Highlights
-
•
Bipolar II is an independent existence from bipolar I.
-
•
Shared and unique dALFF alterations were detected between the subtypes.
-
•
The dALFF changes can be the potential biomarkers for diagnostic differentiation.
Keywords: Bipolar type I, Bipolar type II, Dynamic amplitude of low-frequency fluctuation, Resting-state functional MRI
Abstract
Background
Bipolar disorder is a chronic and highly recurrent mental disorder that can be classified as bipolar type I (BD I) and bipolar type II (BD II). BD II is sometimes taken as a milder form of BD I or even doubted as an independent subtype. However, the fact that symptoms and severity differ in patients with BD I and BD II suggests different pathophysiologies and underlying neurobiological mechanisms. In this study, we aimed to explore the shared and unique functional abnormalities between subtypes.
Methods
The dynamic amplitude of low-frequency fluctuation (dALFF) was performed to compare 31 patients with BD I, 32 with BD II, and 79 healthy controls (HCs). Global dALFF was calculated using sliding-window analysis. Group differences in dALFF among the 3 groups were compared using analysis of covariance (ANCOVA), with covariates of age, sex, years of education, and mean FD, and Bonferroni correction was applied for post hoc analysis. Pearson and Spearman's correlations were conducted between clusters with significant differences and clinical features in the BD I and BD II groups, after which false error rate (FDR) was used for correction.
Results
We found a significant decrease in dALFF values in BD patients compared with HCs in the following brain regions: the bilateral-side inferior frontal gyrus (including the triangular, orbital, and opercular parts), inferior temporal gyrus, the medial part of the superior frontal gyrus, middle frontal gyrus, anterior cingulum, insula gyrus, lingual gyrus, calcarine gyrus, precuneus gyrus, cuneus gyrus, left-side precentral gyrus, postcentral gyrus, inferior parietal gyrus, superior temporal pole gyrus, middle temporal gyrus, middle occipital gyrus, superior occipital gyrus and right-side fusiform gyrus, parahippocampal gyrus, hippocampus, middle cingulum, orbital part of the medial frontal gyrus and superior frontal gyrus. Unique alterations in BD I were observed in the right-side supramarginal gyrus and postcentral gyrus. In addition, dALFF values in BD II were significantly higher than those in BD I in the right superior temporal gyrus and middle temporal gyrus. The variables of dALFF correlated with clinical characteristics differently according to the subtypes, but no correlations survived after FDR correction.
Limitations
Our study was cross-sectional. Most of our patients were on medication, and the sample was limited.
Conclusions
Our findings demonstrated neurobiological characteristics of BD subtypes, providing evidence for BD II as an independent existence, which could be the underlying explanation for the specific symptoms and/or severity and point to potential biomarkers for the differential diagnosis of bipolar subtypes.
1. Introduction
Bipolar disorder is a chronic and highly recurrent mental disorder characterized by episodes of depressed, manic, or hypomanic and mixed mood states (American Psychiatric Association, 2013), which affects 0.4–1.1 % of the population worldwide (Alonso et al., 2011). The two main subtypes are bipolar type I (BD I) and bipolar type II (BD II), which are hinged on the classification of mania or hypomania (American Psychiatric Association, 2013). However, BD II is sometimes taken as a milder form of BD I because it never experiences a full-blown mania episode and there is a general observation of less cognitive impairment compared to BD I (Bora et al., 2011). Separating BD II as an independent subtype of disease entity is arguable to some researchers due to a lack of solid evidence to support its validity and specificity as a distinct subtype (Malhi et al., 2016, Malhi et al., 2019a, Malhi et al., 2019b). However, the fact that symptomatic and biological alterations differ in patients with BD I and BD II suggests different pathophysiologies and underlying neurobiological mechanisms. Goldberg proposed a different position of antidepressant monotherapy in BD I and BD II (Goldberg, 2012), which was in accordance with different hierarchical rankings of treatment recommended by the 2018 guidelines for BD (Yatham et al., 2018), indicating the significance of differential diagnosis for precise treatment.
Previous research revealed some divergences between BD I and BD II, with respect to clinical features, course of illness, comorbidity, treatment, and gender distribution (Arnold, 2003, Baek et al., 2011, Karanti et al., 2020). Moreover, patients with BD II were found to endure more prodromal symptoms (Zhao et al., 2021) and higher mood instability for depression (Faurholt-Jepsen et al., 2019) compared to BD I. Dervic et al. reported more trait-impulsivity and lifetime aggression in BD I compared with BD II, whereas the latter had more hostility (Dervic et al., 2015). Gene research revealed that BD I is associated with schizophrenia in genetics, while BD II is more strongly associated with major depressive disorder (Mullins et al., 2021, Stahl et al., 2019). Research on mitochondrial DNA copy number also reported a significantly lower copy number in BD I than in BD II (Chung et al., 2020). A decrease in serotonin transporter binding in BD I compared with BD II was also observed in a previous study (Chou et al., 2010).
Functional magnetic resonance imaging (fMRI), as a noninvasive technique, is the mainstay of neuroimaging in the study of the brain and has been extensively applied in various studies to reveal the potential differences between BD I and BD II. Research on cortical thickness and cognitive function reported different correlations between groups (Abé et al., 2018). Fractional anisotropy (FA) differs between BD I and BD II (Foley et al., 2018), and fiber impairments appear in different regions according to subtypes, in which fiber alteration is related to cognitive dysfunction in BD I, whereas that in BD II is associated with both cognitive and emotional processing (Liu et al., 2010). Maller et al. reported volumetric, thickness, and white matter integrity differences between subtypes and indicated that volumetric reduction may underlie the pathophysiology of BD I, while alteration of white matter integrity is more associated with BD II (Maller et al., 2014). Decreased cortical thickness and volume in temporal and medial prefrontal areas as unique alterations of BD I were also observed (Abé et al., 2016). However, other studies have indicated that structural alterations are more severe in BD II (Ambrosi et al., 2016, Woo et al., 2021).
Most direct comparisons of previous studies between BD I and BD II are mainly based on structural analysis, and functional methods are conducted for different subtypes individually or combined indiscriminately. BD I was observed with abnormal functional connectivity (FC) within and between networks, and the FC value is correlated with symptoms and executive function (Zhu et al., 2021). Significant cerebellar dysconnectivity in BD II was reported in previous research. A topological study disclosed an alteration in functional connectivity strength (Wang et al., 2018) and regional connectivity (Wang et al., 2017) in BD II. Many studies reported functional abnormalities in the frontal, temporal, occipital, and subcortical (insular, striatum, caudate, putamen, and anterior cingulum) regions in BD (Gong et al., 2020b, Xu et al., 2014, Zhang et al., 2021b) but failed to specifically discriminate subtypes.
These conventional indicators are temporally stationary, of which linear dependence measures are computed over the entire scan. However, unlike sleeping, the participants enduring resting-state fMRI scanning are on stand-by, in which the patterns of brain activity are distinguishable from those in sleep or during goal-directed activity (Deco et al., 2014, Mennes et al., 2011). The sliding-window technique is constantly sensitive to functional activation changes during the entire scan (Gembris et al., 2000), so it can precisely depict the dynamic features of brain activity according to time. Recently, this method has been widely applied to research on BD (Du et al., 2021, Shunkai et al., 2022, Tian et al., 2021, Wang et al., 2022), but is not used to distinguish BD I from BD II, and most methods are based on dynamic functional connectivity. Unlike FC, the amplitude of low-frequency fluctuation (ALFF) is an indicator of regional spontaneous neuronal activity, which can be implicated in underlying pathophysiology in a specific region (Zang et al., 2007).
