Abstract
Water-in-oil emulsion incomplete Freund’s adjuvant (IFA) has been used as an adjuvant in preventive and therapeutic vaccines since its development. New generation, highly purified modulations of the adjuvant, Montanide incomplete seppic adjuvant (ISA)-51 and Montanide ISA-720, were developed to reduce toxicity. Montanide adjuvants are generally considered to be safe, with adverse events largely consisting of antigen and adjuvant dose-dependent injection site reactions (ISRs). Peptide vaccines in Montanide ISA-51 or ISA-720 are capable of inducing both high antibody titers and durable effector T cell responses. However, an efficient T cell response depends on the affinity of the peptide to the presenting major histocompatibility complex class I molecule, CD4+ T cell help and/or the level of co-stimulation. In fact, in the therapeutic cancer vaccine setting, presence of a CD4+ T cell epitope seems crucial to elicit a robust and durable systemic T cell response. Additional inclusion of a Toll-like receptor ligand can further increase the magnitude and durability of the response. Use of extended peptides that need a processing step only accomplished effectively by dendritic cells (DCs) can help to avoid antigen presentation by nucleated cells other than DC. Based on recent clinical trial results, therapeutic peptide-based cancer vaccines using emulsions in adjuvant Montanide ISA-51 can elicit robust antitumor immune responses, provided that sufficient tumor-specific CD4+ T cell help is given in addition to CD8+ T cell epitopes. Co-treatment with PD-1 T cell checkpoint inhibitor, chemotherapy or other immunomodulatory drugs may address local and systemic immunosuppressive mechanisms, and further enhance efficacy of therapeutic cancer peptide vaccines in IFA and its modern variants. Blinded randomized placebo-controlled trials are critical to definitively prove clinical efficacy. Mineral oil-based adjuvants for preventive vaccines, to tackle spread and severity of infectious disease, induce immune responses, but require more studies to reduce toxicity.
Keywords: Adjuvants, Immunologic; Immunogenicity, Vaccine; Adaptive Immunity; Review; Immunotherapy
Introduction
Origin of incomplete Freund’s adjuvant (IFA)
In 1916, Le Moignic and Pinoy found that a suspension of killed Salmonella typhimurium in Vaseline oil with lanolin was capable of boosting immune responses.1 Based on this finding, Jules Freund developed complete Freund’s adjuvant (CFA). CFA is a potent adjuvant consisting of mineral oil with killed mycobacteria. Later, Freund discovered that including the killed mycobacteria was not crucial for adjuvant function and that antigen in water-in-paraffin oil emulsion was just as effective in increasing and extending specific antibody production.2 This led to the development of IFA, which is a water-in-oil emulsion without killed mycobacteria. A large follow-up study in 1953 with 18,000 US Army personnel, immunized against influenza in mineral oil adjuvant, showed no long-term effect of IFA on collagen diseases, neoplasia or death.3–5 However, the adjuvant induced a high incidence of vaccine-site toxicity and allergy manifestations. Since then, new generations of IFA have been developed to reduce injection site toxicity using more highly purified mineral oils, including Montanide incomplete seppic adjuvants (ISA), such as Montanide ISA-51 and Montanide ISA-720, which are commonly used as adjuvants in therapeutic cancer vaccines.6 Montanide ISA-720 is a water-in-oil emulsion which has been an effective vaccine adjuvant in animals for inducing antibody responses. There is also experience using it in human clinical trials with vaccines against infectious agents. Numerous cancer vaccines have been developed with Montanide ISA-51 as a water-in-oil emulsion. This approach continues to be used extensively in clinical trials of cancer vaccines in humans. In the present review, we focus on the use of IFA in cancer vaccines: Montanide ISA-51 and Montanide ISA-720 will be mentioned when the exact formulation was used in studies, whereas IFA refers broadly to IFA-based water-in-oil emulsions.
Mechanism of action of IFA/Montanide ISA-51
The classic mechanism of action of a water-in-oil emulsion is the formation of a depot which supports slow release of antigen at the injection site (figure 1). In mice, this depot can remain present at the vaccination site and continue to release antigen for months after immunization.7 8 However, IFA can also induce an adjuvant effect when injected separately from the antigen, even at distant sites, although with a lower potency than when injected simultaneously.6 Antibody responses after peptide vaccination in IFA partially depend on NOD2, but not MyD88, signaling.9 It is speculated that this effect is due to cellular damage induced by the injection, although this remains to be proven. In general, water-in-oil emulsions induce inflammation and recruit immune cells, including antigen-presenting cells (APCs) and lymphocytes. Due to the nature of the emulsion and interactions with cell membranes, IFA may facilitate antigen uptake by APC.10 Immunization in IFA can effectively induce antibody and T cell responses.7 9 However, there have been conflicting perspectives on the effectiveness of vaccination with peptides in IFA for induction of T cell responses in particular. It has been suggested that its effectiveness may depend on the route of immunization and the length, immunogenicity and/or CD4+ T helper cell requirement of the peptide(s) in question. These issues are discussed in detail below.
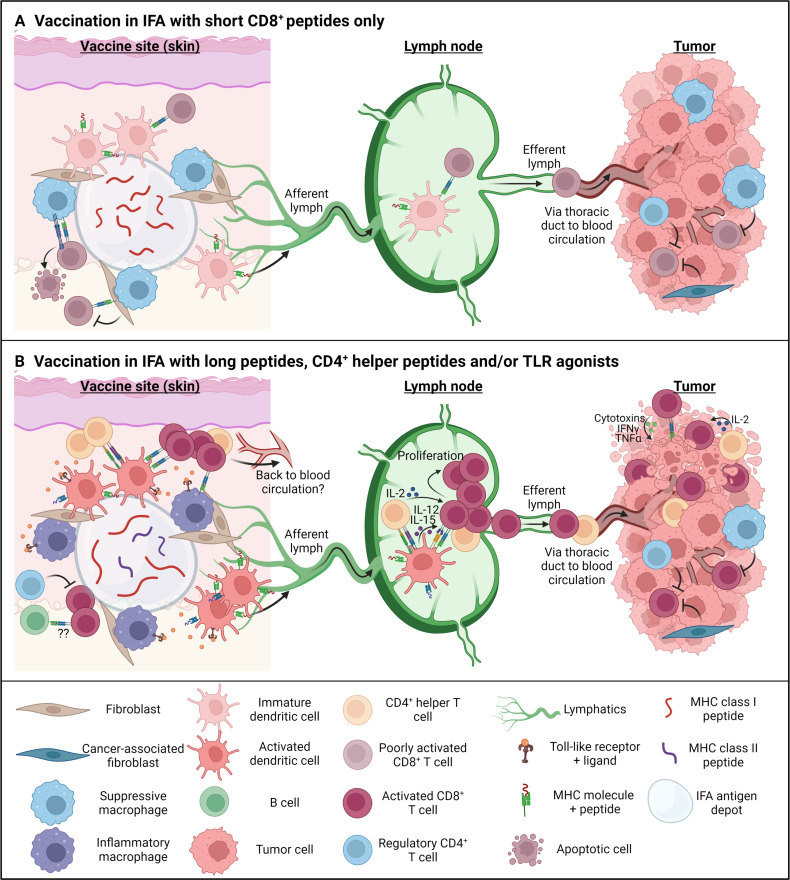
Figure 1.
Mechanism of action for incomplete Freund’s adjuvant (IFA) as a peptide vaccine adjuvant under different vaccination strategies. (Immunization with short major histocompatibility complex (MHC) class I peptides in an IFA antigen depot causes slow release of the antigen into the vaccine site microenvironment (VSME). Due to the poor immunogenicity of IFA alone, dendritic cells (DCs) will remain immature or poorly activated and, in the absence of co-stimulation and CD4+ T cell help, cause a poorly activated or even tolerogenic CD8+ T cell response in the vaccine-draining lymph node (VDLN). Furthermore, the nature of the short peptide antigen allows for direct deposition on MHC class I molecules, without the need of antigen processing and causes accumulation and FasL-mediated apoptosis of tumor-specific T cells at the vaccine site. Due to the poor activation and specific deletion at the vaccine sites, few CD8+ cells likely end up in the tumor microenvironment, where they will have to overcome additional suppression by cancer-associated fibroblasts, suppressive macrophages, and regulatory T cells. (B)Immunization with synthetic long peptides, short peptides including both MHC class I and class II epitopes in IFA, and/or adding a Toll-like receptor (TLR) agonist as additional adjuvant, creates a more inflammatory VSME. DCs receive additional maturation signals through the TLR agonists, which induces expression of MHC molecules and co-stimulatory receptors, leading to proper CD8+ T cell priming in the lymph node. The addition of CD4+ T cell epitopes provides CD4 help locally at the VSME/tumor site and also during priming both in the lymph node and at the effector stage in the tumor. Inflammatory signals will furthermore create inflammatory macrophages and enhance the expression of inflammatory cytokines and chemokines in the VSME, causing the accumulation of B and T cells. T cells in this nursery likely can return to the blood circulation and traffic to the tumor site. On arrival, properly activated T cells can recognize and kill tumor cells through cytotoxins and cytokines. However, suppressive mechanisms remain present in the tumor microenvironment and may have to be targeted separately to ensure full efficacy of therapeutic cancer vaccines. IFN-γ, interferon-γ; TNF-α, tumor necrosis factor-α. Figure made with Biorender.com.
Lessons from using IFA as a vaccine adjuvant in mice
In mice, comprehensive studies have been performed to compare the effects of different vaccine parameters on the cytotoxic T cell response, when using IFA as an adjuvant. There has been a wide variation in findings, with studies reporting induction of tolerance,11–17 induction of a transient CD8+ T cell response8 18–21 or induction of a robust and durable CD8+ T cell response.22–28 From these studies, it becomes clear that IFA, as a vaccine adjuvant in mice, may induce both immune protection and immune tolerance, depending on the model system. In this section, we aim to dissect the different components contributing to the induction of tolerance or protection using IFA, in the interest of guiding decisions about the use of IFA in future clinical trials.
