Abstract
This experiment was conducted to investigate the antibacterial effects of essential oils (EO) in vitro and the influence of EO on growth performance, intestinal morphology and oxidation resistance and cecal microflora of yellow-feathered broilers. A total of 720 one-day-old male yellow feather broilers were randomly assigned into 4 treatments with 6 replicate cages of 30 broilers each. The groups were as follows: CON group (basal diet), EO200 group (basal diet + 200 mg/kg EO), EO400 group (basal diet + 400 mg/kg EO), and EO600 group (basal diet + 600 mg/kg EO). The experiment lasted for 48 d. Results showed that the growth and biofilm formation of avian pathogenic E. coli O78 and Salmonella pullorum were limited by adding EO to the diet (P < 0.05). Besides, birds fed with EO had greater (P < 0.05) average daily feed intake (ADFI), average daily gain (ADG), and body weight (BW) during d 1 to 21, 22 to 42, and 1 to 48 and lower (P < 0.05) feed: gain (F:G) than those fed with basal diet during d 22 to 42 and 1 to 48. Moreover, the activity of antioxidant enzyme and the intestinal permeability were improved in the EO400 and EO600 groups rather than the CON group on d 21 (P < 0.05). There were significant differences in cecal microbial composition and enrichment of metabolic pathways of birds among all groups by 16S-based sequencing. In summary, some dose of EO improved bacteriostatic ability, antioxidant ability, and intestinal health of broilers which contributed to the growth performance improvement of yellow-feathered broilers, which can be a promising antibiotic alternative for improving poultry production.
Key words: essential oils, bacterial growth and biofilm formation, yellow-feathered broilers, growth performance, cecal microbial
INTRODUCTION
Escherichia coli (E. coli) and Salmonella are popular pathogenic bacteria which cause a disturbed gut microbiota and a low production performance of broilers (Kabir, 2010; Ghunaim et al., 2014). The way to solve those problems was generally added antibiotics to feed in the past. What's more, antibiotic growth promoters (AGP) have been used in animal production for many years to improve intestinal health, promote growth performance, and prevent diseases (Dibner and Richards, 2005). However, with the long-term abuse of AGP, antibiotic drug residues, and resistant bacteria has emerged and became a severe problem that pollute the environment and harm human health (Yang et al., 2019). Consequently, in recent years, many countries have issued regulations banning the use of antibiotics in feed (Barug et al., 2006). For instance, the use of AGP in animal feed has been banned in China since July 1, 2020. The prohibition of AGP leads to the decline of animal performance and disease resistance, which seriously affects the economic benefit of animal husbandry. Therefore, the development of safe and effective antibiotic substitutes has become the focus of animal nutrition research.
Essential oils (EO), an aromatic oily liquids, extracted from plants material has been known to contain mainly thymol and carvacrol, whose antibacterial properties have encouraged their usage as natural antibiotic alternatives for animal production (Zeng et al., 2015). Studies have found that the thymol and carvacrol can be digested and absorbed by animals, which is the basis of its promoting role (Michiels et al., 2008; Austgulen et al., 2010; Ocel'Ová et al., 2016). The past few years, the efficacy of EO on reducing the colonization of E. coli, Clostridium perfringens, and Campylobacter jejuni have been extensively investigated in broiler (Hashemipour et al., 2016; Pham et al., 2020). Peng et al. (2016) found that adding 600 mg/kg EO has significantly increased the average daily gain (ADG) during the whole period and weight at 21 and 42 d of AA broilers, and reduced the feed conversion rate (FCR) at d 1 to 21. Mohiti-Asli and Ghanaatparast-Rashti (2016) showed that adding 500 mg/kg EO has markedly improved the growth performance and feed conversion ratio of broilers, and alleviated intestinal damage caused by coccidiosis. Besides, the gut microbiota of broilers has altered by adding the plant extracts containing thymol and carvacrol to the diet (Zhu et al., 2019).
However, the antibacterial effect of EO in vitro on common pathogenic bacteria in poultry production and whether it can improve the growth performance of poultry by improving the gut microbiota structure and composition are rarely reported. Therefore, this study was conducted to investigate the in vitro antibacterial effects of EO on common poultry pathogens and the effects of EO on growth performance, serum biochemistry, intestinal morphology, antioxidant, and intestinal microbial composition of yellow-feathered broilers.
MATERIALS AND METHODS
The animal handling and all procedures of this study received approval from the Animal Care and Use Ethics Committee of the Hunan Agricultural University (Changsha).
Experimental Design, Animals, and Diets
A total of 720 one-day-old male yellow feather broilers were randomly assigned into 4 treatments with 6 replicate cages of 30 broilers each. The groups were as follows: CON group (basal diet), EO200 group (basal diet + 200 mg/kg essential oils), EO400 group (basal diet + 400 mg/kg essential oils), and EO600 group (basal diet + 600 mg/kg essential oils). Essential oils (product name:Wisdem Essential oil, light yellow to yellow, free-flowing powder) was purchased from GuangZhou Wisdom Bio-Technology Co., Ltd, (Guangzhou, China), in which thymol was not less than 12 g/kg, carvacrol was not less than 23 g/kg, and the carrier was rice husk powder and silica.
The temperature was controlled at 33°C in the first week, 28 to 31°C in the second week, and kept at 24 to 26°C thereafter. The relative humidity should be controlled at 60 to 65% during the 1st to 7th days, and then the natural humidity. The light lasted 22 h a day. The broilers were provided with ad libitum access to mash feed and water. The experiment lasted for 48 d, including 1 to 21 d in the early stage, 22 to 42 d in the middle stage, and 43 to 48 d in the late stage. The compositions of basal diets and nutrients level are presented in Table 1.
Table 1.
Composition and nutrient levels of basal diets (%, as-fed basis).
| Ingredients | D 1-21 | D 22-42 | D 43-48 |
|---|---|---|---|
| Corn | 59.03 | 63.14 | 69.66 |
| Soybean meal, 43% | 29.40 | 24.40 | 18.50 |
| Corn gluten meal | 3.00 | 5.00 | 5.00 |
| Fish meal | 2.00 | - | - |
| Soybean oil | 2.10 | 3.00 | 2.70 |
| Limestone | 1.30 | 1.28 | 1.21 |
| Calcium phosphate | 1.45 | 1.50 | 1.25 |
| Salt | 0.35 | 0.35 | 0.35 |
| Choline chloride, 50% | 0.10 | 0.10 | 0.10 |
| L-Lysine hydrochloride, 98.5% | 0.09 | 0.16 | 0.17 |
| DL-Methionine | 0.17 | 0.07 | 0.02 |
| L-Threonine, 98.5% | 0.01 | - | 0.04 |
| Vitamin-mineral premix1 | 1.00 | 1.00 | 1.00 |
| Total | 100 | 100 | 100 |
| Nutrient content2 | |||
| Metabolizable energy, Kcal/kg | 3,000.00 | 3,100.00 | 3,150.00 |
| Crude protein, % | 21.00 | 19.00 | 17.00 |
| Salt, % | 0.46 | 0.40 | 0.39 |
| Sodium, % | 0.18 | 0.15 | 0.15 |
| Chlorine, % | 0.28 | 0.26 | 0.26 |
| Calcium, % | 1.00 | 0.90 | 0.80 |
| Total phosphorus, % | 0.67 | 0.61 | 0.55 |
| Available phosphorus, % | 0.45 | 0.40 | 0.35 |
| Lysine, % | 1.15 | 1.00 | 0.87 |
| Total methionine, % | 0.54 | 0.42 | 0.34 |
| Threonine, % | 0.81 | 0.72 | 0.68 |
Provided per kilogram of diet: Vitamin A, 15,000 IU; Vitamin D3, 3,900 IU; Vitamin E, 30 IU; Vitamin K, 33 mg; Vitamin B1 (thiamin), 12.4 mg; Vitamin B2 (riboflavin), 28 mg; Vitamin B6 (pyridoxine HCl) 20.02 mg, Vitamin B12 (cobalamin) 0.010 mg, pantothenic acid, 25 mg, Nicotinic acid, 45 mg, folic acid, 1.2 mg, biotin, 0.18 mg, choline, 700 mg, copper, 8 mg; iron, 80 mg; zinc, 80 mg; manganese, 100 mg; selenium, 0.15 mg; iodine, 0.35 mg.
