Abstract
This review is an update of an earlier narrative review published in 2015 on developments in the clinical management of cutaneous leishmaniasis (CL) including diagnosis, treatment, prevention and control measurements. CL is a vector-borne infection caused by the protozoan parasite Leishmania. The vector is the female sandfly. Globally, CL affects 12 million cases and annually 2 million new cases occur. CL is endemic in almost 100 countries and the total risk population is approximately 350 million people. WHO lists CL an emerging and uncontrolled disease and a neglected tropical disease. Local experience-based evidence remains the mainstay for the management of CL. Whereas intralesional therapeutic options are the first treatment option for most CL patients, those with mucocutaneous and disseminated involvement require a systemic therapeutic approach. Moreover, different Leishmania species can vary in their treatment outcomes. Therefore, species determination is critical for optimal CL clinical management. New DNA techniques allow for relatively easy Leishmania species determination, yet they are not easily implemented in resource-limited settings. There is a desperate need for novel, less toxic, and less painful treatment options, especially for children with CL. Yet, the large and well conducted studies required to provide the necessary evidence are lacking. To further control and potentially eliminate CL, we urgently need to improve vector control, and diagnostics, and we require efficient and safe vaccines. Alas, since CL primarily affects poor people, biotechnical companies dedicate little investment into the research programs that could lead to diagnostic, pharmaceutical, and vaccine innovations.
Key Points
| WHO acknowledges cutaneous leishmaniasis is an emerging, uncontrolled, and neglected infection affecting millions yearly. |
| Leishmania species determination based on molecular diagnostics is key in the clinical management of cutaneous leishmaniasis, but is unavailable in many low- and middle-income endemic settings. |
| The required evidence for novel, less toxic, and less painful cutaneous leishmaniasis management is currently lacking. |
| To contain and ultimately eliminate cutaneous leishmaniasis, we need comprehensive research programs including vector control, diagnostics, and vaccines. |
Introduction
Leishmaniasis is a vector-borne infection caused by the protozoan parasite of the genus Leishmania. The vectors are female sandflies (Phlebotomus and Lutzomyia). The World Health Organization (WHO) has designated leishmaniasis a neglected tropical disease (NTD), thus emphasizing its considerable impact not only on health, but on societies at large with a high economic burden [1]. Leishmaniasis is endemic in almost 100 countries and the estimated total risk population is approximately 350 million people. Each year, an estimated 2 million new cases occur and the overall prevalence is 12 million cases. In most countries, the incidence numbers are probably underestimated since cases are not recognized and reporting is not mandatory [2, 3]. This is the case in many sub-Saharan African countries, for example, where burden estimations are lacking, and as a result essential control measures cannot be effected [4].
Depending on the Leishmania species, the disease can cause three main clinical manifestations: (1) localized cutaneous leishmaniasis (CL) characterized by cutaneous ulcers, sometimes accompanied by satellite lesions and/or nodular lymphangitis; (2) muco-cutaneous leishmaniasis (MCL) involving mucosa, and underlying connective tissues such as cartilage structures in combination with CL disease; and (3) visceral leishmaniasis (VL) affecting internal organs, like liver, spleen, and bone marrow. VL can be lethal, just like MCL, albeit the latter less frequently [5]. American tegumentary leishmaniasis (ATL) can be considered as a fourth syndrome caused by New World Leishmania species, containing CL and MCL manifestations for the most part, but also much rarer forms such as diffuse and disseminated CL [6].
With 600,000 to 1 million new cases annually worldwide, CL is the most prevalent clinical leishmanial manifestation. Moreover, only eight countries contribute to 90% of cases: Afghanistan, Algeria, Brazil, Iran, Pakistan, Peru, Saudi Arabia, and Syria [7]. Conflicts, such as in Syria recently, caused CL outbreaks due to healthcare disruption, and potential human to human transmission due to massive overcrowding [8]. Outbreaks occurred not only in the actual war zones, but also among refugees sheltered in safe countries such as Turkey, Jordan, and Lebanon [9].
Based on the European world view, Leishmania parasites are divided into two dominant groups: (1) Old World species found in the Mediterranean basin, the Middle East, the horn of Africa and the Indian subcontinent, such as L. (L.) major, L. infantum, and L. (L.) tropica; and (2) the New World species that consists of species found in Middle and South America, such as L. (L.) amazonensis, L. (L.) chagasi, L. mexicana L, L. (Viannia.) naiffi, L. (V.) braziliensis, and L. (V.) guyanensis. Old World species predominantly cause self-limiting ulcers, whereas New World species can be severely destructive and even cause death, mostly in relation to MCL disease.
Not all Leishmania species are susceptible to the currently available array of therapeutic options [10]. Therefore, species determination is key for the clinical outcome of patients with CL or MCL caused by an unknown species. This is often the case in ill returning travelers (e.g., backpackers) who visited multiple leishmaniasis endemic regions where a range of species with varying susceptibility patterns reside. Based on the successful molecular Leishmania species determination, novel parasite species-driven disease manifestations have been unveiled in different regions of the world [11]. For example, L. donovani complex, generally associated with VL, is now also associated with a CL disease presentation [12]. Overlap of VL and CL clinical presentations were described earlier for L. infantum [13].
The two recently updated Cochrane reviews on the treatment for Old [14], and New World CL [15] once again demonstrate that the sparse number of clinical trials available are often poorly designed and/or reported upon. As a result, we lack the evidence for potentially beneficial treatments. In part, this can be explained by the absence of pharmaceutical industry interest in the development of novel anti-leishmanial therapeutics, since it is widely believed that the return on investment will be risky, as the disease mainly affects people in low- and middle-income countries that lack financial resources. Conducting drug trials for CL can be challenging because CL is mainly endemic in remote areas. As a result, patient follow-up is often difficult, and many studies are severely affected by a large amount of loss to follow up data. To inform clinical practice, we urgently need large trials that can produce the necessary data on the long-term effects of current and promising experimental therapies. The establishment of an international platform has been suggested to improve the quality and standardization of future trials.
With the slogan “Small bite, big threat”, WHO launched its initiative in 2014 to fight the emerging threat of vector-borne diseases, including leishmaniasis [16]. WHO considers leishmaniasis an emerging, uncontrolled, and severely neglected disease. Containment of further incidence and morbidity, and more research programs for the improvement of vector control, novel diagnostics, and the extension of the current therapeutic arsenal are required. Here, we will focus on current developments in the diagnosis, treatment, prevention, and strategies for the management and control of CL caused by Old and New World species.
We performed a literature search for articles in PubMed published between 01 January 2015 (end date of our previous search [17]) and 22 February 2022 and filtered on the MESH terms ‘humans’ and ‘cutaneous leishmaniasis’ or ‘cutaneous leishmania’. The following publication languages were included: English, French, Spanish, and Portuguese. The search was narrowed down by using the following items: Prevention or control or therapy/narrow[filter] or diagnosis/broad[filter] or clinical trial[ptyp] or classical article[ptyp] or comparative study[ptyp] or clinical trial, phase i[ptyp] or clinical trial, phase ii[ptyp] or clinical trial, phase iii[ptyp] or clinical trial, phase iv[ptyp] or controlled clinical trial[ptyp] or evaluation studies[ptyp] or guideline[ptyp] or multicenter study[ptyp] or review[ptyp] or practice guideline[ptyp] or randomized controlled trial[ptyp] or systematic[sb] or validation studies[ptyp]).
Laboratory Diagnosis
The diagnosis of CL is based on clinical features (supported by epidemiological data) and laboratory testing. Numerous diagnostic methods have been described with a huge variation in diagnostic accuracy, including direct parasitological examination (microscopy, histopathology, and parasite culture), and/or indirect testing with serology and molecular diagnostics [18]. The selection of the diagnostic test employed often depends on the available infrastructure and resources of the diagnostic facility, and not so much on diagnostic accuracy. Here we selected generally employed diagnostic methodologies only, for discussion.
Direct Microscopy, Histopathology, and Culture
Direct parasitological diagnosis is still considered the gold standard in leishmaniasis diagnosis because of its high specificity, although there are concerns about its sensitivity [18]. Furthermore, this strategy requires expert health staff to collect the diagnostic specimen and to perform the parasitological tests. Furthermore, direct diagnosis can be time consuming (hours to weeks) depending on the methodology used and laboratory structure [19]. Direct parasitological diagnosis can be performed by histopathological analysis of formalin embedded tissue, or in vitro parasite culture from specimens from suspected lesions. With the help of light microscopy, amastigotes can be detected directly in lesional smears of biopsies, scrapings, or impression smears stained by Giemsa’s method. Amastigotes are identified as round or oval bodies 2–4 μm in diameter, with typical nuclei and kinetoplasts (Fig. 1). With the aid of immunohistochemical staining (e.g., CD1a antibodies), amastigotes can be detected more easily [18]. Specimens from the ulcer base usually result in the highest yield. To detect amastigotes and microgranuloma, fine needle aspiration (FNA) cytology is superior to scraping smears, with more patient comfort [20]. A simplified collection method is the Press–Imprint–Smear (PIS). When compared with histopathology, PIS was positive in 85.3% in study cases suspected of CL, and histopathology was only positive in 44%. PIS is considered a rapid and relatively sensitive method for the diagnosis of CL [21].
Fig. 1.
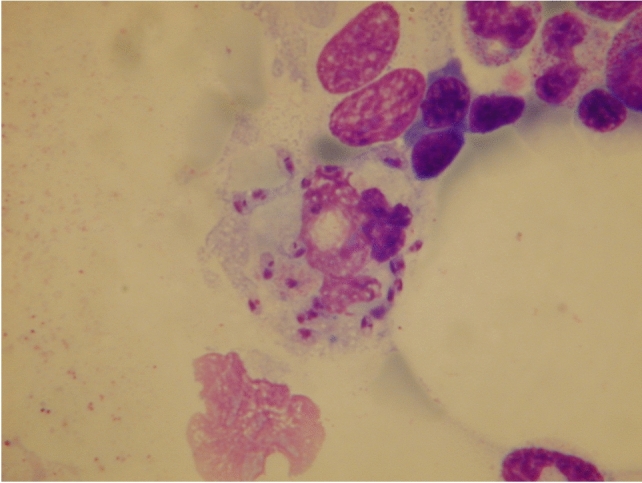
Leishmaniasis amastigotes. Amastigotes appear in skin biopsies as round or oval structures with characteristic nuclei and kinetoplasts, about 2–4 μm in diameter
Parasite culture in tubes containing Novy–MacNeal–Nicolle medium from suspected lesions is difficult, requires significant technical expertise, is prone to contamination, and is time consuming [22]. The sensitivity of culture tends to be low and highly variable [23]. Simplicity and (needle) pain-free sampling are additional advantages of culture. Mini-culture and micro-culture technologies are less costly since they require less culture medium; they also provide easier application and higher sensitivity, even in samples with a low parasite yield [22]. Yet, micro-culture does not allow additional species determination, which as described above is often required to prescribe proper treatment.
Indirect Serological Diagnostic Methods
Indirect fluorescent antibody (IFA), enzyme-linked immunosorbent assay (ELISA), western blot, and lateral flow assay are the mainstay formats used as serological CL tests. However, serology is not used widely to diagnose CL, since the humoral response provoked by the infection is poor and the sensitivity thus low [18, 24, 25]. In fact, recent guidelines do not recommend the use of serology for the diagnosis of CL.
Yet, there are still attempts made to improve the sero-diagnosis of CL. A relative successful approach seems to be the use of crude antigens derived from amastigotes of local Leishmania strains [26]. In contrast, a systematic review by Zanetti et al. revealed that more specific (recombinant) antigens yielded better diagnosis results, that is, sensitivity (93.8–100%) and specificity (82.5–100%) for ATL, compared with crude soluble antigen-based ELISAs [27]. More than 70 different antigens have been evaluated as ELISA diagnostic markers for (M)CL [28]. For example, an ELISA based on a recombinant conserved Leishmania hypothetical protein (which showed a high amino acid sequence homology between CL- as well as VL-causing Leishmania species) was found to be more specific and sensitive in detecting (M)CL patients than an ELISA based on L. braziliensis soluble antigens [29].
Given its complexity and infrastructural requirements, alternatives for ELISA and IFA are sought in the form of point of care (PoC) or rapid diagnostic tests (RDTs). Several RDTs have been developed for VL, mainly based on (variants of) a 39 amino acid sequence repeat of a kinesin-related protein of L. donovani, with relatively good diagnostic performance, except for East Africa [30]. In contrast, only few have been developed for CL. The CL Detect™ Rapid Test (a membrane-based qualitative immunoassay using polyclonal antibodies against amastigote peroxidoxin) is the most prominent example used for the detection of all clinically relevant Leishmania species that cause CL in skin samples. This RDT has been evaluated in Tunisia, Morocco [31], Afghanistan [32], Suriname [33], and Ethiopia [33], with variable diagnostic accuracy rates. The CL Detect™ Rapid Test was initially developed to detect L. major (which often comes at a relative high parasite density) peroxidoxin antigen. As a result, the discrepant lower sensitivities found in several studies (Afghanistan, Suriname, and Ethiopia) could either be caused by differences in non-L. major species involved, that may be less abundant, or have a lower expression of the peroxidoxin antigen, and/or produce a different variant of the antigen [34].
