Abstract
Objective
To examine sex differences in prevalence, volume and distribution of vascular brain lesions on MRI among patients with atrial fibrillation (AF).
Methods
In this cross-sectional analysis, we included 1743 patients with AF (27% women) from the multicentre Swiss Atrial Fibrillation study (SWISS-AF) with available baseline brain MRI. We compared presence and total volume of large non-cortical or cortical infarcts (LNCCIs), small non-cortical infarcts, microbleeds (MB) and white matter hyperintensities (WMH, Fazekas score ≥2 for moderate or severe degree) between men and women with multivariable logistic regression. We generated voxel-based probability maps to assess the anatomical distribution of lesions.
Results
We found no strong evidence for an association of female sex with the prevalence of all ischaemic infarcts (LNCCI and SNCI combined; adjusted OR 0.86, 95% CI 0.67 to 1.09, p=0.22), MB (adjusted OR 0.91, 95% CI 0.68 to 1.21, p=0.52) and moderate or severe WMH (adjusted OR 1.15, 95% CI 0.90 to 1.48, p=0.27). However, total WMH volume was 17% larger among women than men (multivariable adjusted multiplicative effect 1.17, 95% CI 1.01 to 1.35; p=0.04). Lesion probability maps showed a right hemispheric preponderance of ischaemic infarcts in both men and women, while WMH were distributed symmetrically.
Conclusion
Women had higher white matter disease burden than men, while volume and prevalence of other lesions did not differ. Our findings highlight the importance of controlling risk factors for cerebral small vessel disease in patients with AF, especially among women.
Keywords: Atrial Fibrillation, STROKE, Atrial Flutter
WHAT IS ALREADY KNOWN ON THIS TOPIC
Clinically overt stroke, clinically unrecognised infarcts, microbleeds or other vascular brain lesions are commonly found on brain MRI in patients with atrial fibrillation (AF) and have a negative impact on cognitive function. The prevalence of covert brain lesions is increased in women in the general population.
WHAT THIS STUDY ADDS
Our study provides data on differences in vascular brain lesions between men and women in a large sample of patients with AF, due to comprehensive quantity and quality of brain MRI at baseline. We observed a higher white matter disease burden in women than in men, despite well-anticoagulated study population. Volume and prevalence of other lesions showed no sex differences.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Our findings emphasise the importance of targeting risk factors for cerebral small vessel disease among patients with AF, especially in women.
Introduction
Atrial fibrillation (AF) is among the most common causes of overt ischaemic stroke worldwide and women with AF and more than one risk factor appear to be at greater risk of stroke than men.1–3 A recent study claims that female sex should be considered a risk modifier rather than an overall risk factor for stroke in patients with AF.4 Observations suggest that hormone therapy, menopause, haemodynamic and coagulatory mechanisms, cardiovascular remodelling, inflammation and less optimal treatment of vascular risk factors among female patients may contribute to a higher stroke risk in women with AF.5
In addition to clinically overt stroke, clinically unrecognised (ie, covert) infarcts, microbleeds (MB) or other vascular brain lesions are commonly found on brain MRI in patients with AF and have a negative impact on cognitive function.6–8 The prevalence of covert brain lesions is increased in women in the general population.9 However, data on differences in vascular brain lesions between men and women with AF are lacking. Such evidence may improve our understanding of sex-specific differences in vascular brain disease among patients with AF and help specifically target risk factors among women.
Methods
Study design and participants
This cross-sectional analysis used data of the Swiss Atrial Fibrillation cohort study (Swiss-AF), an ongoing prospective, observational cohort study in which patients with documented AF were enrolled between 2014 and 2017 across 14 centres in Switzerland. Detailed information on the study design has been published previously.10 In brief, 2165 patients ≥65 years and 250 patients aged 45–65 years with documented AF were recruited. Main exclusion criteria were short reversible forms of AF, acute illness within the last 4 weeks and inability to sign informed consent. Of 653 (27%) patients had to be excluded due to missing brain MRI at baseline. Nineteen patients were excluded because of other missing data, thus leaving 1743 patients for this analysis.
Standard protocol approvals, registrations and patient consents
Patients or the public were not involved in the design, conduct, reporting or dissemination plans of our research.
Clinical assessment
AF was classified as paroxysmal, persistent and permanent according to recommended definitions.11 Information about patient characteristics, comorbidities, lifestyle factors and current medications (antihypertensive medication, such as angiotensin-converting enzyme inhibitors, beta blockers, angiotensin—1-receptor blockers, calcium antagonists, diuretics, renin antagonists and aldosterone antagonists; oral anticoagulants including vitamin K antagonists and direct oral anticoagulants; statins and antiplatelet therapy) was acquired by standardised case report forms and validated questionnaires. Body height and weight were measured using standardised devices and body mass index was calculated as weight in kilograms divided by height in metres squared. Study physicians performed three consecutive blood pressure measurements at baseline, and the mean of them was used for this analysis.
Brain MRI
MRI was done on 1.5 Tesla or 3.0 Tesla scanners. The standardised protocol consisted of a three-dimensional T1-weighted magnetisation-prepared rapid gradient echo (MPRAGE; spatial resolution 1.0×1.0×1.0 mm3), a two-dimensional axial fluid-damped inversion recovery (FLAIR; spatial resolution 1.0×1.0×3.0 mm3), and a two-dimensional axial diffusion-weighted imaging sequence (spatial resolution 1.0×1.0×3.0 mm3) with whole brain coverage and without interpolation. In addition, either a two-dimensional axial susceptibility-weighted imaging (spatial resolution 1.0×1.0×3.0 mm3) or a two-dimensional axial T2*-weighted gradient echo sequence (spatial resolution 1.0×1.0×3.0 mm3) was applied.
