Abstract
Objectives
To investigate the influence of gender on disease outcomes in patients with spondyloarthritis (SpA), including across SpA subtypes.
Methods
Data from 4185 patients of 23 countries with a diagnosis of axial SpA (axSpA), peripheral SpA (pSpA) or psoriatic arthritis (PsA) from the Assessment of SpondyloArthritis International Society (ASAS)-perSpA study were analysed. Associations between gender and disease activity (Ankylosing Spondylitis Disease Activity Score (ASDAS), Bath Ankylosing Spondylitis Disease Activity Score (BASDAI), C-reactive protein (CRP)), function (Bath Ankylosing Spondylitis Functional Index (BASFI)) and overall health (ASAS Health Index (ASAS HI), European Quality of Life Five Dimension (EQ-5D)) outcomes were investigated. Multilevel multivariable linear mixed models adjusted for relevant confounders (and stratified by disease subtype in case of a relevant interaction) were used.
Results
In total, 65%, 10% and 25% of patients had axSpA, pSpA and PsA, respectively. axSpA was more frequent in males (68%), whereas pSpA and PsA were more frequent in females (53% and 52%, respectively). A significant interaction between gender and disease subtype was found for ASDAS, BASDAI and BASFI. While being female independently contributed to higher BASDAI across the three disease subtypes (with varying magnitude), female gender was only associated with higher ASDAS in pSpA (β (95% CI): 0.36 (0.15 to 0.58)) and PsA (0.25 (0.12 to 0.38)) but not in axSpA (0.016 (−0.07 to 0.11)). No associations were observed between gender and CRP levels. Female gender was associated with higher ASAS HI and EQ-5D, without differences across disease subtype.
Conclusion
Female gender is associated with less favourable outcome measures across the SpA spectrum. However, while female gender influences BASDAI across the three subtypes, ASDAS is associated with gender only in pSpA and PsA but not in axSpA. Therefore, ASDAS is an appropriate instrument both for females and males with axSpA.
Keywords: spondylitis, ankylosing; arthritis, psoriatic; patient reported outcome measures
WHAT IS ALREADY KNOWN ON THIS TOPIC
There is increasing evidence revealing that spondyloarthritis (SpA) manifests differently in females and males.
While gender-related clinical differences in axial SpA (axSpA) have been better described, data are limited and inconsistent in patients with peripheral SpA or psoriatic arthritis.
How gender influences different outcome measures is currently unknown.
WHAT THIS STUDY ADDS
This worldwide study provides information on the frequency of SpA subtypes between males and females, as well as their clinical characteristics.
Female patients consistently reported worse outcomes than male patients across the SpA spectrum in terms of disease activity, functional disability and overall health.
The influence of gender on disease outcomes was not similar among the three disease subtypes.
Female gender influenced Bath Ankylosing Spondylitis Disease Activity Score across all subtypes while Ankylosing Spondylitis Disease Activity Score (ASDAS) was not associated with gender in axSpA.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
These findings may be a stepping-stone for future research aiming to diminish the gap between females and males.
Our findings underline the strengths of the ASDAS for disease activity assessment as it is not influenced by gender in axSpA.
ASDAS should therefore be preferred over other instruments that are more influenced by gender in this disease subtype.
Introduction
Spondyloarthritis (SpA) encompasses different phenotypic presentations, such as axial spondyloarthritis (axSpA), peripheral spondyloarthritis (pSpA) and psoriatic arthritis (PsA), which affect both females and males.1 Understanding gender differences across subtypes is critical to unveil the disease impact in patients over the entire SpA spectrum.
There is a growing body of research revealing that axSpA manifests differently in females and males.2 Compared with males, females with an axSpA diagnosis tend to have more frequently peripheral and extramusculoskeletal manifestations (EMM), such as enthesitis and inflammatory bowel disease (IBD). However, males with axSpA present more radiographic damage and objective signs of inflammation.3 Despite the increasing evidence on gender-related differences in axSpA, most studies highlighting these differences have been performed in one single country and raised some methodological concerns. In addition, scientific evidence on gender-related clinical differences is limited and inconsistent in patients with pSpA or PsA. In pSpA, published data evaluating gender-related differences are scarce.4 A study showed that females with PsA tended to have more frequently polyarthritis as the main joint pattern and to present higher swollen joint count compared with males.5 A more recent study observed that females with PsA had higher symptom duration and body mass index (BMI), and more tender and swollen joint counts, while the articular pattern, and EMM (including uveitis) were quite similar between both genders.6 Despite these differences on clinical presentations, no gender-specific diagnosis or management strategies have been proposed in clinical practice.7 8
Several instruments are available to assess outcomes in patients with SpA. In this regard, previous research has identified a higher burden of disease in females with axSpA as compared with males according to different outcomes.9 Thus, females with axSpA thoroughly report poorer patient-reported outcomes (PROs) for disease activity and functional impairment while males with axSpA usually have more objective signs of inflammation and structural damage.10 In PsA, studies have shown that females had higher disease activity, acute phase reactants and poorer physical activity and fatigue while psoriasis extension and severity were higher in male patients.6 These findings raise the question of whether differences are similar for all the monitoring tools and in all disease subtypes, which may be relevant to select the most appropriate tool to assess patients with SpA.
In this regard, the Assessment of SpondyloArthritis International Society (ASAS)-perSpA study included patients with the three SpA subtypes, and therefore provides a unique opportunity to address research questions concerning gender-related differences across the disease spectrum. Hence, the main aim of this study was to investigate the influence of gender on disease outcomes in patients with SpA including different disease subtypes (axSpA, pSpA and PsA) and whether this influence differed across the SpA subtypes.
Methods
Study design and patients
This analysis includes data from the ASAS-perSpA study, an observational, cross-sectional multicentre and international study, with 24 participating countries (23 actively involved) across four continents (Africa, America, Asia and Europe). Patients diagnosed with axSpA, PsA or pSpA by their rheumatologist were recruited consecutively between July 2018 and February 2020.11 Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Data collection
Data were collected during a dedicated visit at each centre.
Outcome variables
The following disease outcomes were included in the analysis: (1) disease activity, which was assessed by Ankylosing Spondylitis Disease Activity Score (ASDAS)-C-reactive protein (CRP),12 Patient Global Assessment of the disease,13 Bath Ankylosing Spondylitis Disease Activity Score (BASDAI)14 and CRP elevation at any time during the course of the disease; (2) function: Bath Ankylosing Spondylitis Functional Index (BASFI)15; (3) overall health and functioning: ASAS Health Index (ASAS HI),16 European Quality of Life Five Dimension (EQ-5D).17 Further details regarding outcome measures are available in the study main manuscript.11
The main variable of interest (main predictor) was gender.
Other variables of interest that were included in the models and tested as potential confounders were age, educational level, marital status, smoking status, BMI, HLA-B27 carriership, presence of axial involvement (according to the rheumatologist), history of peripheral arthritis or enthesitis, psoriasis, the presence of concomitant fibromyalgia (according to the treating rheumatologist), use of non-steroidal anti-inflammatory drugs during last month, history of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) and biologic disease-modifying antirheumatic drugs (bDMARDs) and current steroids intake.
Statistical analysis
First, descriptive analyses were performed, initially over the entire sample. Then, patients were stratified according to SpA subtype, and the percentage of females for each of these groups was determined. Comparisons between genders for each disease subtype were performed for sociodemographic and clinical characteristics, as well as for disease outcomes. Results were summarised in terms of means and standard deviation (SD) for continuous variables and as frequency and percentage for categorical variables. Comparisons between groups were performed with t-test, Mann-Whitney U test, χ2 test and Fisher’s exact test, as appropriate. In addition, standardised difference scores were calculated. These are intuitive indexes which measure the effect size between two groups. Compared with a t-test or Wilcoxon rank-sum test, they are independent of sample size. Absolute values of effect size of 0.2, 0.5 and 0.8 can be used to represent small, medium and large effect sizes, respectively. A small effect of 0.2 is not so small as to be trivial.18 19
Second, the independent association between gender and each disease outcome was investigated through univariable and multivariable regression models including gender as the main independent variable and the explored outcome as the dependent variable. All models were adjusted for potential confounders, which were selected a priori for each outcome according to previous knowledge. Specifically, multilevel mixed-effects logistic and linear regression models were applied, as suitable, with patients nested according to their country of residence in the analysis. Mixed-effects model analysis allowed to account simultaneously for the within-country and between-country variances, by including specific means for each country of residence (random intercept).20 All variables with a p value <0.2 in the univariable models were included in the multivariable model and retained if significantly contributing to explain the outcome (p<0.05) or if they were confounders of the main relationship of interest. Separate models were created for different disease outcomes to avoid collinearity. In order to assess whether the relationship between gender and disease outcomes varied according to disease subtype, interactions between gender and disease subtype were tested. Interaction terms with p<0.15 indicated a significant effect modification by disease subtype and models were stratified for subtype in this case. Odds ratios (ORs) or regression coefficients were used as measures of association for categorical and continuous outcomes, respectively, together with 95% CIs.
