Abstract
Background:
Overdose education and naloxone distribution (OEND) trains people who use opioids (PWUO) in how to intervene in cases of opioid overdose but best practices have not been assessed empirically.
Methods:
PWUO along with a significant other (SO) were randomized to one of three training conditions. In the Treatment-as-Usual (TAU) condition, participants were randomized to receive minimal overdose-related education. In the extended training (ET) condition, PWUO received an extended training, while their SO received no overdose training. In the final condition, both the participant and SO received the extended overdose training (ETwSO). Outcome measures were naloxone use and overdose knowledge and competency assessed immediately before and after training, and at 1-, 3-, 6-, and 12-month timepoints following training.
Results:
Three hundred and twenty-one PWUO (w/ a SO) were randomized. All intensities of OD training were associated with sustained increases in OD knowledge/ competency (versus pre-training baseline p’s < 0.01). PWUO intervened in 166 ODs. The 12-month incidence of naloxone use did not significantly differ between groups. Extended training (ET + ETwSO) compared to TAU resulted in significantly greater naloxone utilization by: 30 days (10.1% vs 4.1%, p = 0.041), 60 days (16.4% vs 5.2%, p<0.001) and 90 days (17.9% vs 9.5%, p = 0.039).
Conclusions:
All intensities of OD training were associated with sustained increases in OD knowledge and competency, and equivalent rates of successful naloxone use. More extensive training increased naloxone utilization during the first 3 months. However, the benefits of more comprehensive training should be balanced against feasibility.
Keywords: Naloxone, Opioid used disorder, Overdose, Heroin, Harm reduction
1. Introduction
Worldwide, an estimated 27 million people have opioid use disorder (OUD). Overdose is the leading cause of premature mortality among individuals with OUD, people with OUD typically experience several non-fatal overdose events, and some studies estimate that over 90% have witnessed an overdose (Degenhardt et al., 2013; Darke et al., 2011; Lagu et al., 2006; Strang et al., 2000; Tracy et al., 2005; Smyth et al., 2007; Wagner et al., 2015; World Health Organization, 2018). In an effort to reduce overdose mortality, peer-based overdose education and naloxone distribution (OEND) has grown to become a widely used harm reduction practice in the United States (U.S.) and around the world (Davis et al., 2017). Currently, all 50 states in the U.S. have laws to increase access to naloxone among those at risk of experiencing or witnessing an opioid overdose, with some states having additional legislation providing immunity from civil and criminal liability to lay-persons who administer naloxone (Prescription Drug Abuse Policy System, 2019). Though several studies have shown that OEND programs are effective, the dire need for opioid overdose harm reduction necessitated the rapid introduction of OEND training and the widespread implementation of practices that had not been evaluated empirically (Albert et al., 2011; Keane et al., 2018; McDonald and Strang, 2016; Walley et al., 2013).
Typically, naloxone distribution programs include training in recognizing the signs and symptoms of opioid overdose, and instructions in how to intervene (e.g., call 911, perform rescue breathing, administer naloxone, etc. Davis et al., 2017; Clark et al., 2014; Mueller et al., 2015). However, programs vary in length of training (5 min–1 h or longer) and how information is disseminated (video, pamphlets, one-on-one, small/large group (Dunne, 2018; Green et al., 2008; Pietrusza et al., 2018; Seal et al., 2005; Tobin et al., 2009)). Some studies suggest that greater efforts could be directed toward improving the training programs themselves. More specifically, Gaston et al., (2009) found that training people who use opioids significantly improved their identification of risk factors and signs of opioid overdose (in comparison to pre-training assessment). However, the investigators observed a slight and steady deterioration in knowledge, which began at 1-month post-training and continued throughout the 6-month observation period. Additionally, other adaptations of training have evolved, such as training a drug-using partner or significant other (SO) in recognizing and managing opioid overdose (Kim et al., 2009; McAuley et al., 2010; Sherman et al., 2009; Williams et al., 2014).