Lesion segmentation
Scans were analysed centrally (MIAC AG; Medical Image Analysis Center AG, Basel, Switzerland). The blinded experts did not know any personal characteristics and standardised the lesions by marking and segmenting them according to an internal procedure approved for international clinical trials. Board-certified neuroradiologists confirmed all evaluations.6 10
Large non-cortical or cortical infarcts (LNCCIs) were defined as large non-cortical infarcts with a diameter >20 mm on axial sections as well as cortical infarcts characterised as hyperintense lesions on FLAIR involving the cortex regardless of their size and whether they also affect subcortical areas. Small non-cortical infarcts (SNCI) were defined as hyperintense lesions on FLAIR ≤20 mm in diameter on axial sections and not involving the cortex, compatible with ischaemic infarction in the area of a perforating arteriole (located in the white matter, subcortical grey matter or brainstem).12 Hyperintense lesions on FLAIR in either the periventricular or deep white matter region, brain stem or cerebellum, not meeting the criteria for LNCCIs or SNCIs, were identified as white matter hyperintensities (WMH) of presumed vascular origin,12 henceforth referred to as WMH. Perivascular spaces were differentiated from WMH by their tubular morphology, and subsequently excluded. WMH severity was classified using the Fazekas scale with a score of ≥2 and was defined as moderate to severe white matter disease.6 12 MBs were characterised as small (2 mm to 10 mm) areas of signal void with associated blooming seen on T2*-weighted MRI or other sequences that are sensitive to susceptibility effects (SWI-sequence).13 T2-weighted volumes of SNCIs, LNCCIs and WMH were segmented and quantified semiautomatically in mm3 using Amira (Mercury Computer Systems, Chelmsford, Massachusetts). Lesions with a central FLAIR hypointense core were segmented in total without differentiating between hyperintense and hypointense lesion areas. The normalised brain volume was estimated in cm3 on MPRAGE using SIENAX.14
Image registration
FLAIR lesion masks of LNCCIs and SNCIs were registered linearly to a T1-weighted brain template using FSL (V.5.0, FMRIB’s Software Library; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki). Then, the FLAIR-to-T1w images were non-linearly registered to an age-specific standard brain template. The same image processing steps were applied to the WMH masks. We did not map MBs due to their small size. Image registration was successful in 1716 (98.5%; 27% females) patients.
Voxel-based probability maps were computed by overlaying the normalised lesion masks of all patients—separately for infarcts (LNCCIs and SNCIs combined) and WMH. The percentage of lesions in the different vascular territories (calculated by the proportion of lesional voxels per vascular territory per patient) were computed for women and men.
Statistical analysis
Baseline characteristics were compared between male and female patients. Depending on the distribution of the variables, continuous variables were presented as mean±SD or median (IQR). Categorical data were presented as numbers (percentage). Data were compared using χ2 test, t test or Mann-Whitney U test, as appropriate. Univariable and multivariable logistic and linear regression models were built to investigate the association of female sex with the prevalence and volume of brain lesions. Due to the skewed distribution, the volume of brain lesions was used as log-transformed variable and only analysed in patients with a prevalent ischaemic infarct or WMH. The OR (for the prevalence of brain lesions) and the β coefficient (for the log-transformed volume of brain lesions) were calculated with the corresponding 95% CIs, and female patients representing the reference group. Due to the log-transformed outcome variable (volume of brain lesions), β coefficients (95% CI) were reported as multiplicative effect (e ∧ β coefficient). Violin plots were built to show the distribution comparison of ischaemic lesions and WMH volume between female and male patients. P value was not adjusted for multiple testing. The regression analyses were adjusted for age, smoking status, body mass index, AF type, systolic blood pressure, history of hypertension, history of diabetes mellitus, history of sleeping apnoea, history of coronary heart disease, history of heart failure, oral anticoagulants, antiplatelet therapy, statin intake and antihypertensive treatment. Linear regression models using the volume of ischaemic lesions or WMH as the outcome variable were additionally adjusted for the total brain volume, to consider potential sex differences. For WMH volume, we tested an interacting effect of female sex and systolic blood pressure by including the respective interaction term in the model. As a sensitivity analysis, all analyses were repeated in patients without history of stroke or transient ischaemic attack (TIA).
Lesion probability maps were obtained for infarcts (LNCCI and SNCI combined) and WMH and compared between female and male patients. Patients with missing brain MRI or insufficient voxel analysis data have been excluded. For the voxel-based analyses, non-parametric and descriptive data like voxel percentage were reported as median and IQR. The Wilcoxon-matched pair rank test was used to compare median differences of voxel percentages within the vascular territory of anterior cerebral artery, middle cerebral artery, posterior cerebral artery, brainstem and cerebellum and to differ between the left and right hemispheres. Only patients with presence of any ischaemic infarcts and WMH were included in the analysis of the vascular territory distribution. Maximal overlay was calculated as maximum number of lesions per total of patients as a percentage occurred in a voxel. The numbers of the voxel-based mapping figures have been adjusted for the total remaining 1716 patients.
All analyses were performed on an available data basis and conducted using R V.4.0.3 (R Core Team, 2018, R Foundation, Vienna, Austria).