Peptide formulation
CD8+ T cells recognize small peptides usually containing 8–10 amino acids, presented in the binding pocket of MHC class I molecules.29 These may be defined as ‘short’ peptides, when they exactly fit the MHC class I molecule. When vaccinating with these short peptides, they can directly bind to and be presented by MHC class I molecules on professional APC such as dendritic cells (DCs) without the need for antigen processing by DC. However, such short peptides can also directly bind the empty pockets of MHC class I molecules in non-professional APC that express MHC class I, that is, basically all nucleated cells, most of which do not express co-stimulatory molecules (eg, CD80, CD86) and which can be tolerizing.11–16 On the other hand, longer peptides, containing more amino acids than the CD8+ T cell epitope alone, can only be processed and cross-presented efficiently by DC, and thus do not directly bind the MHC pocket on cells other than APC.16 21 30 Thus, peptide length can have a significant effect on immunogenicity. Furthermore, binding affinity and stability of the peptide:MHC class I complex affects the immunogenicity of the peptide.31
When considering the effectiveness of IFA as an antigen depot after vaccination, these different aspects of peptide formulation have been evaluated for their impact on immunogenicity. For certain peptides, there was no difference in the immune response whether the epitope was contained in a short or longer peptide.23–25 Peptide vaccination with a Sendai parainfluenza virus peptide nucleoprotein (NP)321-336 in IFA generated a strong CD8+ cytotoxic T lymphocyte (CTL) response that protected mice against a lethal dose of Sendai virus.23 A very similar protective effect was obtained by vaccinating with the exact H-2 Kb MHC class I-binding 9-mer peptide NP324-332.24 However, when a tolerogenic response was induced with short peptide vaccination in IFA, such as with two unrelated adenovirus peptides,13 14 this could be overcome by long peptide vaccines19 21 or by vaccination with activated DC loaded ex vivo before vaccination with the short exact MHC-binding peptide.13
Another situation in which an undesirable effect of short peptide vaccination was noted is the death of PMEL-1 gp100-specific transgenic T cells that accumulated at the vaccination site following short peptide vaccination after adoptive transfer of large numbers of naïve PMEL-1 T cells.16 17 The inclusion of CD40-specific antibodies, TLR7 agonist Imiquimod and systemic high-dose recombinant IL-2 in this context did not rescue the response. However, vaccination with a long peptide incorporating the same CTL epitope induced far less T cell accumulation at the vaccine site and led to a robust systemic T cell response and a strong antitumor response.16 This type of result may be peptide-specific or may reflect details of that experimental model, because it did not occur in the experiments of Kast et al24 who observed equally strong protection with a short or long Sendai virus peptide in IFA, nor did it occur in the experiments of Ossendorp et al,32 who also noted excellent protection by a short Moloney virus peptide in IFA, although this protection could be further improved by combination with a helper epitope.24 32 Also, in the recent clinical study with short melanoma-associated HLA class I-binding peptides in Montanide ISA-51, excellent systemic T cell responses against melanoma-associated peptides were induced.33 Importantly, this study clearly shows that the subcutaneous vaccine sites served as a nursery for T cells rather than as a T cell sink. Again, in this study a tetanus helper peptide was also included and may have had an impact. Furthermore, there always are challenges in translating the murine dose to a human dose, but the volume of the vaccine emulsion and of the IFA likely have a role in the extent to which T cells may be recruited to that site. In almost all murine studies, each mouse received a 100–200 µL emulsion including 50–100 µL IFA per mouse: on a volume/body weight basis, this may be comparable to a human dose of 200–400 mL emulsion containing 100–200 mL of Montanide ISA-51. Thus, the mouse dose of IFA was equivalent to 100–200 times what is used in humans. In summary, the results of T cell death at the vaccine site16 17 may have been caused by the combined effects of the use of T cell receptor transgenic T cells, use of this particular short peptide, lack of productive T cell help, sustained peptide presentation by cells other than professional APC, causing TCR triggering in the absence of co-stimulation, use of a very high peptide vaccine dose and/or use of a very high dose of IFA.
Immunization route and role of helper epitopes
Intraperitoneal injection of lymphocytic choriomeningitis virus (LCMV) NP118-132 peptide in IFA or high doses of peptide injected subcutaneously induced tolerance, whereas subcutaneous injection of the same peptide at a lower dose induced proper CD8+ cytotoxic T cell responses.12 22 34 Repeated subcutaneous immunization with low-dose peptide furthermore reduced the magnitude of the T cell response compared with a one-time vaccination. Systemic, long-term presence of peptide through the IFA-mediated antigen depot blunted the memory response, which could be rescued when the memory CD8+ T cells were adoptively transferred into naïve mice.8 However, these results may again have been influenced by the lack of appropriate CD4+ T helper cell epitopes in the peptide vaccine. Regardless, route of immunization will play a role in the composition of a local response at the vaccine site, and thereby affect the systemic and/or long-term availability of antigen. In clinical trials testing cancer peptide vaccines in Montanide ISA-51, patients are predominantly treated subcutaneously (table 1); however, when vaccines are used as preventative vaccines against viral infections or parasites, mucosal or intramuscular immunization is preferred to trigger the immune response directly at the site of infection or optimize antibody responses, respectively. For improved therapeutic effects against mucosal tumors, non-specific immune stimulation of the mucosal site by, for example, CpG may be applied, even though the specific vaccine is delivered subcutaneously, inducing a systemic T cell response.35
Table 1.
Current active trials according to clinicaltrials.gov on May 13, 2022
| Therapeutic cancer vaccine trials | ||||||||||
| NCT number | Disease | Phase | Vaccine type | Antigen | Epitope specificity | Additional adjuvant | Additional treatment(s) | Trial design | Route | Primary endpoint |
| NCT04864418 | Advanced solid cancer | I | Peptide | TAA | CD4+ Th1 cell | GM-CSF | N/A | Sequential assignment (dose-escalation) | s.c. | Toxicity |
| NCT04106115 | Bladder cancer | I/II | Peptide | TAA | CD8+ T cell | N/A | Durvalumab | Single group assignment | s.c. | Toxicity and progression-free survival |
| NCT02229084 | Breast cancer | I/II | Peptide | TAA | Pan-T cell | N/A | Doxorubicin, cyclophosphamide, docetaxel/paclitaxel | Non-randomized, sequential assignment | s.c. | Safety/tolerability, clinical response |
| NCT02938442 | Breast cancer | I/II | Peptide | TAA | Pan-T cell | N/A | Doxorubicin, cyclophosphamide, paclitaxel | Randomized, parallel assignment | s.c. | Safety/tolerability, clinical response |
| NCT04270149 | Breast cancer | I | Peptide | TAA | Pan-T cell | GM-CSF | N/A | Single group assignment | s.c. | Toxicity |
| NCT03362060 | Breast cancer | I | Peptide | TAA | CD8+ T cell | Poly-ICLC | Pembrolizumab | Single group assignment | Not specified | Immune response |
| NCT05243862 | Colorectal cancer | II | Peptide | TAA | Pan-T cell | N/A | Atezolizumab | Single group assignment | s.c. | Safety and toxicity |
| NCT05350501 | Colorectal cancer | II | Peptide | TAA | Pan-T cell | N/A | Nivolumab | Single group assignment | Not specified | Response to treatment |
| NCT02795988 | Gastric cancer | I/II | Peptide | TAA | B cell | N/A | Standard-of-care chemotherapy | Randomized, parallel assignment | Not specified | Safety, recommended dose, clinical efficacy |
| NCT04842513 | Glioblastoma | I | Peptide | Not specified | Pan-T cell | XS15 | Radiation therapy and temozolomide | Single group assignment | s.c. | Toxicity and immunogenicity |
| NCT05163080 | Glioblastoma | II | Peptide | TAA | Pan-T cell | GM-CSF | Temozolomide | Randomized, double-blind, placebo-controlled | s.c. | Overall survival |
| NCT04280848 | Glioblastoma | I/II | Peptide | Telomerase | CD4+ Th1 cell | N/A | N/A | Single group assignment | s.c. | T cell responses in blood |
| NCT02455557 | Glioblastoma | II | Peptide | TAA | Pan-T cell | GM-CSF | Temozolomide | Single group assignment | s.c. | Progression-free survival |
| NCT04013672 | Glioblastoma | II | Peptide | TAA | Pan-T cell | GM-CSF | Pembrolizumab | Non-randomized, parallel assignment | Not specified | Progression-free survival |
| NCT03893903 | Glioma | I | Peptide | TAA | B and T cell | N/A | Avelumab | Randomized, Parallel assignment | s.c. | Safety and tolerability |
| NCT02193347 | Glioma | I | Peptide | TAA | Pan-T cell | GM-CSF | Temozolomide, tetanus/diphtheria vaccine | Single group assignment | i.d. | Toxicity |
| NCT05320809 | Hematologic malignancies | I | Peptide | TAA | Pan-T cell | N/A | N/A | Single group assignment | s.c. | Toxicity and immunogenicity |
| NCT03946358 | HPV positive cancers | II | Peptide | Telomerase | CD4+ Th1 cell | N/A | Atezolizumab | Single group assignment | s.c. | Objective response rate |
| NCT04298606 | Lung cancer | Early I | Protein | EGF | B cell | N/A | N/A | Single group assignment | s.c. | Antibody titer, biomarker analysis, toxicity |
| NCT02818426 | Lung cancer | I/II | Peptide | Telomerase | CD4+ Th1 cell | N/A | N/A | Single group assignment | s.c. | Dose-limiting toxicity and immunogenicity |
| NCT04432207 | Lung cancer | I | Peptide | PD-1 | B cell | Measles-specific T cell peptide | N/A | Sequential assignment (dose-escalation) | s.c. | Safety and optimal biological dose |
| NCT02818426 | Lung cancer | I/II | Peptide | Telomerase | CD4+ Th1 cell | N/A | N/A | Single group assignment | s.c. | Toxicity and immunogenicity |
| NCT02955290 | Lung or head and neck cancer | I/II | Protein | EGF | B cell | N/A | Nivolumab, pembrolizumab | Non-randomized, Parallel assignment | i.m. | Toxicity and overall survival |
| NCT03929029 | Melanoma | Ib | Peptide | TAA | Not specified | Poly-ICLC | Nivolumab, ipilimumab | Single group assignment | Not specified | Rate of DLT |
| NCT03617328 | Melanoma | I/II | Peptide | TAA | CD4+ Th1 cell | Poly-ICLC | CDX-1127 anti-CD27 | Randomized, Parallel assignment | s.c. | Safety and immunogenicity |
| NCT02382549 | Melanoma | Early I | Peptide | TAA | CD4+ Th1 cell | N/A | Dabrafenic and trametinib | Single group assignment | i.d./s.c. | Toxicity and CD4+ T cell response in blood |
| NCT03047928 | Melanoma | I/II | Peptide | PD-L1, IDO | Pan-T cell | N/A | Nivolumab | Single group assignment | Not specified | Safety |
| NCT05155254 | Melanoma | III | Peptide | PD-L1, IDO | Pan-T cell | N/A | Pembrolizumab | Randomized, parallel assignment | s.c. | Progression-free survival |
| NCT02334735 | Melanoma | II | Peptide | TAA | B and T cell | Poly-ICLC | N/A | Randomized, parallel assignment | s.c. | Humoral and T cell response |
| NCT01176474 | Melanoma | I | Peptide | TAA | CD8+ T cell | N/A | Nivolumab, ipilimumab | Non-randomized, parallel assignment | s.c. | Time to relapse |
| NCT01176461 | Melanoma | I | Peptide | TAA | CD8+ T cell | N/A | Nivolumab | Non-randomized, parallel assignment | s.c. | Best overall response rate |
| NCT02126579 | Melanoma | I/II | Peptide | TAA | Pan-T cell | Tetanus helper peptide, poly ICLC, Resiquimod | N/A | Randomized, parallel assignment | i.d./s.c. | Adverse events, T cell response in blood |
| NCT05280314 | Melanoma or head and neck cancer | II | Peptide | PD-L1, IDO | Pan-T cell | N/A | Pembrolizumab | Non-randomized, parallel assignment | s.c. | Major pathologic response |
| NCT04040231 | Mesothelioma | I | Peptide | TAA | Pan-T cell | GM-CSF | Nivolumab | Single group assignment | Not specified | Maximum tolerated dose |
| NCT01376505 | Metastatic solid tumors | I | Peptide | TAA | B cell | Measles-specific T cell peptide | N/A | Non-randomized, parallel assignment | i.m. | Immune response and clinical benefit |
| NCT02334865 | Multiple myeloma | I | Peptide | TAA | Pan-T cell | GM-CSF | Lenalidomide | Non-randomized, parallel assignment | s.c. | Toxicity |
| NCT04051307 | Myeloproliferative neoplasms | I/II | Peptide | PD-L1, ARG1 | Pan-T cell | N/A | N/A | Single group assignment | Not specified | Immune response (T cell specific) |
| NCT03879694 | Neuroendocrine tumors | I | Peptide | TAA | Pan-T cell | GM-CSF | Octreotide acetate | Single group assignment | s.c. | Safety and toxicity |
| NCT04713514 | Ovarian cancer | II | Peptide | TAA | CD8+ T cell | N/A | Pembrolizumab | Randomized, parallel assignment | s.c. | Progression-free survival |
| NCT02737787 | Ovarian cancer | I | Peptide | TAA | B and T cell | Poly-ICLC | Nivolumab | Non-randomized, sequential assignment | Not specified | Dose-limiting toxicity |
| NCT05096481 | Pediatric brain malignancies | II | Peptide | CMV | B and T cell | N/A | Temozolomide, tetanus/diphtheria vaccine | Single group assignment | i.d. | Progression-free and overall survival |
| NCT04978727 | Pediatric brain malignancies | I | Peptide | TAA | Pan-T cell | GM-CSF | N/A | Non-randomized, parallel assignment | s.c. | Regimen-limiting toxicity |
| NCT02960230 | Pedriatic glioma | I/II | Peptide | TAA | CD8+ T cell | Tetanus helper peptide, poly-ICLC | Nivolumab | Non-randomized, parallel assignment | s.c. | Adverse events, overall survival |
| NCT04114825 | Prostate cancer | II | Peptide | TAA | Pan-T cell | N/A | N/A | Randomized, double-blind, placebo-controlled | s.c. | Time to PSA progression |
| Other trials | ||||||||||
| NCT number | Disease | Phase | Vaccine type | Antigen | Epitope specificity | Additional adjuvant | Additional treatment(s) | Trial design | Route | Primary endpoint |
| NCT04739917 | Malaria | II | Peptide | Malaria antigen | B and T cell | N/A | N/A | Randomized, double-blind, placebo-controlled | Not specified | Frequency of first case of Plasmodium vivax malaria |
| NCT04447898 | Knee osteoarthritis | I | Peptide | IL-6 | B cell | N/A | N/A | Randomized, double-blind, placebo-controlled | s.c. | Toxicity |
CMV, cytomegalovirus; EGF, epidermal growth factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; i.d., intradermal; IL-6, interleukin-6; i.m., intramuscular; s.c., subcutaneous; TAA, tumor-associated antigens.
