Abstract
Commercial dentin adhesive systems are applied to restorations due to their resistant bonding properties, but they suffer from the lack of bioactivity and are prone to hydrolysis. Therefore, to overcome these limitations, an eco-friendly natural monomer, urushiol, was adopted to be a primer in dentin bonding due to its interaction with collagen and antibacterial activity, preventing further hydrolysis development. First, urushiol was determined to be capable of improving the biological stability of dentin collagen through cross-linking. Using high-fidelity analytical chemistry techniques, such as Fourier transform infrared spectroscopy, we quantified the effects of urushiol on collagen molecules. It could also effectively decrease weight loss after collagenase ingestion by improving the stability of dentin. Moreover, urushiol inhibited Streptococcus mutans growth as well as its biofilm formation. Finally, we demonstrated that the urushiol primer could improve the bonding strength, particularly after aging. The cross-linking and antibacterial functions of urushiol have provided promising developmental prospects for biomaterials in dentin adhesion.
Keywords: urushiol, antibacterial activity, cross-linking, primer, bonding strength
1. Introduction
A hybrid layer, composed of infiltrated resin monomers and demineralized dentin collagen is the key to dentin bonding.1−5 In the etch-and-rinse mode, however, this resin–dentin composite is vulnerable to enzymatic degradation with time as the collagen fibrils that are incompletely infiltrated by monomers6−9 and the current bonding resins are not able to totally remove the free and slackly bound water within the collagen matrix.10 Moreover, the cured adhesive layer may act as a semipermeable membrane that allows water diffusion from the bound dentine to the hybrid layer, leading to the reliable long-term dentin bonding being challenged.11,12 Endogenous proteases, including the matrix metalloproteinases (MMPs) and cysteine cathepsins, are exposed and reactivated after etching, and progressively degrades collagen and results in the loss of adhesive restoration retention.2,13−15
From the relationship between the dentin structure and adhesive, the collagen structure of the demineralized dentin is unavoidably subjected to degradation, whatever the diverse situations in oral. Enhancement of collagen cross-links has been proven to increase the resin bonding.16−19 External collagen cross-linking agents may cause supplementary inter- and intramolecular cross-links, improving the bonding durability of the demineralized collagen network in coronal dentin7,20−23 along with its resistance to enzymatic degradation.16,24 Moreover, the conservation of the collagen fibrils may not only act as a barrier to acid dispersion and mineral loss but also as a facilitator of mineral deposition during the remineralization process. The catechol group of natural collagen cross-linkers has an effect on proteins to trigger cross-linking by different mechanisms including covalent interactions, ionic interactions, hydrogen bonding, and hydrophobic interactions.19,25,26
Dental caries is a common disease caused by biofilms with a significant effect of Streptococcus mutans.27 Antibacterial components have been studied that were added to the adhesive or as the composition of the new monomer.28,29 As a result of the increasing tolerance to antibiotics, the safety, availability, and relatively low costs of natural materials have been used for protecting against caries and merged into dental materials. The lacquer sap was broadly applied as coatings and adhesive by Neolithic humans at the Kuahuqiao site thousands of years ago.30 Urushiol, a natural monomer obtained from the raw lacquer sap that has a catechol group and a linear unsaturated side chain,31 not only has antibacterial and antioxidant properties32 but also excellent corrosion and water resistance.33,34 Its antibacterial activity may be related to attacking the outer membrane and bleb formation on bacteria.35
Moreover, the catechol group of urushiol may have the ability to promote collagen cross-linking with its intrinsic antibacterial activity, which can be used in dentistry. Although urushiol has been used for many years, almost no study has explored its effects as a primer on adhesive bonding and the structure of demineralized dentin. In addition, a primer should have comparable interests, and more profitably the benefit is available to etch-and-rinse adhesive, as collagen is an important part of the interface. The motivation of this research is the potentials of the promotion effect of the collagen cross-linking primer in dentin bonding. Herein, we established for the first time an urushiol primer that can simultaneously exert collagen cross-linking and antibacterial effects to achieve durable dentin bonding in a complex oral environment even after aging. The antibacterial activity of urushiol was demonstrated against S. mutans. Furthermore, to obtain a more representative estimation of the urushiol primer, we performed the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay on the S. mutans biofilm on the dentin surface treated by the urushiol primer. The outcomes indicate that pretreating the dentin surface with the novel multifunctional primers provides a potential reliable operative strategy for dentists to enhance the durability of dentin bond strength.
2. Materials and Methods
2.1. Materials
Urushiol was purchased from Wuhan National Lacquer Co., Ltd. (Wuhan, China). Collagenase with a molecular weight of ∼110 kDa was obtained from Sigma-Aldrich (Beijing, China). Ethanol, dimethyl sulfoxide (DMSO), glutaraldehyde (GA), CaCl2, sodium azide, and phosphate buffer saline were purchased from Aladdin (Shanghai, China). The S. mutans ACTT UA159 (Maryland) was incubated in the brain heart infusion (BHI) broth from QingDaoHopebio-Technology Co., Ltd. (Qingdao, China) supplemented with 5% sucrose at 37 °C with 5% CO2 for 48 h. The hydroxyproline assay kit was purchased from Solarbio (Shanghai, China). Resin composite Filtex Z350 XT and Adper Single Bond 2 were obtained from 3 M (St.Paul).
2.2. Collagen Cross-Linking
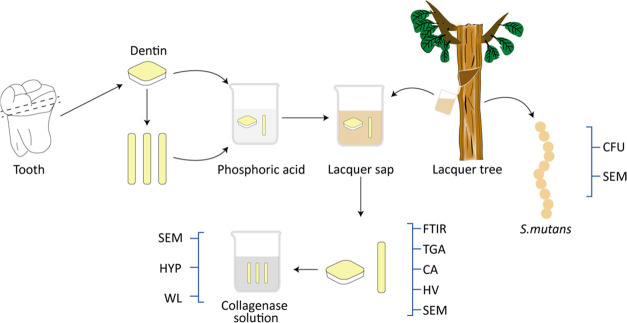
The collagen cross-linking test process is illustrated in Figure 1.
Figure 1.
Schematic of the collagen cross-linking tests.
2.2.1. Preparation of the Urushiol Solution
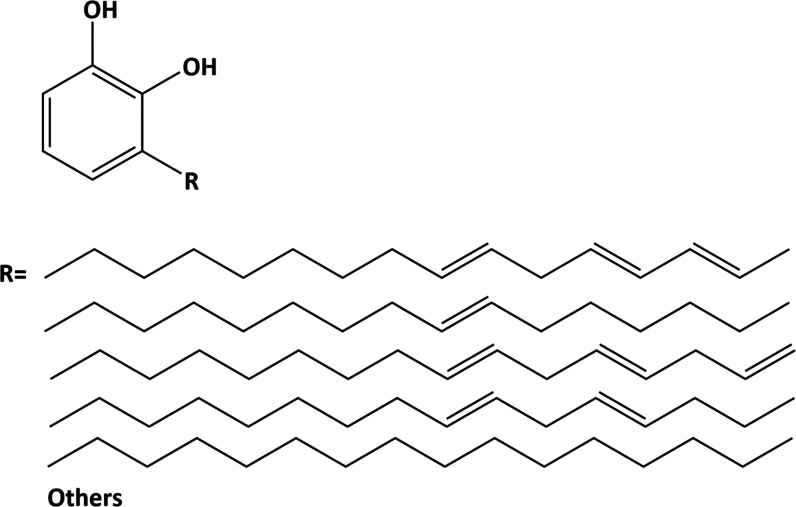
Urushiol (0.5, 1, and 3 wt %) was dissolved in ethanol. The structure of urushiol is shown in Figure 2. Glutaraldehyde was dissolved at 5 wt % in distilled water as the positive control group. Deionized water was the blank control group. The solutions were buffered to pH 7.2.
Figure 2.
Chemical configuration of urushiol; R indicates the long saturated or unsaturated alkyl side chain.
2.2.2. Preparation of the Dentin Specimens
Non-carious human third molars were obtained from the patients of certain ages (18 < age < 30 years) and approved by the Ethics Committee of the Stomatology Hospital of Jilin University. The root and occlusal portions of teeth were cut away by a slow-speed water-cooled diamond saw (Kejing, China) to obtain a 1.2 mm thick slab in the middle, which was cut into beams (1 mm × 2 mm × 6 mm). Dentin slabs and beams were immersed in 10% phosphoric acid for 48 h to be completely demineralized and then characterized by digital radiography. The samples were randomly distributed into 7 groups and then immersed in 1 mL of each solution (n = 15). After biomodification, the samples were rinsed with excessive deionized water and dried in a desiccator for 24 h.
2.2.3. Fourier Transform Infrared (FTIR) Spectra
The demineralized dentin collagen beams were respectively soaked in deionized water and in the experimental urushiol solutions for 1 min and 24 h, and then rinsed and dried. The biomodification effect of samples was detected by Fourier transform infrared (FTIR) spectra at a resolution of 4 cm–1. The slabs were placed on the diamond crystal top plate of an attenuated total reflectance (ATR) accessory with a gauge force of 100 N. The range of all spectra was between 650 and 3700 cm–1.
2.2.4. Thermogravimetric Analysis (TGA) Test
The beams were treated with distilled water (control), GA and urushiol solutions for 24 h. Thermogravimetric analysis was performed under a nitrogen atmosphere using a thermogravimetric analyzer (TA instrument). Temperature increased at the rate of 10 °C/min from 35 to 700 °C.
2.2.5. Contact Angle (CA)
The hydrophilicity of the slabs (n = 5) was determined with a goniometer (Dataphysics OCA-20, Germany) by dropping 6 μL of deionized water onto the slab surface, which showed both left and right contact angles from the shape of the water drop at a room temperature of 25 °C.
2.2.6. Hardness
The Vickers hardness numbers (VHNs) of the samples were gauged using a Vickers hardness tester with a load of 50 g for 15 s. Each sample had five indentations on the surface, which were spaced at least 100 μm apart.
2.2.7. Swelling Ratio
The specimens were swollen by immersion in water at room temperature and equilibrated overnight in phosphate-buffered saline (PBS) (pH 7.4). The samples were weighed immediately after removing excess water from the surface with filter paper. Dentin specimens were then immersed in a large amount of deionized water to remove the buffer salts and dried to a constant weight. The swelling ratio was the weight of the swollen sample compared to the weight of the dried sample.
2.2.8. Collagenase Treatment
TESCA buffer was prepared by adding 11.5 g of N-tris(hydroxymethyl)-methyl-2-aminoethanesulfonic acid, 53 mg of CaCl2·2H2O, and 50 mg of sodium azide in distilled water to 1000 mL of deionized water and adjusting the pH to 7.4. Then, 100 μg of collagenase with a molecular mass of ∼110 kDa was added to the TESCA buffer at the final concentration of 0.1% (w/v).