Results
Baseline characteristics stratified by sex are presented in table 1. Mean age of the population was 73±8 years, 27.4% of participants were women and 90.1% were on oral anticoagulation (59.5% on new oral anticoagulants and 40.4% on Vitamin K antagonists). Compared with men, women were older (74 vs 72 years), had higher systolic blood pressure (138 mm Hg vs 134 mm Hg) and had more often paroxysmal AF (54.0 vs 42.7%). Men suffered more often from diabetes (17.5 vs 11.9%), coronary artery disease (32.0 vs 12.6%), sleep apnoea syndrome (15.4 vs 8.4%) and were more often treated with statins (52.3 vs 35.4%).
Table 1.
Baseline characteristics stratified by sex
| Variable | Women (n=478) | Men (n=1265) | P Value |
| Age (years) | 74±8 | 72±9 | <0.001 |
| Body mass index (kg/m2) | 26.7 (23.5, 31.2) | 26.9 (24.6, 30.1) | 0.35 |
| Blood pressure systolic/diastolic (mm Hg) | 138±21/78±12 | 134±18/79±12 | <0.001/0.08 |
| Smoking status, n (%) | <0.001 | ||
| Current | 39 (8.2) | 92 (7.3) | |
| Past | 163 (34.1) | 680 (53.8) | |
| Never | 276 (57.7) | 491 (38.9) | |
| Average alcohol intake (drinks/day) | 0.1 (0.0, 0.6) | 0.7 (0.2, 1.6) | <0.001 |
| Regular weekly physical activity, n (%) | 229 (47.9) | 617 (48.9) | 0.77 |
| Health Perception Score (0–100) * | 70±17 | 74±17 | <0.001 |
| Atrial fibrillation type, n (%) | <0.001 | ||
| Paroxysmal | 258 (54.0) | 540 (42.7) | |
| Persistent | 140 (29.3) | 406 (32.2) | |
| Permanent | 80 (16.7) | 319 (25.1) | |
| CHA2DS2-VASc score | 4.0±1.5 | 3.1±1.7 | <0.001 |
| History of diabetes, n (%) | 57 (11.9) | 220 (17.5) | 0.01 |
| History of hypertension, n (%) | 327 (68.4) | 879 (69.5) | 0.71 |
| History of heart failure, n (%) | 92 (19.3) | 287 (22.7) | 0.15 |
| History of coronary artery disease, n (%) | 60 (12.6) | 405 (32.0) | <0.001 |
| History of clinical stroke, n (%) | 70 (14.6) | 160 (12.6) | 0.31 |
| History of TIA, n (%) | 50 (10.5) | 110 (8.7) | 0.27 |
| History of major bleeding, n (%) | 28 (5.9) | 69 (5.5) | 0.83 |
| History of sleep apnoea, n (%) | 40 (8.4) | 195 (15.4) | <0.001 |
| History of renal failure, n (%) | 81 (17.0) | 236 (18.7) | 0.48 |
| History/current hormonal therapy, n (%) | 29 (6.1) | 0 (0.0) | – |
| Cardiovascular medication, n (%) | |||
| Antihypertensive medication | 433 (90.6) | 1100 (87.0) | 0.05 |
| Statins | 169 (35.4) | 662 (52.3) | <0.001 |
| Antiarrhythmics class Ic and III | 108 (22.6) | 256 (20.2) | 0.29 |
| Oral anticoagulant intake, n (%) | 432 (90.4) | 1139 (90.0) | 0.90 |
| NOAC | 276 (63.9) | 659 (57.9) | 0.04 |
| Vitamin K antagonist | 156 (36.1) | 479 (42.1) | 0.05 |
| Antiplatelet (including aspirin), n (%) | 57 (11.9) | 250 (19.8) | <0.001 |
Values are mean ± SD, median (interquartile range) or n (%). The p value compares women and male patients. CHA2DS2-VASc score was defined as follows: female sex = 1 point; age ≥65 and <75 years = 1 point; age ≥75 years = 2 points, history of stroke or TIA = 2 points; history of heart failure = 1 point; hypertension = 1 point; diabetes = 1 point; vascular disease, consisting of history of myocardial infarction, history of percutaneous coronary intervention, history of coronary artery bypass graft surgery or periphery artery disease = 1 point.
*Health perception score is a self-assessment concerning their current state of health, evaluated in a scale from 0 to 100. Missing values: blood pressure systolic (n=11), diastolic (n=11), smoking (n=2), alcohol consumption (n=2), physical activity (n=2), CHA2DS2-VASc (n=2), heart failure (n=2), TIA (n=1), sleeping apnoea (n=1), renal failure (n=1). Antihypertensive medication includes angiotensin-converting-enzyme inhibitors, beta blockers, angiotensin-1-receptor-blockers, calcium antagonists, diuretics, renin antagonists, aldosterone antagonists.
NOAC, new oral anticoagulants; TIA, transient ischaemic attack.