The nutrient levels were calculated values.
Sample Collection
On d 21 and 48, one bird per cage with the body weight close to the mean body weight of the cage (6 birds per treatment) was selected. Blood samples were collected by puncturing the vein of wings and clot in polypropylene tubes. Serum samples were separated after blood samples were centrifuged at 3,000 r/min for 10 min at 4°C and stored at −20°C until analysis. After blood sample collection, birds were slaughtered by dislocation of the neck vertebrae and bleeding of the carotid artery. Subsequently, 2 cm segments from the median sections of the jejunum were collected and preserved in 4% paraformaldehyde solution for 24 h for intestine morphological measurements. The remaining portion of the jejunum about 10 cm was opened longitudinally and the mucosa was scraped from the middle portion; and stored in sterile centrifuges tube, snap-frozen in liquid nitrogen, and stored at −80°C for the assay of antioxidant indices. The cecum tract digesta was collected to evaluate microbial populations.
Bacterial Culture of Avian pathogenic E. coli O78 and Salmonella pullorum
Avian pathogenic E. coli O78 (CVCC1490) and Salmonella pullorum (CVCC519) were purchased from China Veterinary Microbial Species Preservation Center (Beijing, China). Thymol (analytical standard, HPLC ≥ 98%) and carvacrol (analytical standard, HPLC ≥ 98%) were purchased from Source Leaf Biological Co., LTD (Shanghai, China). The purchased Avian pathogenic E. coli O78 and Salmonella pullorum were dissolved in LB broth medium, resuscated and cultured at 37°C and 150 rpm for 24 h, and activated for 2 to 3 generations before reserve.
Referring to Franklin and Cockerill, 2011, the MICs of thymol or carvacrol against Avian pathogenic E. coli O78 and Salmonella pullorum were determined using broth microdilution method. Avian pathogenic E. coli O78 and Salmonella pullorum were cultured to logarithmic stage and the bacterial concentration was adjusted to 1 × 106 CFU/mL for reserve. Add 100 µL LB broth and 100 µL bacterial solution to the first well of the 96-well plate, and 200 µL LB broth to the second well as negative control. A series of 2-fold gradient dilution of thymol or carvacrol (at concentrations of 32, 16, 8, 4, 2, 1, 0.5, 0.25, 0.125, 0.0625 mg/mL, 100 µL per well) was carried out in LB broth from the third to the twelfth well, and 100 µL bacterial solution was added from the third to the twelfth well. Then, the turbidity was observed in a 37°C constant temperature incubator for 24 h, and the minimum concentration without turbidity was recorded as the minimum inhibitory concentration.
Avian pathogenic E. coli O78 and Salmonella pullorum were cultured to logarithmic stage and the bacterial concentration was adjusted to 1 × 106 CFU/mL for reserve. According to the MIC of thymol or carvacrol against Avian pathogenic E. coli O78 and Salmonella pullorum measured, LB broth was used as concentration dilution in 96-well plates. Thymol or carvacrol 100 μL with final concentrations of 2 MIC, MIC, 1/2 MIC, 1/4 MIC, 1/8 MIC, and 1/16 MIC were obtained in the well, and the control well was set without drug addition. Add 100 μL bacterial solution to each well, shake well gently, culture at 37°C, 130 RPM for 15 h, the OD600 value was measured every 1.5 h with a full-wavelength microplate reader.
The inhibition of thymol or carvacrol on the formation of Avian pathogenic E. coli O78 biofilm was determined by crystal violet staining with 96-well plates. Gradient dilution of thymol or carvacrol with LB broth medium was carried out on 96-well plate, and the final concentrations of thymol or carvacrol were 2 MIC, MIC, 1/2 MIC, 1/4 MIC, 1/8 MIC and 1/16 MIC 100 μL in the well. Then 100 μL of Avian pathogenic E. coli O78 and Salmonella pullorum at 104 CFU were added, and placed in an incubator at 37°C for incubation for 36 h. After culture, suck out the culture medium, add 200 μL 0.01 M sterile PBS to each well and wash gently three times. After cleaning, add 100 μL methanol to each well and fix for 15 min, then suck out and dry naturally. Then add 200 μL 0.1% crystal violet solution to each well, stain for 5 min, wash with 200 μL 0.01 M sterile PBS for three times to remove excess crystal violet, and dry at room temperature. Finally, add 200 μL 33% glacial acetic acid to each well to dissolve crystal violet, and then read the OD600 value on a full-wavelength microplate reader at 37°C for 30 min. The control group is the one without thymol or carvacrol. Inhibition rate (%) = (1-Treated absorbance value/Untreated absorbance value) × 100%.
Growth Performance
Individual body weight and feed consumption per cage were recorded on d 1, 21, 42, and 48 after 12-h fast to calculate the average daily gain (ADG), average daily feed intake (ADFI), and feed/gain (F/G), and these parameters were corrected for mortality.
Serum Biochemical Indexes and DAO Content
Total protein (TP), total cholesterol (TC), triglyceride (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), and aspartate aminotransferase (AST) in serum were determined by automatic biochemical analyzer. All the above indicators were determined using kits provided by Shanghai Kehua Biological Co., LTD (Shanghai, China). The content of DAO in serum was determined using chicken DIAMine oxidase (DAO) ELISA kit from Jiangsu Enzyme Tag Biotechnology Co., LTD. The specific operations were carried out according to the kit instructions.
Anti-Oxidation of Jejunum Mucosa
Jejunum mucosa was homogenized in icecold phosphate-buffered saline (PBS) and then centrifuged at 10,000 £ ɡ at 4°C for 10 min, and the supernatant was stored at −80°C. The activities of total antioxidant capacity (T-AOC), superoxide dismutase (SOD), catalase (CAT), and the level of MDA in the supernatant of the intestine homogenate were determined using commercially available assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). All procedures were performed according to the manufacturer's instructions.
RNA Isolation and Real-Time Quantitative PCR
Total RNA was extracted from the ileum mucosa using EasyPureTM RNA kit (Beijing Transgene Biotech Ltd., Beijing, China) following the manufacturer's instructions. The purity and concentration of the total RNA were measured by Epoch Microplate Spectrophotometer (BioTek Instruments, Inc., VT). The cDNA samples were obtained by reverse transcription of the total RNA using the first-strand synthesis kit (TransGen Biotech Co., Ltd., Beijing, China). Real-time PCR for analysis of the gene expression was performed using SYBR Green (Thermo Fisher Scientific, MA) on an ABI 6 flex real-time PCR instrument (Thermo Fisher Scientific). Primer sequences used in this study are shown in Supplementary Table 1. The reaction conditions were as follows: 50°C for 2 min, 95°C for 10 min; 40 cycles of 95°C for 15 s, 60°C for 1 min. Melt curve analysis was performed to confirm the PCR amplification specificity. Each sample was measured in duplicate and the relative mRNA expression levels were analyzed using β-actin as an internal control by the 2−ΔΔCt method (Livak and Schmittgen, 2001).
Morphological Measurements of Jejunum
Paraffin sections of tissues were observed with a microscope, multiple discontinuous fields were randomly selected, and representative fields were selected and photographed. The average intestinal villus height (VH) and crypt depth (CD) were measured using Minmei microscopic digital measurement and analysis system V1.6.1, and the villus height/crypt depth (VH/CD) was calculated.