Considering clinical distinguishing of the subtypes is confusing and subjective while in the depressive state, and it can be beneficial for the patients to be diagnosed more precisely under this state, we, therefore, aimed to investigate shared and unique diagnosis-related functional abnormalities between BD I and BD II using dynamic ALFF (dALFF) in the depressive state. We hypothesized that BD II is an independent subtype and can be explicitly distinguished from BD I.
2. Methods and materials
2.1. Subjects
The regional alteration across the diagnosis is the primary variable for sample calculation. The effect size of resting state fMRI on BD I and BD II was seldom reported, according to a meta-analysis on structural imaging of BD, the effect size was 0.39(Kempton et al., 2008). As such, a total sample of 69 was calculated to detect the group differences with α = 0.05 and a power of 0.8 (β = 0.2).
We recruited a total sample of 163 participants, and 21 were excluded for quality control, of which 1 was due to pituitary tumor, and the other 20 were out of head motion or artifact. Thus, 142 participants (BD I: 31; BD II: 32; and HC: 79) were included in the final analysis. All BD patients were recruited from the Department of Psychiatry of the First Hospital of China Medical University and Shenyang Mental Health Center. All the patients were previously diagnosed by experienced psychiatrists working for at least 10 years with standard clinical interviews and were in a depressive episode at the scan. Healthy controls were recruited via advertisements from the local area, with no personal or family history of mental illness. For diagnosis and suitability for inclusion, well-trained researchers further interviewed participants ≥ 18 years with the Structured Clinical Interview for DSM-V (SCID) (Phillips, 2020) and participants < 18 years with the Schedule for Affective Disorders and Schizophrenia for School-Age Children. For all participants, the Young Mania Rating Scale (YMRS) (Young et al., 1978), 17-item Hamilton Depression Rating Scale (HAMD-17) (Hamilton, 1960), Hamilton Anxiety Rating Scale (HAMA) (Hamilton, 1959), and Wisconsin Card Sorting Test (WCST) (Grant and Berg, 1948) were performed at the time of scanning. However, not all participants finished the WCST coordinately; only 26 BD I, 28 BD II, and 57 HCs completed the full test.
The inclusion criteria were as follows: (a) aged between 13 and 55 years old; (b) with no neurological diseases and no history of head trauma with consciousness disturbances; (c) with no major medical conditions or medications that may affect mental health; and (d) with no contraindications for MRI scanning.
Written informed consent was obtained from all participants. The study was approved by the Ethics Committee of the First Hospital of China Medical University.
2.2. MRI data acquisition
MRI scans for all participants were performed on the same GE Signa HD 3.0 T MRI scanner with a standard 8-channel head coil at the First Hospital of China Medical University, Shenyang, China. Resting state fMRI scanning was with a spin echo-planar imaging (EPI) sequence (repetition time (TR) / echo time (TE) = 2000 / 40 ms, flip angle 90°, field of view (FOV) = 24 cm × 24 cm, acquisition matrix = 64 × 64, slice thickness = 3 mm, spacing between slices = 3 mm, slices = 35, and scan time = 400 s). Participants were provided with foam pads and earplugs to minimize noise and were required to close their eyes and stay awake during the scan.
2.3. MRI data preprocessing
DPABI (Yan et al., 2016) was used for imaging data preprocessing. The first 10 images of scanned data were removed due to the instability of the initial signal. The remaining images were then adjusted using slice-timing correction and realignment for head movement correction. Participants with head motion ≥ 3 mm or rotation ≥ 3° in each direction were excluded. We calculated the mean framewise displacement (FD) according to Power (Power et al., 2012). In addition, the mean FD was analyzed as a covariate to minimize the effect of head motion. The functional image was normalized into a standard EPI template of Montreal Neurological Institute (MNI) space, resampled to 3 × 3 × 3 mm3, and smoothed with a 4-mm full width at half-maximum (FWHM) Gaussian kernel. Regression out of the nuisance covariates from the functional signal was performed, including 24 head motion parameters (Friston et al., 1996), white matter signal, cerebrospinal fluid signal, and linear trend. The global signal was not regressed to avoid the introduction of anticorrelations (Murphy et al., 2009) in our main results, while the regressed results were reported in Supplementary (Table 4, and Fig. 1).
Fig. 1.
Significant differences in dALFF across BD I, BD II and HCs. The significance of the voxel-level threshold was set at p < 0.001 with Gaussian random field (GRF) theory for correction, and the cluster-level threshold was set to p < 0.05.
2.4. Dynamic ALFF acquisition
DPABI 4.1 was used to compute the dALFF values. For dynamic analysis, we selected a window length of 100 s (50TRs) for computation of temporal variability of ALFF according to Leonardi (Leonardi and Van De Ville, 2015), which should not be too long or too short to avoid spurious signals. Each time-course had 190 samples (time-points), and the window step size was set as 1 TR (2 s), which resulted in 141 (190–50 + 1) (Sakoğlu et al., 2010) windows for each participant. We also calculated the ALFF values for each sliding window. The time series were transformed to a frequency domain with a fast Fourier transform (FFT) for each given voxel, and then we obtained the ALFF value by computing and summing the square root of the power spectrum across 0.01–0.08 Hz (Zang et al., 2007). Then, the standard deviation (SD) of ALFF values (dALFF) at each voxel across all 141 windows was computed to quantify the temporal dynamic characteristics of ALFF. In addition, the corrected dALFF (dALFF / global mean dALFF) of each voxel within a group mask was used to reduce the global effects of temporal variability.
2.5. Statistical analysis
SPSS v 26.0 (SPSS, Chicago, IL, USA) was used for statistical analyses. Demographic and clinical variables as well as cognitive tests and head motion parameters were analyzed by analysis of variance (ANOVA) or χ2 tests. For all comparisons (Bonferroni corrected for post hoc analysis), p < 0.05 was set as significant. We employed DAPBI v 4.1 to assess group differences in dALFF among the 3 groups with analysis of covariance (ANCOVA), with age, sex, years of education and mean FD as covariates. The statistical significance of the voxel-level threshold was set at p < 0.001 with Gaussian random field (GRF) theory for correction, and the cluster-level threshold was set to p < 0.05. We then performed Bonferroni’s post-hoc test to compare dALFF values extracted from clusters of regions with significant differences, with a significance level set at p < 0.05. Independent sample t-tests were conducted to compare patients with and without medication to correct for the use of medication, with a significance level of p < 0.05 (2 tails). To avoid the confounding impact of clinical characteristics, ANCOVA (HAMD-17, HAMA, YMRS, and WCST score as covariates) was carried out to confirm the results. G*Power 3 (Faul et al., 2007) was applied for sample size calculation. We computed partial η2 for the effect size of dALFF values among the 3 groups (Lakens, 2013). Pearson and Spearman's correlations were conducted between clusters with significant differences and clinical characteristics (HAMD-17, HAMA, YMRS, WCST scores, and medications) in the BD I and BD II groups, after which false error rate (FDR) was used for correction.
