Abstract
Introduction
Studies have reported that antidiabetic medications (ADMs) were associated with lower risk of dementia, but current findings are inconsistent. This study compared the risk of dementia onset in patients with type 2 diabetes (T2D) treated with sulfonylurea (SU) or thiazolidinedione (TZD) to patients with T2D treated with metformin (MET).
Research design and methods
This is a prospective observational study within a T2D population using electronic medical records from all sites of the Veterans Affairs Healthcare System. Patients with T2D who initiated ADM from January 1, 2001, to December 31, 2017, were aged ≥60 years at the initiation, and were dementia-free were identified. A SU monotherapy group, a TZD monotherapy group, and a control group (MET monotherapy) were assembled based on prescription records. Participants were required to take the assigned treatment for at least 1 year. The primary outcome was all-cause dementia, and the two secondary outcomes were Alzheimer’s disease and vascular dementia, defined by International Classification of Diseases (ICD), 9th Revision, or ICD, 10th Revision, codes. The risks of developing outcomes were compared using propensity score weighted Cox proportional hazard models.
Results
Among 559 106 eligible veterans (mean age 65.7 (SD 8.7) years), the all-cause dementia rate was 8.2 cases per 1000 person-years (95% CI 6.0 to 13.7). After at least 1 year of treatment, TZD monotherapy was associated with a 22% lower risk of all-cause dementia onset (HR 0.78, 95% CI 0.75 to 0.81), compared with MET monotherapy, and 11% lower for MET and TZD dual therapy (HR 0.89, 95% CI 0.86 to 0.93), whereas the risk was 12% higher for SU monotherapy (HR 1.12 95% CI 1.09 to 1.15).
Conclusions
Among patients with T2D, TZD use was associated with a lower risk of dementia, and SU use was associated with a higher risk compared with MET use. Supplementing SU with either MET or TZD may partially offset its prodementia effects. These findings may help inform medication selection for elderly patients with T2D at high risk of dementia.
Keywords: Dementia, Preventive Medicine
WHAT IS ALREADY KNOWN ON THIS TOPIC.
Common pathophysiological patterns of type 2 diabetes (T2D) and dementia have inspired research on repurposing antidiabetic medications for dementia prevention and treatment.
Animal studies have reported cognitive-reverse capacity of metformin (MET) and thiazolidinediones (TZDs).
Large population studies and clinical trials for this topic are limited, and current findings are inconclusive.
WHAT THIS STUDY ADDS
Using large-scale real-world medical records, this study compared the effects of long-term MET, TZDs, and sulfonylurea (SU) use on the risk of dementia in a T2D population.
We found TZDs monotherapy associated with a 22% reduction in the risk of dementia, whereas SU monotherapy was associated with a 12% elevated risk, compared with MET-exclusive treatment.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Future studies for repurposing oral antidiabetic agents for dementia prevention may consider prioritizing TZDs.
SUs users could be at an elevated risk of dementia, compared with MET or TZD users. Thus, monitoring cognitive functions regularly is more important to this population.
Introduction
Dementia is a progressive neurodegenerative disorder which affected 55 million people globally in 2015 and increases by nearly 10 million cases per year.1 Type 2 diabetes (T2D) is associated with elevated risk of all-cause dementia, including its two main subtypes, Alzheimer’s disease (AD) and vascular dementia (VaD).2 3 The complex pathophysiology underlying this relationship may involve insulin resistance (IR), hyperglycemia, neuroinflammation, and altered energy homeostasis.4 There have been investigations of the use of antidiabetic medications (ADMs) for dementia (especially AD) prevention and treatment. Use of metformin (MET),5 thiazolidinedione (TZD),6 7 and intranasal insulin was reported to improve cognitive function, while use of sulfonylurea (SU) and regular insulin was associated with increased risks of dementia.8 9 However, these associations were not consistently observed.5–7 10–12 Inconsistencies among previous study findings may be due to small sample sizes, short follow-up time, heterogeneity in study populations or treatment group comparisons, and inadequate adjustment for confounding.5–7 10–12 In addition, patient characteristics such as degree of obesity may modify the treatment effects but have not been well examined.13 14
Using the VA electronic health records (EHRs), we compared the effects of three commonly prescribed oral ADMs, MET, SU, and TZD on dementia onset among veterans with T2D. Given that untreated patients with diabetes are in small numbers and with healthier phenotypes, we employed MET monotherapy as the active comparator. This setting also makes our study comparable with previous research. Our findings provide evidence for medication selections for patients with mild or moderate T2D who are at high risk of dementia.