Previous randomized trials have assessed the effects of behavioral/educational interventions on overdose risk behaviors and the frequency of overdose events experienced by participants themselves (Bohnert et al., 2016; Coffin et al., 2017). Additionally, a secondary data analysis of a randomized controlled trial (n = 80) evaluated the effects of a personally tailored opioid overdose prevention education on overdose knowledge and naloxone usage (Winhusen et al., 2020). As such, no large-scale, prospective, randomized, controlled trials have compared different methods of providing the training that typically accompanies the prescribing of naloxone on overdose intervention outcomes (e.g., naloxone utilization). Thus, the goal of the current trial was to compare the effects of comprehensive and basic OEND training on overdose intervention attempts. Furthermore, this study examined the potential benefits of receiving training with a SO. The investigators hypothesized that being trained with a SO may increase participants’ OEND engagement and decision making in overdose scenarios, and thus may affect naloxone utilization. Deciding to intervene in someone else’s overdose may be seen as a social decision since it clearly affects others and not just the responders themselves. Being trained with a SO as a form of social learning may facilitate perspective-taking, and thereby may make it easier to eventually decide to intervene in response to a suspected overdose event (Akers, 2009).
2. Methods
2.1. Participant recruitment and screening
Potential participants were recruited at harm reduction sites, as well as through advertisements in local newspapers. Following a telephone pre-screen, in-person screening procedures were conducted at the New York State Psychiatric Institute (NYSPI) or NYU-Bellevue Hospital. Screening included various self-report and clinician-administered assessments (Addiction Severity Index (McLellan et al., 1992a), Treatment Service Review (McLellan et al., 1992b); Risk Assessment Battery: Navaline et al., 1994). To be eligible for the trial, participants had to be aged 21–65 years, in otherwise good mental health, and have met DSM-IV criteria for opioid dependence within the past 6 months, and be able to identify a SO (i.e., spouse, relative, friend) willing to attend an OEND training with them. Participants were excluded if they had an active psychiatric disorder that might have affected participation or made participation hazardous (e.g., DSM-IV psychotic disorder, active bipolar disorder with mania, a significant history of violent behavior). Participants were also excluded if they had received OEND, cardiopulmonary resuscitation training, and/or basic cardiac life support training within the past 2 years. Participants who were in treatment with extended-release naltrexone for opioid or alcohol dependence were also excluded, as they would be less likely to experience opioid overdose while on this medication. Study procedures were approved by the NYSPI and NYU Institutional Review Boards.
2.2. Randomization to interventions
Once enrolled, all participants received basic opioid overdose education training at NYSPI or Bellevue, consisting of a ~10 min video and PowerPoint presentation developed by the New York City Department of Health and Mental Hygiene. This training included the New York State Department of Health (NYSDOH) required topics of: opioid overdose risk factors, how to identify an opioid overdose, and how to intervene in an overdose event. Following training, participants were given a choice between an overdose response kit containing two doses of either intramuscular or intranasal naloxone.
Within approximately one week of receiving the standard training, all participants were invited to NYSPI for a second visit and required to bring a significant other (SO: e.g., spouse, relative, or friend). Participants were randomized into one of 3 groups: (1) the participant and the SO received no additional education related to overdose/naloxone, but watched educational videos related to hepatitis and HIV prevention (TAU; Treatment as Usual); (2) both the participant and the SO received an additional in-depth psychosocial education (ETwSO; Extensive Training with Significant Other) focusing on recognition of opioid overdose, prevention of opioid overdose, and appropriate use of naloxone; and (3) the participant received the in-depth training and the SO watched educational videos (ET group; Extensive Training group). The TAU condition served as a control condition and the non-overdose-related educational videos were used to standardize the length of the study visit across the three groups (Table 1).
Table 1.
Randomization conditions and training.
| Randomized Groups | Overdose Training Received | |
|---|---|---|
| Primary Participant* | Significant Other | |
| Arm 1: TAU | Basic | None |
| Arm 2: ET | Basic + Extended | None |
| Arm 3: ETwSO | Basic + Extended | Extended |
-TAU: Treatment as usual; ET = Extended training; ETwSO = Extended training for primary participant and significant other.
-Basic = NYSDOH opioid overdose training (~10 min video plus naloxone kit distribution).
-Extended = Experimental opioid overdose training (1.5–2 h plus naloxone kit distribution).
Person Who Uses Opioids.