2.2.9. Mass Change
Demineralized dentin beams were respectively soaked in different solutions for 24 h and then weighed (M0). Further weight measurements were performed after collagenase degradation (M1) for 48 h. After treatment, the beams should be dried in a vacuum desiccator with silica gel at room temperature for 72 h. The mass change (W%) was described by the percentage weight loss of individual samples, calculated by the following formula
| 1 |
2.2.10. Hydroxyproline Release
The collagenolytic activity was evaluated by measuring the hydroxyproline content in collagenase solution. Measurement of hydroxyproline in each sample was performed according to the instructions. Briefly, a 0.06 mL sample (collagenase solution) was mixed with 0.06 mL of reagent A. After a 20 min interval, 0.06 mL of reagent B and 0.12 mL of deionized water were added. Then, the samples were incubated at 60 °C for 20 min and then 0.2 mL of sample solution of each group was dropped into a 96-well plate. By the microplate reader, the absorbance was read at 560 nm.
2.2.11. Scanning Electron Microscopy (SEM)
Specimens that were treated by different solutions and after collagenase degradation were air-dried and then sprayed with gold. The micro-morphologies images of samples were observed by scanning electron microscopy (SEM).
2.3. Antibacterial Activities
2.3.1. Bacterial Strains and Cultivation Characteristics
S. mutans (UA159) was used for measuring the antibacterial activities of 0, 0.1, 0.5, 0.7, and 1 wt % urushiol. After 24 h of culture with the brain heart infusion (BHI), the bacterial strain was diluted to 1 × 108 CFU/mL.
2.3.2. Colony Counting Assay
The growth conditions of bacteria were assessed by the colony counting method. S. mutans were inoculated in the BHI broth with or without different concentrations of urushiol at 37 °C. Approximately 4 × 108 bacterial cells were inoculated into 1 mL of BHI medium with different concentrations of urushiol. At 0, 30, 60, and 90 min of bacterial incubation, the BHI agar plates were coated with the medium and colony-forming units (CFU) were counted. The test was repeated three times.
2.3.3. Antibacterial Evaluation by SEM
Scanning electron microscopy (SEM) graphs were analyzed using 24-well plates with glass slides. Before SEM imaging, S. mutans was treated with urushiol for 48 h at room temperature. The medium was extracted, and the wells were gently washed twice with sterilized deionized water. Samples were placed in a 2.5% glutaraldehyde solution overnight. Then, the specimens were dehydrated in different concentrations of ethanol washes (50, 70, 90, and 100% for 30 min) and air-dried. Before being visualized by SEM (S4800, Tokyo, Japan), the samples were sprayed with gold. The samples were observed in three views randomly for imaging.
2.4. Urushiol as the Primer
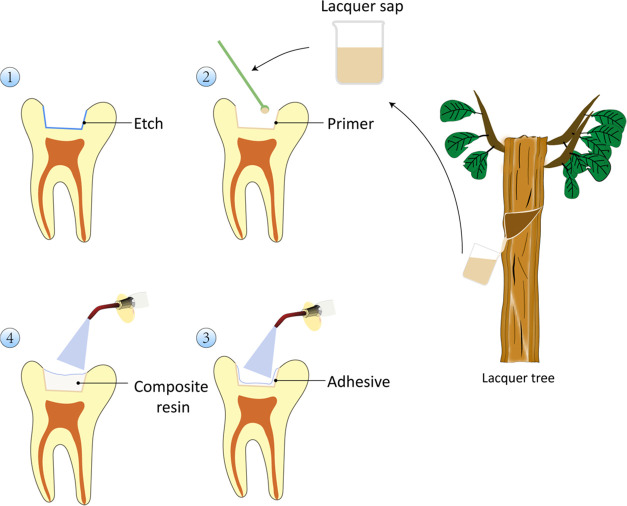
Figure 3 presents the process of applying the primer.
Figure 3.
Schematic of the primer application process.
2.4.1. Preparation of the Dentin Specimens and Primer Agents
Fifty freshly non-carious molars stored in 0.5% chloramine-T at 4 °C were randomly divided into eleven groups (n = 10). The occlusal portion of the teeth was cut away using a diamond saw to expose mid-depth dentin. The standard smear layer was prepared by grinding the dentin surface with 600-grit silicon carbide sandpaper. Dentin surfaces were etched with 37% phosphate gel for 15 s and then deeply rinsed with excessive deionized water.
Urushiol (0.1, 0.5, 0.7, and 1 wt %) dissolved in ethanol and DMSO were used as the primer agents.
2.4.2. Degree of Conversion
The effect of urushiol primers on the polymerization of commercial adhesive was explored by the degree of conversion (DC) test with a Fourier transform infrared spectrometer (n = 5). After urushiol pretreatment for 1 min, adhesives were applied and cured according to instructions, which were then immediately tested by FTIR. The DC values were estimated in accordance with the variation of the absorbance peak intensity of carbon–carbon double bonds (C=C, peak at 1636 cm–1) before and after curing, using the phenyl (peak at 1608 cm–1) as the internal reference. The DC was estimated by the following formula
| 2 |
2.4.3. Contact Angle
The dentin slabs were polished with 1000-grit silicon carbide sandpaper and ultrasonically rinsed twice for 5 min to eliminate the residue. The samples were etched with phosphate gel for 15 s and washed with deionized water for 15 s. Then, 6 μL of deionized water was dropped onto the slab surface using a contact angle meter (DataPhysics OCA-20).
2.4.4. Microtensile Bond Strength Test
As the control group, Adper Single Bond 2 was cured for 20 s followed the steps of the manufacturer’s instructions. Then, a resin composite build-up was constructed with two 2-mm increments and was light-cured for 40 s. The samples were stored in deionized water for 24 h at 37 °C. The specimens were cut parallel to the long axis into small rods with cross-sectioned area of 1.0 mm2 using a diamond saw under flowing water, and at least 6 small rods were expected to be cut out of each tooth. Sample rods were attached to the microtensile molds and the test was performed by a universal testing machine at a crosshead speed of 1 mm/min until failure. The cross-sectional area of the rods was measured with a digital caliper to the nearest 0.01 mm, and the microtensile strength (MPa) was the ratio of the maximum load to the cross-sectional area of the sample.
2.4.5. Aging
Half of the bonded samples were randomly selected to be aged (5 °C for 30 s and 55 °C for 30 s) for 5000 cycles in the thermocycling device (PTC2c; Proto-tech, Portland, ME). The μTBS test was performed as described above.
2.4.6. Nanoleakage
After thermocycling, the bonded samples were cut into 1 mm thick pieces parallel to the long axis of the tooth, and the middle two pieces were selected to be submerged in the 50% ammoniated silver nitrate solution for 24 h. The specimens were rinsed with deionized water, and then were submerged in the photo development solution with fluorescent irradiation for 8 h. After polished with SiC sandpapers (1000-, 1200-, 1500-, 2000- and 2500-grit), samples were then ultrasonically washed with deionized water for 30 min. The samples were air-dried after dehydration and then observed by SEM. In addition, the surfaces of the samples were determined by energy dispersive spectrometry (EDS).
2.4.7. Antibacterial Activity of Urushiol Primer
2.4.7.1. Preparation of the Dentin Disk
In accordance with the μTBS test, ethanol-0.7 wt % urushiol and DMSO-0.7 wt % urushiol groups were chosen to test the antibacterial activity of the urushiol primers. The root and occlusal portions of the non-carious teeth were cut away by the slow-speed diamond saw to obtain 0.5 mm thick dentin disks, which were then polished with 600-, 1200-, 2000-, 3000-grit SiC sandpapers under water and diamond paste (0.5 μm) and washed ultrasonically.
2.4.7.2. Bacterial Culture and Biofilm Preparation
Dentin disks were etched by 37% phosphate gel for 15 s, and then disinfected with UV for 1 h each side before biofilm preparation. The samples were pretreated with two primers for 1 min in 24-well plates and 1 mL of S. mutans suspension was added dropwise which was diluted to 108 CFU/mL in advance. After culture in an incubator to form biofilm-coated specimens at 37 °C for 24 h, the specimens were gently rinsed with sterile phosphate buffer saline (PBS) twice.
2.4.7.3. Antibacterial Evaluation by MTT Assay
The biofilm-coated specimens (n = 9) were transferred to a new 24-well plate and incubated at 37 °C after adding 1 mL of 0.5 mg/mL MTT solution dropwise to each well to estimate the biofilm metabolic activity. The same volume of dimethyl sulfoxide (DMSO) was added dropwise after the removal of MTT solution. After shaking for 30 min in the darkness, the absorbances of plates were measured using a microplate reader at 570 nm with five readings for each sample. The experiment was repeated three times.
2.4.7.4. SEM
The biofilm-coated specimens (n = 3) were fixed overnight by 2.5% glutaraldehyde solution and dried with ethanol gradient (50, 70, 90, and 100% for 20 min), then sprayed with gold. The samples were observed under SEM in three randomly selected fields of view for imaging.
2.4.8. Cytotoxicity
The primers were diluted 1000, 2000, and 4000 times with Dulbecco’s modified Eagle medium (DMEM) medium containing 10% fetal bovine serum (FBS, Gibco, CA), 1% penicillin, and 100 μg/mL streptomycin to culture L929 cells for CCK-8 assay. The density of 5000 cells per well was laid out in a 96-well plate with 100 μL of medium. After 24 h of incubation in DMEM medium, the mediums containing the diluted primer were replaced and cultured for 24 and 48 h. The test was performed according to the CCK-8 kit instructions and the absorbance was read at 450 nm with the microplate reader (Gene 5; Biotek Instruments, Winooski, VT).
2.5. Statistical Analyses
All statistical analyses were calculated by SPSS (IBM SPSS Statistics 20, Armonk, NY). The μTBS test results were submitted to two-way (variables: different urushiol concentrations and aging) analysis of variance (ANOVA) and Tukey’s test. The other test results were calculated using one-way analysis of variance (ANOVA) followed by Tukey’s test. The level of significance was set to 0.05.
3. Results
3.1. Collagen Cross-Linking
3.1.1. Effect of Urushiol on the Space Structure of Collagen
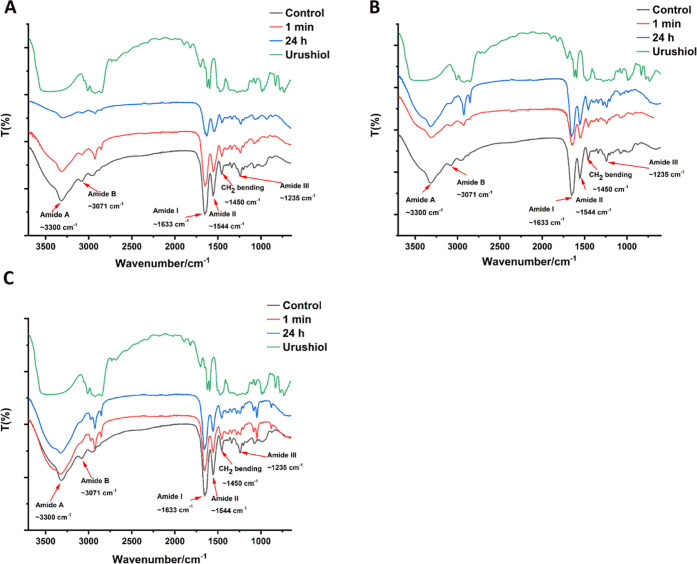
Figures 4 and 5 show the FTIR spectra of untreated dentin collagen and urushiol-treated dentin collagens. The amide A and B bands at 3300 and 3071 cm–1, generally corresponded to the stretching vibrations of N–H groups, respectively. The peak at 1633 cm–1 was attributed chiefly to the stretching vibrations of C=O peptide groups in the amide I band. C–N stretching at 1544 cm–1 was observed in amide II. Amide III band centered at 1235 cm–1 was attributed to the C–N stretching and N–H bending vibrations from amide linkages, in addition to the wagging vibrations of CH2 groups in the glycine backbone and proline side chains.36,37 It should be noted that the peak of the amide I band related to the triple helix structure of collagen retained its position. However, for amide A, I and II bands were broadened to some extent.