Methods
Data sources and participants
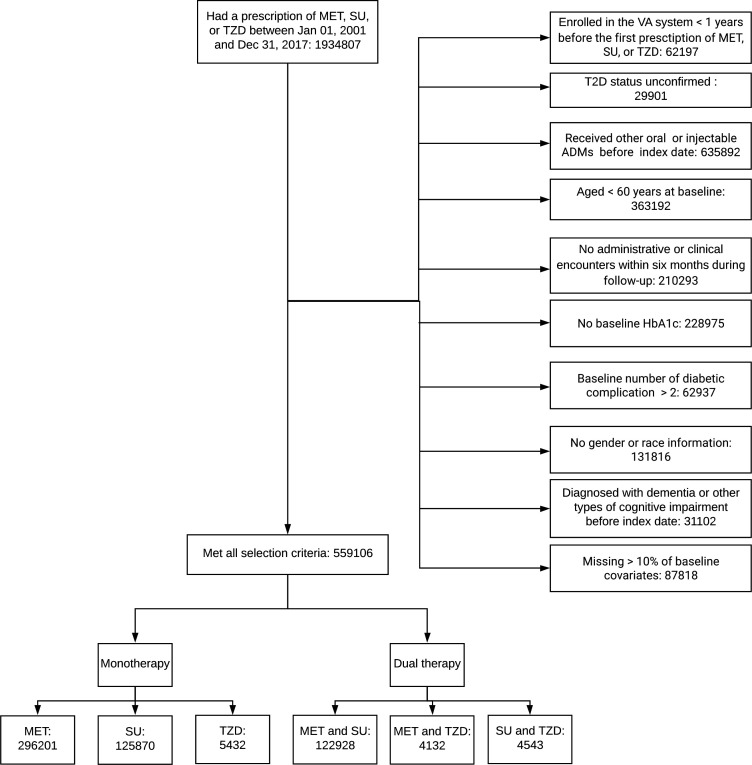
In this prospective cohort study, we used EHRs from the national Veteran Affairs (VA) Health System between January 2000 and December 2019. Data included participants’ demographics, lab results, prescriptions, and diagnoses using the International Classification of Diseases, 9th Revision (ICD-9), or the International Classification of Diseases, 10th Revision (ICD-10), codes in outpatient and inpatient healthcare settings. Figure 1 illustrates our cohort curation process. We included T2D participants who received their first MET, SU (tolbutamide, glimepiride, glipizide, or glyburide), or TZD (rosiglitazone or pioglitazone) prescription between January 2001 and December 2017. To identify new users, eligible participants were enrolled in the VA Health System for at least 1 year without any oral or injectable ADM prescription before receiving their first MET, SU, or TZD prescription. We define a baseline period as 12 months preceding and the 6 months following the first ADM prescription. This permitted 1.5 years to collect baseline characteristics and avoid ADM switches. Treatment assignment was determined according to the ADM received after the baseline period.15 An exposure period was defined as the initial 1-year (primary analysis) or 2-year period (secondary analysis) following the baseline period, and included participants who were only those using MET, SU, or TZD for glucose control during the period. Follow-up for outcomes started 1 year (or 2 years for the secondary analysis) after the end-of-baseline period and was referred to as the index date. We required enrolled participants to have two T2D diagnosis encounters at least 30 days apart (ICD-9-CM 250.xx, except 250.x1 and 250.x3; ICD-10-CM E11.9, E10, or E14) or two glycated hemoglobin (HbA1c) measures above 7% from separate visits during 1 year before the baseline. Additional baseline inclusion criteria were (1) ≥60 years old; (2) having HbA1c, race, and gender information; and (3) having ≤2 diabetes complications.16 Participants who had dementia, other cognitive conditions, brain injury before the index date, or missing information for over 10% of baseline covariates were excluded.17
Figure 1.
Cohort assembling process for the primary analysis. HbA1c, glycated hemoglobin; MET, metformin; SU, sulfonylurea; T2D, type 2 diabetes; TZD, thiazolidinedione; VA, Veteran Affairs.
Exposure
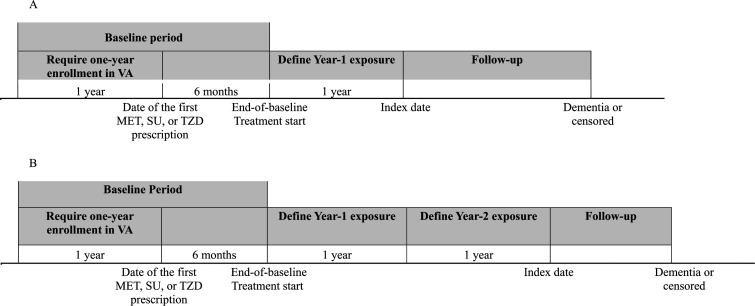
In the primary analysis, we compared the effects of at least 1 year of MET, SU, or TZD monotherapy or two-drug therapies (in combination or prescribed sequentially) on the risk of incident dementia. Exposure was defined as using a medication (determined by days of coverage) longer than one-third of days from the end-of-baseline period to the index date, that is, total days of exposure (figure 2A).18 Treatment groups were mutually exclusive. Patients who did not meet this exposure threshold or were exposed to more than two ADMs were excluded. We extended the 1-year exposure period to 2 years in the secondary analysis and re-evaluated the treatment effects (figure 2B). Participants were required to be dementia-free and stayed on the same treatment in the first and the second exposure years.
Figure 2.
Timeline and schema of the study. MET, metformin; SU, sulfonylurea; TZD, thiazolidinedione; VA, Veteran Affairs.
