, bypassing the conscious, effortful cognitive requirements demanded of a nonclinician user to interpret questions and make decisions on how to respond [67]. Moreover, clinicians are highly educated and not representative of a real-world system user population, including people with eHealth literacy challenges. This study concluded that diversity of user personal experiences can influence how real-world users respond to questions presented by an mHealth system and, ultimately, the recommendation they receive, thus presenting a challenge to developers. For this reason, it is suggested that clinical testing of mHealth systems using vignettes is best used as a precursor to real-world usability testing comprising a representative sample of potential system users.
We found no relationship between age and eHealth literacy, with older participants equally able to arrive at a recommendation as the younger participants. Although this finding should be treated with caution because of the small number of older participants, it could suggest that the perceived ability to seek and use health information is more related to the frequency of internet use rather than age and that differences in eHealth literacy are less likely to be between user group demographics but rather socioeconomic variables between individuals within them [68]. A recent report showed continued growth of internet use in the United Kingdom, with a 6% increase in households with internet access between 2018 and 2020. In the same period, the increase in the number of households with a single adult aged >65 years who accessed the internet within a 3-month period rose from 59% to 80% [39], challenging perceptions about potential mHealth user demographics. An ESOL participant who had assistance from her daughter told us she often sought help from family or neighbors to use the internet, that this was common practice within her community, and that DART could be used effectively in this way. Web-based “surrogate seeking” is now a widespread practice, with significant numbers of internet health information seekers accessing advice on behalf of someone else [69]. However, some studies still link the use of web-based symptom checkers to younger and more highly educated populations [60] and self-referrals for the assessment of musculoskeletal conditions generally [70].
Limitations
Recruitment during the COVID-19 pandemic proved challenging, particularly for people who were not daily users of the internet, as they typically do not engage with social media or advertisements sent via email. All data had to be gathered remotely, affecting the recruitment of people not confident in using web-based video call technology. Although the full recruitment quota was not met for infrequent internet users, this was partially addressed, and the feedback from these participants was particularly valuable in highlighting usability problems. This recruitment challenge could be an indicator of self-selection for DART user adoption related to internet use.
During the DART tests, most participants recalled past conditions that had been resolved or that had been present for some time and had changed since the first onset. At times, this created a problem for participants regarding how to respond to questions; for example, current symptoms versus symptoms they had at the beginning of their problem when they first sought clinical advice. This could be addressed in future studies by only recruiting participants with current problems who had not received medical advice; however, this could potentially exclude participants with chronic MSDs and more complex conditions, thus limiting generalizability.
Although a generic internet system assessment tool, the SUS was chosen as a measure of DART user satisfaction in the absence of a more specific validated mHealth usability measure. As a result, not all the questions were matched to DART in its role as a single-use assessment system with no additional integrated functionality. Other usability assessment tools were considered, including those that measure domains such as loyalty, trust, credibility, and appearance; however, these were designed for the assessment of transactional business systems and included questions inappropriate for DART, such as purchasing and confidence in concluding business [37]. Other tools measured the usability of mHealth systems that support the therapeutic management of conditions over time, with repeated patient use and different integrated functions and features, meaning that the domains assessed were not directly applicable [71].
Future Work
The purpose of this usability study was to optimize usability before proceeding to a trial evaluating the safety and effectiveness of DART against a usual care comparator. A protocol for an initial pilot study has been published and will explore the key aspects of the trial methodology; assess the procedures; and collect exploratory data to inform the design of a definitive, randomized, crossover, noninferiority trial to assess DART safety [36]. DART is currently deployed in a controlled live clinical environment where we use system data, as well as user and clinician feedback, to further refine the algorithms and system usability. A quality improvement study, where DART is integrated into an existing public health service, is also in the design phase.
