Abstract
A new generation of bioreactors with integrated six degrees of freedom (6 DOF) aims to mimic more accurately the natural intervertebral disc (IVD) load. We developed and validated in a biological and mechanical study a specimen holder and corresponding ex vivo IVD organ model according to the bioreactor requirements for multiaxial loading and a long-term IVD culture. IVD height changes and cell viability were compared between the 6 DOF model and the standard 1 DOF model throughout the 3 weeks of cyclic compressive loading in the uniaxial bioreactor. Furthermore, the 6 DOF model and holder were loaded for 9 days in the multiaxial bioreactor under development using the same conditions, and the IVDs were evaluated for cell viability. The interface of the IVD model and specimen holder, enhanced with fixation screws onto the bone, was tested in compression, torsion, lateral bending, and tension. Additionally, critical motions such as tension and bending were assessed for a combination of side screws and top screws or side screws and adhesive. The 6 DOF model loaded in the uniaxial bioreactor maintained similar cell viability in the IVD regions as the 1 DOF model. The viability was high after 2 weeks throughout the whole IVD and reduced by more than 30% in the inner annulus fibrous after 3 weeks. Similarly, the IVDs remained highly viabile when cultured in the multiaxial bioreactor. In both models, IVD height changes after loading were in the range of typical physiological conditions. When differently directed motions were applied, the holder-IVD interface remained stable under hyper-physiological loading levels using a side screw approach in compression and torsion and the combination of side and top screws in tension and bending. We thus conclude that the developed holding system is mechanically reliable and biologically compatible for application in a new generation of multiaxial bioreactors.
Keywords: bioreactor, intervertebral disc, multiaxial loading, organ model, specimen holder, 6 DOF
Introduction
Axial compression, tension, lateral bending, and torsion are loads that arise in the intervertebral disc (IVD) as a result of the physiological function of the spine. The continuous load exerted on the IVD makes it highly susceptible to herniation and degeneration processes.1,2 The magnitudes of mechanical loading and mechanisms that lead to IVD failure through changes in metabolic activity and structural integrity are still not fully clarified.3 Although efforts were put into exploring innovative in vitro approaches like organ-on-chips,4,5 bioreactors for ex vivo culture of IVD organ models remain the main platform for the investigation of the effect of mechanical loading on IVD health and degeneration.6 The bioreactors are designed to exert mechanical loads on IVD in the independent motion axes, so-called degrees of freedom (DOF). Currently available bioreactors have mainly integrated 1 or 2 DOF, namely, axial compression and torsion6,7 and have widely been used to study IVD biology and degeneration processes.8−12 Whole IVDs with a cartilaginous endplate (CEP) and a minimum of the bony structure have typically been cultured in these bioreactors.7 The 6 DOF spine simulators, capable of actively performing translations in x, y, and z axes and rotations about x, y, and z axes, have only recently been implemented in research practice13−16 as advanced tools for more accurate mimicking of the natural mechanical loads on the IVD. Thereby whole motion segments, including elements of vertebral bone, have generally been used. The effect of such simulators on the IVD has mainly been assessed through mechanical parameters13,14 as they lack controlled conditions for ex vivo IVD maintenance and hence biological evaluation.
The development of a new generation of 6 DOF bioreactors for long-term organ model culture is currently ongoing in our laboratory intending to further advance in vitro IVD research and reduce preclinical studies on animals. The development of such a system requires the implementation of a specimen holder, which can efficiently transmit the complex loads from the bioreactor onto the specimen. The holder must provide a tight grip on the specimen without damaging the tissue. The holder material should be biocompatible as well as sterilizable, and the structure porous to allow medium access to the sample. We have designed a circular holder made of stainless steel that meets all the criteria. It is based on a key-keyhole principle implemented as a complementary cross pattern in the holder and specimen and further improved with side screws tightened onto the bony part of the sample. Accordingly, we adjusted the standard bovine ex vivo IVD organ model that has been used for uniaxial loading7,17 (hereafter 1 DOF model) to complement the holder design and requirements for multiaxial loading (hereafter 6 DOF model). The 6 DOF model was adjusted by keeping more bone for cross-machining and fixation with side screws while maintaining the access of the nutrients to the pores of the CEP via a hole machined in the center of the bone. As part of the bioreactor development, this study aimed to validate the biological relevancy of the 6 DOF organ model in the existing system (i.e., uniaxial bioreactor) and mechanically test the resistance of the corresponding specimen holder to motions that will be applied in the multiaxial bioreactor. Accordingly, the first objective was to test whether the new, 6 DOF model could retain a high level of cell viability during 3 weeks of physiological compressive loading when compared to the 1 DOF model. Additionally, we reproduced the study by loading the 6 DOF model for 9 days in the multiaxial bioreactor under development. The second objective was to mechanically test whether the implemented holding system with side screws can sufficiently transmit compression, tension, torsion, and bending loads onto the IVD specimen. Furthermore, we investigated if the mechanical capacity of the holder could be improved with approaches like adhesive applied at the interface between the holder and specimen or additional screws tightened to the top surface of the bone.
