Abstract
Myxoedema can have a variety of presentations, from mild cognitive impairment to psychosis, to overt coma. While majority of cases have primary hypothyroidism as the underlying aetiology, very few cases have central hypothyroidism. We report two patients who presented with myxoedema and were diagnosed with central hypothyroidism. A man in his 50s with a history of panhypopituitarism presented with hypotension, slurring of speech and psychosis that worsened to coma. He was initially treated as adrenal crisis, and on failing to improve he was later treated correctly as myxoedema coma. A woman in her 30s presented with bradykinesia and shock and was diagnosed with Sheehan’s syndrome based on hormonal and imaging features. Both patients improved with a loading dose of oral thyroxine and intravenous steroids. Central hypothyroidism presenting with myxoedema is often complicated by coexisting pituitary hormone deficiencies. A high index of suspicion is required for better treatment outcomes.
Keywords: Pituitary disorders, Thyroid disease
Background
Myxoedema coma represents the most extreme form of hypothyroidism, so severe as to readily progress to death unless diagnosed promptly and treated vigorously.1 The term ‘myxedema coma’ is a misnomer as most of the patients present with an altered mental status more frequently than overt coma. Majority of the cases reported are of primary hypothyroidism, with only a few case reports of central hypothyroidism. In a recently published series of patients presenting with myxoedema, 17% had a central aetiology.2 Since it is a rare presentation and there are no clinical guidelines except a few case reports, patients presenting with myxoedema coma due to central hypothyroidism pose a diagnostic as well as a therapeutic challenge. We report two cases presenting with myxoedema and were found to have secondary hypothyroidism on evaluation.
Case presentation
Case 1
A man in his 50s presented to the emergency department (ED) with slurring of speech. He was previously diagnosed with panhypopituitarism secondary to empty sella following a road traffic accident 8 years ago. Since the diagnosis, he has been on hormonal supplementation (oral levothyroxine (LT4), prednisolone, monthly injection of testosterone depot), but from the last 4 months he had stopped taking his medications. He had a history of fever 1 week ago, followed by decreased oral intake. He also had complaints of easy fatigability, weight gain and increased frequency of urination from the last few months. A loss of interest in daily activities was also noticed by the family members. At presentation, his systolic blood pressure was 70 mm Hg. He was alert and although he had mild slurring of speech there was no focal neurological deficit. Considering the possibility of adrenal crisis, he was started on intravenous fluids, injection hydrocortisone and his previous oral dose of LT4. After initial resuscitation, he was shifted to the endocrinology ward. His haemodynamic parameters improved, but his sensorium worsened progressively from a psychotic state to near-coma and he was then shifted to the intensive care unit. He also developed bradycardia and hypothermia on subsequent days. With these new findings, myxoedema coma was considered a possibility and a loading dose of oral LT4 was given by means of a nasogastric tube (300 μg stat followed by 125 μg daily). Hypothermia was managed by passive rewarming. His sensorium started improving after a week of continued treatment. He later developed polyuria with rising sodium and was initiated on desmopressin tablets.
Routine biochemistry, radiology and cerebrospinal fluid analysis were done and ruled out sepsis or electrolyte imbalance. Electroencephalogram showed grade 2B generalised cerebral dysfunction. The patient’s hormonal testing done on the day of admission and MRI of the brain with pituitary protocol are depicted in table 1 and figure 1A, respectively.
Table 1.
Baseline haematological and biochemical findings in cases 1 and 2 (at the time of admission)
| Case 1 | Case 2 | Reference range (SI units) | |
| Haemoglobin, g/dL (g/L) | 9.6 (96) | 9 (90) | 13–16 (130–160) |
| Serum sodium, mEq/L (mmol/L) | 132 (132) | 128 (128) | 135–145 (135–145) |
| Serum cortisol (08:00), µg/dL (nmol/L) | – | 1.5 (41.4) | 5–12 (138–331.2) |
| Serum FT4, ng/dL (pmol/L) | 0.44 (5.66) | <0.4 (<5.15) | 0.8–1.85 (10.29–23.8) |
| Serum TSH, mIU/L | – | 0.31 | 0.5–5 |
| Serum prolactin, ng/mL | 6.8 | 9.2 | <25 ng/ml |
| Serum LH, mIU/mL (IU/L) | – | 1.5 (1.5) | 3–12 (3–12) |
| Serum FSH, mIU/mL (IU/L) | – | 3.44 (3.44) | 3–12 (3–12) |
FSH, follicle stimulating hormone; FT4, free thyroxine; LH, luteinising hormone; TSH, thyroid stimulating hormone.