Conclusions
This study suggests the DART mHealth system has the potential to be offered as an alternative to primary care physician–led or physiotherapist-led triage as part of an MSD pathway. Participants found DART easy to use and would trust and act on the routing recommendation they were given. With all significant usability problems addressed, DART can proceed to the next stage of validation—a randomized controlled trial to assess the safety and effectiveness against a usual care comparator. The inclusion of real-world participants revealed important usability problems and solutions that were not identified during the development or expert panel review stages and highlights the importance of a more sophisticated approach to mHealth system usability testing. The iterative, convergent mixed methods design proved to be highly effective for system development and evaluation and could provide a blueprint for other researchers of mHealth systems.
Acknowledgments
This study was funded by Optima Health.
Abbreviations
- CL
confidence level
- DART
Digital Assessment Routing Tool
- eHEALS
eHealth Literacy Scale
- ESOL
English for speakers of other languages
- ISO
International Organization for Standardization
- mHealth
mobile health
- MSD
musculoskeletal disorder
- NHS
National Health Service
- SID
systemic inflammatory disease
- SUS
System Usability Scale
Visual joint display (researcher version).
Footnotes
Authors' Contributions: CL and DM designed the study. MB collected quantitative data, whereas CL collected qualitative data, and both performed data analysis and interpretation. CL drafted the manuscript. DM and WM reviewed the manuscript and provided the final approval. CL takes responsibility for the integrity of the data analysis.
Conflicts of Interest: Optima Health has developed the Digital Assessment Routing Tool system and is the owner of the associated intellectual property. The principal investigator (CL) is an employee of Optima Health and a PhD research student at Queen Mary University of London.
References
- 1.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017 Sep 16;390(10100):1211–59. doi: 10.1016/S0140-6736(17)32154-2. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(17)32154-2 .S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018 Nov 10;392(10159):1859–922. doi: 10.1016/S0140-6736(18)32335-3. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(18)32335-3 .S0140-6736(18)32335-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sebbag E, Felten R, Sagez F, Sibilia J, Devilliers H, Arnaud L. The world-wide burden of musculoskeletal diseases: a systematic analysis of the World Health Organization Burden of Diseases Database. Ann Rheum Dis. 2019 Jun;78(6):844–8. doi: 10.1136/annrheumdis-2019-215142.annrheumdis-2019-215142 [DOI] [PubMed] [Google Scholar]
- 4.Musculoskeletal health. World Health Organization. 2022. Jul 14, [2022-08-22]. https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions .
- 5.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018 Nov 10;392(10159):1789–858. doi: 10.1016/S0140-6736(18)32279-7. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(18)32279-7 .S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Impact of Musculoskeletal Disorders on Americans – Opportunities for Action. Bone and Joint Initiative USA. 2016. [2022-08-22]. http://www.boneandjointburden.org/docs/BMUSExecutiveSummary2016.pdf)
- 7.Board assurance prompt: improving the effectiveness of musculoskeletal health for NHS providers. Good Governance Institute. 2019. [2022-08-22]. https://www.good-governance.org.uk/wp-content/uploads/2019/04/MSK-BAP-draft-providers-AW-GW.pdf .
- 8.Ahmed N, Ahmed F, Rajeswaran G, Briggs TR, Gray M. The NHS must achieve better value from musculoskeletal services. Br J Hosp Med (Lond) 2017 Oct 02;78(10):544–5. doi: 10.12968/hmed.2017.78.10.544. [DOI] [PubMed] [Google Scholar]
- 9.Musculoskeletal conditions. National Health Service England. 2020. [2020-05-31]. https://www.england.nhs.uk/ourwork/clinical-policy/ltc/our-work-on-long-term-conditions/musculoskeletal/
- 10.Equipsme. 2021. [2022-08-22]. https://www.equipsme.com/blog/up-to-four-months-to-see-a-physiotherapist/#:~:text=The%20shortest%20wait%20for%20physiotherapy%20was%20in%20Northern,and%20just%201%20day%20for%20an%20urgent%20appointment .