3. Results
3.1. Clinical and demographic features
There were no significant differences in age, sex, years of education, handedness, mean FD values, treatment status, WCST correct response score, hypnotics, or antidepressants taken across groups (p > 0.05), but a trend of HC > BD I > BD II in WCST correct response score was observed. However, the HAMD-17, HAMA, YMRS scores and medication, mood stabilizer, and antipsychotic treatment status were significantly different (p < 0.05, Table 1). No significant differences were observed between BD I and BD II in the HAMD-17, HAMA, and YMRS scores after post hoc analysis (Bonferroni correction). Regarding medication, participants with BD I were more likely to take mood stabilizers and antipsychotics than those with BD II.
Table 1.
Demographic and clinical characteristics of patients with BD I, BD II and HCs.
| BD I (n = 31) | BD II (n = 32) | HC (n = 79) | F / χ2 | p | |
|---|---|---|---|---|---|
| Demographic features | |||||
| Age at scan, years | 25.90 (8.91) | 23.91 (5.63) | 27.18 (6.97) | 2.40a | 0.095 |
| Education, years | 13.74 (3.01) | 13.28 (3.29) | 14.35 (2.24) | 1.98a | 0.142 |
| Male | 7 (22.58 %) | 14 (43.75 %) | 37 (46.83 %) | 5.57a | 0.062 |
| Right handedness | 28 (90.3 %) | 32 (100 %) | 74 (93.7) | 2.99a | 0.560 |
| Clinical features | |||||
| First episode, yes | 12 (38.71 %) | 15 (46.88 %) | N/A | 0.43b | 0.513 |
| Medication, yes | 27 (87.10 %) | 17 (53.13 %) | N/A | 8.62b | 0.003 |
| Mood Stabilizer | 19 (61.29 %) | 8 (25.00 %) | N/A | 8.46b | 0.004 |
| Antidepressants | 18 (58.06 %) | 14 (43.75 %) | N/A | 1.29b | 0.256 |
| Antipsychotics | 18 (58.06 %) | 5 (15.63 %) | N/A | 12.24b | <0.001 |
| Hypnotics | 2 (6.45 %) | 4 (12.5) | N/A | 0.67b | 0.414 |
| HAMD-17 | 17.13 (7.73) | 15.72 (6.48) | 1.49 (2.66) | 149.48a | <0.001 |
| HAMA | 14.55 (8.77) | 13.38 (7.34) | 1.05 (2.37) | 91.16a | <0.001 |
| YMRS | 1.29 (1.64) | 1.16 (1.82) | 0.20 (0.98) | 9.92a | <0.001 |
| Cognitive function | |||||
| WCST corrected response | 29.85 (10.87) | 31.64 (10.88) | 34.30 (9.87) | 1.80a | 0.170 |
| Head motion parameters | |||||
| Mean FD | 0.12 (0.07) | 0.11 (0.04) | 0.11 (0.05) | 0.35a | 0.705 |
Note: Data are presented as either numbers (%) or means (standard deviations). ANOVA was conducted for continuous variables and chi-square test was conducted for categorical variables.
Abbreviations: BD I: bipolar disorder type I; BD II: bipolar disorder type II; HCs: healthy control; N/A: not applicable; FD: framewise displacement; YMRS: Young Mania Rating Scale, HAMD-17: 17-item Hamilton Depression Rating Scale, HAMA: Hamilton Anxiety Rating Scale, WCST: Wisconsin Card Sorting Test.
a: Examinations among BD I, BD II and HCs.
b: Examinations between BD I and BD II.
3.2. Dynamic ALFF variability differences among BD I, BD II and HCs
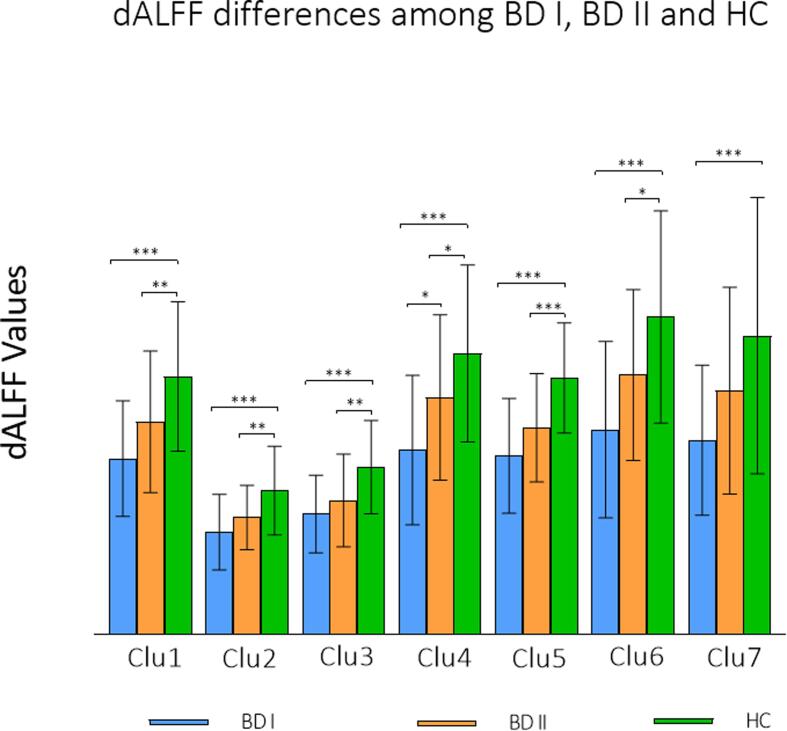
Seven clusters with significant differences across the 3 groups were found using ANCOVA. Post hoc analyses revealed that both the BD I and BD II groups exhibited a significant decrease in dALFF values compared with HCs in the following brain regions: the bilateral-side inferior frontal gyrus (including triangular, orbital, and opercular part), inferior temporal gyrus, the medial part of superior frontal gyrus, middle frontal gyrus, anterior cingulum, insula gyrus, lingual gyrus, calcarine gyrus, precuneus gyrus, cuneus gyrus, and the left-side precentral gyrus, postcentral gyrus, inferior parietal gyrus, superior temporal pole gyrus, middle temporal gyrus, middle occipital gyrus, superior occipital gyrus, and the right-side fusiform gyrus, parahippocampal gyrus, hippocampus, middle cingulum, orbital part of medial frontal gyrus and superior frontal gyrus. The dALFF variables were also found to be significantly higher in HCs than in BD I in the right supramarginal gyrus and postcentral gyrus, but no significant differences were found between BD II and HCs. Dynamic ALFF values in BD II were significantly higher than those in BD I in the right superior temporal gyrus and middle temporal gyrus, which revealed a graded change as HC > BD II > BD I (Fig. 1, Fig. 2, Table 2). To correct for the use of medication, dALFF values between medication users and nonusers in 7 clusters were compared, and no significant difference was found (Supplementary Table 1–3).
Fig. 2.