Outcomes
The primary outcome was all-cause dementia diagnosed during follow-up, defined as having at least two outpatient diagnosis codes at least 30 days apart. The date of the first diagnosis was referred to as the event date. We used the ICD-9 list developed by the VA Dementia Steering Committee, validated by recent studies.16 17 We converted the list to ICD-10. Secondary outcomes were VaD and AD, requiring two diagnosis codes at least 30 days apart. online supplemental table S1 in the online supplemental material provides outcome ICD codes.
bmjdrc-2022-002894supp001.pdf (606.4KB, pdf)
Other covariates
Most patients’ characteristics were extracted during the 1.5-year baseline period, while age, sex, race, household income, and height were extracted within 5 years. Due to the low numbers of non-black minorities, race was aggregated as white, black, and other. Covariates included age, sex, race, household income, calendar year of individual baseline, baseline biomarkers (HbA1c, lipid levels, body mass index (BMI), and systolic and diastolic blood pressure), statin use, number of diabetes complications in the Diabetes Complications Severity Index, and 27 selected Elixhauser’s comorbidities (online supplemental table S1).19 20 Several Elixhauser’s comorbidities, including drug abuse, psychoses, depression, hypothyroidism, renal failure, non-dementia neurological diseases, and each cardiovascular condition, were treated as independent covariates because of their close association with cognition. In contrast, others were aggregated as a weighted sum according to Elixhauser’s algorithm,19 20 as applied before.11 12
Statistical analysis
A propensity score (PS) indicating a participant’s likelihood of receiving MET monotherapy was calculated by multivariable logistic regressions using all covariates listed previously. To avoid results driven by PS extremes (ie, very small PS), we stabilized the weights (inverse of PS) by multiplying them by the marginal probability of receiving MET monotherapy.21 Each PS model showed good discriminatory accuracy (all C-statistics >0.85). The PS between groups were overlapping before stabilization (online supplemental figure S1), but the overlap was substantially increased with stabilization (online supplemental figure S2).
Participants were followed up until events of interest occurred or until death, December 31, 2019, or no clinical encounter in 6 months (referred to as loss of follow-up), whichever occurred first. An inverse probability treatment weighted (IPTW) Cox proportional hazards model was used to assess the treatment effects on the risk of all-cause dementia, AD, and VaD with MET monotherapy group serving as the reference. Models were adjusted for baseline HbA1c, calendar year of individual baseline, comorbidity counts, statin use, and BMI, which remained unbalanced after weighting (defined as having a standardized mean differences higher than 0.1 after IPTW in any pair of comparisons (see online supplemental table S2 in the online supplemental material for details).22 All variables met the proportional hazards assumption evaluated by Schoenfeld residuals.23
To assess whether ADMs and incident dementia were linked through glucose control and use of medical resources, we compared temporal patterns of annual HbA1c levels, frequency of clinical encounters, and hypoglycemia events during follow-up among treatment groups by linear mixed models. Hypoglycemia was defined as having a diagnosis record or having a blood glucose measure of <0.7 g/L.24 To explore whether ADMs’ impact on the risk of dementia was associated with prevention of vascular diseases, we conducted survival analysis to evaluate the risk of a vascular composite outcome (ie, myocardial infarction (MI) or atherosclerosis of arteries). Death was served as a competing risk.
Subgroup analyses were conducted for a set of pre-specified conditions (baseline age, HbA1c, and BMI). When the interactions with treatment arms were statistically significant, treatments were compared with MET monotherapy in each stratum, and between-stratum differences were evaluated by Student’s t-test for dependent samples. Risks of secondary outcomes were not assessed for interactions due to the limited number of cases. Underweight (BMI <18.5 kg/m2) participants were excluded when assessing BMI due to the small sample size.
Sensitivity analysis
As dementia is commonly underdiagnosed, we expanded the primary outcome to include having two fills of one or more types of antidementia drugs (donepezil, rivastigmine, galantamine, memantine, or aducanumab) within 6 months. We examined whether longer drug supplies (ie, cumulative supply of ADM for more than two-thirds of days per exposure year) affected treatment effects as a dose–response analysis. We also repeated the primary analyses after excluding patients with congestive heart failure (CHF) and after excluding patients with myocardial ischemic (MI) or atherosclerosis of arteries at baseline, as TZD may be specifically avoided in this condition.25 The main analyses were also repeated by treating the death as a competing risk in the Cox models to avoid potential non-informative censoring bias. Finally, using cancer as a negative control outcome that is not believed to cause dementia, we re-evaluated the 1-year treatment effect and further investigated bias and confounding factors associated with our study.
Analyses were conducted using SAS V.9.4. A two-sided p value of <0.05 was considered statistically significant.
Results
Of the potential participants, 559 106 (6.7%) met the selection criteria (figure 1) and were largely white (76.8%), male (96.9%), and obese (63.1%) (online supplemental table S3). The mean age was 65.7 (SD 8.7) years old, and the mean HbA1c was 6.8% (SD 1.0) at baseline and age was lowest in the MET group. The cohort incidence rate of all-cause dementia of 8.2 cases per 1000 person-years was highest in the two-drug group of SU and TZD (13.4 cases per 1000 person-years) and lowest in the MET monotherapy group (6.2 cases per 1000 person-years). Approximately 10.1% and 8.2% of all-cause dementia (31 125) could be attributed to AD and VaD, respectively. The MET monotherapy group had the highest loss to follow-up rate (64.1%), and the TZD monotherapy group had the highest mortality rate (39.2%) (online supplemental table S4).