- 11.Deslauriers S, Déry J, Proulx K, Laliberté M, Desmeules F, Feldman DE, Perreault K. Effects of waiting for outpatient physiotherapy services in persons with musculoskeletal disorders: a systematic review. Disabil Rehabil. 2021 Mar;43(5):611–20. doi: 10.1080/09638288.2019.1639222. [DOI] [PubMed] [Google Scholar]
- 12.Lewis AK, Harding KE, Snowdon DA, Taylor NF. Reducing wait time from referral to first visit for community outpatient services may contribute to better health outcomes: a systematic review. BMC Health Serv Res. 2018 Nov 20;18(1):869. doi: 10.1186/s12913-018-3669-6. https://bmchealthservres.biomedcentral.com/articles/10.1186/s12913-018-3669-6 .10.1186/s12913-018-3669-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steel N, Ford JA, Newton JN, Davis AC, Vos T, Naghavi M, Glenn S, Hughes A, Dalton AM, Stockton D, Humphreys C, Dallat M, Schmidt J, Flowers J, Fox S, Abubakar I, Aldridge RW, Baker A, Brayne C, Brugha T, Capewell S, Car J, Cooper C, Ezzati M, Fitzpatrick J, Greaves F, Hay R, Hay S, Kee F, Larson HJ, Lyons RA, Majeed A, McKee M, Rawaf S, Rutter H, Saxena S, Sheikh A, Smeeth L, Viner RM, Vollset SE, Williams HC, Wolfe C, Woolf A, Murray CJ. Changes in health in the countries of the UK and 150 English Local Authority areas 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018 Nov 03;392(10158):1647–61. doi: 10.1016/S0140-6736(18)32207-4. https://linkinghub.elsevier.com/retrieve/pii/S0140-6736(18)32207-4 .S0140-6736(18)32207-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briggs T. A national review of adult elective orthopaedic services in England: Getting It Right First Time. British Orthopaedic Association. 2015. Mar, [2022-08-22]. https://gettingitrightfirsttime.co.uk/wp-content/uploads/2017/06/GIRFT-National-Report-Mar15-Web.pdf .
- 15.Joseph C, Morrissey D, Abdur-Rahman M, Hussenbux A, Barton C. Musculoskeletal triage: a mixed methods study, integrating systematic review with expert and patient perspectives. Physiotherapy. 2014 Dec;100(4):277–89. doi: 10.1016/j.physio.2014.03.007.S0031-9406(14)00034-0 [DOI] [PubMed] [Google Scholar]
- 16.Oakley C, Shacklady C. The clinical effectiveness of the extended-scope physiotherapist role in musculoskeletal triage: a systematic review. Musculoskeletal Care. 2015 Dec;13(4):204–21. doi: 10.1002/msc.1100. [DOI] [PubMed] [Google Scholar]
- 17.Bornhöft L, Larsson ME, Thorn J. Physiotherapy in Primary Care Triage - the effects on utilization of medical services at primary health care clinics by patients and sub-groups of patients with musculoskeletal disorders: a case-control study. Physiother Theory Pract. 2015 Jan;31(1):45–52. doi: 10.3109/09593985.2014.932035. [DOI] [PubMed] [Google Scholar]
- 18.Bornhöft L, Thorn J, Svensson M, Nordeman L, Eggertsen R, Larsson ME. More cost-effective management of patients with musculoskeletal disorders in primary care after direct triaging to physiotherapists for initial assessment compared to initial general practitioner assessment. BMC Musculoskelet Disord. 2019 May 01;20(1):186. doi: 10.1186/s12891-019-2553-9. https://bmcmusculoskeletdisord.biomedcentral.com/articles/10.1186/s12891-019-2553-9 .10.1186/s12891-019-2553-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Transforming musculoskeletal and orthopaedic elective care services: a handbook for local health and care systems. National Health Service England. 2017. [2022-08-22]. https://www.england.nhs.uk/wp-content/uploads/2017/11/msk-orthopaedic-elective-care-handbook-v2.pdf .