SPSS software V.24.0 was employed for descriptive analyses and Stata SE V.14 for regression analyses.
Results
A total of 4185 patients with SpA study was included, among whom 2719 (65%) had a diagnosis of axSpA, 433 (10%) pSpA and 1033 (25%) PsA. Patients with axSpA had an average age at the study visit of 42 years, patients with pSpA of 44 years and patients with PsA of 52 years. Overall, the proportion of females was lower in axSpA (32%) but slightly higher in pSpA (53%) and PsA (52%). Percentages of females stratified by region are shown in figure 1. In general, female proportion was higher in Middle East and Africa as compared with the rest of the regions. Across disease subtypes, female patients were less frequently current smokers compared with male patients and had a lower alcohol intake. Concomitant fibromyalgia was more frequent in female patients for all subtypes (axSpA 17% vs 3%, pSpA 18% vs 3%, PsA 19% vs 3%). Additionally, no significant differences between genders were found for the use of bDMARDs. A summary of gender-related differences by subtype of SpA is shown in tables 1–3.
Figure 1.
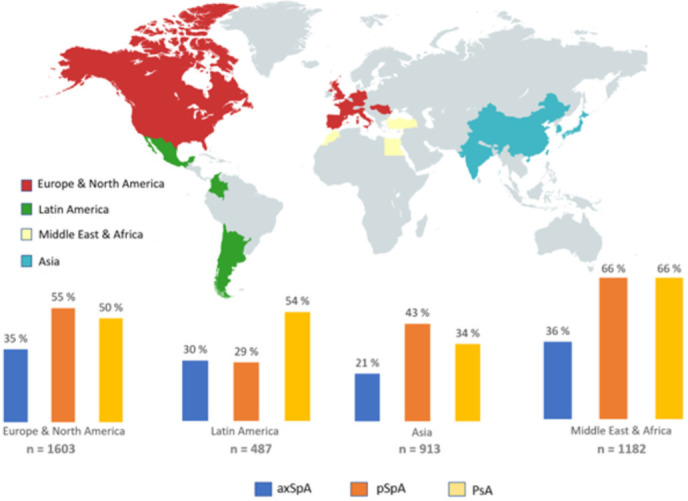
Percentage of females stratified by region and disease subtype in the Assessment of SpondyloArthritis International Society-perSpA study. axSpA, axial spondyloarthritis; pSpA, peripheral spondyloarthritis; PsA, psoriatic arthritis.
Table 1.
Demographic, disease characteristics and treatments of patients with axSpA stratified by gender
| Total axSpA n=2719 | Male n=1858 (68.3) |
Female n=861 (31.7) |
P value | Standardised difference | |
| Age at study visit | 41.9 (13.1) | 41.4 (13.2) | 42.9 (12.6) | <0.01 | −0.116 |
| Smoking habit (ever) | 1185 (43.6) | 910 (49.0) | 275 (31.9) | <0.001 | 0.354 |
| Alcohol (ever) | 1089 (40.1) | 886 (47.7) | 203 (23.6) | <0.001 | 0.52 |
| Body mass index (kg/m2) | 25.9 (5.1) | 25.8 (4.7) | 26.0 (5.8) | 0.56 | −0.038 |
| University education | 1178 (43.4) | 830 (44.7) | 348 (40.4) | 0.04 | 0.087 |
| Family history of SpA | 964 (35.5) | 628 (33.8) | 336 (39.0) | <0.01 | −0.108 |
| Diagnosis delay (years) since the first symptom | 5.8 (7.7) | 5.6 (7.6) | 6.2 (7.9) | 0.08 | −0.077 |
| Symptom duration | 14.4 (11.1) | 14.8 (11.4) | 13.4 (10.4) | <0.01 | 0.128 |
| Axial involvement according to the rheumatologist | 2651 (97.5) | 1816 (97.7) | 835 (97.0) | 0.24 | 0.044 |
| Inflammatory back pain (ASAS criteria) | 2544 (93.6) | 1733 (93.3) | 811 (94.2) | 0.35 | −0.037 |
| Sacroiliitis on imaging. n/N (%) by: | |||||
| xRay mNY criteria | 2042/2645 (75.4) | 1507/1812 (83.2) | 535/833 (64.2) | <0.001 | 0.442 |
| MRI-SIJ, ASAS def | 1469/1783 (82.3) | 950/1146 (82.9) | 519/637 (81.5) | 0.45 | 0.037 |
| mNY criteria or ASAS def | 2499/2694 (92.8) | 1746/1841 (94.8) | 753/853 (88.3) | <0.001 | 0.235 |
| HLA-B27 positive | 1709/2178 (78.8) | 1238/1490 (83.1) | 471/677 (69.6) | <0.001 | 0.322 |
| Elevated CRP (>5 mg/L) | 1902 (70.0) | 1352 (75.4) | 550 (65.6) | <0.001 | 0.216 |
| CRP (mg/L) | 11.7 (26.6) | 12.5 (27.6) | 9.8 (24.2) | 0.01 | 0.104 |
| Peripheral arthritis | 978 (36.0) | 637 (34.3) | 341 (39.6) | <0.01 | −0.11 |
| Enthesitis | 1113 (40.9) | 725 (39.0) | 388 (45.1) | <0.01 | −0.124 |
| Dactylitis | 164 (6.0) | 105 (5.7) | 59 (6.9) | 0.22 | −0.049 |
| Psoriasis | 187 (6.9) | 109 (5.9) | 78 (9.1) | <0.01 | −0.122 |
| IBD | 132 (4.9) | 81 (4.4) | 51 (5.9) | 0.08 | −0.068 |
| Uveitis | 588 (21.6) | 406 (21.9) | 182 (21.1) | 0.67 | 0.019 |
| Concomitant fibromyalgia according to: | |||||
| Treating rheumatologist | 212 (7.8) | 62 (3.3) | 150 (17.4) | <0.001 | −0.476 |
| FiRST questionnaire | 427 (17.2) | 219 (13.1) | 208 (25.8) | <0.001 | −0.325 |
| csDMARD (ever) | 1402 (51.6) | 930 (50.1) | 472 (54.8) | <0.05 | −0.094 |
| bDMARD (ever) | 1613 (59.3) | 1121 (60.3) | 492 (57.1) | 0.12 | 0.065 |
Results are shown as absolute numbers (percentages) or mean (SD). Estimates with p<0.05 are highlighted in bold. Standardised difference scores of 0.2, 0.5 and 0.8 represent small, medium and large effect sizes, respectively.
ASAS, Assessment of SpondyloArthritis International Society; axSpA, axial spondyloarthritis; bDMARD, biological disease-modifying antirheumatic drug; CRP, C reactive protein; csDMARD, conventional synthetic disease-modifying antirheumatic drug; FiRST, Fibromyalgia Rapid Screening Tool; IBD, inflammatory bowel disease.
Table 2.
