Abstract
Introduction
Increased efforts to optimize outcomes for early stage NSCLC through the investigation of novel perioperative treatment strategies are ongoing. An emerging question is the role of pathologic response and its association with long-term clinical outcomes after neoadjuvant therapy.
Methods
To investigate the association of pathologic complete response (pCR) and event-free survival (EFS) and overall survival (OS), we performed a systematic review and meta-analysis identifying studies reporting on the prognostic impact of pCR after neoadjuvant chemotherapy or chemoradiotherapy. To evaluate this prognostic value, an aggregated data (AD) meta-analyses was conducted to estimate the pooled hazard ratios (HRs) of EFS and OS for pCR. Using reconstructed individual patient data (IPD), pooled Kaplan-Meier curves were obtained to estimate this association in a more granular fashion. Subgroup analyses were conducted to further explore the impacts of study-level characteristics.
Results
A total of 28 studies comprising 7011 patients were included in the AD meta-analysis, of which, IPD was available for 6274 patients from 24 studies. Results from our AD meta-analysis revealed a pooled pCR rate of 18% (95% confidence interval [CI]: 15%–21%), including significant improvements in OS (HR = 0.50, 95% CI: 0.45–0.56) and EFS (HR = 0.46, 95% CI: 0.37–0.57) on the basis of pCR status. Our IPD analysis revealed a 5-year OS rate of 63% (95% CI: 59.6–67.4) for patients with a pCR compared with 39% (95% CI: 34.5–44.5) for those without a pCR.
Conclusions
pCR after neoadjuvant chemotherapy plus or minus radiotherapy is associated with significant improvements in EFS and survival for patients with resectable NSCLC.
Keywords: Pathologic complete response, Lung cancer, Neoadjuvant, Chemotherapy, Radiotherapy
Introduction
Lung cancer remains the leading cause of cancer death globally on the basis of recent available data.1 For patients with earlier stage, resectable disease, the risk of recurrence after surgery is greater than 50%.2 In light of these sobering statistics, increased emphasis on the treatment of early stage NSCLC has led to a flurry of exciting new neoadjuvant trials, each hoping to address this serious clinical need and keep pace with advances in the metastatic setting.
Increasingly, pathologic assessments of response have been incorporated into neoadjuvant clinical trial designs to assess the activity of study regimens. Thresholds of pathologic response, including major pathologic response (MPR) and pathologic complete response (pCR), have been used as primary end points for several ongoing and recently reported chemoimmunotherapy trials.3,4 Such pathologic end points provide early indicators of therapy response and represent an efficient clinical trial design for evaluating the efficacy of novel neoadjuvant therapies. Previous studies have suggested the positive correlation between pathologic response, including pCR and MPR, and more established clinical end points, such as event-free survival (EFS) and overall survival (OS).5 Nevertheless, questions have been raised over the appropriateness of these pathologic end points as surrogates for long-term clinical outcomes. Therefore, further research is needed to effectively quantify the association between pathologic responses, such as pCR, and long-term outcomes, including EFS and OS.
Although long-term clinical outcomes from recent neoadjuvant trials incorporating immune checkpoint inhibitors (ICIs) are still maturing, we can draw on more extensive experiences from neoadjuvant chemotherapy (CT) and chemoradiotherapy (CRT) to effectively quantify the prognostic value of pCR. Establishing this association has significant clinical implications in both patient management and clinical trial design. The prognostic information of pathologic responses allows clinicians to more effectively counsel patients and tailor subsequent management. Such an association is important for researchers designing future neoadjuvant ICI trials in NSCLC. Given the recent approval of neoadjuvant chemoimmunotherapy, both scenarios will increasingly be encountered, making this both a relevant and urgent clinical need. In addition, considering many neoadjuvant trials, completed or ongoing, are not fully powered to detect a difference in OS, a well-understood association between pathologic responses and long-term outcomes will substantially accelerate evidence generation.
To address this question, we have performed a comprehensive systematic review and meta-analysis of available literature quantifying the relationship between pathologic response and long-term clinical outcomes for patients with NSCLC who were treated with neoadjuvant CT or CRT. In this study, we included key subgroup analyses, such as stage and neoadjuvant treatment regimen, to explore the relationship between pCR and clinical outcomes in various patient populations.
Materials and Methods
Study Identifications
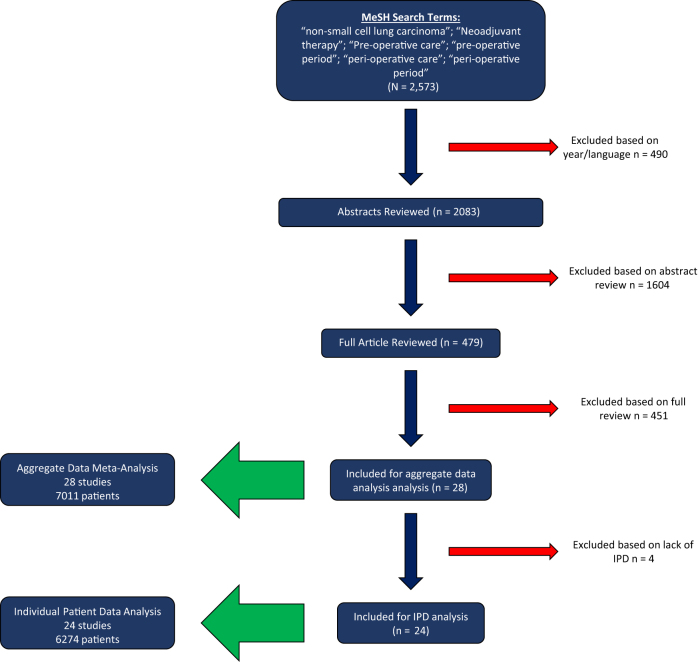
We performed our systematic search using the PubMed database to identify potentially eligible studies. This search was carried out in March 2022. The search used a combination of MESH terms including “Carcinoma, Non-Small-Cell Lung,” “neoadjuvant therapy,” “preoperative care,” “preoperative period,” “peri-operative care,” and “peri-operative period.” We included all trials published after 1997 which coincided with the publication of the fifth edition of TNM classification for lung cancer.6 Eligibility included studies that reported rates of pCR or MPR and their relationship with long-term clinical outcomes, such as EFS, or its equivalent, or OS. We included all relevant retrospective cohort, prospective cohort, and randomized controlled trials. We restricted eligibility to those publications written in English. Eligible neoadjuvant regimens included those that incorporated platinum-based CT with or without radiotherapy (XRT). Additional references were identified by searching for publications that cited the articles we included and reviewing related systematic literature reviews. Articles were initially screened by one member of the study team (SR) and then reviewed by the remainder of the team (CL, PF, CH), to ensure eligibility was met. If two or more publications reported on the same cohorts, only the most recent publication was included. A Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram, summarizing our article screening process, is presented in Figure 1.
Figure 1.