- 20.World Health Organization. Geneva, Switzerland: World Health Organization; 2011. [2022-08-22]. mHealth: new horizons for health through mobile technologies: second global survey on eHealth. https://apps.who.int/iris/handle/10665/44607 . [Google Scholar]
- 21.Personalised Health and Care 2020 – Using Data and Technology to Transform Outcomes for Patients and Citizens: A Framework for Action. National Information Board. 2014. Nov, [2022-08-22]. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/384650/NIB_Report.pdf .
- 22.Babatunde OO, Bishop A, Cottrell E, Jordan JL, Corp N, Humphries K, Hadley-Barrows T, Huntley AL, van der Windt DA. A systematic review and evidence synthesis of non-medical triage, self-referral and direct access services for patients with musculoskeletal pain. PLoS One. 2020 Jul 6;15(7):e0235364. doi: 10.1371/journal.pone.0235364. https://dx.plos.org/10.1371/journal.pone.0235364 .PONE-D-20-05712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashwood JS, Mehrotra A, Cowling D, Uscher-Pines L. Direct-to-consumer telehealth may increase access to care but does not decrease spending. Health Aff (Millwood) 2017 Mar 01;36(3):485–91. doi: 10.1377/hlthaff.2016.1130.36/3/485 [DOI] [PubMed] [Google Scholar]
- 24.Zapata BC, Fernández-Alemán JL, Idri A, Toval A. Empirical studies on usability of mHealth apps: a systematic literature review. J Med Syst. 2015 Feb;39(2):1. doi: 10.1007/s10916-014-0182-2. [DOI] [PubMed] [Google Scholar]
- 25.Jones RB, Ashurst EJ, Trappes-Lomax T. Searching for a sustainable process of service user and health professional online discussions to facilitate the implementation of e-health. Health Informatics J. 2016 Dec;22(4):948–61. doi: 10.1177/1460458215599024. https://journals.sagepub.com/doi/10.1177/1460458215599024?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed .1460458215599024 [DOI] [PubMed] [Google Scholar]
- 26.Greenhalgh T, Wherton J, Papoutsi C, Lynch J, Hughes G, A'Court C, Hinder S, Fahy N, Procter R, Shaw S. Beyond adoption: a new framework for theorizing and evaluating nonadoption, abandonment, and challenges to the scale-up, spread, and sustainability of health and care technologies. J Med Internet Res. 2017 Nov 01;19(11):e367. doi: 10.2196/jmir.8775. https://www.jmir.org/2017/11/e367/ v19i11e367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krebs P, Duncan DT. Health app use among US mobile phone owners: a national survey. JMIR Mhealth Uhealth. 2015 Nov 04;3(4):e101. doi: 10.2196/mhealth.4924. https://mhealth.jmir.org/2015/4/e101/ v3i4e101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evidence standards framework for digital health technologies. National Institute for Health and Care Excellence. 2019. Mar, [2022-08-22]. https://www.nice.org.uk/Media/Default/About/what-we-do/our-programmes/evidence-standards-framework/digital-evidence-standards-framework.pdf .
- 29.Guidance: a guide to good practice for digital and data-driven health technologies. Department of Health and Social Care, United Kingdom Government. 2021. Jan 19, [2022-08-22]. https://www.gov.uk/government/publications/code-of-conduct-for-data-driven-health-and-care-technology/initial-code-of-conduct-for-data-driven-health-and-care-technology .
- 30.Ergonomics of human-system interaction — Part 210: Human-centred design for interactive systems (ISO 9241-210:2019) International Organization for Standardization. 2019. [2022-08-22]. https://www.iso.org/standard/77520.html .
- 31.Information technology — Development of user interface accessibility — Part 1: Code of practice for creating accessible ICT products and services (ISO/IEC 30071-1:2019) International Organization for Standardization. 2019. [2022-08-22]. https://www.iso.org/standard/70913.html .
- 32.Green Paper on mobile health ("mHealth") – SWD135 final. European Commission. 2014. [2022-08-22]. https://ec.europa.eu/newsroom/dae/redirection/document/5147 .