Primary analysis (1-year treatment)
Compared with MET monotherapy, SU monotherapy was associated with a 12% higher risk of all-cause dementia (HR 1.12, 95% CI 1.09% to 1.15%) and a 14% higher risk of VaD (HR 1.14, 95% CI 1.04% to 1.24%) (table 1). In contrast, TZD monotherapy was associated with a 22% lower risk of all-cause dementia (HR 0.78, 95% CI 0.75% to 0.81%), an 11% lower risk of AD (HR 0.89, 95% CI 0.79% to 0.99%) and a 57% lower risk of VaD (HR 0.43, 95% CI 0.37% to 0.51%). Additionally, the two-drug therapy of MET and TZD lowered the risk of all-cause dementia, but SU-involved therapies increased the risks of all-cause dementia and VaD. When we accounted for death as a competing risk, TZD’s protective effects were slightly attenuated by 2%–5%, while SU’s hazards increased by 5%–10%. Thus, including death as a competing risk did not change the significance of the reported treatment effects.
Table 1.
Estimated HRs and 95% CIs of patients with incident dementia for T2D treated with SU, TZD, MET and SU, MET and TZD, and SU and TZD compared with MET monotherapy*
| All-cause dementia | AD | VaD | |||||||
| Treatment | Event | Unadjusted HR (95% CI) | Adjusted HR (95% CI)† | Event | Unadjusted HR (95% CI) | Adjusted HR (95% CI)† | Event | Unadjusted HR (95% CI) | Adjusted HR (95% CI)† |
| Primary analysis: 1-year exposure | |||||||||
| SU | 11 689 | 1.14 (1.11 to 1.16) | 1.12 (1.09 to 1.15) | 1200 | 0.99 (0.92 to 1.08) | 0.97 (0.90 to 1.05) | 922 | 1.14 (1.04 to 1.25) | 1.14 (1.04 to 1.24) |
| TZD | 450 | 0.81 (0.77 to 0.83) | 0.78 (0.75 to 0.81) | 62 | 0.93 (0.83 to 1.05) | 0.89 (0.79 to 0.99) | 19 | 0.44 (0.37 to 0.52) | 0.43 (0.37 to 0.51) |
| MET and SU | 6725 | 1.17 (1.14 to 1.2) | 1.14 (1.11 to 1.18) | 634 | 1.10 (1.01 to 1.21) | 1.04 (0.95 to 1.14) | 611 | 1.09 (0.99 to 1.20) | 1.14 (1.04 to 1.26) |
| MET and TZD | 247 | 0.95 (0.91 to 0.98) | 0.89 (0.86 to 0.93) | 22 | 0.89 (0.79 to 0.99) | 0.81 (0.72 to 0.92) | 11 | 0.51 (0.44 to 0.59) | 0.48 (0.41 to 0.56) |
| SU and TZD | 493 | 1.11 (1.07 to 1.15) | 1.04 (1.01 to 1.08) | 55 | 0.93 (0.83 to 1.04) | 0.85 (0.76 to 0.95) | 30 | 1.13 (1.00 to 1.27) | 1.11 (0.99 to 1.25) |
| Exploratory analysis: 2-year exposure | |||||||||
| SU | 7031 | 1.19 (1.15 to 1.22) | 1.14 (1.11 to 1.18) | 689 | 1.09 (0.98 to 1.21) | 1.03 (0.93 to 1.14) | 557 | 1.20 (1.07 to 1.35) | 1.18 (1.05 to 1.33) |
| TZD | 285 | 0.62 (0.60 to 0.65) | 0.65 (0.62 to 0.68) | 36 | 0.73 (0.64 to 0.83) | 0.77 (0.67 to 0.89) | 5 | 0.27 (0.21 to 0.34) | 0.28 (0.22 to 0.36) |
| MET and SU | 3944 | 0.88 (0.85 to 0.91) | 0.91 (0.88 to 0.95) | 369 | 0.81 (0.73 to 0.91) | 0.84 (0.75 to 0.93) | 346 | 0.90 (0.80 to 1.02) | 0.91 (0.81 to 1.04) |
| MET and TZD | 151 | 0.66 (0.63 to 0.69) | 0.69 (0.66 to 0.72) | 14 | 0.86 (0.75 to 0.98) | 0.89 (0.78 to 1.01) | 7 | 0.14 (0.10 to 0.19) | 0.14 (0.10 to 0.20) |
| SU and TZD | 317 | 0.88 (0.84 to 0.91) | 0.9 (0.87 to 0.94) | 32 | 0.55 (0.48 to 0.65) | 0.57 (0.49 to 0.66) | 19 | 1.06 (0.92 to 1.23) | 1.08 (0.94 to 1.25) |
Boldfaced values representing estimated HRs are statistically different from those with a p value of <0.05.
*In the 1-year MET monotherapy group, the numbers of incident all-cause dementia, AD, and VaD were 11 521, 1169, and 949, respectively, and in the 2-year MET monotherapy group, the numbers of events were 7352, 719, and 583.
†Models were adjusted on the calendar year of individual baseline, baseline age, statin use, HbA1c, systolic blood pressure, diastolic blood pressure, and congestive heart failure history collected at baseline, because these variables remained unbalanced after matching, at least on the comparison.
AD, Alzheimer’s disease; HbA1c, glycated hemoglobin; MET, metformin; SU, sulfonylurea; T2D, type 2 diabetes; TZD, thiazolidinedione; VaD, vascular dementia.