Leishmania Skin Test
The Leishmania intradermal skin test (LST) or Montenegro skin test (MST) is a marker of cellular immune response (a delay-type hypersensitivity response) and can be used for an indirect CL diagnosis because of its simple use and because of its high predictive value, being positive in more than 90% of CL cases with good sensitivity and specificity values [35, 36]. In addition, the LST can be useful in epidemiological studies to monitor exposure and immunity to Leishmania as well as in vaccine studies as a surrogate marker of immunity [37]. The test, comparable to the well-known Mantoux tuberculosis test, is based on the intradermal injection of Leishmania extracts (leishmanin) and results in a cutaneous reaction. If the skin reaction to LST is ≥ 5 mm, the test is considered positive, and if < 5 mm, negative. Otherwise diagnosed CL patients with a negative LST have a predictive value for relapse or treatment failure, probably because of an inadequate T-cell mediated response [35, 38].
The LST has been available for over a century, but despite its clinical value, its use has greatly declined in the last decade due to unavailability of standardised antigen. As yet, there is no production under good manufacturing practices conditions. The immune response against Leishmania is cell mediated, and depends on interferon (IFN)-γ induced activation of macrophages. Potentially, leishmanin can be used in a yet to be developed in vitro IFN-γ release assay (IGRA) with similar prognostic value as the LST test, but with the advantages of improved standardization and patient comfort [37].
Nucleic Acid Amplification Tests
Polymerase Chain Reaction (PCR)
Molecular approaches, and in particular PCR, have revolutionized CL diagnostics since they have superior test characteristics (both in sensitivity and specificity) in comparison with the previously mentioned traditional diagnostic methods. Moreover, molecular tests can be used in combination with less invasive sampling [39]. The main PCR sequence targets used for diagnostic assays in the recent past are the ribosomal DNA internal transcribed spacer 1 sequence [40–42], or sequences within the kinetoplast DNA (kDNA) of Leishmania genus [43, 44]. Head-to-head comparisons of different PCR tests are lacking, and there are no defined general accepted protocols, since almost every laboratory applies its own in-house method [39]. However, a recent systematic review and meta-analysis clearly confirmed that PCR is the most accurate diagnostic approach for CL, but the number of studies (n = 13) that could be included for this review was limited [45]. An important observation from this study was that simple smear samples are sufficient to get a reliable PCR result, whereas older non-molecular techniques would require more invasive sampling methods such as needle aspirates or skin biopsies. Furthermore, it is noteworthy that quantified PCR (qPCR) is suggested to monitor treatment responses in CL patients and may even be used to predict treatment failures [46].
Isothermal Platforms
A disadvantage of PCR diagnostics is the requirement for advanced laboratory infrastructure and technicians. This makes PCR-based platforms in general less suitable for disease endemic countries, that often have to rely on resource-restricted laboratories. In an attempt to simplify PCR requirements, isothermal diagnostic platforms have been recently developed and evaluated. Where PCR platforms require strictly controlled and varying temperature cycles, isothermal tests can be performed under a fixed temperature.
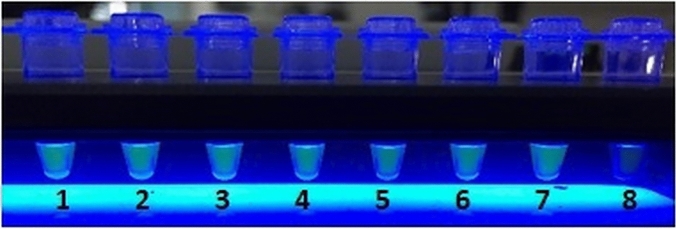
Loop-mediated isothermal reaction (LAMP) is an example of an isothermal molecular method that is performed at 60 °C and 65 °C, uses only one enzyme (Bst DNA polymerase) for amplification, and is able to produce large amounts of DNA within 30–60 min. LAMP specificity is high because it uses six primers and the end product can be visualized directly using simple detection methods, such as naked eye reading (turbidity) and lateral flow strips (Fig. 2) [47, 48]. LAMP is therefore widely adopted in CL diagnostics.
Fig. 2.
Loop-mediated isothermal reaction (LAMP) molecular diagnostics. Many molecular diagnostic techniques require adequate infrastructure and technically skilled operators, making these tests less suitable for resource-restricted laboratories in disease endemic countries. LAMP is an isothermal diagnostic platform that partly circumvents these requirements. The picture shows visualization of LAMP results under UV light. Positive samples (1–7) show turbidity, whereas the negative sample (8) has remained transparent
The initial reverse transcriptase (RT-)LAMP test for CL targeted the conserved region of the 18S ribosomal RNA (rRNA) gene. The pre-amplification addition of a fluorescent detection reagent and a simple UV lamp visualized the amplification when the target was present in the samples of patients with lesions containing between 10 and 100 parasites per mL with 98% sensitivity [47]. This platform was further developed into a LAMP test detecting in principal all CL- and VL-causing Leishmania species based on primers specific for the 18S ribosomal DNA and the conserved region of mini circle kinetoplast DNA (kDNA). RT-LAMP was marketed through a collaboration between Eiken Company (Japan) and the Foundation for Innovative New Diagnostics (Switzerland) [49]. Many research groups have now taken this platform or modified versions using different targets, like cysteine proteinase b gene, to further it for various applications [50–52]. A systematic review and meta-analysis on LAMP assays reported a sensitivity ranging from 83 to 99% and specificity from 31 to 94% for LAMP, compared with microscopy, and a sensitivity ranging from 80 to 99% and specificity from 91 to 98% compared with various PCR platforms [48]. The application of LAMP on swab samples opens up the possibility to develop a simple and rapid PoC molecular diagnostic method for CL in resource-limited settings [53, 54].
Recombinase polymerase amplification (RPA) is a second isothermal technology that has the potential for application in resource-limited settings, because of its low infrastructural demands. The RPA process employs three essential enzymes: a recombinase, a single-stranded DNA-binding protein, and strand-displacing polymerase. The RPA reaction can be performed at 37–42 °C in, for example, a water bath or heat block. An interesting feature of the RPA reaction is that it can detect RNA as well as DNA, without the need for a separate step to produce cDNA [55]. RPA uses standard PCR detection methods, such as real-time fluorescent detection, gel electrophoresis, and lateral flow strip detection. RPA assays have now also been developed for CL, but a systematic review of its diagnostic performance is not yet available. Most RPA for CL exploit kDNA as a target, often in combination with a lateral flow (LF) stick read-out [56–58]. It is also possible to design your own primers and probes by using software from the RPA manufacturer and using the TriTryp database [59, 60]. So far, observed diagnostic sensitivity and specificity of employed RPA for CL are slightly lower compared with (q)PCR or LAMP [58, 60]. However, a recent evaluation of an RPA lateral flow test for CL in a clinic in the Peruvian Amazon basin found a good 91.2% sensitivity (confidence interval [CI] 86.5–94.4), and a 93% (CI 88.6–95.8) positive predictive value compared with kDNA-PCR. It highly surpassed microscopy as a diagnostic method, strongly suggesting the possibility to enhance the performance of RPA [57].
Sampling for Molecular Biology
Rapid and affordable CL tests will have to rely on non-invasive sampling. To further the evaluation of the clinical accuracy of the most optimal molecular platform, unification of the sample source, the method of DNA recovery, and the type of molecular test is required [61]. Adams and co-workers found that a lesional swab processed with Qiagen® DNA extraction was the most efficient, sensitive, and specific recovery method for Leishmania DNA, when compared with aspirate samples, which were also more painful and more complicated to obtain [61]. This approach has now been further validated in several other studies that support the use of cotton swabs for the non-invasive collection of diagnostic material for subsequent PCR analysis [62–65]. Although rubbing over the lesion with cotton swabs potentially yields a low parasitic load, several studies have indicated that the choice of the subsequent nucleic acid extraction and amplification method influences the results obtained with cotton swab collection [61, 63]. Moreover, the minimally invasive cotton swab collection method could be useful for children or in cases where the cosmetic outcome is of importance, such as in patients with facial lesions [65].
To collect specimens for PCR tests, FTA cards (Whatman filter paper cards) have also been successfully used. Apart from being minimally invasive for patients, FTA cards are easy to handle for medical personnel and easily transported under dry conditions, whereas for cotton swabs, a storage buffer might be needed for transportation [66, 67]. Several studies have recently confirmed good results with the FTA card sampling method [68, 69].
Another attractive non-invasive DNA sampling method is the use of tape strips to obtain tissue from the surface of ulcerative or closed skin lesions of CL patients. DNA recovered from infected skin was about 10 times higher than that obtained from healthy skin, and molecular analysis by PCR after conventional DNA isolation with a kit resulted in 100% sensitivity [70].
Species Determination
Leishmania species cannot be distinguished from each other by light microscopy. Thus, other strain identification methods have been developed [10, 71]. The first attempts for strain identification were performed with isoenzyme analysis (or multi locus enzyme electrophoreses, MLEE) and led to the construction of phylogenetic classifications [72]. MLEE was even capable of the differentiation of anthroponotic variants from zoonotic variants within a single species [73]. The MLEE technique uses the variation in the electrophoretic mobility of Leishmanial enzymes, and strains are classified along so-called zymodemes. Currently, MLEE is only performed in a few reference laboratories, because it requires large amounts of cultured promastigotes, making it a costly and time consuming procedure [74].
Another culture-dependent method is the use of monoclonal antibodies to identify Leishmania species. The method depends on parasite isolation and subsequent culturing of promastigotes. The antibodies are mostly used for specification of New World Leishmania species and to a much lesser extend the Old World species [75].
Given the technical difficulties with large-scale culturing of parasites, alternative typing methods are being developed, and in particular based on genetic characteristics of the parasite. Molecular methods offer robust species typing. Van der Auwera and DuJardin have provided a state of the art overview of the currently deployed and available Leishmania species typing assays, with a focus on methods that are applicable globally rather than validated locally [76]. Furthermore, this review outlines the consensus taxonomy for the genus Leishmania, including debatable species attributions.
Nowadays, species discrimination methods use PCR (to amplify the DNA required for typing) in combination with restriction fragment length polymorphism (RFLP) analysis or sequencing. Currently, the mini-exon gene, crucial in the trans-splicing process of nuclear mRNA, is an excellent genotyping marker that is present as 100–200 tandem repeated copies per nuclear genome. Each mini-exon repeat consists of three major parts [77]. As a result of the variability in sizes of the amplification products, preliminary discrimination between the major complexes (i.e., Old World Leishmania, New World Leishmania, and New World Viannia complexes) is possible [77, 78]. WHO reference strains of Leishmania and cultured isolates from patients were used to validate the mini-exon PCR-RFLP genotyping scheme [77, 78]. The mini-exon method is now widely accepted as a high resolution, sensitive, and specific tool that can help in the clinical management of CL [10, 79–81].
Alternative gene targets for typing have been identified and are extensively reviewed by Van der Auwera and Dujardin, such as cytochrome B (encoded on the kDNA maxi circles), glycoprotein 63, cysteine proteinase B, Heat Shock Protein 70 and glucose-6-phosphate dehydrogenase [76]. The choice of target and method will strongly depend on the species and question (epidemiology, treatment etc.) addressed.
Species-Based Clinical Management
Most CL patients are identified as probable cases, solely based on patient history and physical examination, since they are found in endemic regions lacking the resources of laboratory confirmation tests. Apart from a typical clinical presentation, a probable case can be identified based on a number of findings such as younger age, typical professions such as mining, farming, military and hunting, not using bed nets, residing in deforested, rural, sub-urbane sites (Fig. 3), living close to pets and cattle, a history of travelling such as tourism or labor migration (where immunologically naïve cases enter a CL endemic area), and the season of exposure (rainy seasons and El Niño are associated with CL) [82–86]. Typical CL cutaneous lesions are ulcers covered with hemorrhagic crusts on unexposed skin of the extremities and face, accompanied by satellite lesions and/or lymphangitis (Fig. 4). In MCL, the ear nose and throat tract, especially the mucosal tissue and underlying structures such as cartilage bones are affected.
Fig. 3.

Deforestation in Godo-olo, district Sipaliwini (Suriname). Human interference can cause leishmaniasis outbreaks due to the disruption of the natural reservoir-vector transmission cycle of Leishmania parasites and the introduction of an immune-naïve labor force in the deforested area.