DNA Extraction and Analysis of Cecal Microbiota
Total genomic DNA of 24 digesta samples was extracted using a Stool DNA Isolation Kit (Tiangen Biotech Co., Ltd., Beijing, China) following the manufacturer's instructions. The quantity and quality of extracted DNAs were measured using a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific) and agarose gel electrophoresis, respectively. The genes of bacteria 16S ribosomal RNA in the region of V4-V5 were amplified by using polymerase chain reaction (PCR) with primers with barcode (F: CCTACGGGNGGCWGCAG, R: GGACTACHVGGGTATCTAAT). Electrophoresis was applied to analyze the integrity of PCR amplicons by using a Tapestation Instruction (Agilent technologies, California, USA). AxyPrep DNA Gel 122Extraction Kit was chosen to extract and purify PCR amplicons using 2% agarose gels (Axygen 123Biosciences, Union City, CA) and then the production was quantified using QuantiFluor -ST and sequenced on an Illumina MiSeq system. QIIME software was used to demultiplex and quality-filtered raw Illumina fastq files. Operational taxonomic units (OTUs) were defined as a similarity threshold of 0.97 using UPARSE. Then UCHIME was applied to identify and delete the unnormal gene sequences. RDP database (http://rdp.cme.msu.edu/) was also referenced to take the taxonomy-based analysis for OUTs using RDP classifier at a 90% confidence level. The α-diversity indices including Simpson and Chao1 were analyzed by Version 1.9.1. PCoA tools in R language were used for principal co-ordinates analysis (PCoA). The histogram of linear discriminant analysis (LDA) distribution was implemented using LDA effect size analysis (LEfSe) software. In Spearman correlation analysis, corr. Test function of PSYCH package in R was used to calculate Spearman correlation values of species and environmental factors and test their significance. Then, Pheatmap function in Pheatmap package was used for visualization. Feature comments select FAPROTAX database.
Statistical Analysis
Data were analyzed by the one-way analysis of variance (ANOVA) procedure and differences were examined using Duncan's multiple range test using IBM SPSS Statistics 25.0 (SPSS Inc., Chicago, IL), and each chicken was regarded as a statistical unit. The linear and quadratic effects of dietary EO supplementation dose were evaluated by regression analysis. Data are showed as mean values with standard error of the total mean (SEM). For all tests, P < 0.05 was considered as significant difference, while 0.05 < P < 0.10 as a tendency.
RESULTS
The Growth and Biofilm Formation of Avian Pathogenic E. coli O78, and Salmonella pullorum
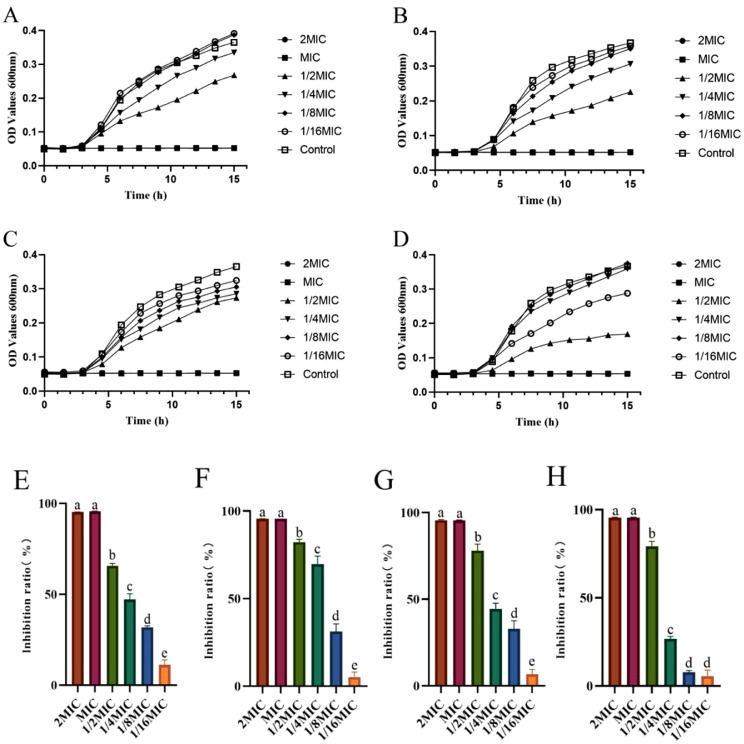
The MIC of thymol and carvacrol on the against avian pathogenic E. coli O78 and Salmonella pullorum were 0.25 mg/mL and 0.5 mg/mL, respectively (Supplementary Table 2). The effects of different concentrations of thymol or carvacrol on the growth curve and biofilm formation of Avian pathogenic E. coli O78 and Salmonella pullorum in the next moment. It was shown that the OD values of Avian pathogenic E. coli O78 and Salmonella pullorum in 2MIC and MIC groups remained stable during the whole test period regardless of the species, but the OD values of the remaining test groups were lower than those of the control group after 5-h culture (Figures 1A–1D). Besides, the inhibition rate of biofilm formation of Avian pathogenic E. coli O78 and Salmonella pullorum has reached 95% by adding the thymol or carvacrol in the 2MIC and MIC groups (Figures 1E–1H). As the concentration of thyme or carvacrol gradually decreased, the inhibition rate of biofilm formation of Avian pathogenic E. coli O78 and Salmonella pullorum also gradually decreased (Figures 1E–1H).
Figure 1.
Effects of different concentrations of thymol or carvacrol on the growth curve and biofilm formation of Avian pathogenic E. coli O78 and Salmonella pullorum. The effects of thymol (A) and carvacrol (B) on growth curve of Avian pathogenic E. coli O78, thymol (C), and carvacrol (D) on growth curve of Salmonella pullorum, thymol (E), and carvacrol (G) on the formation of Avian pathogenic E. coli O78 biofilm, thymol (F) and carvacrol (H) on the formation of Salmonella pullorum. Abbreviation: MIC, minimum inhibitory concentration. a-e Mean value within a role with no common superscript differ significantly (P < 0.05).
Growth Performance
As shown in Table 2, EO supplementation linearly and quadratically increased the ADFI, ADG, and BW during the d 1 to 21 (P < 0.05). And compared with the CON group, the ADFI, ADG and BW were significantly increased in the treatment groups during the d 1 to 21 (P < 0.05). During d 22 to 42, the ADFI in the EO200 group and the F:G, BW in all treatment groups was decreased, but the ADG in the EO400 was enhanced comparison with the CON group (P < 0.05). The ADFI, F:G, and FBW in all treatment groups were reduced during d 43 to 48 and 1 to 48, but the ADG was increased during d 1 to 48 compared with the CON group (P < 0.05).
Table 2.
Effects of dietary EO supplementation on growth performance of yellow-feather broilers.
|
P-value |
||||||||
|---|---|---|---|---|---|---|---|---|
| Items | CON | EO200 | EO400 | EO600 | SEM | Treatment | Linear | Quadratic |
| D 1–2,139 | ||||||||
| IBW | 38.65 | 38.39 | 38.49 | 38.90 | 0.091 | 0.167 | 0.252 | 0.053 |
| ADFI, g/d | 31.81b | 33.80a | 34.34a | 34.47a | 0.28 | <0.001 | <0.001 | 0.005 |
| ADG, g/d | 21.77b | 23.57a | 24.00a | 23.79a | 0.24 | <0.001 | <0.001 | 0.002 |
| BW, g | 496.09 ± 14.54b | 533.35±17.08a | 538.52±12.42a | 539.36 ± 7.55a | 5.09 | <0.001 | <0.001 | 0.002 |
| FCR | 1.46 | 1.44 | 1.43 | 1.45 | 0.01 | 0.439 | 0.542 | 0.145 |
| D 22–42 | ||||||||
| ADFI, g/d | 96.67ab | 93.78c | 98.51a | 94.63bc | 0.58 | 0.009 | 0.723 | 0.582 |
| ADG, g/d | 49.07b | 50.25b | 52.22a | 49.68b | 0.37 | 0.011 | 0.149 | 0.005 |
| BW, g | 1,535.13 ± 30.21b | 1,577.88 ± 38.37a | 1,614.99 ± 34.62a | 1,585.84 ± 22.26a | 10.77 | 0.001 | 0.002 | 0.009 |
| FCR | 1.97a | 1.87b | 1.89b | 1.91b | 0.01 | 0.005 | 0.042 | 0.004 |
| D 43–48 | ||||||||
| ADFI, g/d | 148.87a | 141.51b | 145.18ab | 141.74b | 1.18 | 0.019 | 0.019 | 0.4 |
| ADG, g/d | 58.52 | 58.34 | 57.85 | 57.20 | 0.40 | 0.69 | 0.246 | 0.785 |
| FBW, g | 1,878.17 ± 22.55c | 1,925.92 ± 37b | 1,972.36 ± 24.08a | 1,926.17 ± 33.13b | 9.16 | 0.001 | 0.004 | 0.002 |
| FCR | 2.55 | 2.43 | 2.51 | 2.46 | 0.02 | 0.105 | 0.262 | 0.309 |
| D 1–48 | ||||||||
| ADFI, g/d | 75.94 | 73.72 | 75.37 | 74.35 | 0.37 | 0.139 | 0.327 | 0.403 |
| ADG, g/d | 38.44b | 39.53a | 39.97a | 39.31ab | 0.19 | 0.017 | 0.041 | 0.013 |
| FCR | 1.98a | 1.87b | 1.89b | 1.89b | 0.01 | <0.001 | <0.001 | <0.001 |
Abbreviations: ADFI, average daily feed intake; ADG, average daily gain; BW, body weight; FBW, final body weight; FCR, feed conversion ratio; IBW, initial body weight.