Demographic, disease characteristics and treatments in patients with pSpA stratified by gender
| Total pSpA n=433 | Male n=203 (46.8) |
Female n=230 (53.2) |
P value | Standardised difference | |
| Age at study visit | 44.1 (14.4) | 41.6 (15.3) | 46.3 (13.1) | <0.01 | −0.33 |
| Smoking habit (ever) | 128 (29.6) | 86 (42.6) | 42 (18.3) | <0.001 | 0.547 |
| Alcohol (ever) | 179 (41.4) | 111 (55.0) | 68 (29.6) | <0.001 | 0.532 |
| Body mass index (kg/m2) | 26.3 (5.4) | 25.9 (5.1) | 26.7 (5.6) | 0.10 | −0.149 |
| University education | 197 (45.5) | 89 (43.8) | 108 (47.0) | 0.52 | −0.064 |
| Family history of SpA | 125 (28.9) | 65 (32.0) | 60 (26.1) | 0.17 | 0.13 |
| Family history of PsO | 63 (15.9) | 31 (16.2) | 32 (15.7) | 0.89 | 0.014 |
| Diagnosis delay (years) since the first symptom | 4.3 (6.6) | 3.8 (6.1) | 4.7 (7.1) | 0.17 | −0.136 |
| Symptom duration | 10.1 (9.5) | 10.5 (10.0) | 9.7 (8.9) | 0.35 | 0.085 |
| Axial involvement according to the rheumatologist | 238 (55.0) | 124 (61.1) | 79 (38.9) | 0.02 | 0.455 |
| Inflammatory back pain (ASAS criteria) | 240 (55.4) | 126 (62.1) | 114 (49.6) | <0.05 | 0.254 |
| Sacroiliitis on imaging, n/N (%) by: | |||||
| xRay mNY criteria | 146/398 (36.7) | 83/191 (43.5) | 63/207 (30.4) | <0.01 | 0.274 |
| MRI-SIJ, ASAS def | 126/282 (44.6) | 66/135 (48.8) | 60/141 (42.6) | 0.29 | 0.125 |
| mNY criteria or ASAS def | 198/407 (48.6) | 101/192 (52.6) | 97/215 (45.1) | 0.14 | 0.15 |
| HLA-B27 positive | 197/319 (62.3) | 116/167 (69.5) | 91/149 (54.4) | <0.001 | 0.315 |
| Elevated CRP (>5 mg/L) | 297 (68.6) | 149 (74.9) | 148 (65.5) | 0.04 | 0.207 |
| CRP (mg/L) | 13.9 (25.4) | 14.9 (26.8) | 13.1 (24.0) | 0.46 | 0.014 |
| Peripheral arthritis | 410 (94.7) | 194 (95.6) | 216 (93.9) | 0.44 | 0.076 |
| Enthesitis | 248 (57.3) | 118 (58.1) | 130 (56.5) | 0.74 | 0.032 |
| Dactylitis | 100 (23.1) | 44 (21.7) | 56 (24.3) | 0.51 | −0.062 |
| Psoriasis | 64 (14.8) | 30 (14.8) | 34 (14.8) | 0.99 | 0 |
| IBD | 25 (5.8) | 7 (3.4) | 18 (7.8) | 0.05 | −0.192 |
| Uveitis | 75 (17.3) | 33 (16.3) | 42 (18.3) | 0.58 | −0.053 |
| Concomitant fibromyalgia according to: | |||||
| Treating rheumatologist | 48 (11.1) | 6 (3.0) | 42 (18.3) | <0.001 | −0.512 |
| FiRST questionnaire | 69 (17.6) | 19 (10.6) | 50 (23.7) | <0.01 | −0.353 |
| csDMARD (ever) | 384 (88.7) | 177 (87.2) | 207 (90.0) | 0.36 | −0.088 |
| bDMARD (ever) | 223 (51.5) | 107 (52.7) | 116 (50.4) | 0.64 | 0.046 |
Results are shown as absolute numbers (percentages) or mean (SD). Estimates with p<0.05 are highlighted in bold. Standardised difference scores of 0.2, 0.5 and 0.8 represent small, medium and large effect sizes, respectively.
ASAS, Assessment of SpondyloArthritis International Society; bDMARD, biological disease-modifying antirheumatic drug; CRP, C reactive protein; csDMARD, conventional synthetic disease-modifying antirheumatic drug; FiRST, Fibromyalgia Rapid Screening Tool; IBD, inflammatory bowel disease; PsO, psoriasis; pSpA, peripheral spondyloarthritis.
Table 3.
Demographic, disease characteristics and treatments in patients with PsA stratified by gender
| Total PsA n=1033 | Male n=501 (48.5) |
Female n=532 (51.5) |
P value | Standardised difference | |
| Age at study visit | 51.8 (13.0) | 52.3 (13.3) | 51.3 (12.7) | <0.05 | 0.077 |
| Smoking habit (ever) | 494 (47.9) | 278 (55.6) | 216 (40.6) | <0.001 | 0.304 |
| Alcohol (ever) | 451 (43.7) | 315 (63.0) | 136 (25.6) | <0.001 | 0.813 |
| Body mass index (kg/m2) | 28.0 (5.9) | 27.3 (4.6) | 28.7 (6.8) | <0.001 | −0.241 |
| University education | 320 (31.0) | 177 (35.4) | 143 (26.9) | <0.01 | 0.184 |
| Family history of SpA | 375 (36.3) | 159 (31.7) | 216 (40.6) | <0.01 | −0.186 |
| Family history of PsO | 341 (36.1) | 142 (30.7) | 199 (41.2) | <0.01 | −0.22 |
| Diagnosis delay (years) since the first symptom | 9.1 (11.1) | 9.1 (11.0) | 9.0 (11.2) | 0.87 | 0.009 |
| Symptom duration | 16.8 (12.3) | 17.3 (12.1) | 16.2 (12.4) | 0.15 | 0.09 |
| Axial involvement according to the rheumatologist | 367 (35.5) | 196 (39.1) | 171 (32.1) | 0.02 | 0.147 |
| Inflammatory back pain (ASAS criteria) | 366 (35.4) | 188 (37.5) | 178 (33.5) | 0.30 | 0.084 |
| Sacroiliitis on imaging, n/N (%) by: | |||||
| xRay mNY criteria | 212/855 (24.8) | 115/417 (27.6) | 97/438 (22.1) | 0.07 | 0.128 |
| MRI-SIJ, ASAS def | 150/589 (25.5) | 72/294 (24.5) | 78/295 (26.4) | 0.59 | −0.044 |
| mNY criteria or ASAS def | 273/877 (31.1) | 141/431 (32.7) | 132/446 (29.6) | 0.34 | 0.067 |
| HLA-B27 positive | 86/474 (18.1) | 49/234 (20.9) | 37/240 (15.4) | 0.26 | 0.143 |
| Elevated CRP (>5 mg/L) | 584 (58.8) | 281 (58.5) | 303 (59.1) | 0.87 | −0.012 |
| CRP (mg/L) | 11.4 (28.6) | 11.7 (33.3) | 11.2 (23.4) | 0.81 | 0.017 |
| Peripheral arthritis | 938 (90.8) | 456 (91.0) | 482 (90.6) | 0.82 | 0.014 |
| Enthesitis | 473 (45.8) | 212 (42.3) | 261 (49.1) | <0.05 | −0.137 |
| Dactylitis | 382 (37.0) | 190 (37.9) | 192 (36.1) | 0.54 | 0.037 |
| Psoriasis | 946 (91.6) | 466 (93.0) | 480 (90.2) | 0.11 | 0.101 |
| IBD | 6 (0.6) | 3 (0.6) | 3 (0.6) | 0.94 | 0 |
| Uveitis | 27 (2.6) | 11 (2.2) | 16 (3.0) | 0.41 | −0.05 |
| Fibromyalgia according to: | |||||
| Treating rheumatologist | 120 (11.6) | 17 (3.4) | 103 (19.4) | <0.001 | −0.52 |
| FiRST questionnaire | 245 (24.9) | 73 (15.1) | 172 (34.4) | <0.001 | −0.459 |
| csDMARD (ever) | 959 (92.8) | 465 (92.8) | 494 (92.9) | 0.98 | −0.004 |
| bDMARD (ever) | 668 (64.7) | 331 (66.1) | 337 (63.3) | 0.36 | 0.059 |
Results are shown as absolute numbers (percentages). Estimates with p<0.05 are highlighted in bold. Standardised difference scores of 0.2, 0.5 and 0.8 represent small, medium and large effect sizes, respectively.
ASAS, Assessment of SpondyloArthritis International Society; bDMARD, biological disease-modifying antirheumatic drug; CRP, C reactive protein; csDMARD, conventional synthetic disease-modifying antirheumatic drug; FiRST, Fibromyalgia Rapid Screening Tool; IBD, inflammatory bowel disease; PsA, psoriatic arthritis; PsO, psoriasis.