Materials and Methods
IVD Organ Model Preparation
Fresh bovine tails from slaughtered calves of age six to twelve months were dissected according to the standard procedure for IVD explant preparation.7 Skin, connective tissues, and bone elements, such as spinous and transverse processes, were removed. IVDs were isolated with a band saw (300 CL model; Exakt, Norderstedt, Germany) with two parallel and even bone cuts. The 1 DOF organ model was cut 0.5 mm above the highest point of the CEP and was kept without a growth plate (GP) to allow the nutrition infusion, as previously described.7,17,18 The 6 DOF organ model was cut 7 mm above the CEP (or around 4 mm above the GP) to retain enough bone for cross-machining outside the GP region (Figure 1a). A 2.6 mm wide and 2.2 mm deep cross was drilled in the center of the specimen (Figure 1c). To maintain a good nutrition infiltration in the 6 DOF model, a 6.6 × 6.6 mm wide and 5 mm deep hole was additionally machined to remove the bone and GP in the center of a specimen (Figure 1c). For study in the uniaxial bioreactor, the cross and the central hole were manually drilled to approximate sizes with a high-speed drill (Foredom, Bethel, CT, USA). For mechanical tests and study in the multiaxial bioreactor, the procedure was further improved with a milling machine (MF70 model; Proxxon, Föhren, Germany) mounted with a custom-made clamping tool (Figure 1b). The approximate time to make the cross and hole patterns in the bone was 15 min per specimen. During all the steps of processing, samples were continuously cooled with Ringer’s solution (Braun, Melsungen, Germany) connected to an Intrafix administration set (Braun) and a needle (Figure 1b). The cutting and bone machining were performed in the open air using sterile machine parts and sterile Ringer’s solution and set for irrigation. Blood and marrow clots were removed from the bone with a jet lavage system (Pulsavac, Zimmer Biomet, Warsaw, IN, USA), as previously described.18 Specimens were washed for 12 min on a shaker in 10% penicillin and streptomycin solution (Pen-Strep; Life Technologies, Carlsbad, CA, USA) prepared in phosphate-buffered saline and for 2 min in 1% Pen-Strep. Finally, IVDs were cultured in the medium containing DMEM with 4.5 g/L glucose supplemented with sodium bicarbonate and pyruvate, 1% Pen-Strep, 2% fetal calf serum (FCS; Corning, Corning, NY, USA), 1% ITS+ (Corning), 1% nonessential amino acids (Gibco, Life Technologies), 25 mmol/L HEPES (Gibco, Life Technologies), 50 μg/mL ascorbate-2-phosphate, and 50 μg/mL Primocin (InvivoGen, San Diego, CA, USA). For the biological study in the uniaxial bioreactor, two IVDs of diameter between 16 and 21 mm were randomly assigned to 1 DOF and 6 DOF model groups and to 3 time points (week 1, 2, and 3). Samples assigned to a time point originated from the same tail. The unloaded control group (day 0) was represented with three samples isolated from each investigated tail and prepared as the 1 DOF model. For the study in the multiaxial bioreactor, four IVDs of diameter between 17 and 20 mm were randomly assigned to an unloaded control group (day 0) and a loaded group. For mechanical tests, in total, 24 samples of diameter between 16 and 18 mm were used and 3 were assigned to each of the groups. The side screw approach was tested for compression, tension, bending, and torsion and the top screw and adhesive approaches for tension and bending. Mechanical tests using the side screw approach were performed 1–3 days after IVD harvesting, and for the top screw approach, 10 days after harvesting. For mechanical tests with adhesive, the time points varied between 1 and 8 days because only one pair of holders was available, and the adhesive application required an overnight incubation in the medium for better adherence. Between the harvesting and mechanical tests, the IVDs were kept at 37 °C in a medium composed of DMEM with 4.5 g/L glucose supplemented with sodium bicarbonate and pyruvate, 1% Pen-Strep, 10% FCS, and 50 μg/mL Primocin.
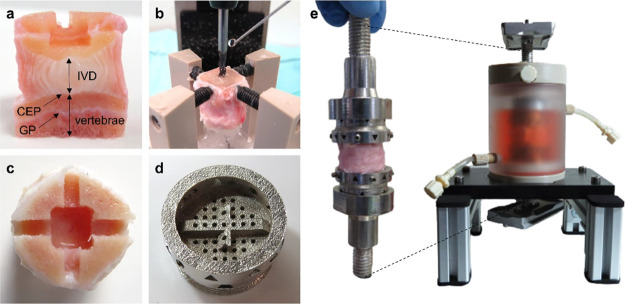
Figure 1.
IVD organ model and specimen holder designed for multiaxial loading in the new generation of spine bioreactors. Images show (a) longitudinally cut section of an ex vivo IVD bovine model with 7 mm of bone (vertebrae) preserved on both sides of the specimen, including cartilaginous endplate (CEP) and growth plate (GP), with cross and the central hole made on one side of the sample, (b) customized clamping tool mounted on a milling machine for accurate bone machining, (c) cross-pattern (2.6 mm wide and 2.2 mm deep) and a central hole for IVD nutrition (6.6 × 6.6 mm wide and 5 mm deep) machined in the bony part of the sample, (d) circular, porous specimen holder made of stainless steel with the cross of the same dimensions as its counterpart in the IVD, containing openings for nutrient access, (e) assembly of IVD specimen and holder tightened with side screws onto the bone and mechanical interface tightened onto the holder with a ring, shown individually and when inserted in the custom-made chamber for sterile IVD culture. The chamber is positioned on a customized rack and contains side tubes for medium exchange via a pump and a top opening that can be replaced by a filter for gas exchange. Two silver plates at the ends of the interface are used to clamp the chamber to the bioreactor (not shown).
6 DOF Organ Model Validation in the Uniaxial Bioreactor
IVD specimens were loaded in the uniaxial bioreactor in a chamber without the holders, but were placed between top and bottom sintered plates to allow uniform transmission of the loads and nutrition.12 Physiological IVD conditions were simulated by 2 h of daily loading in a chamber under a compressive loading regime (0.02–0.2 MPa, 0.2 Hz) and overnight free swelling in a well plate for a between-cycle recovery, in 5 mL of medium.19 The conditions were at all times maintained at 37 °C, 85% humidity, and 5% CO2. The specimens were loaded for 1, 2, and 3 weeks, and the medium was changed every day to prevent oscillations in the pH that may occur due to the release of cell metabolites in a small volume of the media. A nonloaded positive control (day 0) was cultured in free swelling conditions overnight.
The specimen height (including the bone) was measured at two positions with a caliper immediately after dissection, and daily following loading and free swelling culture.9 IVD height change after loading or swelling was in both models calculated relative to the initial IVD height after dissection. For the 6 DOF model, the bone thickness (excluding 0.5 mm on each side that is comparable with the 1 DOF model) was manually measured and deducted from the initial specimen height.