PRISMA flowchart for study selection. On the basis of our preset eligibility criteria, 2083 abstracts were reviewed. From these, 479 underwent full text review, leading to 28 studies that were included for our aggregate data meta-analysis and 24 studies with available individual patient data. MeSH, Medical Subject Headings; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Evaluation of Bias
Only manuscripts published in peer-reviewed journals were included, and conference proceedings and abstracts were therefore not included. Demographic, clinical, pathologic, and treatment information of each study were extracted and summarized, to ensure the broadest representation of patients. Data were collected in a predefined file in which we reported the PubMed identification, first author, journal, publication year, and other key variables as detailed previously. Studies were included regardless of funding sources. Demographic information on the final study lists for both the individual patient data and aggregate data analyses is presented in Table 1 and Supplementary Table 1.2, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33
Table 1.
Summary of Study and Patient Characteristics, Organized According to the Type of Analysis They Were Incorporated Under (i.e., Aggregate Data or Individual Patient Data Meta-Analysis)
| Study Characteristics | Aggregate Data |
Individual Patient Data |
||
|---|---|---|---|---|
| Publications Included |
Sample Size |
Publications Included |
Sample Size |
|
| n = 28 | n = 7011 | n = 24 | n = 6274 | |
| Study type, n (%) | ||||
| Prospective cohort | 1 (4) | 27 (0) | 1 (4) | 27 (0) |
| Prospective trial | 6 (21) | 292 (4) | 6 (25) | 292 (5) |
| RCTs | 1 (4) | 492 (7) | 1 (4) | 492 (8) |
| Retro cohort | 20 (71) | 6200 (88) | 16 (67) | 5463 (87) |
| Geographic region, n (%) | ||||
| Asia-Pacific | 12 (43) | 1659 (24) | 10 (42) | 1099 (18) |
| Europe | 7 (25) | 1126 (16) | 5 (21) | 949 (15) |
| Middle East | 1 (4) | 124 (2) | 1 (4) | 124 (2) |
| North America | 8 (29) | 4102 (59) | 8 (33) | 4102 (65) |
| Publication date, median (range) | 2014 (2000–2021) | 2016 (2000–2021) | ||
| Follow-up time (mo), median (range) | 42 (18–97) | 42.2 (18–97) | ||
| Median age (ys), median (range) | 59 (55–67) | 59 (55–67) | ||
| Proportion of stage 3 patients, median (range) | 100% (0%–100%) | 100% (0%–100%) | ||
| pCR rate, median (range) | 17% (4%–38%) | 19% (4%–38%) | ||
| pCR definition, n (%) | ||||
| T0N0 | 15 (54) | 4504 (64) | 13 (54) | 4173 (67) |
| T0 | 6 (21) | 1540 (22) | 5 (21) | 1185 (19) |
| Not defined | 7 (25) | 967 (14) | 6 (25) | 916 (15) |
| AJCC edition | ||||
| Fifth | 2 (7) | 212 (3) | 2 (8) | 212 (3) |
| Sixth | 6 (21) | 2887 (41) | 6 (25) | 2887 (46) |
| Seventh | 9 (32) | 2769 (39) | 7 (29) | 2438 (39) |
| Eight | 1 (4) | 92 (1) | 1 (4) | 92 (1) |
| Not reported | 10 (36) | 1051 (15) | 8 (33) | 645 (10) |
| Evaluated association between pCR and OS, n (%) | 27 (96) | 6979 (100) | 23 (96) | 6292 (100) |
| Evaluated association between pCR and EFS, n (%) | 9 (32) | 1649 (24) | 7 (29) | 1530 (24) |
| Recurrence definition, n (%) | ||||
| RFS | 4 (14) | 834 (12) | 4 (17) | 834 (13) |
| PFS | 5 (18) | 595 (8) | 3 (12) | 189 (3) |
| RFS/PFS | 1 (4) | 32 (0) | 1 (4) | 32 (1) |
| DFS | 2 (7) | 584 (8) | 2 (8) | 584 (9) |
| DFS/DSS | 1 (4) | 122 (2) | 1 (4) | 122 (2) |
| Distant recurrence | 1 (4) | 85 (1) | 1 (4) | 85 (1) |
| TTP | 1 (4) | 157 (2) | 1 (4) | 157 (3) |
| Not included | 13 (46) | 4602 (66) | 11 (46) | 4271 (68) |
| EFS start date, n (%) | ||||
| Diagnosis | 2 (7) | 1868 (27) | 2 (8) | 1868 (30) |
| First treatment | 8 (29) | 1373 (20) | 7 (29) | 1018 (16) |
| Enrollment | 1 (4) | 48 (1) | 1 (4) | 48 (1) |
| Surgery | 11 (39) | 1724 (25) | 9 (38) | 1547 (25) |
| Not defined | 6 (21) | 1998 (28) | 5 (21) | 1793 (29) |
| Reported adjuvant therapy use, n (%) | ||||
| Yes | 9 (32) | 2763 (39) | 6 (25) | 2077 (33) |
| No | 19 (68) | 4248 (61) | 18 (75) | 4197 (67) |
| % patients who received adjuvant treatment | 83.1% (13.7%–100%) | 65.5% (13.7%–100%) | ||
| Preop cycles, n (%) | ||||
| ≤2 | 12 (43) | 1409 (20) | 11 (46) | 1204 (19) |
| ≥3 | 8 (29) | 1321 (19) | 6 (25) | 1144 (18) |
| Not reported | 8 (29) | 4281 (61) | 7 (29) | 3926 (63) |
| XRT dose, n (%) | ||||
| <50 Gy | 12 (43) | 1914 (27) | 11 (46) | 1559 (25) |
| ≥50 Gy | 10 (36) | 2878 (41) | 8 (33) | 2622 (42) |
| Not reported | 6 (21) | 2219 (32) | 5 (21) | 2093 (33) |
Note: The relative proportions of both the number of studies and patient sample size for each variable were included.
AJCC, American Joint Committee on Cancer; CI, confidence interval; DFS, disease-free survival; DSS, disease-specific survival; EFS, event-free survival; HR, hazard ratio; Op, operative; OS, overall survival; pCR, pathologic complete response; PFS, progression-free survival; RCT, randomized controlled trial; Retro, retrospective; RFS, recurrence-free survival; TTP, time to progression; XRT, radiotherapy.
End Points
The primary clinical end points collected were cancer recurrence and overall survival. These time-to-event measurements varied between studies and were therefore captured by our study team. For the recurrence end point, studies used a variety of measurements, including EFS, disease-free survival, progression-free survival, and recurrence-free survival. Each of these end points was treated as equivalent for our pooled analysis and referred to EFS generically for simplicity. The primary pathologic end point collected was pCR, defined as 0% viable tumor on surgical pathology specimen.34 Variability in the definition of pCR was noted and considered for this analysis, including studies that assessed both primary tumor and nodal metastases (pT0N0), primary tumor alone (T0), or studies that did not include a clear definition of pCR (“not defined”).
Statistical and Data Extraction Methods
To evaluate the prognostic value of pCR, aggregated data (AD) meta-analysis and individual patient-level data (IPD) meta-analysis were both conducted to estimate the hazard ratio (HRs) of EFS and OS for pCR versus no pCR, respectively. HR less than 1.0 indicates that pCR was associated with better EFS or survival, relative to those who did not.