CON, basic diet; EO200, basic diet contain 200 mg/kg essential oils; EO400, basic diet contain 400 mg/kg essential oils; EO600, basic diet contain 600 mg/kg essential oils.
Mean value within a role with no common superscript differ significantly in the treatment (P < 0.05).
Serum Biochemical Indexes
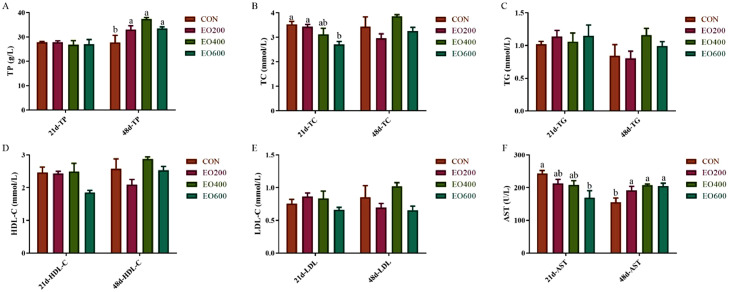
It was shown that the effects of dietary EO supplementation on serum biochemical indexes of yellow-feather broilers (Figure 2). The serum concentration of TC and AST were reduced in the EO600 group on d 21 (Figures 2B and 2F), whereas the TP and AST were enhanced in the treatment groups on d 48 compared with the CON group (P < 0.05; Figures 2A and 2F).
Figure 2.
Effects of dietary EO supplementation on serum biochemical indexes of yellow-feather broilers. (A) Total protein (TP), (B) total cholesterol (TC), (C) triglyceride (TG), (D) high density lipoprotein cholesterol (HDL-C), (E) low density lipoprotein cholesterol (LDL-C), (F) glutamic-pyruvic transaminase (ALT) of serum in yellow-feather broilers on d 21 and 48 were measured by an automatic biochemical analyzer. CON, basic diet; EO200, basic diet contain 200 mg/kg essential oils; EO400, basic diet contain 400 mg/kg essential oils; EO600, basic diet contain 600 mg/kg essential oils. a, b Mean value within a role with no common superscript differ significantly (P < 0.05).
Antioxidation of Jejunum Mucosa
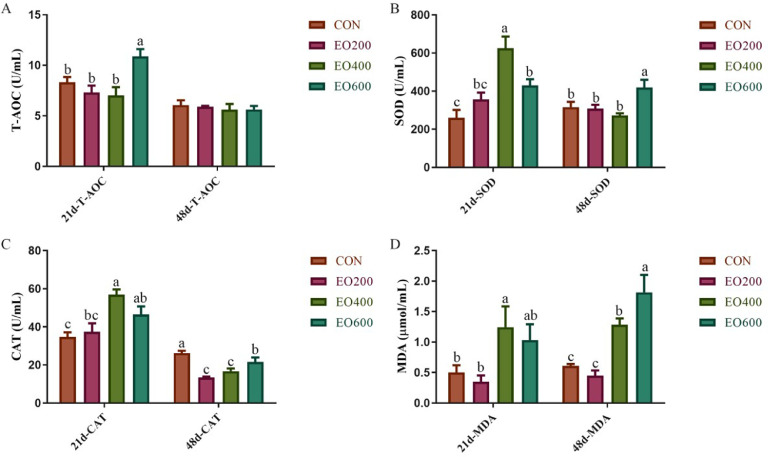
As shown in Figure 3, compared with the CON group, the activity of T-AOC in the EO600 group, the activities of SOD and CAT in the EO400 and EO600 groups, as well as the level of MDA in the EO400 group were increased on d 21 (P < 0.05). In addtion, on d 48, the activity of SOD in the EO600 group and the level of MDA in the EO400 and EO600 groups were enhanced, but the activity of CAT in all treatment was reduced comparison with the CON group (P < 0.05).
Figure 3.
Effects of dietray EO supplementation on antioxidation of jejunum mucosa in yellow-feather broilers. (A) Total antioxidant capacity (T-AOC), (B) superoxide dismutase (SOD), (C) catalase (CAT), (D) malondialdehyde (MDA) of jejunum mucosa in yellow-feather broilers on d 21 and 48. CON, basic diet; EO200, basic diet contain 200 mg/kg essential oils; EO400, basic diet contain 400 mg/kg essential oils; EO600, basic diet contain 600 mg/kg essential oils. a, b, c Mean value within a role with no common superscript differ significantly (P < 0.05).
Intestinal Permeability
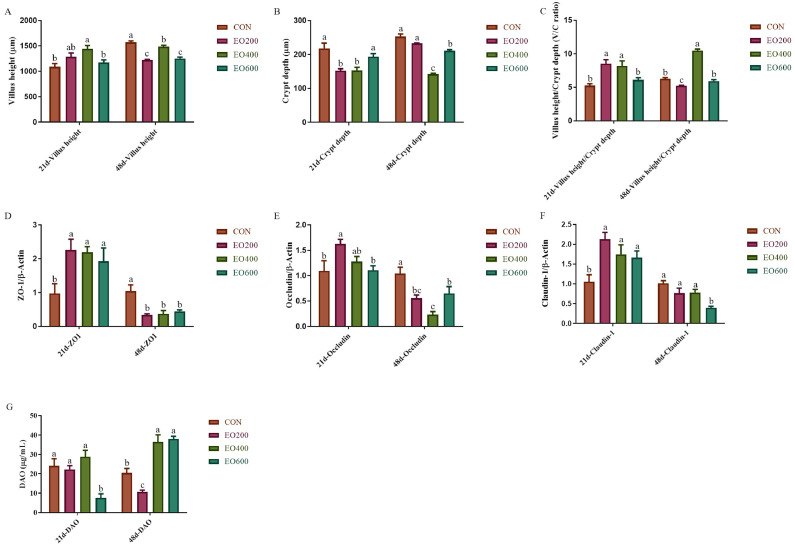
As indicated in Figure 4, dietary EO supplement at some dose improved the jejunal morphology of yellow-feather broilers and affected the relative expression of tight junction protein and level of serum DAO of yellow-feather broilers. On d 21, the VH and the VH/CD of jejunum were increased in the EO200 and EO400 groups, whereas the CD was decreased (P < 0.05; Figures 4A–4C). On d 48, the VH of jejunum with dietary EO supplementation and the CD in the EO400 and EO600 groups and the VH/CD in the EO200 group were reduced, but the VH/CD in the EO400 group was increased compared with the CON group (P < 0.05; Figures 4A–4C). Furthermore, the relative expression of ZO-1, Occludin, and Claudin-1 were enhanced on d 21 but decreased on d 48 with dietary EO supplementation (P < 0.05; Figures 4D–4F). Besides, the concentration of serum DAO were decreased in the EO600 group on d 21 and in the EO200 group on d 48, but increased in the EO400 and EO600 groups on d 48 (P < 0.05; Figure 4G).
Figure 4.