Figure 1.
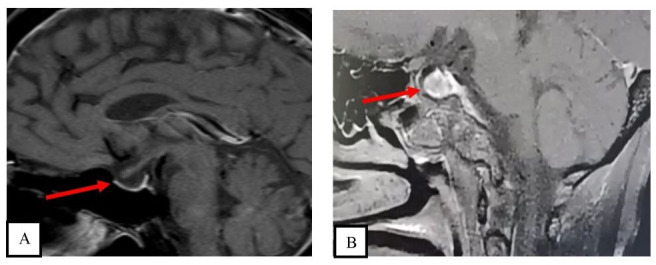
Sagittal sections of contrast-enhanced MRI showing (A) partial empty sella (arrow) in case 1 and (B) pituitary gland (12×10×9 mm) (arrow) with multiple enhancing areas in the anterior pituitary, with normal peripheral rim enhancement and with central thinned-out pituitary stalk in case 2.
Case 2
A woman in her 30s presented to the ED with vomiting, burning micturition and decreased oral intake for the past 15 days. On a detailed history-taking, it was found that the patient has been amenorrhoeic since her last delivery 1 year ago, which had resulted in a stillbirth. There was no history of severe postpartum blood loss or blood transfusion. There was history of slowness in daily activities, delay in speech and multiple hospital admissions in view of symptomatic hypoglycaemia since then. At presentation to our institute, she had pallor, coarse dry skin, slowness of speech and delayed tendon reflexes. On the second day she developed hypotension with worsening of sensorium. Biochemical investigations sent on the day of admission revealed hyponatraemia, anaemia and panhypopituitarism (table 1). The possibility of myxoedema coma due to secondary hypothyroidism and adrenal crisis was considered. The patient was given a loading dose of LT4 500 μg, through the nasogastric tube and injection hydrocortisone 100 μg intravenously followed by oral LT4 125 μg once a day and injection hydrocortisone 50 mg 6 hourly. She was given empiric antibiotic coverage and passive warming was done through blankets. After 3 days of treatment in the endocrinology ward, the patient exhibited improvement in sensorium with reversal of shock. MRI was performed after stabilisation and revealed bulky pituitary and features suggestive of sequelae to pituitary necrosis (figure 1B).
Outcome and follow-up
Both patients improved with oral thyroxine given in a loading dose followed by maintenance dose. The first patient was discharged after 2 weeks of presentation on oral LT4, steroids, depot testosterone and desmopressin. He has been on regular follow-up with us for a year and is doing well. The second patient was also discharged after 10 days on oral LT4 and steroids. She is on regular outpatient follow-up and was started on cyclical oestrogen and progesterone therapy.
Discussion
The two cases discussed here highlight the diagnostic and therapeutic challenge posed by myxoedema due to central hypothyroidism.
A number of factors have been known to precipitate myxoedema coma: sepsis, sedatives, hyponatraemia, hypoxia, cerebrovascular accidents and heart failure.1 Our first patient had a history of fever, likely viral fever, which precipitated the adrenal crisis and myxoedema. In the second case there was a history of urinary tract infection which precipitated the myxoedema. The neurological presentation in myxoedema coma can vary from lethargy to stupor, to coma.1 Our first patient presented with psychosis, with repetition of words and slurring, which then progressed to a rigid akinetic state, while the second patient presented with slurred speech progressing to a coma, highlighting the varied presentation of this clinical condition. Atypical presentation like psychosis led to delayed treatment initiation in the first case. Myxoedema was suspected when he developed coma, along with bradycardia and hypothermia. Altered sensorium in the background history of slurring of speech and slowness of activities led to a suspicion of myxoedema in the second case. As there are no clear-cut criteria available to diagnose myxoedema, there should be a high index of suspicion in any patient presenting with altered mental sensorium, confusion and lethargy. Treatment can be initiated based on clinical suspicion without waiting for laboratory results.