- 33.Maramba I, Chatterjee A, Newman C. Methods of usability testing in the development of eHealth applications: a scoping review. Int J Med Inform. 2019 Jun;126:95–104. doi: 10.1016/j.ijmedinf.2019.03.018.S1386-5056(18)31318-2 [DOI] [PubMed] [Google Scholar]
- 34.Alwashmi MF, Hawboldt J, Davis E, Fetters MD. The iterative convergent design for mobile health usability testing: mixed methods approach. JMIR Mhealth Uhealth. 2019 Apr 26;7(4):e11656. doi: 10.2196/11656. https://mhealth.jmir.org/2019/4/e11656/ v7i4e11656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowe C, Hanuman Sing H, Browne M, Alwashmi MF, Marsh W, Morrissey D. Usability testing of a digital assessment routing tool: protocol for an iterative convergent mixed methods study. JMIR Res Protoc. 2021 May 18;10(5):e27205. doi: 10.2196/27205. https://www.researchprotocols.org/2021/5/e27205/ v10i5e27205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowe C, Hanuman Sing H, Marsh W, Morrissey D. Validation of a musculoskeletal digital assessment routing tool: protocol for a pilot randomized crossover noninferiority trial. JMIR Res Protoc. 2021 Dec 13;10(12):e31541. doi: 10.2196/31541. https://www.researchprotocols.org/2021/12/e31541/ v10i12e31541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sauro J, Lewis JR. Standardized usability questionnaires. In: Sauro J, Lewis JR, editors. Quantifying the User Experience. 2nd edition. Burlington, MA, USA: Morgan Kaufman; 2016. pp. 185–248. [Google Scholar]
- 38.Ritchie J, Lewis J, McNaughton Nicholls CM, Ormston R. Qualitative Research Practice: A Guide for Social Science Students and Researchers. 2nd edition. Thousand Oaks, CA, USA: Sage Publications; 2013. [Google Scholar]
- 39.Internet access – households and individuals, Great Britain: 2020. Office for National Statistics. 2020. [2021-10-12]. https://www.ons.gov.uk/peoplepopulationandcommunity/householdcharacteristics/homeinternetandsocialmediausage/bulletins/internetaccesshouseholdsandindividuals/2020 .
- 40.Norman CD, Skinner HA. eHEALS: the eHealth literacy scale. J Med Internet Res. 2006 Nov 14;8(4):e27. doi: 10.2196/jmir.8.4.e27. https://www.jmir.org/2006/4/e27/ v8i4e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alhadreti O, Mayhew P. Rethinking thinking aloud: a comparison of three think-aloud protocols. Proceedings of the 2018 CHI Conference on Human Factors in Computing Systems; CHI '18; April 21-26, 2018; Montreal, Canada. 2018. pp. 1–12. [DOI] [Google Scholar]
- 42.Brooke J. SUS: a 'quick and dirty' usability scale. In: Jordan PW, Thomas B, McClelland IL, Weerdmeester B, editors. Usability Evaluation in Industry. London, UK: CRC Press; 1996. pp. 189–94. [Google Scholar]
- 43.Bangor A, Kortum PT, Miller JT. An empirical evaluation of the system usability scale. Int J Human Comput Interact. 2008 Jul 30;24(6):574–94. doi: 10.1080/10447310802205776. http://dx.doi.org.proxy-ub.rug.nl/10.1016/j.jsat.2009.06.002 .S0740-5472(09)00096-8 [DOI] [Google Scholar]
- 44.Brooke J. SUS: a retrospective. J Usability Stud. 2013;8(2):29–40. https://uxpajournal.org/sus-a-retrospective/ [Google Scholar]
- 45.Fetters MD, Curry LA, Creswell JW. Achieving integration in mixed methods designs-principles and practices. Health Serv Res. 2013 Dec;48(6 Pt 2):2134–56. doi: 10.1111/1475-6773.12117. https://europepmc.org/abstract/MED/24279835 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sauro J. A Practical Guide to the System Usability Scale: Background, Benchmarks & Best Practices. Scotts Valley, CA, USA: CreateSpace; 2012. [Google Scholar]
- 47.Joffe H. Thematic analysis. In: Harper D, Thompson AR, editors. Qualitative Research Methods in Mental Health and Psychotherapy: A Guide for Students and Practitioners. Hoboken, NJ, USA: Wiley Online Library; 2021. pp. 209–23. [DOI] [Google Scholar]
- 48.Spencer L, Ritchie J, Ormston R, O'Connor W, Barnard M. Analysis: principles and processes. In: Ritchie J, Lewis J, McNaughton Nicholls C, Ormston R, editors. Qualitative Research Practice: A Guide for Social Science Students and Researchers. Thousand Oaks, CA, USA: Sage Publications; 2014. pp. 269–95. [Google Scholar]
- 49.Applying Human Factors and Usability Engineering to Medical Devices: Guidance for Industry and Food and Drug Administration Staff. U.S. Department of Health and Human Services, Food and Drug Administration. 2016. Feb 3, [2022-08-22]. https://www.fda.gov/media/80481/download .