Effects of dietary EO on intestinal permeability in yellow-feather broilers. (A) Villus height, (B) crypt depth, and (C) villus height/crypt depth (V/C value) of jejunum in yellow-feather broilers on d 21 and 48 was detected and observed. The related mRNA expression of (D) ZO-1, (E) occludin, and (F) claudin-1 of jejunum in yellow-feather broilers on d 21 and 48 by the RT-PCR. (G) The serum DAO content of 21-day and 48-day-old yellow-feather broilers was also measured by ELISA Kit. CON, basic diet; EO200, basic diet contain 200 mg/kg essential oils; EO400, basic diet contain 400 mg/kg essential oils; EO600, basic diet contain 600 mg/kg essential oils. Abbreviation: DAO, diamine oxidase. a,b,c Mean value within a role with no common superscript differ significantly (P < 0.05).
Cecal Microbiota Diversity, Composition, Function Prediction, and Correlation Analysis
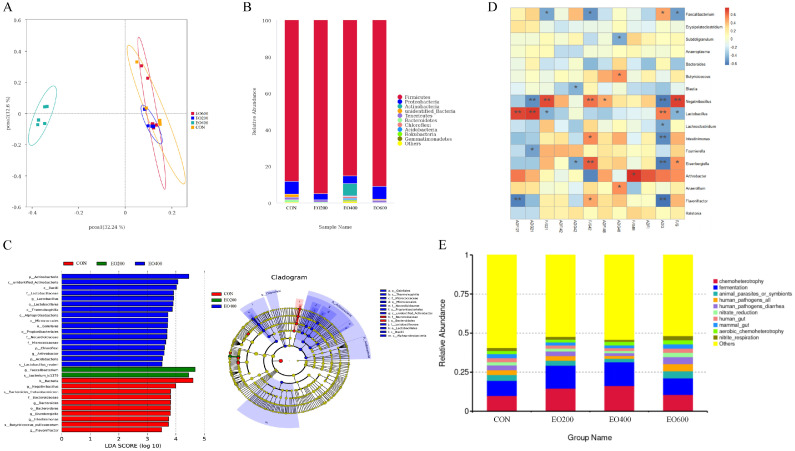
A total of 3,771 OTUs were detected in all samples, and 416 OTUs were shared by the four groups. 816, 699, 1,344, and 912 OTUs were obtained in CON, EO200, EO400, and EO600 groups, respectively, wherein 198, 143, 713, and 255 OTUs have been identified exclusively in CON, EO200, EO400, and EO600 groups, respectively (Supplementary Figure 1). The α-diversity of cecal microbiota including Ace, Chao1 index, Shannon and Simpson index were presented in Table 3. The EO200 group induced higher Ace and Chao1 index of cecum than other groups (P < 0.05). The β-diversity of bacterial community among all groups was presented with PCoA, showing a significantly different clustering of microbial communities in cecum between the treatment groups and CON group (P < 0.05; Figure 5A).
Table 3.
Effects of dietary EO supplementation on alpha diversity of cecal microbes in 21-day-old yellow-feathered broilers.
| Items | CON | EO200 | EO400 | EO600 | SEM |
P-value |
||
|---|---|---|---|---|---|---|---|---|
| Treatment | Linear | Quadratic | ||||||
| Ace | 497.16b | 497.84b | 886.73a | 552.60b | 42.15 | <0.001 | 0.021 | 0.004 |
| Chao1 | 907.82b | 850.63b | 2,257.35a | 1,118.42b | 178.22 | 0.008 | 0.112 | 0.067 |
| Shannon | 6.34 | 6.32 | 6.58 | 6.23 | 0.07 | 0.301 | 0.920 | 0.226 |
| Simpson | 0.97 | 0.96 | 0.96 | 0.97 | 0.07 | 0.878 | 0.860 | 0.440 |
CON, basic diet; EO200, basic diet contain 200 mg/kg essential oils; EO400, basic diet contain 400 mg/kg essential oils; EO600, basic diet contain 600 mg/kg essential oils.
Mean value within a role with no common superscript differ significantly in the treatment (P < 0.05).
Figure 5.
Effects of dietary EO on the composition of cecal microbe, function prediction, and correlation analysis of cecal microbial abundance and growth performance index of yellow-feather broilers. (A) The β-diversity of cecum in the yellow-feather broilers on d 21 was analyzed by the principal component analysis (PCA) based on binary_jaccard algorithm among samples of different groups. Each point represents one sample. (B) The relative abundance of cecal microbiota on phylum level was analyzed. (C) LEfSe analysis of 21-d cecal microbiota of yellow-feather broilers among the treatment groups. (D) Correlation analysis of cecal microbial abundance and growth performance index of yellow-feather broilers. (E) The metabolic function of cecal microflora in 21-d yellow-feathered broilers was predicted based on FAPROTAX database. CON, basic diet; EO200, basic diet contain 200 mg/kg essential oils; EO400, basic diet contain 400 mg/kg essential oils; EO600, basic diet contain 600 mg/kg essential oils. * Indicates a significant difference (P < 0.05).
The percentage relative abundance of the top 10 phyla detected in respect to the total bacterial sequences for each treatment are presented in Figure 5B. Firmicutes, proteobacteria, and actinobacteria were the dominant bacteria in cecum of yellow-feathered broilers at 21 d of age, with a total abundance of more than 95%. Proteobacteria is the largest group of bacteria, including many pathogenic bacteria, such as E. coli, Salmonella, vibrio cholerae, and so on. A lower relative abundance of proteobacteria in the EO200 and EO400 groups was displayed than that in the CON and EO600 groups in Figure 5B. The lefse analysis was used to identify the significantly different bacteria in the cecum between the treatment groups and the CON group from the phylum to genus level (Figure 5C). Results as shown in Figure 5C, adding EO in the diet of yellow-feathered broilers mainly changed 29 bacterial groups (19 increased and 10 decreased) in cecal microbiota at 21 d of age. At the genus level, the relative abundance of Lactobacillus, Arthrobacter, and Faecalibacterium of cecum were enhanced in yellow-feathered broilers fed with the EO supplemental diet, whereas the relative abundance of Negativibacillus, Eisenbergiella, Intestinimonas, and Flavonifractor were decreased (Figure 5C).
Spearman correlation analysis found that there was a correlation between the microbial abundance of the cecum of yellow-feathered broilers and the growth performance indicators of yellow-feathered broilers (Figure 5D). Faecalibacterium was significantly negatively correlated with F/G during d 1 to 21 and 22 to 42 of the yellow-feathered broilers. Lactobacillus was positively correlated with ADFI during d 1 to 21 and ADG during d 1 to 21 and 1 to 48, and negatively correlated with F/G during d 1 to 21 and 1 to 48. Negativibacillus was positively correlated with F/G during d 1 to 21, 22 to 42, and 1 to 48 and ADFI during d 42 to 48, and negatively correlated with ADGs during d 1 to 1 and 1 to 48. Intestinimonas was positively correlated with ADG during d 22 to 42 and negatively correlated with ADG during d 1 to 48. Flavonifractor is negatively correlated with ADFI during d 1 to 21, ADG during d 1 to 48, and F/G during d 22 to 42. Based on the FACOPEX database, the metabolic function of the cecum flora of yellow-feathered broilers was predicted. The results showed that the EO400 group enhanced the metabolic pathways of the 21-day-old cecum flora of yellow-feathered broilers, chemoheterotrophy, and fermentation.
DISCUSSION
E. coli and Salmonella,bacterias that live in human and animal intestines, are popular pathogenic bacteria (Mead and Griffin, 1998). A lot of antibiotics were used to prevent the development of these pathogens, which cause many pathogens to become resistant to antibiotics. Biofilms are reported to be a possible means to increase the resistance against antibiotic by promoting horizontal gene transfers (Fux et al., 2005). Essential oil (90% of pure oregano, thymol, cavacrol) has been confirmed that essential oils have some interesting effect on anti-biofilm formation of E. coli and Salmonella strains from pig feces and thus inhabited the bacterial growth (Oh et al., 2017). MIC is a common indicator to evaluate the antibacterial activity of antibacterial substances. The smaller the MIC value is, the stronger the antibacterial effect is. The smaller the MIC value is, the stronger the antibacterial effect is. Interestingly, in the present study, we found that the thymol or carvacrol also cloud limite the proliferation and biofilm formation of avian pathogenic E. coli O78 and Salmonella pullorum.