When exploring the underlying mechanisms of the results, treatment groups did not significantly differ from MET monotherapy in terms of HbA1c changes, clinical encounter frequencies (on average 13 visits/year, IQR 7–25) and hypoglycemia rates (on average 1.2 event/year, IQR 0–3) during follow-up. The differences in HbA1c changing rates during the follow-up period were under 0.02% per year (online supplemental figure S3). Moreover, we found that the risks of MI or atherosclerosis were lower in TZD users and higher in SU users, which had the same patterns as risks for any dementia (online supplemental table S5). In a sensitivity analysis that identified all-cause dementia by ICD codes or use of antidementia medication, all-cause dementia incidence increased at similar levels across groups (ranged 0.6%–1.8%). Still, the overall patterns remained the same as in the primary analysis (online supplemental table S6). Excluding patients with CHF or excluding patients with MI or atherosclerosis at baseline did not affect the overall conclusion (online supplemental table S7). Negative control outcome analysis did not identify the presence of residual bias (online supplemental table S5).
Secondary analysis and subgroup analyses
Extending the ADM exposure to 2 years did not change the patterns of all-cause dementia risks with SU monotherapy and two-drug therapy of MET and TZD, while TZD monotherapy became more protective. The two-drug therapy of MET and SU became protective for all-cause dementia (HR 0.91, 95% CI 0.88 to 0.95). Previous patterns of AD or VaD risks across groups remained similar (table 1).
We detected interactions between ADM therapies and baseline age, HbA1c, and BMI for risk of all-cause dementia. Thus, the risk was reassessed in six subgroups, defined by cut-offs derived from previous studies or clinical guidelines. Compared with 1 year MET monotherapy, the protective effects of 1-year TZD monotherapy or the two-drug therapy of MET with TZD were more significant in participants ≤75 years old with BMI of >25 kg/m2 than in participants >75 years old and with normal BMI, respectively (table 2). Compared with MET monotherapy, the risk of dementia with SU use was stronger in higher BMI participants. TZD use was associated with reduced dementia risk among 2-year treatment comparisons, and the risk reduction was even greater in overweight or obese participants. The two-drug therapy of MET and SU now became protective (table 2). Analysis based on ADM supply days also indicated that more consistent TZD use (as dual therapy with MET or as monotherapy) was associated with lower risks of all-cause dementia, while more consistent monotherapy SU use was linked to higher risks (online supplemental table S8).
Table 2.
Subgroup analysis of the risk of incident dementia among ADM treatment comparisons (MET monotherapy served as the reference)
| Age | BMI | HbA1c | |||||||
| ≤75 years | >75 years | P value* | 18.5–25 kg/m2 | ≥25 kg/m2 | P value* | ≤7% | >7% | P value* | |
| n=466 855 | n=92 251 | n=37 476 | n=521 082 | n=175 806 | n=383 300 | ||||
| Primary analysis: 1-year exposure† | |||||||||
| SU | 1.12 (1.08 to 1.16) | 1.11 (1.07 to 1.16) | 0.14 | 1.07 (0.99 to 1.15) | 1.14 (1.09 to 1.18) | 0.06 | 1.14 (1.10 to 1.17) | 1.09 (1.03 to 1.15) | 0.04 |
| TZD | 0.67 (0.64 to 0.71) | 0.91 (0.86 to 0.96) | 0.01 | 0.98 (0.88 to 1.08) | 0.72 (0.68 to 0.77) | 0.01 | 0.79 (0.76 to 0.82) | 0.71 (0.65 to 0.78) | 0.02 |
| MET and SU | 1.15 (1.11 to 1.19) | 1.16 (1.11 to 1.22) | 0.26 | 0.98 (0.90 to 1.06) | 1.17 (1.12 to 1.23) | 0.01 | 1.15 (1.11 to 1.19) | 1.14 (1.08 to 1.20) | 0.53 |
| MET and TZD | 0.86 (0.82 to 0.9) | 0.91 (0.86 to 0.96) | 0.01 | 0.90 (0.81 to 1.00) | 0.89 (0.84 to 0.94) | 0.69 | 0.93 (0.89 to 0.97) | 0.82 (0.76 to 0.90) | 0.01 |
| SU and TZD | 1.12 (1.07 to 1.17) | 1.05 (0.99 to 1.11) | 0.01 | 1.06 (0.96 to 1.17) | 0.90 (0.85 to 0.96) | 0.19 | 1.00 (0.96 to 1.04) | 1.13 (1.05 to 1.22) | 0.01 |
| Exploratory analysis: 2-year exposure† | |||||||||
| SU | 1.13 (1.08 to 1.18) | 1.12 (1.06 to 1.17) | 0.53 | 1.02 (0.93 to 1.12) | 1.15 (1.10 to 1.21) | 0.01 | 1.11 (1.11 to 1.19) | 1.13 (1.06 to 1.21) | 0.19 |
| TZD | 0.69 (0.65 to 0.73) | 0.72 (0.67 to 0.78) | 0.02 | 0.74 (0.65 to 0.84) | 0.63 (0.59 to 0.68) | 0.32 | 0.67 (0.64 to 0.71) | 0.60 (0.53 to 0.67) | 0.04 |
| MET and SU | 0.98 (0.94 to 1.03) | 1.07 (1.01 to 1.14) | 0.01 | 0.79 (0.71 to 0.88) | 0.9 (0.86 to 0.96) | 0.01 | 0.93 (0.89 to 0.97) | 0.88 (0.83 to 0.94) | 0.04 |
| MET and TZD | 0.69 (0.65 to 0.74) | 0.81 (0.75 to 0.88) | 0.01 | 0.84 (0.73 to 0.96) | 0.74 (0.69 to 0.80) | 0.01 | 0.67 (0.64 to 0.71) | 0.75 (0.67 to 0.83) | 0.02 |
| SU and TZD | 1.03 (0.97 to 1.08) | 0.93 (0.87 to 1.01) | 0.01 | 1.02 (0.91 to 1.15) | 0.88 (0.82 to 0.94) | 0.01 | 0.84 (0.80 to 0.88) | 1.04 (0.94 to 1.13) | 0.01 |
Boldfaced values representing estimated HRs were statistically different from 0 with p value of <0.05.