Group differences in dALFF values across BD I, BD II and HCs. Significant differences were set at p < 0.05, Bonferroni for post hoc. Clu1: left inferior frontal gyrus (triangular part), left inferior frontal gyrus (opercular part), left postcentral gyrus, left inferior frontal gyrus (orbital part), left precentral gyrus, left inferior parietal gyrus, left insula, left superior temporal pole gyrus; Clu2: right inferior temporal gyrus, right fusiform gyrus, right parahippocampal gyrus, right hippocampus; Clu3: right inferior frontal gyrus (orbital part), right insula; Clu4: right superior temporal gyrus, right middle temporal gyrus; Clu5: bilateral superior frontal gyrus (medial part), bilateral middle frontal gyrus, bilateral anterior cingulum, right inferior frontal gyrus (triangular part), right superior frontal gyrus, right middle cingulum, right inferior frontal gyrus (orbital part), right medial frontal gyrus (orbital part); Clu6: bilateral lingual gyrus, bilateral calcarine gyrus, left middle temporal gyrus, left middle occipital gyrus, bilateral cuneus gyrus, left superior occipital gyrus, bilateral precuneus gyrus, left inferior temporal gyrus; Clu7: right postcentral gyrus and right supramarginal gyrus. Note: 0.01 < p < 0.05 (*), 0.001 < p < 0.01 (**), p < 0.001 (***).
Table 2.
Dynamic ALFF differences across BD I, BD II and HCs.
| Clusters | Brain regions voxels) | Hemisphere | Peak MNI coordinates | Post-hoc analysis (Bonforroni, p < 0.05) | Fvalues | p | Partial η2 | ||
|---|---|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||||
| 1 | Inferior triangular frontal gyrus (1 5 0) | L | −54 | 12 | 21 | BD I < HC, BD II < HC | 16.340 | < 0.001 | 0.190 |
| Inferior opercular frontal gyrus (1 0 4) | |||||||||
| Postcentral gyrus (97) | |||||||||
| Inferior orbital frontal gyrus (82) | |||||||||
| Precentral gyrus (78) | |||||||||
| Inferior parietal gyrus (75) | |||||||||
| Insula (74) | |||||||||
| Superior temporal pole gyrus (56) | |||||||||
| 2 | Inferior temporal gyrus (55) | R | 27 | −39 | −6 | BD I < HC, BD II < HC | 13.466 | < 0.001 | 0.162 |
| Fusiform gyrus (45) | |||||||||
| ParaHippocampal gyrus (43) | |||||||||
| Hippocampus (38) | |||||||||
| 3 | Inferior orbital frontal gyrus (69) | R | 30 | 18 | −21 | BD I < HC, BD II < HC | 14.580 | < 0.001 | 0.173 |
| Insula (54) | |||||||||
| 4 | Superior temporal gyrus (2 0 9) | R | 60 | −24 | 12 | BD I < HC, BD II < HC, BD I < BD II | 15.063 | < 0.001 | 0.178 |
| Middle temporal gyrus (1 0 1) | |||||||||
| 5 | Medial superior frontal gyrus (2 0 7) | L | 6 | 18 | 27 | BD I < HC, BD II < HC | 25.086 | < 0.001 | 0.265 |
| Middle frontal gyrus (1 3 8) | |||||||||
| Anterior cingulum (93) | |||||||||
| Middle frontal gyrus (1 3 0) | R | ||||||||
| Inferior triangular frontal gyrus (1 6 3) | |||||||||
| Medial superior frontal gyrus (80) | |||||||||
| Anterior cingulum (77) | |||||||||
| Superior frontal gyrus (73) | |||||||||
| Middle cingulum (66) | |||||||||
| Inferior opercular frontal gyrus (58) | |||||||||
| Medial orbital frontal gyrus (51) | |||||||||
| 6 | Lingual gyrus (2 0 7) | L | 21 | −93 | 21 | BD I < HC, BD II < HC | 15.468 | < 0.001 | 0.182 |
| Calcarine gyrus (1 5 8) | |||||||||
| Middle temporal gyrus (1 4 9) | |||||||||
| Middle occipital gyrus (1 3 8) | |||||||||
| Cuneus gyrus (1 1 2) | |||||||||
| Superior occipital gyrus (1 0 7) | |||||||||
| Precuneus gyrus (95) | |||||||||
| Inferior temporal gyrus (76) | |||||||||
| Lingual gyrus (1 4 5) | R | ||||||||
| Calcarine gyrus (1 0 1) | |||||||||
| Cuneus gyrus (95) | |||||||||
| Precuneus gyrus (62) | |||||||||
| 7 | Postcentral gyrus (90) | R | 60 | −12 | 7 | BD I < HC | 9.088 | < 0.001 | 0.116 |
| SupraMarginal gyrus (36) | |||||||||
Note: ALFF: amplitude of low-frequency fluctuations; BD I: bipolar disorder type I; BD II: bipolar disorder type II; HCs: healthy control; MNI: Montreal Neurological Institute; R: right; L: left.
To avoid the confounding impact of clinical characteristics, ANCOVA (HAMD-17, HAMA, YMRS, WCST scores as covariates) was carried out, and the results were consistent.
3.3. Correlations between dALFF and clinical characteristics
Group-based differences in correlations of clinical characteristics and dALFF were observed in our research. However, no significant correlations survived after FDR correction (Supplementary Table 4).
4. Discussion
To the best of our knowledge, this is the first study using dALFF to distinguish BD I from BD II. Through this study, we revealed some shared temporal variables of ALFF in both BD I and BD II compared with HCs, but BD II was found to be closer to HCs than to BD I. Additionally, we also found unique changes in dALFF in BD I and some specific modifications to distinguish BD I from BD II. Correlations between dALFF values and clinical variables in different subtypes were observed as well, but failed to survive after FDR correlation.
In our study, both BD I and BD II showed a significant decrease in dALFF values encompassing the frontal, parietal, occipital, and temporal cortices and limbic system, which underlies the shared alterations of BD. Similarly, functional and structural abnormalities in BD were also reported in previous studies (Nabulsi et al., 2020, Strakowski et al., 2005, Zhong et al., 2019). Intrinsic alterations of ALFF in BD were proposed in a meta-analysis in the bilateral inferior frontal gyrus (involving the orbital part), bilateral insula, right superior frontal gyrus (including the prefrontal part), occipital cortex, right fusiform and bilateral precuneus (Gong et al., 2020b). Multiscale entropy (MSE) was introduced to quantify the complexity of physiologic time series (Costa et al., 2002), which was applied in analysis across different time scales in BD, and found that BD patients exhibited variations of complexity in the calcarine, precuneus, lingual gyrus, hippocampus, middle temporal and middle frontal gyrus (Zhang et al., 2021a). Thompson et al. also indicated that BD was related to decreased cortical thickness in the bilateral temporal, parietal and frontal regions, especially in the left fusiform gyrus and rostral middle frontal cortex (Thompson et al., 2020). Spatiotemporal consistency alterations were also observed in pediatric BD patients in the left triangular inferior frontal gyrus, left precentral gyrus, right postcentral gyrus, and right postcentral gyrus (Gao et al., 2021). Modifications of brain function and structure were also discovered in various studies (Lv et al., 2016, Malhi et al., 2007, Qiu et al., 2018, Wang et al., 2008, Yang et al., 2021, Zuliani et al., 2009), including the left opercular and triangular parts of the inferior frontal gyrus, left temporal pole, left postcentral gyrus, left inferior parietal gyrus, bilateral anterior cingulum, bilateral inferior temporal gyrus, and right parahippocampal gyrus. Together with our study, the extensive shared alterations suggested a congruously abnormal pattern of activity in both BD I and BD II.
