Abstract
Introduction
Autologous skin cell suspension (ASCS) significantly reduces donor skin requirements versus conventional split-thickness skin grafts (STSG) for thermal burn treatment. In analyses using the Burn-medical counter measure Effectiveness Assessment Cost Outcomes Nexus (BEACON) model, ASCS was associated with shorter hospital length of stay (LOS) and cost savings versus STSG. This study hypothesized that daily practice data from the USA would support these findings.
Methods
Electronic medical record data from 500 healthcare facilities (January 2019–August 2020) were used to match adult patients who received inpatient burn treatment with ASCS (± STSG) to patients treated with STSG alone on the basis of sex, age, percent total body surface area (TBSA), and comorbidities. Based on BEACON analyses, LOS was assumed to represent 70% of total costs and used as a proxy to assess the data. Mean LOS, costs, and the incremental revenue associated with inpatient capacity changes were calculated.
Results
A total of 151 ASCS and 2443 STSG patients were identified: 63.0% were male and average age was 44.5 years. Eight-one matches were made between cohorts. LOS was 21.7 days with ASCS and 25.0 days with STSG alone (difference 3.3 days [13.2%]). LOS was lower with ASCS than STSG in four of five TBSA intervals. The LOS difference led to hospital bed cost savings of $25,864 per ASCS patient; overall cost savings were $36,949 per patient. Similar cost savings were observed in TBSA groupings < 20% and ≥ 20%. The reduced LOS with ASCS translated into an increased capacity of 2.2 inpatients/bed annually, which increased hospital revenue by $92,283/burn unit bed annually.
Conclusions
Real-world data show that ASCS (± STSG) is associated with reduced LOS and cost savings versus STSG alone across all burn sizes, supporting the validity of the BEACON analyses. ASCS use may also increase patient capacity and throughput, leading to increased hospital revenue.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02306-y.
Keywords: Burn injury, Real-world data, Autologous skin cell suspension, Length of stay, Cost savings
Plain Language Summary
Autologous skin cell suspension (ASCS) is a treatment for thermal skin burn injuries that can be used alone or in combination with split-thickness skin grafts (STSG), the conventional standard of care. Projections using the Burn-medical counter measure Effectiveness Assessment Cost Outcomes Nexus (BEACON) model indicate that ASCS leads to shorter hospital length of stay (LOS) and overall cost savings compared with STSG alone. These model findings are supported by benchmarking study data from a limited sample of US burn centers. The current study aimed to understand whether the BEACON projections are supported by daily clinical practice data from US healthcare facilities. Using electronic medical record data, we matched patients who received ASCS ± STSG from January 2019 to August 2020 to those receiving STSG alone on the basis of demographic and clinical factors. Data analysis showed that hospital LOS was shorter (3.3 days) with ASCS ± STSG than STSG alone, a difference associated with a hospital bed cost savings of $25,864 per ASCS patient. Overall cost savings, which included nursing time and other costs, were $36,949 per patient. Analysis of patients with burns comprising total body surface areas less than 20% or at least 20% showed cost savings in both groups. The reduced LOS with ASCS also translated into the ability to treat 2.2 more patients per hospital bed per year, which was projected to increase hospital earnings. These real-world findings support those of modeling analyses, indicating that use of ASCS ± STSG is associated with meaningful clinical and economic benefits compared with use of STSG alone.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02306-y.
Key Summary Points
| The BEACON economic model projections showed that hospital length of stay (LOS) and costs were reduced with use of autologous skin cell suspension (ASCS) versus split-thickness skin grafts (STSG) in the treatment of thermal burn injuries. |
| This study hypothesized that data from real-world US clinical practice would show similar benefits with use of ASCS ± STSG compared with STSG alone among patients with a range of burn sizes. |
| Analysis of healthcare utilization data and costs showed that ASCS ± STSG was associated with reductions in LOS and cost savings compared with STSG alone, regardless of burn injury size, as well as increases in inpatient capacity and hospital revenue. |
| These real-world findings support the validity of the BEACON model projections, underscoring the clinical and economic benefits of ASCS in the treatment of a range of thermal burn sizes. |
Introduction
In 2017 in the USA, approximately 489,000 patients received medical treatment for non-fatal burns in emergency departments (ED), accounting for approximately 1.2% of all non-fatal injuries nationwide and leading to about 50,000 hospital admissions [1–3]. Most burn hospitalizations occur at the 130 plus burn centers located throughout the country, with approximately 200 admissions annually per center [3, 4]; in contrast, acute care hospitals (n = 4500) average fewer than three burn admissions per year [4]. The average length of stay (LOS) of admissions for burn treatment ranges from 8.5 to 10 days [3], leading to a high cost burden: a study of two privately insured US populations found that the mean total medical costs associated with treating surviving burn patients ranged from approximately $131,000 to $157,000 per patient. Overall, the resource and economic burden of burns is substantial to patients, the healthcare system, and society [5, 6].