Figure 4.
FTIR spectra of collagen treated with 0.5 wt % (A), 1 wt % (B), and 3 wt % urushiol (C).
Figure 5.
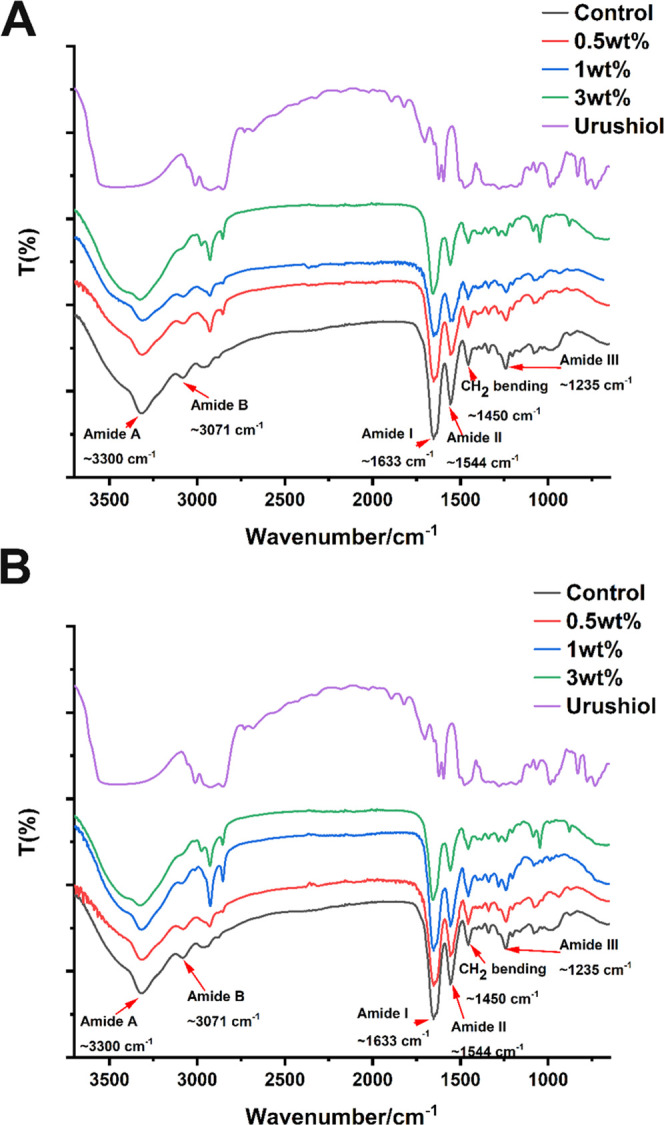
FTIR spectra of collagen with or without urushiol treatment for 1 min (A) and 24 h (B).
Meanwhile, besides the above changes, the FTIR absorption ratios of CH2 scissoring to 1450 cm–1, attributed to AIII/A1450 thereafter, are also regarded as a measure of protection of the integrity of collagen triple helixes.38 As is shown in Figure 4, the ratios for 3% urushiol-treated collagen slightly decreased from 1.0 for pure collagen.
3.1.2. Thermal Stability of Collagen with Urushiol Treatment
The thermal stability of collagen with urushiol treatment was evaluated by TGA analysis. As shown in Figure 6, the mass loss of collagen without urushiol treatment at 350 °C was about 44.6%, but the weight losses were 39.2 and 40.3% of 3 wt %-urushiol and GA groups. The results for urushiol-modified collagen films of the GA group and 3 wt % urushiol group had the least weight loss at 350 °C.
Figure 6.
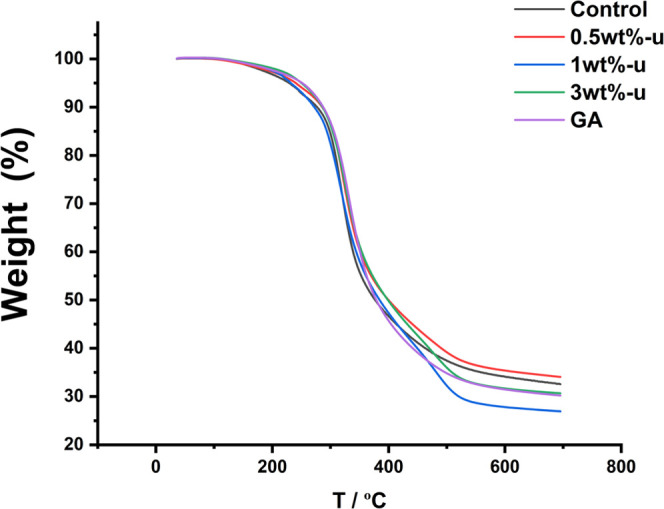
TGA thermograms of collagen/urushiol films treated with different concentrations of urushiol and GA. GA represents glutaraldehyde.
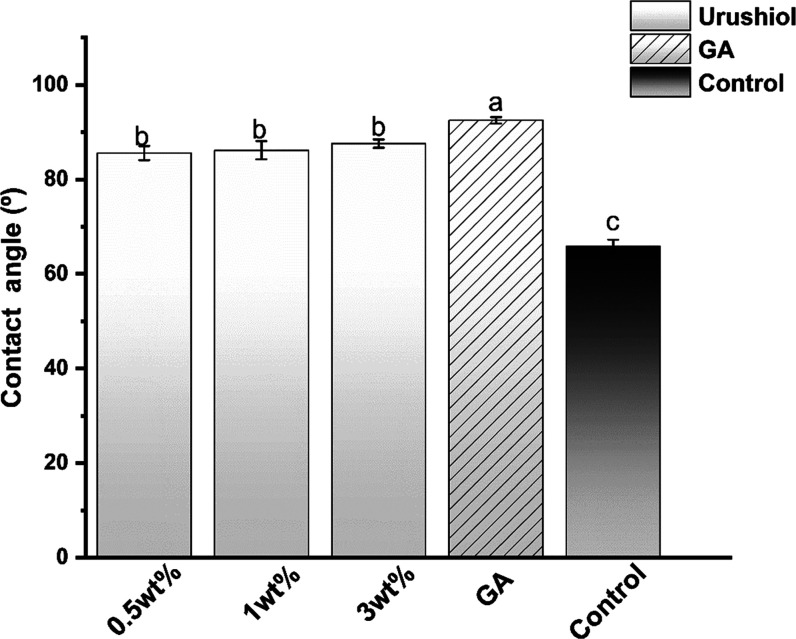
3.1.3. Contact Angles
As is shown in Figure 7, the addition of urushiol caused the initial contact angle to increase by approximately 20°. The GA-treated collagen had the highest contact angle (P < 0.05). All of the treated collagen had a higher contact angle than pure collagen (P < 0.05).
Figure 7.
Contact angle (CA) of collagen/urushiol slabs with a function time of 24 h. Different letter cases represent significant differences at P < 0.05. GA represents glutaraldehyde.
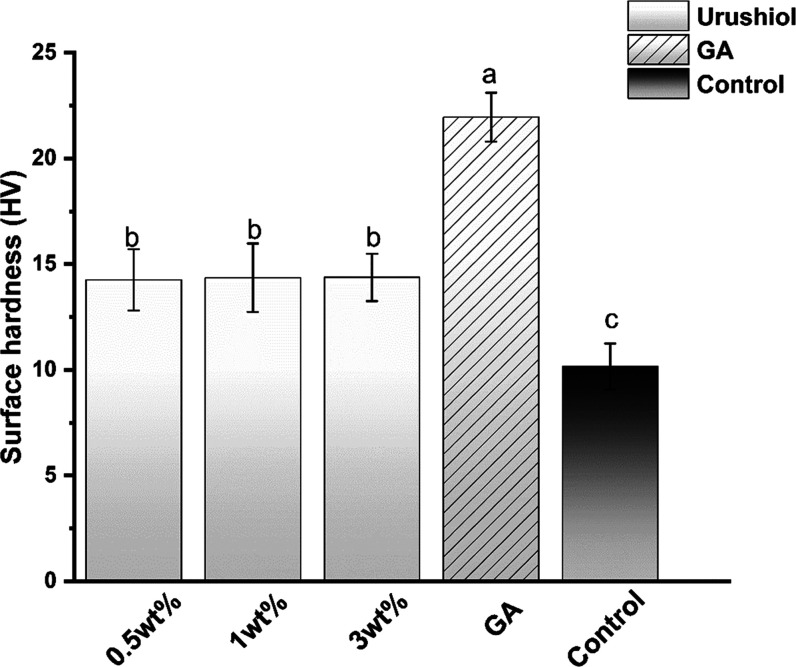
3.1.4. Hardness
Figure 8 illustrates the surface hardness measurements of the urushiol-treated collagen at different concentrations. The hardness value of the GA group was the highest of all groups (P < 0.05). The specimens treated with the urushiol solutions exhibited significantly higher HVs than the control group (P < 0.05). No significant differences were revealed in HV between different urushiol-treated groups.
Figure 8.
Dentin surface hardness of the collagen/urushiol films with a function time of 24 h. Different letter cases represent significant differences at P < 0.05. GA represents glutaraldehyde.
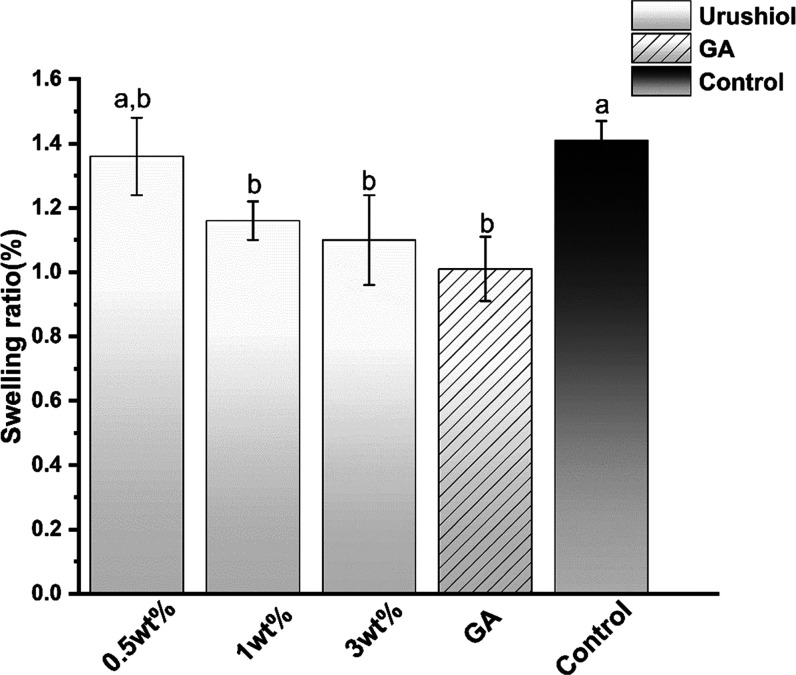
3.1.5. Swelling Ratio
Figure 9 presents the swelling ratio of the urushiol-treated and untreated collagen beams. The use of urushiol solution at different concentrations significantly decreased the swelling ratio (P < 0.05) except for the 0.5 wt % urushiol group. The GA group mostly reduced the swelling ratio (P < 0.05).