Decreased temporal variations in the right postcentral and supramarginal gyrus were specific to BD I in our research. Among the limited direct comparison of BD I and BD II, we failed to find similar results in resting-state fMRI studies, which implies our results as a novel finding to the distinctive alterations in BD I. Unique modifications in BD I were reported in the bilateral uncinate fasciculus in task-state fMRI research (Foley et al., 2018). In addition, research on offspring of the subtypes and neuroinflammation also confirmed our findings. Offspring of BD I were reported to have a thinner cortex in the right supramarginal gyrus (Hanford et al., 2016), and a higher level of neuroinflammation was observed to be related to a lower volume in the right supramarginal gyrus in BD I (Tsai et al., 2021). Simonetti et al. proposed reduced thickness in the right supramarginal gyrus and a correlation with the subscales (anger and hostility) of aggression questionnaire in pediatric BD (Simonetti et al., 2021), the type of which was not divided, although majorities were BD I. Therefore, this may explain why BD I has a higher impulsivity rate than BD II (Izci et al., 2016). Diminished functional connectivity between the postcentral gyrus and other brain regions in BD I have been repeatedly reported in previous research (Liu et al., 2021, Zhang et al., 2020). The primary somatosensory cortex is located on the postcentral gyrus (Kaas et al., 1979), which is essential to motor function, empathy, and emotion regulation (Kropf et al., 2019, Morrison et al., 2013). Cognitive empathy deficits appear in both manic and depressive patients. However, indices of affective empathy are much higher in manic episodes and positively correlated with manic symptoms, which may be related to disturbances in emotion inhibition and extreme bouts of expansive and persistent positive feelings (Bodnar and Rybakowski, 2017, Gruber, 2011). As a result, we think the functional alterations of the postcentral gyrus may be the underlying interpretation for affective dysregulation and misbehaviors in BD I, especially in mania episodes.
We found a significant decrease in dALFF values in the right superior and middle temporal gyrus in BD I compared with BD II, which may indicate potential differential diagnostic neuroimaging biomarkers for BD subtyping. In accordance with our findings, dALFF abnormalities in the right superior temporal gyrus were also observed in the limited research on dALFF in BD (Gong et al., 2020a). Significantly lower metabolite levels (Atagün et al., 2018) and white matter abnormalities (Ha et al., 2011) in temporal cortices in BD I also infer a critical role of temporal cortices in distinguishing the subtypes. The right superior temporal area is mandatory for audiomotor and audiovisual speech integration (Komeilipoor et al., 2017). Our finding of dALFF alterations in this region may underlie BD I exhibiting decreased temporal activations than BD II in cerebral attentional function during external emotional stimuli (Zhang et al., 2019). Middle temporal cortices are involved in the neural circuitry in the escalation of anger and aggression (Potegal, 2012), and impulsivity scores were also found to be positively correlated with the local gyrification index (an index representing cortical folding) in the right temporal cortex (Hirjak et al., 2017). As a result, our observation of functional variations in this area may explain why BD I scores were higher on impulsivity than BD II (Xu et al., 2015), which is similar to previous research on juvenile violent offenders, where decreased functional connectivity in the right middle temporal was found compared with HCs (Sun et al., 2021).
Diagnosis-based clinical characteristics were observed to correlate with dALFF in our research. However, no significant correlations survived after FDR correction, which may be due to our small sample size. Different associations between executive functions and structural alterations in BD I and BD II were reported previously (Abé et al., 2018). Therefore, larger-sample studies are expected to provide more reliable and replicable evidence in the future (Marek et al., 2022).
Our findings demonstrated distinct neural mechanisms of BD I and BD II respectively, providing a neuroimaging foundation for both diagnosis and varying treatment of different subtypes. Only half of antidepressant-related mood elevation was observed in BD II than in BD I in both acute and maintenance stages (Bond et al., 2008), supporting the previous viewpoint of potential benefits of monotherapy of antidepressants to BD II (Goldberg, 2012). Considering the higher rate of depression and greater frequency of suicide in BD II (Karanti et al., 2020), early diagnosis of the subtypes and prompt antidepressant treatment can be essential for the clinic.
5. Limitation
There are some limitations to be considered. First, it was a cross-sectional study, so it failed to determine the progressive abnormalities during the course of illness. All BD patients were in a depressive state, and whether our results are applicable to euthymic or even premorbid states remains to be explored in future research. A longitudinal study with repeated scans is necessary to answer this question. Second, most participants were on medication, although the medication usage was matched between BD I and BD II, and no meaningful effects of medication status on our findings (see Supplementary Table 1–3). It is still unclear whether dALFF variations are of different nature in unmedicated patients. Finally, even though the sample size was adequate, a larger sample replication of our results is necessary. A larger sample size study with no medication usage is expected in the future.
6. Conclusion
In conclusion, we demonstrated neurobiological characteristics of BD I and BD II in dynamic intrinsic brain activities, showing some shared and distinct alterations, and providing evidence for BD II as an independent existence. Our findings could be the underlying explanation for the specific symptoms and/or severity and serve as potential biomarkers for the differential diagnosis of bipolar subtypes.
Funding
This work was supported by the National Key R&D Program of China (Grant #2018YFC1311600 and 2016YFC1306900 to Yanqing Tang), Liaoning Revitalization Talents Program (Grant #XLYC1808036 to Yanqing Tang), Natural Science Foundation of Liaoning Province (2020-MS-176 to Xiaowei Jiang), National Key R&D Program “Science and Technology Winter Olympics” (2021YFF0306503 to Feng Wu), Joint Fund of National Natural Science Foundation of China (U1808204 to Feng Wu), Natural Science Foundation of Liaoning Province (2019-MS-05 to Feng Wu).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.103184.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The authors are unable or have chosen not to specify which data has been used.
References
- Abé C., Ekman C.J., Sellgren C., Petrovic P., Ingvar M., Landén M. Cortical thickness, volume and surface area in patients with bipolar disorder types I and II. J. Psychiatry Neurosci. 2016;41(4):240–250. doi: 10.1503/jpn.150093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abé C., Rolstad S., Petrovic P., Ekman C.J., Sparding T., Ingvar M., et al. Bipolar disorder type I and II show distinct relationships between cortical thickness and executive function. Acta Psychiatr. Scand. 2018;138(4):325–335. doi: 10.1111/acps.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso J., Petukhova M., Vilagut G., Chatterji S., Heeringa S., Üstün T.B., et al. Days out of role due to common physical and mental conditions: results from the WHO World Mental Health surveys. Mol. Psychiatry. 2011;16(12):1234–1246. doi: 10.1038/mp.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosi E., Chiapponi C., Sani G., Manfredi G., Piras F., Caltagirone C., et al. White matter microstructural characteristics in Bipolar I and Bipolar II Disorder: a diffusion tensor imaging study. J. Affect. Disord. 2016;189:176–183. doi: 10.1016/j.jad.2015.09.035. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association., 2013. American Psychiatric Association: Diagnostic and Statistical Manuel of Mental Disorders, 5th ed. In: Washington, DC.