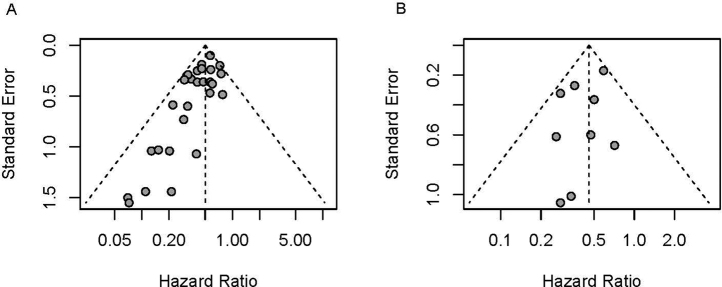
For AD meta-analysis, the fixed-effects model and random-effects model with DerSimonian-Laird method were applied to estimate the pooled HR. HRs and 95% confidence intervals (CIs) for each study were directly extracted from the literature. Publication bias was explored using the funnel plot (Supplementary Fig. 1). If HRs were not reported, Guyot’s algorithm35 was used to reconstruct pseudo-IPD from published Kaplan-Meier curves (KMCs) of EFS and OS by pCR status, then study-level HRs and 95% CI were estimated by Cox proportional hazards model on the basis of the pseudo-IPD. For studies with no events observed in the pCR group, Firth's penalized maximum likelihood bias reduction method for Cox PH model was used for HR estimation.36 I2 statistic and Cochrane’s Q test were conducted to assess the heterogeneity of HRs across studies, and fixed-effects models were reported over random-effects models if there was no strong evidence of heterogeneity suggested. Forest plots were presented to visualize the results on individual studies and the degree of heterogeneity across studies.
In addition, an IPD meta-analysis was conducted to evaluate the association between pCR status and survival outcomes (EFS and OS) in a granular fashion. For the subset of studies that published KMCs of EFS and OS by pCR status, pseudo-IPD was reconstructed using Guyot’s algorithm. Shared frailty semiparametric Cox PH model,37 a random effect Cox proportional hazard model of individual time-to-event data that accounts for interstudy heterogeneity, was used to obtain the pooled HR for pCR across individuals from all selected studies. Across-study variations were explicitly modeled through a common baseline hazard function, multiplying a Gamma distributed frailty term (Gamma frailty). To account for the potential nonrandom heterogeneity from study-specific HRs for pCR, an additive frailty model38 was further applied to calculate the corresponding pooled HRs. If additive frailty models suggested that explicitly modeling the nonrandom heterogeneity in HRs was not required, only shared frailty model results were provided. Semiparametric penalized likelihood estimation method was used on the hazard function. During the estimation, hazard functions were modeled as cubic M-spline functions with seven knots, and smoothing parameter kappa was selected using cross-validation.
To assess the reliability of pseudo-IPD, we compared the survival probabilities from published KMCs with those estimated from pseudo-IPD. Root-mean square error and mean absolute error are presented to summarize the comparison.
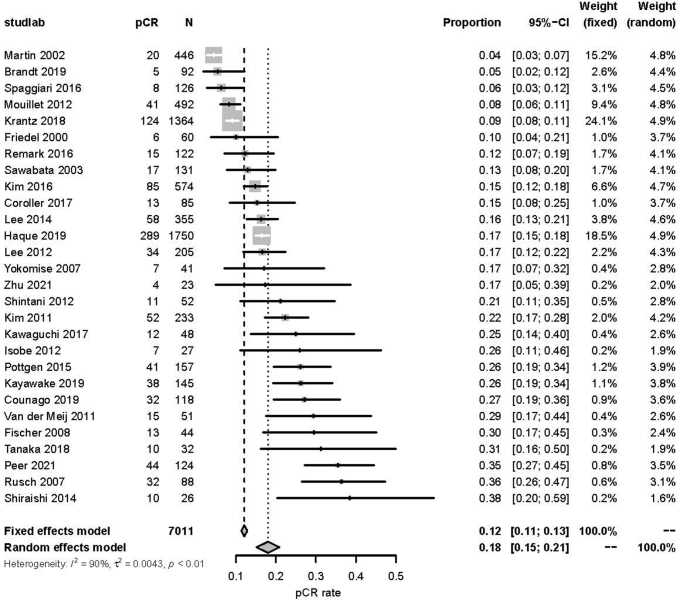
In addition, in AD meta-analysis, pCR rate was pooled using fixed-effect meta-analysis, or random effect meta-analysis if substantial heterogeneity existed according to Cochran’s Q test and I2 statistic.
These analyses were conducted using R version 4.1.1 (R Institute for Statistical Computing, Vienna, Austria) and packages meta version 5.1-1, frailtypack version 3.5.0, and coxphf version 1.13.1.
Subgroup Analysis
Subgroup analyses were conducted in both AD meta-analysis and IPD meta-analysis to further assess the consistency and explore the impacts of study-level characteristics, including type of therapy, geographic region, study type, study definition of pCR, use of adjuvant therapy, proportion of patients on stage III, number of preoperative CT cycles (>/<2 cycles), and radiation dose (>/<50 Gy), on the association between pCR and survival outcomes (EFS and OS).
Results
Search Results
A total of 2083 citations with abstracts were reviewed. Of these, 479 articles underwent full-text review. From these, 451 were excluded for not meeting preset eligibility criteria, leaving 28 studies that were included for our aggregate data meta-analysis. Of these 28 studies, 24 had individual patient data available for extraction which comprised our cohort of articles included in the IPD analysis. Ultimately, 7011 patients were included in the AD meta-analysis and 6274 patients were included in the IPD analysis. Figure 1 depicts the Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart detailing study selection.
Study Characteristics
The selected studies were published from 2000 to 2021, with median publication date of 2014. They featured a broad range of geographic regions including North America, Asia-Pacific, Europe, and the Middle East. Most studies came from retrospective cohort analyses, although additional study types were included, such as randomized controlled trials and nonrandomized prospective studies. A range of American Joint Committee on Cancer TNM staging classifications were used, including fifth edition to the current eighth edition. Of the 28 studies in AD analysis and 24 in IPD analysis, nine (32%) and six (25%) studies reported the use of postoperative therapy, which comprised 39% of the AD patient population and 33% of the IPD patients. Study characteristics and breakdown for all 28 studies included in the AD analysis and the 24 IPD studies are detailed further in Table 1.