6 DOF Organ Model Validation in the Multiaxial Bioreactor
The 6 DOF organ model was assembled in the hood with sterile holders, interface and chamber. Briefly, a specimen was placed onto the bottom holder using the press-fit method. Four headless screws were added on the holder side and manually tightened onto the bone with a screwdriver. Screws were firmly tightened, but penetration and damage to the bone were avoided. The same procedure was applied to connect the top holder. The holders were assembled with the mechanical interface and inserted in a custom-made polycarbonate chamber (external diameter 60 mm, internal 40 mm; Figure 1e) through a silicon membrane and sealed with a nut. The interface was connected to the bioreactor via flat plates tightened at interface ends. The chamber was filled with 45-50 mL of medium prepared as indicated above and was changed every 3 days via side tubes (Figure 1e) connected to a peristaltic pump. Specimens were loaded for 9 days under the same physiological conditions as described above, and were kept in the chamber during overnight free swelling with a filter for gas exchange added through an opening on the top of the chamber (Figure 1e). The chamber conditions were maintained at 37 °C, 85% humidity, and 5% CO2 during the recovery phase only (∼22 h). A nonloaded positive control (day 0) prepared as a 1 DOF model was cultured in free swelling conditions overnight.
Cell Viability Analysis
Specimens were centrally and longitudinally cut into two halves, and again transversally through the IVD center, snap-frozen in liquid nitrogen, and sliced with cryotome (NX70 model; Thermo Fisher Scientific, Waltham, MA, USA) to 10 μm sections. Cell viability was assessed with the combined staining method for visualization of lactate dehydrogenase activity in alive cells and ethidium homodimer-1 binding to nuclei of dead cells and cells cut open during sectioning.20 Four random regions of interest (ROI) were analyzed in the outer annulus fibrosus (AF) regions. Inner AF and nucleus pulposus (NP) cells were counted on four (study in uniaxial bioreactor) or six (multiaxial bioreactor) ROIs. The sections were viewed with light microscopes (Zeiss, Oberkochen, Germany and Olympus, Tokio, Japan) under transmitted and fluorescence light. Cells stained blue and blue/red were assigned to living cells, and cells that stained red to dead cells. The number of alive and dead cells was counted using the ImageJ program and expressed as a measure of cell viability per ROI (0.39 mm2 uniaxial, 0.23 mm2 multiaxial).
Mechanical Tests
The mechanical properties of the holder-IVD specimen interface were measured in tension, lateral bending, compression, and axial torsion (Figure 2a) using the prototype of specimen holder setup with side screws (Figures 2b and S1). Complementary to the holder with side screws, tensile and lateral bending properties were measured for (i) a setup with side screws and 400 mg of Tetranite adhesive (RevBio Inc., Lowell, MA, USA) prepared according to manufacturer’s instructions and applied at the interface between the holder and sample, avoiding the cross area; and (ii) a setup with side screws and four additional top screws vertically inserted 4 mm through the holder onto the bone (Figure 2b). Tensile, lateral bending (four-point-bending setup), and compressive properties were measured with Instron 5866 (Instron, Norwood, MA, USA) equipped with a 1 kN load cell using a velocity of 0.1 mm/s. Axial torsion was measured with Instron 5943 (Instron) equipped with a 1 kN/25 Nm load cell, with an angular velocity of 1 degree/s. All tests were carried out as single ramp-to-failure tests and the maximum value of the force or moment attained during the test was recorded. The failure was observed as a clear drop in the load signal. Reference values were defined for all loading modes. For compression, we targeted a magnitude of IVD average stress of 0.8 MPa, which is considered degenerative for young bovine IVDs.21 For tension, we targeted 0.5 MPa, which is adequate for the measurement of the linear region in tension.22 For axial torsion, the linear region of the moment-rotation curve and a rotation of 12 degrees were targeted, which was previously shown to induce degeneration.23 For lateral bending, in the absence of degenerative loading data in the literature, we targeted to attain the linear region.
Figure 2.
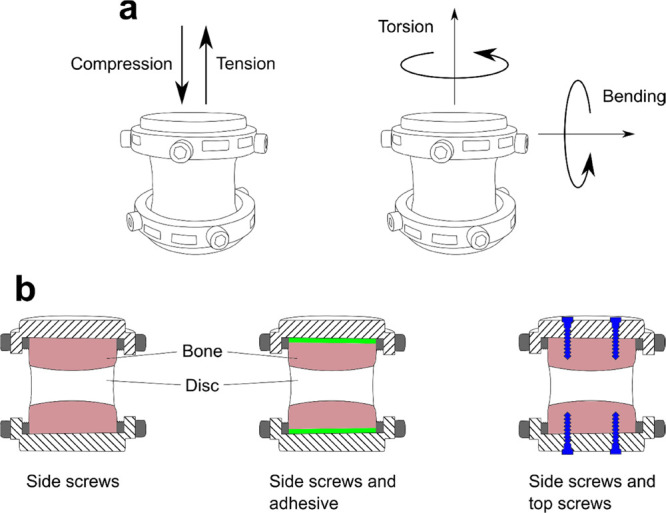
Set up for mechanical tests. Measurements were conducted in compression, tension, axial torsion, and lateral bending (a). Holder prototype and IVD assembly used in these tests are shown in Figure S1. Compression and torsion properties were measured for holder setup with side screws. Tensile and lateral bending properties were measured for the setup with side screws, side screws and adhesive at the interface, and side screws with additional top screws (b).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 8 software (GraphPad, San Diego, CA, USA). The normality of the cell viability data was analyzed using D’Agostino and Pearson tests. In data sets from day 0 (outer AF and NP) from the study in uniaxial bioreactor and all data sets except day 0 (outer AF) from the study in the multiaxial bioreactor, the normality test failed to confirm the normal distribution of data. It stems from the fact that these data sets contain at least one outlier point falling from the mean ± 2 standard deviations (SD), whereas all other points fall within mean ± SD. In all other data sets, the D’Agostino and Pearson’s test confirmed the normal distribution of data sets (p > 0.05). To compare normally distributed data assuming similar SDs, we performed an unpaired parametric t-test. An unpaired nonparametric Kolmogorov–Smirnov t-test was performed between not normally distributed groups. Relative height change was analyzed using a multiple t-test based on the Holm–Sidak method. Data were considered statistically significant when p < 0.05.