- Arnold L.M. Gender differences in bipolar disorder. Psychiatr. Clin. N. Am. 2003;26(3):595–620. doi: 10.1016/s0193-953x(03)00036-4. [DOI] [PubMed] [Google Scholar]
- Atagün M., Şıkoğlu E.M., Can S.S., Uğurlu G.K., Kaymak S.U., Çayköylü A., et al. Neurochemical differences between bipolar disorder type I and II in superior temporal cortices: a proton magnetic resonance spectroscopy study. J. Affect. Disord. 2018;235:15–19. doi: 10.1016/j.jad.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek J.H., Park D.Y., Choi J., Kim J.S., Choi J.S., Ha K., et al. Differences between bipolar I and bipolar II disorders in clinical features, comorbidity, and family history. J. Affect. Disord. 2011;131(1–3):59–67. doi: 10.1016/j.jad.2010.11.020. [DOI] [PubMed] [Google Scholar]
- Bodnar A., Rybakowski J.K. Increased affective empathy in bipolar patients during a manic episode. Braz. J. Psychiatry. 2017;39(4):342–345. doi: 10.1590/1516-4446-2016-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond D.J., Noronha M.M., Kauer-Sant'Anna M., Lam R.W., Yatham L.N. Antidepressant-associated mood elevations in bipolar II disorder compared with bipolar I disorder and major depressive disorder: a systematic review and meta-analysis. J. Clin. Psychiatry. 2008;69(10):1589–1601. doi: 10.4088/jcp.v69n1009. [DOI] [PubMed] [Google Scholar]
- Bora E., Yücel M., Pantelis C., Berk M. Meta-analytic review of neurocognition in bipolar II disorder. Acta Psychiatr. Scand. 2011;123(3):165–174. doi: 10.1111/j.1600-0447.2010.01638.x. [DOI] [PubMed] [Google Scholar]
- Chou Y.H., Wang S.J., Lin C.L., Mao W.C., Lee S.M., Liao M.H. Decreased brain serotonin transporter binding in the euthymic state of bipolar I but not bipolar II disorder: a SPECT study. Bipolar Disord. 2010;12(3):312–318. doi: 10.1111/j.1399-5618.2010.00800.x. [DOI] [PubMed] [Google Scholar]
- Chung J.K., Ahn Y.M., Kim S.A., Joo E.J. Differences in mitochondrial DNA copy number between patients with bipolar I and II disorders. J. Psychiatr. Res. 2020 doi: 10.1016/j.jpsychires.2020.11.016. [DOI] [PubMed] [Google Scholar]
- Costa M., Goldberger A.L., Peng C.K. Multiscale entropy analysis of complex physiologic time series. Phys. Rev. Lett. 2002;89(6) doi: 10.1103/PhysRevLett.89.068102. [DOI] [PubMed] [Google Scholar]
- Deco G., Hagmann P., Hudetz A.G., Tononi G. Modeling resting-state functional networks when the cortex falls asleep: local and global changes. Cereb. Cortex. 2014;24(12):3180–3194. doi: 10.1093/cercor/bht176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dervic K., Garcia-Amador M., Sudol K., Freed P., Brent D.A., Mann J.J., et al. Bipolar I and II versus unipolar depression: clinical differences and impulsivity/aggression traits. Eur. Psychiatry. 2015;30(1):106–113. doi: 10.1016/j.eurpsy.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Du M., Zhang L., Li L., Ji E., Han X., Huang G., et al. Abnormal transitions of dynamic functional connectivity states in bipolar disorder: a whole-brain resting-state fMRI study. J. Affect. Disord. 2021;289:7–15. doi: 10.1016/j.jad.2021.04.005. [DOI] [PubMed] [Google Scholar]
- Faul F., Erdfelder E., Lang A.G., Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Faurholt-Jepsen M., Frost M., Busk J., Christensen E.M., Bardram J.E., Vinberg M., et al. Differences in mood instability in patients with bipolar disorder type I and II: a smartphone-based study. Int. J. Bipolar. Disord. 2019;7(1):5. doi: 10.1186/s40345-019-0141-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley S.F., Bracher-Smith M., Tansey K.E., Harrison J.R., Parker G.D., Caseras X. Fractional anisotropy of the uncinate fasciculus and cingulum in bipolar disorder type I, type II, unaffected siblings and healthy controls. Br. J. Psychiatry. 2018;213(3):548–554. doi: 10.1192/bjp.2018.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Williams S., Howard R., Frackowiak R.S., Turner R. Movement-related effects in fMRI time-series. Magn. Reson. Med. 1996;35(3):346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Gao W., Cui D., Jiao Q., Su L., Lu G., Yang R. Altered spatiotemporal consistency in pediatric bipolar disorder patients with and without psychotic symptoms. BMC Psychiatry. 2021;21(1):506. doi: 10.1186/s12888-021-03524-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gembris D., Taylor J.G., Schor S., Frings W., Suter D., Posse S. Functional magnetic resonance imaging in real time (FIRE): sliding-window correlation analysis and reference-vector optimization. Magn. Reson. Med. 2000;43(2):259–268. doi: 10.1002/(sici)1522-2594(200002)43:2<259::aid-mrm13>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Goldberg J.F. In: Bipolar II Disorder: Modeling, Measuring and Managing. 2nd ed. Parker G., editor. Cambridge University Press; Cambridge/New York: 2012. The role of antidepressants in managing bipolar II disorder; pp. 100–113. [Google Scholar]
- Gong J., Chen G., Zhou M., Jia Y., Zhong S., Chen F., et al. Characteristics of temporal dynamics of intrinsic brain activity in unmedicated bipolar disorder with suicidality. Aust. N. Z. J. Psychiatry. 2020;54(11):1115–1124. doi: 10.1177/0004867420948960. [DOI] [PubMed] [Google Scholar]
- Gong J., Wang J., Qiu S., Chen P., Luo Z., Wang J., et al. Common and distinct patterns of intrinsic brain activity alterations in major depression and bipolar disorder: voxel-based meta-analysis. Transl. Psychiatry. 2020;10(1):353. doi: 10.1038/s41398-020-01036-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant D.A., Berg E.A. A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. J. Exp. Psychol. 1948;38(4):404–411. doi: 10.1037/h0059831. [DOI] [PubMed] [Google Scholar]
- Gruber J. A review and synthesis of positive emotion and reward disturbance in bipolar disorder. Clin. Psychol. Psychother. 2011;18(5):356–365. doi: 10.1002/cpp.776. [DOI] [PubMed] [Google Scholar]