Interestingly, vaccination with a 19-mer helper CD4+ T cell epitope-containing peptide alone in IFA protected mice against an MHC class II negative Moloney murine leukemia virus-induced lymphoma and also against sarcoma induction by Moloney sarcoma virus. The protective effector cells also included CD8+ T cells, induced indirectly in vivo by the helper peptide vaccination.32 Vaccination with an 8-mer immunodominant MHC class I-binding Moloney virus peptide in IFA also protected against lymphoma outgrowth, but optimal protection was obtained by simultaneous subcutaneous vaccination in IFA with the 19-mer and 8-mer peptides combined in IFA.32 Meanwhile, it has become evident that CD4+ T cell responses are essential for optimal CD8+ effector cell responses and CD8+ memory and that the main influence of CD4+ T cells requires DC as an intermediate cell that transmits the CD4+ cognate interactions by becoming activated and expressing the necessary co-stimulatory signals for optimal CD8+ T cell function (figure 1).36 37 Thus, where vaccination with adenovirus-derived peptide in IFA induces tolerance, co-administration of this vaccine with anti-CD40 antibodies to mimic the CD4+ helper T cell response, broke tolerance and instead induced a strong CTL response.18 38 Similarly, vaccination with short human papilloma virus (HPV) E7 49-57 peptide in IFA, which already induces an immune response capable of controlling E7 expressing tumors, can be further potentiated by adding anti-CD40 antibodies.25 38 Conversely, as mentioned before, subcutaneous vaccination with LCMV NP118-132 peptide induces robust CTL responses, which is strongly reduced after CD4+ T cell depletion in vivo.34 The provision of tumor-specific CD4+ T cell help is clearly more effective than that of non-specific help, because tumor-specific CD4+ helper cells travel to the tumor microenvironment (TME) and can exert multiple beneficial effects for tumor rejection on DCs, CD8+ T cells and macrophages. In one murine tumor study, the mechanism of this was interferon (IFN)-γ-dependent local production of chemokines as well as enhanced interleukin (IL)-2-driven CD8+ T cell proliferation and upregulation of granzyme B in the TME.39 In a detailed study of the interaction between tumor-specific CD4+ T cells, common DC type 1 (cDC1) and tumor-specific CD8+ T cells, early priming of CD4+ T cells against tumor-derived antigens required cDC1.40 Genetic deletion of either major histocompatibility complex (MHC) class II or CD40 in cDC1 impaired tumor rejection, consistent with a role for cognate CD4+ T cell interactions and CD40 signaling in cDC1 activation for proper CD8+ T cell priming. In this study, CD40 signaling in cDC1 was critical not only for CD8+ T cell priming, but also for initial CD4+ T cell activation. Thus, in the setting of tumor-derived antigens, cDC1 cells function as an autonomous platform capable of antigen processing and priming for both CD4+ and CD8+ T cells and of the direct orchestration of their cross-talk that is required for optimal antitumor immunity.40 In a clinical study of vaccination of patients with established melanoma with a NY-ESO-1 class I epitope-containing peptide alone or co-delivered with a specific NY-ESO-1 HLA DR-epitope-containing peptide, both in Montanide-ISA-51+CpG 7909, the CD8+ CTL response induced by vaccination with the combined peptides was clearly superior as established by improved IFN-γ release and lytic activity by vaccine-induced CD8+ T cells.41 The vaccination-induced T cells expressed high levels of PD-1 and Tim-3, and a plea was made to therefore combine this type of peptide vaccination with systemic PD-1 and Tim-3 blocking, since the CD8+ T cells functioned much better on ex vivo incubation with inhibitory antibodies against PD-1 and Tim-3.41 In a very recent study, CD4+, PD1+ CXCL13+ tumor-specific T cells served as a major interacting hub with DC in the TME of patients with non-small cell lung cancer. In a murine tumor model, these investigators showed that such tumor-specific CD4+ T cells were primed in the tumor-draining lymph nodes by DC presenting the specific model tumor antigen ovalbumin.42 Quite apart from these effects on DC and CD8+ T cells, tumor-specific CD4+ T cells can directly kill MHC class II-positive tumor cells through perforin and granzyme B-mediated mechanisms.43 In addition, tumor-specific CD4+ T cells, through IFN-γ secretion and possibly CD40 licensing, can also activate macrophages to kill closely apposed myeloma tumor cells in the bone marrow TME, regardless of tumor MHC expression.44 A comprehensive review is available on mechanistic aspects of the role of CD4+ helper cells in inducing optimal CD8+ T cell responses and CD8+ T cell memory.37
Few studies have evaluated the impact of vaccines on the local vaccine site microenvironment (VSME) or in the vaccine-draining lymph node (VDLN) in humans. In particular, the impact of IFA in those immune compartments is only beginning to be elucidated. In prior work, we have found that CD8+ T cell responses to peptide vaccines in IFA can be detected at higher magnitude and in higher frequency in the VDLN,45 46 suggesting that vaccines injected in the skin supports T cell responses in the VDLN. Interestingly, however, recent data suggest that the vaccine site itself may be a critically important location contributing to vaccine immunity rather than just the draining lymph node, that IFA induces a favorable VSME, with TLR agonist being most beneficial early in the vaccine course, and that same-site injections lead to persistent stimulation of immune pathways that may be beneficial in eliciting antigen-specific T cell expansion (figure 1).47
Addition of other adjuvants
In studies where vaccination with peptides in IFA causes tolerogenic or transient responses, this can furthermore be overcome by the co-administration of Toll-like receptor (TLR) agonists. Vaccination with peptide in IFA plus the TLR9 agonist CpG has a favorable outcome in terms of robust, specific CTL responses and tumor control, compared with vaccination with peptide in IFA alone.48 In a clinical trial vaccinating against the melanoma antigen Melan-A, addition of CpG to the peptide/IFA emulsion increased the magnitude of CD8+ T cell responses in patients detected directly ex vivo by more than a log, compared with those vaccinated with the peptide in IFA, without CpG.49 Also, in randomized trials with a CD4+ T helper cell peptide vaccine, addition of a TLR3 agonist to IFA significantly increased the percent of patients with CD4+ T cell responses detected directly ex vivo.50 Addition of TLR agonists likely induces more robust DC activation, causing increased expression CC-chemokine receptor (CCR)7, MHC class II and co-stimulatory molecules, which would greatly improve T cell activation in the lymph node (figure 1).51 Vaccination with peptides with agonists to TLR3 or TLR4 was less immunogenic for CD8+ T cell responses than adding IFA to those TLR agonists,33 and in another trial with long peptides ex vivo T cell responses were greater in vaccines with IFA (with or without TLR agonists) than vaccination with TLR agonists alone.52
In summary, the induction of a successful CTL response after vaccination with peptides in IFA in mice thus depends on the affinity of the peptide to MHC class I molecules, systemic versus local presence of peptide, DC-focused antigen presentation, CD4+ T cell help and co-stimulation (figure 1). The individual importance of each component furthermore seems to depend on the presence or strength of the others.