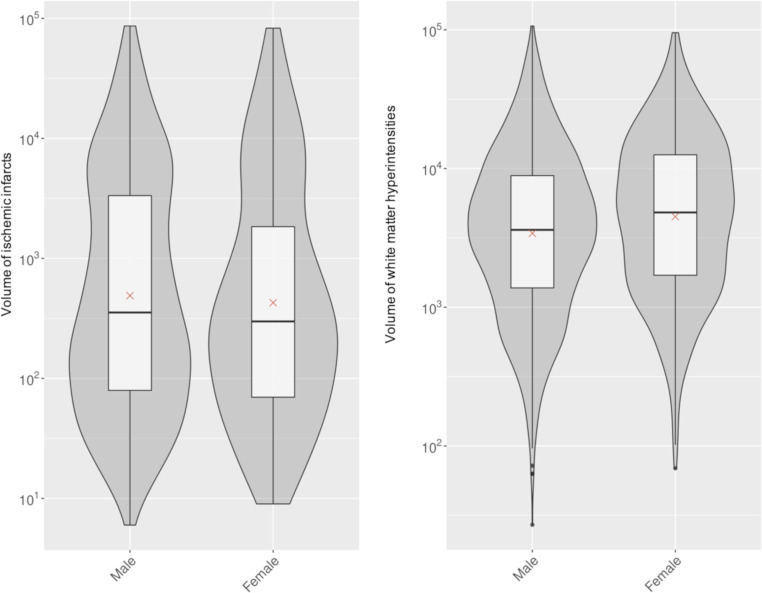
The prevalence and volume of vascular brain lesions are presented in table 2, as violin plots in figure 1 and unadjusted as well as adjusted comparisons thereof between women and men in table 3 and table 4. In the unadjusted analysis, we found no strong evidence for a difference between women and men in presence and volume of LNCCI (prevalence 20.3 vs 24.0%), SNCI (21.1 vs 22.8%), any ischaemic infarcts (LNCCI and SNCI combined, 35.6 vs 38.8%) or in prevalence of MB (21.0 vs 22.7%). Moderate or severe white matter disease was more common in women than in men (59.0 vs 51.7%, unadjusted p=0.006) and likewise, total WMH volume was greater in women (4779 mm3 vs 3609 mm3; multiplicative effect (1.32, 95% CI 1.2 to 1.5, unadjusted p<0.001).
Table 2.
Prevalence and volume of brain lesions stratified by sex
| Variable | Women (n=478) | Men (n=1265) |
| Large noncortical and cortical infarcts | ||
| Prevalence, n (%) | 97 (20.3) | 303 (24.0) |
| Volume (mm3), median (IQR) | 1230 (270, 7800) | 1680 (258, 6971) |
| Small noncortical infarcts | ||
| Prevalence, n (%) | 101 (21.1) | 289 (22.8) |
| Volume (mm3), median (IQR) | 69 (30, 195) | 69 (30, 189) |
| Any ischaemic infarcts (LNCCI or SNCI) | ||
| Prevalence, n (%) | 170 (35.6) | 491 (38.8) |
| Volume (mm3), median (IQR) | 299 (70, 1837) | 354 (80, 3335) |
| Microbleeds | ||
| Prevalence, n (%) | 98 (21.0) | 277 (22.7) |
| Counts (number) | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) |
| White matter hyperintensities | ||
| Prevalence, Fazekas scale ≥2, n (%) | 282 (59.0) | 653 (51.7) |
| Volume total (mm3), median (IQR) | 4779 (1707, 12546) | 3609 (1368, 8859) |
Values are median (IQR) or n (%). Only the volume of patients showing the presence of lesions was taken into account. Missing values: microbleed count (n=55), white matter hyperintensities (n=1).
LNCCI, large noncortical and cortical infarcts (including acute lesions); SNCI, small noncortical infarcts (including acute lesions).
Figure 1.
Violin plot of the log-transformed volume of ischaemic infarcts (LNCCI and SNCI combined) and WMH stratified by sex. The figure shows the distribution of ischaemic infarct volume (LNCCI and SNCI combined) and WMH volume in the SWISS-AF patients with successful co-registration (n=1716) compared between men and women. LNCCI, large non-cortical or cortical infarct; SNCI, small non-cortical infarct; SWISS-AF, Swiss Atrial Fibrillation study; WMH, white matter hyperintensity.
Table 3.
Association between female sex and the prevalence of brain lesions
| Prevalence | Univariable | Age adjusted model | Multivariable adjusted model |
| All patients (n=1743) OR (95% CI) | All patients (n=1743) OR (95% CI) | All patients (n=1727) OR (95% CI) | |
| Large noncortical and cortical infarcts | 0.81 (0.62 to 1.04) p=0.11 | 0.73 (0.56 to 0.95) p=0.02 | 0.86 (0.65 to 1.14) p=0.28 |
| Small noncortical infarcts | 0.90 (0.70 to 1.17) p=0.44 | 0.78 (0.59 to 1.01) p=0.06 | 0.82 (0.62 to 1.09) p=0.18 |
| Ischaemic lesions (LNCCI and SNCI) | 0.87 (0.70 to 1.08) p=0.21 | 0.75 (0.60 to 0.94) p=0.01 | 0.86 (0.67 to 1.09) p=0.22 |
| Microbleeds | 0.91 (0.70 to 1.17) p=0.47 | 0.81 (0.62 to 1.06) p=0.13 | 0.91 (0.68 to 1.21) p=0.52 |
| White matter hyperintensities, Fazekas≥2 | 1.35 (1.09 to 1.67) p=0.006 | 1.11 (0.88 to 1.40) p=0.37 | 1.15 (0.90 to 1.48) p=0.27 |
Data are presented as OR and 95% CI; predictor of interest: female sex; multivariable adjusted model was adjusted for age, body mass index, smoking status, AF type (paroxysmal vs non-paroxysmal), systolic blood pressure, hypertension, diabetes mellitus, heart failure, coronary heart disease, sleep apnoea, statin therapy, antihypertensive medication, oral anticoagulation, antiplatelet therapy. Missing values: microbleeds count (n=55); white matter hyperintensities (n=1); covariates (n=16).
AF, atrial fibrillation; LNCCI, large noncortical and cortical infarcts (including acute lesions); SNCI, small noncortical infarcts (including acute lesions).
Table 4.