An independent biostatistician developed a stratified randomization schedule. Group assignment was based upon the following strata: sex (male/female), substance use disorder severity (severe/non-severe, as assessed by their current Addiction Severity Index (McLellan et al., 1992a) drug use composite score (<0.4, or ≥0.4: range 0–1)), and OUD treatment status [i.e., recently detoxified, active non-medical opioid user, or receiving medications for the treatment of OUD (MOUD)]. The goal of the stratification was to equalize the relative risk of witnessing and experiencing an overdose event across the three conditions.
The extensive training (ET) developed by the research team was informed by an in-depth training developed by another group of investigators (Seal et al., 2005) and also by the major components of general training curricula being used in the U.S. at the time (Boyer, 2012; Enteen et al., 2010; Green et al., 2008; Substance Abuse and Mental Health Services Administration, 2013). These components included training on: (a) overdose risk factors, (b) methods of assessing potential overdose victims, (c) notification of emergency medical services, (d) how to perform rescue breathing, and (e) how to administer naloxone. The ET also included psychosocial components designed to increase: knowledge, skills, attention, beliefs about capabilities, optimism, and reinforcement related to providing aid to others. The resulting 1.5/2 h training included a one-on-one didactic session with the trainer, audiovisual segments, and hands-on skills training.
2.3. Assessment and measures
Study assessments were administered at the following time points: before the basic OEND training (pre-training), immediately after basic OEND training (post-training), immediately after randomization education group training (Timepoint 0, T0), as well as 1-, 3-, 6-, and 12-months post-training (T1, T3, T6, and T12). Follow-up time points of 1, 3, 6, and 12 months were selected based on previous research showing that overdose knowledge begins to decrease as soon as 1 month after training (Gaston et al., 2009). Participants were compensated $25 for the BL/T0 visit and $25 for each follow-up visit. In the later years of the trial the compensation was increased to $50 per visit. The SO was only asked to attend the BL/T0 visit during which they received the overdose education curriculum to which they were randomized. Primary outcome measures were overdose knowledge and retention, as well as rates of overdose intervention throughout the 1-year follow-up period. The following measures were used to evaluate overdose knowledge and characterize overdose experiences throughout the trial:
Overdose Tracking (1°): The frequency of naloxone utilization was a primary outcome measure. Participants were instructed to contact staff members if they experienced, observed, or intervened in an overdose event. Participants were also asked about overdose encounters at each follow-up visit. The NYSDOH Opioid Overdose Reporting Form was used to detail the circumstances of the overdose event. Staff also completed a Naloxone Adverse Effect form that asked participants to describe any adverse effects following the naloxone administration and their severity.
Brief Overdose Recognition & Response Assessment (1°) (BORRA: Green et al., 2008): Participants are asked to read 16 putative overdose scenarios and decide whether the presenting symptoms are an opioid overdose and if naloxone should be administered (Range: 0–32).
Opioid Overdose Attitudes Scale (1°) (OOAS: Williams et al., 2013): This 28-item questionnaire assessed participants’ attitudes about intervening during an overdose event (Range: 28–140).
Opioid Overdose Knowledge Scale (1°) (OOKS Williams et al., 2013): This 14-item questionnaire assessed participants’ knowledge of risk factors and symptoms associated with opioid overdose and how to intervene during an overdose (Range: 0–45).
Secondary outcome measures included incidents of participant OD, intervention success rates, along with assessments of participants’ drug use and psychiatric health. Some of these data have been reported in previous manuscripts (Martinez et al., 2020, 2021; Jones et al., 2017, 2020). Urine samples were collected at each study visit to assess opioid and other drug use. Outcome measures were not assessed for the SOs due to the practical challenges associated with doubling our sample size.
2.4. Retention
At the T0 visit, participants completed a Locator Form to maintain contact. Participants were called by telephone periodically between visits and they received a reminder text message the day before each scheduled time point. If a follow-up visit was not completed within a pre-specified window (T1: 3 days before, 10 days after, T3: ± 2 weeks, T6: ± 3 weeks, and T12: ± 4 weeks), the visit was considered “missed.” However, a participant was not considered to be discontinued from the trial unless they withdrew consent and contact efforts were continued for subsequent follow-up visits. If the participant missed a time point, three attempts were made to contact the participant by telephone (voice, text) and email. If a participant did not respond, a research assistant would then call the secondary contacts. Two letters were mailed to the participant’s home address reminding them of their future appointments. As a last resort, staff members would attempt a home visit.