Sociodemographic, disease characteristics and treatments of the 2719 patients with axSpA are shown in table 1. In the population with axSpA, 861 (32%) patients were female. The two groups differed in relevant characteristics. Female patients were older, had less frequently university education and more frequently family history of SpA as compared with males. With regard to disease characteristics, while female patients presented shorter disease duration, male patients presented more HLA-B27 positivity. Female patients had more frequently peripheral arthritis, enthesitis and psoriasis, also using more csDMARDs than male patients.
Regarding pSpA, among the 433 patients, 230 (53%) were female. The characteristics of these patients are shown in table 2. Of note, females with pSpA were older at the time of the study visit compared with males. There were no differences between genders for most disease characteristics, including family history of SpA, diagnostic delay since the first symptoms, dactylitis, IBD and uveitis; however, while males had more inflammatory back pain and were more HLA-B27 positive, females had more fibromyalgia.
Of the 1033 patients with PsA, 532 (51.5%) were female (table 3). Females with PsA had younger age at the time of the study visit, a higher BMI and less university education, compared with males. Family history of SpA was more frequent among females. No significant differences were found for most of the disease characteristics, although enthesitis and fibromyalgia were more frequent in females.
Assessment of the impact of gender on outcome measures
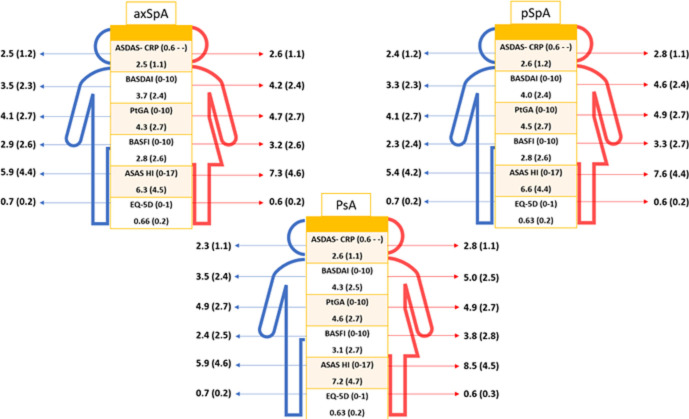
Overall, females presented worse outcomes across the whole disease spectrum (figure 2), including across components of indices such as BASDAI (online supplemental table S1). Female patients with axSpA reported significantly higher disease activity, more functional disability and worse overall health than male patients. Conversely, females presented less often elevated CRP (65.6% vs 75.4%, p<0.001). Similarly, females with pSpA also reported higher disease activity and more functional disability, and worse overall health than males, while they presented less often elevated CRP (74.9% vs 65.5%, p<0.001). Similarly, females with PsA reported worse outcomes, except that there were no differences in the percentage of elevated CRP in patients with PsA between genders (59.1% of females vs 58.5% of males, p=0.87).
Figure 2.
Disease outcomes in patients with axial spondyloarthritis (axSpA), peripheral spondyloarthritis (pSpA) and psoriatic arthritis (PsA) stratified by gender. ASDAS, Ankylosing Spondylitis Disease Activity Score; BASDAI, Bath Ankylosing Spondylitis Disease Activity Score; BASFI, Bath Ankylosing Spondylitis Functional Index; CRP, C reactive protein; EQ-5D, European Quality of Life Five Dimension; HI, Health Index; PtGA, Patient Global Assessment of the disease.
rmdopen-2022-002514supp001.pdf (158.5KB, pdf)
Multivariable models for each outcome, stratified by disease subtype, where appropriate, are shown in table 4. A significant interaction between gender and disease subtype was found for ASDAS, BASDAI and BASFI. Being female independently contributed to a higher BASDAI across the three disease subtypes with varying magnitude. Hence, in patients with axSpA, being female contributed to an increase of an average of 0.39 units (95% CI: 0.20 to 0.58) in BASDAI; in patients with pSpA 1.22 (95% CI: 0.77 to 1.69) units and in patients with PsA 0.88 (95% CI: 0.59 to 1.16) units (complete model in online supplemental table S2). However, female gender was only associated with higher ASDAS in pSpA (β (95% CI): 0.36 (0.15 to 0.58)) and PsA (0.25 (0.12 to 0.38)) but not in axSpA (0.016 (−0.07 to 0.11)) (online supplemental table S3). In addition, female gender was likewise associated with higher BASFI in PsA (0.46 (0.20 to 0.72)) (online supplemental table S4). No associations were observed between gender and CRP levels. Finally, female gender was associated with higher ASAS HI (0.90 (0.70 to 1.10)) and EQ-5D (−0.02 (−0.03 to –0.01)), without significant differences across disease subtypes (online supplemental table S5).
Table 4.
Multivariable multilevel regression models for the association between gender and disease outcomes (stratified by disease subtype in case of interaction)
| Disease subtype | Determinant of interest | Outcome | |||||
| ASDAS*(0.6–) | BASDAI† (0–10) | BASFI‡ (0–10) | CRP§ (0–) | ASAS HI¶ (0–17) | EQ-5D** (−0.654–1) | ||
| axSpA | Gender (female vs male) |
0.02 (−0.07 to 0.11) | 0.39 (0.20 to 0.58) | 0.01 (−0.14 to 0.17) | −1.36 (−3.17 to 0.44) | 0.90 (0.70 to 1.10) | −0.02 (−0.03 to –0.01) |
| pSpA | 0.36 (0.15 to 0.58) | 1.22 (0.77 to 1.69) | 0.30 (−0.12 to 0.71) | ||||
| PsA | 0.25 (0.12 to 0.38) | 0.88 (0.59 to 1.16) | 0.46 (0.20 to 0.72) | ||||
All models are adjusted by age, gender and education. Each cell represents the results of one model. All results are expressed in β (95% CI). Estimates with p<0.05 are highlighted in bold. Full models are presented in the online supplemental material.
*Also adjusted for marital status, BMI, smoking, axial involvement, peripheral arthritis, enthesitis, fibromyalgia, NSAIDs, steroids, csDMARDs, bDMARDs.
†Also adjusted for marital status, BMI, smoking, axial involvement, peripheral arthritis, enthesitis, psoriasis, fibromyalgia, NSAIDs, bDMARDs.
‡Also adjusted for marital status, BMI, ASDAS, radiographic damage, fibromyalgia, NSAIDs, bDMARDs.
§Also adjusted for marital status, BMI, radiographic damage, concomitant NSAIDs, steroids, csDMARDs.
¶Also adjusted for smoking, ASDAS, BASFI, peripheral arthritis, enthesitis, fibromyalgia.
**Also adjusted for BMI, smoking, ASDAS, BASFI, radiographic damage, HLA-B27, enthesitis, fibromyalgia.
ASAS HI, Assessment of SpondyloArthritis International Society Health Index; ASDAS, Ankylosing Spondylitis Disease Activity Score; axSpA, axial spondyloarthritis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; bDMARDs, biologic disease-modifying antirheumatic drugs; BMI, body mass index; CRP, C reactive protein; csDMARDs, conventional synthetic disease-modifying antirheumatic drugs; EQ-5D, European Quality of Life Five Dimension; NSAIDs, non-steroidal anti-inflammatory drugs; PsA, psoriatic arthritis; pSpA, peripheral spondyloarthritis.
Discussion
This study investigates differences between genders across the SpA spectrum within the same cohort, especially related to disease outcomes. First, it provides an overall distribution of gender across SpA subtypes, namely axSpA, pSpA and PsA. While sociodemographic and clinical differences between genders varied across the disease spectrum, female patients consistently reported worse outcomes. More specifically, females with axSpA, pSpA and PsA reported higher disease activity, functional limitation and worse overall health than males. However, the influence of gender on disease outcomes was heterogeneous among the three disease subtypes. In this respect, female gender influenced BASDAI across all subtypes whereas ASDAS was not influenced by gender in axSpA.