Figure 9.
Swelling ratio of the collagen/urushiol beams with a function time of 24 h. Different case letters represent significant differences at P < 0.05. GA represents glutaraldehyde.
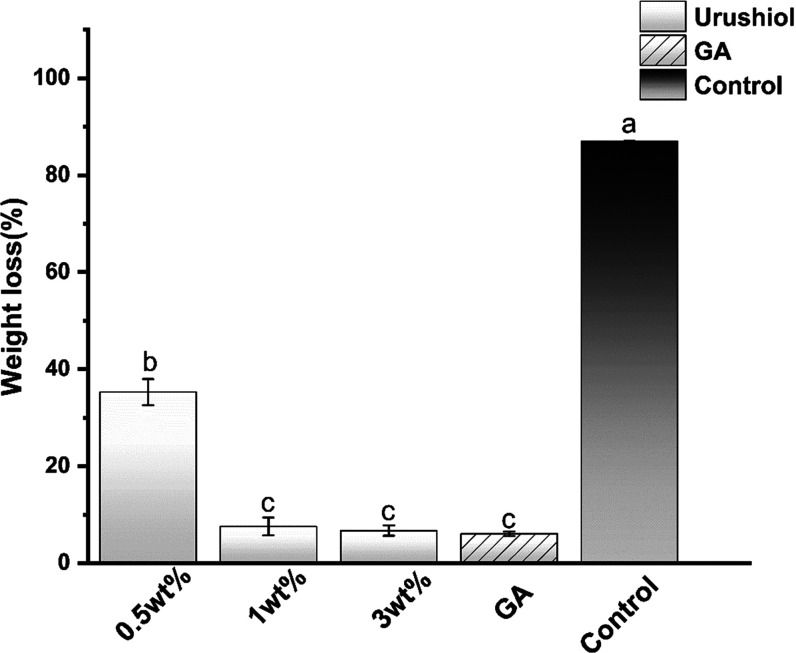
3.1.6. Mass Change
All of the urushiol-treated groups and GA-treated groups exhibited significantly decreased weight loss (P < 0.05) (Figure 10). As is shown in Figure 10, with increasing urushiol content, the samples exhibited a lower weight loss than the control group (P < 0.05). Nevertheless, when the concentration increased to 1 wt %, no significant difference among the higher urushiol content groups was observed. The weight loss of the control group was the highest (P < 0.05).
Figure 10.
Weight loss of collagen/urushiol beams with a function time of 24 h. Different letter cases represent significant differences at P < 0.05. GA represents glutaraldehyde.
3.1.7. Hydroxyproline
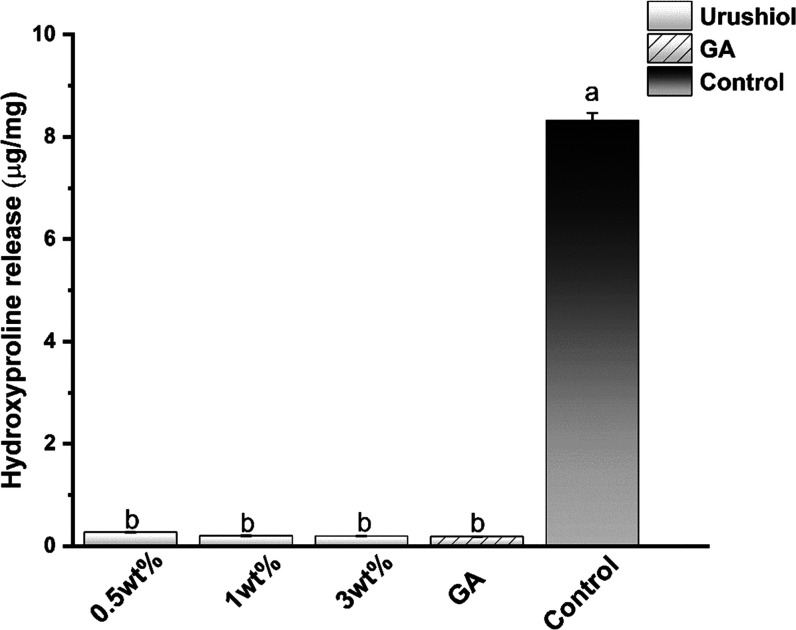
An in vitro biodegradation assay indicated that the proteins of the control group exposed to bacterial collagenase were almost entirely lost after 48 h. The urushiol- and GA-treated collagen were strongly resistant to collagenase degradation (Figure 11). The HYP release values of the urushiol-treated collagen were lower than that of the untreated collagen regardless of the concentration (P < 0.05). Among all groups, the HYP release of the 3 wt % urushiol group and the GA group was the lowest.
Figure 11.
Hydroxyproline release of the collagen/urushiol beams with a function time of 24 h. Different letter cases represent significant differences at P < 0.05. GA represents glutaraldehyde.
3.1.8. SEM Evaluations
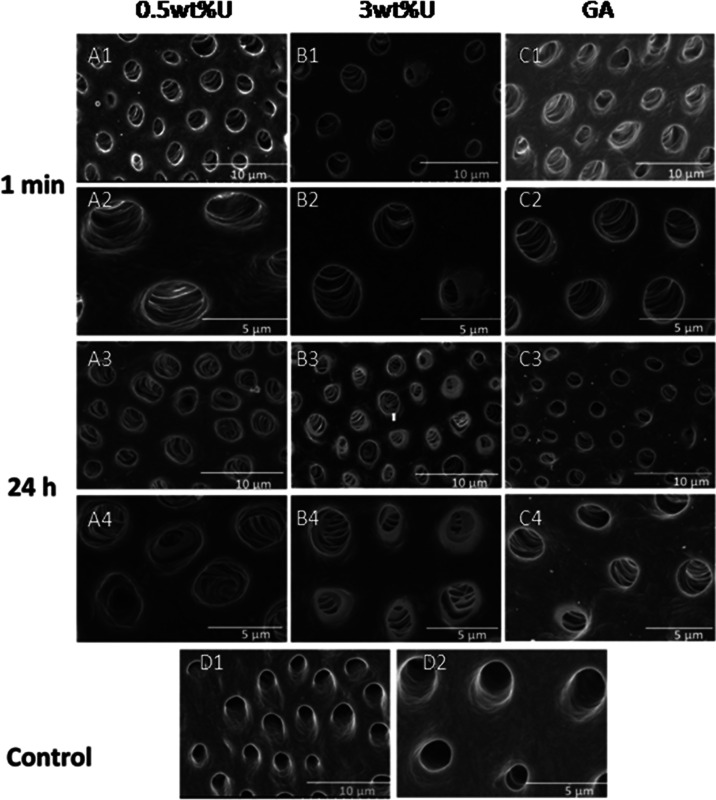
The representative SEM images of dentine surfaces from each group after 1 min or 24 h urushiol ingestion with the lowest and highest concentrations are presented in Figure 12. In pictures D1 and D2 of the control group, the dentin tubules were clear and smooth. After 0.5 wt % urushiol treatment for 1 min, the walls of dentin tubules were wrinkled, as shown in pictures A1 and A2. However, after 24 h, all of the treated collagen showed distinct collagen cross-linking.
Figure 12.
Representative SEM images of the dentine surfaces of different groups after 1 min and 24 h treatment. GA represents glutaraldehyde.
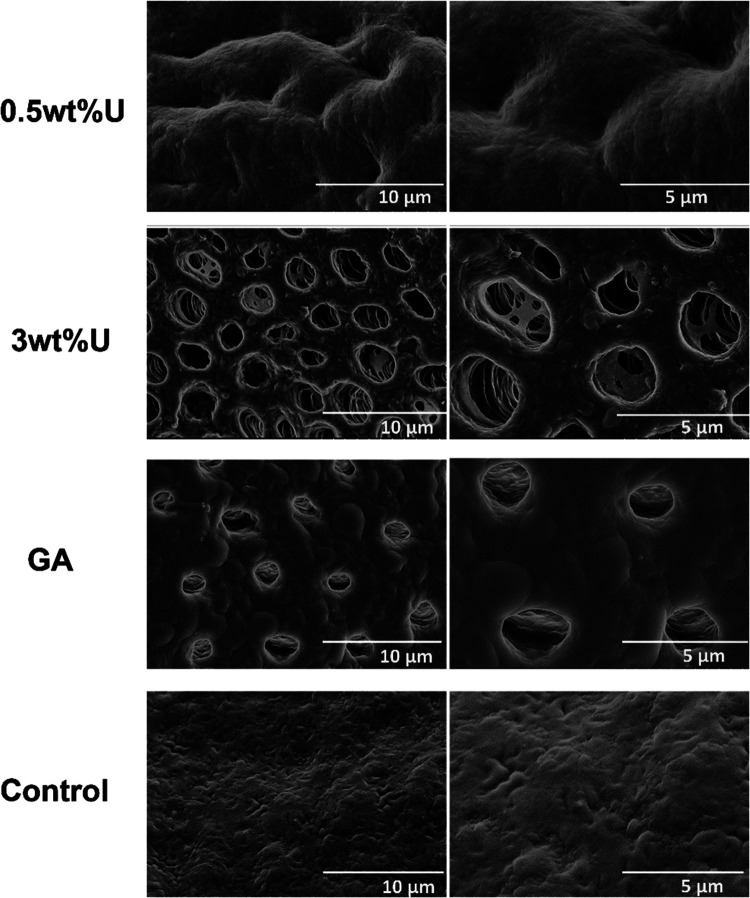
After collagenase digestion, the collagen matrix of the control group appeared to collapse, and the dentin structure of the control group could not be seen. However, in the pictures of the 0.5 wt % urushiol group, dentin tubules could be identified, but the dentin collagen was destroyed (Figure 13). Specimens treated with a 3 wt % urushiol preconditioner showed a relatively complete dentin tubule structure.
Figure 13.
SEM graphs of the dentin surfaces of different groups after collagenase ingestion. GA represents glutaraldehyde.
3.2. Antibacterial Test
3.2.1. Antibacterial Activity Test
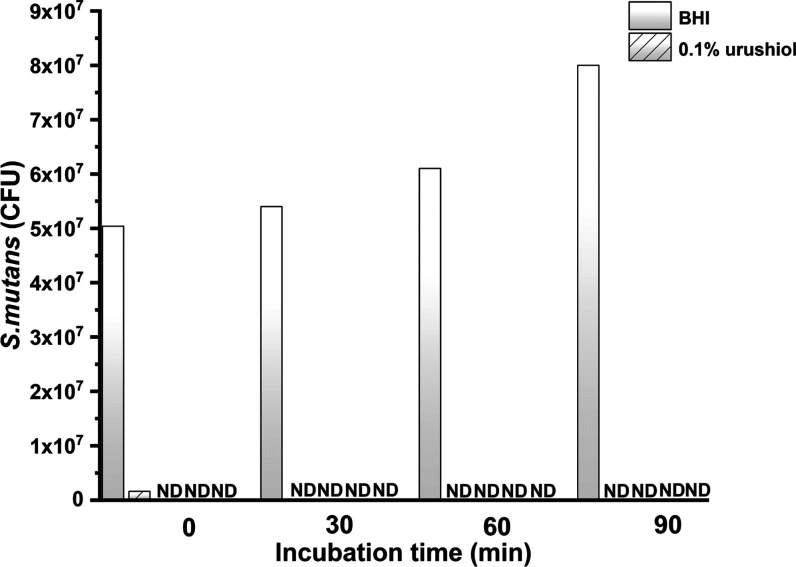
As is shown in Figure 14, no viable S. mutans microorganisms were discovered after a 30 min culture with four concentrations of urushiol.