Results
IVD Height Changes after Loading in the Uniaxial Bioreactor
Following compressive daily loading in the uniaxial bioreactor, IVD height was reduced up to a maximum of 8 and 11% after 3 weeks of culture in the 1 DOF and 6 DOF models, respectively, but was not significantly different between the two models (Figure 3). Following free swelling, IVD height increased in the 1 DOF model up to 3% during 2 weeks of culture and 6% after 3 weeks. The maximal increase for the 6 DOF model was 8% after 2 weeks and 15% after 3 weeks of IVD culture and was significantly higher than that in the 1 DOF model.
Figure 3.
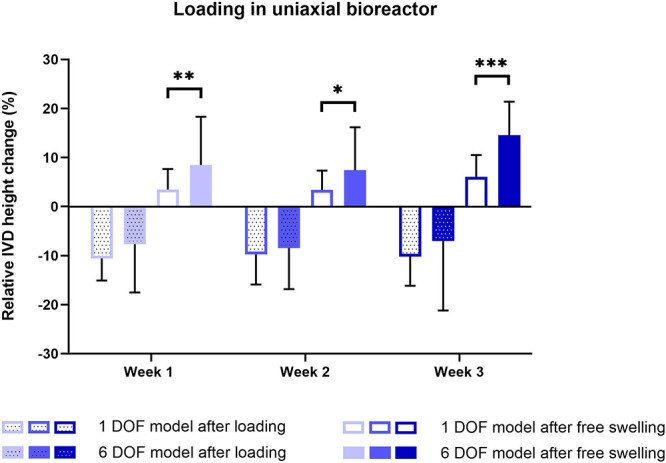
Relative height changes during 3 weeks of IVD culture under physiological conditions in the uniaxial bioreactor. The height was measured daily after loading and free swelling recovery and was calculated relative to the initial IVD height after dissection. Data are shown as the mean of two biological sample replicates measured throughout 7 days + standard deviation. Statistical analysis was performed using a multiple two-sample t test, where p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***) were statistically significant.
Cell Viability after Loading in the Uniaxial Bioreactor
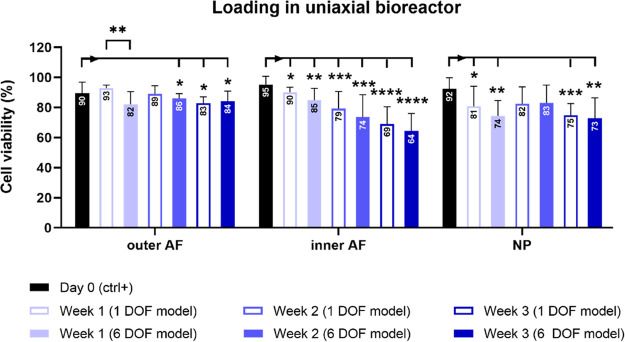
After 3 weeks of alternating physiological loading in the uniaxial bioreactor and free swelling culture, the number of alive cells significantly decreased in all IVD regions when compared to the day 0 samples (Figures 4 and 5). The most significant reduction was in the inner AF region after 3 weeks in both 1 DOF and 6 DOF models. No significant difference in the cell viability between the model groups was detected throughout the whole culture period, except in the outer AF tissue after the first week of culture.
Figure 4.
Cell viability during 3 weeks of IVD culture under physiological conditions in the uniaxial bioreactor. The viability was assessed with lactate dehydrogenase and ethidium homodimer staining and quantified in 1 DOF and 6 DOF models in outer annulus fibrosus (AF), inner AF, and nucleus pulposus (NP) regions on IVD sections. Day 0 represents a nonloaded positive control group. Data are shown as the mean of four regions of interest from 2 (week 1, 2, and 3) or 3 (day 0) biological replicates + standard deviation. Statistical analysis was performed using a nonparametric and parametric t-test, where p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), and p < 0.0001 (****) were statistically significant. Asterisks above a bar indicate a significant difference between day 0 and the loaded groups, and asterisks between the bars indicate a significant difference between the 1 DOF and 6 DOF models.
Figure 5.
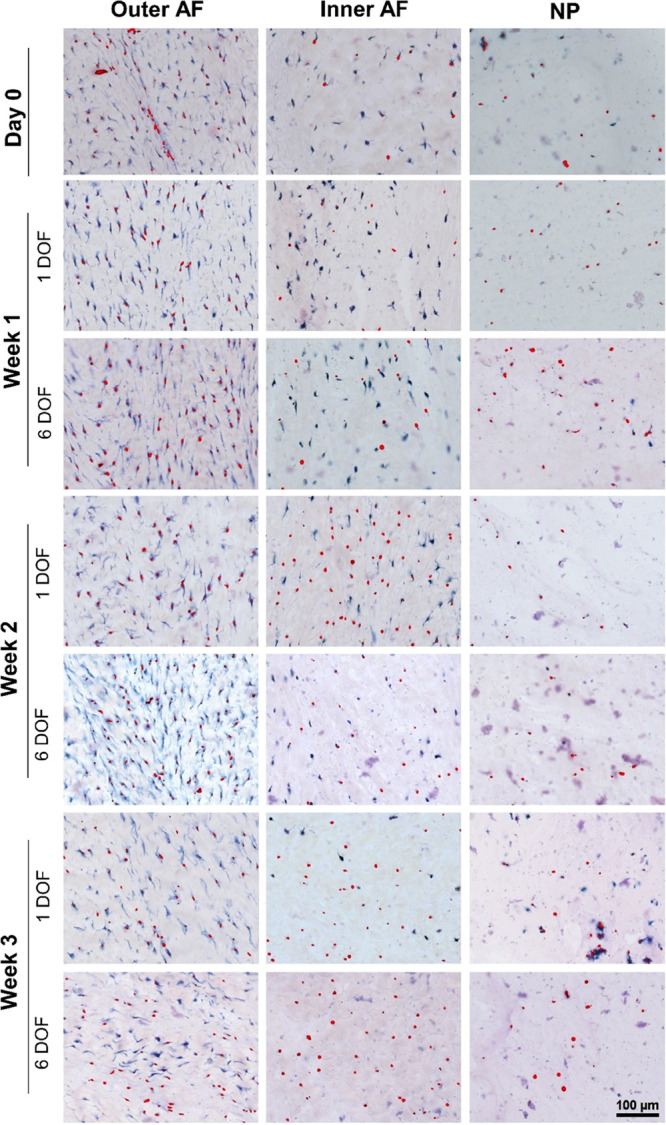
Representative images of different IVD regions on histology sections assessed with lactate dehydrogenase and ethidium homodimer-1 staining. Sections show 1 DOF and 6 DOF model groups from week 1 to week 3, and day 0 nonloaded control group. Cells stained with blue and blue/red indicate alive cells, and cells stained with red indicate dead cells.