Vaccine platforms in which IFA has been used with advantage in clinical studies
In humans, Montanide ISA-51 has been used as a vaccine adjuvant in thousands of patients in clinical trials for therapeutic vaccination against cancer, and for preventive vaccination against viruses and parasites.53
Adverse events (AEs) and safety of Montanide ISA-51
A prior systematic review of the safety and tolerability of Montanide ISA-51 identified 462 studies of vaccines using Montanide ISA-51 and selected 91 for systematic review, where Montanide ISA-51 was administered subcutaneously, intradermally and/or intramuscularly.53 In cancer vaccine studies with a control group, the most common AEs linked to the Montanide ISA-51 adjuvant were local reactions at the injection site, fatigue and influenza-like symptoms. Less frequent AEs, occurring in at least two of the evaluated controlled cancer vaccine trials, were lymphadenopathy, myalgia and rash (NCT00273910).54 55 In general, most AEs were grade 1–2. Grade 3 and 4 AEs were reported in a subset of trials, but details on attribution to the vaccine were not provided for most of these higher grade AEs. For one trial testing an HIV vaccine, severe AEs were reported for five subjects, but none were judged to be vaccine-related.56 Injection site reactions (ISRs) and rash were more frequent in patients receiving antigen+Montanide ISA-51 than either antigen or Montanide ISA-51 alone, suggesting that these AEs were enhanced by the combination rather than Montanide ISA-51 itself, and may reflect the immunogenicity of the antigens included in the vaccines.53 Two other controlled trials testing an HIV or malaria vaccine in healthy subjects were stopped prematurely due to unacceptable AEs.57 58 The HIV trial was stopped due to the development of sterile abscess in four volunteers, which have also occurred in cancer peptide vaccine trials using Montanide ISA-51 as an adjuvant.53 58 The malaria trial was stopped because 2 out of 10 volunteers, receiving the higher dose of pvs25 malaria antigen, developed erythema nodosum-like symptoms.57 The erythema nodosum was likely caused by the combination of antigen and Montanide ISA-51, however, and has not been observed in other trials using Montanide ISA-51 as adjuvant. Importantly, a study (NCT00273910) correlating different techniques for emulsification of the peptide and Montanide ISA-52 showed that, although both techniques induced ISRs, only vortex mixing, but not two-syringe mixing, induced more rare AEs such as myalgia, decreased leukocyte counts, gastrointestinal disorders and general disorders. A comparison study in mice also recommends two-syringe mixing instead of vortex mixing, when comparing emulsion stability and immunogenicity of the vaccine in vivo.59 Both Wu et al and Graham et al mixed the emulsions with a homogenizer, instead of two-syringe mixing, which could be another explanation for the observed high number of AEs.57 58
Preventive vaccination against viruses and parasites
HIV
To prevent or to delay the development of AIDS, vaccination strategies in HIV-infected individuals have been explored extensively. Vaccination with inactivated gp120-depleted HIV-1 immunogen in IFA significantly increased antibody levels, compared with IFA placebo control, and levels remained elevated for up to 2 years after the last vaccine dose.60–62 Additionally, T cell responses and IFN-γ production were increased in vaccinated individuals.62 Together, this led to consistently reduced HIV-1 viral load in the vaccinated group.
SARS-CoV-2
With the emergence of the COVID-19 pandemic caused by SARS-CoV-2, the need for vaccines inducing long-lasting immunity was high. Many different approaches have been and are being developed, including a multipeptide vaccine using Montanide ISA-51 as adjuvant. In this phase I trial, 36 adults were immunized, which was considered safe without any severe AEs.63 One single vaccination, with addition of a TLR1/2 ligand in the emulsion with Montanide ISA-51, induced robust Th1 CD4+ and effector CD8+ T cell responses, and shows promise as a (complementary) vaccine. Induction of T cell responses to conserved T cell epitopes in multiple SARS-CoV-2 proteins may protect patients with B cell malignancies and severe autoimmune diseases treated with B cell-depleting therapies such as rituximab (anti-CD20), who fail to respond to the currently available preventive vaccines against SARS-CoV-2, which all depend on the induction of virus-neutralizing antibodies.
Malaria
Malaria is an infectious disease caused by Plasmodium parasites, and the most advanced vaccines target the sporozoite stage of disease. However, due to the complex life cycle of Plasmodium parasites, targeting one stage of disease is not sufficient. Therefore, transmission blocking vaccines hold promise to further reduce spread. These vaccines target antigens expressed when the parasite is in the midgut of the mosquito. The host produces antibodies, which are taken up by the mosquito when it feeds from the vaccinated host. Through this mechanism, the development of the parasites will be halted at the mosquito stage. The most commonly targeted antigens at this stage are pfs25 and pfs48/45 and antibodies against these targets can successfully block transmission. Vaccination with either Pfs25 or Pfs48/45, in Montanide ISA-51, induced high antibody titers with transmission-blocking activity in monkeys. However, a phase I trial testing the safety and immunogenicity of Pfs25 in Montanide ISA-51 in humans was stopped due to AEs. Despite these AEs, high antibody titers were observed in all five volunteers who received two doses of 5 µg Pfs25/ISA 51, which remained detectable 1 year after vaccination. Furthermore, sera of these volunteers could reduce parasite levels in mosquitos by >90%. This suggests that, if adverse reactivity can be reduced, vaccination with Pfs25/ISA 51 is a promising strategy to halt the spread of Malaria.
A separate vaccine strategy to prevent malaria has been developed to target Circumsporozoite (CS) protein during the sporozoite stage. Immunization with recombinant CS protein in Montanide ISA-720 induced high and long-lasting antibody responses and partial protection against challenge with parasites in mice.64 Immunization with recombinant CS protein in Montanide ISA-51 is tested in malaria-naïve and semi-immune volunteers in an ongoing trial (NCT04739917, Table 1).
Therapeutic cancer vaccines
The only registered cancer vaccine is the Provenge (Sipuleucel-T) vaccine for the treatment of patients with hormone-resistant prostate cancer. This vaccine is a cellular product of autologous blood monocytes in peripheral blood mononuclear cell (PBMC) generated by their culture with a fusion protein of recombinant prostate-specific phosphatase (PAP) coupled to granulocyte-macrophage CSF (GM-CSF). It was approved in the USA and Europe on the basis of its capacity to prolong overall survival of patients with hormone-resistant prostate cancer by an average of 3 months.65 However, this treatment has never reached great popularity because of logistical challenges to create the product and the modest clinical effects. Attempts at generating efficacy by direct intramuscular vaccination (up to 13 doses) with recombinant MAGE-A3 protein adjuvanted with AS15, an immunostimulatory adjuvant cocktail consisting of a liposomal formulation containing TLR4 ligand MPL and saponin QS-21 mixed with TLR9-ligand CpG 7909, failed to show efficacy in phase III double blind, placebo-controlled, randomized trials. These trials were conducted either in patients with histologically proven completely resected stage IIIB or IIIC MAGE-A3 positive cutaneous melanoma with macroscopic lymph node involvement66 or in patients with completely resected MAGE-A3-positive non-small cell lung cancer who did or did not receive adjuvant chemotherapy. A clue to the failure to reach efficacy in these trials may be the observation that in these trials or in phase I/II trials with a similarly adjuvanted PRAME protein vaccine in patients with PRAME+ cancers only relatively modest CD4+ T cell responses against the MAGE-A3 or PRAME proteins were demonstrable, but no CD8+ T cell responses against predefined MAGE-A3 epitopes.66–69 T cell responses to MAGE-A3 protein vaccination in AS15 were detectable only after in vitro stimulation, and even with in vitro stimulation, CD8+ T cell responses were detected in less than 10% of patients in one study.70 This may reflect limitations of that vaccine preparation, which did not include Montanide ISA-51. These weak T cell responses may also reflect a basic limitation of recombinant protein vaccines that can be traced back to the observation that DCs that need to initiate CD8+ CTL responses are very inefficient in taking up and processing of recombinant proteins for presentation in HLA class I molecules. Indeed, in a study with both murine DC and human DC, we observed no processing and presentation by DC of recombinant proteins to CD8+ T cell clones in comparison with equimolar amounts of much shorter synthetic long peptides (SLPs). The processing and presentation of recombinant proteins to CD4+ T cells in this study was weakly positive, but still much less efficient than that of equimolar amounts of long peptides.71
According to clinicaltrials.gov, there are currently 44 active trials (May 13, 2022) using Montanide ISA-51 or Montanide ISA-720 as an adjuvant in therapeutic cancer vaccines for treatment of several cancer types, one preventative vaccine trial for malaria, and one therapeutic vaccine trial for knee osteoarthritis. Surprisingly, most trials use no co-adjuvant strategy, and, if used, GM-CSF or poly I:C LC are predominant (table 1). Most trials, however, included peptides with either tumor-associated CD4+ T cell helper epitopes or non-specific CD4+ T cell helper epitopes and/or employed B cell epitopes to induce antibody responses (table 1). Additionally, many trials study the combination of a therapeutic peptide vaccine with other immune therapies, including checkpoint blockade inhibitors or anti-CD27 (table 1). Current trials thus predominantly focus on the inclusion of CD4+ T cell help to the peptide vaccines with IFA to boost effective CTL responses, and the combination of therapeutic vaccines with checkpoint blockade to further enhance the CTL response by reducing suppression in the TME. Inclusion of CD4+ T helper epitopes in the vaccine is indeed supported by the overwhelming evidence of the role of CD4+ T cell help for optimal and durable CD8+ effector T cell responses and for CD8+ T cell memory.36 37
Therapeutic vaccines for melanoma
T cell response
In clinical trials, vaccination with peptides in Montanide ISA-51 supports induction of effector CD8+ T cell responses to melanoma-associated antigens,72–74 many of which induce CD8+ and/or CD4+ T cell responses that are readily and commonly detected ex vivo.75–78 In trials with a vaccine containing a set of 12 Class I MHC-restricted peptides (12MP) plus a tetanus helper peptide restricted by class II MHC, addition of IFA significantly enhanced circulating specific CD8+ T cell responses, detected ex vivo, in both magnitude and durability, compared with vaccination with the same peptides combined with a TLR3 or a TLR4 agonist alone.33 This effect is greatest when the Montanide ISA-51 was included with each vaccine rather than just one vaccine dose.33 Vaccination with 12MP induced CD8+ T cell responses in 100% of patients when evaluated after in vitro stimulation and when the adjuvant was Montanide ISA-51 plus GM-CSF.79 However, in a randomized study assessing the value of including GM-CSF, the CD8+ T cell responses were significantly greater when GM-CSF was omitted, and the only adjuvant was Montanide ISA-51, such that T cell responses were detected ex vivo in greater than 70% of patients, and CD4+ T cell responses to a tetanus helper peptide in that vaccine were also greater with Montanide ISA-51 as the only adjuvant, detected in 95% of patients.75 Also, when Montanide ISA-51 was administered with NY-ESO protein and poly I:C LC, vaccine-induced T cells had higher avidity for their antigens than with protein and poly I:C LC alone.80 The combination of Montanide ISA-51+poly I:C LC with class II MHC-restricted peptides has also produced more durable CD4+ responses than with peptides and poly I:C LC alone.50 Interestingly, Melan-A peptide vaccination in IFA induced better CD8+ T cell responses in the blood than Melan-A peptide vaccination with QS21 and TLR4 ligand MPL.81 Administration of Melan-A peptide in Montanide ISA-51 showed improved peptide-specific T cell responses in the blood with inclusion of TLR9 agonists CpG as an adjuvant, compared with no CpG.49 Combined, these trials show that peptide vaccination with Montanide ISA-51 as an adjuvant induces significant T cell responses in patients with cancer, and that adding Montanide ISA-51 to a TLR agonist further improves T cell responses.