Both cases had normocytic normochromic anaemia on presentation that could be explained by severe hypothyroidism, coexisting sex steroids and glucocorticoid deficiency.3 Hyponatraemia was earlier considered to be a commonly associated electrolyte abnormality mostly due to increased water retention, but recent data suggest that the two may not be associated.4 5 Recurrent hypoglycaemia can be due to deficiency in growth hormone and cortisol or severe hypothyroidism, all of which can lead to impaired gluconeogenesis and depletion of glycogen stores.6 Our second patient had hyponatraemia and recurrent hypoglycaemia which improved after hydrocortisone supplementation. Patients with severe hypothyroidism can have various cardiac function and rhythm abnormalities.7 Bradycardia was seen in our first patient during admission and resolved after biochemical euthyroidism was achieved. Coexisting central diabetes insipidus may be masked by adrenal insufficiency in patients with panhypopituitarism and unmasked after glucocorticoid replacement, a finding which was seen in our first patient, who developed polyuria later on.8 Coexisting adrenal insufficiency can confound the clinical picture as happened in our first patient, which led to delay in making a diagnosis and initiating the appropriate dose of LT4.
Guidelines by the American Thyroid Association (ATA) recommend intravenous LT4 at a loading dose of 200–400 μg followed by maintenance dose as the primary treatment modality for myxoedema.9 Oral absorption can be impaired in patients with severe hypothyroidism.10 There are no trials comparing intravenous versus oral LT4 and a few reports have shown improvement with oral LT4 alone even in patients with severe hypothyroidism.11 Both our patients were treated with oral LT4, as intravenous preparations were not available at our centre, and both showed complete recovery. Conversion of LT4 to liothyronine (LT3) can be impaired in patients with severe hypothyroidism, so whether LT3 is appropriate for patients whose mental status does not improve in 24–48 hours after administration of LT4 is still controversial.11 The ATA suggests supplementing LT3, but a high dose should be avoided as it has been shown to increase mortality.12 Coexisting adrenal insufficiency needs to be ruled out in patients with secondary hypothyroidism with myxoedema coma. The ATA recommends empiric glucocorticoid coverage with intravenous glucocorticoids at appropriate stress doses (injection hydrocortisone 100 mg intravenous stat followed by 100 mg every 6 hours) for patients with myxoedema, which is to be continued until therapeutic endpoints are achieved, such as improvement in mental status and in cardiac and pulmonary function.9 Patients with coexisting adrenal insufficiency should be shifted to physiological doses of glucocorticoids (15–25 mg hydrocortisone or equivalent in divided doses) after achieving therapeutic endpoints.13 The aetiology of hypothyroidism had no influence on the outcome, as suggested in a recently published meta-analysis.2 Other supportive care for hypothermia, hypoventilation and empiric antibiotics in suspected sepsis should be given.9
Empty sella is a common finding on pituitary imaging in patients with central hypothyroidism after a pituitary insult.14 Our first patient had empty sella after a trauma, but in our second case MRI showed bulky pituitary with multiple hypoenhancing areas secondary to necrosis. A few reports have shown normal to enlarged pituitary during the evolving phase of Sheehan’s syndrome, suggesting the need for a sequential scan to further clarify the aetiology.15 Another differential in our second patient could be pituitary necrosis in a pre-existing adenoma, although a clear-cut adenoma was not discernible on the present MRI.
The reported rate of mortality in myxoedema is variable (20%–50%), with a decreasing trend due to advances in critical care.1 2 16 Both our patients recovered completely and are doing well on follow-up, emphasising better outcomes with early recognition and treatment.
Patient’s perspective.
My father used to feel ill and would skip work on most of the days before we brought him to the hospital. His speech was slurred and we were not able to understand his words. When he became unresponsive, we were scared. For almost 3–4 days in the hospital he did not respond. After a few days he started recognizing us and began to ask for food and water. After discharge now, he is taking all his medications on time, he is eating well and attending to his work. (Son of the first patient)
Learning points.
Central hypothyroidism as a cause of myxoedema is rare.
It can have varied neurological presentations.
Clinical presentation is often complicated by coexisting pituitary hormone deficiencies.
High index of suspicion is required in any patient presenting with altered mental sensorium with a history of pituitary insult.