Collection Ramdas, S., 2009
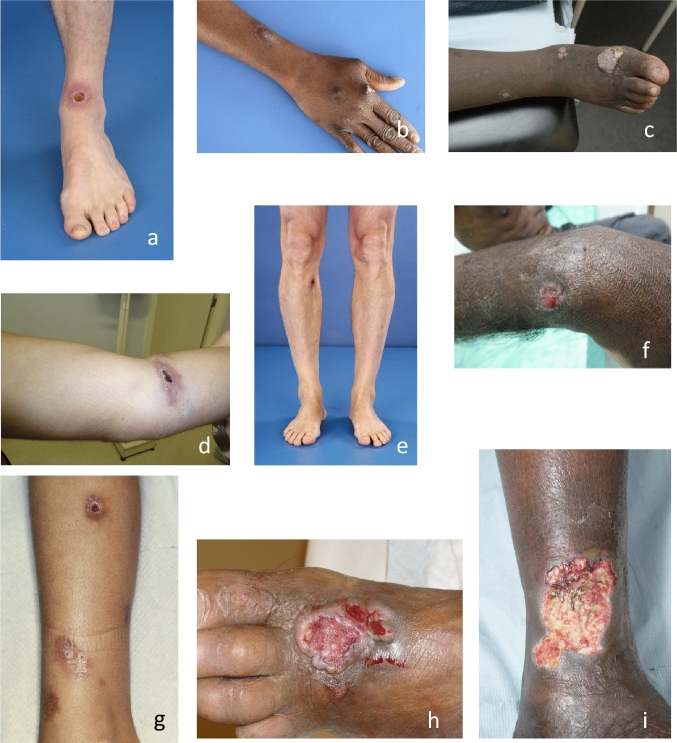
Fig. 4.
Various manifestations of cutaneous leishmaniasis. a A cutaneous leishmaniasis ulcer with a satellite lesion on the lower arm. b Same patient as in a, healed after treatment with cryotherapy and intralesional antimonials. c Non-ulcerative cutaneous leishmaniasis lesion on the elbow. Multibacillary leprosy was considered but disproved in this patient. d Crusty cutaneous leishmaniasis ulcer with lymphangitis on the upper leg. e Typical cutaneous leishmaniasis lesion as often seen in children with Leishmania infantum contracted in North Africa. f Chiclero ulcer, typically affecting the ear with cartilage destruction. g Disseminated cutaneous lesions in a patient with mucocutaneous leishmaniasis caused by L. guyanensis. h Intralesional treatment of cutaneous leishmaniasis with antimonials.
Photo credits: Department of Dermatology, Amsterdam UMC, Amsterdam, The Netherlands
Direct diagnostic tests (such as light microscopy) can help to establish a more definite diagnosis and aid clinical management. As discussed above, microscopy does not allow for species determination, and as such cannot guide the choice of therapy [87]. In many endemic regions, one species dominates the CL epidemic, and first-line treatment can be based on (trial and error-based) local experience. The situation can be more complicated when multiple Leishmania species prevail in the same region, each requiring different therapeutic options. This is the case throughout Middle and South America where the geographical spread of species causing both CL and MCL overlap in large areas [88–90]. CL and MCL require a different clinical approach (see next paragraph), thus the identification of the responsible species is key for the choice of treatment and follow-up [91, 92].
Cheap, reliable, easy to perform, and rapid species-specific diagnostic tests are urgently needed to guide clinical management and prevent unnecessary treatment failure and late complications. PoC near patient rapid diagnostics are especially helpful in low resource endemic settings since they allow for timely treatment (preferably during the first clinic visit), and a reduction of return visits to prevent loss to follow-up. In non-endemic settings, clinical management based on CL species identification is of importance when dealing with returning travelers with CL, especially when multiple endemic regions with a variety of causative species have been visited [10, 84]. Lastly, in clinical trials where well defined cases are crucial for the quality of generated data, species determination is also necessary.
Clinical Dilemmas: Co-Infections and Unusual Presentations
Asymptomatic infection is the most common outcome after Leishmania inoculation [93]. This is based on epidemiological studies where individuals with a positive Montenegro skin test proved resistant and capable of controlling the infection without evidence of tissue damage. The mechanisms responsible for controlling the parasite are not fully understood, but might aid the development of effective vaccines.
Diffuse CL and disseminated CL are rare presentations that are often missed initially since they mimic other generalized skin infections such as leprosy and fungal infections [94]. Diffuse CL is caused by anergy to Leishmania and a lack of T-cell mediated immunity. As a result, the response to the LST is negative or poor, and the number of parasites found in lesions/smears is massive. Diffuse CL is characterized by nodules, papules, plaques, and erythema and resembles lepromatous leprosy (in fact, lepromatous leprosy is also characterized by the absence of T-mediated immunity against Mycobacterium leprae). Mucocutaneous involvement is rarely seen. The response to therapy is poor in diffuse CL, and relapses occur frequently.
Disseminated CL is characterized by a strong T-cell hypersensitivity immune response, a strong positive LST reaction, and scanty parasites in lesions. In disseminated CL, necrotic ulcers can be found (yet not in diffuse CL), and the response to therapy is in general good (Fig. 4). Mucosal involvement is frequent seen. The dichotomy in the immunological responses of diffuse CL on the one hand and disseminated CL and MCL on the other hand has led to the proposition that leishmaniasis is a spectrum disease comparable with leprosy [95].
Many CL endemic regions may have geographical overlap with other highly prevalent infections such as helminthiasis, leprosy, trypanosomiasis, and fungal infections. Diagnostic problems may occur when concurrent infections cause similar lesions (e.g., CL and leprosy, Fig. 5), when different pathogens are present in the same lesions (e.g., Leishmania and Sporothrix schenckii), or when similarities between phylogenetically close pathogens affect accuracy of diagnostic tests (e.g., serology for leishmaniasis and Chagas’ disease) [96]. This may lead to diagnostic errors and delays, and it can influence the effectiveness and safety of treatment. HIV co-infection in CL patients can increase the risk of CL recurrence and treatment failure [97]. Vice versa, CL can interfere with innate immunity, thus facilitating HIV progression [98].
Fig. 5.
Skin diseases that can mimic the clinical picture of cutaneous leishmaniasis. a Buruli ulcer caused by Mycobacterium ulcerans on the lower leg. b Atypical mycobacterial infection on the hand with lymphangitis caused by M. marinum infection. c Hyperkeratotic lesions caused by deep mycosis of the lower leg with lymphangitis in an immunosuppressed patient. d Ulcerative lesion caused by sporotrichosis with a satellite lesion on the arm. e Rickettsiosis on the lower leg with an eschar. f Ulcerative lesion in a patient with multibacillary leprosy. g Ulcerative lesions in a patient with necrobiosis lipoidica in a sporotrichoid dissimination pattern. h Squamous cell carcinoma on the dorsal foot. i Pyoderma gangrenosum ulcer with ragged margins and yellow necrosis on the lower leg.
Photo credits: Department of Dermatology, Amsterdam UMC, Amsterdam, The Netherlands
Besides HIV infection, immunosuppression in general can lead to atypical clinical CL presentations [99]. Therefore, one should consider CL, especially in endemic regions, when granulomatous and tumorous skin diseases such as subcutaneous and deep mycosis, cutaneous lymphoma, pseudolymphoma, and basal and squamous cell carcinoma are deliberated. In a non-endemic setting, medical and travel history can help clinicians to consider CL in the differential diagnosis of complex and unusual appearing dermatoses.
Treatment
In most cases, CL is self-limiting. Nonetheless, irreversible disfiguring scar formation often occurs, and nodular lymphangitis and MCL can complicate the outcome with lasting disability and permanent unsightly destruction. The recovery period can be hindered by secondary bacterial infections and can take months to years, during which time patients have to deal with function impairment. In the absence of sound evidence, most therapy options are based on expert opinion [10]. Moreover, when species identification is not available, therapy options have to rely on local expertise. CL has been largely neglected for drug development because it affects poor people in poor regions of the world [100]. Most of the current drugs used to treat CL are decades old and have many limitations such significant toxicity and side effects [101]. Costs are another challenge in low- and middle-income endemic settings, especially with the systemic treatment options. A clinician with in-depth knowledge of the outcome of CL and the therapeutic options should make a tailored management plan in close discussion with the patient (shared decision making). A risk–benefit assessment of therapeutic options should be made, and in mild and indolent cases a wait-and-see policy can sometimes be the best option. Moreover, drug resistance is an emerging problem in the control of CL [87].
Several treatment options for CL are available. Pentavalent antimonials (sodium stibogluconate—Pentostam® or meglumine antimoniate—Glucantime®) remain the first treatment option for CL in most countries. Alternative treatment regimens include miltefosine, pentamidine isethionate, amphotericin B, antifungal agents (e.g., ketoconazole, fluconazole [102], itraconazole, paromomycin [103], granulocyte macrophage colony-stimulating factor [104], and heat therapy or cryotherapy [105–107]. Although attempts are being made to discover novel drugs against CL (e.g., via smart ex vivo compounds screening technology), promising therapeutic breakthroughs for CL are decades away from the clinic [108].
Treatment of Old World Cutaneous Leishmaniasis (CL)
Based on expert opinion, local therapy is preferred in patients with fewer than five lesions [10]. Compared with intralesional antimony or cryotherapy monotherapy, a combination of the two options proved more effective, even though differences were quite small [109–111]. Heat therapy is also effective [112–115], yet recently failed in over 90% of patients with CL caused by L. tropica [116]. The ratio behind both heat and cryotherapy is to kill parasites locally, and to induce an inflammatory response to indirectly eliminate parasites. Whereas liquid nitrogen is used for cryotherapy, heat therapy can be applied with different methods including infrared, ultrasound, exothermic crystallization thermotherapy, radiofrequency, and microwave. A major drawback of most local therapeutic options is the pain induced by the required intradermal injections. This makes local options especially challenging in children with CL.
Systemic treatment should be considered in patients with multiple lesions, potentially disfiguring facial involvement, or lesions at locations less fit for topical treatment. Miltefosine is an orally administered systemic treatment option that works well in patients with complicated Old World CL (L. major) lesions [117, 118]. Especially in children with Old World CL (caused by L tropica or L major), miltefosine seems a safe and effective alternative for painful local treatment options [119].
In a 2017 Cochrane review on therapeutic interventions for Old World CL, 40 new studies were identified since the previous update from 2008 [120]. Most studies were at unclear or high risk for most bias domains, and almost 40% lacked blinding. Only two additional comparisons could be added in the update: itraconazole cured more CL versus placebo; and there was no difference in the cured number of participants between paromomycin ointment versus vehicle arms. Both interventions caused mild side effects.
A number of experimental local treatments such as infiltration with hypertonic salt solutions [121], trichloroacetic acid [122], intralesional metronidazole [123], diphencyprone [124], carbon dioxide laser [125], intense pulsed light [126], photodynamic therapy [127], and systemic treatments such as cimetidine [128] in combination with antimoniate and chloroquine [129, 130] have been tried in small single-centered studies with varying results that need further evidence to conclude on their therapeutic value.
Treatment of American CL
In a 2019 Cochrane review on therapeutic interventions for American CL and mucocutaneous leishmaniasis (ACML), 37 new studies were identified since the previous update from 2009 [15]. In total, 75 studies were analyzed and the most assessed drugs were oral miltefosine and antimonials (particularly meglumine antimoniate). Most studies comprised participants with ulcerative CL lesions on the extremities, yet none included cases with diffuse cutaneous or disseminated CL. Most studies were hampered by unclear or a high risk of bias in at least one domain. Following are the most remarkable conclusions from the Cochrane review:
Intramuscular meglumine antimoniate given for 20 days to treat L. braziliensis and L. panamensis infections in ACML may increase the likelihood of complete cure.
Compared with placebo, at 6-month follow-up, oral miltefosine given for 28 days to treat L. mexicana, L. panamensis, and L. braziliensis infections in American cutaneous leishmaniasis (ACL) probably improves the likelihood of complete cure, with gastrointestinal side effects reported.
Comparing intramuscular meglumine antimoniate and miltefosine to treat L. braziliensis, L. panamensis, L. guyanensis, and L. amazonensis infections in ACML shows little to no difference to the likelihood of complete cure.