Humoral response
Vaccination with six melanoma helper peptides (6MHP) in Montanide ISA-51 in humans has induced strong antibody responses. Very high and durable circulating IgG antibody responses to peptide antigens were induced when vaccinating with those peptides in Montanide ISA-5182 83 and responses appeared greater than when vaccinating with TLR agonists alone. However, the combination of Montanide ISA-51 plus a TLR agonist further enhanced antibody responses to the peptides in the vaccines.50 52 Patients who were vaccinated with NY-ESO-1 protein, poly I:C LC, and Montanide ISA-51 developed more NY-ESO-1-specific antibodies after three to four vaccines than patients who only received antigen and poly I:C LC.80
Vaccine site microenvironment induced by Montanide ISA-51
The most direct effect of vaccines, including IFA, are in the VSME. We have evaluated immune cell accumulation and gene expression profiling in the VSME after injection with peptides in IFA, with or without TLR agonists. Peptide vaccination with IFA induced greater CD8+ T cell and B cell accumulation at the vaccine site, compared with peptide vaccination with a TLR agonist.84 85 One vaccination dose with peptides in IFA+peptides markedly enhanced expression of markers of DC activation and maturation (CD80, CD83, CD86). When Montanide ISA-51 was injected at the same skin site three times with peptides each week, there was evidence of an indirect effect on CD8+ T cell numbers through activation of CD4+ T cells, enhancing their expression of CD40L,84 which can support APC licensing and enhanced antigen presentation.36 37 RNA sequencing analysis of the VSME tissues identified enhanced antigen processing and presentation, chemokine signaling, phagosome and cell adhesion signaling, while repeated vaccination with peptides in IFA also enhanced leukocyte transendothelial migration, B cell receptor signaling, NOD-like receptor signaling, JAK-STAT signaling, and other immune related pathways.84 Also, repeated vaccination with peptides in IFA induced dramatic accumulation of T cells and other immune cells, a relative increase in Tbet:Gata3 ratio and markedly enhanced IFN-γ and STAT1 expression. A subset of patients vaccinated in this way formed tertiary lymphoid structures (TLS) in the VSME and enhanced expression of chemokines associated with TLS formation.84 85 Interestingly, these vaccines induced a marked reduction in arginase 1 (ARG1) expression when repeated weekly for 3 weeks. They also enhanced expression of TLR adapter molecules TICAM-1 (TRIF) and MYD88.
As expected in any inflammatory environment, there was also induction of regulatory gene expression, and accumulation of regulatory FoxP3+ cells.84 85 IFA also induced CD8+ T cell inhibitory pathways including PD-L1 and IDO at the VSME as well as components of the FAS-mediated apoptosis pathway.85 PD-1 expression has also been found to increase, but only after multiple same site vaccinations with IFA and peptides.84
Thus, in contrast to murine studies, human studies showed that Montanide ISA-51 decreased myeloid-derived suppressor cells (MDSC)-related genes including GITR and Syndecan-4, as well as ARG1, suggesting downregulation of MDSC.84 85 Interestingly, repeat same site vaccinations with Montanide ISA-51 with or without peptides activated signaling through the TLR pathway as seen by increased MyD88 and TRIF expression, even without the addition of a TLR agonist.84
The signs of a VSME supportive of T cell survival and stimulation correspond with the extensive evidence from numerous trials that clinical peptide vaccination in Montanide ISA-51 is associated with the induction of robust and durable systemic T cell responses as evident from specific T cell response measurements in PBMC.33 80 81 86–90 Hence, these VSME sites appear to serve as nurseries for robust and durable systemic T cell responses.
Th1/Th2 ratio
A higher Th1/Th2 ratio is believed to favor antitumor immune responses. Th2 dominance has previously been described at the VSME shown by a predominance of GATA3 staining.85 However, same site vaccination, for three times once a week, with peptides in IFA decreases GATA3 and enhances Th1 cell transcription factors TBX21, IFN-γ and STAT1, thereby supporting a more Th1-dominant microenvironment.84 91
Tertiary lymphoid structures
TLS, which include B cells, T cells and mature DCs, are important for recruiting and activating T cells and are induced under chronic inflammatory conditions.92–94 Vaccination with Montanide ISA-51 induces TLS at the vaccine site, especially with repeated vaccinations at the same site. After three vaccinations, the expression of 16/18 TLS-associated genes increased significantly compared with normal tissue, including expression of CXCL13, a gene crucial to TLS formation.84 85 In comparison, vaccination with TLR agonists produced a transient chemokine response, with only 8 out of 18 genes significantly increased compared with normal skin.
Clinical activity of a helper peptide vaccine administered in IFA (Montanide ISA-51)
In one study, 37 participants with stage III-IV melanoma were administered a 6MHP vaccine intradermally and subcutaneously in an emulsion with IFA. There was an ex vivo T cell response, demonstrable in peripheral blood mononuclear cells (PBMCs) and/or a VDLN, in 81% of the participants. Of the 17 patients with measurable advanced melanomas, objective partial clinical responses were observed in 2 (12%), and stable disease was observed in another 2 patients (12%). Those responses (partial response or stable disease) persisted for at least 1 year, and as long as 7 years, representing a 24% durable disease control rate.82 88 Also, in a larger clinical trial, a 7% overall response rate was observed with this vaccine.89 Thus, this vaccine with six helper peptides in IFA has clinical activity as monotherapy.
Also, among patients with stage III-IV melanoma, patients with both PBMC T cell and serum antibody responses had better survival than those who did not.83 This was true both for those patients with measurable disease (p<0.033); for patients with no evidence of disease (p<0.015). Further, patients with stage IV melanoma who enrolled in 6MHP/IFA vaccine trials had significantly better overall survival than those in a matched group who did not enroll in a 6MHP/IFA vaccine trial, despite meeting criteria to be eligible for the vaccine trials.95 In fact, for those who received the 6MHP/IFA vaccines after having had all their tumor removed, 5-year survival was 74%, which is similar to survival reported with PD-1 antibody therapies.95
Immunogenicity and clinical activity with a long peptide vaccine administered in IFA (Montanide ISA-51), combined with systemic PD-1 blockade
A novel vaccine strategy has been to induce T cell responses to two key immune checkpoint molecules, PD-L1 and IDO-1, and this approach was combined with systemic PD-1 antibody therapy.96 Immune responses were reported to IDO-1 in 33% of patients with metastatic melanoma at baseline, and this increased to over 90% after vaccination. Similar but slightly lower T cell response rates to PD-L1 were also reported. Among the 30 treated patients, a very high clinical response rate of 80% was observed, with 43% having complete responses. These data further support immunogenicity and clinical activity for vaccination with these peptides in Montanide ISA-51.
Therapeutic vaccination against diseases caused by high-risk human papilloma virus type 16 (HPV16)
AEs and safety of vaccination with SLP vaccine in Montanide ISA-51 in patients with cancer or premalignant disease caused by HPV16
The first studies with a therapeutic SLP vaccine against the oncoproteins E6 and E7 of HPV16 were conducted in patients with cervical cancer. The antigenic component of the vaccine consisted of 13 SLP, 25–35 amino acids long, which together spanned the complete amino acid sequence of E6 and E7 oncoproteins. This vaccine, called HPV16-SLP,86 87 at a subcutaneous dose of 300 µg/peptide in an emulsion with Montanide ISA-51, caused mainly grade 2 ISRs. In two studies of high-grade vulvar intraepithelial neoplasia (VIN3) caused by HPV16, the same HPV16-SLP vaccine again mainly caused grade 1–2 local ISRs, characterized by swelling and redness in 100% of patients, low-grade skin rash in a minority of patients and fever in the majority of patients.97 In a later study in which that vaccine at the same dose (300 µg/peptide) was administered subcutaneously to VIN3 patients, with or without adding the TLR7 agonist imiquimod on the skin overlaying the vaccine site, the side effects were similar. In this study, 7 out of 34 patients developed injection site ulcers, 2 of whom required special treatment.98 In a recent study in 77 patients with recurrent or metastatic cervical cancer, local ISRs to 20, 40, 100, or 300 µg/peptide were less frequent and less severe at the lower doses.90 The HPV16-specific T cell responses of PBMC induced by doses of 40 and 100 µg/peptide were as robust as those induced by the 300 µg/peptide. The dose of 100 µg/peptide was also used in an investigator-sponsored study exploring potential synergy of the HPV-16 SLP vaccine and anti-PD-1 nivolumab. The treatment scheme started with a dose of HPV16-SLP vaccine, followed a week later by the first dose of nivolumab. Two additional vaccine doses were given on the same day as nivolumab doses that continued for up to 2 years or disease progression. Vaccine-associated side effects were again limited largely to grade 1–2 ISRs.99
T cell responses
The HPV16-SLP vaccine in Montanide ISA-51 has been highly immunogenic in patients with cervical cancer, generating both CD4+ and CD8+ T cell responses against HPV16 E6/E7, measurable in PBMC by IFN-γ Elispot assay.86 87 When all 13 SLP were injected in one subcutaneous site, the T cell response against E6 dominated that to E7. However, when the four E7 SLP, together with two E6 SLP and the remaining seven E6 SLP were injected in two different subcutaneous sites (in two different limbs), the T cell response to E7 prominently surged from almost undetectable to very strong as measured in PBMC.86 This suggests that among the 13 SLP antigenic competition may occur at the level of the VDLN, which can be avoided by administering separated subcutaneous injections of 6 and 7 SLP, each in Montanide ISA-51. This is one of the advantages of using peptides as a vaccine platform. Another crucial advantage of SLP over proteins is the fact that DCs much more efficiently process and present SLP compared with intact proteins for presentation to both CD4+ and CD8+ T cells.71
Clinical responses
The spontaneous regression rate of VIN3 lesions is less than 2%.100 In the monotherapy studies in VIN3 patients with the SLP vaccine against HPV16 E6/E7 in Montanide ISA-51, greater than 50% of patients experienced partial or complete regressions of disease.97 98 Addition of imiquimod to the vaccine site did not improve the T cell or clinical response.98 Strikingly, in both studies the size of the vaccine-induced T cell response correlated significantly with the clinical response. The weak vaccine-induced T cell response in patients with late stage cervical cancer, treated with the HPV16-directed SLP vaccine in Montanide ISA-51, were not noticeably associated with a clinical response. When combined with standard of care chemotherapy, the SLP vaccine-induced T cell responses increased to very high levels and again, a strong correlation between the size of the vaccine-induced T cell response and the clinical response was observed.90 Patients with a higher than median vaccine-induced T cell response lived substantially longer than those with a lower than median induced T cell response.90 Thus, in three independent studies, the size of the systemic vaccine-induced T cell response positively correlated with the clinical response.90 97 98 This makes vaccine-induced T cell responses, as measured by IFN-γ Elispot assay of PBMC, a potentially powerful biomarker predicting clinical responses after this vaccine. A promising signal of clinical benefit was also observed in patients with HPV16+ oropharyngeal cancer (OPC) who received combination therapy of nivolumab and HPV16-SLP in Montanide-ISA-51. While the overall response rate in patients with recurrent or metastatic HPV16+ OPC was 16%,101 the overall response rate in patients with HPV16+ OPC receiving nivolumab and HPV16-SLP in a separate single-arm study of 24 patients was 34%. Also the median overall survival in the Ferris et al study was approximately 9 months, whereas the median overall survival in the Massarelli et al study for this category of patients with OPCwas almost 18%.99 101 In a long-term follow-up of this study, two out of two patients with a complete response still had an ongoing complete response after 46 months and of six patients with a partial response, one died of an unrelated vascular event without evidence of disease progression after 38 months.102 These data show that a peptide vaccine in Montanide ISA-51 can be both immunogenic and therapeutic for human solid tumors.