2.5. Statistical methods
The primary aims were two-fold. First, we sought to compare the rate of naloxone use (i.e., cumulative incidence across one year) between participants receiving TAU and those receiving extended training, either alone (ET) or with a significant other (ETwSO), per the a priori analysis plan. In order to take advantage of the richness of the data, we also examined these results as a function of the combined extended training conditions (ET & ETwSO) vs control (TAU) conditions, and group differences in kit use at each time point post-randomization (i.e., T1, T3, T6, and T12). Second, we sought to investigate the effects of opioid overdose education on opioid overdose attitudes (OOAS) and knowledge (OOKS) and whether they change over time.
The use of naloxone in response to suspected cases of an opioid overdose was analyzed using both time-to-event and generalized linear regression models. Kaplan-Meier was used to estimate the cumulative incidence of time until first kit use. Participants who never used a kit were censored at their last visit date or 365 days, whichever was earlier. Participants who died during the trial were censored at their last visit date. Differences in the cumulative incidence of kit use between randomized treatment groups at 30, 60, 90, 180, and 365 days were tested using the fixed-point test (Klein et al., 2007) implemented in the R package ComparisonSurv (Lyu et al., 2020). Additionally, a generalized linear model was used to test for differences in the overall number of naloxone kits used by treatment group including an offset term (log follow-up time) to control for differential follow-up. For a better fit of the data (based on Bayesian information criterion), a negative binomial distribution was selected over Poisson.
Paired t-tests were used to measure changes in knowledge and attitudes (i.e., OOKS and OOAS scores) before and after training. Then, linear repeated measures models were used to analyze trajectories of knowledge and attitude scores (OOKS, OOAS, and BORRA) as well as differences in scores between extended training groups over the one-year follow-up period. Baseline demographics, including current treatment status and overdose history, were compared across groups for balance using chi-square tests. Generalized linear and linear repeated measures models were performed using SAS software version 9.4 (SAS Institute, Cary, NC). Statistical tests were two-sided with a significance criterion α = 0.05.