Association between female sex and the log-transformed volume of brain lesions
| Volume | Univariable | Age adjusted model | Multivariable adjusted model |
| Multiplicative effect (95% CI) | Multiplicative effect (95% CI) | Multiplicative effect (95% CI) | |
| Large noncortical and cortical infarcts | 0.96 (0.61 to 1.53) p=0.88 | 0.96 (0.60 to 1.53) p=0.86 | 1.13 (0.64 to 1.98), p=0.67 |
| Small noncortical infarcts | 0.98 (0.75 to 1.29) p=0.89 | 0.98 (0.75 to 1.29) p=0.89 | 1.20 (0.89 to 1.62), p=0.24 |
| Ischaemic lesions (LNCCI and SNCI) | 0.87 (0.59 to 1.29), p=0.50 | 0.88 (0.59 to 1.30), p=0.51 | 1.18 (0.76 to 1.85), p=0.46 |
| White matter hyperintensities, total | 1.32 (1.15 to 1.53) p<0.001 | 1.14 (1.01 to 1.30) p=0.04 | 1.17 (1.01 to 1.35), p=0.04 |
Data are presented as multiplicative effect and 95% CI; multiplicative effect=e∧ β-coefficient (due to log-transformed outcome variable); Only patients with the respective lesion were taken into account for this analysis; predictor of interest: female sex; multivariable adjusted model was adjusted for age, body mass index, smoking status, AF type (paroxysmal vs non-paroxysmal), systolic blood pressure, hypertension, diabetes mellitus, heart failure, coronary heart disease, sleep apnoea, statin therapy, antihypertensive medication, oral anticoagulation, antiplatelet therapy and normalised brain volume. Missing values multivariable adjusted models: LNCCI n=3; SNCI n=2; ischaemic lesions n=4; WMH n=16. Number of patients in the multivariable adjusted model (including brain volume): LNCCI: n=333; SNCI: n=350; ischaemic lesions: n=569; white matter lesions: n=1491.
AF, atrial fibrillation; LNCCI, large noncortical and cortical infarcts (including acute lesions); SNCI, small noncortical infarcts (including acute lesions).
In the age-adjusted comparison, the prevalence of LNCCI (OR 0.73, 95% CI 0.56 to 0.95, p=0.02) alone and of any infarct (LNCCI and SNCI combined, OR 0.75, 95% CI 0.60 to 0.94, p=0.01) was lower in women than in men, but the differences were no longer statistically significant after adjustment for additional patient characteristics (table 3). The association between female sex and presence of moderate or severe white matter disease was no longer significant after adjusting for age (OR 1.11, 95% CI 0.88 to 1.40, p=0.37) and after adjusting for additional patient characteristics (OR 1.15, 95% CI 0.90 to 1.48, p=0.27; table 3). However, women had greater total WMH volume than men both in the age-adjusted (multiplicative effect 1.14, 95% CI 1.01 to 1.30, p=0.04) and in the multivariable-adjusted model (multiplicative effect 1.17, 95% CI 1.01 to 1.35, p=0.04; table 4). There was no association between sex and volume of LNCCI, SNCI or any infarct. In a post hoc analysis, there was no interaction between female sex and systolic blood pressure and WMH volume as the outcome variable.
In the subgroup of patients without a history of stroke or TIA, the prevalence and volume of vascular brain lesions were lower than in the full study population, both among men and women (online supplemental table e-1). The associations between female sex and brain lesions were nearly similar to the full study population (online supplementary table e-2, e-3).
openhrt-2022-002033supp001.pdf (90.3KB, pdf)
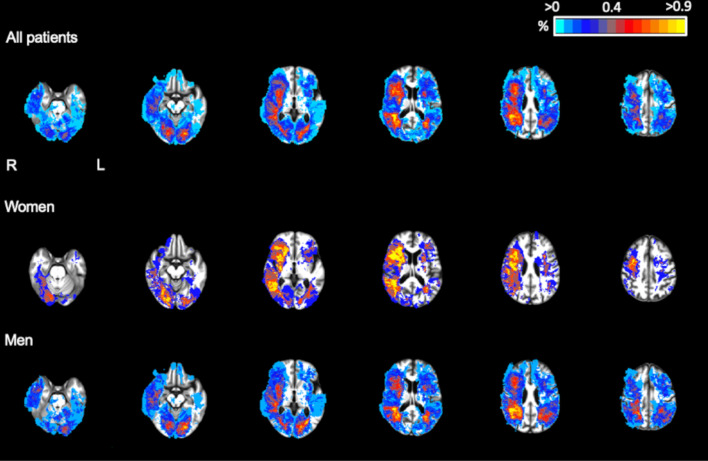
Maximal achievable overlay of ischaemic lesions in a voxel was 1.3% overall (1.7% for women and 1.5% for men). Voxels affected by ischaemic lesions were similarly distributed in women and men, with a right-hemispheric predominance of infarcts in both sexes (figure 2). Total WMH maximal overlay was 65.3%, in women 76.4% and men 61.2%. An equal distribution for WMH was observed over the two hemispheres in men and women (online supplemental figure e-1).
Figure 2.
Localisation of ischaemic lesions (LNCCI and SNCI combined) stratified by sex. The figure shows the distribution of ischaemic lesions (LNCCI and SNCI, except acute lesions) in the SWISS-AF patients with successful co-registration (n=1716) compared between men and women in a standard space. The colour indicates that a voxel is affected by an ischaemic lesion in this percentage (%) of patients. LNCCI, large non-cortical or cortical infarct; SNCI, small non-cortical infarct; SWISS-AF, Swiss Atrial Fibrillation study.
openhrt-2022-002033supp002.pdf (1.1MB, pdf)
Percentages of voxels affected by infarcts and WMH within different vascular territories and brain regions by sex are listed in online supplemental table e-4 in the online supplement. There were no differences between men and women in the voxel-based prevalence of infarcts in any territory.