Cell Viability after Loading in the Multiaxial Bioreactor
After 9 days of physiological loading in the multiaxial bioreactor, the cell viability in outer and inner AF was similar to that in the day 0 control (Figure 6). In comparison to the control group, the viability significantly decreased in the NP region by 16%.
Figure 6.
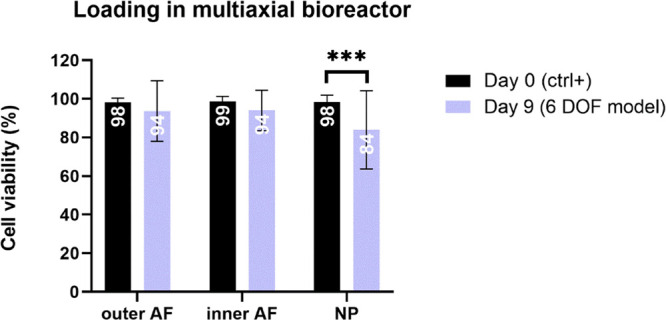
Cell viability after 9 days of IVD culture under physiological conditions in the multiaxial bioreactor. The viability in the 6 DOF model was assessed with lactate dehydrogenase and ethidium homodimer staining and quantified in outer AF, inner AF, and NP regions on IVD sections. Day 0 represents a nonloaded positive control group. Data are shown as the mean of 4 (outer AF) or 6 (inner AF and NP) regions of interest from 4 biological replicates + standard deviation. Statistical analysis was performed with an unpaired nonparametric t-test, where p < 0.001 (***) was statistically significant.
Mechanical Properties of the Holder–IVD Specimen Interface
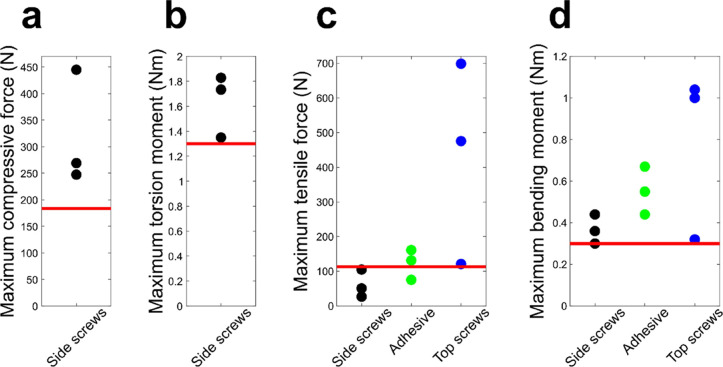
The maximum compressive force attained with the side screw setup was substantially higher than the reference value (0.8 MPa) indicated with a red line (Figure 7a). All the samples in the compressive test failed by NP herniation through the CEP in the central hole (not shown). In torsion tests, all three individual measurements attained the linear region and exhibited torsion moment values higher than the reference value (the moment at 12 degrees of rotation; Figure 7b). All torsion samples failed by vertebral bone cracking at the holder interface. For tension, the setups with top screws and adhesive showed higher tensile strength than the setup with side screws (Figure 7c). The corresponding tensile strength values achieved with the adhesive (tensile strength of the adhesive) were 0.75, 1.13, and 1.32 MPa. The top screw setup exhibited the highest forces regarding the targeted tensile value, although with variable outcomes. In tension, the samples failed by slipping from the holder (side screws), at the adhesive-holder interface (adhesive) or by bone cracking (top screws). The linear region in bending was attained at 0.3 Nm and was considered as the reference. All three approaches were performed at or above the reference value. However, the maximum bending moment values indicated that the top screw setup performed better than the two other setups but exhibited some variation (Figure 7d). In bending, the samples failed by slipping from the holder or by bone cracking (side screws), at the adhesive-holder interface (adhesive) or by bone cracking (top screws).
Figure 7.
Mechanical properties of the holder-IVD specimen interface. The graphs indicate maximum compressive force and maximum torsion moment for the specimen holder setup with side screws and maximum tensile force and bending moment for all holder setups. Red lines indicate targeted reference values,21−23 which represent loading magnitudes at the linear region for tension and bending, and degenerative loading for compression and torsion.
Discussion
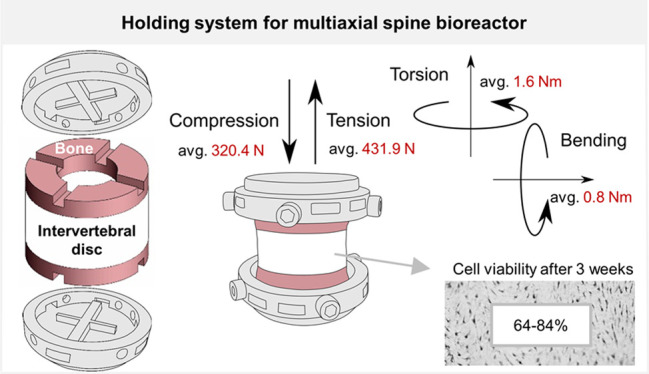
We have successfully designed and evaluated a customized holding system integrated into a corresponding 6 DOF organ model for IVD studies intended to apply multiaxial loading in a new generation of spine bioreactors. In long-term physiological culture conditions, the 6 DOF model maintained similar viability and disc height changes as the 1 DOF model. A validation study in the multiaxial bioreactor confirmed maintenance of high cell viability after 9 days of loading. The holder–IVD interface was able to withstand degenerative loads in compression, tension, torsion, and bending.