AD Meta-Analysis: Pooled HR of OS/EFS for pCR Versus No pCR
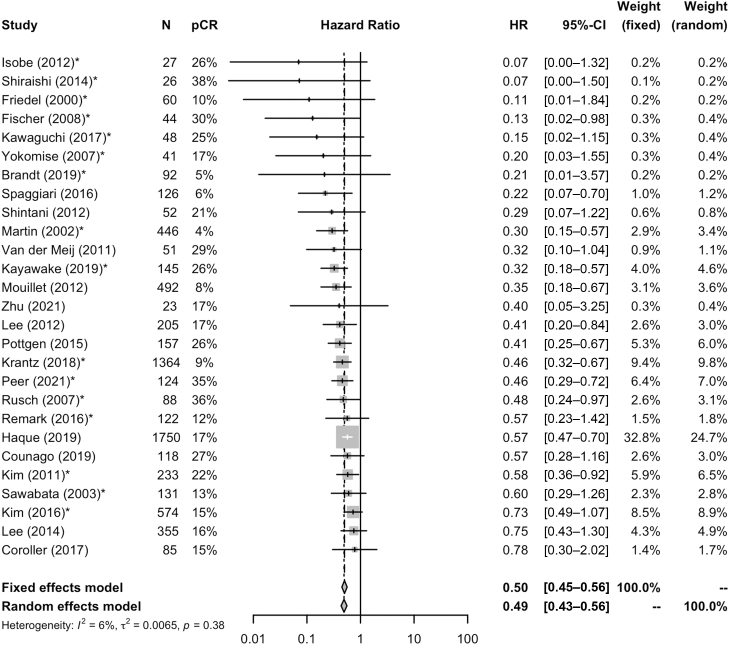
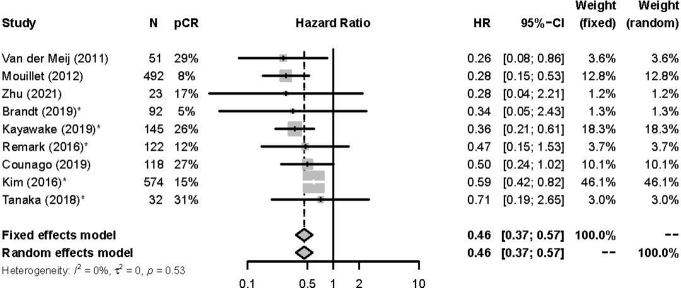
For the 28 studies and 7011 patients included in the aggregate data meta-analysis, the pooled pCR rate was 18% (95% CI: 15%–21%) (Supplementary Fig. 2). Figure 2 illustrates the estimated OS HR and 95% CI for pCR from each of 28 studies and the pooled HR on the basis of fixed-effect meta-analysis. Overall, patients who achieved a pCR had a significantly better OS (HR = 0.50, 95% CI: 0.45–0.56) when compared with patients without a pCR (Fig. 2). Similar findings were noted for EFS, where patients with a pCR had improved EFS (HR = 0.46, 95% CI: 0.37–0.57) (Supplementary Fig. 3). Minimal heterogeneity was noted for both OS (I2 = 6%, τ2 = 0.0065, Cochrane’s Q test p = 0.38) and EFS (I2 = 0%, τ2 = 0, Cochrane’s Q test p = 0.53).
Figure 2.
Forest plot representation of the overall HR estimates with 95% confidence intervals for the association of pCR with overall survival, by study and pooled on the basis of aggregated data meta-analysis. CI, confidence interval; HR, hazard ratio; pCR, pathologic complete response.
As exploratory sensitivity analysis, we conducted subgroup analysis to assess the consistency of estimated HRs of OS for pCR across treatment type (CRT versus CT versus CRT/CT), study type (retrospective cohort, prospective cohort, randomized controlled trials), and percent of patients on stage III included (<50%, 50%–99%, 100%). In general, considering the potentially small number of studies used, estimated OS HRs for pCR in subgroups were numerically similar across subgroups defined by the number of preoperative CT cycles (≤2 versus >2), reported administration of adjuvant therapy, or dose of XRT given (<50 Gy versus ≥50 Gy) for CRT studies. Focusing on the interaction of stage and OS on the basis of presence of pCR, a numerical trend was noted, where studies with lower percentages of patients on stage III had stronger associations between pCR and survival. Additional results from subgroup analysis for OS/EFS by pCR status are detailed in Supplementary Table 2.
IPD Meta-Analysis: Estimated OS/EFS by pCR Status
Pseudo-IPD was reconstructed from 24 eligible studies, comprising 6274 patients. As found in Supplementary Table 3, the KMCs on the basis of reconstructed IPD are in reasonable agreement with those from original manuscripts.
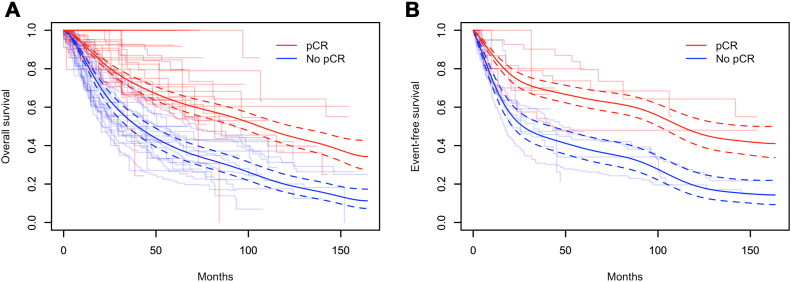
KMCs for both OS and EFS, stratified by pCR status from the 24 eligible studies, are found in Figure 3. Using the frailty model detailed previously, the estimated pooled OS and EFS rates over time between pCR and no pCR are found in Figure 3A and B. On the basis of this analysis, pCR after neoadjuvant CT plus or minus XRT had a significant prognostic effect on OS (HR = 0.49, 95% CI: 0.43–0.55). Similar findings were appreciated in terms of EFS, where pCR was again found to be a significant prognostic factor for EFS (HR = 0.46, 95% CI: 0.36–0.58). Pooled 5-year OS rates for patients with and without pCR were 63% (95% CI: 59.6–67.4) and 39% (95% CI: 34.5–44.5), respectively.
Figure 3.
Kaplan-Meier estimates of (A) OS and (B) EFS for patients with or without a pCR, by study and pooled on the basis of individual patient data meta-analysis. The dashed lines represent the 95% CI for their respective colors. CI, confidence interval; EFS, event-free survival; pCR, pathologic complete response.
Subgroup Analysis From IPD
Using analysis from pseudo-IPD, associations between pCR and long-term clinical outcomes were evaluated on the basis of pertinent subgroups. Similar to the subgroup analysis from the AD meta-analysis, the association between pCR and survival outcomes (EFS and OS) was reasonably consistent across subgroups. In an exploratory and hypothesis-generating nature, we note some numerical differences in estimated HR for pCR and OS on the basis of treatment type, study type, and percentage of patients on stage III included. Similar to observations noted from subgroup analysis of the aggregate pooled data, a numerical trend was appreciated whereby studies with lower percentages of patients on stage III corresponded with stronger associations of pCR and OS. In terms of estimated HR for pCR and EFS, in addition to these three subgroups, the dose of radiation administered, number of preoperative CT cycles, and adjuvant treatment status had more appreciable differences, albeit with fewer eligible studies (n = 7).
Focusing on interactions with adjuvant therapy, again, most IPD studies did not report on or offer postoperative treatment. When comparing the estimated HR for pCR on EFS, there was a greater difference between studies with adjuvant therapy versus those without adjuvant treatment, with results favoring the use of adjuvant treatment. Nevertheless, this difference was reduced when assessing the estimated HR for the association between pCR and OS. A similar trend was observed in terms of XRT dose and number of CT cycles, where differences in HR for pCR and EFS were more pronounced compared with OS. Full subgroup analysis results from our IPD, for both EFS and OS, are found in Table 2.
Table 2.