The interactions between treatment and baseline age, BMI, and HbA1c were all statistically significant with a p value of <0.001.
*P value for testing the interaction effects between treatment and baseline age, BMI, and HbA1c categories.
†Models were adjusted for age, calendar year of individual baseline, statin use, HbA1c, systolic blood pressure, diastolic blood pressure, and congestive heart failure history collected at baseline because these variables remained unbalanced after matching, in at least one comparison.
ADM, antidiabetic medication; BMI, body mass index; HbA1c, glycated hemoglobin; MET, metformin; SU, sulfonylurea; TZD, thiazolidinedione.
Discussions
We found that TZD monotherapy was associated with reductions in risk of all-cause dementia compared with use of MET or SU among participants with T2D. The use of TZD with MET or SU showed a lower risk for all-cause dementia than MET monotherapy. In addition, TZD-related treatments were associated with much lower risks of VaD. This is consistent with the prior reports that TZDs can reduce carotid atherosclerosis and incident strokes.26 27 Vascular diseases increase the risk of AD,28 so TZD’s reduction in VaD may also reduce AD development. Some studies comparing TZD with either placebo or standard care within patients with T2D have reported reduced risk of AD.6 7 Comparing with MET monotherapy helps provide a relevant active comparator as it is the most used ADM, is a middle-of-the-road glucose-lowering drug, and has not been linked with increased incident dementia. In contrast, comparisons with untreated patients with diabetes would be complicated by their small numbers and healthier phenotype.
Subgroup analyses show that participants aged ≤75 years benefited more from TZD use than older participants, perhaps highlighting the difficulty of successfully intervening in more advanced disease stages and the importance of early prevention for dementia. TZD use also appeared to be more protective in overweight or obese participants. This may result as TZD reduces central obesity,29 a recognized risk factor for dementia.13
Our results add substantially to the literature concerning the effects of ADM on dementia where previous findings have been inconsistent.5–7 11 Studies with follow-up time less than 3 years have mainly reported null associations, while studies with longer follow-up time typically yielded protective findings.5–7 11 12 With a mean follow-up time of 6.8 years, we had a sufficient duration to detect treatment differences. Another strength of this study was that we required ADM supplies for more than one-third of the time per exposure year and observed similar patterns of dementia risks as studies that controlled for treatment doses or frequency.7 11 We also found that treatment effects increased with treatment durations and drug supply days, which implies a dose–response relationship. Finally, we controlled the treatment misclassification rate by setting a 6-month drug adaptative period.15
The complex pathways linking T2D with incident dementia require studies to control many confounders.30 31 Traditional multivariate regression is ineffective and even invalid in this situation.32 We used stabilized IPTW with a comprehensive list of covariates to address the issue. Orkaby et al compared MET with SU uses with similar statistical strategies as our study and reported findings consistent with ours.11 To assess surveillance bias in dementia detection during the long follow-up period, we examined patterns of HbA1c during follow-up, clinical visit frequencies, and hypoglycemia event frequencies. These factors did not explain the observed differences in treatment effects.
Dementia misclassification is possible using EHR-based observational studies because dementia is commonly underdiagnosed.1 Since IPTW balanced participants’ characteristics, we assumed equal rates of dementia underestimation across each group. To strengthen the robustness of our results, we broadened the dementia diagnosis to include the use of antidementia medications. With dementia cases increased by up to 8%, this did not affect the results. However, phenotyping algorithms using EHRs to distinguish dementia subtypes remain challenging. In our study, less than 20% of all-cause dementia could be coded as AD and VaD, lower than the real-world rate.3 This may reflect the higher frequency of initial dementia diagnoses by primary care providers and their tendency to provide more non-specific codes for dementia than neurologists and geriatricians.33 Newcomer et al reported that 80% of AD cases were underdiagnosed when using a single year of medical claims,34 but the percent declined to 13% after extending the claims-extraction period to 5 years.35 For VaD detection, a 75% sensitivity and a 74% specificity of ICD-10 codes were reported.36 In our study, the misclassification rate of AD could be higher than VaD, but the long follow-up time may partially offset the difference. Additionally, we excluded participants diagnosed with cognitive conditions or brain injury before follow-up, making results less biased by pre-existing conditions. We observed a high loss to follow-up rate among MET monotherapy users, which may lead to underestimating dementia rates in this population. Combined with the relatively low loss to follow-up rate in TZD users, our results provided a conservative estimation of the TZD treatment effects for dementia.