Conclusions and discussion
IFA or IFA-like vaccine adjuvants such as Montanide ISA-51 have been and are being extensively used in both healthy subjects for disease prevention and in patients with cancer and other diseases for therapeutic vaccination. A murine study has raised concerns that vaccination with a short melanoma peptide in IFA may deplete vaccine-reactive T cells16 17 after recruiting them to the vaccine site. Other murine studies have also raised concerns of negative effects of IFA as an adjuvant,11–15 21 in particular with short peptides. However, many human studies, summarized here, provide ample evidence that peptide vaccines in IFA induce DC activation locally at the vaccine sites, TLS at those sites, strong T-cell and antibody responses systemically, and control of viral infections and solid tumors.
IFA-like adjuvants are generally safe but vaccines with immunogenic peptides plus IFA can induce ISRs that can require treatment,53 98 and they commonly induce mild transient systemic toxicities. Both the antigen and IFA/Montanide ISA-51 contribute to the ISRs. The mixing procedure is crucial in decreasing AEs and emulsification by syringe extrusion is recommended to prevent unstable solutions.6 Reduction of antigen and/or adjuvant dose may further reduce AEs, while preserving immunogenicity.
Preclinical studies have identified several factors that drive T cell responses to vaccines, including affinity of the peptide to the presenting MHC class I molecule, CD4+ T cell help, and the level of co-stimulation. A convenient way to achieve optimal co-stimulation is to use extended peptides requiring a processing step by DC, thereby avoiding direct peptide loading on MHC molecules of nucleated cells other than DC. Inclusion of peptides that can stimulate CD4+ helper T cells enhances immunogenicity of peptide vaccines in IFA, preventing early demise of non-helped CD8+ T cells.34 Additional inclusion of a TLR ligand such as CpG or poly I:C LC can further increase the magnitude and durability of the response.
Therapeutic vaccines with Montanide ISA-51 as monotherapy have shown clinical benefit in melanoma and VIN3 patients, associated with robust systemic T cell responses. An interesting aspect of the use of peptide vaccines delivered subcutaneously as an emulsion with Montanide ISA-51 is the creation of a VSME characterized by an inflammatory landscape, including tertiary lymphoid architecture. These sites appear to serve as a nursery for T cell induction and expansion, resulting in durable systemically circulating CD4+ and CD8+ T cells with tumoricidal activity. However, for the T cells to remain functional in a hostile, suppressive TME and in the face of systemic immunosuppression, characteristic of late stage recurrent/metastatic cancer, additional therapies are required. Currently investigated strategies aim to reprogram, eliminate and/or inhibit suppressive cell types within the TME, including tumor-associated macrophages, MDSCs, cancer-associated fibroblasts and regulatory T cells (figure 1).103 104 Combining these targeted therapies with peptide vaccines may create a less hostile environment for the vaccine-induced T cells and likely improve cytotoxic killing of tumor cells.
Co-treatment with checkpoint blockade therapy, such as anti-PD-1 or anti-PD-L1 antibodies, or with standard of care chemotherapy (eg, carboplatin and paclitaxel) have shown encouraging results and need to be further explored in randomized clinical trials. An overview of ongoing trials testing some of these combination treatments is provided in table 1. Interestingly, three current vaccine trials directly target immune suppressive mechanisms by the vaccine itself. This is achieved by targeting ARG1, IDO-1 or PD-L1 with peptide vaccines, which can reinvigorate pre-existing T cell responses, as well as make the environment more inflammatory and permissive for new infiltration. In one of these trials (NCT03047928), metastatic melanoma patients received IDO-1 and PD-L1 peptide vaccination in Montanide ISA-51 in combination with nivolumab. Patients who received this treatment showed an unprecedented overall response rate of 80% (n=30 patients), complete response rate of 43% and a mean progression-free survival of 26 months.96
In conclusion, there appears to be an exciting future for therapeutic peptide-based cancer vaccines using emulsions in adjuvant Montanide ISA-51, provided sufficient CD4+ T cell help is provided. Extensive recurrent or metastatic disease may require additional co-treatment with anti-PD-1 antibody, chemotherapy or other immunomodulatory drugs to address local and systemic immunosuppressive mechanisms. However, clinical activity has been observed with peptides in IFA, but blinded randomized placebo-controlled trials are required to definitively prove clinical efficacy. Vaccine monotherapy using peptides in IFA may be most effective for patients with pre-malignant disease or minimal residual disease following successful debulking.
Acknowledgments
We thank Anna Dimberg at Uppsala University for the Biorender laboratory license to assemble figure 1.
Footnotes
Twitter: @maritmelssen
Contributors: MMM, CTF, CLS and CJMM drafted and/or substantially revised the manuscript. MMM assembled the figures. All authors read and approved the final manuscript.
Funding: This work was supported by a Cancer Research Institute Clinical Laboratory Integration Project (CLIP) award and the Rebecca C. Harris Fellowship.
Competing interests: CLS has the following disclosures: Research support to the University of Virginia from Celldex (funding, drug), Glaxo-Smith Kline (funding), Merck (funding, drug), 3M (drug), Theraclion (device staff support); funding to the University of Virginia from Polynoma for PI role on the MAVIS Clinical Trial; funding to the University of Virginia for roles on Scientific Advisory Boards for Immatics and CureVac. Also CLS receives licensing fee payments through the UVA Licensing and Ventures Group for patents for peptides used in cancer vaccines. CJMM is the Chief Scientific Officer (CSO) of ISA Pharmaceuticals, a biotech company aiming at development and registration of synthetic long peptide vaccines against cancer-associated antigens for treatment of cancer. In this function he receives a salary as CSO and is participant in an ISA management participation plan, equivalent to a shareholder position. No potential conflicts of interest were disclosed by the other authors.
Provenance and peer review: Commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Le Moignic E. Pinoy. Les vaccins en emulsion dans les corps gras ou “lipo-vaccins. Comptes Rendus la Soc Biol 1916;79:201–3. [Google Scholar]
- 2.Freund J, Casals J, Hosmer EP. Sensitization and antibody formation after injection of tubercle bacilli and paraffin oil. Exp Biol Med 1937;37:509–13. 10.3181/00379727-37-9625 [DOI] [Google Scholar]
- 3.Salk JE, Contakos M, Laurent AM, et al. Use of adjuvants in studies on influenza immunization. III. degree of persistence of antibody in human subjects two years after vaccination. J Am Med Assoc 1953;151:1169–75. 10.1001/jama.1953.02940140013005 [DOI] [PubMed] [Google Scholar]
- 4.Beebe GW, Simon AH, Vivona S. Follow-up study on army personnel who received adjuvant influenza virus vaccine 1951-1953. Am J Med Sci 1964;247:385–406. 10.1097/00000441-196404000-00001 [DOI] [PubMed] [Google Scholar]
- 5.Jensen FC, Savary JR, Diveley JP, et al. Adjuvant activity of incomplete freund's adjuvant. Adv Drug Deliv Rev 1998;32:173–86. 10.1016/s0169-409x(98)00009-x [DOI] [PubMed] [Google Scholar]
- 6.Aucouturier J, Dupuis L, Deville S, et al. Montanide Isa 720 and 51: a new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev Vaccines 2002;1:111–8. 10.1586/14760584.1.1.111 [DOI] [PubMed] [Google Scholar]
- 7.Herbert WJ. The mode of action of mineral-oil emulsion adjuvants on antibody production in mice. Immunology 1968;14:301–18. [PMC free article] [PubMed] [Google Scholar]
- 8.den Boer AT, van Mierlo GJD, Fransen MF, et al. The tumoricidal activity of memory CD8+ T cells is hampered by persistent systemic antigen, but full functional capacity is regained in an antigen-free environment. J Immunol 2004;172:6074–9. 10.4049/jimmunol.172.10.6074 [DOI] [PubMed] [Google Scholar]
- 9.Moreira LO, Smith AM, DeFreitas AA, et al. Modulation of adaptive immunity by different adjuvant-antigen combinations in mice lacking NOD2. Vaccine 2008;26:5808–13. 10.1016/j.vaccine.2008.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khong H, Overwijk WW. Adjuvants for peptide-based cancer vaccines. J Immunother Cancer 2016;4:56. 10.1186/s40425-016-0160-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyburz D, Aichele P, Speiser DE, et al. T cell immunity after a viral infection versus T cell tolerance induced by soluble viral peptides. Eur J Immunol 1993;23:1956–62. 10.1002/eji.1830230834 [DOI] [PubMed] [Google Scholar]
- 12.Aichele BP, Brduscha-riem K, Zinkernagel RM. T cell priming versus T cell tolerance induced by synthetic peptides peptide-induced priming of LCMV-specific CTL 1995;182. 10.1084/jem.182.1.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toes RE, Offringa R, Blom RJ, et al. Peptide vaccination can lead to enhanced tumor growth through specific T-cell tolerance induction. Proc Natl Acad Sci U S A 1996;93:7855–60. 10.1073/pnas.93.15.7855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Toes RE, van der Voort EI, Schoenberger SP, et al. Enhancement of tumor outgrowth through CTL tolerization after peptide vaccination is avoided by peptide presentation on dendritic cells. J Immunol 1998;160:4449–56. [PubMed] [Google Scholar]
- 15.Aichele P, Brduscha-Riem K, Oehen S, et al. Peptide antigen treatment of naive and virus-immune mice: antigen-specific tolerance versus immunopathology. Immunity 1997;6:519–29. 10.1016/s1074-7613(00)80340-4 [DOI] [PubMed] [Google Scholar]
- 16.Hailemichael Y, Dai Z, Jaffarzad N, et al. Persistent antigen at vaccination sites induces tumor-specific CD8⁺ T cell sequestration, dysfunction and deletion. Nat Med 2013;19:465–72. 10.1038/nm.3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hailemichael Y, Woods A, Fu T, et al. Cancer vaccine formulation dictates synergy with CTLA-4 and PD-L1 checkpoint blockade therapy. J Clin Invest 2018;128:1338–54. 10.1172/JCI93303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.den Boer AT, Diehl L, van Mierlo GJ, et al. Longevity of antigen presentation and activation status of APC are decisive factors in the balance between CTL immunity versus tolerance. J Immunol 2001;167:2522–8. 10.4049/jimmunol.167.5.2522 [DOI] [PubMed] [Google Scholar]
- 19.Zwaveling S, Ferreira Mota SC, Nouta J, et al. Established human papillomavirus type 16-expressing tumors are effectively eradicated following vaccination with long peptides. J Immunol 2002;169:350–8. 10.4049/jimmunol.169.1.350 [DOI] [PubMed] [Google Scholar]