- Ha T.H., Her J.Y., Kim J.H., Chang J.S., Cho H.S., Ha K. Similarities and differences of white matter connectivity and water diffusivity in bipolar I and II disorder. Neurosci. Lett. 2011;505(2):150–154. doi: 10.1016/j.neulet.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Hamilton M. The assessment of anxiety states by rating. Br. J. Med. Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23(1):56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanford L.C., Sassi R.B., Minuzzi L., Hall G.B. Cortical thickness in symptomatic and asymptomatic bipolar offspring. Psychiatry Res. Neuroimag. 2016;251:26–33. doi: 10.1016/j.pscychresns.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Hirjak D., Thomann A.K., Kubera K.M., Wolf R.C., Jeung H., Maier-Hein K.H., et al. Cortical folding patterns are associated with impulsivity in healthy young adults. Brain Imaging Behav. 2017;11(6):1592–1603. doi: 10.1007/s11682-016-9618-2. [DOI] [PubMed] [Google Scholar]
- Izci F., Fındıklı E.K., Zincir S., Zincir S.B., Koc M.I. The differences in temperament-character traits, suicide attempts, impulsivity, and functionality levels of patients with bipolar disorder I and II. Neuropsychiatr. Dis. Treat. 2016;12:177–184. doi: 10.2147/ndt.S90596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas J.H., Nelson R.J., Sur M., Lin C.S., Merzenich M.M. Multiple representations of the body within the primary somatosensory cortex of primates. Science. 1979;204(4392):521–523. doi: 10.1126/science.107591. [DOI] [PubMed] [Google Scholar]
- Karanti A., Kardell M., Joas E., Runeson B., Pålsson E., Landén M. Characteristics of bipolar I and II disorder: a study of 8766 individuals. Bipolar Disord. 2020;22(4):392–400. doi: 10.1111/bdi.12867. [DOI] [PubMed] [Google Scholar]
- Kempton M.J., Geddes J.R., Ettinger U., Williams S.C., Grasby P.M. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch. Gen. Psychiatry. 2008;65(9):1017–1032. doi: 10.1001/archpsyc.65.9.1017. [DOI] [PubMed] [Google Scholar]
- Komeilipoor N., Cesari P., Daffertshofer A. Involvement of superior temporal areas in audiovisual and audiomotor speech integration. Neuroscience. 2017;343:276–283. doi: 10.1016/j.neuroscience.2016.03.047. [DOI] [PubMed] [Google Scholar]
- Kropf E., Syan S.K., Minuzzi L., Frey B.N. From anatomy to function: the role of the somatosensory cortex in emotional regulation. Braz. J. Psychiatry. 2019;41(3):261–269. doi: 10.1590/1516-4446-2018-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front. Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi N., Van De Ville D. On spurious and real fluctuations of dynamic functional connectivity during rest. Neuroimage. 2015;104:430–436. doi: 10.1016/j.neuroimage.2014.09.007. [DOI] [PubMed] [Google Scholar]
- Liu J.X., Chen Y.S., Hsieh J.C., Su T.P., Yeh T.C., Chen L.F. Differences in white matter abnormalities between bipolar I and II disorders. J. Affect. Disord. 2010;127(1–3):309–315. doi: 10.1016/j.jad.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Liu M., Wang Y., Zhang A., Yang C., Liu P., Wang J., et al. Altered dynamic functional connectivity across mood states in bipolar disorder. Brain Res. 2021;1750 doi: 10.1016/j.brainres.2020.147143. [DOI] [PubMed] [Google Scholar]
- Lv D., Lin W., Xue Z., Pu W., Yang Q., Huang X., et al. Decreased functional connectivity in the language regions in bipolar patients during depressive episodes but not remission. J. Affect. Disord. 2016;197:116–124. doi: 10.1016/j.jad.2016.03.026. [DOI] [PubMed] [Google Scholar]
- Malhi G.S., Lagopoulos J., Owen A.M., Ivanovski B., Shnier R., Sachdev P. Reduced activation to implicit affect induction in euthymic bipolar patients: an fMRI study. J. Affect. Disord. 2007;97(1–3):109–122. doi: 10.1016/j.jad.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Malhi G.S., Byrow Y., Boyce P., Bassett D., Fitzgerald P.B., Hopwood M., et al. Why the hype about subtype? Bipolar I, bipolar II–it's simply bipolar, through and through! Aust. N. Z. J. Psychiatry. 2016;50(4):303–306. doi: 10.1177/0004867416641541. [DOI] [PubMed] [Google Scholar]
- Malhi G.S., Irwin L., Outhred T. Counting the days from bipolar II to bipolar true! Acta Psychiatr. Scand. 2019;139(3):211–213. doi: 10.1111/acps.12999. [DOI] [PubMed] [Google Scholar]
- Malhi G.S., Outhred T., Irwin L. Bipolar II disorder is a myth. Can. J. Psychiatry. 2019;64(8):531–536. doi: 10.1177/0706743719847341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller J.J., Thaveenthiran P., Thomson R.H., McQueen S., Fitzgerald P.B. Volumetric, cortical thickness and white matter integrity alterations in bipolar disorder type I and II. J. Affect. Disord. 2014;169:118–127. doi: 10.1016/j.jad.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Marek S., Tervo-Clemmens B., Calabro F.J., Montez D.F., Kay B.P., Hatoum A.S., et al. Reproducible brain-wide association studies require thousands of individuals. Nature. 2022;603(7902):654–660. doi: 10.1038/s41586-022-04492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennes M., Zuo X.N., Kelly C., Di Martino A., Zang Y.F., Biswal B., et al. Linking inter-individual differences in neural activation and behavior to intrinsic brain dynamics. Neuroimage. 2011;54(4):2950–2959. doi: 10.1016/j.neuroimage.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison I., Tipper S.P., Fenton-Adams W.L., Bach P. “Feeling” others' painful actions: the sensorimotor integration of pain and action information. Hum. Brain Mapp. 2013;34(8):1982–1998. doi: 10.1002/hbm.22040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins N., Forstner A.J., O'Connell K.S., Coombes B., Coleman J.R.I., Qiao Z., et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat. Genet. 2021;53(6):817–829. doi: 10.1038/s41588-021-00857-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K., Birn R.M., Handwerker D.A., Jones T.B., Bandettini P.A. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44(3):893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabulsi L., McPhilemy G., Kilmartin L., Whittaker J.R., Martyn F.M., Hallahan B., et al. Frontolimbic, frontoparietal, and default mode involvement in functional dysconnectivity in psychotic bipolar disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2020;5(2):140–151. doi: 10.1016/j.bpsc.2019.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips, M. R. t. 2020. Adapted Chinese Version of Structured Clinical Interview for DSM-5 Disorders, Research Version (SCID-5-RV) by Micahel B. First, Jane B.W. Williams, Rhonda S. Karg, and Robert L. Spitzer. Beijing: Peking University Press.