The study is also subject to residual confounding due to missing data (eg, kidney function) or unavailable information (eg, risk genes) in the database. The Food and Drug Administration (FDA) restricted TZD use in 2010 and again eased its use later, which changed clinicians’ prescription patterns.37 Although we did not find TZD’s treatment effects were mediated by year of treatment initiation (estimated by year of baseline), future studies may consider stratifying the analysis by the timing of FDA’s announcement. Although our use of MET as the comparator does not allow us to identify the specific relationship of MET use with dementia, its common use, mid-range effects on glucose control, apparently relatively neutral impact on dementia provides important advantages for comparison of early-stage diabetes medications. Given the predominately white and male VA population, future studies among more diverse populations are needed to confirm the findings.
In summary, TZD users had a lower risk of dementia, and SU users had a higher risk of dementia than MET users among T2D participants. The protective effects of TZD were more substantial for overweight or obese patients. Our findings provide additional information to aid clinicians’ selection of ADMs for patients with mild or moderate T2D and are at high risk of dementia.
Footnotes
Contributors: Guarantor: XT and JJZ
Conceptualization and design: RDB, ZC, LVF, YK, PR, KR, RM, XT, and JJZ. Resources: PR and JJZ. Acquisition, analysis, or interpretation of data: ZC, PR, RM, XT, and JJZ. Drafting of the manuscript: XT and JJZ. Critical revision of the manuscript for important intellectual content: PR, RM, XT, and JJZ. Supervision: JJZ.
Funding: This research was partially funded by grants from the National Human Genome Research Institute (R01HG006139 JJZ), the National Science Foundation (DMS-2054253 JJZ), the National Institute of Diabetes and Digestive and Kidney Disease (K01DK106116 JJZ), and the National Heart, Lung, and Blood Institute (R21HL150374 JJZ and PDR; R21HL150268 to P.D.R.).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Analysis results that are mentioned in the manuscript but are not shown in tables are available upon request. Aggregated and deidentified participant data that are not reported in the manuscript are available upon request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study was approved by the Phoenix VA Institutional Review Board.
References
- 1. Organization WH . Global status report on the public health response to dementia 2021.
- 2. Cheng G, Huang C, Deng H, et al. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta-analysis of longitudinal studies. Intern Med J 2012;42:484–91. 10.1111/j.1445-5994.2012.02758.x [DOI] [PubMed] [Google Scholar]
- 3. Ott A, Stolk RP, van Harskamp F, et al. Diabetes mellitus and the risk of dementia: the Rotterdam study. Neurology 1999;53:1937–42. 10.1212/WNL.53.9.1937 [DOI] [PubMed] [Google Scholar]
- 4. Sims-Robinson C, Kim B, Rosko A, et al. How does diabetes accelerate Alzheimer disease pathology? Nat Rev Neurol 2010;6:551–9. 10.1038/nrneurol.2010.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hsu C-C, Wahlqvist ML, Lee M-S, et al. Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J Alzheimers Dis 2011;24:485–93. 10.3233/JAD-2011-101524 [DOI] [PubMed] [Google Scholar]
- 6. Heneka MT, Fink A, Doblhammer G. Effect of pioglitazone medication on the incidence of dementia. Ann Neurol 2015;78:284–94. 10.1002/ana.24439 [DOI] [PubMed] [Google Scholar]
- 7. Chou P-S, Ho B-L, Yang Y-H. Effects of pioglitazone on the incidence of dementia in patients with diabetes. J Diabetes Complications 2017;31:1053–7. 10.1016/j.jdiacomp.2017.01.006 [DOI] [PubMed] [Google Scholar]
- 8. editors.;:.Scherrer JF, Salas J, Floyd JS, et al. Metformin and sulfonylurea use and risk of incident dementia. Mayo clinic proceedings. Elsevier, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reger MA, Watson GS, Frey WH, et al. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging 2006;27:451–8. 10.1016/j.neurobiolaging.2005.03.016 [DOI] [PubMed] [Google Scholar]
- 10. Lu C-H, Yang C-Y, Li C-Y, et al. Lower risk of dementia with pioglitazone, compared with other second-line treatments, in metformin-based dual therapy: a population-based longitudinal study. Diabetologia 2018;61:562–73. 10.1007/s00125-017-4499-5 [DOI] [PubMed] [Google Scholar]
- 11. Orkaby AR, Cho K, Cormack J, et al. Metformin vs sulfonylurea use and risk of dementia in US veterans aged ≥65 years with diabetes. Neurology 2017;89:1877–85. 10.1212/WNL.0000000000004586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cheng C, Lin C-H, Tsai Y-W, et al. Type 2 diabetes and antidiabetic medications in relation to dementia diagnosis. J Gerontol A Biol Sci Med Sci 2014;69:1299–305. 10.1093/gerona/glu073 [DOI] [PubMed] [Google Scholar]
- 13. Whitmer RA, Gustafson DR, Barrett-Connor E, et al. Central obesity and increased risk of dementia more than three decades later. Neurology 2008;71:1057–64. 10.1212/01.wnl.0000306313.89165.ef [DOI] [PubMed] [Google Scholar]
- 14. Akomolafe A, Beiser A, Meigs JB, et al. Diabetes mellitus and risk of developing Alzheimer disease: results from the Framingham study. Arch Neurol 2006;63:1551–5. 10.1001/archneur.63.11.1551 [DOI] [PubMed] [Google Scholar]