- 20.Torréns I, Mendoza O, Batte A, et al. Immunotherapy with CTL peptide and VSSP eradicated established human papillomavirus (HPV) type 16 E7-expressing tumors. Vaccine 2005;23:5768–74. 10.1016/j.vaccine.2005.07.049 [DOI] [PubMed] [Google Scholar]
- 21.Bijker MS, van den Eeden SJF, Franken KL, et al. Cd8+ CTL priming by exact peptide epitopes in incomplete Freund's adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J Immunol 2007;179:5033–40. 10.4049/jimmunol.179.8.5033 [DOI] [PubMed] [Google Scholar]
- 22.Aichele P, Hengartner H, Zinkernagel RM, et al. Antiviral cytotoxic T cell response induced by in vivo priming with a free synthetic peptide. J Exp Med 1990;171:1815–20. 10.1084/jem.171.5.1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kast WM, Roux L, Curren J, et al. Protection against lethal sendai virus infection by in vivo priming of virus-specific cytotoxic T lymphocytes with a free synthetic peptide. Proc Natl Acad Sci U S A 1991;88:2283–7. 10.1073/pnas.88.6.2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kast WM, Brandt RM, Melief CJ. Strict peptide length is not required for the induction of cytotoxic T lymphocyte-mediated antiviral protection by peptide vaccination. Eur J Immunol 1993;23:1189–92. 10.1002/eji.1830230534 [DOI] [PubMed] [Google Scholar]
- 25.Feltkamp MC, Smits HL, Vierboom MP, et al. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol 1993;23:2242–9. 10.1002/eji.1830230929 [DOI] [PubMed] [Google Scholar]
- 26.Mandelboim O, Vadai E, Fridkin M, et al. Regression of established murine carcinoma metastases following vaccination with tumour-associated antigen peptides. Nat Med 1995;1:1179–83. 10.1038/nm1195-1179 [DOI] [PubMed] [Google Scholar]
- 27.Ossevoort MA, Feltkamp MC, van Veen KJ, et al. Dendritic cells as carriers for a cytotoxic T-lymphocyte epitope-based peptide vaccine in protection against a human papillomavirus type 16-induced tumor. J Immunother Emphasis Tumor Immunol 1995;18:86–94. 10.1097/00002371-199508000-00002 [DOI] [PubMed] [Google Scholar]
- 28.Li R, Zheng C, Wang Q, et al. Identification of an immunogenic DKK1 long peptide for immunotherapy of human multiple myeloma. Haematologica 2021;106:838–46. 10.3324/haematol.2019.236836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Falk K, Rötzschke O, Stevanovié S, et al. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature 1991;351:290–6. 10.1038/351290a0 [DOI] [PubMed] [Google Scholar]
- 30.Bijker MS, van den Eeden SJF, Franken KL, et al. Superior induction of anti-tumor CTL immunity by extended peptide vaccines involves prolonged, DC-focused antigen presentation. Eur J Immunol 2008;38:1033–42. 10.1002/eji.200737995 [DOI] [PubMed] [Google Scholar]
- 31.Bijker MS, Melief CJM, Offringa R, et al. Design and development of synthetic peptide vaccines: past, present and future. Expert Rev Vaccines 2007;6:591–603. 10.1586/14760584.6.4.591 [DOI] [PubMed] [Google Scholar]
- 32.Ossendorp F, Mengedé E, Camps M, et al. Specific T helper cell requirement for optimal induction of cytotoxic T lymphocytes against major histocompatibility complex class II negative tumors. J Exp Med 1998;187:693–702. 10.1084/jem.187.5.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melssen MM, Petroni GR, Chianese-Bullock KA, et al. A multipeptide vaccine plus toll-like receptor agonists LPS or polyICLC in combination with incomplete freund’s adjuvant in melanoma patients. J Immunother Cancer 2019;7:1–13. 10.1186/s40425-019-0625-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fayolle C, Deriaud E, Leclerc C. In vivo induction of cytotoxic T cell response by a free synthetic peptide requires CD4+ T cell help. J Immunol 1991;147:4069–73. [PubMed] [Google Scholar]
- 35.Domingos-Pereira S, Galliverti G, Hanahan D, et al. Carboplatin/paclitaxel, E7-vaccination and intravaginal CpG as tri-therapy towards efficient regression of genital HPV16 tumors. J Immunother Cancer 2019;7:1–7. 10.1186/s40425-019-0593-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melssen M, Slingluff CL. Vaccines targeting helper T cells for cancer immunotherapy. Curr Opin Immunol 2017;47:85–92. 10.1016/j.coi.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borst J, Ahrends T, Bąbała N, et al. CD4+ T cell help in cancer immunology and immunotherapy. Nat Rev Immunol 2018;18:635–47. 10.1038/s41577-018-0044-0 [DOI] [PubMed] [Google Scholar]
- 38.Diehl L, den Boer AT, Schoenberger SP, et al. Cd40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat Med 1999;5:774–9. 10.1038/10495 [DOI] [PubMed] [Google Scholar]
- 39.Bos R, Sherman LA. Cd4+ T-cell help in the tumor milieu is required for recruitment and cytolytic function of CD8+ T lymphocytes. Cancer Res 2010;70:8368–77. 10.1158/0008-5472.CAN-10-1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ferris ST, Durai V, Wu R, et al. cDC1 prime and are licensed by CD4+ T cells to induce anti-tumour immunity. Nature 2020;584:624–9. 10.1038/s41586-020-2611-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fourcade J, Sun Z, Pagliano O, et al. Pd-1 and Tim-3 regulate the expansion of tumor antigen-specific CD8⁺ T cells induced by melanoma vaccines. Cancer Res 2014;74:1045–55. 10.1158/0008-5472.CAN-13-2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cohen M, Giladi A, Barboy O, et al. The interaction of CD4+ helper T cells with dendritic cells shapes the tumor microenvironment and immune checkpoint blockade response. Nat Cancer 2022;3:303–17. 10.1038/s43018-022-00338-5 [DOI] [PubMed] [Google Scholar]
- 43.Quezada SA, Simpson TR, Peggs KS, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med 2010;207:637–50. 10.1084/jem.20091918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haabeth OAW, Hennig K, Fauskanger M, et al. Cd4+ T-cell killing of multiple myeloma cells is mediated by resident bone marrow macrophages. Blood Adv 2020;4:2595–605. 10.1182/bloodadvances.2020001434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slingluff CL, Yamshchikov GV, Hogan KT, et al. Evaluation of the sentinel immunized node for immune monitoring of cancer vaccines. Ann Surg Oncol 2008;15:3538–49. 10.1245/s10434-008-0046-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamshchikov GV, Barnd DL, Eastham S, et al. Evaluation of peptide vaccine immunogenicity in draining lymph nodes and peripheral blood of melanoma patients. Int J Cancer 2001;92:703–11. [DOI] [PubMed] [Google Scholar]
- 47.Meneveau MO, Kumar P, Lynch KT, et al. The vaccine-site microenvironment: impacts of antigen, adjuvant, and same-site vaccination on antigen presentation and immune signaling. J Immunother Cancer 2022;10:e003533. 10.1136/jitc-2021-003533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maynard SK, Marshall JD, MacGill RS, et al. Vaccination with synthetic long peptide formulated with CpG in an oil-in-water emulsion induces robust E7-specific CD8 T cell responses and TC-1 tumor eradication. BMC Cancer 2019;19:540. 10.1186/s12885-019-5725-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Speiser DE, Liénard D, Rufer N, et al. Rapid and strong human CD8+ T cell responses to vaccination with peptide, IFA, and CpG oligodeoxynucleotide 7909. J Clin Invest 2005;115:739–46. 10.1172/JCI23373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Slingluff CL, Petroni GR, Chianese-Bullock KA, et al. Trial to evaluate the immunogenicity and safety of a melanoma helper peptide vaccine plus incomplete freund's adjuvant, cyclophosphamide, and polyICLC (Mel63). J Immunother Cancer 2021;9:e000934. 10.1136/jitc-2020-000934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sallusto F, Lanzavecchia A. The instructive role of dendritic cells on T-cell responses. Arthritis Res 2002;4 Suppl 3:127–32. 10.1186/ar567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel SP, Petroni GR, Roszik J, et al. Phase I/II trial of a long peptide vaccine (LPV7) plus Toll-like receptor (TLR) agonists with or without incomplete freund's adjuvant (IFA) for resected high-risk melanoma. J Immunother Cancer 2021;9:3220. 10.1136/jitc-2021-003220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Doorn E, Liu H, Huckriede A, et al. Safety and tolerability evaluation of the use of montanide ISA™51 as vaccine adjuvant: a systematic review. Hum Vaccin Immunother 2016;12:159–69. 10.1080/21645515.2015.1071455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sabbatini P, Tsuji T, Ferran L, et al. Phase I trial of overlapping long peptides from a tumor self-antigen and poly-ICLC shows rapid induction of integrated immune response in ovarian cancer patients. Clin Cancer Res 2012;18:6497–508. 10.1158/1078-0432.CCR-12-2189 [DOI] [PubMed] [Google Scholar]
- 55.Goldinger SM, Dummer R, Baumgaertner P, et al. Nano-particle vaccination combined with TLR-7 and -9 ligands triggers memory and effector CD8⁺ T-cell responses in melanoma patients. Eur J Immunol 2012;42:3049–61. 10.1002/eji.201142361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boffito M, Fox J, Bowman C, et al. Safety, immunogenicity and efficacy assessment of HIV immunotherapy in a multi-centre, double-blind, randomised, placebo-controlled phase Ib human trial. Vaccine 2013;31:5680–6. 10.1016/j.vaccine.2013.09.057 [DOI] [PubMed] [Google Scholar]
- 57.Wu Y, Ellis RD, Shaffer D, et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide Isa 51. PLoS One 2008;3:e2636. 10.1371/journal.pone.0002636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Graham BS, McElrath MJ, Keefer MC, et al. Immunization with cocktail of HIV-derived peptides in montanide ISA-51 is immunogenic, but causes sterile abscesses and unacceptable reactogenicity. PLoS One 2010;5:e1199. 10.1371/journal.pone.0011995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Koh YT, Higgins SA, Weber JS, et al. Immunological consequences of using three different clinical/laboratory techniques of emulsifying peptide-based vaccines in incomplete freund's adjuvant. J Transl Med 2006;4:42. 10.1186/1479-5876-4-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levine AM, Groshen S, Allen J, et al. Initial studies on active immunization of HIV-infected subjects using a gp120-depleted HIV-1 immunogen: long-term follow-up. J Acquir Immune Defic Syndr Hum Retrovirol 1996;11:351–64. 10.1097/00042560-199604010-00005 [DOI] [PubMed] [Google Scholar]
- 61.Trauger RJ, Ferre F, Daigle AE, et al. Effect of immunization with inactivated gp120-depleted human immunodeficiency virus type 1 (HIV-1) immunogen on HIV-1 immunity, viral DNA, and percentage of CD4 cells. J Infect Dis 1994;169:1256–64. 10.1093/infdis/169.6.1256 [DOI] [PubMed] [Google Scholar]