3. Results
3.1. Participant baseline data and retention
A total of 321 participants attended the baseline appointment (BL) and were randomized to one of the following treatment groups: TAU (n = 109), ET (n = 108) or ETwSO (n = 104). Seven participants did not complete their T0 appointment during which they would have received their experimental training, and per our a priori analysis plan, they were no longer followed (Fig. 1). Of the 314 participants who completed T0, follow-up rates at the respective time points were: T1: 85%, T3: 80%, T6: 76%, and T12: 72%. At baseline there were no statistically significant differences in demographic (e.g., age, sex) or drug-use (e.g., OUD severity, treatment status) variables among the three treatment groups (Table 2). Across the three treatment conditions, there were no significant differences in their naloxone choice, type of SO, or the amount of time the participant had known their SO (mean = 5.1 years).
Fig. 1.
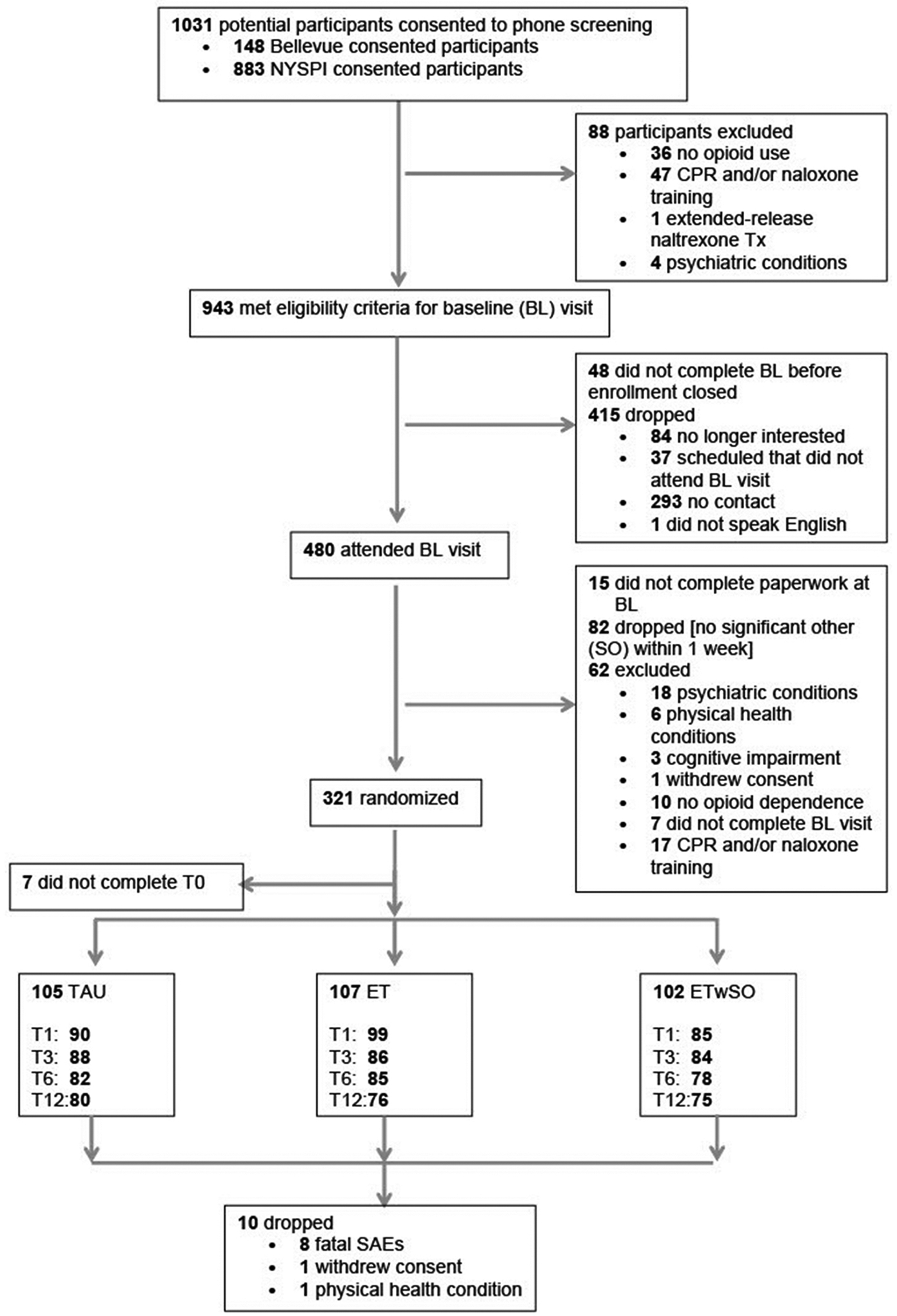
Subject disposition flow chart.
Table 2.
Participant demographics and trial retention.
| Treatment Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TAU (N = 109) | ET (N = 108) | ETwSO (N = 104) | ||||||||
| Baseline Variables | Total n | % | n | % | n | % | n | % | Chi Sq. | p |
| Sex | 321 | 1.22 | 0.54 | |||||||
| Male | 250 | 77.9 | 83 | 76.2 | 88 | 81.5 | 79 | 75.9 | ||
| Female | 71 | 22.1 | 26 | 23.9 | 20 | 18.5 | 25 | 24.0 | ||
| Age | 316 | 4.31 | 0.37 | |||||||
| Under 40 | 75 | 23.7 | 31 | 29.3 | 24 | 22.4 | 20 | 19.4 | ||
| 40–50 | 100 | 31.7 | 35 | 33.0 | 31 | 28.9 | 34 | 33.0 | ||
| Over 50 | 141 | 44.6 | 40 | 37.7 | 52 | 48.6 | 49 | 47.6 | ||
| Race/Ethnicity | 314 | 10.26 | 0.11 | |||||||
| Non-Hispanic White | 59 | 18.8 | 29 | 27.4 | 15 | 14.2 | 15 | 14.7 | ||
| Non-Hispanic Black | 136 | 43.3 | 39 | 36.8 | 50 | 47.2 | 47 | 46.1 | ||
| Hispanic/Latin | 99 | 31.5 | 31 | 29.3 | 32 | 30.2 | 36 | 35.3 | ||
| Other/Multiracial | 20 | 6.4 | 7 | 6.6 | 9 | 8.5 | 4 | 3.9 | ||
| OUD TX Status | 321 | 0.27 | 0.99 | |||||||
| Not Seeking Tx | 146 | 45.5 | 48 | 44.0 | 51 | 47.2 | 47 | 45.2 | ||