New burn care interventions seek to improve clinical and economic outcomes through reductions in the number of surgeries, time to wound closure, infection rates, and rehabilitation needs, all of which can impact the LOS of patients in hospital [7, 8]. In 2018, the RECELL® Autologous Cell Harvesting Device (ACHD) (AVITA Medical, Valencia, CA, USA) was initially approved by the US Food and Drug Administration (FDA) for the treatment of adult patients with deep partial thickness (DPT) or full thickness (FT)/mixed depth acute thermal burns with a total body surface area (TBSA) less than 50%; in 2021, this indication was expanded to include adults with all burn sizes and pediatric patients [9]. Using RECELL®, an autologous skin cell suspension (ASCS) treatment is prepared that can be applied either as a primary intervention for DPT burns with confluent dermis or used in combination with a meshed split-thickness graft (STSG) for acute FT or mixed depth burns [9].
By enabling rapid preparation of an ASCS at the point of care [9, 10], clinical trials, compassionate use studies, and analyses of real-world data (i.e., data obtained outside of clinical trial and academic settings) have shown clinical and economic benefits with ASCS compared with conventional STSG in burn care. For example, recent clinical trials show that ASCS treatment significantly reduces donor skin harvesting requirements compared with STSG, enhances re-epithelialization of widely meshed skin grafts, and may decrease the need for follow-up reconstructive procedures (e.g., for contractures) [10–13]. Furthermore, analyses of real-world burn center records indicate that use of ACHD-generated ASCS, in isolation or in combination with STSG, can reduce hospital LOS for severe burn patients compared with use of STSG alone [7, 14, 15].
Three recent studies have analyzed the healthcare resource utilization needs and costs associated with the treatment of acute thermal burns [7, 15, 16]. In the first study, Kowal and colleagues used the Burn-MCM (medical counter measure) Effectiveness Assessment Cost Outcomes Nexus (BEACON) hospital-perspective model, which incorporated sequential decision trees to depict acute burn care pathways and predict relative LOS and cost differences between ASCS ± STSG and STSG alone [7]. The model derived clinical inputs from randomized clinical trials, analyses of the American Burn Association’s (ABA) National Burn Repository (NBR) dataset (Version 8.0; 2002–2011) and the National Health and Nutrition Examination Survey (2014–2016), and surveys and interviews conducted with burn surgeons. Unit cost and hospital resource use inputs were obtained from three US burn centers, the best available data at the time of the analysis. The study found that use of ASCS ± STSG was associated with reduced LOS compared with use of STSG alone, which was projected to result in substantial hospital cost savings across various patient profiles and scenarios [7]. In the second study, a representative sample of 14 US burn centers was surveyed (June–December 2019) regarding patient and burn characteristics, resource use, LOS, and costs [15]. The results of this survey, which reflected more recent and robust estimates of burn-related outcomes than those used in the study by Kowal and colleagues, were used in a benchmarking analysis conducted using the BEACON model [16]. Even with the inclusion of more representative input data, the results of the benchmarking analysis supported those of the first BEACON model analysis, projecting a substantial reduction in hospital LOS and cost savings with ASCS ± STSG.
Although the findings of the BEACON model analyses are in agreement regarding the LOS and cost benefits of ASCS, it is of interest to understand whether their projections are supported by the real-world clinical experience with burn treatment in the USA. Given that ICD-10-PCS codes became available for RECELL® in October of 2019, the current study sought to compare these outcomes for ASCS ± STSG versus STSG alone in a population of patients with TBSA < 50% using real-world data derived from a large cohort of US facilities. It was hypothesized that similar to the modeling studies, use of ASCS ± STSG would be associated with reduced LOS and cost savings compared with use of STSG alone.
Methods
Data Source
De-identified electronic medical record (EMR) data for patient LOS were collected from a closed sample of 500 US facilities (including burn centers) using a large, commercially available EMR system (Decision Resources Group/Clarivate, Toronto, Canada). Patient records were not accessed by the study investigators. A 20-month period (January 2019 to August 2020 inclusive) was considered for data collection. The dataset encompassed each facility’s entire chargemaster (e.g., the hospital-specific compendia of all items that a hospital bills), including LOS. Key coded data fields included ICD-10-PCS codes, age, sex, ICD-10-CM codes by TBSA, and LOS.