- 50.Nielsen J. Estimating the number of subjects needed for a thinking aloud test. Int J Human Comput Stud. 1994 Sep;41(3):385–97. doi: 10.1006/ijhc.1994.1065. doi: 10.1006/ijhc.1994.1065. [DOI] [Google Scholar]
- 51.Malterud K, Siersma VD, Guassora AD. Sample size in qualitative interview studies: guided by information power. Qual Health Res. 2016 Nov;26(13):1753–60. doi: 10.1177/1049732315617444.1049732315617444 [DOI] [PubMed] [Google Scholar]
- 52.Sauro J, Dumas JS. Comparison of three one-question, post-task usability questionnaires. Proceedings of the SIGCHI Conference on Human Factors in Computing Systems; CHI '09; April 4-9, 2009; Boston, MA, USA. 2009. pp. 1599–608. [DOI] [Google Scholar]
- 53.Bangor A, Kortum P, Miller J. Determining what individual SUS scores mean: adding an adjective rating scale. J Usability Stud. 2009;4(3):114–23. https://uxpajournal.org/determining-what-individual-sus-scores-mean-adding-an-adjective-rating-scale/ [Google Scholar]
- 54.Suresh E. Diagnosis of early rheumatoid arthritis: what the non-specialist needs to know. J R Soc Med. 2004 Sep;97(9):421–4. doi: 10.1258/jrsm.97.9.421. https://europepmc.org/abstract/MED/15340020 .97/9/421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rheumatoid arthritis | Causes, symptoms, treatments. Versus Arthritis. 2022. [2022-08-22]. https://www.versusarthritis.org/media/24663/rheumatoid-arthritis-information-booklet-2022.pdf .
- 56.Diagnosis of rheumatoid arthritis (RA) National Rheumatoid Arthritis Society. 2022. [2022-08-22]. https://nras.org.uk/resource/diagnosis/
- 57.Powley L, McIlroy G, Simons G, Raza K. Are online symptoms checkers useful for patients with inflammatory arthritis? BMC Musculoskelet Disord. 2016 Aug 24;17(1):362. doi: 10.1186/s12891-016-1189-2. https://bmcmusculoskeletdisord.biomedcentral.com/articles/10.1186/s12891-016-1189-2 .10.1186/s12891-016-1189-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Monti S, Montecucco C, Bugatti S, Caporali R. Rheumatoid arthritis treatment: the earlier the better to prevent joint damage. RMD Open. 2015 Aug 15;1(Suppl 1):e000057. doi: 10.1136/rmdopen-2015-000057. https://rmdopen.bmj.com/lookup/pmidlookup?view=long&pmid=26557378 .rmdopen-2015-000057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA. 2018 Oct 02;320(13):1360–72. doi: 10.1001/jama.2018.13103.2705192 [DOI] [PubMed] [Google Scholar]
- 60.Semigran HL, Linder JA, Gidengil C, Mehrotra A. Evaluation of symptom checkers for self diagnosis and triage: audit study. BMJ. 2015 Jul 08;351:h3480. doi: 10.1136/bmj.h3480. http://www.bmj.com/lookup/pmidlookup?view=long&pmid=26157077 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chambers D, Cantrell AJ, Johnson M, Preston L, Baxter SK, Booth A, Turner J. Digital and online symptom checkers and health assessment/triage services for urgent health problems: systematic review. BMJ Open. 2019 Aug 01;9(8):e027743. doi: 10.1136/bmjopen-2018-027743. https://bmjopen.bmj.com/lookup/pmidlookup?view=long&pmid=31375610 .bmjopen-2018-027743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hill MG, Sim M, Mills B. The quality of diagnosis and triage advice provided by free online symptom checkers and apps in Australia. Med J Aust. 2020 Jun;212(11):514–9. doi: 10.5694/mja2.50600. [DOI] [PubMed] [Google Scholar]