Gender distribution in our study, where patients were consecutively recruited, shows that axSpA is more frequent in males whereas the proportion of female gender is higher in pSpA and PsA. Concerning other sociodemographic and disease characteristics in patients with axSpA, most results are in accordance with the current literature.21 Thus, female with axSpA were older, presented less HLA-B27 positivity, had more peripheral manifestations and more fibromyalgia, which is aligned with previous studies.10 22 On the other hand, while a recent meta-analysis showed that the diagnostic delay in axSpA is longer for females compared with males (8.8 vs 6.5 years),23–25 our analysis only observed a short difference (6.2 vs 5.6 years), which was not statistically significant. Relating to pSpA and gender differences, previous data were very scarce, and limited to non-adjusted comparisons.4 26 The evaluation of gender differences in PsA is more difficult than in axSpA due to the heterogeneity of the disease, with different articular patterns.27 The main accepted difference across gender in PsA concerns axial involvement, observed generally more frequently in males with PsA.6 28 Nevertheless, in previous studies, comparisons using gender as the stratifying variable were established throughout univariate analysis, which hinders the interpretation of disease characteristics within each subtype.
Our analysis shows that females consistently report worse PROs across the SpA spectrum as compared with males. In this sense, evidence in axSpA has shown that the burden of disease is higher for females than for males, with more affected disease activity and PRO measures.10 29 Similar to patients with axSpA, females in our study reported consistently worse outcomes for all the measures in pSpA and PsA. To our knowledge, this had not been previously assessed in adjusted analyses through the entire SpA spectrum. Moreover, even unadjusted comparisons, which may be subject to bias and therefore be misleading, are very scarce in patients with pSpA. In the ESPeranza study, in which female patients showed higher rates of functional limitation, while a more recent publication on the real-world Spondyloarthritis Italian Registry: Evidence from a National Pathway (SIRENA) study showed that female patients presented higher disease activity, and worse PROs compared with male patients.4 30 Regarding PsA, a recent study showed gender differences in the clinical burden, with more affected disease in females regarding disease activity, pain and functional capacity.28 In the same line, another study reported that females seem to be more affected in daily activities and report higher disability and fatigue scores.28 These remarkable differences in PROs are aligned with the ones reported in our analysis; hence, these insights might serve as a starting point for considering future research and subsequent actions aiming to diminish the gap between females and males, such as an adjustment in measures to promote balanced healthcare.
As a potential explanation of worse outcomes in females, it has been argued that the presence of concomitant fibromyalgia in females with axSpA may bias PROs towards worse outcomes.31 Notwithstanding, our models were adjusted by concomitant fibromyalgia, leading to findings that are independent of presenting the condition. Some contributing factors may affect differently to gender and could contribute to explaining the gender differences observed in outcomes, as it has been suggested with central obesity.32 Interestingly, there is a vast amount of literature that shows that females report higher levels of pain, regardless of the type of pain.33 34 Several differences between females and males may explain this divergence in pain experience; although there is evidence pointing that this may be neurologically or hormonally driven, it is also plausible that a social factor plays a role in differential experience of pain.35 36 In this sense, a different pattern of social norms has been described that distinguished female and male perceptions and coping with pain, in line with a stereotype of masculine behaviour. Hence, while female gender has been presented as more sensitive to pain and more likely report it, male gender has been portrayed as stoic and more reluctant to express vulnerability or pain.37 38 Moreover, previous data suggested that gender-related treatment inequities (leading to females having less access to biologics) might explain higher disease activity in some regions.39
To get a better insight on how gender influences disease outcomes in each disease subtype, the usage of multilevel multivariable analyses in our study is an important strength. Thus, our analysis takes relevant confounders into consideration as well as nest patients within their country of residence, which is an important explanatory factor of the outcomes in a worldwide setting. Obtained effect of gender on outcomes is thus independent of confounders. As reported, being female contributed to higher BASDAI across all disease subtypes whereas it was only associated with higher ASDAS in pSpA and PsA but not in axSpA. This has important consequences for the management of disease activity in SpA. First, ASDAS is nowadays the recommended instrument for measuring disease activity in axSpA, as this has shown better psychometric properties compared with BASDAI.12 Our analysis shows that ASDAS is not affected by gender, and this holds true despite a higher proportion of female patients with fibromyalgia. Therefore, it can be concluded that ASDAS provides a reliable and valid assessment both for males and females with axSpA. Second, this finding highlights the need for improvement of instruments to assess predominantly peripheral disease, in which all current measurement tools investigated in this study are affected by gender. In this sense, more research is needed to understand why females report poorer outcomes as compared with males.
This study presents some potential limitations. First, most of the instruments used to assess different disease domains (eg, disease activity) have been validated in axSpA but not in pSpA or PsA. Besides, other validated instruments for PsA, such as Psoriatic Arthritis Impact of Disease were not used. However, given the similarities across disease spectrum and the limitation on the assessment of other specific instruments for these subtypes, we consider these tools may add information for this research purpose and acknowledge that other instruments may be more appropriate to assess certain outcomes in pSpA and PsA. In fact, they have been used and validated to some extent in pSpA and PsA studies.40–43 It would have been relevant to assess treatment effectiveness; in this sense, although there is some evidence that female patients with axSpA who receive bDMARD therapy have shown reduced rates of therapeutic response as compared with male patients, there is a need for studies in this regard.44 45 However, the cross-sectional design of this study does not allow to assess properly treatment effectiveness and the use of outcomes for this purpose. Another limitation was that data to identify the gender of the included patients were reported as either female or male, and therefore transgender population was not specified. Of note, diagnosis was established by the disease that better described the patient predominant symptoms at the study visit according to the rheumatologist, while some of the disease characteristics referred to the presence of current or past symptoms. This may have overestimated the presence of some symptoms such as inflammatory back pain. Moreover, most centres were tertiary institutions with ASAS members specialised in SpA. This may have led to a selection bias, with a meticulous management of patients with SpA and a higher prevalence of axSpA and pSpA as compared with PsA that might not be generalisable. As patients were recruited consecutively, people may extrapolate these results as the actual prevalence of the disease subtypes, which is not accurate. On the other hand, such a large sample of patients with SpA including the most relevant disease subtypes from a multinational setting is undoubtedly robust evidence that helps understand gender-related differences in the whole spectrum of SpA.
To summarise, there are several relevant gender-related differences in disease outcomes across SpA subtypes. Female gender was associated with less favourable outcomes across the SpA spectrum, except for CRP in which there were no differences between gender. While female gender influenced BASDAI across the three disease subtypes, ASDAS was not associated with gender in axSpA. These results demonstrate that ASDAS is an appropriate instrument in clinical practice both for females and males with axSpA and therefore should be preferred over other instruments, which are affected by gender. Further research that evaluates whether gender-specific management can improve outcomes will help to promote balanced healthcare while heading towards precision-based decision-making in rheumatology.