Summary of Subgroup Analysis, Incorporating Individual Patient Data From 24 Available Studies, Evaluating Key Variables and Their Association With OS and EFS Based on the Presence or Absence of pCR, Represented by Estimated HRs With Corresponding 95% CIs
| Subgroup Analyses | OS |
EFS |
||||||
|---|---|---|---|---|---|---|---|---|
| N Studies | Sample size | HR | 95% CI | n Studies | Sample size | HR | 95% CI | |
| Overall | 23 | 6292 | 0.49 | (0.43–0.55) | 7 | 1530 | 0.46 | (0.36–0.58) |
| Treatment type | ||||||||
| CT | 4 | 779 | 0.39 | (0.23–0.64) | 4 | 779 | 0.31 | (0.19–0.53) |
| CT/CRT | 6 | 2328 | 0.44 | (0.35–0.54) | 1 | 145 | 0.36 | (0.21–0.60) |
| CRT | 13 | 3185 | 0.52 | (0.45–0.60) | 2 | 606 | 0.58 | (0.42–0.80) |
| Geographic region | ||||||||
| Asia-Pacific | 9 | 1067 | 0.45 | (0.34–0.60) | 4 | 774 | 0.51 | (0.38–0.67) |
| Europe | 5 | 949 | 0.41 | (0.30–0.57) | 2 | 614 | 0.31 | (0.18–0.53) |
| Middle East | 1 | 124 | 0.46 | (0.29–0.71) | 0 | 0 | N/A | N/A |
| North America | 8 | 4152 | 0.51 | (0.44–0.60) | 1 | 142 | 0.34 | (0.05–2.42) |
| Study type | ||||||||
| Retro cohort | 16 | 5513 | 0.51 | (0.45–0.58) | 4 | 983 | 0.51 | (0.39–0.67) |
| Prospective trial | 5 | 260 | 0.29 | (0.16–0.52) | 2 | 55 | 0.49 | (0.17–1.43) |
| Prospective cohort | 1 | 27 | 0.07 | (0.00–1.32) | 0 | 0 | N/A | N/A |
| RCTs | 1 | 492 | 0.35 | (0.19–0.66) | 1 | 492 | 0.28 | (0.15–0.53) |
| pCR definition | ||||||||
| T0N0 | 12 | 4141 | 0.51 | (0.44–0.58) | 2 | 154 | 0.50 | (0.21–1.15) |
| T0 | 5 | 1185 | 0.52 | (0.38–0.71) | 3 | 1089 | 0.48 | (0.36–0.64) |
| Not defined | 6 | 966 | 0.40 | (0.29–0.54) | 2 | 287 | 0.37 | (0.23–0.62) |
| Adjuvant therapy | ||||||||
| Yes | 5 | 2045 | 0.42 | (0.32–0.56) | 3 | 547 | 0.31 | (0.18–0.54) |
| No | 18 | 4247 | 0.50 | (0.44–0.57) | 4 | 983 | 0.51 | (0.39–0.67) |
| % of stage III patients | ||||||||
| 100% | 15 | 4657 | 0.51 | (0.45–0.59) | 4 | 751 | 0.56 | (0.41–0.76) |
| 50%–100% | 4 | 909 | 0.46 | (0.34–0.60) | 1 | 145 | 0.36 | (0.21–0.60) |
| <50% | 4 | 726 | 0.28 | (0.16–0.50) | 2 | 634 | 0.29 | (0.16–0.52) |
| Number of preoperative chemotherapy cycles | ||||||||
| ≥3 | 6 | 1194 | 0.45 | (0.35–0.60) | 2 | 634 | 0.29 | (0.16–0.52) |
| ≤2 | 10 | 1172 | 0.43 | (0.33–0.57) | 5 | 896 | 0.50 | (0.38–0.66) |
| Not reported | 7 | 3926 | 0.52 | (0.45–0.60) | 0 | 0 | N/A | N/A |
| Radiation dose | ||||||||
| ≥50 Gy | 7 | 2590 | 0.52 | (0.44–0.61) | 1 | 32 | 0.71 | (0.19–2.63) |
| <50 Gy | 11 | 1559 | 0.47 | (0.38–0.58) | 2 | 719 | 0.51 | (0.38–0.67) |
| Not reported | 5 | 2143 | 0.43 | (0.32–0.58) | 4 | 779 | 0.31 | (0.19–0.53) |
CI, confidence interval; CRT, chemoradiotherapy; CT, chemotherapy; EFS, event-free survival; HR, hazard ratio; N/A, not applicable; OS, overall survival; pCR, pathologic complete response; RCT, randomized controlled trial; Retro, retrospective.
Definition of pCR (pT0N0 versus T0) was not associated with differences in HR estimates for pCR and OS or EFS. Nevertheless, studies without a clear definition (labeled “not defined”) did have nominal improvements in both OS and EFS for patients with pCR.
Discussion
To date, there are limited data establishing the prognostic relationship between pCR after neoadjuvant systemic therapy and long-term clinical outcome, making it an area of debate among clinical investigators. In the era of neoadjuvant ICI, only short-term clinical data are available from recently reported studies. One such study by Provencio et al.39 reported on 24-month progression-free survival rate of 96.2% for the 26 patients with pCR. The two-year survival rate for patients with pCR and MPR in this study was 100%; however, at the reported interim analysis, there was no statistically significant difference in survival compared with patients with incomplete pathologic response, and authors did not comment on differences in outcomes for pathologic nonresponders. More recently, we have found a strong association between pCR after neoadjuvant chemoimmunotherapy and EFS, as evidenced by results from CheckMate-816—a phase 3 clinical trial that compared neoadjuvant nivolumab plus CT versus neoadjuvant CT.3 Although overall patient numbers were small among the different subgroups, there was a clear improvement in EFS for patients who experienced pCR after chemoimmunotherapy (HR = 0.13, 95% CI: 0.05–0.37). The small number of patients (4 of 179) who had a pCR after neoadjuvant CT also did well. Nevertheless, final conclusions regarding pathologic response and OS will require more mature data. Results such as those presented in this report provide key insight for providers managing patients in the perioperative setting.
In addition to performing a standard meta-analysis of studies evaluating pathologic response after neoadjuvant therapy and long-term clinical outcomes in NSCLC5—where once again a strong and favorable association with survival was noted—we have included more in-depth IPD analysis to further characterize this relationship. By extracting IPD and reconstructing KMCs from available studies, we are better able to capture the significant association between pCR and long-term clinical outcomes of EFS and OS. Similar statistical tools and modeling have been used in breast cancer40, 41, 42 and have reinforced the prognostic value of these pathologic end points in terms of recurrence and survival. Using the example of breast cancer, on the basis of the strong association between pathologic response and survival, clinical management is now tied to the level of pathologic response after neoadjuvant therapy in HER-2–positive breast cancer,43 highlighting the important role this pathologic end point can play in the management of perioperative patients.