In order to investigate whether these 2 substances can also improve performance by inhibiting the proliferation of harmful bacteria in poultry, 1-day-old yellow-feathered broilers were selected and fed diets supplemented with a certain proportion of EO (thymol and carvacrol ≥35 g/kg), and the broilers were slaughtered at 21 and 48 d of age, and the related indexes were determined. The results of this study shown that the ADFI of broilers during d 1 to 21 and the ADG of broilers during d 1 to 21 and 22 to 42 and 1 to 48 were improved in the diet supplement with EO. Besides, the F/G of broilers during d 22 to 42 and 1 to 48 was reduced in the EO groups. Similar results have been found in previous studies. Peng et al. (2016) found that the addition of 600 mg/kg EO could significantly increase the whole ADG of AA broilers and reduce the F/G during d 1 to 21. Mohiti-Asli and Ghanaatparast-Rashti (2016) showed that adding 500 mg/kg EO could reduce F/G. Ramirez et al. (2021) found that adding oregano extract to the diet of laying hens can reduce F/G (Ramirez et al., 2021).
Serum TP is the sum of serum albumin and globulin, which has the combined transport of various substances and immune effects. Its synthesis site is mainly in the liver, and its content directly reflects the feed condition and the growth and physiological state of animals (Akrami et al., 2015). In this study, the concentration of TP of broilers was increased in the EO groups at d 48, which may be because EO can improve immune function and increase albumin level (Farouk et al., 2020). TC and TG in serum are important indicators of lipid metabolism, while HDL-C and LDL-C are indicators of lipid transport in the body. The concentration of TC of broilers was decreased by adding the OE the diet at d 21 in the present study, while the level of TG, HDL-C, and LDL-C on broilers has no difference between the CON group and EO groups. Previous studies have shown that the EO has a small influence on the lipid metabolism of the body (Abouelezz et al., 2019; Farouk et al., 2020). However, the specific mechanism of the small effect on lipid metabolism remains to be further explored. In addition, the activities of ALT and AST are indicators to evaluate liver health, and liver cell damage will lead to increased levels of ALT and AST (Li et al., 2014). Farouk et al. (2020) showed that the addition of excessive doses of EO increased the levels of AST and ALT of Japanese quails at 42 d of age (Farouk et al., 2020). Similarly, in this study, the activity of serum AST of yellow-feathered broilers was increased in the diet supplement with 200, 400, and 600 mg/kg EO at 48 d of age but reduced in the diet supplement with 600 mg/kg EO at 21 d of age, which indicate that dose and duration of action of EO could affect the liver function of broilers.
Excessive oxidative stress can affect not only the production performance of broilers but also the quality of meat (Zhang et al., 2011), and antioxidant enzymes are known to be the key molecules that reduce oxidative stress in the body (Zima et al., 1996; Mccord, 2000; Yesilbag et al., 2011). Furthermore, MDA is the main oxidation product of polyunsaturated fatty acids and an important index of lipid peroxidation, which can reflect the degree of oxidative damage in the body (Gao et al., 2020). Dong et al. (2021) found that the supplementation of EO enhanced the glutathione peroxidase activity and T-AOC in jejunum and ileum mucosa of Qingyuan chickens, and reduced the content of MDA in jejunum mucosa. Likewise in present study, the activities of T-AOC, SOD, and CAT of jejunum mucosa in yellow-feather broilers at d 21 and SOD at d 48 were increased by feeding the diet with EO. But differently, the content of MDA of jejunum mucosa was also enhanced in yellow-feather broilers at d 21 and 48. However, these results have on discovered on Peking duck and rabbit (Placha et al., 2013; Ding et al., 2020). These results reflected that adding EO to the diet can improve the antioxidant capacity of jejunal mucosa in broilers, but the effect is affected by time and dose.
Intestinal permeability is closely related to intestinal health. The good degree of intestinal morphology is related to the stability and health of the organism (Forte et al., 2016). Longer villi may increase the surface area for nutrient absorption (Paiva et al., 2014). The cells of intestinal crypt constantly differentiate to villi to form new cells to replace the exfoliated or damaged epithelial cells. The shallow crypt depth indicates that the cell generation rate of crypt decreases and the maturation rate of epithelial cells increases, thus indicating that the intestinal nutrient absorption function is enhanced. VH/CD is a suitable indicator for assessing nutrient digestion and absorption capacity (Montagne et al., 2004). In present study, the VH and VH/CD of jejunum in yellow-feather broilers at 21 d of age have enhanced in the EO groups. Meanwhile, CD of jejunum in yellow-feather broilers at 21 d of age has increased in the EO groups. But, compared with the CON group, villus height and crypt depth of jejunum in yellow-feather broilers at 48 d of age had different changes in EO group. These results may explain to some extent why EO can improve the growth performance of yellow-feather broilers in early stage.
Besides, Zo-1, Occludin, and Claudin-1, as the 3 most important tight junction proteins, are important protein molecules that constitute the integrity of intestinal mucosal barrier and determine intestinal permeability (Mazzon et al., 2002; Yang et al., 2005). Similarly to the results of intestinal morphology, the relative expression of Zo-1, Occludin, and Claudin-1 of jejunum in yellow-feather broilers at 21 d of age has increased in the EO groups, whereas decreased at 48 d of age. Futhermore, Liu et al. (2018) found that the gene expression of Occludin, Claudin-1, Claudin-5, ZO-1, and ZO-2 in the intestinal mucosa of Ross 308 broilers was significantly increased by feeding the diets containing 200, 300, and 400 μL of carvonol essential oil orally for 2 wk. On the other hand, DAO is an intracellular enzyme in the upper villi of intestinal mucosa, which can reflect the integrity and damage degree of intestinal mechanical barrier (Tian et al., 2013). Under normal physiological conditions, the content of DAO in serum is low, and when intestinal mucosa is damaged, it will increase (Ghaisas et al., 2015). In this study, the concentration of serum DAO of yellow-feather broilers at 21 d of age was reduced, but the DAO at 48 d of age was enhanced, which manifested that the effect of EO on intestinal barrier function of yellow-feathered broilers may be dose and time dependent (Wei et al., 2015).
Recent studies have found that the effects of plant extracts largely depend on the regulation of intestinal flora (Di et al., 2019; Zhao et al., 2019). Previous studies have shown that the intestinal flora structure of livestock and poultry can be affected by EO, which can improve the relative abundance of beneficial bacteria and reduce the relative abundance of harmful bacteria in general (He et al., 2017; Mohammed et al., 2019; Dong et al., 2021). The higher alpha diversity of cecal microbes in the EO groups and the significant differences in gut microbiota composition were observed in this study. Besides, the beneficial bacterium, such as Lactobacillus and Faecalibacterium, were improved in the EO groups. The only known species of Faecalibacterium, a new generation of probiotics, are Faecalibacterium prausnitzii and Faecalibacterium prausnitzii, which can produce butyrate and other short-chain fatty acids by fermentation of dietary fiber (Martín et al., 2017). Otherwise, in present study, the harmful bacterium was reduced in the EO groups such as Bacteroides. There are a variety of opportunistic pathogens in the genus Bacteroides, resistant to a variety of antibiotics, including β-lactam and aminoglycosides, which can lead to endogenous infections (Madigan, 2012). Bacteroides_thetaiotaomicron belongs to Bacteroides, which is an opportunistic pathogen that can promote the growth and virulence gene expression of pathogenic bacteria (Curtis et al., 2014). Spearman correlation analysis showed that there was a potential correlation between cecal microflora and growth performance indexes of yellow-feathered broilers, wherein Lactobacillus was positively correlated with ADFI and ADG but negatively correlated with F/G. Negativibacillus, Intestinimonas, and Flavonifractor were negatively correlated with the ADG of yellow-feathered broilers in the study, which revealed that EO may promote the growth of yellow-feathered broilers by increasing the proportion of beneficial bacteria and decreasing the proportion of harmful bacteria (He et al., 2017; Mohammed et al., 2019; Dong et al., 2021). The metabolic function of cecal microflora of yellow-feathered broilers was predicted based on FAPROTAX database. The results showed that EO400 group enhanced the chemoheterotrophy and fermentation metabolic pathways of cecal microflora of yellow-feathered broilers compared with the CON group. These results suggest that EO may improve the growth performance of yellow-feathered broilers by enhancing the metabolic capacity of cecal microflora.