Figure 14.
Numbers of CFUs in terms of the culture time of S. mutans ND: S. mutans not discovered, CFU: colony-forming unit.
3.2.2. SEM
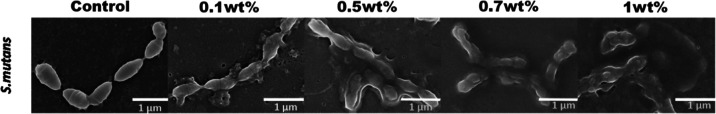
Figure 15 shows typical morphological micrographs of S. mutans of the control and urushiol groups. SEM microscopy provided the images of S. mutans. Urushiol (0.1 wt %) affected the membranes of the bacteria. With the increasing concentrations of urushiol, the internal substance of bacteria leaked out. Moreover, the content of normal bacteria decreased. In the control group, the bacteria had a complete shape. When bacteria were treated with 0.7 wt % urushiol, almost none of the bacteria were alive. Samples treated with urushiol exhibited marked differences in morphology compared with control samples.
Figure 15.
SEM micrographs of S. mutans treated with different concentrations of urushiol and the control group.
3.3. Urushiol as a Primer
3.3.1. Degree of Conversion
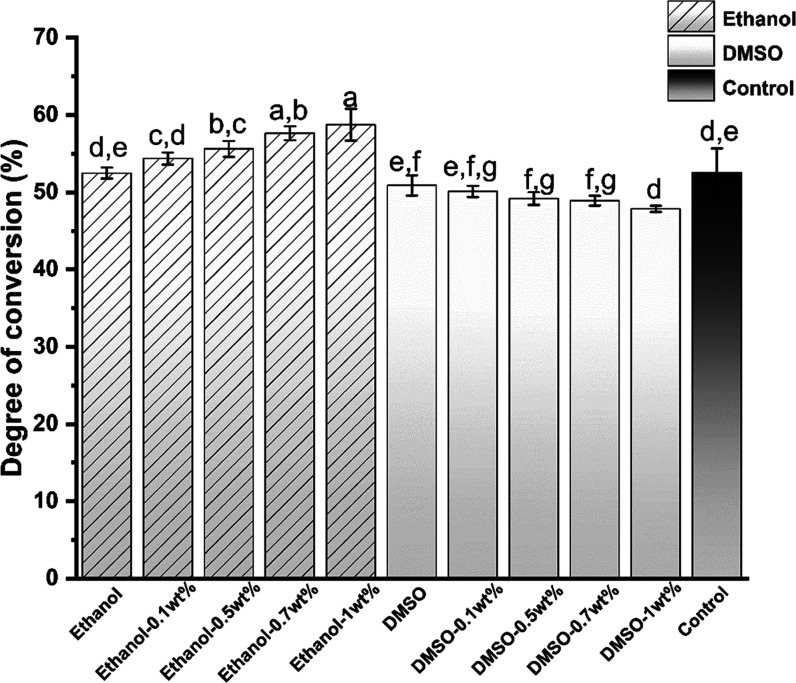
As shown in Figure 16, the DC of the primer showed just over 50% polymerization throughout the analysis. The degree of conversion of the ethanol samples increased slowly to approximately 57%. However, that of the DMSO groups decreased gradually. The DC of the ethanol-1% urushiol group was 59.73 ± 0.73% and the DC of the DMSO-1 wt % urushiol group was 47.86 ± 0.41%. There was a significant difference between the control group and other experimental groups (P < 0.05).
Figure 16.
Degree of conversion of experimental groups and the control group. Different letter cases represent significant differences at P < 0.05.
3.3.2. Contact Angle Test
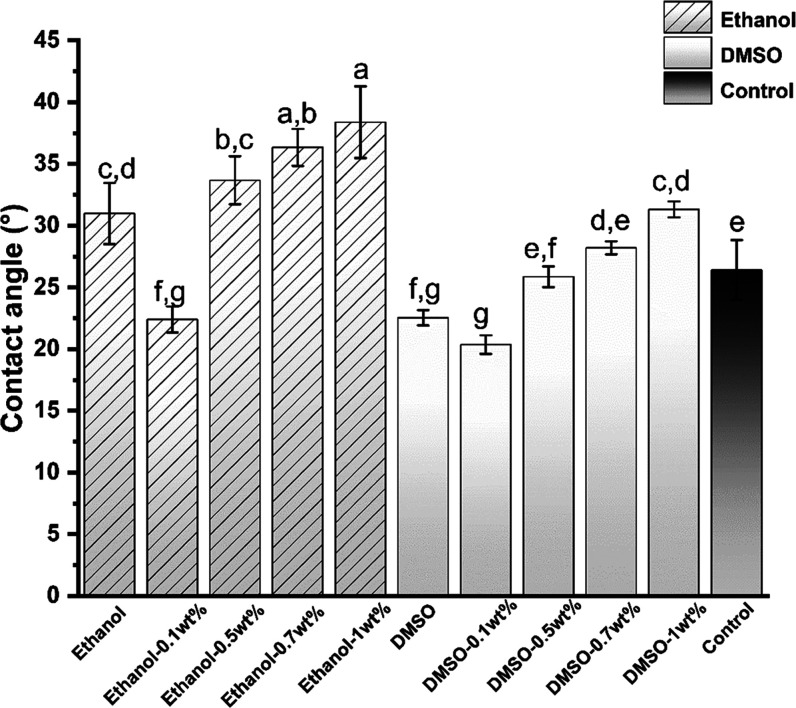
As Figure 17 shows, the average contact angles (mean ± standard deviation, SD) for the control, 1 wt % urushiol in ethanol and DMSO solvents were 26.4 ± 2.41°, 39.38 ± 1.02°, and 31.12 ± 0.65°, respectively. Compared with the blank control group, the contact angle of the pure ethanol-treated dentin increased significantly (P < 0.05). However, the contact angle of the pure DMSO group was lower than pure collagen (P < 0.05). The 0.1 wt % urushiol groups had a lower contact angle than the other three urushiol-treated groups that showed gradually increasing contact angles.
Figure 17.
Contact angle of the experimental and control groups. Different letter cases represent significant differences at P < 0.05.
3.3.3. μTBS
Table 1 shows the μTBS data of all groups. In the immediate groups, the DMSO-1 wt % urushiol group has the highest bond strength (P < 0.05). Nevertheless, among the thermocycling groups, there were no significant differences between the 1 wt % urushiol in the ethanol and DMSO groups. After aging the bond strengths of the ethanol-0.7 wt % and ethanol-1 wt % treated groups were not affected. After 5000 thermocycling cycles of the aging test, the bond strength of all groups decreased to different degrees. The control group had the lowest bond strength among all groups.
Table 1. μTBS of the Experimental and Control Groups at 24 h and 6 ma.
| group | immediate | aging |
|---|---|---|
| ethanol | 44.73 ± 2.91Af | 37.13 ± 2.25Bf |
| ethanol-0.1% | 54.73 ± 2.89Ad,e | 50.37 ± 1.43Be |
| ethanol-0.5% | 56.58 ± 1.47Ac,d,e | 52.97± 0.87Bd,e |
| ethanol-0.7% | 58.66 ± 1.20Ab,c,d | 57.77±1.58Ab,c |
| ethanol-1% | 59.95 ± 0.95Aa,b,c | 58.69 ± 0.61Aa,b,c |
| DMSO | 53.05 ± 2.96Ae | 38.05 ± 1.38Bf |
| DMSO-0.1% | 56.46 ± 1.82Ac,d,e | 49.75 ± 1.56Be |
| DMSO-0.5% | 59.84 ± 3.15Ab,c | 55.10 ± 0.59Bc,d |
| DMSO-0.7% | 63.53± 1.46Aa,b | 60.16 ± 1.26Ba,b |
| DMSO-1% | 65.10 ± 1.14Aa | 61.64± 2.51Ba |
| control | 27.53 ± 3.28Ag | 22.87 ± 3.32Bg |
MPa, mean ± SD.
Different lowercase letters in the same column imply statistically significant differences (P < 0.05; vertical comparisons); different capital letters in the same line imply statistically significant differences (P < 0.05; horizontal comparisons).
3.3.4. Nanoleakage
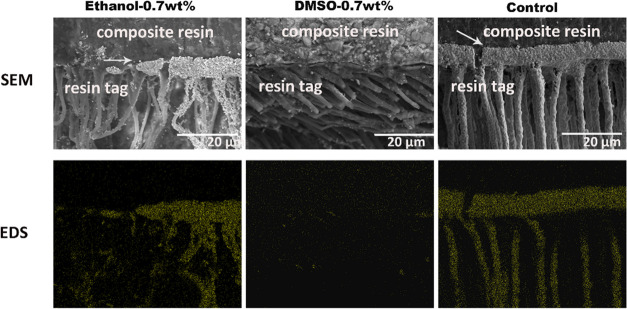
After thermocycling, the Ag had infiltrated into the hybrid layer and the resin tag of the control group, which was confirmed by EDS mapping. As shown in Figure 18, The ethanol-0.7 wt % and DMSO-0.7 wt % groups had less nanoleakage, especially the DMSO primer group.
Figure 18.
Nanoleakage images of the 0.7 wt % urushiol primer groups and the control group.
3.3.5. Antibacterial Activity by the MTT Assay
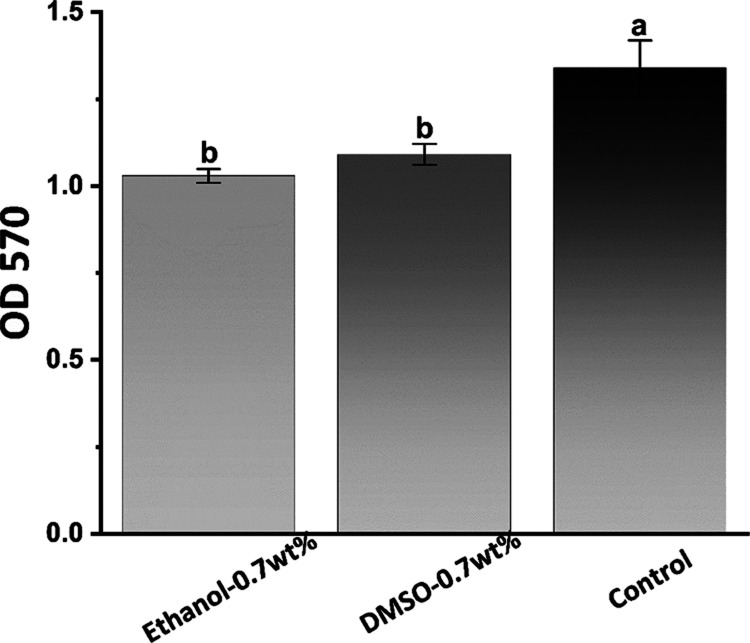
The results of the MTT assay are shown in Figure 19. The S. mutans biofilm of the 0.7 wt % urushiol-containing ethanol/DMSO pretreated groups had lower metabolic activity (P < 0.05).