The 1 DOF model for the application of dynamic compression in the uniaxial bioreactor retains only the CEP and a thin layer of bone.7,24 A challenge in the design of an ex vivo IVD model for multiaxial loading was to keep enough bony elements to secure the specimen yet retain enough mobility for 6 DOF motions like torsion and bending and, most importantly, to avoid compromising the IVD nutrition. The 6 DOF model has 7 mm of bone on each side of the IVD, including the CEP and GP, except in the central region where most of the bone is removed to enable nutrition infiltration. Such a high bone volume was preserved not only to provide a good fit to the holder but also to avoid making a cross at the level of the GP, which has shown to be susceptible to cracking in our preliminary tests. IVD organ culture models with preserved adjacent vertebral bone are often unable to achieve long-term IVD cell viability.17,25 Removal of GP and implementation of loading or a glucose-enhanced medium can increase the chances of long-term IVD survival.10,25,26 The 6 DOF model, despite a partially preserved GP, additional machining procedure for cross drilling, and a narrower surface for nutrient access that is crucial for IVD survival,24,25 could in our study maintain similar levels of cell viability as the 1 DOF model with a minimum bone. A uniaxial IVD bioreactor exerting compressive force was an obvious choice for biological validation of the 6 DOF model, as it has been widely used in our research. Because we have recently advanced with the multiaxial bioreactor development to the phase where it is possible to perform loading tests, we applied the same physiological loading protocol on the 6 DOF model. The loading in the multiaxial bioreactor maintained higher cell viability in the 6 DOF model (94% in outer and inner AF, and 84% in NP) than when it was loaded in the uniaxial bioreactor for 1 week (82% in outer AF, 85% inner AF, and 74% NP). Good viability in the 6 DOF model was achieved by multiple accesses to medium through small holes and side openings in the holder design. As evident from this data and the previous research conducted by our group,8,27 the region where it usually comes to the fastest decline in cell viability is the center of the IVD. The viability in the NP region after 3 weeks of loading in the uniaxial bioreactor was in our study 75 and 73% in the 1 DOF and 6 DOF organ models, respectively, which we consider a good outcome after a long period of in vitro organ culture. However, cell viability in the inner AF region was after 3 weeks maintained at only 69 and 64% in 1 DOF and 6 DOF models, respectively, which can be considered as a significant reduction. Given that the decline was observed in both models, we can discard the possibility that the partial retention of vertebral bone next to the central hole in the 6 DOF model compromises the infusion of the nutrients toward inner AF, whereas the NP and outer AF zones are supplied through the central hole and direct contact with the medium, respectively. However, the changes could be related to the reduction of nutrients toward the inner AF, irrespective of the model, as well as to the different magnitudes of strain distribution throughout the IVD. As previously shown on human IVDs and quantified with MRI,28 when 1000 N compression was applied, magnitudes of axial and radial strains were higher in the inner AF than in other IVD regions, indicating that inner AF may be more susceptible to cell death and disc damage after longer culture periods. The loading parameters could therefore be adjusted to exert less compressive stress on the inner AF and NP regions. Because the interface between inner AF and NP is the region where the IVD changes in morphological and compositional properties occur, it is likely that in the previous research, this region was neglected in cell counting. However, because of the high cell death observed there, the choice of regions for counting should be reconsidered in future studies. Despite a decrease in cell viability throughout the whole tissue, we can conclude that the IVD maintained its viscoelastic properties and responded to loading and recovery within the expected range of height changes, which is up to 10% for physiological loading.9
Surprisingly, the 6 DOF model showed more capacity to recover the height between loading cycles. Height changes in the 6 DOF model were measured by deducting the bone from the total specimen height. The concave shape of bovine CEPs6 makes it difficult to precisely define the interface between IVD and bone. Inaccurate measurements (i.e., deducting more bone) may have indicated larger swelling capacities than is the reality. However, it is most likely that the 1 DOF model with minimum bone is more exposed to the liquid uptake during the IVD preparation process. This may have led to an increase in the initial IVD volume and smaller changes in swelling during overnight recovery after loading cycles.
The holding system is a crucial part of an IVD bioreactor as it must enable a successful transmission of loads onto the sample while maintaining biological requirements like previously mentioned nutrient access. An obstacle in choosing a proper holder design is the geometry of bovine IVDs, which often differ in diameter, thickness, and shape.29 An ideal holder should be adaptable to these differences and provide the reproducibility of motions for various IVD shapes. We have introduced a circular holder that supports IVD dimensions of a maximum of 25 mm in diameter. Its design makes it adaptable to specimen variations in shape. For example, irregular IVD shapes can be compensated using side screws of different lengths attached to the bone in 4 or 6 positions, depending on the holder size, closer or further away from the holder. Additionally, such a holder is easy to manufacture in smaller or bigger sizes to adapt better to a model. To enhance the robustness of fixation, we adapted the top surface of the bone by introducing a cross pattern of the same dimensions as its counterpart in the holder. Such an approach stabilizes the sample in the center regardless of size and shape. Reproducibility of the cross-making is important; we, therefore, introduced a tool with a custom-made clamping system for IVD to machine a cross of consistent dimensions. In addition to the cross pattern, the specimen was secured by tightening screws to the side of the bone. This approach can easily withstand degenerative compressive force as the values obtained in mechanical tests were substantially higher than those targeted. Additionally, the samples failed via herniation of NP through the CEP, suggesting that the maximum compression was not limited by the specimen holder. Similarly, the approach with side screws performed well when torsion was applied. However, the mechanical tests implied that fixation with side screws only may not be sufficient to provide a tight grip for critical motions such as tension or bending. We, therefore, showed that the system could be adapted with adhesive or additional top screws to enhance the interface performance, thus expanding the range of loads that can be applied to the IVD. The specimen holder setup with top screws performed the best in resisting tensile force and lateral bending, but the difference to the other setups was more evident in tensile loading. The fixation strength for the adhesive may have been limited to its application across a small bone area; therefore, future testing could look to maximize the adhesive’s contact with the available bone surface area and/or its use to augment the screw fixation strength to the bone. We thus conclude that when applying tensile and lateral bending loads, the top screw setup should be used, whereas for compressive and torsional loads, the setup with side screws is adequate for loading in bioreactor. However, the variability of outcomes observed between individual samples, which could be related to the density of bony elements or uneven tightening of the screws onto the bone, as these were the failure locations, implies that the mechanical capacity of the holder–IVD interface should be further assessed in the bioreactor setting including more samples. A limitation of the mechanical testing of the holder is that the performance was evaluated based on failure loads only. As evidenced in earlier research,30 relative movements could occur between the bone and holder before failure, potentially affecting the mechanical evaluation of the IVD. A comparison with common techniques, such as potting in PMMA or the use of specimen-specific 3D-printed holder inserts,30 could give further insight into the mechanical capacities of the holder. In addition, the reference values for loads were based on literature data or by targeting the linear region of the load-deformation curve. However, we are currently working on the development of computational models that would more accurately predict the range of loads necessary to retain physiological homeostasis or induce degeneration.