In conclusion, the bacterial increment rate and biofilm formation of Avian pathogenic E. coli O78 and Salmonella pullorum after thymol and carvacrol treatment were limited. Furthermore, supplementation of EO could enhance growth by improving antioxidant enzyme activities, intestinal barrier function and intestinal microbiome composition of yellow-feathered broilers in the young age. Moreover, dietary 400 mg/kg EO addition showed better performance as a growth promoter and an antioxidant. Therefore, it can be concluded that the EO may play a pivotal role in replacing antibiotics in broilers; however, more studies are needed to assess the mechanism of action of EO on the growth performance of broilers.
ACKNOWLEDGMENTS
The authors are thankful to the National Key Research and Development Program of Intergovernmental Key Projects of China (Grant No: 2018YFE0101700), Support plan for scientific and technological innovation and entrepreneurship team of enterprises in Hunan Province (2020) and China Agriculture Research System of MOF and MARA (CARS-41-Z08) for providing fund support to complete this article.
DISCLOSURES
The authors declare that they have no conflict of interest to this work.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2022.102087.
Appendix. Supplementary materials
REFERENCES
- Abouelezz K., Abou-Hadied M., Yuan J., Elokil A.A., Wang G., Wang S., Wang J., Bian G. Nutritional impacts of dietary oregano and Enviva essential oils on the performance, gut microbiota and blood biochemicals of growing ducks. Animal. 2019;13:2216–2222. doi: 10.1017/S1751731119000508. [DOI] [PubMed] [Google Scholar]
- Akrami R., Gharaei A., Mansour M.R., Galeshi A. Effects of dietary onion (Allium cepa) powder on growth, innate immune response and hemato-biochemical parameters of Beluga (Huso huso linnaeus,1754) juvenile. Fish Shellfish Immuno. 2015;45:828–834. doi: 10.1016/j.fsi.2015.06.005. [DOI] [PubMed] [Google Scholar]
- Austgulen L.O., Solheim E., Scheline R.R. Metabolism in rats of pymene derivatives: carvacrol and thymol. Pharmacol. Toxicol. 2010;30:716–721. doi: 10.1111/j.1600-0773.1987.tb01783.x. [DOI] [PubMed] [Google Scholar]
- Barug D., De Jong J., Kies A.K., Verstegen M.W.A. 1st ed. Vol. 5. Wageningen Academic Publishers; the Netherlands: 2006. (Pages 14–18 in Antimicrobial Growth Promoters: Where Do We Go from Here?). [Google Scholar]
- Curtis M.M., Hu Z., Klimko C., Narayanan S., Deberardinis R., Sperandio V. The gut commensal bacteroides thetaiotaomicron exacerbates enteric infection through modification of the metabolic landscape. Cell Host Microbe. 2014;16:759–769. doi: 10.1016/j.chom.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Meo F., Margarucci S., Galderisi U., Crispi S., Peluso G. Curcumin, gut microbiota, and neuroprotection. Nutrients. 2019;11:426. doi: 10.3390/nu11102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner J.J., Richards J.D. Antibiotic growth promoters in agriculture: history and mode of action. Poult. Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Ding X., Wu X., Zhang K., Bai S., Wang J., Peng H.W., Xuan Y., Su Z., Zeng Q. Dietary supplement of essential oil from oregano affects growth performance, nutrient utilization, intestinal morphology and antioxidant ability in Pekin ducks. J. Anim. Physiol. Anim. Nutr. (Berl) 2020;104:1067–1074. doi: 10.1111/jpn.13311. [DOI] [PubMed] [Google Scholar]
- Dong R., Fan Q., Fouad A.M., Sun Y., Huang S., Wu A., L C., Kuang Z., Zhang C., Jiang S. Effects of dietary oregano essential oil supplementation on growth performance, intestinal antioxidative capacity, immunity and intestinal microbiota in yellow-feathered chickens. J. Anim. Sci. 2021;99 doi: 10.1093/jas/skab033. skab033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farouk S.M., Yusuf M.S., El Nabtiti A.A.S., Abdelrazek H.M.A. Effect of oregano essential oil supplementation on performance, biochemical, hematological parameters and intestinal histomorphometry of Japanese quail (Coturnix coturnix Japonica) Vet. Res. Forum. 2020;11:219–227. doi: 10.30466/vrf.2019.97574.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte C., Acuti G., Manuali E., Proietti P.C., Pavone S., Trabalza-Marinucci M., Moscati L., Onofri A., Lorenzetti C., Franciosini M.P. Effects of two different probiotics on microflora, morphology, and morphometry of gut in organic laying hens. Poult. Sci. 2016;95:2528–2535. doi: 10.3382/ps/pew164. [DOI] [PubMed] [Google Scholar]
- Fux C.A., Costerton J.W., Stewart P.S., Stoodley P. Survival strategies of infectious biofilms. Trends Microbiol. 2005;13:34–40. doi: 10.1016/j.tim.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Gao S., Li R., Heng N., Chen Y., Wang L., Li Z., Guo Y., Sheng X., Wang X., Xing K., Ni H., Qi X. Effects of dietary supplementation of natural astaxanthin from Haematococcus pluvialis on antioxidant capacity, lipid metabolism, and accumulation in the egg yolk of laying hens. Poult. Sci. 2020;99:5874–5882. doi: 10.1016/j.psj.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaisas S., Maher J., Kanthasamy A. Gut microbiome in health and disease: Lnking the microbiome-gut-brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol. Ther. 2015;158:52–62. doi: 10.1016/j.pharmthera.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghunaim H., Abu-Madi M.A., Kariyawasam S. Advances in vaccination against avian pathogenic Escherichia coli respiratory disease: potentials and limitations. Vet. Microbiol. 2014;172:13–22. doi: 10.1016/j.vetmic.2014.04.019. [DOI] [PubMed] [Google Scholar]
- Hashemipour H., Veldkamp T., Rubio L.A., Veldkamp T., Van Krimpen M.M. Effect of feed supplementation with a thymol plus carvacrol mixture, in combination or not with an NSP-degrading enzyme, on productive and physiological parameters of broilers fed on wheat-based diets. Anim. Feed Sci. Technol. 2016;211:117–131. [Google Scholar]
- He X., Hao D., Liu C., Zhang X., Wu R. Effect of supplemental oregano essential oils in diets on production performance and relatively intestinal parameters of laying hens. Am. J. Mol. Biol. 2017;07:73–85. [Google Scholar]
- Kabir S.M.L. Avian colibacillosis and salmonellosis: a closer look at epidemiology, pathogenesis, diagnosis, control and public health concerns. Int. J. Environ. Res. Public Health. 2010;7:89–114. doi: 10.3390/ijerph7010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.F., Tian H.Y., Zhang D.D., Jiang G.Z., Liu W.B. Feeding frequency affects stress, innate immunity and disease resistance of juvenile blunt snout bream Megalobrama amblycephala. Fish Shellfish Immunol. 2014;38:80–87. doi: 10.1016/j.fsi.2014.03.005. [DOI] [PubMed] [Google Scholar]
- Liu S.D., Song M., Yun W., Lee J., Lee C., Kwak W., Han N., Kim H., Cho J. Effects of oral administration of different dosages of carvacrol essential oils on intestinal barrier function in broilers. J. Anim. Physiol. Anim. Nutr. (Berl) 2018;102:1257–1265. doi: 10.1111/jpn.12944. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Madigan M.T., Martinko J.M., Stahl D., Clark D.P. 14th edn. Benjamin Cummings; Boston, MA: 2012. Brock Biology of Microorganisms. [Google Scholar]