- 62.Moss RB, Trauger RJ, Giermakowska WK, et al. Effect of immunization with an inactivated gp120-depleted HIV-1 immunogen on β-chemokine and cytokine production in subjects with HIV-1 infection. J Acquir Immune Defic Syndr Hum Retrovirology 1997;14:343–50. 10.1097/00042560-199704010-00006 [DOI] [PubMed] [Google Scholar]
- 63.Heitmann JS, Bilich T, Tandler C, et al. A COVID-19 peptide vaccine for the induction of SARS-CoV-2 T cell immunity. Nature 2022;601:617–22. 10.1038/s41586-021-04232-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gimenez AM, Salman AM, Marques RF, et al. A universal vaccine candidate against Plasmodium vivax malaria confers protective immunity against the three PvCSP alleles. Sci Rep 2021;11:1–14. 10.1038/s41598-021-96986-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411–22. 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- 66.Dreno B, Thompson JF, Smithers BM, et al. Mage-A3 immunotherapeutic as adjuvant therapy for patients with resected, MAGE-A3-positive, stage III melanoma (DERMA): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2018;19:916–29. 10.1016/S1470-2045(18)30254-7 [DOI] [PubMed] [Google Scholar]
- 67.Vansteenkiste JF, Cho BC, Vanakesa T, et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2016;17:822–35. 10.1016/S1470-2045(16)00099-1 [DOI] [PubMed] [Google Scholar]
- 68.Gutzmer R, Rivoltini L, Levchenko E, et al. Safety and immunogenicity of the PRAME cancer immunotherapeutic in metastatic melanoma: results of a phase I dose escalation study. ESMO Open 2016;1:1–7. 10.1136/esmoopen-2016-000068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pujol J-L, De Pas T, Rittmeyer A, et al. Safety and immunogenicity of the PRAME Cancer Immunotherapeutic in patients with resected non-small cell lung Cancer: a phase I dose escalation study. J Thorac Oncol 2016;11:2208–17. 10.1016/j.jtho.2016.08.120 [DOI] [PubMed] [Google Scholar]
- 70.Slingluff CL, Petroni GR, Olson WC, et al. A randomized pilot trial testing the safety and immunologic effects of a MAGE-A3 protein plus AS15 immunostimulant administered into muscle or into dermal/subcutaneous sites. Cancer Immunol Immunother 2016;65:25–36. 10.1007/s00262-015-1770-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosalia RA, Quakkelaar ED, Redeker A, et al. Dendritic cells process synthetic long peptides better than whole protein, improving antigen presentation and T-cell activation. Eur J Immunol 2013;43:2554–65. 10.1002/eji.201343324 [DOI] [PubMed] [Google Scholar]
- 72.Valmori D, Dutoit V, Ayyoub M. Simultaneous CD8+ T cell responses to multiple tumor antigen epitopes in a multipeptide melanoma vaccine. Cancer Immun 2003;3:1–14. [PubMed] [Google Scholar]
- 73.Meijer SL, Dols A, Jensen SM, et al. Induction of circulating tumor-reactive CD8+ T cells after vaccination of melanoma patients with the gp100 209-2M peptide. J Immunother 2007;30:533–43. 10.1097/CJI.0b013e3180335b5e [DOI] [PubMed] [Google Scholar]
- 74.Baumgaertner P, Rufer N, Devevre E, et al. Ex vivo detectable human CD8 T-cell responses to cancer-testis antigens. Cancer Res 2006;66:1912–6. 10.1158/0008-5472.CAN-05-3793 [DOI] [PubMed] [Google Scholar]
- 75.Slingluff CL, Petroni GR, Olson WC, et al. Effect of granulocyte/macrophage colony-stimulating factor on circulating CD8+ and CD4+ T-cell responses to a multipeptide melanoma vaccine: outcome of a multicenter randomized trial. Clin Cancer Res 2009;15:7036–44. 10.1158/1078-0432.CCR-09-1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Slingluff CL, Petroni GR, Chianese-Bullock KA, et al. Randomized multicenter trial of the effects of melanoma-associated helper peptides and cyclophosphamide on the immunogenicity of a multipeptide melanoma vaccine. J Clin Oncol 2011;29:2924–32. 10.1200/JCO.2010.33.8053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mauldin IS, Wages NA, Stowman AM, et al. Topical treatment of melanoma metastases with imiquimod, plus administration of a cancer vaccine, promotes immune signatures in the metastases. Cancer Immunol Immunother 2016;65:1201–12. 10.1007/s00262-016-1880-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mauldin IS, Wages NA, Stowman AM, et al. Intratumoral interferon-gamma increases chemokine production but fails to increase T cell infiltration of human melanoma metastases. Cancer Immunol Immunother 2016;65:1189–99. 10.1007/s00262-016-1881-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Slingluff CL, Petroni GR, Chianese-Bullock KA, et al. Immunologic and clinical outcomes of a randomized phase II trial of two multipeptide vaccines for melanoma in the adjuvant setting. Clin Cancer Res 2007;13:6386–95. 10.1158/1078-0432.CCR-07-0486 [DOI] [PubMed] [Google Scholar]
- 80.Pavlick A, Blazquez AB, Meseck M, et al. Combined vaccination with NY-ESO-1 protein, Poly-ICLC, and montanide improves humoral and cellular immune responses in patients with high-risk melanoma. Cancer Immunol Res 2020;8:70–80. 10.1158/2326-6066.CIR-19-0545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liénard D, Rimoldi D, Marchand M, et al. Ex vivo detectable activation of Melan-A-specific T cells correlating with inflammatory skin reactions in melanoma patients vaccinated with peptides in IFA. Cancer Immun 2004;4:4:4. [PubMed] [Google Scholar]
- 82.Slingluff CL, Petroni GR, Olson W, et al. Helper T-cell responses and clinical activity of a melanoma vaccine with multiple peptides from MAGE and melanocytic differentiation antigens. J Clin Oncol 2008;26:4973–80. 10.1200/JCO.2008.17.3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reed CM, Cresce ND, Mauldin IS, et al. Vaccination with melanoma helper peptides induces antibody responses associated with improved overall survival. Clin Cancer Res 2015;21:3879–87. 10.1158/1078-0432.CCR-15-0233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pollack KE, Meneveau MO, Melssen MM, et al. Incomplete freund’s adjuvant reduces arginase and enhances Th1 dominance, TLR signaling and CD40 ligand expression in the vaccine site microenvironment. J Immunother Cancer 2020;8:e0005:e000544. 10.1136/jitc-2020-000544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Melssen MM, Pollack KE, Meneveau MO, et al. Characterization and comparison of innate and adaptive immune responses at vaccine sites in melanoma vaccine clinical trials. Cancer Immunol Immunother 2021;70:2151–64. 10.1007/s00262-020-02844-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kenter GG, Welters MJP, Valentijn ARPM, et al. Phase I immunotherapeutic trial with long peptides spanning the E6 and E7 sequences of high-risk human papillomavirus 16 in end-stage cervical cancer patients shows low toxicity and robust immunogenicity. Clin Cancer Res 2008;14:169–77. 10.1158/1078-0432.CCR-07-1881 [DOI] [PubMed] [Google Scholar]
- 87.Welters MJP, Kenter GG, Piersma SJ, et al. Induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin Cancer Res 2008;14:178–87. 10.1158/1078-0432.CCR-07-1880 [DOI] [PubMed] [Google Scholar]
- 88.Chianese-Bullock KA, Irvin WP, Petroni GR, et al. A multipeptide vaccine is safe and elicits T-cell responses in participants with advanced stage ovarian cancer. J Immunother 2008;31:420–30. 10.1097/CJI.0b013e31816dad10 [DOI] [PubMed] [Google Scholar]
- 89.Slingluff CL, Lee S, Zhao F, et al. A randomized phase II trial of multiepitope vaccination with melanoma peptides for cytotoxic T cells and helper T cells for patients with metastatic melanoma (E1602). Clin Cancer Res 2013;19:4228–38. 10.1158/1078-0432.CCR-13-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Melief CJM, Welters MJP, Vergote I, et al. Strong vaccine responses during chemotherapy are associated with prolonged cancer survival. Sci Transl Med 2020;12:eaaz8235. 10.1126/scitranslmed.aaz8235 [DOI] [PubMed] [Google Scholar]
- 91.Schaefer JT, Patterson JW, Deacon DH, et al. Dynamic changes in cellular infiltrates with repeated cutaneous vaccination: a histologic and immunophenotypic analysis. J Transl Med 2010;8:1–13. 10.1186/1479-5876-8-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peske JD, Thompson ED, Gemta L, et al. Effector lymphocyte-induced lymph node-like vasculature enables naive T-cell entry into tumours and enhanced anti-tumour immunity. Nat Commun 2015;6:7114. 10.1038/ncomms8114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rodriguez AB, Peske JD, Woods AN, et al. Immune mechanisms orchestrate tertiary lymphoid structures in tumors via cancer-associated fibroblasts. Cell Rep 2021;36:109422. 10.1016/j.celrep.2021.109422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schumacher TN, Thommen DS. Tertiary lymphoid structures in cancer. Science 2022;375:eabf9419. 10.1126/science.abf9419 [DOI] [PubMed] [Google Scholar]
- 95.Hu Y, Kim H, Blackwell CM, et al. Long-term outcomes of helper peptide vaccination for metastatic melanoma. Ann Surg 2015;262:456–62. 10.1097/SLA.0000000000001419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kjeldsen JW, Lorentzen CL, Martinenaite E, et al. A phase 1/2 trial of an immune-modulatory vaccine against IDO/PD-L1 in combination with nivolumab in metastatic melanoma. Nat Med 2021;27:2212–23. 10.1038/s41591-021-01544-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kenter GG, Welters MJP, Valentijn ARPM, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N Engl J Med 2009;361:1838–47. 10.1056/NEJMoa0810097 [DOI] [PubMed] [Google Scholar]
- 98.van Poelgeest MIE, Welters MJP, Vermeij R, et al. Vaccination against oncoproteins of HPV16 for noninvasive Vulvar/Vaginal lesions: lesion clearance is related to the strength of the T-cell response. Clin Cancer Res 2016;22:2342–50. 10.1158/1078-0432.CCR-15-2594 [DOI] [PubMed] [Google Scholar]
- 99.Massarelli E, William W, Johnson F, et al. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol 2019;5:67–73. 10.1001/jamaoncol.2018.4051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van Seters M, van Beurden M, de Craen AJM. Is the assumed natural history of vulvar intraepithelial neoplasia III based on enough evidence? A systematic review of 3322 published patients. Gynecol Oncol 2005;97:645–51. 10.1016/j.ygyno.2005.02.012 [DOI] [PubMed] [Google Scholar]
- 101.Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–67. 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sousa LGde, Rajapakshe K, Rodriguez Canales J, et al. ISA101 and nivolumab for HPV-16+ cancer: updated clinical efficacy and immune correlates of response. J Immunother Cancer 2022;10:e004232. 10.1136/jitc-2021-004232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Laplagne C, Domagala M, Le Naour A, et al. Latest advances in targeting the tumor microenvironment for tumor suppression. Int J Mol Sci 2019;20:471. 10.3390/ijms20194719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saxena M, van der Burg SH, Melief CJM, et al. Therapeutic cancer vaccines. Nat Rev Cancer 2021;21:360–78. 10.1038/s41568-021-00346-0 [DOI] [PubMed] [Google Scholar]