To bolster our analysis, we included studies using both CT and CRT neoadjuvant treatments. Although platinum-based CT was incorporated for all neoadjuvant strategies, this interaction between XRT and pathologic response is important to note. We found that numerical differences in HR estimated for pCR and EFS, including OS, emerged on the basis of whether CT versus CRT was used, to the extent that pCR after neoadjuvant CT might have a stronger association with survival. Further reinforcing this finding, when evaluating studies that incorporated a mixture of neoadjuvant CT or CRT (labeled CT/CRT), estimated HRs of pCR and survival fell in between the boundaries of both modalities. Evaluating possible explanations for this finding, we can consider the effect radiation may have on both achieving tumor necrosis in the corresponding radiation field44 and subsequent interpretation of pathologic response45—perhaps increasing the rate of pCR among the respective cohort without a parallel systemic effect. In addition, the dose of CT used in combination with radiation is often lower and “radio-sensitizing,” affecting the systemic impact of these agents in eliminating micrometastatic disease, a postulated benefit of neoadjuvant therapy.46 As such, having a pCR after neoadjuvant CT may carry a more favorable prognostic impact on survival, when compared with CRT—though this requires further study.
Another pertinent question is the role of adjuvant therapy in further improving outcomes after neoadjuvant treatment. In our subgroup analysis, limited by study heterogeneity and number of studies, there seemed to be a more pronounced interaction between clinical outcomes and pCR for studies that included adjuvant therapy, with a stronger effect on EFS compared with OS. Currently, interim National Comprehensive Cancer Network guidelines do not recommend further adjuvant ICI for patients who received neoadjuvant ICI. The Food and Drug Administration label in the United States for neoadjuvant nivolumab plus CT is silent in terms of postoperative therapy and CheckMate 816 did not mandate or exclude specific postoperative therapies. As neoadjuvant chemoimmunotherapy for resectable NSCLC is increasingly incorporated into practice, future investigation will be needed to answer the question of optimal perioperative treatment duration. Integral to this will be the identification of clinically relevant biomarkers, such as circulating tumor DNA, to measure postoperative minimal residual disease status,47 including concordance of such noninvasive measurements with pathologic response. Adaptive treatment strategies on the basis of pathologic and molecular response to neoadjuvant therapy, analogous to strategies in early stage breast cancer, will be an increasing area of interest. Trials such as MERMAID-2 will be key to addressing this clinical need.48
When evaluating the interaction of stage on associations between pCR and OS, we appreciated a consistent numerical trend favoring improved outcomes after pCR for studies comprising a lower percentage of patients on stage III. These results should be interpreted cautiously and may in fact just represent inferior clinical outcomes associated with a higher risk group of patients. The impact of stage on selection of optimal neoadjuvant treatment for resectable NSCLC is an active question. Results from studies evaluating neoadjuvant chemoimmunotherapy suggest increased pathologic response and EFS benefit for patients with higher risk stage IIIA disease, compared with CT alone.3,49 Future research will be needed to explore whether stage has an impact on the prognostic value of pCR in the setting of neoadjuvant chemoimmunotherapy.
For the purposes of this study, we focused our analysis on pCR as opposed to MPR. Nevertheless, the optimal pathologic cutoff point is an area of active research, particularly in the setting of neoadjuvant ICI. On the basis of our findings, pCR seems to be a favorable prognostic marker after neoadjuvant CT, a conclusion we feel will extend to more novel neoadjuvant strategies, including ICI and chemoimmunotherapy. Nevertheless, given differences in mechanism of action between CT and ICI, these analyses may not fully capture the interaction of ICI and pathologic response. Indeed, although the cytotoxic effects of CT are largely static, longstanding antitumor activity is one of the hallmarks of ICI, where durable responses may be appreciated even in patients without complete pathologic eradication of the tumor. This may be analogous to findings in the metastatic setting where patients treated with ICI may enjoy durable clinical benefits despite only having partial or even stable radiographic disease.50
There was a concerted effort to account for study variability in our analysis, including definition of pCR, definition of time point zero, and definition of recurrence end point. It is important to recognize these variations when interpreting results. Although effects of each variable were unable to be measured in terms of pCR and clinical outcomes, we did evaluate the role of pCR definition. As mentioned, there did not seem to be an overwhelming or unreasonable difference in association of pCR and clinical outcomes on the basis of this measure alone; however, not all studies provided a clear pCR definition, potentially influencing this finding.
This meta-analysis has several potential limitations. First, the heterogeneity of study-specific outcomes and reporting on certain variables may have affected our analysis and findings. For example, as previously stated, definitions such as recurrence-specific end points, pCR, and the start of OS/EFS (time 0) varied across studies. Although we attempted to capture and correct for this variability, there were still several studies where these variables were either ill defined or excluded from the publication entirely. This was represented by the number of papers available for EFS analysis, where only seven studies with IPD included a defined EFS end point, ultimately limiting our available study pool. Second, there was variability in neoadjuvant treatment regimen between studies, particularly in terms of radiation course and dose. There were also some retrospective studies that included a mixture of both CT and CRT regimens. Studies with CRT also varied as to whether patients received concurrent CRT or sequential, albeit most used concurrent dosing. Our study worked to capture these differences within our subgroup analysis by categorizing major variables such as radiation dose, number of CT cycles, or treatment regimen, and evaluating for differences in clinical outcomes accordingly. Finally, to answer our primary objective, we only included studies that reported on the prognostic impact of pCR, which excluded several high-impact randomized, controlled trials and limited much of our study pool to retrospective studies, which carry their own inherit bias.
In conclusion, this meta-analysis helps to synthesize the currently available data from both prospective and retrospective studies evaluating the prognostic impact of complete pathologic response on long-term clinical outcomes. We used statistical techniques and modeling to reconstruct survival curves on the basis of individual patient data from available studies, quantifying the improvement in clinical outcomes based on the presence of pCR after neoadjuvant therapy. To our knowledge, this is the first meta-analysis incorporating such techniques to evaluate this association in early stage NSCLC. Given the changing landscape of neoadjuvant therapy away from CT to chemoimmunotherapy, these findings associating pCR with survival should be replicated and validated once long-term clinical data become available, as extrapolating directly from our results may have inherit limitations. Nevertheless, although results from definitive prospective studies evaluating various neoadjuvant immunotherapy treatment strategies in NSCLC are still maturing, this meta-analysis may offer important insight for clinicians and patients, while also providing further validity to the use of complete pathologic response as a surrogate end point for future neoadjuvant studies.
CRediT Authorship Contribution Statement
Samuel Rosner: Conceptualization, Investigation, Data curation, Visualization, Interpretation, Manuscript writing.
Chunnan Liu: Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Manuscript writing.
Patrick Forde: Conceptualization, Study interpretation, Supervision, Manuscript writing.
Chen Hu: Conceptualization, Formal Analysis, Methodology, Interpretation, Supervision, Manuscript writing.
Footnotes
Drs. Rosner and Liu contributed equally to this work.
Drs. Forde and Hu contributed equally to this work as co-senior authors.
Disclosure: Dr. Forde reports receiving payments to his institution from AstraZeneca, Bristol-Myers Squibb, Novartis, Corvus, Array, and Kyowa; receiving consulting fees from Amgen, AstraZeneca, Bristol-Myers Squibb, Genentech, G1, Surface, F Star, Iteos, Janssen, Novartis, Daichii Sankyo, and Sanofi; having advisory board participation with Polaris and Flame; and having participation with LUNGevity, MARF, and Cancer Trials Ireland. Dr. Hu is supported in part by the National Institutes of Health under Grant Nos. U10-CA180822 and P30-CA006973 and Johns Hopkins-Allegheny Health Network Cancer Research Fund.