Early recognition and appropriate management are key to good clinical outcome.
Footnotes
Contributors: KKau and KKad were involved in writing the manuscript. NB was involved in patient care and collecting patient data. KS was involved in critically revising the manuscript. All authors approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Wartofsky L. Myxedema coma. Endocrinol Metab Clin North Am 2006;35:vii–viii:687–98. 10.1016/j.ecl.2006.09.003 [DOI] [PubMed] [Google Scholar]
- 2.Dutta P, Bhansali A, Masoodi SR, et al. Predictors of outcome in myxoedema coma: a study from a tertiary care centre. Crit Care 2008;12:R1. 10.1186/cc6211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishioka H, Haraoka J. Hypopituitarism and anemia: effect of replacement therapy with hydrocortisone and/or levothyroxine. J Endocrinol Invest 2005;28:528–33. 10.1007/BF03347241 [DOI] [PubMed] [Google Scholar]
- 4.Wolf P, Beiglböck H, Smaijs S, et al. Hypothyroidism and hyponatremia: rather coincidence than causality. Thyroid 2017;27:611–5. 10.1089/thy.2016.0597 [DOI] [PubMed] [Google Scholar]
- 5.Sun GEC, Pantalone KM, Hatipoglu B. Hypothyroidism as a cause of hyponatremia: fact or fiction? Endocr Pract 2012;18:894–7. 10.4158/EP12130.OR [DOI] [PubMed] [Google Scholar]
- 6.Samaan NA. Hypoglycemia secondary to endocrine deficiencies. Endocrinol Metab Clin North Am 1989;18:145–54. 10.1016/S0889-8529(18)30393-1 [DOI] [PubMed] [Google Scholar]
- 7.Polikar R, Burger AG, Scherrer U, et al. The thyroid and the heart. Circulation 1993;87:1435–41. 10.1161/01.CIR.87.5.1435 [DOI] [PubMed] [Google Scholar]
- 8.Yang L-Y, Lin S, Xie Q-B, et al. Central diabetes insipidus unveiled by glucocorticoid therapy in a patient with an empty sella: a case report and literature review. Medicine 2020;99:e22939. 10.1097/MD.0000000000022939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonklaas J, Bianco AC, Bauer AJ, et al. Guidelines for the treatment of hypothyroidism: prepared by the American thyroid association Task force on thyroid hormone replacement. Thyroid 2014;24:1670–751. 10.1089/thy.2014.0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Read DG, Hays MT, Hershman JM. Absorption of oral thyroxine in hypothyroid and normal man. J Clin Endocrinol Metab 1970;30:798–9. 10.1210/jcem-30-6-798 [DOI] [PubMed] [Google Scholar]
- 11.Arlot S, Debussche X, Lalau JD, et al. Myxoedema coma: response of thyroid hormones with oral and intravenous high-dose L-thyroxine treatment. Intensive Care Med 1991;17:16–18. 10.1007/BF01708403 [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto T, Fukuyama J, Fujiyoshi A. Factors associated with mortality of myxedema coma: report of eight cases and literature survey. Thyroid 1999;9:1167–74. 10.1089/thy.1999.9.1167 [DOI] [PubMed] [Google Scholar]
- 13.Bornstein SR, Allolio B, Arlt W, et al. Diagnosis and treatment of primary adrenal insufficiency: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab 2016;101:364–89. 10.1210/jc.2015-1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiloiro S, Giampietro A, Bianchi A, et al. Empty sella syndrome: multiple endocrine disorders. Handb Clin Neurol 2021;181:29–40. 10.1016/B978-0-12-820683-6.00003-8 [DOI] [PubMed] [Google Scholar]
- 15.Morani A, Parmar H, Ibrahim M. Teaching NeuroImages: sequential MRI of the pituitary in Sheehan syndrome. Neurology 2012;78:e3. 10.1212/WNL.0b013e31823ed07d [DOI] [PubMed] [Google Scholar]
- 16.Rodríguez I, Fluiters E, Pérez-Méndez LF, et al. Factors associated with mortality of patients with myxoedema coma: prospective study in 11 cases treated in a single institution. J Endocrinol 2004;180:347–50. 10.1677/joe.0.1800347 [DOI] [PubMed] [Google Scholar]