Based on expert opinion, local treatment can be considered in patients with a small number of lesions caused by American strains that are not associated with MCL like L. naiffi, L. chagasi, and L. mexicana [131, 132]. In patients with CL caused by L. mexicana, miltefosine treatment often fails due to antimicrobial resistance shown in both in vitro and in vivo studies; here, antimony (either locally or systemically administered) should be preferred [133, 134]. Provided CL patients affected by L. panamensis or L. amazonensis can be followed up closely, local treatment (e.g., combination local therapy of antimony and cryotherapy) could be considered, since these strains rarely cause MCL [10, 135, 136]. Although MCL due to L. guyanensis is not as rare as formerly thought, in patients with a few uncomplicated CL lesions, local therapy can also be considered [137]. Nonetheless, in Suriname and Brazil, systemic pentamidine is the preferred treatment option for L. guyanensis infections [138, 139], even though in Manaus, Brazil, efficacy proved lower with 50–60% success rates [140]. As a rule of thumb, strict follow-up is advisable in patients with American CL, to exclude treatment failure or progression towards MCL manifestations [141, 142]. The role of intralesional pentamidine has been studied in a small number of patients and requires additional evidence to prove its efficacy [143].
Because of the considerable risk of MCL seen with L. braziliensis infections, systemic antimony is the gold standard, and in general local therapy is not recommended. Yet again, when strict follow-up can be guaranteed, local treatment options for L. braziliensis infections can be considered in limited and uncomplicated cases [106, 135]. In some South American regions, miltefosine shows comparable treatment outcomes to antimony, whereas in other regions, miltefosine is inferior [144–147]. Treatment outcome variations are likely related to differences in susceptibility among local L. braziliensis strains. Although systemic amphotericin B is at least non-inferior to antimony to treat L. braziliensis MCL, costs and the severity of side effects make it the second-line treatment option [148–151]. Local amphotericin options currently do not seem to increase cure rates [152].
Because of the lack of trials evaluating the treatment of MCL caused by L. panamensis, L. amazonensis, and L. guyanensis, it is as advised to follow the L. braziliensis MCL treatment recommendations [10]. The combination of antimony and pentoxifylline is likely not more effective than antimony monotherapy in the treatment of CL caused by L. braziliensis [153]. Similarly, adding tamoxifen to antimony does not seem to improve cure rates [154].
Prevention
The old paradigm, prevention is better than cure, also applies to CL, not only for the patient but also for the community at large. Primary prevention consists of interventions against infected fly bites and should be offered to affected key populations such as farmers, hunters, military and mining laborers in CL endemic areas [82, 155, 156]. Here, an important intervention is sleeping under bed nets impregnated with insecticide and a maze that should be three times smaller than the bed nets used in the fight against malaria. To further reduce the chance of sand fly bites, bed nets can be impregnated with permethrin or other effective insect repellents [155]. This approach can be supplemented with indoor residual spraying [156], which has shown to be a (cost) effective measure for the prevention of CL [157]. However, emerging resistance of sand flies against insecticides is a concern in keeping these measures effective [158–160].
Intervention programs targeting reservoir-to-human transmission lack sound evidence on their effectiveness [161]. Although it is evident that the potential reservoir for zoonotic transmission is large and its full size not yet completely known, the exact contribution of mammalian species to disease transmission remains to be better established [162]. Canines are considered to be one of the main reservoirs and vaccination as well as culling strategies or the use of insecticide-impregnated dog collars have been explored to reduce transmission, however with very variable success [155, 163].
A good understanding of community perceptions of the disease and healthcare-seeking behavior are essential to the further design of (cost-effective) preventive measures [85, 164, 165]. A recent review by Alidosti et al. revealed that providing educational programs to strengthen positive beliefs and understanding of the diseases and to correct negative beliefs and misunderstandings about CL will be useful to improve relevant behavioral changes towards prevention and control of CL [166]. Providing information on CL to key populations can improve the correct uptake of preventive measures, ameliorate risk behavior, and improve help-seeking behavior.
Early recognition of CL cases is of great importance, both for the individual patients, but also for public health purposes. In remote, sparsely populated, and underserved areas, mobile app-based care, and tele-dermatology applications can help in clinical management of suspected CL cases and surveillance [167].
Within the WHO NTD program, leishmaniasis is further integrated in the context of skin-related neglected tropical diseases (skin NTDs) [168]. Skin NTDs have in common that they are associated with stigmatization, discrimination, and socioeconomic problems. Moreover, skin NTDs often affect the same population. This offers opportunities such as integrated approaches to cover a number of skin NTDs. Integration is defined as a mode to optimize the use of limited resources, disability management, and community engagement, in addition to simplification of treatment pathways and the minimisation of stigma, through the implementation of activities to prevent two or more diseases simultaneously in the same community [167, 169]. Examples are the integration of screening programs, training of health workers and community volunteers, and the use of common laboratory and case management infrastructure.
Future Preventive Measures, Vaccination
The development (and delivery) of a vaccine against CL would ultimately be one of the most effective measures to control or even eliminate the disease [170]. It has been estimated that an effective vaccine against CL (70% efficacy, 10 years’ protection) could prevent 41,000–141,000 cases of CL in Latin America for a smaller financial amount than needed to cover treatment of these cases [170, 171]. The road towards development of an effective vaccine against CL is difficult and complicated due to the complex interaction between the Leishmania parasites and the host immune system. However, naturally recovered CL infection mostly induces life-long immunity against the species that caused the primary infection, and this suggests that vaccination in theory should be possible [170, 172, 173]. This is further supported by the fact that ‘leishmanization’ (i.e., immunization of individuals with living parasites on an inconspicuous part of the body to protect against disfiguration lesions caused by a natural infection) indeed confers protection against CL [174]. The practice of leishmanization has nowadays been greatly abandoned because of safety issues and problems with standardization of the ‘vaccine’ [170, 174]. Still, the option of using a live attenuated vaccine, because of its ability to induce a protective Th1 response, is an attractive approach that is currently being explored [174]. Next to live attenuated vaccines, many experimental vaccines based on the use of (components of) dead parasites, recombinant proteins or DNA have been explored, but so far none have led to a licensed CL vaccine for use in humans [175]. The recent advances in vaccine development (fueled especially by the Covid-19 pandemic) will hopefully also be applied to NTDs in the near future, including leishmaniasis. Finally, deforestation, migration, and climate change, including global warming, may all contribute to spread of the vector and CL outside the currently known endemic areas [176].
Conclusions
Globally, CL affects millions. Most CL cases occur in low- to middle-income countries, which are often burdened by other ailments like malaria, tuberculosis, and HIV. At the same time, their governments have limited healthcare budgets and often have to rely on poor healthcare infrastructure. Because of lacking disease management and public health control interventions, CL is emerging and threatens to become an uncontrollable disease. Since it primarily affects poor people, pharmaceutical companies do not develop CL pipelines, because they fear their investments might not be returned. As a consequence, industry dedicates little investments and research into innovative diagnostics, treatments, and vaccines for CL. With the current molecular techniques, Leishmania species can be identified more easily, and species identification enables rational therapy management. Current CL treatment guidelines lack the required sound evidence and are mostly based on improperly designed and ill-conducted trials. Thus, there is an urgent need for large standardized and state-of-the-art trials that can evaluate potentially beneficial treatments, including less toxic drugs, and for children, painless modalities without injections. To further contain CL incidence and morbidity, we need intensified preventive research programs into improved vector control, vaccines, and diagnostics.
Declarations
Funding
No additional sources of funding were used for this manuscript.
Conflict of interest
HJCdV and HDS have no conflicts of interest to declare.
Author contributions
HJCdV wrote the first version and approved the final version of the manuscript, HDS added significant contributions.
Ethics approval
Not applicable.
Data availability
Not applicable.
Informed consent
Not applicable.
References
- 1.Control of Neglected Tropical Diseases. World Health Organization. Geneva: WHO; 2020. http://www.who.int/neglected_diseases/diseases/en/#. Accessed 14 Dec 2020.
- 2.McGwire BS, Satoskar AR. Leishmaniasis: clinical syndromes and treatment. QJM Mon J Assoc Physicians. 2014;107(1):7–14. doi: 10.1093/qjmed/hct116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leihmaniasis Geneva: WHO. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis. Accessed Sep 7 2022.
- 4.Sunyoto T, Verdonck K, El Safi S, Potet J, Picado A, Boelaert M. Uncharted territory of the epidemiological burden of cutaneous leishmaniasis in sub-Saharan Africa—a systematic review. PLoS Negl Trop Dis. 2018;12(10):e0006914. doi: 10.1371/journal.pntd.0006914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey MS, Lockwood DN. Cutaneous leishmaniasis. Clin Dermatol. 2007;25(2):203–211. doi: 10.1016/j.clindermatol.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Martins AL, Barreto JA, Lauris JR, Martins AC. American tegumentary leishmaniasis: correlations among immunological, histopathological and clinical parameters. An Bras Dermatol. 2014;89(1):52–58. doi: 10.1590/abd1806-4841.20142226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burza S, Croft SL, Boelaert M. Leishmaniasis. Lancet. 2018;392(10151):951–970. doi: 10.1016/S0140-6736(18)31204-2. [DOI] [PubMed] [Google Scholar]
- 8.Ergönül Ö, Tülek N, Kayı I, Irmak H, Erdem O, Dara M. Profiling infectious diseases in Turkey after the influx of 3.5 million Syrian refugees. Clin Microbiol Infect. 2020;26(3):307–312. doi: 10.1016/j.cmi.2019.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ozaras R, Leblebicioglu H, Sunbul M, Tabak F, Balkan II, Yemisen M, et al. The Syrian conflict and infectious diseases. Expert Rev Anti Infect Ther. 2016;14(6):547–555. doi: 10.1080/14787210.2016.1177457. [DOI] [PubMed] [Google Scholar]
- 10.Hodiamont CJ, Kager PA, Bart A, de Vries HJ, van Thiel PP, Leenstra T, et al. Species-directed therapy for leishmaniasis in returning travellers: a comprehensive guide. PLoS Negl Trop Dis. 2014;8(5):e2832. doi: 10.1371/journal.pntd.0002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thakur L, Singh KK, Shanker V, Negi A, Jain A, Matlashewski G, et al. Atypical leishmaniasis: a global perspective with emphasis on the Indian subcontinent. PLoS Negl Trop Dis. 2018;12(9):e0006659. doi: 10.1371/journal.pntd.0006659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elamin EM, Guizani I, Guerbouj S, Gramiccia M, El Hassan AM, Di Muccio T, et al. Identification of Leishmania donovani as a cause of cutaneous leishmaniasis in Sudan. Trans R Soc Trop Med Hyg. 2008;102(1):54–57. doi: 10.1016/j.trstmh.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Aoun K, Bouratbine A. Cutaneous leishmaniasis in North Africa: a review. Parasite. 2014;21:14. doi: 10.1051/parasite/2014014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heras-Mosteiro J, Monge-Maillo B, Pinart M, Lopez Pereira P, Reveiz L, Garcia-Carrasco E, et al. Interventions for Old World cutaneous leishmaniasis. Cochrane Database Syst Rev. 2017;11(11):Cd005067. doi: 10.1002/14651858.CD005067.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinart M, Rueda JR, Romero GA, Pinzón-Flórez CE, Osorio-Arango K, Silveira Maia-Elkhoury AN, et al. Interventions for American cutaneous and mucocutaneous leishmaniasis. Cochrane Database Syst Rev. 2020;8(8):004834. doi: 10.1002/14651858.CD004834.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savioli L, Velayudhan R. Small bite, big threat: World Health Day 2014. East Mediterr Health J (La revue de sante de la Mediterranee orientale al-Majallah al-sihhiyah li-sharq al-mutawassit) 2014;20(4):217–218. [PubMed] [Google Scholar]