Patients who had received ASCS ± STSG for burn treatment were identified using ICD-10-PCS codes for skin graft procedures (0HR_X72, 0HR_X73, and 0HR_X74; see supplementary material, Table S1). Hospital inpatients meeting the FDA-approved indication for the ASCS device at the time of the study were eligible for inclusion: at least 21 years of age with DPT or FT/mixed depth acute thermal burns of less than 50% TBSA (ICD-10-CM range T31.0–T31.44; see supplementary material, Table S2) [9]. To ensure inclusion of patients who were similar in terms of baseline characteristics, the ASCS ± STSG patients were matched 1:1 by age, sex, and percent TBSA to those treated with STSG alone. The criteria for age matching between cohorts were based on the same age stratification of 10-year increments (i.e., 20–29, 30–39, etc.) used in the 2019 NBR report [3], where patients falling within the same stratification were considered a match. Burn injury severity was matched on the basis of the ICD-10-CM diagnosis codes, which use the following TBSA intervals: < 10%, 10–19%, 20–29%, 30–39%, and 40–49%. In the event that multiple exact matches were possible on the basis of age, sex, and percent TBSA, patients with the closest non-burn comorbidities, such as diabetes, obesity, substance abuse, mental disorders, and other disease states, were matched and included in the analysis.
Cost Inputs, Calculations, and Statistical Analyses
All statistical analyses were performed using R 3.5.0 (R Core Team, 2018). Mean LOS and standard deviations (SDs) were calculated for each treatment group and by TBSA interval. A Shapiro–Wilk’s test was used to understand whether the data were normally distributed (level of significance, p < 0.0001) and a Wilcoxon test was subsequently performed to determine statistical differences (95% confidence interval) in LOS between treatment groups.
Given that reductions in LOS contributed to approximately 70% of the cost savings associated with ASCS ± STSG versus STSG in the BEACON model analyses [7], LOS was used as a single-measure proxy to determine any differences in costs between use of ASCS ± STSG and STSG alone. The remaining 30% of costs were attributed to operating room, nurse, and scrub tech time, physical and occupational therapy, and contracture surgery, among others [7]. Data from a recent survey of US burn centers [15], which reported a mean bed cost per day of $7554 for patients with TBSA < 20% and $8362 for patients with TBSA ≥ 20% (2019 data), were used to calculate cost differences between the ASCS ± STSG and STSG alone groups (both per-patient and in the matched-population) for LOS and overall. Calculations were also undertaken to understand whether the cost of the ASCS device ($7500 per unit [7]) would be offset by any LOS-related savings associated with the use of ASCS ± STSG. As each ASCS device treats approximately 10% TBSA for an average-sized adult burn patient [9, 17], it was assumed that patients with TBSA < 20% would require two ASCS devices and those with TBSA ≥ 20% would require four devices.
The study also investigated whether inpatient capacity gains could translate into additional hospital revenue. Using the difference in LOS between treatment cohorts, the analysis estimated the number of additional burn patients who could receive inpatient treatment by projecting the annual utilization of a hospital burn unit bed. Full occupancy of each hospital burn unit bed was assumed (i.e., when one patient was discharged, another was immediately admitted). Incremental annual hospital revenue was estimated by multiplying the increased number of treated patients by the diagnosis-related group (DRG) payment for the stay. The unadjusted national amount for MS-DRG 928 (i.e., FT burn with skin graft or inhalation injury with comorbidity or complication/major comorbidity or complication) of $41,947 (2020) was used to estimate revenue per burn patient stay. Annual incremental revenue per patient was calculated by dividing the estimated change in revenue by the estimated number of patients treated annually and compared between ASCS ± STSG and STSG alone.
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Results
Patient Characteristics
Based on the study inclusion criteria, data derived from the EMR system encompassed information from US healthcare facilities located in 14 states: 7 states for ASCS ± STSG patients and all 14 states for STSG alone. A total of 151 ASCS ± STSG and 2443 STSG cases were obtained and reviewed. Patient matching by age, sex, TBSA interval, and comorbidities (as required) yielded a total of 81 matches across the two groups. Across all matched patients, 63.0% were male and the average age was 44.5 years (Table 1). In terms of burn size, 33.3% had TBSA < 10% and 77.7% had TBSA < 20%.
Table 1.