Figure 19.
Average OD570 values after S. mutans incubation on dentin surfaces for 24 h. Different letter cases represent significant differences at P < 0.05.
3.3.6. SEM
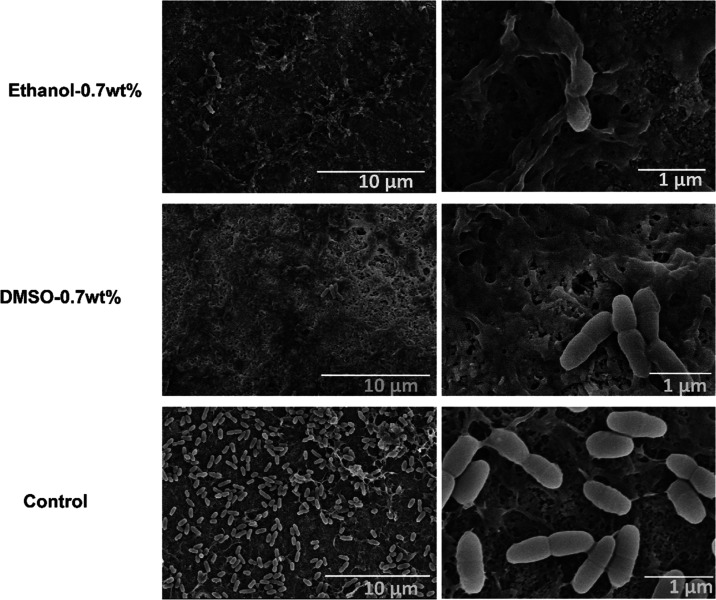
Figure 20 shows typical SEM photos of S. mutans biofilms in the control and 0.7 wt % urushiol groups. The bacterial biofilm was observed to cover the entire dentin surface in the control group. After the treatment of dentin samples with 0.7 wt % urushiol-containing ethanol/DMSO primers, few intact bacteria grew, and they were dispersed along the dentin surface. More destroyed bacteria could be seen on the surface.
Figure 20.
SEM images of the dentin surface with cultured S. mutans of the 0.7 wt % urushiol groups and the control group.
3.3.7. Cytotoxicity
The toxicity of the urushiol primer toward L929 cells was assessed. As is illustrated in Figure 21, the relative cell viabilities of different groups were all higher than 75%. The cell culture time of 48 h had higher cell viability than the 24 h culture time.
Figure 21.
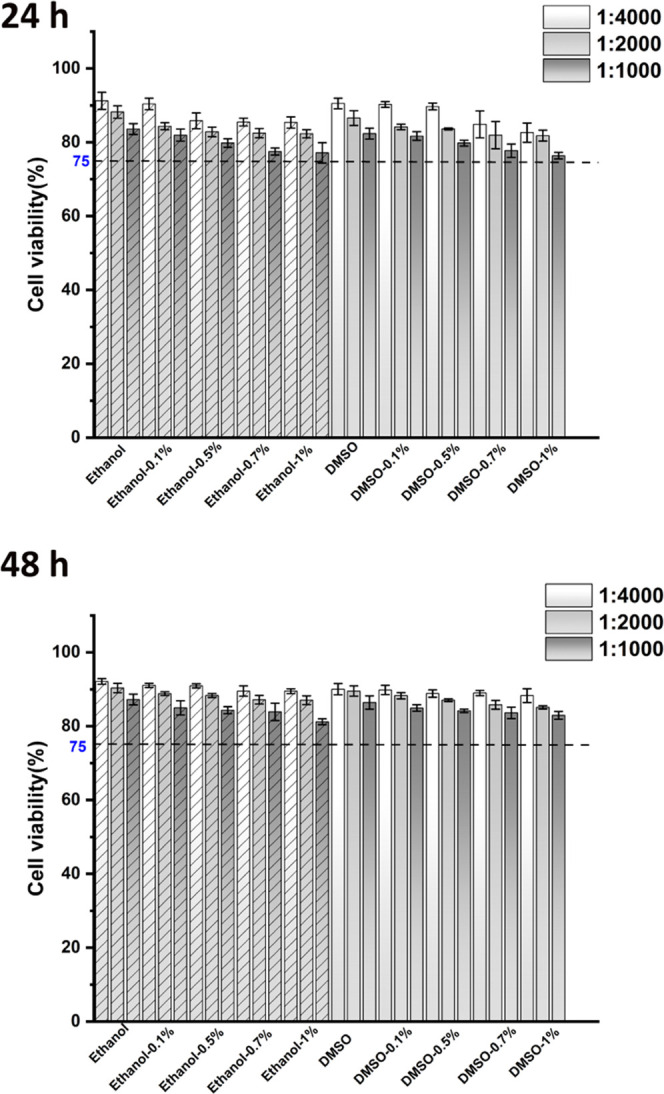
Cytotoxicity of primers with a culture time of 24 and 48 h was confirmed by CCK-8 assay.
4. Discussion
In the present study, urushiol dissolved in ethanol and DMSO as primers improves the resin–dentin bond strength, especially after aging. The desirable functions of urushiol are achieved.
The FTIR spectra of proteins can correspond to their structures.39 The triple helix of collagen was maintained due to the unchanged peak at 1633 cm–1, which suggested that the treatment did not destroy the structure of collagen. As is shown in the FTIR spectra, the amide A, I and II were broadened, which might involve hydrogen bonding interactions between urushiol and collagen.36 The mechanism of urushiol interaction with the collagen molecule by hydrogen bonds is shown in Figure 22. Hydrogen bonds have an important effect on thermal stability and triple helix conformation of collagen.39 The FTIR spectra in Figures 4 and 5 demonstrate that the different concentrations of urushiol treatment have significant effects on cross-linking. In FTIR spectra, the effect of time on collagen cross-linking was not obvious.
Figure 22.
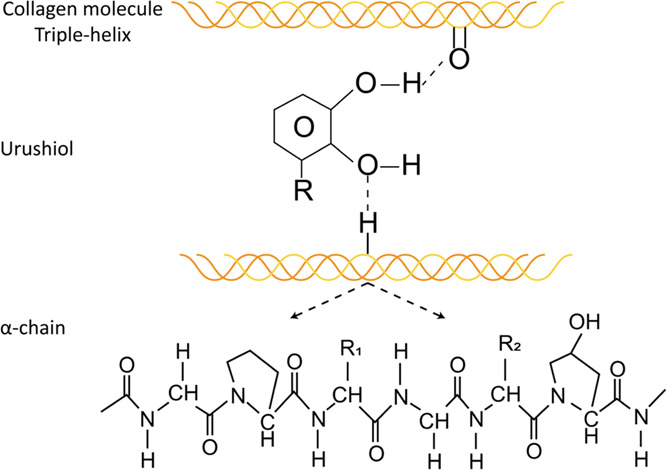
Hydrogen bonds between the urushiol and collagen molecules.
The range of 200–500 °C is involved in the degradation of the collagen chains, which is related to the preservation of the triple helix conformation of collagen, which is essential for the mechanical properties and biocompatibility of collagen-based materials.40 With urushiol treatment, the stability of treated collagen is enhanced, which indicates that improving the cross-linking of urushiol on collagen has distinct effects.
To investigate the relevance between the microstructure of urushiol-modified collagen and its surface properties, the contact angle of the dentin surface was tested. Urushiol contains phenol hydroxyl groups, which combine with the carboxyl and amino groups in collagen to form hydrogen bonds. Following this, the hydrophilic groups of collagen decrease and the hydrophobicity of collagen increases. The cross-linking of collagen leads to dehydration, which causes a higher contact angle of the dentin surface, and dehydration is the real reason why the cross-linking effect could enhance the stability of collagen.41
The results of the surface hardness measurements manifest that urushiol could improve the mechanical properties of collagen by the cross-linking effect. All treated collagen samples had lower water sorption than the untreated collagen except for the 0.5 wt % urushiol group (Figure 9). The collagen fibers stimulated by exogenous cross-linking are dense leading to the reduction in the swelling ratio. The treatment of urushiol could affect the water distribution of the dentin matrix and significantly decrease water sorption.
Owing to the presence of the triple helix structure, collagen fiber has a certain ability to resist endogenous proteases. The binding site of collagenase is situated at a deep gap with a width of approximately 0.5 nm. Some proteolytic enzymes cannot enter such a narrow region.42Figure 10 indicates that although the concentration of urushiol is low, treated collagens are durable and could withstand collagenase degradation. Type I collagen consists of approximately 10% hydroxyproline in quality, while most other proteins hardly contain this amino acid.43 As a result, the hydroxyproline release test is widely used.44 In this study, upon the urushiol treatment of the collagen, the release of HYP decreased significantly regardless of the concentration. The results are in accordance with the weight loss test. The SEM images indicate after collagenase degradation, urushiol-treated collagen presents a preservation effect as in the images in Figure 13.
Urushiol is a kind of herbal medicine for the treatment of bacteria and cancer.45−47S. mutans is chosen for study because it is widely known as the major bacteria in caries.48 No S. mutans was discovered after 30 min of culture with urushiol (Figure 14). In the primer part, on the basis of MTT results, the metabolism of the S. mutans biofilm in 0.7 wt % urushiol dissolved in two solvents groups was lower in comparison with the control group. Based on the result and SEM images of Figures 19 and 20 show that urushiol has antibacterial activity even at low concentrations.
In fact, the mechanism of several antimicrobial drugs in clinical treatment involves preventing the synthesis of the bacterial cell wall.49 Urushiol is a polyphenol with a catechol group. The cell wall of bacteria seems to be the major molecular target for the antibacterial effect of most polyphenols.50 Polyphenols can destroy the morphology of bacteria and structural integrity of intracellular matrix. One underlying mechanism related to the degradation of the bacterial cell wall could be the accumulation of certain essential surface proteins that leads to its inactivation. The targets of polyphenols are not only proteins but also their ability to form various noncovalent interactions such as hydrogen bonds, van der Waals forces, and hydrophobic interactions with proteins and other molecules. Moreover, possible targets for polyphenols are membrane-bound enzymes, cell surface adhesion proteins, and cell wall polypeptides.51 The antibacterial activities of urushiol derivatives with alkyl chains of different lengths were tested in other studies. The results showed that alkyl side chains in urushiol derivatives inhibited food spoilage and microbial growth.52 In conclusion, the mechanism of the antibacterial activity of urushiol is related to the combined effects of the catechol group and the alkyl chain.