- Martín R., Miquel S., Benevides L., Bridonneau C., Robert V., Hudault S., Chain F., Berteau O., Azevedo V., Chatel J.M., Sokol H., Bermúdez-Humarán L.G., Thomas M., Langella P. Functional characterization of novel faecalibacterium prausnitzii strains isolated from healthy volunteers: a step forward in the use of F. prausnitzii as a next-generation probiotic. Front. Microbiol. 2017;8:1226. doi: 10.3389/fmicb.2017.01226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzon E., Sturniolo G.C., Puzzolo D., Frisina N., Fries W. Effect of stress on the paracellular barrier in the rat ileum. Gut. 2002;51:507–513. doi: 10.1136/gut.51.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccord J.M. The evolution of free radicals and oxidative stress. Am. J. Med. 2000;108:652–659. doi: 10.1016/s0002-9343(00)00412-5. [DOI] [PubMed] [Google Scholar]
- Mead P.S., Griffin P.M. Escherichia coli O157: H7. Lancet. 1998;352:1207–1212. doi: 10.1016/S0140-6736(98)01267-7. [DOI] [PubMed] [Google Scholar]
- Michiels J., Missotten J., Dierick N., Fremaut D., Maene P., Smet S.D. In vitro degradation and in vivo passage kinetics of carvacrol, thymol, eugenol and trans-cinnamaldehyde along the gastrointestinal tract of piglets. J. Sci. Food Agric. 2008;88:2371–2381. [Google Scholar]
- Mohammed K., Abou-Hadied M., Yuan J., Elokil A.A., Wang G., Wang S., Wang J., Bian G. Nutritional impacts of dietary oregano and Enviva essential oils on the performance, gut microbiota and blood biochemicals of growing ducks. Animal. 2019;13:2216–2222. doi: 10.1017/S1751731119000508. [DOI] [PubMed] [Google Scholar]
- Mohiti-Asli M., Ghanaatparast-Rashti M. Comparing the effects of a combined phytogenic feed additive with an individual essential oil of oregano on intestinal morphology and microflora in broilers. J. Appl. Anim. Res. 2016;46:184–189. [Google Scholar]
- Montagne L., Cavaney F.S., Hampson D.J., Lallès J.P., Pluske J.R. Effect of diet composition on postweaning colibacillosis in piglets. J. Anim. Sci. 2004;82:2364–2374. doi: 10.2527/2004.8282364x. [DOI] [PubMed] [Google Scholar]
- Yang R.K., Harada T., Li J., Uchiyama T., Han Y., Englert J.A., Fink M.P. Bile modulatesintestinal epithelial barrier function via an extracellularsignal related kinase 1/2 dependent mechanism. Intensive Care Med. 2005;31:709–717. doi: 10.1007/s00134-005-2601-9. [DOI] [PubMed] [Google Scholar]
- Yang X., Liu Y., Yan F., Yang C., Yang X. Effects of encapsulated organic acids and essential oils on intestinal barrier, microbial count, and bacterial metabolites in broiler chickens. Poult. Sci. 2019;98:2858–2865. doi: 10.3382/ps/pez031. [DOI] [PubMed] [Google Scholar]
- Yesilbag D., Eren M., Agel H., Kovanlikaya A., Balci F. Effects of dietary rosemary, rosemary volatile oil and vitamin E on broiler performance, meat quality and serum SOD activity. Br. Poult. Sci. 2011;52:472–482. doi: 10.1080/00071668.2011.599026. [DOI] [PubMed] [Google Scholar]
- Ocel'Ová V., Chizzola R., Pisarčíková J., Novak J., Ivanišinoviá O., Faix Š. Effect of thyme essential oil supplementation on thymol content in blood plasma, liver, kidney and muscle in broiler chickens. Nat. Prod. Commun. 2016;11:1545–1550. [PubMed] [Google Scholar]
- Oh S.Y., Yun W., Lee J.H., Lee C.H., Kwak W.K., Cho J.H. Effects of essential oil (blended and single essential oils) on anti-biofilm formation of Salmonella and Escherichia coli. J. Anim. Sci. Technol. 2017;59:4. doi: 10.1186/s40781-017-0127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva D., Walk C., McElroy A. Dietary calcium, phosphorus, and phytase effects on bird performance, intestinal morphology, mineral digestibility, and bone ash during a natural necrotic enteritis episode. Poult. Sci. 2014;93:2752–2762. doi: 10.3382/ps.2014-04148. [DOI] [PubMed] [Google Scholar]
- Peng Q.Y., Li J.D., Li Z., Duan Z.Y., Wu Y.P. Effects of dietary supplementation with oregano essential oil on growth performance, carcass traits and jejunal morphology in broiler chickens. Anim. Feed. Sci. Technol. 2016;214:148–153. [Google Scholar]
- Pham V.H., Kan L., Huang J., Geng Y., Zhen W., Guo Y., Abbas W., Wang Z. Dietary encapsulated essential oils and organic acids mixture improves gut health in broiler chickens challenged with necrotic enteritis. J. Anim. Sci. Biotechnol. 2020;11:18. doi: 10.1186/s40104-019-0421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placha I., Chrastinova L., Laukova A., Cobanova K., Takacova J., Strompfova V., Chrenkova M., Formelova Z., Faix S. Effect of thyme oil on small intestine integrity and antioxidant status, phagocytic activity and gastrointestinal microbiota in rabbits. Acta Vet. Hung. 2013;61:197–208. doi: 10.1556/AVet.2013.012. [DOI] [PubMed] [Google Scholar]
- Ramirez S.Y., Peñuela-Sierra L.M., Ospina M.A. Effects of oregano (Lippia origanoides) essential oil supplementation on the performance, egg quality, and intestinal morphometry of Isa Brown laying hens. Vet. World. 2021;14:595–602. doi: 10.14202/vetworld.2021.595-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian R., Tan J.T., Wang R.L., Xie H., Qian Y.B., Yu K.L. The role of intestinal mucosa oxidative stress in gut barrier dysfunction of severe acute pancreatitis. Eur. Rev. Med. Pharmacol. Sci. 2013;17:349–355. [PubMed] [Google Scholar]
- Wei H.K., Chen G., Wang R.J., Peng J. Oregano essential oil decreased susceptibility to oxidative stress-induced dysfunction of intestinal epithelial barrier in rats. J. Funct. Foods. 2015;2015:1191–1199. [Google Scholar]
- Zeng Z., Zhang S., Wang H., Piao X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: a review. J. Anim. Sci. Biotechnol. 2015;6:7. doi: 10.1186/s40104-015-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Xiao S., Lee E.J., Ahn D.U. Consumption of oxidized oil increases oxidative stress in broilers and affects the quality of breast meat. J. Agric. Food Chem. 2011;59:969–974. doi: 10.1021/jf102918z. [DOI] [PubMed] [Google Scholar]
- Zhao H., Cheng N., Zhou W., Chen S., Wang Q., Gao H., Xue X., Wu L., Cao W. Honey polyphenols ameliorate DSS-Induced ulcerative colitis via modulating gut microbiota in rats. Mol. Nutr. Food Res. 2019;63 doi: 10.1002/mnfr.201900638. [DOI] [PubMed] [Google Scholar]
- Zhu N., Wang J., Yu L., Zhang Q., Chen K., Liu B. Modulation of growth performance and intestinal microbiota in chickens fed plant extracts or virginiamycin. Front. Microbiol. 2019;10:1333. doi: 10.3389/fmicb.2019.01333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zima T., Spicka I., Stípek S., Crkovská J., Pláteník J., Merta M., Nĕmecek K., Tesar V. [Lipid peroxidation and activity of antioxidative enzymes in patients with multiple myeloma] Casopís Lékar Ceskych. 1996;135:14–17. [PubMed] [Google Scholar]
- Franklin R., Cockerill M.D., III. Performance standards for antimicrobial susceptibility testing, twenty-first informational supplement M100-S21. Clinical and Laboratory Standards Institute (CLSI; Wayne, PA, USA: 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.