Patient characteristics in the current study of real-world data (overall and matched) and the 2019 NBR report
| Patient characteristic | Current study of ASCS | 2019 NBR report n = 161,585 |
||
|---|---|---|---|---|
| All patients | Matched patients n = 81a |
|||
| ASCS ± STSG n = 151 |
STSG alone n = 2443 |
|||
| Male sex, n (%)a | 88 (58.3) | 1581 (64.7) | 51 (63.0) | 102,687 (63.5) |
| Age, years, n (%)a | ||||
| 20–29 | 31 (20.5) | 337 (13.8) | 17 (21.0) | 32,749 (20.3) |
| 30–39 | 35 (23.2) | 435 (17.8) | 19 (23.5) | 29,476 (18.2) |
| 40–49 | 26 (17.2) | 408 (16.7) | 20 (24.7) | 30,583 (18.9) |
| 50–59 | 22 (14.6) | 447 (18.3) | 9 (11.1) | 31,245 (19.3) |
| 60–69 | 19 (12.6) | 432 (17.7) | 9 (11.1) | 20,188 (12.5) |
| 70–79 | 7 (4.6) | 254 (10.4) | 4 (4.9) | 10,513 (6.5) |
| Above 80 | 11 (7.3) | 130 (5.3) | 3 (3.7) | 6831 (4.2) |
| TBSA, n (%) | ASCS ± STSG n = 151 |
STSG alone n = 2443 |
Matched patients n = 81a |
2019 NBR report n = 177,348 |
|---|---|---|---|---|
| 0.1–9.9% | 35 (23.2) | 1484 (60.7) | 27 (33.3) | 139,960 (78.9) |
| 10–19.9% | 48 (31.8) | 542 (22.2) | 36 (44.4) | 23,458 (13.2) |
| 20–29.9% | 31 (20.5) | 216 (8.8) | 11 (13.6) | 7668 (4.3) |
| 30–39.9% | 15 (9.9) | 96 (3.9) | 5 (6.2) | 3888 (2.2) |
| 40–49.9% | 10 (6.6) | 44 (1.8) | 2 (2.5) | 2374 (1.3) |
| > 50%b | 12 (8.0) | 61 (2.5) | – | – |
ASCS autologous skin cell suspension, NBR National Burn Repository, STSG split-thickness skin graft alone, TBSA total body surface area
aIncludes both ASCS ± STSG and STSG alone patients
bPatients with TBSA >50% were included in the original dataset but were not considered for matching in the current analysis due to labeling for ASCS at the time of the study (see Methods)
Length of Stay Outcomes
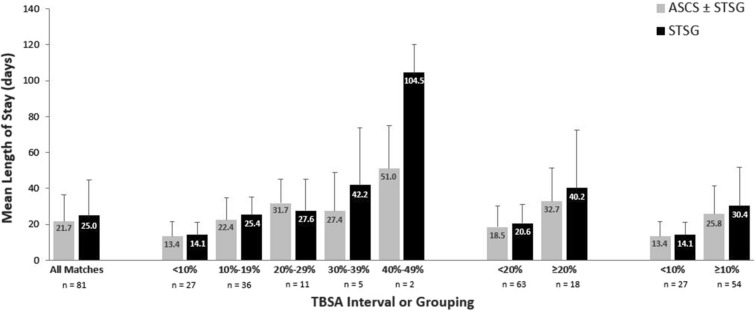
Across all patient matches, ASCS ± STSG was associated with a shorter average LOS (SD) than STSG alone, at 21.7 (14.7) days versus 25.0 (19.5) days, respectively (Fig. 1). This 3.3-day difference represented a 13.2% reduction in LOS with ASCS ± STSG (p = 0.0627). LOS was shorter with ASCS ± STSG than with STSG alone in 67% of patient matches. This reduction translated into a total of 266 hospital bed days saved across all treated patients.
Fig. 1.
Comparison of LOS associated with ASCS ± STSG or STSG alone by TBSA interval or grouping. ASCS autologous skin cell suspension, LOS length of stay, STSG split-thickness skin graft, TBSA total body surface area
Across the TBSA intervals (10% increments), four of the five TBSA groups had shorter LOS with ASCS ± STSG than STSG alone (Fig. 1). The reductions in LOS with ASCS ± STSG ranged from 0.7 days for TBSA < 10% (n = 27) to 53.5 days for TBSA 40–49% (n = 2); the differences between treatment groups were not statistically significant given small sample sizes. When the TBSA intervals were combined, LOS was 2.1 days lower with ASCS ± STSG for TBSA < 20% (mean [SD] 18.5 [11.57] days vs. 20.6 [10.33] days with STSG alone) and 7.6 days lower with ASCS ± STSG for TBSA ≥ 20% (32.7 [18.73] days vs. 40.2 [32.29] days); neither difference was statistically significant. In terms of mean (SD) LOS per percent TBSA, ASCS ± STSG ranged from 0.78 (0.61) to 2.69 (1.66) days and STSG alone ranged from 1.11 (0.69) to 2.83 (1.37) days across the TBSA intervals (Table 2).
Table 2.