Urushiol can fulfill the role as a priming agent on dentin due to its bioactive functions such as collagen cross-linking enhancement and its antibacterial activity. Commonly with polymer restorative materials, a high degree of conversion has an important effect on the mechanical properties.53,54 The higher permeability caused by residual monomers accelerates hybrid layer degradation and reduces its sealing ability.55,56 According to the DC results, although urushiol has an inhibitory effect on polymerization, low concentrations have no distinct effect on the adhesive. After pure ethanol was applied to collagen, water on the dentin surface evaporated with the ethanol leading to a higher contact angle. DMSO is a water-soluble solvent; as a result, the contact angle is lower than pure collagen. However, with increasing concentrations, the contact angle increased, which might indicate that the collagen cross-linking effect was effective.
In this study, compared with controls the μTBS results demonstrate that the values of urushiol-pretreated groups are significantly higher, especially the DMSO-1 wt % and ethanol-0.7 wt % urushiol groups after aging. DMSO has the ability to modify the collagen structure57 and improve dentin wettability58 to enhance bonding effectiveness even under dry conditions. As DMSO is capable of breaking the water self-associative inclination, it could improve the dried collagen re-expansion.57 To some extent, the use of DMSO as a pretreatment could reduce the sensitivity of the technique.59 Moreover, DMSO-pretreatments significantly reduced the activity of endogenous collagenase in dentin. The ethanol wet-bonding (EWB) theory suggests that the water in the dentin matrix could be replaced by ethanol and enhance the penetration of hydrophobic resin monomers into the collagen network. Therefore, the high-grade hydrophobic hybrid layer can be formed to develop bonding strength and durability. Ethanol was chosen in this study as a solvent. The compound urushiol and ethanol may withstand the interfacial biodegradation resulting from intraoral temperature changes to improve bonding durability. Nanoleakage was first described as an occurrence in which porosity can be seen all around the base of the hybrid layer. The nanoleakage of the dentin adhesive interface after aging suggests hydrolysis, product exudation, and destruction of collagen fibers after polymerization.60 With the urushiol primer treatment, there were obviously lower sliver particles deposited on the hybrid layer, which indicated that the primer application had better interface sealing.
According to the CCK-8 results (Figure 21), the biocompatibility of primers was acceptable, and with increasing culture time, the cytotoxicity of primers decreased. Owing to the pressure of dentinal tubules, it may be hard for urushiol to penetrate the long dentin tubules, which prevents it from affecting the pulp cells.
Because of the multiple effects of urushiol, it may have great potential in dentistry. In our study, the effect of the urushiol primer on adhesive–dentin bonding was assessed. The results show that the pretreatment of urushiol solutions could enhance the aging resistance of the interface of dentin bonding, which may result from improving collagen cross-links and inhibiting the growth of S. mutans. It is of great significance to promote the long-term stability of resin restoration.
5. Conclusions
Urushiol (0.5, 0.7, and 1 wt %) showed obvious antibacterial activity and collagen cross-linking. Moreover, primers of ethanol-0.7 wt % and DMSO-1 wt % solutions applied for 1 min were effective at improving the integrity of the bonding interface and bond strength after aging. Therefore, urushiol may have promising applications in dentin adhesion.
Acknowledgments
This research was supported by grant No. 20200201425JC from the Scientific and Technological Development Scheme of Jilin Province. Authors were grateful to all the people who devoted to this study.
The authors declare no competing financial interest.
References
- Agee K. A.; Prakki A.; Abu-Haimed T.; Naguib G. H.; Nawareg M. A.; Tezvergil-Mutluay A.; Scheffel D. L. S.; Chen C.; Jang S. S.; Hwang H.; Brackett M.; Grégoire G.; Tay F. R.; Breschi L.; Pashley D. H. Water distribution in dentin matrices: Bound vs. unbound water. Dent. Mater. 2015, 31, 205–216. 10.1016/j.dental.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breschi L.; Maravic T.; Cunha S. R.; Comba A.; Cadenaro M.; Tjaderhane L.; Pashley D. H.; Tay F. R.; Mazzoni A. Dentin bonding systems: From dentin collagen structure to bond preservation and clinical applications. Dent. Mater. 2018, 34, 78–96. 10.1016/j.dental.2017.11.005. [DOI] [PubMed] [Google Scholar]
- Nakabayashi N.; Nakamura M.; Yasuda N. Hybrid layer as a dentin-bonding mechanisms. J. Esthet. Dent. 1991, 3, 133–138. 10.1111/j.1708-8240.1991.tb00985.x. [DOI] [PubMed] [Google Scholar]
- Nakabayashi N. In The Hybrid Layer a Resin-Dentin Composite, Proceedings of the Finnish Dental Society, Division of Organic Materials, 1992; pp 321–329. [PubMed]
- Matuda L. S. d. A.; Marchi G. M.; Aguiar T. R.; Leme A. A.; Ambrosano G. M.; Bedran-Russo A. K. Dental adhesives and strategies for displacement of water/solvents from collagen fibrils. Dent. Mater. 2016, 32, 723–31. 10.1016/j.dental.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Spencer P. Hybridization efficiency of the adhesive/dentin interface with wet bonding. J. Dent. Res. 2003, 82, 141–145. 10.1177/154405910308200213. [DOI] [PubMed] [Google Scholar]
- Mazzoni A.; Angeloni V.; Comba A.; Maravic T.; Cadenaro M.; Tezvergil-Mutluay A.; Pashley D. H.; Tay F. R.; Breschi L. Cross-linking effect on dentin bond strength and MMPs activity. Dent. Mater. 2018, 34, 288–295. 10.1016/j.dental.2017.11.009. [DOI] [PubMed] [Google Scholar]
- Nakabayashi N.; Kojima K.; Masuhara E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J. Biomed. Mater. Res. 1982, 16, 265–273. 10.1002/jbm.820160307. [DOI] [PubMed] [Google Scholar]
- Perdigão J.; Reis A.; Loguercio A. D. Dentin Adhesion and MMPs: A Comprehensive Review. J. Esthet. Restor. Dent. 2013, 25, 219–241. 10.1111/jerd.12016. [DOI] [PubMed] [Google Scholar]
- Kim J.; Arola D. D.; Gu L.; Kim Y. K.; Mai S.; Liu Y.; Pashley D. H.; Tay F. R. Functional biomimetic analogs help remineralize apatite-depleted demineralized resin-infiltrated dentin via a bottom-up approach. Acta Biomater. 2010, 6, 2740–2750. 10.1016/j.actbio.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pashley D. H.; Tay F. R. Single-step adhesives are semi-permeable membranes. I. Nanoleakage and fluid conductance evidence. J. Dent. Res. 2002, 81, A468–A468. [DOI] [PubMed] [Google Scholar]
- Suh B. I.; Tay F. R.; Pashley D. H.; Carvalho R. M. Single-step adhesives are semi-permeable membranes. II. Morphologic and bond strength evidence. J. Dental Res. 2002, 81, A469. 10.1177/154405910208100707. [DOI] [Google Scholar]
- Frassetto A.; Breschi L.; Turco G.; Marchesi G.; Di Lenarda R.; Tay F. R.; Pashley D. H.; Cadenaro M. Mechanisms of degradation of the hybrid layer in adhesive dentistry and therapeutic agents to improve bond durability—A literature review. Dent. Mater. 2016, 32, e41–e53. 10.1016/j.dental.2015.11.007. [DOI] [PubMed] [Google Scholar]
- Nakabayashi N. Bonding of restorative materials to dentine: the present status in Japan. Int. Dental J. 1985, 35, 145–154. [PubMed] [Google Scholar]
- Pereira P. N. R.; Bedran-de-Castro A. K. B.; Duarte W. R.; Yamauchi M. Removal of noncollagenous components affects dentin bonding. J. Biomed. Mater. Res., Part B- 2007, 80, 86–91. 10.1002/jbm.b.30572. [DOI] [PubMed] [Google Scholar]
- Al-Ammar A.; Drummond J. L.; Bedran-Russo A. K. The Use of Collagen Cross-Linking Agents to Enhance Dentin Bond Strength. J. Biomed. Mater. Res., Part B 2009, 91B, 419–424. 10.1002/jbm.b.31417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedran-Russo A. K. B.; Pashley D. H.; Agee K.; Drummond J. L.; Miescke K. J. Changes in stiffness of demineralized dentin following application of collagen crosslinkers. J. Biomed. Mater. Res., Part B 2008, 86, 330–334. 10.1002/jbm.b.31022. [DOI] [PubMed] [Google Scholar]
- Sung H. W.; Chang W. H.; Ma C. Y.; Lee M. H. Crosslinking of biological tissues using genipin and/or carbodiimide. J. Biomed. Mater. Res., Part A 2003, 64, 427–438. 10.1002/jbm.a.10346. [DOI] [PubMed] [Google Scholar]
- Han B.; Jaurequi J.; Tang B. W.; Nimni M. E. Proanthocyanidin: A natural crosslinking reagent for stabilizing collagen matrices. J. Biomed. Mater. Res., Part A 2003, 65, 118–124. 10.1002/jbm.a.10460. [DOI] [PubMed] [Google Scholar]
- Parise Gré C.; Lise D. P.; Ayres A. P.; De Munck J.; Tezvergil-Mutluay A.; Seseogullari-Dirihan R.; Lopes G. C.; Van Landuyt K.; Van Meerbeek B. Do collagen cross-linkers improve dentin’s bonding receptiveness?. Dent. Mater. 2018, 34, 1679–1689. 10.1016/j.dental.2018.08.303. [DOI] [PubMed] [Google Scholar]
- De-Paula D. M.; Lomonaco D.; Ponte A. M. P.; Cordeiro K. E.; Moreira M. M.; Mazzetto S. E.; Feitosa V. P. Influence of collagen cross-linkers addition in phosphoric acid on dentin biomodification and bonding of an etch-and-rinse adhesive. Dent. Mater. 2020, 36, E1–E8. 10.1016/j.dental.2019.11.019. [DOI] [PubMed] [Google Scholar]
- Mazzoni A.; Angeloni V.; Sartori N.; Duarte S. Jr.; Maravic T.; Tjaderhane L.; Pashley D. H.; Tay F. R.; Breschi L. Substantivity of Carbodiimide Inhibition on Dentinal Enzyme Activity over Time. J. Dent. Res. 2017, 96, 902–908. 10.1177/0022034517708312. [DOI] [PubMed] [Google Scholar]