Cite this article as: Rosner S, Liu C, Forde P, Hu C. Association of pathologic complete response and long-term survival outcomes among patients treated with neoadjuvant chemotherapy or chemoradiotherapy for NSCLC: a meta-analysis. JTO Clin Res Rep. 2022;3:100384.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org/ and at 10.1016/j.jtocrr.2022.100384.
Supplementary Data
Supplemental Figure 1.
Supplemental Figure 2.
Supplemental Figure 3.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Martin J., Ginsberg R.J., Venkatraman E.S., et al. Long-term results of combined-modality therapy in resectable non–small-cell lung cancer. J Clin Oncol. 2002;20:1989–1995. doi: 10.1200/JCO.2002.08.092. [DOI] [PubMed] [Google Scholar]
- 3.Forde P.M., Spicer J., Lu S., et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386:1973–1985. doi: 10.1056/NEJMoa2202170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cascone T., William W.N., Weissferdt A., et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med. 2021;27:504–514. doi: 10.1038/s41591-020-01224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waser N.A., Adam A., Schweikert B., et al. Pathologic response as early endpoint for survival following neoadjuvant therapy (NEO-AT) in resectable non-small cell lung cancer (rNSCLC): systematic literature review and meta-analysis. Ann Oncol. 2020;31:S806. [Google Scholar]
- 6.Sobin L.H., Fleming I.D. TNM classification of malignant tumors. 5th ed. Cancer. 1997;80:1803–1804. doi: 10.1002/(sici)1097-0142(19971101)80:9<1803::aid-cncr16>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 7.Rusch V.W., Giroux D.J., Kraut M.J., et al. Induction chemoradiation and surgical resection for superior sulcus non–small-cell lung carcinomas: long-term results of Southwest Oncology Group Trial 9416 (intergroup trial 0160) J Clin Oncol. 2007;25:313–318. doi: 10.1200/JCO.2006.08.2826. [DOI] [PubMed] [Google Scholar]
- 8.Kawaguchi K., Yokoi K., Niwa H., et al. A prospective, multi-institutional phase II study of induction chemoradiotherapy followed by surgery in patients with non-small cell lung cancer involving the chest wall (CJLSG0801) Lung Cancer. 2017;104:79–84. doi: 10.1016/j.lungcan.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 9.Peer M., Azzam S., Cyjon A., et al. Major pulmonary resection after neoadjuvant chemotherapy or chemoradiation in potentially resectable stage III non-small cell lung carcinoma. Sci Rep. 2021;11 doi: 10.1038/s41598-021-99271-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedel G., Hruska D., Budach W., et al. Neoadjuvant chemoradiotherapy of stage III non-small-cell lung cancer. Lung Cancer. 2000;30:175–185. doi: 10.1016/s0169-5002(00)00151-3. [DOI] [PubMed] [Google Scholar]
- 11.Zhu J., Zhang Y., Wang M., et al. Outcomes in 36 patients with stage IIIA-N2 squamous cell carcinoma of the lung treated with nab-paclitaxel plus carboplatin as neoadjuvant therapy: a prospective study from a single center. Med Sci Monit. 2021;27 doi: 10.12659/MSM.930738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawabata N., Keller S.M., Matsumura A., et al. The impact of residual multi-level N2 disease after induction therapy for non-small cell lung cancer. Lung Cancer. 2003;42:69–77. doi: 10.1016/s0169-5002(03)00245-9. [DOI] [PubMed] [Google Scholar]
- 13.Krantz S.B., Mitzman B., Lutfi W., et al. Neoadjuvant chemoradiation shows no survival advantage to chemotherapy alone in stage IIIA patients. Ann Thorac Surg. 2018;105:1008–1016. doi: 10.1016/j.athoracsur.2017.10.056. [DOI] [PubMed] [Google Scholar]
- 14.Kayawake H., Okumura N., Yamanashi K., et al. Non-small cell lung cancer with pathological complete response: predictive factors and surgical outcomes. Gen Thorac Cardiovasc Surg. 2019;67:773–781. doi: 10.1007/s11748-019-01076-9. [DOI] [PubMed] [Google Scholar]
- 15.Couñago F., Montemuiño S., Martin M., et al. Prognostic factors in neoadjuvant treatment followed by surgery in stage IIIA-N2 non-small cell lung cancer: a multi-institutional study by the Oncologic Group for the Study of Lung Cancer (Spanish Radiation Oncology Society) Clin Transl Oncol. 2019;21:735–744. doi: 10.1007/s12094-018-1976-3. [DOI] [PubMed] [Google Scholar]
- 16.Yokomise H., Gotoh M., Okamoto T., et al. Induction chemoradiotherapy (carboplatin-taxane and concurrent 50-Gy radiation) for bulky cN2, N3 non–small cell lung cancer. J Thorac Cardiovasc Surg. 2007;133:1179–1185. doi: 10.1016/j.jtcvs.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 17.Van Der Meij B.S., Phernambucq E.C.J., Fieten G.M., et al. Nutrition during trimodality treatment in stage III non-small cell lung cancer: not only important for underweight patients. J Thorac Oncol. 2011;6:1563–1568. doi: 10.1097/JTO.0b013e3182208e90. [DOI] [PubMed] [Google Scholar]
- 18.Coroller T.P., Agrawal V., Huynh E., et al. Radiomic-based pathological response prediction from primary tumors and lymph nodes in NSCLC. J Thorac Oncol. 2017;12:467–476. doi: 10.1016/j.jtho.2016.11.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer S., Darling G., Pierre A.F., et al. Induction chemoradiation therapy followed by surgical resection for non-small cell lung cancer (NSCLC) invading the thoracic inlet. Eur J Cardio Thorac Surg. 2008;33:1129–1134. doi: 10.1016/j.ejcts.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Haque W., Verma V., Butler E.B., Teh B.S. Pathologic nodal clearance and complete response following neoadjuvant chemoradiation for clinical N2 non-small cell lung cancer: predictors and long-term outcomes. Lung Cancer. 2019;130:93–100. doi: 10.1016/j.lungcan.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Isobe K., Hata Y., Sakaguchi S., et al. Pathological response and prognosis of stage III non-small cell lung cancer patients treated with induction chemoradiation. Asia Pac J Clin Oncol. 2012;8:260–266. doi: 10.1111/j.1743-7563.2012.01529.x. [DOI] [PubMed] [Google Scholar]
- 22.Kim H.K., Cho J.H., Choi Y.S., et al. Outcomes of neoadjuvant concurrent chemoradiotherapy followed by surgery for non-small-cell lung cancer with N2 disease. Lung Cancer. 2016;96:56–62. doi: 10.1016/j.lungcan.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Kim A.W., Liptay M.J., Bonomi P., et al. Neoadjuvant chemoradiation for clinically advanced non-small cell lung cancer: an analysis of 233 patients. Ann Thorac Surg. 2011;92:233–243. doi: 10.1016/j.athoracsur.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Lee H.Y., Lee K.S., Park J., et al. Baseline SUVmax at PET-CT in stage IIIA non-small-cell lung cancer patients undergoing surgery after neoadjuvant therapy. Acad Rad. 2012;19:440–445. doi: 10.1016/j.acra.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Lee H., Ahn Y.C., Pyo H., et al. Pretreatment clinical mediastinal nodal bulk and extent do not influence survival in N2-positive stage IIIA non-small cell lung cancer patients treated with trimodality therapy. Ann Surg Oncol. 2014;21:2083–2090. doi: 10.1245/s10434-014-3540-x. [DOI] [PubMed] [Google Scholar]