Discussion
First, we did not find significant sex-related differences in the prevalence and volume of LNCCIs, SNCIs and MB. Second, women more often had moderate or severe white matter disease and larger volumes of WMH compared with men, the difference in the latter remaining significant after adjustment for baseline characteristics. Third, we did not observe any differences in regional distribution of vascular brain lesions between women and men, with infarcts predominantly occurring in the right hemisphere, and symmetrical location of white matter disease in both sexes.
Covert vascular brain lesions are present in a substantial proportion of patients with AF. We previously reported that 22% of patients in the Swiss-AF cohort study had LNCCIs and 21% SNCIs on MRI at study enrolment, despite the fact that most of the patients were taking oral anticoagulants (89%).6 Even after exclusion of patients with a history of stroke or TIA, these proportions were still 15% and 18%, respectively. Large-scale studies on sex-specific differences in vascular brain lesions among patients with AF have been lacking and the current evidence is largely limited to the risk of clinically overt stroke.
In the absence of other risk factors (ie, in the absence of non-sex related points of the CHA2DS2-VASc score), the risk of thromboembolic events in women with AF is low. However, female sex increases risk across the higher risk strata (especially in the presence of ≥2 non-sex-related stroke risk factors).4 In our study, the median CHA2DS2VASC score among women was 4±2% and 81% had ≥2 non-sex-related stroke risk factors. Nonetheless, the age-corrected prevalence of ischaemic infarcts was lower in women than in men. When correcting for additional patient characteristics and cardiovascular risk factors in the multivariable model, this difference was no longer significant. Hence, we found no sex differences of ischaemic brain infarcts on MRI. Both in women and men, the majority of these infarcts were covert, that is, present in patients without a history of stroke or TIA.
An important finding of our study was that women with AF had a higher burden of white matter disease than men. The median volume WMH was about a third larger in women. The Rotterdam Scan Study reported that women had a trend to a higher WMH burden than men in the general population.15 WMH by the definition used in our study are considered markers of cerebral small vessel disease.12 Age, hypertension and diabetes are among the most important risk factors for cerebral small vessel disease. In our cohort, women were older, less often diabetic but had higher baseline blood pressure than men. Nonetheless, in our study, the association between female sex and WMH volume persisted after correction for these as well as other risk factors and patient characteristics.
Although 91% of all women and 87% of all men in our cohort were on antihypertensive medication at baseline, systolic blood pressure at baseline was significantly higher in women than in men with a difference in median of 4 mm Hg. An important implication of our study is, therefore, that women with AF might be undertreated for hypertension. The Framingham study showed that women over the age of 60 have higher blood pressure than men, probably because menopause accelerates the onset of arterial stiffness, especially in those with history of hypertension.16 A randomised controlled trial showed that patients with a systolic blood pressure goal of <120 mm Hg had less increase in WMH volume over time compared with patients with a target of <140 mm Hg.17 The Rotterdam Scan Study observed in the general population that WMH and covert brain infarcts independently increase the risk of clinically manifest stroke in the future,18 reduce cognitive performance and are associated with brain volume loss.19 Our findings highlight that optimal care of patients with AF must go beyond anticoagulation and rhythm control and encompass vascular comorbidities causing vascular brain injury unrelated to embolism. Women appear to be vulnerable to cerebral small vessel disease, which might warrant lower targets for blood pressure control and other cardiovascular risk factors. Further studies may be needed to explore the influence of covert brain lesions and cardiovascular risk factors such as diabetes or smoking in women and men.
We found no difference in the regional distribution of vascular brain lesions between men and women. We found that in both sexes, ischaemic infarcts (LNCCI and SNCI) were more often located in the right hemisphere than in the left hemisphere. Physical properties of cardiac emboli, flow dynamics in the aortic arch, the more proximal origin of the brachiocephalic trunk compared with the left common carotid artery as well as the more rectilinear path of an embolus to the right compared with the left hemisphere may each contribute to this finding.20 21
Our study has limitations. The cross-sectional design precludes an assessment of causality or directionality of effect. Participants in our study were mostly white, and all were enrolled in Switzerland, limiting the generalisability of our findings to other populations. We only had data on prevalence of diabetes in our population, but no data on glycaemic control. The most important limitation was the low number of females participating in our cohort, a problem which is common to virtually all cardiovascular disease studies.22
In conclusion, we observed no strong differences between women and men with AF in the prevalence and volume of brain infarcts on MRI, the majority of which were clinically covert. However, women had a higher burden of white matter disease than men. Our findings emphasise the importance of targeting risk factors for cerebral small vessel disease among patients with AF, especially in women.
Acknowledgments
This paper was presented as an abstract and oral presentation at the Swiss Society of Cardiology annual meeting in June 2021 and European Stroke Conference in September 2021.
Footnotes
Collaborators: All SWISS-AF investigators are listed in online supplemental file 1.
Contributors: Conception and design—LHB, SC, SA, MK and SO. Analysis and interpretation of the data—SC, SA, AA, LHB and MC; all authors provided input in the interpretation of the data. Drafting of the manuscript—SC, SA and LHB. Critical revision for important intellectual content—all authors. Final approval of the manuscript—all authors. Guarantor—LHB.
Funding: The Swiss-AF study is supported by grants of the Swiss National Science Foundation (Grant numbers 33CS30_148474, 33CS30_177520, 32473B_176178, 32003B_197524), the Swiss Heart Foundation, the Foundation for Cardiovascular Research Basel and the University of Basel. David Conen holds a McMaster University Department of Medicine Mid-Career Research Award.