Acknowledgments
This study was conducted under the umbrella of the International Society for Spondyloarthritis Assessment (ASAS). We would like to thank all the patients and investigators who participated in this research. We would like to thank all the collaborators who participated in the study: José Maldonado-Cocco (Buenos Aires University School of Medicine, Buenos Aires, Argentina), Hernán Maldonado Ficco (Hospital San Antonio de Padua, Rio Cuarto, Argentina), Rodolfo Pérez Alamino (Hospital Dr Nicolás Avellaneda, Tucumán, Argentina), Emilio Buschiazzo (Hospital Señor del Milagro, Salta, Argentina), Romina Calvo (Hospital Provincial Dr José M Cullen, Santa Fé, Aregntina), Vanesa Duarte (Clínica Monte Grande, Buenos Aires, Argentina), Maria Victoria Martire (Instituto Médico Platense, La Plata, Argentina), Diego Baenas (Hospital Privado de Córdoba, Córdoba, Argentina), Dora Pereira (Hospital Ricardo Gutiérrez, La Plata, Argentina), Adrian Salas (Consultorio Reumatológico, La Plata, Argentina), Juan Manuel Bande (Hospital General de Agudos Dr E Tornú, Buenos Aires, Argentina), Alberto Berman (Centro Médico Privado de Tucumán, Tucumán, Argentina), Walter P Maksymowych (University of Alberta, Canada), Stephanie Belton (University of Alberta, Canada), Sebastián Ibáñez (Facultad de Medicina Clínica Alemana—Universdidad del Desarrollo, Santiago de Chile, Chile), María Paz Poblete (Facultad de Medicina Clínica Alemana—Universidad del Desarrollo, Santiago de Chile, Chile), Francisca Valenzuela (Facultad de Medicina Clínica Alemana—Universidad del Desarrollo, Santiago de Chile, Chile), Wilson Bautista-Molano (University Hospital Fundación Santa Fé de Bogotá, Bogotá, Colombia), Jieruo Gu (Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China), Min Xiao (Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China), CS Lau (Hong-Kong University, China), Ho Yin Chung (Hong-Kong University, China), Bassel Elzorkany (Cairo University, Cairo, Egypt), Sherif Gamal (Cairo University, Cairo, Egypt), Catherine Lebourlout (Cochin Hospital, Paris, France), Daniel Wendling (CHU Besançon, Besançon, France), Clément Prati (CHU Besançon, Besançon, France), Frank Verhoeven (CHU Besançon, Besançon, France), Martin Soubrier (CHU Clermont-Ferrand, Clermont-Ferrand, France), Carine Savel (CHU Clermont-Ferrand, Clermont-Ferrand, France), Trigui Alia (CHU Clermont-Ferrand, Clermont-Ferrand, France), Fan Angélique (CHU Clermont-Ferrand, Clermont-Ferrand, France), Pascal Claudepierre (Henri Mondor Hospital, Créteil, France), Valerie Farrenq (Henri Mondor Hospital, Créteil, France), Kamelia Faramarz (Henri Mondor Hospital, Créteil, France), Uta Kiltz (Rheumazentrum Ruhrgebiet, Herne, Germany), Isabella Sieber (Rheumazentrum Ruhrgebiet, Herne, Germany), Dories Morzeck (Rheumazentrum Ruhrgebiet, Herne, Germany), Fabian Proft (Charité University, Berlin, Germany), Pál Geher (Semmelweis University, Budapest, Hungary), Edit Toth (Flór Ferenc Hospital, Kistarcsa, Hungary), Katalin Nagy (Markhot Ferenc Hospital, Eger, Hungary), Attila Kovacs (MÁV Hospital, Szolnok, Hungary), Meghna Gavali (Nizam’s Institute of Medical Sciences, Hyderabad, India), Liza Rajasekhar (Nizam’s Institute of Medical Sciences, Hyderabad, India), Sapan Pandya (Smt NHL Medical College and Sardar Vallabhbhai Patel Hospital and Vedanta Institute of Medical Sciences, Ahmedabad, India), Bhowmik Meghnathi (Sri Sai Siri Hospital and Prathima Institue of Medical Sciences, Karimnagar, India), Carlomaurizio Montecucco (Fondazione IRCCS Policlinico San Matteo, Pavia, Italia), Sara Monti (Fondazione IRCCS Policlinico San Matteo, Pavia, Italia), Alessandro Biglia (Fondazione IRCCS Policlinico San Matteo, Pavia, Italia), Mitsumasa Kishimoto (Kyorin University School of Medicine, Tokyo, Japan), Akihiko Asahina (The Jikei University School of Medicine, Japan), Masato Okada (St Luke’s International University and Hospital, Japan), Tadashi Okano (Osaka City University, Japan), Yuko Kaneko (Keio University School of Medicine, Japan), Hideto Kameda (Toho University, Japan), Yoshinori Taniguchi (Kochi University, Japan), Naoto Tamura (Juntendo University School of Medicine, Japan), Shigeyoshi Tsuji (National Hospital Organization Osaka Minami Medical Center, Japan), Hiroaki Dobashi (Kagawa University Faculty of Medicine, Japan), Yoichiro Haji (Daido Hospital, Japan), Akimichi Morita (Nagoya City University, Japan), Nelly Ziade (Saint-Joseph University, Beirut, Lebanon), Nelly Salloum (Saint-Joseph University, Beirut, Lebanon), Rubén Burgos-Vargas (Hospital General de México Eduardo Liceaga, Mexico City, Mexico), Graciela Meza (CLIDITER), Julio Casasola-Vargas (Hospital General de Mexico, Mexico), César Pacheco-Tena (Hospital General Dr Salvador Zubirán, Chihuahua, Mexico), Greta Reyes-Cordero (Hospital General Dr Salvador Zubirán, Chihuahua, Mexico), César Ramos-Remus (Unidad de Investigación de Enfermedades Crónico Degenerativas, Jalisco, Mexico), J Dionisio Castillo (Unidad de Investigación de Enfermedades Crónico Degenerativas, Jalisco, Mexico), Laura González-López (Universidad de Guadalajara, Jalisco, Mexico), Iván Gámez-Nava (Unidad de Investigación Biomédica 02, Hospital de Especialidades, Centro Médico Nacional de Occidente, IMSS Guadalajara, Jalisco, Mexico), Najia Hajjaj-Hassouni (International University of Rabat (UIR), Rabat, Morocco), Fadoua Allali (University Mohammed V, CHU Ibn Sina, Rabat, Morocco), Hanan Rkain (University Mohammed V, CHU Ibn Sina, Rabat, Morocco), Lahcen Achemlal (University Mohammed V, CHU Ibn Sina, Rabat, Morocco), Taoufik Harzy (University Sidi Mohammed Benabdellah, CHU Hassan II, Fès, Morocco), Fernando M Pimentel-Santos (Universidade NOVA de Lisboa, Lisboa, Portugal), Santiago Rodrigues-Manica (Universidade NOVA de Lisboa, Portugal), Agna Neto (Universidade NOVA de Lisboa, Portugal), Jose Marona (Universidade NOVA de Lisboa, Portugal), Mª Joao Gonçalves (Universidade NOVA de Lisboa, Portugal), Ana Filipa Mourao (Universidade NOVA de Lisboa, Portugal), Rita Pinheiro Torres (Universidade NOVA de Lisboa, Portugal), Ruxandra Schiotis (Iuliu Hatieganu University of Medicine, Cluj-Napoca, Romania), Simona Rednic (Iuliu Hatieganu University of Medicine, Cluj-Napoca, Romania), Siao-Pin Simon (Iuliu Hatieganu University of Medicine, Cluj-Napoca, Romania), Laura Muntean (Iuliu Hatieganu University of Medicine, Cluj-Napoca, Romania), Ileana Filipescu (Iuliu Hatieganu University of Medicine, Cluj-Napoca, Romania), Maria Tamas (Iuliu Hatieganu University of Medicine, Cluj-Napoca, Romania), Laura Damian (Iuliu Hatieganu University of Medicine, Cluj-Napoca, Romania), Ioana Felea (Iuliu Hatieganu University of Medicine, Cluj-Napoca, Romania), Dana Fodor (Second Medical Clinic, Emergency Conty Hospital, Cluj-Napoca, Romania), Tae-Jong Kim (Chonnam National University Medical School and Hospital, South Korea), Hyun-Yi Kook (Chonnam National University Medical School and Hospital, South Korea), Hyun-Ju Jung (Chonnam National University Medical School and Hospital, South Korea), Tae-Hwan Kim (Hanyang University Hospital for Rheumatic Diseases, South Korea), Mireia Moreno (Hospital Parc Taulí, Barcelona, Spain), Eduardo Collantes-Estévez (Hospital Universitario Reina Sofía de Córdoba, Spain), M Carmen Castro-Villegas (Hospital Universitario Reina Sofía, Córdoba, Spain), Cristina Fernández-Carballido (Hospital Universitario San Juan de Alicante, Alicante, Spain), Elizabeth Fernández (Hospital Universtario La Paz, Madrid, Spain), Marta Arévalo (Hospital Parc Taulí, Barcelona, Spain), Shue-Fen Luo (Chang Gung Memorial Hospital-Linkou, Taoyuan, Taiwan), Yeong-Jian Jan Wu (Chang Gung Memorial Hospital at Kee-Lung, Taiwan), Tian-Tsai Cheng (Chang Gung Memorial Hospital at Kao-Hsiung, Taiwan), Cheng-Chung Wei (Chung Sun Medical University, Taiwan), Tuncay Duruöz (Marmara University School of Medicine, Istanbul, Turkey), Servet Akar (Izmir Katip Çelebi University School of Medicine, Turkey), Ilhan Sezer (Akdeniz University School of Medicine, Turkey), Umut Kalyoncu (Hacettepe University School of Medicine, Turkey), Sebnem Ataman (Ankara University School of Medicine, Turkey), Meltem Alkan Melikoglu (Erzurum Atatürk University School of Medicine, Turkey), Sami Hizmetli (Sivas Cumhuriyet University School of Medine, Turkey), Ozgur Akgul (Manisa Celal Bayar University School of Medicine, Turkey), Nilay Sahin (Balikesir University School of Medicine, Turkey), Erhan Capkin (Karadeniz Teknik University School of Medicine, Turkey), Fatima Gluçin Ural (Ankara Yildirim Beyazit University School of Medicine, Turkey), Figen Yilmaz (Istanbul Sisli Etfal Training and Research Hospital), Ilknur Aktas (Istanbul Fatih Sultan Mehmet Training and Research Hospital, Turkey), Floris van Gaalen (Leiden University Medical Center, The Netherlands), Anne Boel (Leiden University Medical Center, The Netherlands), Mirian Starmans-Kool (Zuyderland Medical Center, The Netherlands), Femke Hoekstra-Drost (Zuyderland Medical Center, The Netherlands), Maha Abdelkadir (Maasstad Hospital in Rotterdam, The Netherlands), Angelique Weel (Maasstad Hospital in Rotterdam, The Netherlands), Pedro M Machado (University College of London, London, UK), Marina Magrey (Cases Western Reserve University School of Medicine, Cleveland, Ohio, USA), Darerian Schueller (Cases Western Reserve University School of Medicine, Cleveland, Ohio, USA). Steering committee: Joaquim Sieper (Charité University, Berlin, Germany), Desirée van der Heijde (Leiden University Medical Center, The Netherlands), Robert Landewé (Zuyderland Medical Center, The Netherlands). The present work was presented as a poster at EULAR 2022 Congress: D Benavent, D Capelusnik, S Ramiro, A Moltó, C López-Medina, M Dougados, V Navarro-Compán. POS0972 Most Disease Outcome Measures but Not ASDAS are Influenced by Gender in Patients with Axial SpA: Results From ASAS-perSpA. EULAR 2022 Congress.