- 63.Using Technology to Ease The Burden on Primary Care. Healthwatch Enfield. 2019. [2022-08-22]. https://healthwatchenfield.co.uk/wp-content/uploads/2019/01/Report_UsingTechnologyToEaseTheBurdenOnPrimaryCare.pdf .
- 64.Harris PR, Sillence E, Briggs P. Perceived threat and corroboration: key factors that improve a predictive model of trust in Internet-based health information and advice. J Med Internet Res. 2011 Jul 27;13(3):e51. doi: 10.2196/jmir.1821. https://www.jmir.org/2011/3/e51/ v13i3e51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arthritis Research UK National Primary Care Centre, Keele University Consultations for selected diagnoses and regional problems. Musculoskeletal Matters. 2010. [2022-08-20]. https://viewer.joomag.com/musculoskeletal-matters-2-consultations-for-selected-diagnoses-regional/0458165001492524063 .
- 66.Sillence E, Blythe JM, Briggs P, Moss M. A revised model of trust in Internet-based health information and advice: cross-sectional questionnaire study. J Med Internet Res. 2019 Nov 11;21(11):e11125. doi: 10.2196/11125. https://www.jmir.org/2019/11/e11125/ v21i11e11125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gruppen LD. Clinical reasoning: defining it, teaching it, assessing it, studying it. West J Emerg Med. 2017 Jan;18(1):4–7. doi: 10.5811/westjem.2016.11.33191. https://europepmc.org/abstract/MED/28115999 .wjem-18-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berkowsky RW. Exploring predictors of eHealth literacy among older adults: findings from the 2020 CALSPEAKS survey. Gerontol Geriatr Med. 2021 Dec 14;7:23337214211064227. doi: 10.1177/23337214211064227. https://journals.sagepub.com/doi/10.1177/23337214211064227?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%3dpubmed .10.1177_23337214211064227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cutrona SL, Mazor KM, Vieux SN, Luger TM, Volkman JE, Finney Rutten LJ. Health information-seeking on behalf of others: characteristics of "surrogate seekers". J Cancer Educ. 2015 Mar;30(1):12–9. doi: 10.1007/s13187-014-0701-3. https://europepmc.org/abstract/MED/24989816 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Igwesi-Chidobe CN, Bishop A, Humphreys K, Hughes E, Protheroe J, Maddison J, Bartlam B. Implementing patient direct access to musculoskeletal physiotherapy in primary care: views of patients, general practitioners, physiotherapists and clinical commissioners in England. Physiotherapy. 2021 Jun;111:31–9. doi: 10.1016/j.physio.2020.07.002. https://linkinghub.elsevier.com/retrieve/pii/S0031-9406(20)30388-6 .S0031-9406(20)30388-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garcia A. UX Research | Standardized Usability Questionnaire. User Research. 2013. Nov 27, [2022-08-22]. https://arl.human.cornell.edu/linked%20docs/Choosing%20the%20Right%20Usability%20Questionnaire.pdf .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Visual joint display (researcher version).