- 17.de Vries HJ, Reedijk SH, Schallig HD. Cutaneous leishmaniasis: recent developments in diagnosis and management. Am J Clin Dermatol. 2015;16(2):99–109. doi: 10.1007/s40257-015-0114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goto H, Lindoso JA. Current diagnosis and treatment of cutaneous and mucocutaneous leishmaniasis. Expert Rev Anti Infect Ther. 2010;8(4):419–433. doi: 10.1586/eri.10.19. [DOI] [PubMed] [Google Scholar]
- 19.De Brito RCF, Aguiar-Soares RDO, Cardoso JMO, Coura-Vital W, Roatt BM, Reis AB. Recent advances and new strategies in Leishmaniasis diagnosis. Appl Microbiol Biotechnol. 2020;104(19):8105–8116. doi: 10.1007/s00253-020-10846-y. [DOI] [PubMed] [Google Scholar]
- 20.Hosseinzadeh M, Omidifar N, Lohrasb MH. Use of fine needle aspiration cytology in the diagnosis of cutaneous leishmaniasis: a comparison with the conventional scraping method. Trop Doct. 2012;42(2):112–113. doi: 10.1258/td.2011.110420. [DOI] [PubMed] [Google Scholar]
- 21.Sousa AQ, Pompeu MM, Frutuoso MS, Lima JW, Tinel JM, Pearson RD. Press imprint smear: a rapid, simple, and cheap method for the diagnosis of cutaneous Leishmaniasis caused by Leishmania (Viannia) braziliensis. Am J Trop Med Hyg. 2014;91(5):905–907. doi: 10.4269/ajtmh.14-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boggild AK, Miranda-Verastegui C, Espinosa D, Arevalo J, Martinez-Medina D, Llanos-Cuentas A, et al. Optimization of microculture and evaluation of miniculture for the isolation of Leishmania parasites from cutaneous lesions in Peru. Am J Trop Med Hyg. 2008;79(6):847–852. doi: 10.4269/ajtmh.2008.79.847. [DOI] [PubMed] [Google Scholar]
- 23.Faber WR, Oskam L, van Gool T, Kroon NC, Knegt-Junk KJ, Hofwegen H, et al. Value of diagnostic techniques for cutaneous leishmaniasis. J Am Acad Dermatol. 2003;49(1):70–74. doi: 10.1067/mjd.2003.492. [DOI] [PubMed] [Google Scholar]
- 24.Kar K. Serodiagnosis of leishmaniasis. Crit Rev Microbiol. 1995;21(2):123–152. doi: 10.3109/10408419509113537. [DOI] [PubMed] [Google Scholar]
- 25.Aronson N, Herwaldt BL, Libman M, Pearson R, Lopez-Velez R, Weina P, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH) Am J Trop Med Hyg. 2017;96(1):24–45. doi: 10.4269/ajtmh.16-84256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bracamonte ME, Álvarez AM, Sosa AM, Hoyos CL, Lauthier JJ, Cajal SP, et al. High performance of an enzyme linked immunosorbent assay for American tegumentary leishmaniasis diagnosis with Leishmania (Viannia) braziliensis amastigotes membrane crude antigens. PLoS One. 2020;15(5):e0232829. doi: 10.1371/journal.pone.0232829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zanetti ADS, Sato CM, Longhi FG, Ferreira SMB, Espinosa OA. Diagnostic accuracy of Enzyme-Linked Immunosorbent Assays to detect anti-Leishmania antibodies in patients with American Tegumentary Leishmaniasis: a systematic review. Rev Inst Med Trop Sao Paulo. 2019;61:e42. doi: 10.1590/s1678-9946201961042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freire ML, Rêgo FD, Cota G, Pascoal-Xavier MA, Oliveira E. Potential antigenic targets used in immunological tests for diagnosis of tegumentary leishmaniasis: a systematic review. PLoS One. 2021;16(5):e0251956. doi: 10.1371/journal.pone.0251956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carvalho A, Costa LE, Salles BCS, Santos TTO, Ramos FF, Lima MP, et al. An ELISA immunoassay employing a conserved Leishmania hypothetical protein for the serodiagnosis of visceral and tegumentary leishmaniasis in dogs and humans. Cell Immunol. 2017;318:42–48. doi: 10.1016/j.cellimm.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Hagos DG, Schallig H, Kiros YK, Abdulkadir M, Wolday D. Performance of rapid rk39 tests for the diagnosis of visceral leishmaniasis in Ethiopia: a systematic review and meta-analysis. BMC Infect Dis. 2021;21(1):1166. doi: 10.1186/s12879-021-06826-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennis I, Verdonck K, El Khalfaoui N, Riyad M, Fellah H, Dujardin JC, et al. Accuracy of a rapid diagnostic test based on antigen detection for the diagnosis of cutaneous leishmaniasis in patients with suggestive skin lesions in Morocco. Am J Trop Med Hyg. 2018;99(3):716–722. doi: 10.4269/ajtmh.18-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vink MMT, Nahzat SM, Rahimi H, Buhler C, Ahmadi BA, Nader M, et al. Evaluation of point-of-care tests for cutaneous leishmaniasis diagnosis in Kabul, Afghanistan. EBioMedicine. 2018;37:453–460. doi: 10.1016/j.ebiom.2018.10.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Henten S, Fikre H, Melkamu R, Dessie D, Mekonnen T, Kassa M, et al. Evaluation of the CL detect rapid test in Ethiopian patients suspected for cutaneous leishmaniasis. PLoS Negl Trop Dis. 2022;16(1):e0010143. doi: 10.1371/journal.pntd.0010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schallig H, Hu RVP, Kent AD, van Loenen M, Menting S, Picado A, et al. Evaluation of point of care tests for the diagnosis of cutaneous leishmaniasis in Suriname. BMC Infect Dis. 2019;19(1):25. doi: 10.1186/s12879-018-3634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antonio Lde F, Fagundes A, Oliveira RV, Pinto PG, Bedoya-Pacheco SJ, Vasconcellos Ede C, et al. Montenegro skin test and age of skin lesion as predictors of treatment failure in cutaneous leishmaniasis. Rev Inst Med Trop Sao Paulo. 2014;56(5):375–380. doi: 10.1590/S0036-46652014000500002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braz LMA. Tegumentary leishmaniasis diagnosis: what happened with MST (Montenegro Skin Test) in Brazil? Rev Inst Med Trop Sao Paulo. 2019;61:e17. doi: 10.1590/s1678-9946201961017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carstens-Kass J, Paulini K, Lypaczewski P, Matlashewski G. A review of the leishmanin skin test: a neglected test for a neglected disease. PLoS Negl Trop Dis. 2021;15(7):e0009531. doi: 10.1371/journal.pntd.0009531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Passos VM, Barreto SM, Romanha AJ, Krettli AU, Volpini AC, Lima e Costa MF. American cutaneous leishmaniasis: use of a skin test as a predictor of relapse after treatment. Bull World Health Organ. 2000;78(8):968–974. [PMC free article] [PubMed] [Google Scholar]
- 39.Moreira OC, Yadon ZE, Cupolillo E. The applicability of real-time PCR in the diagnostic of cutaneous leishmaniasis and parasite quantification for clinical management: current status and perspectives. Acta Trop. 2018;184:29–37. doi: 10.1016/j.actatropica.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 40.Odiwuor SO, Saad AA, De Doncker S, Maes I, Laurent T, El Safi S, et al. Universal PCR assays for the differential detection of all Old World Leishmania species. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2011;30(2):209–218. doi: 10.1007/s10096-010-1071-3. [DOI] [PubMed] [Google Scholar]
- 41.Toz SO, Culha G, Zeyrek FY, Ertabaklar H, Alkan MZ, Vardarli AT, et al. A real-time ITS1-PCR based method in the diagnosis and species identification of Leishmania parasite from human and dog clinical samples in Turkey. PLoS Negl Trop Dis. 2013;7(5):e2205. doi: 10.1371/journal.pntd.0002205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monroy-Ostria A, Nasereddin A, Monteon VM, Guzman-Bracho C, Jaffe CL. ITS1 PCR-RFLP diagnosis and characterization of leishmania in clinical samples and strains from cases of human cutaneous leishmaniasis in states of the Mexican Southeast. Interdiscip Perspect Infect Dis. 2014;2014:607287. doi: 10.1155/2014/607287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Satow MM, Yamashiro-Kanashiro EH, Rocha MC, Oyafuso LK, Soler RC, Cotrim PC, et al. Applicability of kDNA-PCR for routine diagnosis of American tegumentary leishmaniasis in a tertiary reference hospital. Rev Inst Med Trop Sao Paulo. 2013;55(6):393–399. doi: 10.1590/S0036-46652013000600004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jara M, Adaui V, Valencia BM, Martinez D, Alba M, Castrillon C, et al. Real-time PCR assay for detection and quantification of Leishmania (Viannia) organisms in skin and mucosal lesions: exploratory study of parasite load and clinical parameters. J Clin Microbiol. 2013;51(6):1826–1833. doi: 10.1128/JCM.00208-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mesa LE, Manrique R, Muskus C, Robledo SM. Test accuracy of polymerase chain reaction methods against conventional diagnostic techniques for Cutaneous Leishmaniasis (CL) in patients with clinical or epidemiological suspicion of CL: systematic review and meta-analysis. PLoS Negl Trop Dis. 2020;14(1):e0007981. doi: 10.1371/journal.pntd.0007981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mans DR, Kent AD, Hu RV, Lai AFEJ, Schoone GJ, Adams ER, et al. Monitoring the response of patients with cutaneous leishmaniasis to treatment with pentamidine isethionate by quantitative real-time PCR, and identification of Leishmania parasites not responding to therapy. Clin Exp Dermatol. 2016;41(6):610–615. doi: 10.1111/ced.12786. [DOI] [PubMed] [Google Scholar]
- 47.Adams ER, Schoone GJ, Ageed AF, Safi SE, Schallig HD. Development of a reverse transcriptase loop-mediated isothermal amplification (LAMP) assay for the sensitive detection of Leishmania parasites in clinical samples. Am J Trop Med Hyg. 2010;82(4):591–596. doi: 10.4269/ajtmh.2010.09-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Erber AC, Sandler PJ, de Avelar DM, Swoboda I, Cota G, Walochnik J. Diagnosis of visceral and cutaneous leishmaniasis using loop-mediated isothermal amplification (LAMP) protocols: a systematic review and meta-analysis. Parasit Vectors. 2022;15(1):34. doi: 10.1186/s13071-021-05133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adams ER, Schoone G, Versteeg I, Gomez MA, Diro E, Mori Y, et al. Development and evaluation of a novel loop-mediated isothermal amplification assay for diagnosis of cutaneous and visceral leishmaniasis. J Clin Microbiol. 2018;56(7):e00386. doi: 10.1128/JCM.00386-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nzelu CO, Gomez EA, Caceres AG, Sakurai T, Martini-Robles L, Uezato H, et al. Development of a loop-mediated isothermal amplification method for rapid mass-screening of sand flies for Leishmania infection. Acta Trop. 2014;132:1–6. doi: 10.1016/j.actatropica.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 51.Karani M, Sotiriadou I, Plutzer J, Karanis P. Bench-scale experiments for the development of a unified loop-mediated isothermal amplification (LAMP) assay for the in vitro diagnosis of Leishmania species' promastigotes. Epidemiol Infect. 2014;142(8):1671–1677. doi: 10.1017/S0950268813002677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verma S, Avishek K, Sharma V, Negi NS, Ramesh V, Salotra P. Application of loop-mediated isothermal amplification assay for the sensitive and rapid diagnosis of visceral leishmaniasis and post-kala-azar dermal leishmaniasis. Diagn Microbiol Infect Dis. 2013;75(4):390–395. doi: 10.1016/j.diagmicrobio.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 53.Mikita K, Maeda T, Yoshikawa S, Ono T, Miyahira Y, Kawana A. The Direct Boil-LAMP method: a simple and rapid diagnostic method for cutaneous leishmaniasis. Parasitol Int. 2014;63(6):785–789. doi: 10.1016/j.parint.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 54.Caliendo AM, Gilbert DN, Ginocchio CC, Hanson KE, May L, Quinn TC, et al. Better tests, better care: improved diagnostics for infectious diseases. Clin Infect Dis Off Publ Infect Dis Soc Am. 2013;57(Suppl 3):S139–S170. doi: 10.1093/cid/cit578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li J, Macdonald J, von Stetten F. Review: a comprehensive summary of a decade development of the recombinase polymerase amplification. Analyst. 2018;144(1):31–67. doi: 10.1039/C8AN01621F. [DOI] [PubMed] [Google Scholar]
- 56.Saldarriaga OA, Castellanos-Gonzalez A, Porrozzi R, Baldeviano GC, Lescano AG, de Los Santos MB, et al. An innovative field-applicable molecular test to diagnose cutaneous Leishmania Viannia spp. infections. PLoS Negl Trop Dis. 2016;10(4):e0004638. doi: 10.1371/journal.pntd.0004638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Travi BL, Delos Santos MB, Shelite TR, Santos RP, Rosales LA, Castellanos-Gonzalez A, et al. Diagnostic efficacy of recombinase-polymerase-amplification coupled with lateral flow strip reading in patients with cutaneous Leishmaniasis from the Amazonas Rainforest of Peru. Vector Borne Zoonotic Dis. 2021;21(12):941–947. doi: 10.1089/vbz.2021.0038. [DOI] [PubMed] [Google Scholar]
- 58.Cossio A, Jojoa J, Castro MDM, Castillo RM, Osorio L, Shelite TR, et al. Diagnostic performance of a recombinant polymerase amplification test-lateral flow (RPA-LF) for cutaneous leishmaniasis in an endemic setting of Colombia. PLoS Negl Trop Dis. 2021;15(4):e0009291. doi: 10.1371/journal.pntd.0009291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.TriTryp database https://tritrypdb.org/, an open database for kinetoplastid informatics resources. Accessed 1 May 2022.