Footnotes
Contributors: DB: statistical analysis, presentation of the data, discussion and interpretation of the findings, writing original draft, guarantor. DC: statistical analysis, presentation of the data, discussion and interpretation of the findings, manuscript revision. SR: study idea, design and set up, methodology, discussion and interpretation of the findings, manuscript revision. AM: study idea, design and set up, methodology, data collection, discussion and interpretation of the findings, manuscript revision. CL-M: study design and set up, data collection, manuscript revision. MD: methodology, manuscript revision. VN-C: study idea, design and set up, methodology, presentation of the data, discussion and interpretation of the findings, manuscript revision, supervision.
Funding: No funding was provided for the current analysis. The ASAS-perSpA study has been conducted under the umbrella of ASAS thanks to unrestricted grants from Pfizer, Lilly, AbbVie, Novartis, UCB, Janssen and Merck.
Disclaimer: The funders did not have any role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript and decision to submit the manuscript for publication.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Competing interests: DB: speakers bureau: Janssen. Grant/Research support from: Novartis. DC: speakers bureau: Bristol Myers Squibb, Pfizer. Grant/Research support from: Pfizer. SR: speakers bureau: Eli Lilly, MSD, Novartis, Pfizer, UCB. Consultant of: AbbVie, Eli Lilly, MSD, Novartis, Pfizer, UCB, Sanofi. Grant/Research support from: AbbVie, Galapagos, Novartis, MSD, Pfizer, UCB. AM: consultant of: AbbVie, UCB, Novartis, Gilead, Pfizer, Lilly and Janssen. Grant/Research support from: UCB. CL-M: speakers bureau: Lilly, Novartis, Janssen, UCB and AbbVie. MD: none declared. VN-C: speakers bureau: AbbVie, Eli Lilly, Janssen, Galapagos, Moonlake, Novartis, Pfizer, UCB Pharma. Consultant of: AbbVie, Eli Lilly, Galapagos, Moonlake, Novartis, Pfizer, UCB Pharma. Grant/Research support from: Novartis.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Data are available on reasonable request. Researchers willing to use data collected during the study should contact the first author, who will send a study proposal template to be completed by the applicant. Thereafter, the steering committee of the ASAS-perSpA study will approve (or not) the proposal and proceed to the data sharing.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
The local ethics committees approved the ASAS-perSpA study protocol. The reference committee was ‘Comitée de Protection des Personnes—Ile de France III’ (code 3584-NI). All participants provided written informed consent.
References
- 1.Navarro-Compán V, Sepriano A, El-Zorkany B, et al. Axial spondyloarthritis. Ann Rheum Dis 2021;80:1511–21. 10.1136/annrheumdis-2021-221035 [DOI] [PubMed] [Google Scholar]
- 2.Rusman T, van Vollenhoven RF, van der Horst-Bruinsma IE. Gender differences in axial spondyloarthritis: women are not so lucky. Curr Rheumatol Rep 2018;20:35. 10.1007/s11926-018-0744-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webers C, Essers I, Ramiro S, et al. Gender-attributable differences in outcome of ankylosing spondylitis: long-term results from the outcome in ankylosing spondylitis International study. Rheumatology 2016;55:419–28. 10.1093/rheumatology/kev340 [DOI] [PubMed] [Google Scholar]
- 4.Chiu C, Shirley W, Lau CS. AB0852 Gender differences in axial and peripheral spondyloarthritis: results from the esperanza cohort. Ann Rheum Dis 2018;77:1554. 10.1136/annrheumdis-2018-eular.3992 [DOI] [Google Scholar]
- 5.Queiro R, Tejón P, Coto P, et al. Clinical differences between men and women with psoriatic arthritis: relevance of the analysis of genes and polymorphisms in the major histocompatibility complex region and of the age at onset of psoriasis. Clin Dev Immunol 2013;2013:1–7. 10.1155/2013/482691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nas K, Capkin E, Dagli AZ, et al. Gender specific differences in patients with psoriatic arthritis. Mod Rheumatol 2017;27:345–9. 10.1080/14397595.2016.1193105 [DOI] [PubMed] [Google Scholar]
- 7.van den Berg R, de Hooge M, van Gaalen F, et al. Percentage of patients with spondyloarthritis in patients referred because of chronic back pain and performance of classification criteria: experience from the spondyloarthritis caught early (space) cohort. Rheumatology 2013;52:1492–9. 10.1093/rheumatology/ket164 [DOI] [PubMed] [Google Scholar]
- 8.Ortolan A, van Lunteren M, Ramiro S, et al. Are gender-specific approaches needed in diagnosing early axial spondyloarthritis? data from the spondyloarthritis caught early cohort. Arthritis Res Ther 2018;20:218. 10.1186/s13075-018-1705-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tournadre A, Pereira B, Lhoste A, et al. Differences between women and men with recent-onset axial spondyloarthritis: results from a prospective multicenter French cohort. Arthritis Care Res 2013;65:1482–9. 10.1002/acr.22001 [DOI] [PubMed] [Google Scholar]
- 10.Wright GC, Kaine J, Deodhar A. Understanding differences between men and women with axial spondyloarthritis. Semin Arthritis Rheum 2020;50:687–94. 10.1016/j.semarthrit.2020.05.005 [DOI] [PubMed] [Google Scholar]
- 11.López-Medina C, Molto A, Sieper J, et al. Prevalence and distribution of peripheral musculoskeletal manifestations in spondyloarthritis including psoriatic arthritis: results of the worldwide, cross-sectional ASAS-PerSpA study. RMD Open 2021;7:e001450. 10.1136/rmdopen-2020-001450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Heijde D, Lie E, Kvien TK, et al. ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis 2009;68:1811–8. 10.1136/ard.2008.100826 [DOI] [PubMed] [Google Scholar]
- 13.Scott PJ, Huskisson EC. Measurement of functional capacity with visual analogue scales. Rheumatol Rehabil 1977;16:257–9. 10.1093/rheumatology/16.4.257 [DOI] [PubMed] [Google Scholar]
- 14.Garrett S, Jenkinson T, Kennedy LG, et al. A new approach to defining disease status in ankylosing spondylitis: the bath ankylosing spondylitis disease activity index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 15.Calin A, Garrett S, Whitelock H, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the bath ankylosing spondylitis functional index. J Rheumatol 1994;21:2281–5. [PubMed] [Google Scholar]
- 16.Kiltz U, van der Heijde D, Boonen A, et al. Development of a health index in patients with ankylosing spondylitis (ASAS HI): final result of a global initiative based on the ICF guided by ASAS. Ann Rheum Dis 2015;74:830–5. 10.1136/annrheumdis-2013-203967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.EuroQol Group . EuroQol--a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199–208. 10.1016/0168-8510(90)90421-9 [DOI] [PubMed] [Google Scholar]
- 18.Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS ®, 2012: 335. [Google Scholar]