Difference in mean LOS per percent TBSA with ASCS ± STSG versus STSG alone
| TBSA interval | n | ASCS ± STSG, days Mean (SD) |
STSG alone, days Mean (SD) |
Difference (average days) |
|---|---|---|---|---|
| < 10% | 27 | 2.69 (1.66) | 2.83 (1.37) | 0.14 |
| 10–19% | 36 | 1.49 (0.81) | 1.63 (0.66) | 0.14 |
| 20–29% | 11 | 1.27 (0.54) | 1.11 (0.69) | − 0.16 |
| 30–39% | 5 | 0.78 (0.61) | 1.21 (0.90) | 0.42 |
| 40–49% | 2 | 1.13 (0.53) | 2.32 (0.34) | 1.19 |
ASCS autologous skin cell suspension, LOS length of stay, SD standard deviation, STSG split-thickness skin graft, TBSA total body surface area
Cost Outcomes
When average costs of $7554 (TBSA < 20%) and $8362 (TBSA ≥ 20%) per day were used, the 3.3-day reduction in average LOS with ASCS ± STSG translated into a savings of $25,864 (13.2%) in hospital bed costs (LOS) per ASCS ± STSG patient (Table 3). Across all 81 matched patients, total hospital bed cost savings were $2,094,998 with ASCS ± STSG. In the subgroup of patients with TBSA < 20%, the average LOS reduction of 2.1 days with ASCS ± STSG decreased hospital bed costs by $15,588 per patient ($982,020 for all matched patients). In the subgroup of patients with TBSA ≥ 20%, the average LOS reduction of 7.5 days with ASCS ± STSG decreased these costs by $62,715 per patient ($1,128,870 for all matched patients). In terms of overall costs (i.e., LOS plus other costs such as nurse, scrub tech, operating room time, etc.), the projected savings with ASCS ± STSG were $36,949 per patient or $2,992,855 for the entire matched cohort including all TBSAs. For patients with TBSA < 20%, overall savings were $22,268 per patient or $1,402,886 across all matched patients in this subgroup; for patients with TBSA ≥ 20%, overall savings were $89,593 per patient or $1,612,671 across all matched patients.
Table 3.
Cost savings (USD) associated with use of ASCS ± STSG versus STSG alone
| Patient group | n | LOS hospital bed cost savings with ASCS ± STSG | Overall cost savings with ASCS ± STSG |
|---|---|---|---|
| All patients | 81 | ||
| Per patient | $25,864 | $36,949 | |
| All matched patients | $2,094,998 | $2,992,855 | |
| Patients with TBSA < 20% | 63 | ||
| Per patient | $15,588 | $22,268 | |
| All matched patients | $982,020 | $1,402,886 | |
| Patients with TBSA ≥ 20% | 18 | ||
| Per patient | $62,715 | $89,593 | |
| All matched patients | $1,128,870 | $1,612,671 |
ASCS autologous skin cell suspension, LOS length of stay, STSG split-thickness skin graft, TBSA total body surface area, USD United States dollars
For patients with TBSA < 20%, the cost of two ASCS devices would be $15,000; for patients with larger burn sizes, assuming use of four ASCS devices, the cost would be $30,000. For both subgroups, the cost savings associated with the difference in LOS between ASCS ± STSG and STSG alone were found to offset the upfront cost of the required ASCS devices (difference $588 for TBSA < 20% and $32,715 for TBSA ≥ 20%).
Based on the reduced LOS associated with ASCS ± STSG, it was projected that the number of patients treated per bed per year would be 16.8 for ASCS ± STSG and 14.6 for STSG alone, an additional 2.2 patients per burn unit bed annually. This increase in patient capacity was estimated to increase hospital revenue by $92,283 per burn unit bed each year (2.2 patients × $41,947 MS-DRG 928 payment) or by $5493 per patient treated with ASCS ± STSG ($92,283 revenue per bed per year/16.8 ASCS ± STSG patients per bed per year). For a hospital with five burn unit beds, total revenue could increase by $461,417 annually ($92,283 revenue per bed per year × 5 beds).
Discussion
To our knowledge, the current study is the first analysis of EMR-derived data for patients treated for a wide range of burn sizes at USA-based centers using ICD-10-PCS codes for ASCS, which first became available on October 1, 2019. It is also the largest analysis conducted to date of the cost savings associated with LOS using actual ASCS data. As such, the study offers valuable insights into the contemporary resource utilization and cost differences associated with ASCS ± STSG versus conventional STSG across a matched sample of US patients with thermal burn wounds.