- 60.Mesa LE, Manrique R, Robledo SM, Tabares J, Pineda T, Muskus C. The performance of the recombinase polymerase amplification test for detecting Leishmania deoxyribonucleic acid from skin lesions of patients with clinical or epidemiological suspicion of cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 2021;115(12):1427–1433. doi: 10.1093/trstmh/trab073. [DOI] [PubMed] [Google Scholar]
- 61.Adams ER, Gomez MA, Scheske L, Rios R, Marquez R, Cossio A, et al. Sensitive diagnosis of cutaneous leishmaniasis by lesion swab sampling coupled to qPCR. Parasitology. 2014;141(14):1891–1897. doi: 10.1017/S0031182014001280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gomes CM, Cesetti MV, de Paula NA, Vernal S, Gupta G, Sampaio RN, et al. Field validation of SYBR Green- and TaqMan-based real-time pcr using biopsy and swab samples to diagnose American Tegumentary Leishmaniasis in an area where Leishmania (Viannia) braziliensis is endemic. J Clin Microbiol. 2017;55(2):526–534. doi: 10.1128/JCM.01954-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silgado A, Armas M, Sanchez-Montalva A, Goterris L, Ubals M, Temprana-Salvador J, et al. Changes in the microbiological diagnosis and epidemiology of cutaneous leishmaniasis in real-time PCR era: a six-year experience in a referral center in Barcelona. PLoS Negl Trop Dis. 2021;15(11):e0009884. doi: 10.1371/journal.pntd.0009884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blaizot R, Simon S, Ginouves M, Prevot G, Blanchet D, Ravel C, et al. Validation of swab sampling and SYBR green-based real-time PCR for the diagnosis of cutaneous Leishmaniasis in French Guiana. J Clin Microbiol. 2021;59(2):e02218. doi: 10.1128/JCM.02218-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daoui O, Ait Kbaich M, Mhaidi I, El Kacem S, Hjiyej Andaloussi L, Akarid K, et al. The role of sampling by cotton swab in the molecular diagnosis of cutaneous leishmaniasis. Transbound Emerg Dis. 2021;68(4):2287–2294. doi: 10.1111/tbed.13886. [DOI] [PubMed] [Google Scholar]
- 66.Kato H, Caceres AG, Mimori T, Ishimaru Y, Sayed AS, Fujita M, et al. Use of FTA cards for direct sampling of patients' lesions in the ecological study of cutaneous leishmaniasis. J Clin Microbiol. 2010;48(10):3661–3665. doi: 10.1128/JCM.00498-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miranda A, Saldana A, Gonzalez K, Paz H, Santamaria G, Samudio F, et al. Evaluation of PCR for cutaneous leishmaniasis diagnosis and species identification using filter paper samples in Panama, Central America. Trans R Soc Trop Med Hyg. 2012;106(9):544–548. doi: 10.1016/j.trstmh.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 68.Rico Rodriguez CP, Oradav F, Yarbmta Pinzon TMV. the imprint as a non-invasive method of sampling for the molecular diagnosis of cutaneous leishmaniasis in Colombia's Military population. Revfacmed [online]. 2018;26(2):15–21. [Google Scholar]
- 69.Al-Jawabreh A, Dumaidi K, Ereqat S, Nasereddin A, Azmi K, Al-Jawabreh H, et al. A comparison of the efficiency of three sampling methods for use in the molecular and conventional diagnosis of cutaneous leishmaniasis. Acta Trop. 2018;182:173–177. doi: 10.1016/j.actatropica.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 70.Taslimi Y, Sadeghipour P, Habibzadeh S, Mashayekhi V, Mortazavi H, Muller I, et al. A novel non-invasive diagnostic sampling technique for cutaneous leishmaniasis. PLoS Negl Trop Dis. 2017;11(7):e0005750. doi: 10.1371/journal.pntd.0005750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wall EC, Watson J, Armstrong M, Chiodini PL, Lockwood DN. Epidemiology of imported cutaneous leishmaniasis at the Hospital for Tropical Diseases, London, United Kingdom: use of polymerase chain reaction to identify the species. Am J Trop Med Hyg. 2012;86(1):115–118. doi: 10.4269/ajtmh.2012.10-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pratlong F, Dereure J, Ravel C, Lami P, Balard Y, Serres G, et al. Geographical distribution and epidemiological features of Old World cutaneous leishmaniasis foci, based on the isoenzyme analysis of 1048 strains. Trop Med Int Health TM IH. 2009;14(9):1071–1085. doi: 10.1111/j.1365-3156.2009.02336.x. [DOI] [PubMed] [Google Scholar]
- 73.Chaara D, Haouas N, Dedet JP, Babba H, Pratlong F. Leishmaniases in Maghreb: an endemic neglected disease. Acta Trop. 2014;132:80–93. doi: 10.1016/j.actatropica.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 74.Azmi K, Schonian G, Schnur LF, Nasereddin A, Ereqat S, Abdeen Z. Development of assays using hexokinase and phosphoglucomutase gene sequences that distinguish strains of Leishmania tropica from different zymodemes and microsatellite clusters and their application to Palestinian foci of cutaneous leishmaniasis. PLoS Negl Trop Dis. 2013;7(9):e2464. doi: 10.1371/journal.pntd.0002464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grimaldi G, McMahon-Pratt D. Monoclonal antibodies for the identification of New World Leishmania species. Mem Inst Oswaldo Cruz. 1996;91(1):37–42. doi: 10.1590/S0074-02761996000100006. [DOI] [PubMed] [Google Scholar]
- 76.Van der Auwera G, Dujardin JC. Species typing in dermal leishmaniasis. Clin Microbiol Rev. 2015;28(2):265–294. doi: 10.1128/CMR.00104-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marfurt J, Nasereddin A, Niederwieser I, Jaffe CL, Beck HP, Felger I. Identification and differentiation of Leishmania species in clinical samples by PCR amplification of the miniexon sequence and subsequent restriction fragment length polymorphism analysis. J Clin Microbiol. 2003;41(7):3147–3153. doi: 10.1128/JCM.41.7.3147-3153.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Marfurt J, Niederwieser I, Makia ND, Beck HP, Felger I. Diagnostic genotyping of Old and New World Leishmania species by PCR-RFLP. Diagn Microbiol Infect Dis. 2003;46(2):115–124. doi: 10.1016/S0732-8893(03)00040-3. [DOI] [PubMed] [Google Scholar]
- 79.Kent AD, Dos Santos TV, Gangadin A, Samjhawan A, Mans DR, Schallig HD. Studies on the sand fly fauna (Diptera: Psychodidae) in high-transmission areas of cutaneous leishmaniasis in the Republic of Suriname. Parasit Vectors. 2013;6(1):318. doi: 10.1186/1756-3305-6-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dorlo TP, van Thiel PP, Schoone GJ, Stienstra Y, van Vugt M, Beijnen JH, et al. Dynamics of parasite clearance in cutaneous leishmaniasis patients treated with miltefosine. PLoS Negl Trop Dis. 2011;5(12):e1436. doi: 10.1371/journal.pntd.0001436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Imani M, Dehkharghani AD, Ghelman M, Mohammadloo M. Molecular technique for detection of Leishmania infantum isolates in Iran. Trop Parasitol. 2014;4(1):35–37. doi: 10.4103/2229-5070.129160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Votypka J, Kasap OE, Volf P, Kodym P, Alten B. Risk factors for cutaneous leishmaniasis in Cukurova region, Turkey. Trans R Soc Trop Med Hyg. 2012;106(3):186–190. doi: 10.1016/j.trstmh.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 83.van Thiel PP, Leenstra T, de Vries HJ, van der Sluis A, van Gool T, Krull AC, et al. Cutaneous leishmaniasis (Leishmania major infection) in Dutch troops deployed in northern Afghanistan: epidemiology, clinical aspects, and treatment. Am J Trop Med Hyg. 2010;83(6):1295–1300. doi: 10.4269/ajtmh.2010.10-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bart A, van Thiel PP, de Vries HJ, Hodiamont CJ, Van Gool T. Imported leishmaniasis in the Netherlands from 2005 to 2012: epidemiology, diagnostic techniques and sequence-based species typing from 195 patients. (Euro surveillance bulletin Europeen sur les maladies transmissibles) Eur Commun Dis Bull. 2013;18(30):20544. doi: 10.2807/1560-7917.es2013.18.30.20544. [DOI] [PubMed] [Google Scholar]
- 85.Saberi S, Zamani A, Motamedi N, Nilforoushzadeh MA, Jaffary F, Rahimi E, et al. The knowledge, attitude, and prevention practices of students regarding cutaneous leishmaniasis in the hyperendemic region of the Shahid Babaie Airbase. Vector Borne Zoonotic Dis. 2012;12(4):306–309. doi: 10.1089/vbz.2010.0259. [DOI] [PubMed] [Google Scholar]
- 86.Chaves LF, Calzada JE, Valderrama A, Saldana A. Cutaneous leishmaniasis and sand fly fluctuations are associated with el nino in panama. PLoS Negl Trop Dis. 2014;8(10):e3210. doi: 10.1371/journal.pntd.0003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Croft SL, Sundar S, Fairlamb AH. Drug resistance in leishmaniasis. Clin Microbiol Rev. 2006;19(1):111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schriefer A, Schriefer AL, Goes-Neto A, Guimaraes LH, Carvalho LP, Almeida RP, et al. Multiclonal Leishmania braziliensis population structure and its clinical implication in a region of endemicity for American tegumentary leishmaniasis. Infect Immun. 2004;72(1):508–514. doi: 10.1128/IAI.72.1.508-514.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lucas CM, Franke ED, Cachay MI, Tejada A, Cruz ME, Kreutzer RD, et al. Geographic distribution and clinical description of leishmaniasis cases in Peru. Am J Trop Med Hyg. 1998;59(2):312–317. doi: 10.4269/ajtmh.1998.59.312. [DOI] [PubMed] [Google Scholar]
- 90.Schriefer A, Guimaraes LH, Machado PR, Lessa M, Lessa HA, Lago E, et al. Geographic clustering of leishmaniasis in northeastern Brazil. Emerg Infect Dis. 2009;15(6):871–876. doi: 10.3201/eid1506.080406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hu RV, Kent AD, Adams ER, van der Veer C, Sabajo LO, Mans DR, et al. First case of cutaneous leishmaniasis caused by Leishmania (Viannia) braziliensis in Suriname. Am J Trop Med Hyg. 2012;86(5):825–827. doi: 10.4269/ajtmh.2012.11-0728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van der Meide W, de Vries H, Pratlong F, van der Wal A, Sabajo L. Leishmaniasis, suriname. Emerg Infect Dis. 2008;14(5):857–859. doi: 10.3201/eid1405.070433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Andrade-Narvaez FJ, Loría-Cervera EN, Sosa-Bibiano EI, Van Wynsberghe NR. Asymptomatic infection with American cutaneous leishmaniasis: epidemiological and immunological studies. Mem Inst Oswaldo Cruz. 2016;111(10):599–604. doi: 10.1590/0074-02760160138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hashiguchi Y, Gomez EL, Kato H, Martini LR, Velez LN, Uezato H. Diffuse and disseminated cutaneous leishmaniasis: clinical cases experienced in Ecuador and a brief review. Trop Med Health. 2016;44:2. doi: 10.1186/s41182-016-0002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Silveira FT, Lainson R, Corbett CE. Clinical and immunopathological spectrum of American cutaneous leishmaniasis with special reference to the disease in Amazonian Brazil: a review. Mem Inst Oswaldo Cruz. 2004;99(3):239–251. doi: 10.1590/S0074-02762004000300001. [DOI] [PubMed] [Google Scholar]
- 96.Martínez DY, Verdonck K, Kaye PM, Adaui V, Polman K, Llanos-Cuentas A, et al. Tegumentary leishmaniasis and coinfections other than HIV. PLoS Negl Trop Dis. 2018;12(3):e0006125. doi: 10.1371/journal.pntd.0006125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.van Griensven J, Carrillo E, Lopez-Velez R, Lynen L, Moreno J. Leishmaniasis in immunosuppressed individuals. Clin Microbiol Infect. 2014;20(4):286–299. doi: 10.1111/1469-0691.12556. [DOI] [PubMed] [Google Scholar]