- 15. Grant RW, Wexler DJ, Watson AJ, et al. How doctors choose medications to treat type 2 diabetes: a national survey of specialists and academic generalists. Diabetes Care 2007;30:1448–53. 10.2337/dc06-2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilkinson T, Ly A, Schnier C, et al. Identifying dementia cases with routinely collected health data: A systematic review. Alzheimers Dement 2018;14:1038–51. 10.1016/j.jalz.2018.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barnes DE, Byers AL, Gardner RC, et al. Association of mild traumatic brain injury with and without loss of consciousness with dementia in US military veterans. JAMA Neurol 2018;75:1055–61. 10.1001/jamaneurol.2018.0815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dailey G, Kim MS, Lian JF. Patient compliance and persistence with antihyperglycemic drug regimens: evaluation of a Medicaid patient population with type 2 diabetes mellitus. Clin Ther 2001;23:1311–20. 10.1016/S0149-2918(01)80110-7 [DOI] [PubMed] [Google Scholar]
- 19. Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27. 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 20. Young BA, Lin E, Von Korff M, et al. Diabetes complications severity index and risk of mortality, hospitalization, and healthcare utilization. Am J Manag Care 2008;14:15–23. [PMC free article] [PubMed] [Google Scholar]
- 21. Robins JM, Hernán Miguel Ángel, Brumback B. Marginal structural models and causal inference in epidemiology. 11. Lww, 2000: 550–60. 10.1097/00001648-200009000-00011 [DOI] [PubMed] [Google Scholar]
- 22. Cohen J. Statistical power analysis for the behavioral sciences. Academic press, 2013. [Google Scholar]
- 23. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515–26. 10.1093/biomet/81.3.515 [DOI] [Google Scholar]
- 24. Ginde AA, Blanc PG, Lieberman RM, et al. Validation of ICD-9-CM coding algorithm for improved identification of hypoglycemia visits. BMC Endocr Disord 2008;8:4. 10.1186/1472-6823-8-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nesto RW, Bell D, Bonow RO, et al. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American heart association and American diabetes association. October 7, 2003. Circulation 2003;108:2941–8. 10.1161/01.CIR.0000103683.99399.7E [DOI] [PubMed] [Google Scholar]
- 26. Kernan WN, Viscoli CM, Furie KL, et al. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med 2016;374:1321–31. 10.1056/NEJMoa1506930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Saremi A, Schwenke DC, Buchanan TA, et al. Pioglitazone slows progression of atherosclerosis in prediabetes independent of changes in cardiovascular risk factors. Arterioscler Thromb Vasc Biol 2013;33:393–9. 10.1161/ATVBAHA.112.300346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dickstein DL, Walsh J, Brautigam H, et al. Role of vascular risk factors and vascular dysfunction in Alzheimer's disease. Mt Sinai J Med 2010;77:82–102. 10.1002/msj.20155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Akazawa S, Sun F, Ito M, et al. Efficacy of troglitazone on body fat distribution in type 2 diabetes. Diabetes Care 2000;23:1067–71. 10.2337/diacare.23.8.1067 [DOI] [PubMed] [Google Scholar]
- 30. Stewart R, Liolitsa D. Type 2 diabetes mellitus, cognitive impairment and dementia. Diabet Med 1999;16:93–112. 10.1046/j.1464-5491.1999.00027.x [DOI] [PubMed] [Google Scholar]
- 31. Geijselaers SLC, Sep SJS, Stehouwer CDA, et al. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol 2015;3:75–89. 10.1016/S2213-8587(14)70148-2 [DOI] [PubMed] [Google Scholar]
- 32. Shah BR, Laupacis A, Hux JE, et al. Propensity score methods gave similar results to traditional regression modeling in observational studies: a systematic review. J Clin Epidemiol 2005;58:550–9. 10.1016/j.jclinepi.2004.10.016 [DOI] [PubMed] [Google Scholar]
- 33. Cho K, Gagnon DR, Driver JA, et al. Dementia coding, workup, and treatment in the Va new England healthcare system. Int J Alzheimers Dis 2014;2014:1–5. 10.1155/2014/821894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Newcomer R, Clay T, Luxenberg JS, et al. Misclassification and selection bias when identifying Alzheimer's disease solely from Medicare claims records. J Am Geriatr Soc 1999;47:215–9. 10.1111/j.1532-5415.1999.tb04580.x [DOI] [PubMed] [Google Scholar]
- 35. Taylor DH, Fillenbaum GG, Ezell ME. The accuracy of medicare claims data in identifying Alzheimer's disease. J Clin Epidemiol 2002;55:929–37. 10.1016/S0895-4356(02)00452-3 [DOI] [PubMed] [Google Scholar]
- 36. Knopman DS, Parisi JE, Boeve BF, et al. Vascular dementia in a population-based autopsy study. Arch Neurol 2003;60:569–75. 10.1001/archneur.60.4.569 [DOI] [PubMed] [Google Scholar]
- 37. Woodcock J, Sharfstein JM, Hamburg M. Regulatory action on rosiglitazone by the U.S. food and drug administration. N Engl J Med 2010;363:1489–91. 10.1056/NEJMp1010788 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2022-002894supp001.pdf (606.4KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Analysis results that are mentioned in the manuscript but are not shown in tables are available upon request. Aggregated and deidentified participant data that are not reported in the manuscript are available upon request.