Overall, the distribution of patient characteristics in the matched population was generally similar to that reported for the NBR [3], a nationally representative registry of burn patients (Table 1). In the 2019 NBR report, male patients represented 63.5% of all cases over the age of 20 and most patients were under the age of 50. In terms of TBSA, the all-patient dataset for STSG alone (n = 2443) in the current study was similar to that of the NBR distribution. Any differences in TBSA distribution between the all-patient ASCS ± STSG dataset (n = 151) and the NBR report—with ASCS ± STSG having relatively more patients with large burns—may be attributable to the donor skin-sparing advantage of ASCS. However, the differences in TBSA distributions were smaller than expected, with more than half of ASCS ± STSG treatments occurring among patients with TBSA < 20%.
The primary goal of the study was to compare differences in LOS and costs between ASCS ± STSG and STSG alone. It was also of interest to understand whether the findings validate those projected by the BEACON model [7, 16]. The study results indeed corroborate the findings of the BEACON model analyses: when real-world treatment data were used, a 13.2% (3.3-day) reduction in average LOS was observed with ASCS ± STSG versus STSG alone across all patient matches, which led to a total cost savings of $36,949 per ASCS ± STSG patient. In the first BEACON model analysis, a similar reduction in annual burn center costs (14–17.3%; $26,600–34,100) was reported for ASCS across conservative and NBR-based scenarios [7]. In the benchmark analysis of the BEACON model [16] that incorporated inputs from a survey of 14 US burn centers [15], use of ASCS was projected to lower overall costs by an estimated $79,600 per patient, a reduction of 17.4%.
The findings for small burns (TBSA < 20%) were also consistent between the current study and the BEACON model analyses [7, 16], showing lower LOS and costs with ASCS ± STSG than STSG alone. Although the cost savings associated with ASCS ± STSG were lower with smaller than larger burn sizes in the current study, the savings with small burns are still important: data from the NBR show that burns with TBSA < 20% comprise 92.1% of inpatient burn admissions [3]. Given the high proportion of patients incurring small burns, the savings associated with ASCS ± STSG can accumulate over a large number of patients, as observed in the current study: the 63 ASCS ± STSG patients with TBSA < 20% represented more than $1.4 million in overall cost savings.
The current study also analyzed LOS per percent TBSA, showing lower values with use of ASCS ± STSG than STSG alone across four of five TBSA intervals. The range of values for this outcome were generally comparable to those from an analysis conducted by Kruger and colleagues, who showed that LOS per percent TBSA ranged from 1.07 to 1.21 days for TBSA 10–50% among adult patients [18]. The current study’s values were similar except in the TBSA < 10% interval, which was not included in the Kruger study, and for STSG alone in the TBSA 40–49% interval, where the result was 2.32 days. Only two patients were included in the 40–49% interval, which may explain this result. It should be noted that several factors can impact the LOS per percent TBSA of burn patients (e.g., age, TBSA) and may have influenced the findings [19]; however, where available, such factors were considered through patient matching in the current study.
Notably, the estimated cost savings associated with the reduced LOS with ASCS ± STSG were found to more than cover the cost of the ASCS devices required for a typical patient. Thus, the initial acquisition cost for the ASCS device would come back to the institution in the form of cost savings, as well as potential revenue increases related to the capacity to treat more patients. Additional savings may also arise from changes in the staffing requirements needed to care for burn patients, given fewer staff members may be required, as well as those related to the learning curve associated with use of ASCS—that is, further improvements in LOS and costs may be realized over time as staff members become more comfortable with its use.
This study additionally sought to understand the potential revenue gains associated with use of ASCS, a benefit that is distinct and separate from its findings of reduced LOS and cost savings. For hospitals with DRG-based revenue generation for burn patients, the increase in patient capacity related to shorter LOS was estimated to increase average revenue by approximately $5493 per ASCS ± STSG-treated patient. Simultaneously, total costs could decrease by $36,949 per patient treated with ASCS ± STSG rather than STSG alone. Considered collectively, use of ASCS ± STSG can provide a substantial budgetary impact given both cost savings and the potential for additional revenue recognition. Moreover, the reduced LOS and improved patient throughput associated with ASCS ± STSG are especially valuable given that labor shortages have negatively impacted clinical care during the COVID-19 pandemic.