- Potegal M. Temporal and frontal lobe initiation and regulation of the top-down escalation of anger and aggression. Behav. Brain Res. 2012;231(2):386–395. doi: 10.1016/j.bbr.2011.10.049. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu M., Zhang H., Mellor D., Shi J., Wu C., Huang Y., et al. Aberrant neural activity in patients with bipolar depressive disorder distinguishing to the unipolar depressive disorder: a resting-state functional magnetic resonance imaging study. Front. Psychiatry. 2018;9:238. doi: 10.3389/fpsyt.2018.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoğlu U., Pearlson G.D., Kiehl K.A., Wang Y.M., Michael A.M., Calhoun V.D. A method for evaluating dynamic functional network connectivity and task-modulation: application to schizophrenia. Magma. 2010;23(5–6):351–366. doi: 10.1007/s10334-010-0197-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shunkai L., Chen P., Zhong S., Chen G., Zhang Y., Zhao H., et al. Alterations of insular dynamic functional connectivity and psychological characteristics in unmedicated bipolar depression patients with a recent suicide attempt. Psychol. Med. 2022;1–12 doi: 10.1017/s0033291722000484. [DOI] [PubMed] [Google Scholar]
- Simonetti A., Kurian S., Saxena J., Verrico C.D., Restaino A., Di Nicola M., et al. Cortical correlates of impulsive aggressive behavior in pediatric bipolar disorder. Front. Psychiatry. 2021;12 doi: 10.3389/fpsyt.2021.674707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl E.A., Breen G., Forstner A.J., McQuillin A., Ripke S., Trubetskoy V., et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat. Genet. 2019;51(5):793–803. doi: 10.1038/s41588-019-0397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strakowski S.M., Delbello M.P., Adler C.M. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol. Psychiatry. 2005;10(1):105–116. doi: 10.1038/sj.mp.4001585. [DOI] [PubMed] [Google Scholar]
- Sun Q., Zhang Y., Zhou J., Wang X. Altered resting-state functional connectivity in the default mode network in male juvenile violent offenders. Brain Imaging Behav. 2021 doi: 10.1007/s11682-021-00535-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson P.M., Jahanshad N., Ching C.R.K., Salminen L.E., Thomopoulos S.I., Bright J., et al. ENIGMA and global neuroscience: a decade of large-scale studies of the brain in health and disease across more than 40 countries. Transl. Psychiatry. 2020;10(1):100. doi: 10.1038/s41398-020-0705-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S., Zhu R., Chattun M.R., Wang H., Chen Z., Zhang S., et al. Temporal dynamics alterations of spontaneous neuronal activity in anterior cingulate cortex predict suicidal risk in bipolar II patients. Brain Imaging Behav. 2021;15(5):2481–2491. doi: 10.1007/s11682-020-00448-7. [DOI] [PubMed] [Google Scholar]
- Tsai S.Y., Sajatovic M., Hsu J.L., Chung K.H., Chen P.H., Huang Y.J. Peripheral inflammatory markers associated with brain volume reduction in patients with bipolar I disorder. Acta Neuropsychiatr. 2021;1–10 doi: 10.1017/neu.2021.39. [DOI] [PubMed] [Google Scholar]
- Wang F., Jackowski M., Kalmar J.H., Chepenik L.G., Tie K., Qiu M., et al. Abnormal anterior cingulum integrity in bipolar disorder determined through diffusion tensor imaging. Br. J. Psychiatry. 2008;193(2):126–129. doi: 10.1192/bjp.bp.107.048793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wang J., Jia Y., Zhong S., Zhong M., Sun Y., et al. Topologically convergent and divergent functional connectivity patterns in unmedicated unipolar depression and bipolar disorder. Transl. Psychiatry. 2017;7(7) doi: 10.1038/tp.2017.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhong S., Chen G., Liu T., Zhao L., Sun Y., et al. Altered cerebellar functional connectivity in remitted bipolar disorder: a resting-state functional magnetic resonance imaging study. Aust. N. Z. J. Psychiatry. 2018;52(10):962–971. doi: 10.1177/0004867417745996. [DOI] [PubMed] [Google Scholar]
- Wang H., Zhu R., Tian S., Zhang S., Dai Z., Shao J., et al. Dynamic connectivity alterations in anterior cingulate cortex associated with suicide attempts in bipolar disorders with a current major depressive episode. J. Psychiatr. Res. 2022;149:307–314. doi: 10.1016/j.jpsychires.2022.03.010. [DOI] [PubMed] [Google Scholar]
- Woo Y., Kang W., Kang Y., Kim A., Han K.M., Tae W.S., et al. Cortical thickness and surface area abnormalities in bipolar I and II disorders. Psychiatry Investig. 2021;18(9):850–863. doi: 10.30773/pi.2021.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., Gao Q., Ma L., Fan H., Mao H., Liu J., et al. The zuckerman-kuhlman personality questionnaire in bipolar I and II disorders: a preliminary report. Psychiatry Res. 2015;226(1):357–360. doi: 10.1016/j.psychres.2015.01.019. [DOI] [PubMed] [Google Scholar]
- Xu K., Liu H., Li H., Tang Y., Womer F., Jiang X., et al. Amplitude of low-frequency fluctuations in bipolar disorder: a resting state fMRI study. J. Affect. Disord. 2014;152–154:237–242. doi: 10.1016/j.jad.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Yan C.G., Wang X.D., Zuo X.N., Zang Y.F. DPABI: data processing & analysis for (Resting-State) brain imaging. Neuroinformatics. 2016;14(3):339–351. doi: 10.1007/s12021-016-9299-4. [DOI] [PubMed] [Google Scholar]
- Yang Y., Cui Q., Pang Y., Chen Y., Tang Q., Guo X., et al. Frequency-specific alteration of functional connectivity density in bipolar disorder depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2021;104 doi: 10.1016/j.pnpbp.2020.110026. [DOI] [PubMed] [Google Scholar]
- Yatham L.N., Kennedy S.H., Parikh S.V., Schaffer A., Bond D.J., Frey B.N., et al. Canadian network for mood and anxiety treatments (CANMAT) and international society for bipolar disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97–170. doi: 10.1111/bdi.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R.C., Biggs J.T., Ziegler V.E., Meyer D.A. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Zang Y.F., He Y., Zhu C.Z., Cao Q.J., Sui M.Q., Liang M., et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29(2):83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Bo Q., Li F., Zhao L., Wang Y., Liu R., et al. Increased ALFF and functional connectivity of the right striatum in bipolar disorder patients. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2021;111 doi: 10.1016/j.pnpbp.2020.110140. [DOI] [PubMed] [Google Scholar]
- Zhang B., Jia Y., Wang C., Shao X., Wang W. Visual event-related potentials in external emotional conditions in bipolar disorders I and II. Neurophysiol. Clin. 2019;49(5):359–369. doi: 10.1016/j.neucli.2019.09.002. [DOI] [PubMed] [Google Scholar]
- Zhang L., Li W., Wang L., Bai T., Ji G.J., Wang K., et al. Altered functional connectivity of right inferior frontal gyrus subregions in bipolar disorder: a resting state fMRI study. J. Affect. Disord. 2020;272:58–65. doi: 10.1016/j.jad.2020.03.122. [DOI] [PubMed] [Google Scholar]
- Zhang N., Niu Y., Sun J., An W., Li D., Wei J., et al. Altered complexity of spontaneous brain activity in schizophrenia and bipolar disorder patients. J. Magn. Reson. Imaging. 2021;54(2):586–595. doi: 10.1002/jmri.27541. [DOI] [PubMed] [Google Scholar]
- Zhao Q., Guo T., Li Y., Zhang L., Lyu N., Wilson A., et al. Clinical characteristic of prodromal symptoms between bipolar I and II disorder among Chinese patients: a retrospective study. BMC Psychiatry. 2021;21(1):275. doi: 10.1186/s12888-021-03295-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S., Chen G., Zhao L., Jia Y., Chen F., Qi Z., et al. Correlation between Intrinsic Brain Activity and Thyroid-Stimulating Hormone Level in Unmedicated Bipolar II Depression. Neuroendocrinology. 2019;108(3):232–243. doi: 10.1159/000497182. [DOI] [PubMed] [Google Scholar]
- Zhu W., Tang W., Liang Y., Jiang X., Li Y., Chen Z., et al. Aberrant functional connectivity of sensorimotor network and its relationship with executive dysfunction in bipolar disorder type I. Front. Neurosci. 2021;15 doi: 10.3389/fnins.2021.823550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuliani R., Moorhead T.W., Job D., McKirdy J., Sussmann J.E., Johnstone E.C., et al. Genetic variation in the G72 (DAOA) gene affects temporal lobe and amygdala structure in subjects affected by bipolar disorder. Bipolar Disord. 2009;11(6):621–627. doi: 10.1111/j.1399-5618.2009.00731.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors are unable or have chosen not to specify which data has been used.