Conclusions
The system presented herein will provide a basis for the further development of a multiaxial IVD bioreactor, intending to be the first such system for the 6 DOF mechano-biological control of IVD specimens in in vitro conditions. It should bring a novel and unique platform for testing engineered biomaterials, biological grafts, or therapies in more realistic physiological conditions of the spine.
Acknowledgments
This work was supported by the Swiss National Science Foundation under grant number 189915; the AO Foundation and AO Spine. We thank Dr. Christoph Sprecher for his help with histology image processing.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsbiomaterials.2c00330.
Figure S1. Prototype of the specimen holder used in mechanical tests (PDF)
Author Contributions
A.Š.: conceptualization, methodology, validation, formal analysis, investigation, writing-original draft, writing- review and editing, visualization. A.R.: conceptualization, methodology, validation, formal analysis, investigation, writing-original draft, writing- review and editing, visualization. S.C.: conceptualization, methodology, formal analysis, investigation. Z.L.: conceptualization, methodology, supervision. A.S.: conceptualization. M.A.: conceptualization, supervision. S.J.F.: funding acquisition, resources, supervision. G.W.: funding acquisition, resources, supervision. S.H.: funding acquisition, resources, conceptualization, validation. D.L.: resources, conceptualization, validation, project administration. S.G.: funding acquisition, project administration, supervision.
The authors declare no competing financial interest.
Supplementary Material
References
- Chan S. C.; Ferguson S. J.; Gantenbein-Ritter B. The effects of dynamic loading on the intervertebral disc. Eur. Spine J. 2011, 20, 1796–1812. 10.1007/s00586-011-1827-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter B. A.; Korecki C. L.; Purmessur D.; Roughley P. J.; Michalek A. J.; Iatridis J. C. Complex loading affects intervertebral disc mechanics and biology. Osteoarthritis Cartilage 2011, 19, 1011–1018. 10.1016/j.joca.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergroesen P. P.; Kingma I.; Emanuel K. S.; Hoogendoorn R. J.; Welting T. J.; van Royen B. J.; van Dieen J. H.; Smit T. H. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthritis Cartilage 2015, 23, 1057–1070. 10.1016/j.joca.2015.03.028. [DOI] [PubMed] [Google Scholar]
- Dai J.; Xing Y.; Xiao L.; Li J.; Cao R.; He Y.; Fang H.; Periasamy A.; Oberhozler J.; Jin L.; et al. Microfluidic Disc-on-a-Chip Device for Mouse Intervertebral Disc-Pitching a Next-Generation Research Platform To Study Disc Degeneration. ACS Biomater. Sci. Eng. 2019, 5, 2041–2051. 10.1021/acsbiomaterials.8b01522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang M. H.; Cho D. H.; Baek S. M.; Lee J. W.; Park J. H.; Yoo C. M.; Shin J. H.; Nam H. G.; Son H. G.; Lim H. J.; et al. Spine-on-a-chip: Human annulus fibrosus degeneration model for simulating the severity of intervertebral disc degeneration. Biomicrofluidics 2017, 11, 064107 10.1063/1.5005010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantenbein B.; Illien-Junger S.; Chan S. C.; Walser J.; Haglund L.; Ferguson S. J.; Iatridis J. C.; Grad S. Organ culture bioreactors--platforms to study human intervertebral disc degeneration and regenerative therapy. Curr. Stem Cell Res. Ther. 2015, 10, 339–352. 10.2174/1574888x10666150312102948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannkuche J. J.; Guo W.; Cui S.; Ma J.; Lang G.; Peroglio M.; Richards R. G.; Alini M.; Grad S.; Li Z. Intervertebral disc organ culture for the investigation of disc pathology and regeneration - benefits, limitations, and future directions of bioreactors. Connect. Tissue Res. 2020, 61, 304–321. 10.1080/03008207.2019.1665652. [DOI] [PubMed] [Google Scholar]
- Lang G.; Liu Y.; Geries J.; Zhou Z.; Kubosch D.; Sudkamp N.; Richards R. G.; Alini M.; Grad S.; Li Z. An intervertebral disc whole organ culture system to investigate proinflammatory and degenerative disc disease condition. J. Tissue Eng. Regener. Med. 2018, 12, e2051–e2061. 10.1002/term.2636. [DOI] [PubMed] [Google Scholar]
- Li Z.; Lezuo P.; Pattappa G.; Collin E.; Alini M.; Grad S.; Peroglio M. Development of an ex vivo cavity model to study repair strategies in loaded intervertebral discs. Eur. Spine J. 2016, 25, 2898–2908. 10.1007/s00586-016-4542-0. [DOI] [PubMed] [Google Scholar]
- Walter B. A.; Illien-Junger S.; Nasser P. R.; Hecht A. C.; Iatridis J. C. Development and validation of a bioreactor system for dynamic loading and mechanical characterization of whole human intervertebral discs in organ culture. J. Biomech. 2014, 47, 2095–2101. 10.1016/j.jbiomech.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junger S.; Gantenbein-Ritter B.; Lezuo P.; Alini M.; Ferguson S. J.; Ito K. Effect of limited nutrition on in situ intervertebral disc cells under simulated-physiological loading. Spine 2009, 34, 1264–1271. 10.1097/BRS.0b013e3181a0193d. [DOI] [PubMed] [Google Scholar]
- Illien-Junger S.; Gantenbein-Ritter B.; Grad S.; Lezuo P.; Ferguson S. J.; Alini M.; Ito K. The combined effects of limited nutrition and high-frequency loading on intervertebral discs with endplates. Spine 2010, 35, 1744–1752. 10.1097/BRS.0b013e3181c48019. [DOI] [PubMed] [Google Scholar]