A primary limitation of the study relates to its retrospective evaluation of administrative datasets—it was assumed that all data (e.g., ICD codes) were abstracted correctly from patient charts. Another limitation was the relatively low number of patient matches (n = 81), particularly among patients with large burn sizes. In general, the study population was restricted by the limited number of ASCS ± STSG patients captured using the ICD-10-PCS codes. Furthermore, at the time of the study, the approved indication for the ASCS device did not include patients under the age of 21 or with TBSA > 50%; the indication has since been expanded to include these populations [9]. Still, considering data from the 2019 NBR report, it is unlikely that use of the expanded indication would have identified many more patients, given that most burn patients have small burns (92.1% TBSA < 20%; 99.9% TBSA < 50%) and are at least 20 years of age (72.9%) [3]. Overall, the limited number of patient matches resulted in a lack of power that prevented robust evaluation of statistical significance. Another study limitation was that only two standard daily costs (based on % TBSA) were applied to patients’ LOS rather than the specific costs incurred at individual treatment facilities; however, such costs were not available. LOS costs vary between patients with smaller versus larger burns, in part because of variations in bed use (i.e., intensive care unit versus surgical) [15]; accordingly, use of actual facility costs would impact the results of this study. Still, the results for reduced LOS and cost savings are in alignment with those reported in previous studies [7, 16] and the lack of observed statistical significance does not mean that the findings are unimportant clinically or financially. A further study limitation is that the dataset did not capture the healthcare setting in which each patient received treatment (i.e., burn vs. non-burn centers). It is probable that included patients received care at a mix of healthcare settings; however, considering the timing of data collection and ASCS product launch, it is assumed that most if not all ASCS ± STSG patients were treated in burn centers, given initial use of this therapy was highly limited to these facilities. For the STSG alone cohort, it is not possible to determine or make broad assumptions about the type of treating facility. Accordingly, some STSG alone-treated patients, especially those with smaller burn sizes, may have received treatment at non-burn center facilities. Given that burn care expertise is more limited at non-burn centers, the LOS of STSG-treated patients may have been prolonged relative to that of the ASCS ± STSG patients. Although the magnitude of this impact could not be evaluated, the observed trend was consistent with the findings of previous studies. Finally, an additional study limitation was that the calculation of revenue changes related to bed capacity assumed that all hospital beds dedicated to burn patients were fully utilized—any days that burn unit beds remain idle would reduce revenue estimates. Furthermore, potential revenue increases were only applicable to hospitals with DRG-based payer contracts and not those with other revenue systems, such as per-diem-based contracts.
Conclusions
This study is the first and largest analysis of real-world utilization data and costs related to inpatient treatment of thermal burns since the availability of ICD-10-PCS codes for ASCS. Its findings support the validity of the BEACON model analyses, showing reductions in LOS and cost savings with ASCS ± STSG when compared to STSG alone. Notably, the projected savings more than cover the cost of the ASCS device, even among the 55% of patients who received treatment for small burns (TBSA < 20%). Beyond the reductions in LOS, additional reductions in the resource utilization associated with ASCS will further improve cost savings. Moreover, given the shorter LOS associated with ASCS ± STSG, significant increases in revenue may be realized related to the improved capacity to treat more burn patients. As additional real-world data become available, new analyses should be conducted in larger patient populations using more granular datasets (e.g., including inputs for inhalation injury, contracture surgery) such that the current findings can be validated and powered to show statistical significance.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study and the journal's Rapid Service Fee were sponsored by AVITA Medical, Valencia, CA, USA.
Medical Writing, Editorial, and Other Assistance
The authors wish to thank Stacey Kowal for her support with strategic direction of this manuscript, as well as Dana L. Anger of WRITRIX Medical Communications Inc. for medical writing support that was funded by AVITA Medical. The authors additionally wish to acknowledge the Biomedical Advanced Research and Development Authority (BARDA) for its support of and work on the BEACON model.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
All authors contributed to study conception and design. Material preparation, data collection, and analysis were performed by Thomas Walsh and Russell Becker. The first draft of the manuscript was written by Russell Becker; all authors commented on previous versions of the manuscript and read and approved the final manuscript.
Disclosures
Jeffrey Carter is a consultant to SpectralMD Inc. and AVITA Medical, a stockholder of PermeaDerm Inc. & SpectralMD Inc., and has research supported by Spirit of Charity Foundation Burn Research Fund. Joshua Carson is a consultant to AVITA Medical, Mallinckrodt Pharmaceutical, and the United States Department of Defense (via General Dynamics). William Hickerson is a consultant to AVITA Medical, Vericel, and Avadim Health Inc, and is a stockholder of PermeaDerm. Lisa Rae and Syed Saquib have no conflicts to disclose. Lisa Wibbenmeyer and Russell Becker are consultants to AVITA Medical, Valencia, CA, USA. Jeremiah Sparks and Thomas Walsh are employees of AVITA Medical, Valencia, CA, USA.
Compliance with Ethics Guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to vendor license limitations on the sharing of proprietary data. However, the dataset is available for acquisition from Decision Resource Group/Clarivate.
References
- 1.Rui P, Kang K. National Hospital Ambulatory Medical Care Survey: 2017 emergency department summary tables. National Center for Health Statistics 2017. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2017_ed_web_tables-508.pdf. Accessed Aug 2021.