- 98.Mock DJ, Hollenbaugh JA, Daddacha W, Overstreet MG, Lazarski CA, Fowell DJ, et al. Leishmania induces survival, proliferation and elevated cellular dNTP levels in human monocytes promoting acceleration of HIV co-infection. PLoS Pathog. 2012;8(4):e1002635. doi: 10.1371/journal.ppat.1002635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meireles CB, Maia LC, Soares GC, Teodoro IPP, Gadelha M, da Silva CGL, et al. Atypical presentations of cutaneous leishmaniasis: a systematic review. Acta Trop. 2017;172:240–254. doi: 10.1016/j.actatropica.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 100.Renslo AR, McKerrow JH. Drug discovery and development for neglected parasitic diseases. Nat Chem Biol. 2006;2(12):701–710. doi: 10.1038/nchembio837. [DOI] [PubMed] [Google Scholar]
- 101.Bezemer JM, van der Ende J, Limpens J, de Vries HJC, Schallig H. Safety and efficacy of allylamines in the treatment of cutaneous and mucocutaneous leishmaniasis: a systematic review. PLoS One. 2021;16(4):e0249628. doi: 10.1371/journal.pone.0249628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Prates FV, Dourado ME, Silva SC, Schriefer A, Guimarães LH, Brito MD, et al. Fluconazole in the treatment of cutaneous leishmaniasis caused by Leishmania braziliensis: a randomized controlled trial. Clin Infect Dis Off Publ Infect Dis Soc Am. 2017;64(1):67–71. doi: 10.1093/cid/ciw662. [DOI] [PubMed] [Google Scholar]
- 103.Soto J, Soto P, Ajata A, Luque C, Tintaya C, Paz D, et al. Topical 15% paromomycin-aquaphilic for bolivian leishmania braziliensis cutaneous leishmaniasis: a randomized, placebo-controlled trial. Clin Infect Dis Off Publ Infect Dis Soc Am. 2019;68(5):844–849. doi: 10.1093/cid/ciy619. [DOI] [PubMed] [Google Scholar]
- 104.Machado PRL, Prates FVO, Boaventura V, Lago T, Guimarães LH, Schriefer A, et al. A double-blind, randomized trial to evaluate miltefosine and topical granulocyte macrophage colony-stimulating factor in the treatment of cutaneous leishmaniasis caused by leishmania braziliensis in Brazil. Clin Infect Dis Off Publ Infect Dis Soc Am. 2021;73(7):e2465–e2469. doi: 10.1093/cid/ciaa1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gonzalez U. Cochrane reviews on neglected diseases: the case of cutaneous leishmaniasis. Cochrane Database Syst Rev. 2013;3:ED000055. doi: 10.1002/14651858.ED000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gonzalez U, Pinart M, Rengifo-Pardo M, Macaya A, Alvar J, Tweed JA. Interventions for American cutaneous and mucocutaneous leishmaniasis. Cochrane Database Syst Rev. 2009;2:CD004834. doi: 10.1002/14651858.CD004834.pub2. [DOI] [PubMed] [Google Scholar]
- 107.Briones Nieva CA, Cid AG, Romero AI, García-Bustos MF, Villegas M, Bermúdez JM. An appraisal of the scientific current situation and new perspectives in the treatment of cutaneous leishmaniasis. Acta Trop. 2021;221:105988. doi: 10.1016/j.actatropica.2021.105988. [DOI] [PubMed] [Google Scholar]
- 108.Lamotte S, Aulner N, Späth GF, Prina E. Discovery of novel hit compounds with broad activity against visceral and cutaneous Leishmania species by comparative phenotypic screening. Sci Rep. 2019;9(1):438. doi: 10.1038/s41598-018-36944-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Asilian A, Sadeghinia A, Faghihi G, Momeni A. Comparative study of the efficacy of combined cryotherapy and intralesional meglumine antimoniate (Glucantime) vs. cryotherapy and intralesional meglumine antimoniate (Glucantime) alone for the treatment of cutaneous leishmaniasis. Int J Dermatol. 2004;43(4):281–283. doi: 10.1111/j.1365-4632.2004.02002.x. [DOI] [PubMed] [Google Scholar]
- 110.Salmanpour R, Razmavar MR, Abtahi N. Comparison of intralesional meglumine antimoniate, cryotherapy and their combination in the treatment of cutaneous leishmaniasis. Int J Dermatol. 2006;45(9):1115–1116. doi: 10.1111/j.1365-4632.2006.02822.x. [DOI] [PubMed] [Google Scholar]
- 111.El Darouti MA, Al Rubaie SM. Cutaneous leishmaniasis Treatment with combined cryotherapy and intralesional stibogluconate injection. Int J Dermatol. 1990;29(1):56–59. doi: 10.1111/j.1365-4362.1990.tb03759.x. [DOI] [PubMed] [Google Scholar]
- 112.Aronson NE, Wortmann GW, Byrne WR, Howard RS, Bernstein WB, Marovich MA, et al. A randomized controlled trial of local heat therapy versus intravenous sodium stibogluconate for the treatment of cutaneous Leishmania major infection. PLoS Negl Trop Dis. 2010;4(3):e628. doi: 10.1371/journal.pntd.0000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Reithinger R, Mohsen M, Wahid M, Bismullah M, Quinnell RJ, Davies CR, et al. Efficacy of thermotherapy to treat cutaneous leishmaniasis caused by Leishmania tropica in Kabul, Afghanistan: a randomized, controlled trial. Clin Inf Dis Off Publ Infect Dis Soc Am. 2005;40(8):1148–1155. doi: 10.1086/428736. [DOI] [PubMed] [Google Scholar]
- 114.Refai WF, Madarasingha NP, Sumanasena B, Weerasingha S, De Silva A, Fernandopulle R, et al. Efficacy, safety and cost-effectiveness of thermotherapy in the treatment of leishmania donovani-induced cutaneous leishmaniasis: a randomized controlled clinical trial. Am J Trop Med Hyg. 2017;97(4):1120–1126. doi: 10.4269/ajtmh.16-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Silva H, Liyanage A, Deerasinghe T, Sumanasena B, Munidasa D, de Silva H, et al. Therapeutic response to thermotherapy in cutaneous leishmaniasis treatment failures for sodium stibogluconate: a randomized controlled proof of principle clinical trial. Am J Trop Med Hyg. 2021;104(3):945–950. doi: 10.4269/ajtmh.20-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kämink S, Abdi A, Kamau C, Ashraf S, Ansari MA, Qureshi NA, et al. Failure of an innovative low-cost, noninvasive thermotherapy device for treating cutaneous leishmaniasis caused by leishmania tropica in Pakistan. Am J Trop Med Hyg. 2019;101(6):1373–1379. doi: 10.4269/ajtmh.19-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mohebali M, Fotouhi A, Hooshmand B, Zarei Z, Akhoundi B, Rahnema A, et al. Comparison of miltefosine and meglumine antimoniate for the treatment of zoonotic cutaneous leishmaniasis (ZCL) by a randomized clinical trial in Iran. Acta Trop. 2007;103(1):33–40. doi: 10.1016/j.actatropica.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 118.van Thiel PP, Leenstra T, Kager PA, de Vries HJ, van Vugt M, van der Meide WF, et al. Miltefosine treatment of Leishmania major infection: an observational study involving Dutch military personnel returning from northern Afghanistan. Clin Infect Dis Off Publ Infect Dis Soc Am. 2010;50(1):80–83. doi: 10.1086/648726. [DOI] [PubMed] [Google Scholar]
- 119.Ollech A, Solomon M, Horev A, Reiss-Huss S, Ben-Amitai D, Zvulunov A, et al. Cutaneous leishmaniasis treated with miltefosine: a case series of 10 paediatric patients. Acta Derm Venereol. 2020;100(18):adv00322. doi: 10.2340/00015555-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Heras-Mosteiro J, Monge-Maillo B, Pinart M, Lopez Pereira P, Reveiz L, Garcia-Carrasco E, et al. Interventions for Old World cutaneous leishmaniasis. Cochrane Database Syst Rev. 2017;12(12):005067. doi: 10.1002/14651858.CD005067.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ranawaka RR, Weerakoon HS, de Silva SH. Randomized, double-blind, controlled, comparative study on intralesional 10% and 15% hypertonic saline versus intralesional sodium stibogluconate in Leishmania donovani cutaneous leishmaniasis. Int J Dermatol. 2015;54(5):555–563. doi: 10.1111/ijd.12685. [DOI] [PubMed] [Google Scholar]
- 122.Banihashemi M, Yazdanpanah MJ, Amirsolymani H, Yousefzadeh H. Comparison of lesion improvement in lupoid leishmaniasis patients with two treatment approaches: trichloroacetic acid and intralesional meglumine antimoniate. J Cutan Med Surg. 2015;19(1):35–39. doi: 10.2310/7750.2014.13193. [DOI] [PubMed] [Google Scholar]
- 123.Somaratne VN, Ranawaka RR, Jayaruwan HM, Wipuladasa DM, de Silva SHP. Randomized, double-blind study on intralesional metronidazole versus intralesional sodium stibogluconate in Leishmania donovani cutaneous leishmaniasis. J Dermatol Treat. 2019;30(1):87–91. doi: 10.1080/09546634.2018.1472738. [DOI] [PubMed] [Google Scholar]
- 124.Nahidi Y, Mashayekhi Goyonlo V, Layegh P, Taghavi F, Najaf NM. Immunomodulatory effects of topical diphencyprone for the treatment of acute urban cutaneous leishmaniasis. J Dermatol Treat. 2021;32(2):220–226. doi: 10.1080/09546634.2019.1642997. [DOI] [PubMed] [Google Scholar]
- 125.Artzi O, Sprecher E, Koren A, Mehrabi JN, Katz O, Hilerowich Y. Fractional ablative CO2 laser followed by topical application of sodium stibogluconate for treatment of active cutaneous leishmaniasis: a randomized controlled trial. Acta Derm Venereol. 2019;99(1):53–57. doi: 10.2340/00015555-3058. [DOI] [PubMed] [Google Scholar]
- 126.Tafazzoli Z, Nahidi Y, Mashayekhi Goyonlo V, Morovatdar N, Layegh P. Evaluating the efficacy and safety of vascular IPL for treatment of acute cutaneous leishmaniasis: a randomized controlled trial. Lasers Med Sci. 2021;36(3):631–640. doi: 10.1007/s10103-020-03102-2. [DOI] [PubMed] [Google Scholar]
- 127.Khan K, Khan AU, Ghufran KA, Khan M, Ahmad I. Fractionated illumination improves the treatment outcomes of photodynamic therapy for high grade cutaneous leishmaniasis. Photodiagn Photodyn Ther. 2020;29:101622. doi: 10.1016/j.pdpdt.2019.101622. [DOI] [PubMed] [Google Scholar]
- 128.Shanehsaz SM, Ishkhanian S. A comparative study between the efficacy of oral cimetidine and low-dose systemic meglumine antimoniate (MA) with a standard dose of systemic MA in the treatment of cutaneous leishmaniasis. Int J Dermatol. 2015;54(7):834–838. doi: 10.1111/ijd.12709. [DOI] [PubMed] [Google Scholar]
- 129.Hanif MM, Akram K, Mustafa G. Intralesional versus oral chloroquine in cutaneous leishmaniasis: comparison of outcome, duration of treatment and total dose of drug. J Coll Physicians Surg Pak. 2016;26(4):260–262. [PubMed] [Google Scholar]
- 130.Malik F, Hanif MM, Mustafa G. Comparing the efficacy of oral chloroquine versus oral tetracycline in the treatment of cutaneous leishmaniasis. J Coll Physicians Surg Pak. 2019;29(5):403–405. doi: 10.29271/jcpsp.2019.05.403. [DOI] [PubMed] [Google Scholar]
- 131.Velasco-Castrejon O, Walton BC, Rivas-Sanchez B, Garcia MF, Lazaro GJ, Hobart O, et al. Treatment of cutaneous leishmaniasis with localized current field (radio frequency) in Tabasco, Mexico. Am J Trop Med Hyg. 1997;57(3):309–312. doi: 10.4269/ajtmh.1997.57.309. [DOI] [PubMed] [Google Scholar]
- 132.Pratlong F, Deniau M, Darie H, Eichenlaub S, Proll S, Garrabe E, et al. Human cutaneous leishmaniasis caused by Leishmania naiffi is wide-spread in South America. Ann Trop Med Parasitol. 2002;96(8):781–785. doi: 10.1179/000349802125002293. [DOI] [PubMed] [Google Scholar]
- 133.Escobar P, Matu S, Marques C, Croft SL. Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), ET-18-OCH(3) (edelfosine) and amphotericin B. Acta Trop. 2002;81(2):151–157. doi: 10.1016/S0001-706X(01)00197-8. [DOI] [PubMed] [Google Scholar]
- 134.Soto J, Arana BA, Toledo J, Rizzo N, Vega JC, Diaz A, et al. Miltefosine for new world cutaneous leishmaniasis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2004;38(9):1266–1272. doi: 10.1086/383321. [DOI] [PubMed] [Google Scholar]
- 135.World Health O. Control of the leishmaniases. World Health Organization technical report series. 949:xii-xiii, 1-186, back cover; 2010. [PubMed]