- Amin D. B.; Lawless I. M.; Sommerfeld D.; Stanley R. M.; Ding B.; Costi J. J. The effect of six degree of freedom loading sequence on the in-vitro compressive properties of human lumbar spine segments. J. Biomech. 2016, 49, 3407–3414. 10.1016/j.jbiomech.2016.09.009. [DOI] [PubMed] [Google Scholar]
- Costi J. J.; Stokes I. A.; Gardner-Morse M.; Laible J. P.; Scoffone H. M.; Iatridis J. C. Direct measurement of intervertebral disc maximum shear strain in six degrees of freedom: motions that place disc tissue at risk of injury. J. Biomech. 2007, 40, 2457–2466. 10.1016/j.jbiomech.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsgrove T. P.; Gheduzzi S.; Gill H. S.; Miles A. W. The development of a dynamic, six-axis spine simulator. Spine J. 2014, 14, 1308–1317. 10.1016/j.spinee.2013.11.045. [DOI] [PubMed] [Google Scholar]
- Wilke H. J.; Kienle A.; Maile S.; Rasche V.; Berger-Roscher N. A new dynamic six degrees of freedom disc-loading simulator allows to provoke disc damage and herniation. Eur. Spine J. 2016, 25, 1363–1372. 10.1007/s00586-016-4416-5. [DOI] [PubMed] [Google Scholar]
- Chan S. C.; Gantenbein-Ritter B. Preparation of intact bovine tail intervertebral discs for organ culture. J. Visualized Exp. 2012, 3490. 10.3791/3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravi B.; Lang G.; Grad S.; Alini M.; Richards R. G.; Schmal H.; Sudkamp N.; Li Z. A Proinflammatory Degenerative Organ Culture Model to Simulate Early-Stage Intervertebral Disc Disease. J. Visualized Exp. 2021, 62100. 10.3791/62100. [DOI] [PubMed] [Google Scholar]
- Li Z.; Gehlen Y.; Heizmann F.; Grad S.; Alini M.; Richards R. G.; Kubosch D.; Sudkamp N.; Izadpanah K.; Kubosch E. J.; et al. Preclinical ex-vivo Testing of Anti-inflammatory Drugs in a Bovine Intervertebral Degenerative Disc Model. Front. Bioeng. Biotechnol. 2020, 8, 583. 10.3389/fbioe.2020.00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadala G.; Russo F.; Pattappa G.; Peroglio M.; Stadelmann V. A.; Roughley P.; Grad S.; Alini M.; Denaro V. A Nucleotomy Model with Intact Annulus Fibrosus to Test Intervertebral Disc Regeneration Strategies. Tissue Eng., Part C 2015, 21, 1117–1124. 10.1089/ten.TEC.2015.0086. [DOI] [PubMed] [Google Scholar]
- Paul C. P.; Schoorl T.; Zuiderbaan H. A.; Zandieh Doulabi B.; van der Veen A. J.; van de Ven P. M.; Smit T. H.; van Royen B. J.; Helder M. N.; Mullender M. G. Dynamic and static overloading induce early degenerative processes in caprine lumbar intervertebral discs. PLoS One 2013, 8, e62411 10.1371/journal.pone.0062411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhitpanichkul M.; Dreischarf M.; Illien-Junger S.; Walter B. A.; Nukaga T.; Long R. G.; Sakai D.; Hecht A. C.; Iatridis J. C. Fibrin-genipin adhesive hydrogel for annulus fibrosus repair: performance evaluation with large animal organ culture, in situ biomechanics, and in vivo degradation tests. Eur. Cell Mater. 2014, 28, 25–37. 10.22203/ecm.v028a03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey-Burgess M.; Gregory D. E. The Effect of Axial Torsion on the Mechanical Properties of the Annulus Fibrosus. Spine 2019, 44, E195–E201. 10.1097/BRS.0000000000002803. [DOI] [PubMed] [Google Scholar]
- Gantenbein B.; Grunhagen T.; Lee C. R.; van Donkelaar C. C.; Alini M.; Ito K. An in vitro organ culturing system for intervertebral disc explants with vertebral endplates: a feasibility study with ovine caudal discs. Spine 2006, 31, 2665–2673. 10.1097/01.brs.0000244620.15386.df. [DOI] [PubMed] [Google Scholar]
- Lee C. R.; Iatridis J. C.; Poveda L.; Alini M. In vitro organ culture of the bovine intervertebral disc: effects of vertebral endplate and potential for mechanobiology studies. Spine 2006, 31, 515–522. 10.1097/01.brs.0000201302.59050.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant M.; Epure L. M.; Salem O.; AlGarni N.; Ciobanu O.; Alaqeel M.; Antoniou J.; Mwale F. Development of a Large Animal Long-Term Intervertebral Disc Organ Culture Model That Includes the Bony Vertebrae for Ex Vivo Studies. Tissue Eng., Part C 2016, 22, 636–643. 10.1089/ten.TEC.2016.0049. [DOI] [PubMed] [Google Scholar]
- Zhou Z.; Cui S.; Du J.; Richards R. G.; Alini M.; Grad S.; Li Z. One strike loading organ culture model to investigate the post-traumatic disc degenerative condition. J. Orthop. Translat. 2021, 26, 141–150. 10.1016/j.jot.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell G. D.; Vresilovic E. J.; Elliott D. M. Human intervertebral disc internal strain in compression: the effect of disc region, loading position, and degeneration. J. Orthop. Res. 2011, 29, 547–555. 10.1002/jor.21232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco L. A.; DeWitte-Orr S. J.; Gregory D. E. A Comparison Between Porcine, Ovine, and Bovine Intervertebral Disc Anatomy and Single Lamella Annulus Fibrosus Tensile Properties. J. Morphol. 2016, 277, 244–251. 10.1002/jmor.20492. [DOI] [PubMed] [Google Scholar]
- Cornaz F.; Burkhard M.; Fasser M. R.; Spirig J. M.; Snedeker J. G.; Farshad M.; Widmer J. 3D printed clamps for fixation of spinal segments in biomechanical testing. J. Biomech. 2021, 125, 110577 10.1016/j.jbiomech.2021.110577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.