- 2.Centers for Disease Control and Prevention. Centers for disease control and prevention national center for injury prevention and control. Web-based Injury Statistics Query and Reporting System (WISQARS) 2021. www.cdc.gov/injury/wisqars/. Accessed Aug 2021.
- 3.American Burn Association. National burn respository 2019 update. Report of data from 2009–2018. Dataset Version 14.0 2020. https://ameriburn.org/. Accessed Aug 2021.
- 4.American Burn Association. Burn Incidence Fact Sheet. Burn incidence and treatment in the United States: 2016 2020. https://ameriburn.org/who-we-are/media/burn-incidence-fact-sheet/. Accessed Aug 2021.
- 5.Centers for Disease Control and Prevention. Centers for disease control and prevention national center for injury prevention and control. Estimated number of nonfatal emergency department visits and average and total lifetime costs, United States, 2010 2021. https://wisqars.cdc.gov:8443/costT/cost_Part1_Intro.jsp. Accessed Aug 2021.
- 6.Centers for Disease Control and Prevention. Centers for disease control and prevention national center for injury prevention and control. Number of deaths and estimated average and total lifetime costs, United States, 2010 2021. https://wisqars.cdc.gov:8443/costT/cost_Part1_Intro.jsp. Accessed Aug 2021.
- 7.Kowal S, Kruger E, Bilir P, et al. Cost-effectiveness of the use of autologous cell harvesting device compared to standard of care for treatment of severe burns in the United States. Adv Ther. 2019;36(7):1715–1729. doi: 10.1007/s12325-019-00961-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith RR, Hill DM, Hickerson WL, Velamuri SR. Analysis of factors impacting length of stay in thermal and inhalation injury. Burns. 2019;45(7):1593–1599. doi: 10.1016/j.burns.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Food and Drug Administration. Instructions for use: RECELL(R) autologous cell harvesting device. AWIFU-030–7 2021. https://www.fda.gov/media/116382/download. Accessed Aug 2021.
- 10.Holmes JH, IV, Molnar JA, Carter JE, et al. A comparative study of the ReCell® device and autologous split-thickness meshed skin graft in the treatment of acute burn injuries. J Burn Care Res. 2018;39(5):694–702. doi: 10.1093/jbcr/iry029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sood R, Roggy DE, Zieger MJ, Nazim M, Hartman BC, Gibbs JT. A comparative study of spray keratinocytes and autologous meshed split-thickness skin graft in the treatment of acute burn injuries. Wounds. 2015;27(2):31–40. [PubMed] [Google Scholar]
- 12.Holmes JH, IV, Molnar JA, et al. Demonstration of the safety and effectiveness of the RECELL® system combined with split-thickness meshed autografts for the reduction of donor skin to treat mixed-depth burn injuries. Burns. 2019;45(4):772–782. doi: 10.1016/j.burns.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Gravante G, Di Fede MC, Araco A, et al. A randomized trial comparing ReCell system of epidermal cells delivery versus classic skin grafts for the treatment of deep partial thickness burns. Burns. 2007;33(8):966–972. doi: 10.1016/j.burns.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Carter JE, Platt B, Tuggle CT., III Reduced length of stay with autologous skin cell suspension reduces burn injuries. J Burn Care Res. 2020;41(1):S37–S38. doi: 10.1093/jbcr/iraa024.060. [DOI] [Google Scholar]
- 15.Carter JE, Amani H, Carter D, et al. Evaluating real-world national and regional trends in definitive closure in U.S. burn care: a survey of U.S. burn centers. J Burn Care Res. 2021;43(1):141–148. doi: 10.1093/jbcr/irab151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster K, Amani A, Carter D, et al. Evaluating health economic outcomes of autologous skin cell suspension (ASCS) for definitive closure in US burn care using contemporary real-world burn center data. J Curr Med Res Opin. 2021;4(11):1042–1054. [Google Scholar]
- 17.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 18.Kruger E, Kowal S, Bilir SP, Han E, Foster K. Relationship between patient characteristics and number of procedures as well as length of stay for patients surviving severe burn injuries: analysis of the American Burn Association National Burn Repository. J Burn Care Res. 2020;41(5):1037–1044. doi: 10.1093/jbcr/iraa040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor SL, Sen S, Greenhalgh DG, Lawless M, Curri T, Palmieri TL. Not all patients meet the 1day per percent burn rule: a simple method for predicting hospital length of stay in patients with burn. Burns. 2017;43(2):282–289. doi: 10.1016/j.burns.2016.10.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to vendor license limitations on the sharing of proprietary data. However, the dataset is available for acquisition from Decision Resource Group/Clarivate.