Competing interests: AA reports no disclosures; DC received speaker fees from Servier, Canada, outside of the current work; JW is an employee of MIAC AG, has received funding from EU (Horizon2020), Else-Kröner-Fresenius Foundation, Novartis Foundation, and consultancy, steering committee, advisory board and speaker honoraria from Actelion, Bayer, Biogen, Idorsia, Roche, Sanofi-Genzyme and Teva; LHB received grants from the Swiss National Science Foundation (PBBSB-116873, 33CM30-124119, 32003B-156658; Berne, Switzerland), The Swiss Heart Foundation (Berne, Switzerland, and the University of Basel (Basel, Switzerland). LHB has received an unrestricted research grant from AstraZeneca, and consultancy or advisory board fees or speaker’s honoraria from Amgen, Bayer, Bristol-Myers Squibb, and Claret Medical, and travel grants from AstraZeneca and Bayer; MC reports no disclosures; MD received honoraria for lectures from Pfizer, Bayer and Sonofi Genzyme, is a consultant for Hovid Berhad and Roche Pharma; MK reports personal fees from Bayer, personal fees from Böhringer Ingelheim, personal fees from Pfizer BMS, personal fees from Daiichi Sankyo, personal fees from Medtronic, personal fees from Biotronik, personal fees from Boston Scientific, personal fees from Johnson&Johnson, grants from Bayer, grants from Pfizer, grants from Boston Scientific, grants from BMS, grants from Biotronik; MRB reports no disclosures; NR received a grant from the Swiss Heart Foundation; SA reports no disclosures; SC reports no disclosures; SE-D reports no disclosures; SN reports no disclosures; SO reports no disclosures; TS reports no disclosures.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Contributor Information
SWISS-AF Investigators:
Stefanie Aeschbacher, Chloé Auberson, Steffen Blum, Leo H Bonati, Selinda Ceylan, David Conen, Simone Evers-Doerpfeld, Ceylan Eken, Marc Girod, Elisa Hennings, Elena Herber, Vasco Iten, Philipp Krisai, Michael Kühne, Mirko Lischer, Christine Meyer-Zürn, Pascal Meyre, Andreas U Monsch, Christian Müller, Stefan Osswald, Anne Springer, Christian Sticherling, Thomas Szucs, Gian Völlmin, Stefan Osswald, Michael Kühne, Drahomir Aujesky, Manuel R Blum, Urs Fischer, Juerg Fuhrer, Laurent Roten, Simon Jung, Heinrich Mattle, Luise Adam, Carole Elodie Aubert, Martin Feller, Axel Loewe, Elisavet Moutzouri, Claudio Schneider, Tanja Flückiger, Cindy Groen, Lukas Ehrsam, Sven Hellrigl, Alexandra Nuoffer, Damiana Rakovic, Nathalie Schwab, Rylana Wenger, Nicolas Rodondi, Christopher Beynon, Roger Dillier, Michèle Deubelbeiss, Franz Eberli, Christine Franzini, Isabel Juchli, Claudia Liedtke, Jacqueline Nadler, Thayze Obst, Jasmin Roth, Fiona Schlomowitsch, Xiaoye Schneider, Katrin Studerus, Noreen Tynan, Dominik Weishaupt, Andreas Müller, Simone Fontana, Silke Kuest, Karin Scheuch, Denise Hischier, Nicole Bonetti, Alexandra Grau, Jonas Villinger, Eva Laube, Philipp Baumgartner, Mark Filipovic, Marcel Frick, Giulia Montrasio, Stefanie Leuenberger, Franziska Rutz, Jürg-Hans Beer, Angelo Auricchio, Adriana Anesini, Cristina Camporini, Giulio Conte, Maria Luce Caputo, Francois Regoli, Tiziano Moccetti, Roman Brenner, David Altmann, Michaela Gemperle, Peter Ammann, Mathieu Firmann, Sandrine Foucras, Martine Rime, Daniel Hayoz, Benjamin Berte, Virgina Justi, Frauke Kellner-Weldon, Brigitta Mehmann, Sonja Meier, Myriam Roth, Andrea Ruckli-Kaeppeli, Ian Russi, Kai Schmidt, Mabelle Young, Melanie Zbinden, Richard Kobza, Luisa Vicari, Giorgio Moschovitis, Georg Ehret, Hervé Gallet, Elise Guillermet, Francois Lazeyras, Karl-Olof Lovblad, Patrick Perret, Philippe Tavel, Cheryl Teres, Dipen Shah, Nathalie Lauriers, Marie Méan, Sandrine Salzmann, Jürg Schläpfer, Andrea Grêt, Jan Novak, Sandra Vitelli, Frank-Peter Stephan, Jane Frangi-Kultalahti, Augusto Gallino, Marcello Di Valentino, Fabienne Witassek, Matthias Schwenkglenks, Jens Würfel, Anna Altermatt, Michael Amann, Marco Düring, Petra Huber, Esther Ruberte, Tim Sinnecker, Vanessa Zuber, Michael Coslovsky, Pascal Benkert, Gilles Dutilh, Milica Markovic, Pia Neuschwander, Patrick Simon, and Ramun Schmid
Data availability statement
Data are available upon reasonable request. Deidentified participant data are available from selinda.ceylan@hotmail.com. Swiss AF protocol is available in the supplementary materials.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study complies with the Declaration of Helsinki, the study protocol has been approved by the university hospital Basel, local ethics committees EKNZ (Ethikkommission Nordwest- und Zentralschweiz) and informed written consent was obtained from each participant. Reference number: EKNZ 2014-067. Participants gave informed consent to participate in the study before taking part.