- 26.Pöttgen C., Stuschke M., Graupner B., et al. Prognostic model for long-term survival of locally advanced non-small-cell lung cancer patients after neoadjuvant radiochemotherapy and resection integrating clinical and histopathologic factors. BMC Cancer. 2015;15:363. doi: 10.1186/s12885-015-1389-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shintani Y., Funakoshi Y., Inoue M., et al. Pathological status of mediastinal lymph nodes after preoperative concurrent chemoradiotherapy determines prognosis in patients with non-small cell lung cancer. Ann Thorac Cardiovasc Surg. 2012;18:530–535. doi: 10.5761/atcs.oa.11.01811. [DOI] [PubMed] [Google Scholar]
- 28.Shiraishi T., Hiratsuka M., Yanagisawa J., et al. Pulmonary resection after chemoradiotherapy for advanced non-small cell lung cancer: the impact of presurgical radiation therapy. Surg Today. 2014;44:123–130. doi: 10.1007/s00595-013-0520-x. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka F., Yokomise H., Soejima T., et al. Induction chemoradiotherapy (50 Gy), followed by resection, for stage IIIA-N2 non-small cell lung cancer. Ann Thorac Surg. 2018;106:1018–1024. doi: 10.1016/j.athoracsur.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 30.Spaggiari L., Casiraghi M., Guarize J., et al. Outcome of patients with pN2 “potentially resectable” nonsmall cell lung cancer who underwent surgery after induction chemotherapy. Semin Thorac Cardiovasc Surg. 2016;28:593–602. doi: 10.1053/j.semtcvs.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Remark R., Lupo A., Alifano M., et al. Immune contexture and histological response after neoadjuvant chemotherapy predict clinical outcome of lung cancer patients. OncoImmunology. 2016;5 doi: 10.1080/2162402X.2016.1255394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mouillet G., Monnet E., Milleron B., et al. Pathologic complete response to preoperative chemotherapy predicts cure in early-stage non–small-cell lung cancer: combined analysis of two IFCT randomized trials. J Thorac Oncol. 2012;7:841–849. doi: 10.1097/JTO.0b013e31824c7d92. [DOI] [PubMed] [Google Scholar]
- 33.Brandt W.S., Yan W., Zhou J., et al. Outcomes after neoadjuvant or adjuvant chemotherapy for cT2-4N0-1 non–small cell lung cancer: a propensity-matched analysis. J Thorac Cardiovasc Surg. 2019;157:743–753. doi: 10.1016/j.jtcvs.2018.09.098. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Travis W.D., Dacic S., Wistuba I., et al. IASLC multidisciplinary recommendations for pathologic assessment of lung cancer resection specimens after neoadjuvant therapy. J Thorac Oncol. 2020;15:709–740. doi: 10.1016/j.jtho.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guyot P., Ades A., Ouwens M.J., Welton N.J. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012;12:9. doi: 10.1186/1471-2288-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heinze G., Schemper M. A solution to the problem of monotone likelihood in cox regression. Biometrics. 2001;57:114–119. doi: 10.1111/j.0006-341x.2001.00114.x. [DOI] [PubMed] [Google Scholar]
- 37.Rondeau V., Commenges D., Joly P. Lifetime Data Anal. 2003;9:139–153. doi: 10.1023/a:1022978802021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rondeau V., Michiels S., Liquet B., Pignon J.P. Investigating trial and treatment heterogeneity in an individual patient data meta-analysis of survival data by means of the penalized maximum likelihood approach. Stat Med. 2008;27:1894–1910. doi: 10.1002/sim.3161. [DOI] [PubMed] [Google Scholar]
- 39.Provencio M., Nadal E., Insa A., et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21:1413–1422. doi: 10.1016/S1470-2045(20)30453-8. [DOI] [PubMed] [Google Scholar]
- 40.Broglio K.R., Quintana M., Foster M., et al. Association of pathologic complete response to neoadjuvant therapy in HER2-positive breast cancer with long-term outcomes. JAMA Oncol. 2016;2:751. doi: 10.1001/jamaoncol.2015.6113. [DOI] [PubMed] [Google Scholar]
- 41.Spring L.M., Fell G., Arfe A., et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. Clin Cancer Res. 2020;26:2838–2848. doi: 10.1158/1078-0432.CCR-19-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang M., O’Shaughnessy J., Zhao J., et al. Association of pathologic complete response with long-term survival outcomes in triple-negative breast cancer: a meta-analysis. Cancer Res. 2020;80:5427–5434. doi: 10.1158/0008-5472.CAN-20-1792. [DOI] [PubMed] [Google Scholar]
- 43.Von Minckwitz G., Huang C.-S., Mano M.S., et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–628. doi: 10.1056/NEJMoa1814017. [DOI] [PubMed] [Google Scholar]
- 44.Eriksson D., Stigbrand T. Radiation-induced cell death mechanisms. Tumor Biol. 2010;31:363–372. doi: 10.1007/s13277-010-0042-8. [DOI] [PubMed] [Google Scholar]
- 45.Macchia G., Gambacorta M.A., Masciocchi C., et al. Time to surgery and pathologic complete response after neoadjuvant chemoradiation in rectal cancer: a population study on 2094 patients. Clin Transl Radiat Oncol. 2017;4:8–14. doi: 10.1016/j.ctro.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Topalian S.L., Taube J.M., Pardoll D.M. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science. 2020;367 doi: 10.1126/science.aax0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pellini B., Chaudhuri A.A. Circulating tumor DNA minimal residual disease detection of non–small-cell lung cancer treated with curative intent. J Clin Oncol. 2022;40:567–575. doi: 10.1200/JCO.21.01929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spigel D.R., Peters S., Ahn M.J., et al. 93TiP MERMAID-2: phase III study of durvalumab in patients with resected, stage II–III NSCLC who become MRD+ after curative-intent therapy. J Thorac Oncol. 2021;16:S745–S746. [Google Scholar]
- 49.Provencio M, Serna-Blasco R, Nadal E, et al. Overall survival and biomarker analysis of neoadjuvant nivolumab plus chemotherapy in operable stage IIIA non–small-cell lung cancer (NADIM phase II trial) [e-pub ahead of print]. J Clin Oncol. 10.1200/JCO.21.02660, accessed May 16, 2022. [DOI] [PMC free article] [PubMed]
- 50.Tan A.C., Emmett L., Lo S., et al. FDG-PET response and outcome from anti-PD-1 therapy in metastatic melanoma. Ann Oncol. 2018;29:2115–2120. doi: 10.1093/annonc/mdy330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.