- 136.López-Carvajal L, Cardona-Arias JA, Zapata-Cardona MI, Sánchez-Giraldo V, Vélez ID. Efficacy of cryotherapy for the treatment of cutaneous leishmaniasis: meta-analyses of clinical trials. BMC Infect Dis. 2016;16:360. doi: 10.1186/s12879-016-1663-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Guerra JA, Prestes SR, Silveira H, Coelho LI, Gama P, Moura A, et al. Mucosal Leishmaniasis caused by Leishmania (Viannia) braziliensis and Leishmania (Viannia) guyanensis in the Brazilian Amazon. PLoS Negl Trop Dis. 2011;5(3):e980. doi: 10.1371/journal.pntd.0000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hu RV, Straetemans M, Kent AD, Sabajo LO, de Vries HJ, Fat RF. Randomized single-blinded non-inferiority trial of 7 mg/kg pentamidine isethionate versus 4 mg/kg pentamidine isethionate for cutaneous leishmaniaisis in Suriname. PLoS Negl Trop Dis. 2015;9(3):e0003592. doi: 10.1371/journal.pntd.0003592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gadelha EPN, Ramasawmy R, da Costa OB, Morais Rocha N, de Oliveira GJA, da Silva GAVR, et al. An open label randomized clinical trial comparing the safety and effectiveness of one, two or three weekly pentamidine isethionate doses (seven milligrams per kilogram) in the treatment of cutaneous leishmaniasis in the Amazon Region. PLoS Negl Trop Dis. 2018;12(10):e0006850. doi: 10.1371/journal.pntd.0006850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Neves LO, Talhari AC, Gadelha EP, Silva Junior RM, Guerra JA, Ferreira LC, et al. A randomized clinical trial comparing meglumine antimoniate, pentamidine and amphotericin B for the treatment of cutaneous leishmaniasis by Leishmania guyanensis. An Bras Dermatol. 2011;86(6):1092–1101. doi: 10.1590/S0365-05962011000600005. [DOI] [PubMed] [Google Scholar]
- 141.Gangneux JP, Sauzet S, Donnard S, Meyer N, Cornillet A, Pratlong F, et al. Recurrent American cutaneous leishmaniasis. Emerg Infect Dis. 2007;13(9):1436–1438. doi: 10.3201/eid1309.061446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dedet JP, Pradinaud R, Gay F. Epidemiological aspects of human cutaneous leishmaniasis in French Guiana. Trans R Soc Trop Med Hyg. 1989;83(5):616–620. doi: 10.1016/0035-9203(89)90375-1. [DOI] [PubMed] [Google Scholar]
- 143.Soto J, Paz D, Rivero D, Soto P, Quispe J, Toledo J, et al. Intralesional pentamidine: a novel therapy for single lesions of bolivian cutaneous leishmaniasis. Am J Trop Med Hyg. 2016;94(4):852–856. doi: 10.4269/ajtmh.15-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Soto J, Rea J, Balderrama M, Toledo J, Soto P, Valda L, et al. Efficacy of miltefosine for Bolivian cutaneous leishmaniasis. Am J Trop Med Hyg. 2008;78(2):210–211. doi: 10.4269/ajtmh.2008.78.210. [DOI] [PubMed] [Google Scholar]
- 145.Chrusciak-Talhari A, Dietze R, Chrusciak Talhari C, da Silva RM, Gadelha Yamashita EP, de Oliveira PG, et al. Randomized controlled clinical trial to access efficacy and safety of miltefosine in the treatment of cutaneous leishmaniasis caused by Leishmania (Viannia) guyanensis in Manaus, Brazil. Am J Trop Med Hyg. 2011;84(2):255–260. doi: 10.4269/ajtmh.2011.10-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Machado PR, Ampuero J, Guimaraes LH, Villasboas L, Rocha AT, Schriefer A, et al. Miltefosine in the treatment of cutaneous leishmaniasis caused by Leishmania braziliensis in Brazil: a randomized and controlled trial. PLoS Negl Trop Dis. 2010;4(12):e912. doi: 10.1371/journal.pntd.0000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Monge-Maillo B, López-Vélez R. Miltefosine for visceral and cutaneous leishmaniasis: drug characteristics and evidence-based treatment recommendations. Clin Infect Dis Off Publ Infect Dis Soc Am. 2015;60(9):1398–1404. doi: 10.1093/cid/civ004. [DOI] [PubMed] [Google Scholar]
- 148.Amato VS, Tuon FF, Imamura R, de Camargo RA, Duarte MI, Neto VA. Mucosal leishmaniasis: description of case management approaches and analysis of risk factors for treatment failure in a cohort of 140 patients in Brazil. J Eur Acad Dermatol Venereol JEADV. 2009;23(9):1026–1034. doi: 10.1111/j.1468-3083.2009.03238.x. [DOI] [PubMed] [Google Scholar]
- 149.Nonata R, Sampaio R, Marsden PD. Mucosal leishmaniasis unresponsive to glucantime therapy successfully treated with Am Bisome. Trans R Soc Trop Med Hyg. 1997;91(1):77. doi: 10.1016/S0035-9203(97)90404-1. [DOI] [PubMed] [Google Scholar]
- 150.Soto J, Toledo J, Valda L, Balderrama M, Rea I, Parra R, et al. Treatment of Bolivian mucosal leishmaniasis with miltefosine. Clin Infect Dis Off Publ Infect Dis Soc Am. 2007;44(3):350–356. doi: 10.1086/510588. [DOI] [PubMed] [Google Scholar]
- 151.Santos CR, Tuon FF, Cieslinski J, de Souza RM, Imamura R, Amato VS. Comparative study on liposomal amphotericin B and other therapies in the treatment of mucosal leishmaniasis: a 15-year retrospective cohort study. PLoS One. 2019;14(6):e0218786. doi: 10.1371/journal.pone.0218786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.López L, Vélez I, Asela C, Cruz C, Alves F, Robledo S, et al. A phase II study to evaluate the safety and efficacy of topical 3% amphotericin B cream (Anfoleish) for the treatment of uncomplicated cutaneous leishmaniasis in Colombia. PLoS Negl Trop Dis. 2018;12(7):e0006653. doi: 10.1371/journal.pntd.0006653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Brito G, Dourado M, Guimarães LH, Meireles E, Schriefer A, de Carvalho EM, et al. Oral pentoxifylline associated with pentavalent antimony: a randomized trial for cutaneous leishmaniasis. Am J Trop Med Hyg. 2017;96(5):1155–1159. doi: 10.4269/ajtmh.16-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Machado PRL, Ribeiro CS, França-Costa J, Dourado MEF, Trinconi CT, Yokoyama-Yasunaka JKU, et al. Tamoxifen and meglumine antimoniate combined therapy in cutaneous leishmaniasis patients: a randomised trial. Trop Med Int Health TM IH. 2018;23(9):936–942. doi: 10.1111/tmi.13119. [DOI] [PubMed] [Google Scholar]
- 155.Stockdale L, Newton R. A review of preventative methods against human leishmaniasis infection. PLoS Negl Trop Dis. 2013;7(6):e2278. doi: 10.1371/journal.pntd.0002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Montenegro Quiñonez CA, Runge-Ranzinger S, Rahman KM, Horstick O. Effectiveness of vector control methods for the control of cutaneous and visceral leishmaniasis: a meta-review. PLoS Negl Trop Dis. 2021;15(5):e0009309. doi: 10.1371/journal.pntd.0009309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Faraj C, Yukich J, Adlaoui EB, Wahabi R, Mnzava AP, Kaddaf M, et al. Effectiveness and cost of insecticide-treated bed nets and indoor residual spraying for the control of cutaneous leishmaniasis: a cluster-randomized control trial in Morocco. Am J Trop Med Hyg. 2016;94(3):679–685. doi: 10.4269/ajtmh.14-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Arzamani K, Vatandoost H, Rassi Y, Abai MR, Akhavan AA, Alavinia M, et al. Susceptibility status of wild population of Phlebotomus sergenti (Diptera: Psychodidae) to different imagicides in a endemic focus of cutaneous leishmaniasis in northeast of Iran. J Vector Borne Dis. 2017;54(3):282–286. doi: 10.4103/0972-9062.217621. [DOI] [PubMed] [Google Scholar]
- 159.Chowdhury R, Das ML, Chowdhury V, Roy L, Faria S, Priyanka J, et al. Susceptibility of field-collected Phlebotomus argentipes (Diptera: Psychodidae) sand flies from Bangladesh and Nepal to different insecticides. Parasit Vectors. 2018;11(1):336. doi: 10.1186/s13071-018-2913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Karakus M, Gocmen B, Ozbel Y. Insecticide susceptibility status of wild-caught sand fly populations collected from two leishmaniasis endemic areas in Western Turkey. J Arthropod Borne Dis. 2017;11(1):86–94. [PMC free article] [PubMed] [Google Scholar]
- 161.González U, Pinart M, Sinclair D, Firooz A, Enk C, Vélez ID, et al. Vector and reservoir control for preventing leishmaniasis. Cochrane Database Syst Rev. 2015;2015(8):Cd008736. doi: 10.1002/14651858.CD008736.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Cardoso L, Schallig H, Persichetti MF, Pennisi MG. New epidemiological aspects of animal leishmaniosis in Europe: the role of vertebrate hosts other than dogs. Pathogens. 2021;10(3):307. doi: 10.3390/pathogens10030307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Werneck GL, Costa CH, de Carvalho FA, Pires ECMS, Maguire JH, Castro MC. Effectiveness of insecticide spraying and culling of dogs on the incidence of leishmania infantum infection in humans: a cluster randomized trial in Teresina, Brazil. PLoS Negl Trop Dis. 2014;8(10):e3172. doi: 10.1371/journal.pntd.0003172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ramdas S, van der Geest S. Not-knowing and the proliferation of assumptions: local explanations of cutaneous leishmaniasis in suriname. Anthropol Med. 2020;27(2):144–159. doi: 10.1080/13648470.2019.1627654. [DOI] [PubMed] [Google Scholar]
- 165.Ramdas S. Cruel disease, cruel medicine: self-treatment of cutaneous leishmaniasis with harmful chemical substances in Suriname. Soc Sci Med. 2012;75(6):1097–1105. doi: 10.1016/j.socscimed.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 166.Alidosti M, Heidari Z, Shahnazi H, Zamani-Alavijeh F. Behaviors and perceptions related to cutaneous leishmaniasis in endemic areas of the world: a review. Acta Trop. 2021;223:106090. doi: 10.1016/j.actatropica.2021.106090. [DOI] [PubMed] [Google Scholar]
- 167.Carrion C, Robles N, Sola-Morales O, Aymerich M, Ruiz Postigo JA. Mobile health strategies to tackle skin neglected tropical diseases with recommendations from innovative experiences: systematic review. JMIR Mhealth Uhealth. 2020;8(12):e22478. doi: 10.2196/22478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.WHO. Control of Neglected Tropical Diseases. World Health Organization. Geneva: WHO; 2020. http://www.who.int/neglected_diseases/diseases/en/#. Accessed 14 Dec 2020.
- 169.WHO. Skin-related neglected tropical diseases Geneva: WHO. https://www.who.int/teams/control-of-neglected-tropical-diseases/skin-ntds.
- 170.Gillespie PM, Beaumier CM, Strych U, Hayward T, Hotez PJ, Bottazzi ME. Status of vaccine research and development of vaccines for leishmaniasis. Vaccine. 2016;34(26):2992–2995. doi: 10.1016/j.vaccine.2015.12.071. [DOI] [PubMed] [Google Scholar]
- 171.Bacon KM, Hotez PJ, Kruchten SD, Kamhawi S, Bottazzi ME, Valenzuela JG, et al. The potential economic value of a cutaneous leishmaniasis vaccine in seven endemic countries in the Americas. Vaccine. 2013;31(3):480–486. doi: 10.1016/j.vaccine.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Alvar J, Croft SL, Kaye P, Khamesipour A, Sundar S, Reed SG. Case study for a vaccine against leishmaniasis. Vaccine. 2013;31(Suppl 2):B244–B249. doi: 10.1016/j.vaccine.2012.11.080. [DOI] [PubMed] [Google Scholar]
- 173.Nagill R, Kaur S. Vaccine candidates for leishmaniasis: a review. Int Immunopharmacol. 2011;11(10):1464–1488. doi: 10.1016/j.intimp.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 174.Zabala-Peñafiel A, Todd D, Daneshvar H, Burchmore R. The potential of live attenuated vaccines against Cutaneous Leishmaniasis. Exp Parasitol. 2020;210:107849. doi: 10.1016/j.exppara.2020.107849. [DOI] [PubMed] [Google Scholar]
- 175.Thomaz-Soccol V, da Costa ESF, Karp SG, Junior LLA, Soccol FT, Soccol CR. Recent advances in vaccines against leishmania based on patent applications. Recent Pat Biotechnol. 2018;12(1):21–32. doi: 10.2174/1872208311666170510121126. [DOI] [PubMed] [Google Scholar]
- 176.Parhizgari N, Piazak N, Mostafavi E. Vector-borne diseases in Iran: epidemiology and key challenges. Future Microbiol. 2021;16(1):51–69. doi: 10.2217/fmb-2019-0306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.