References
- 1. Giralt-Steinhauer E, Cuadrado-Godia E, Ois A, et al. Comparison between CHADS2 and CHA2 DS2 -VASc score in a stroke cohort with atrial fibrillation. Eur J Neurol 2013;20:623–8. 10.1111/j.1468-1331.2012.03807.x [DOI] [PubMed] [Google Scholar]
- 2. Friberg L, Benson L, Rosenqvist M, et al. Assessment of female sex as a risk factor in atrial fibrillation in Sweden: nationwide retrospective cohort study. BMJ 2012;344:e3522–10. 10.1136/bmj.e3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Olesen JB, Lip GYH, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ 2011;342:d124–9. 10.1136/bmj.d124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nielsen PB, Skjøth F, Overvad TF. Female sex is a risk modifer rather than a risk factor for stroke in atrial fibrillation should we use a Cha 2 Ds 2 -VA score rather than Cha 2 Ds 2 -VASc? Circulation 2018;137:832–40. 10.1161/CIRCULATIONAHA.117.029081 [DOI] [PubMed] [Google Scholar]
- 5. Cove CL, Albert CM, Andreotti F, et al. Female sex as an independent risk factor for stroke in atrial fibrillation: possible mechanisms. Thromb Haemost 2014;111:385–91. 10.1160/TH13-04-0347 [DOI] [PubMed] [Google Scholar]
- 6. Conen D, Rodondi N, Müller A, et al. Relationships of overt and silent brain lesions with cognitive function in patients with atrial fibrillation. J Am Coll Cardiol 2019;73:989–99. 10.1016/j.jacc.2018.12.039 [DOI] [PubMed] [Google Scholar]
- 7. Stefansdottir H, Arnar DO, Aspelund T, et al. Atrial fibrillation is associated with reduced brain volume and cognitive function independent of cerebral infarcts. Stroke 2013;44:1020–5. 10.1161/STROKEAHA.12.679381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marfella R, Sasso FC, Siniscalchi M, et al. Brief episodes of silent atrial fibrillation predict clinical vascular brain disease in type 2 diabetic patients. J Am Coll Cardiol 2013;62:525–30. 10.1016/j.jacc.2013.02.091 [DOI] [PubMed] [Google Scholar]
- 9. Vermeer SE, Koudstaal PJ, Oudkerk M, et al. Prevalence and risk factors of silent brain infarcts in the population-based rotterdam scan study. Stroke 2002;33:21–5. 10.1161/hs0102.101629 [DOI] [PubMed] [Google Scholar]
- 10. Conen D, Rodondi N, Mueller A, et al. Design of the Swiss atrial fibrillation cohort study (Swiss-AF): structural brain damage and cognitive decline among patients with atrial fibrillation. Swiss Med Wkly 2017;147:w14467. 10.4414/smw.2017.14467 [DOI] [PubMed] [Google Scholar]
- 11. European Heart Rhythm Association, European Association for Cardio-Thoracic Surgery, Camm AJ, et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of cardiology (ESC). Eur Heart J 2010;31:2369–429. 10.1093/eurheartj/ehq278 [DOI] [PubMed] [Google Scholar]
- 12. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013;12:822–38. 10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greenberg SM, Vernooij MW, Cordonnier C, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 2009;8:165–74. 10.1016/S1474-4422(09)70013-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 2002;17:479–89. 10.1006/nimg.2002.1040 [DOI] [PubMed] [Google Scholar]
- 15. de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. the Rotterdam scan study. J Neurol Neurosurg Psychiatry 2001;70:9–14. 10.1136/jnnp.70.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Franklin SS, Gustin W, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham heart study. Circulation 1997;96:308–15. 10.1161/01.cir.96.1.308 [DOI] [PubMed] [Google Scholar]
- 17. SPRINT MIND Investigators for the SPRINT Research Group, Nasrallah IM, Pajewski NM, et al. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA 2019;322:524–34. 10.1001/jama.2019.10551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vermeer SE, Hollander M, van Dijk EJ, et al. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam scan study. Stroke 2003;34:1126–9. 10.1161/01.STR.0000068408.82115.D2 [DOI] [PubMed] [Google Scholar]
- 19. Schmidt R, Ropele S, Enzinger C, et al. White matter lesion progression, brain atrophy, and cognitive decline: the Austrian stroke prevention study. Ann Neurol 2005;58:610–6. 10.1002/ana.20630 [DOI] [PubMed] [Google Scholar]
- 20. Kim H-J, Song J-M, Kwon SU, et al. Right-left propensity and lesion patterns between cardiogenic and aortogenic cerebral embolisms. Stroke 2011;42:2323–5. 10.1161/STROKEAHA.111.616573 [DOI] [PubMed] [Google Scholar]
- 21. Carr IA, Nemoto N, Schwartz RS, et al. Size-dependent predilections of cardiogenic embolic transport. Am J Physiol Heart Circ Physiol 2013;305:H732–9. 10.1152/ajpheart.00320.2013 [DOI] [PubMed] [Google Scholar]
- 22. Ding EL, Powe NR, Manson JE, et al. Sex differences in perceived risks, distrust, and willingness to participate in clinical trials: a randomized study of cardiovascular prevention trials. Arch Intern Med 2007;167:905–12. 10.1001/archinte.167.9.905 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2022-002033supp001.pdf (90.3KB, pdf)
openhrt-2022-002033supp002.pdf (1.1MB, pdf)
Data Availability Statement
Data are available upon reasonable request. Deidentified participant data are available from selinda.ceylan@hotmail.com. Swiss AF protocol is available in the supplementary materials.