- 19.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–107. 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Twisk JWR. Applied Mixed Model Analysis: A Practical Guide. In: Applied mixed model analysis, 2019. https://www.cambridge.org/core/books/applied-mixed-model-analysis/16BB3849827F848579608B8C788A51F8 [Google Scholar]
- 21.MÁ P-L, Ladehesa-Pineda L, Font-Ugalde P. Distribution of comorbidities in spondyloarthritis with regard to the phenotype and psoriasis: data from the ASAS-COMOSPA study. Ther Adv Musculoskelet Dis 2021;13. 10.1177/1759720X211045263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maguire S, Wilson F, Gallagher P, et al. Worse scores but similar patterns of disease activity: interpreting outcomes in women with axial spondyloarthropathy. Scand J Rheumatol 2022:1–8. 10.1080/03009742.2021.2007609 [DOI] [PubMed] [Google Scholar]
- 23.Jovaní V, Blasco-Blasco M, Ruiz-Cantero MT, et al. Understanding how the diagnostic delay of spondyloarthritis differs between women and men: a systematic review and metaanalysis. J Rheumatol 2017;44:174–83. 10.3899/jrheum.160825 [DOI] [PubMed] [Google Scholar]
- 24.Garrido-Cumbrera M, Poddubnyy D, Gossec L, et al. Gender differences in patient journey to diagnosis and disease outcomes: results from the European map of axial spondyloarthritis (EMAS). Clin Rheumatol 2021;40:2753–61. 10.1007/s10067-020-05558-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan S, Shridharmurthy D, Lapane KL, et al. The disease burden of axial spondyloarthritis: through a gendered lens. Clin Rheumatol 2022;41:1115–24. 10.1007/s10067-021-06008-8 [DOI] [PubMed] [Google Scholar]
- 26.Coates LC, Tillett W. How should we measure peripheral spondyloarthritis? J Rheumatol 2022;49:239–41. 10.3899/jrheum.211043 [DOI] [PubMed] [Google Scholar]
- 27.Moll JM, Wright V. Psoriatic arthritis. Semin Arthritis Rheum 1973;3:55–78. 10.1016/0049-0172(73)90035-8 [DOI] [PubMed] [Google Scholar]
- 28.Eder L, Thavaneswaran A, Chandran V, et al. Gender difference in disease expression, radiographic damage and disability among patients with psoriatic arthritis. Ann Rheum Dis 2013;72:578–82. 10.1136/annrheumdis-2012-201357 [DOI] [PubMed] [Google Scholar]
- 29.Mease PJ, McLean RR, Dube B, et al. Comparison of men and women with axial spondyloarthritis in the US-based corrona psoriatic arthritis/spondyloarthritis registry. J Rheumatol 2021;48:1528–36. 10.3899/jrheum.201549 [DOI] [PubMed] [Google Scholar]
- 30.Zabotti A, Luchetti MM, Selmi CF, et al. An Italian disease-based registry of axial and peripheral spondyloarthritis: the SIRENA study. Front Med 2021;8:711875. 10.3389/fmed.2021.711875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santos-Faria D, Dougados M, Gossec L, et al. Evaluation of the performance of extreme patient-reported outcomes as surrogate markers for fibromyalgia in axial spondyloarthritis. Rheumatol Int 2019;39:141–6. 10.1007/s00296-018-4200-4 [DOI] [PubMed] [Google Scholar]
- 32.Maguire S, Wilson F, Gallagher P, et al. Central obesity in axial spondyloarthritis: the missing link to understanding worse outcomes in women? J Rheumatol 2022;49:577–84. 10.3899/jrheum.211062 [DOI] [PubMed] [Google Scholar]
- 33.Racine M, Tousignant-Laflamme Y, Kloda LA, et al. A systematic literature review of 10 years of research on sex/gender and experimental pain perception - part 1: are there really differences between women and men? Pain 2012;153:602–18. 10.1016/j.pain.2011.11.025 [DOI] [PubMed] [Google Scholar]
- 34.Ruau D, Liu LY, Clark JD, et al. Sex differences in reported pain across 11,000 patients captured in electronic medical records. J Pain 2012;13:228–34. 10.1016/j.jpain.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samulowitz A, Gremyr I, Eriksson E, et al. "Brave men" and "emotional women": a theory-guided literature review on gender bias in health care and gendered norms towards patients with chronic pain. Pain Res Manag 2018;2018:1–14. 10.1155/2018/6358624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fauchon C, Meunier D, Rogachov A, et al. Sex differences in brain modular organization in chronic pain. Pain 2021;162:1188–200. 10.1097/j.pain.0000000000002104 [DOI] [PubMed] [Google Scholar]
- 37.Martel MO, Thibault P, Sullivan MJL. Judgments about pain intensity and pain genuineness: the role of pain behavior and judgmental heuristics. J Pain 2011;12:468–75. 10.1016/j.jpain.2010.10.010 [DOI] [PubMed] [Google Scholar]
- 38.Ahlsen B, Mengshoel AM, Solbrække KN. Troubled bodies--troubled men: a narrative analysis of men's stories of chronic muscle pain. Disabil Rehabil 2012;34:1765–73. 10.3109/09638288.2012.660601 [DOI] [PubMed] [Google Scholar]
- 39.Ibáñez Vodnizza SE, van Bentum RE, Valenzuela O, et al. Patients with axial spondyloarthritis report significant differences between men and women and high impact of the disease: large websurvey analysis. Joint Bone Spine 2020;87:315–9. 10.1016/j.jbspin.2020.02.004 [DOI] [PubMed] [Google Scholar]
- 40.Turina MC, Ramiro S, Baeten DL, et al. A psychometric analysis of outcome measures in peripheral spondyloarthritis. Ann Rheum Dis 2016;75:1302–7. 10.1136/annrheumdis-2014-207235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kılıç G, Kılıç E, Nas K, et al. Comparison of ASDAS and BASDAI as a measure of disease activity in axial psoriatic arthritis. Clin Rheumatol 2015;34:515–21. 10.1007/s10067-014-2734-8 [DOI] [PubMed] [Google Scholar]
- 42.Beckers E, Been M, Webers C, et al. Performance of 3 composite measures for disease activity in peripheral spondyloarthritis. J Rheumatol 2022;49:256–64. 10.3899/jrheum.210075 [DOI] [PubMed] [Google Scholar]
- 43.Taylor WJ, Harrison AA. Could the bath ankylosing spondylitis disease activity index (BASDAI) be a valid measure of disease activity in patients with psoriatic arthritis? Arthritis Rheum 2004;51:311–5. 10.1002/art.20421 [DOI] [PubMed] [Google Scholar]
- 44.Chimenti M-S, Alten R, D'Agostino M-A, et al. Sex-associated and gender-associated differences in the diagnosis and management of axial spondyloarthritis: addressing the unmet needs of female patients. RMD Open 2021;7:e001681. 10.1136/rmdopen-2021-001681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marzo-Ortega H, Navarro-Compán V, Akar S, et al. The impact of gender and sex on diagnosis, treatment outcomes and health-related quality of life in patients with axial spondyloarthritis. Clin Rheumatol 2022. 10.1007/s10067-022-06228-6. [Epub ahead of print: 28 Jun 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002514supp001.pdf (158.5KB, pdf)
Data Availability Statement
Data are available on reasonable request. Data are available on reasonable request. Researchers willing to use data collected during the study should contact the first author, who will send a study proposal template to be completed by the applicant. Thereafter, the steering committee of the ASAS-perSpA study will approve (or not) the proposal and proceed to the data sharing.