- Cadenaro M.; Fontanive L.; Navarra C. O.; Gobbi P.; Mazzoni A.; Di Lenarda R.; Tay F. R.; Pashley D. H.; Breschi L. Effect of carboidiimide on thermal denaturation temperature of dentin collagen. Dent. Mater. 2016, 32, 492–498. 10.1016/j.dental.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Bedran-Russo A. K. B.; Pereira P. N. R.; Duarte W. R.; Drummond J. L.; Yamauchi M. Application of crosslinkers to dentin collagen enhances the ultimate tensile strength. J. Biomed. Mater. Res., Part B 2007, 80, 268–272. 10.1002/jbm.b.30593. [DOI] [PubMed] [Google Scholar]
- Xu L. Q.; Neoh K.-G.; Kang E.-T. Natural polyphenols as versatile platforms for material engineering and surface functionalization. Prog. Polym. Sci. 2018, 87, 165–196. 10.1016/j.progpolymsci.2018.08.005. [DOI] [Google Scholar]
- Feiz A.; Badrian H.; Goroohi H.; Mojtahedi N. The Effect of Synthetic Grape Seed Extract (GSE) on the Shear Bond Strength of composite resin to Dentin. J. Res. Med. Dent. Sci. 2017, 5, 65–70. [Google Scholar]
- Jeon J. G.; Rosalen P. L.; Falsetta M. L.; Koo H. Natural Products in Caries Research: Current (Limited) Knowledge, Challenges and Future Perspective. Caries Res. 2011, 45, 243–263. 10.1159/000327250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W.; Yi L.; Wang Z.; Yang H.; Huang C. Effects of resveratrol/ethanol pretreatment on dentin bonding durability. Mater. Sci. Eng., C 2020, 114, 111000 10.1016/j.msec.2020.111000. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Yu J.; Yao C.; Yang H.; Huang C. New perspective to improve dentin-adhesive interface stability by using dimethyl sulfoxide wet-bonding and epigallocatechin-3-gallate. Dent. Mater. 2020, 36, 1452–1463. 10.1016/j.dental.2020.08.009. [DOI] [PubMed] [Google Scholar]
- Wu M.; Zhang B.; Jiang L.; Wu J.; Sun G. Natural lacquer was used as a coating and an adhesive 8000 years ago, by early humans at Kuahuqiao, determined by ELISA. J. Archaeol. Sci. 2018, 100, 80–87. 10.1016/j.jas.2018.10.004. [DOI] [Google Scholar]
- Hatada K.; Kitayama T.; Nishiura T.; Nishimoto A.; Simonsick W. J.; Vogl O. Structural-Analysis of the Components of Chinese Lacquer Kuro-Urushi. Macromol. Chem. Phys. 1994, 195, 1865–1870. 10.1002/macp.1994.021950533. [DOI] [Google Scholar]
- Suk K. T.; Kim H. S.; Kim M. Y.; Kim J. W.; Uh Y.; Jang I. H.; Kim S. K.; Choi E. H.; Kim M. J.; Joo J. S.; Baik S. K. In vitro Antibacterial and Morphological Effects of the Urushiol Component of the Sap of the Korean lacquer tree (Rhus vernicifera Stokes) on Helicobacter pylori. J. Korean Med. Sci. 2010, 25, 399–404. 10.3346/jkms.2010.25.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. X.; Lohakare J. D.; Chae B. J. Effects of lacquer (Rhus verniciflua) meal supplementation on layer performance. Asian-Australas. J. Anim. Sci. 2007, 20, 82–88. 10.5713/ajas.2007.82. [DOI] [Google Scholar]
- Jeong H.; Cho Y.-A.; Cho Y.; Kang E.; Ahn H.-w.; Hong J. Durable Urushiol-Based Nanofilm with Water Repellency for Clear Overlay Appliances in Dentistry. ACS Biomater. Sci. Eng. 2016, 2, 344–348. 10.1021/acsbiomaterials.5b00440. [DOI] [PubMed] [Google Scholar]
- Suk K. T.; Baik S. K.; Kim H. S.; Park S. M.; Paeng K. J.; Uh Y.; Jang I. H.; Cho M. Y.; Choi E. H.; Kim M. J.; Ham Y. L. Antibacterial Effects of the Urushiol Component in the Sap of the Lacquer Tree (Rhus verniciflua Stokes) on Helicobacter pylori. Helicobacter 2011, 16, 434–443. 10.1111/j.1523-5378.2011.00864.x. [DOI] [PubMed] [Google Scholar]
- Madhan B.; Subramanian V.; Rao J. R.; Nair B. U.; Ramasami T. Stabilization of collagen using plant polyphenol: Role of catechin. Int. J. Biol. Macromol. 2005, 37, 47–53. 10.1016/j.ijbiomac.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Acharya G.; Lee C. H. Effects of dialdehyde starch on calcification of collagen matrix. J. Biomed. Mater. Res., Part A 2011, 99A, 485–492. 10.1002/jbm.a.33209. [DOI] [PubMed] [Google Scholar]
- Albu M. G.; Ghica M. V.; Leca M.; Popa L.; Borlescu C.; Cremenescu E.; Giurginca M.; Trandafir V. Doxycycline Delivery From Collagen Matrices Crosslinked With Tannic Acid. Mol. Cryst. Liq. Cryst. 2010, 523, 97–105. 10.1080/15421401003724159. [DOI] [Google Scholar]
- He L.; Mu C.; Shi J.; Zhang Q.; Shi B.; Lin W. Modification of collagen with a natural cross-linker, procyanidin. Int. J. Biol. Macromol. 2011, 48, 354–359. 10.1016/j.ijbiomac.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Mu C. D.; Liu F.; Cheng Q. S.; Li H. L.; Wu B.; Zhang G. Z.; Lin W. Collagen Cryogel Cross-Linked by Dialdehyde Starch. Macromol. Mater. Eng. 2010, 295, 100–107. 10.1002/mame.200900292. [DOI] [Google Scholar]
- Miles C. A.; Avery N. C.; Rodin V. V.; Bailey A. J. The increase in denaturation temperature following cross-linking of collagen is caused by dehydration of the fibres. J. Mol. Biol. 2005, 346, 551–556. 10.1016/j.jmb.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Nagase H.; Fushimi K. Elucidating the function of non catalytic domains of collagenases and aggrecanases. Connect. Tissue Res. 2008, 49, 169–174. 10.1080/03008200802151698. [DOI] [PubMed] [Google Scholar]
- Bornstein P.; Sage H. Structurally distinct collagen types. Annu. Rev. Biochem. 1980, 49, 957–1003. 10.1146/annurev.bi.49.070180.004521. [DOI] [PubMed] [Google Scholar]
- Bridi E. C.; Leme-Kraus A. A.; Aydin B.; Basting R. T.; Bedran-Russo A. K. Long-term evaluation of the stability of dentin matrix following treatments with aqueous solutions of titanium tetrafluoride at different concentrations. Archiv. Oral Biol. 2018, 91, 51–56. 10.1016/j.archoralbio.2018.04.006. [DOI] [PubMed] [Google Scholar]
- Hong S. H.; Suk K. T.; Choi S. H.; Lee J. W.; Sung H. T.; Kim C. H.; Kim E. J.; Kim M. J.; Han S. H.; Kim M. Y.; Baik S. K.; Kim D. J.; Lee G.-J.; Lee S.-k.; Park S. H.; Ryu O. H. Anti-oxidant and natural killer cell activity of Korean red ginseng (Panax ginseng) and urushiol (Rhus vernicifera Stokes) on non-alcoholic fatty liver disease of rat. Food Chem. Toxicol. 2013, 55, 586–591. 10.1016/j.fct.2013.01.022. [DOI] [PubMed] [Google Scholar]
- Kim J. Y.; Cho J.-Y.; Ma Y. K.; Lee Y. G.; Moon J.-H. Nonallergenic urushiol derivatives inhibit the oxidation of unilamellar vesicles and of rat plasma induced by various radical generators. Free Radical Biol. Med. 2014, 71, 379–389. 10.1016/j.freeradbiomed.2014.03.041. [DOI] [PubMed] [Google Scholar]
- Ryckewaert L.; Sacconnay L.; Carrupt P.-A.; Nurisso A.; Simoes-Pires C. Non-specific SIRT inhibition as a mechanism for the cytotoxicity of ginkgolic acids and urushiols. Toxicol. Lett. 2014, 229, 374–380. 10.1016/j.toxlet.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Kim J.-S.; Shin D.-H. Inhibitory effect on Streptococcus mutans and mechanical properties of the chitosan containing composite resin. Restor. Dent. Endod. 2013, 38, 36–42. 10.5395/rde.2013.38.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S. L. Glutamate racemase as a target for drug discovery. Microb. Biotechnol. 2008, 1, 345–360. 10.1111/j.1751-7915.2008.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez-Martínez F. J.; Barrajon-Catalan E.; Antonio Encinar J.; Carlos Rodriguez-Diaz J.; Micol V. Antimicrobial Capacity of Plant Polyphenols against Gram-positive Bacteria: A Comprehensive Review. Curr. Med. Chem. 2020, 27, 2576–2606. 10.2174/0929867325666181008115650. [DOI] [PubMed] [Google Scholar]
- Naz S.; Siddiqi R.; Ahmad S.; Rasool S.; Sayeed S. A. Antibacterial activity directed isolation of compounds from Punica granatum. J. Food Sci. 2007, 72, M341–M345. 10.1111/j.1750-3841.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- Cho J.-Y.; Park K. Y.; Kim S.-J.; Oh S.; Moon J.-H. Antimicrobial activity of the synthesized non-allergenic urushiol derivatives. Biosci., Biotechnol., Biochem. 2015, 79, 1915–1918. 10.1080/09168451.2015.1061418. [DOI] [PubMed] [Google Scholar]
- Hass V.; Dobrovolski M.; Zander-Grande C.; Martins G. C.; Arana Gordillo L. A.; Rodrigues Accorinte M. L.; Mongruel Gomes O. M.; Loguercio A. D.; Reis A. Correlation between degree of conversion, resin-dentin bond strength and nanoleakage of simplified etch-and-rinse adhesives. Dent. Mater. 2013, 29, 921–928. 10.1016/j.dental.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Eick J. D.; Gwinnett A. J.; Pashley D. H.; Robinson S. J. Current concepts on adhesion to dentin. Crit. Rev. Oral Biol. Med. 1997, 8, 306–335. 10.1177/10454411970080030501. [DOI] [PubMed] [Google Scholar]
- Breschi L.; Mazzoni A.; Ruggeri A.; Cadenaro M.; Di Lenarda R.; De Stefano Dorigo E. Dental adhesion review: aging and stability of the bonded interface. Dent. Mater. 2008, 24, 90–101. 10.1016/j.dental.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Cadenaro M.; Antoniolli F.; Sauro S.; Tay F. R.; Di Lenarda R.; Prati C.; Biasotto M.; Contardo L.; Breschi L. Degree of conversion and permeability of dental adhesives. Eur. J. Oral Sci. 2005, 113, 525–530. 10.1111/j.1600-0722.2005.00251.x. [DOI] [PubMed] [Google Scholar]
- Vishnyakov A.; Lyubartsev A. P.; Laaksonen A. Molecular dynamics simulations of dimethyl sulfoxide and dimethyl sulfoxide-water mixture. J. Phys. Chem. A 2001, 105, 1702–1710. 10.1021/jp0007336. [DOI] [Google Scholar]
- Mehtälä P.; Pashley D. H.; Tjaderhane L. Effect of dimethyl sulfoxide on dentin collagen. Dent. Mater. 2017, 33, 915–922. 10.1016/j.dental.2017.04.018. [DOI] [PubMed] [Google Scholar]
- Pashley D. H.; Tay F. R.; Carvalho R. M.; Rueggeberg F. A.; Agee K. A.; Carrilho M.; Donnelly A.; Garcia-Godoy F. From dry bonding to water-wet bonding to ethanol-wet bonding. A review of the interactions between dentin matrix and solvated resins using a macromodel of the hybrid layer. Am. J. Dent. 2007, 20, 7–20. [PubMed] [Google Scholar]
- Sano H.; Shono T.; Takatsu T.; Hosoda H. Microporous dentin zone beneath resin-impregnated layer. Oper. Dent. 1994, 19, 59–64. [PubMed] [Google Scholar]