| Agonist Maintained | 139 | 43.3 | 49 | 44.9 | 45 | 41.7 | 45 | 43.3 | ||
| Recently Detoxed | 36 | 11.2 | 12 | 11.0 | 12 | 11.1 | 12 | 11.5 | ||
| Addiction Severity | 321 | 0.35 | 0.84 | |||||||
| Severe | 87 | 27.1 | 31 | 28.4 | 30 | 27.8 | 26 | 25.0 | ||
| Non-severe | 234 | 72.9 | 78 | 71.6 | 78 | 72.2 | 78 | 75.0 | ||
| Children | 311 | 6.58 | 0.03 | |||||||
| No | 115 | 36.9 | 46 | 43.4 | 29 | 27.4 | 40 | 40.4 | ||
| Yes | 196 | 63.0 | 60 | 56.6 | 77 | 72.6 | 59 | 59.6 | ||
| Ever Overdosed | 306 | 0.79 | 0.67 | |||||||
| No | 222 | 72.6 | 74 | 70.5 | 73 | 71.6 | 75 | 75.8 | ||
| Yes | 84 | 27.5 | 31 | 29.5 | 29 | 28.4 | 24 | 24.2 | ||
| Ever Witnessed an Overdose | 305 | 4.45 | 0.11 | |||||||
| No | 102 | 33.4 | 37 | 35.2 | 40 | 39.2 | 25 | 25.5 | ||
| Yes | 203 | 66.6 | 68 | 64.8 | 62 | 60.8 | 73 | 74.5 | ||
| Enrollment Year | 321 | 2.28 | 0.89 | |||||||
| 2015 | 77 | 23.9 | 29 | 26.6 | 24 | 22.2 | 24 | 23.1 | ||
| 2016 | 76 | 23.7 | 27 | 24.8 | 26 | 24.1 | 23 | 22.1 | ||
| 2017 | 98 | 30.5 | 30 | 27.5 | 37 | 34.3 | 31 | 29.8 | ||
| 2018 | 70 | 21.8 | 23 | 21.1 | 21 | 19.4 | 26 | 25.0 | ||
| Interest in Drug TX | 321 | 9.49 | 0.30 | |||||||
| Not at all | 155 | 48.3 | 57 | 52.3 | 41 | 37.9 | 57 | 54.8 | ||
| Slightly | 16 | 4.9 | 6 | 5.5 | 5 | 4.6 | 5 | 4.8 | ||
| Moderately | 18 | 5.61 | 7 | 6.4 | 6 | 5.6 | 5 | 4.8 | ||
| Considerably | 41 | 12.8 | 13 | 11.9 | 15 | 13.9 | 13 | 12.5 | ||
| Extremely | 91 | 28.4 | 26 | 23.9 | 41 | 37.9 | 24 | 23.1 | ||
| Naloxone Choice (Intramuscular or Intranasal) | 256 | 1.24 | 0.54 | |||||||
| Intranasal | 204 | 79.7 | 65 | 76.5 | 69 | 79.3 | 70 | 83.3 | ||
| Intramuscular | 52 | 20.3 | 20 | 23.5 | 18 | 20.7 | 14 | 16.7 | ||
| Attended Baseline | 321 | 8.32 | 0.22 | |||||||
| Yes | 321 | 100 | 109 | 100 | 108 | 100 | 104 | 100 | ||
| Attended T0 | 321 | 1.96 | 0.37 | |||||||
| No | 7 | 2.2 | 4 | 3.7 | 1 | 0.93 | 2 | 1.92 | ||
| Yes | 314 | 97.8 | 105 | 96.3 | 107 | 99.1 | 102 | 98.1 | ||
| Attended T1 | 321 | 5.21 | 0.07 | |||||||
| No | 47 | 14.6 | 19 | 17.4 | 9 | 8.3 | 19 | 18.3 | ||
| Yes | 274 | 85.4 | 90 | 82.6 | 99 | 91.7 | 85 | 81.7 | ||
| Attended T3 | 321 | 0.057 | 0.97 | |||||||
| No | 63 | 19.6 | 21 | 19.3 | 22 | 20.4 | 20 | 19.2 | ||
| Yes | 258 | 80.4 | 88 | 80.7 | 86 | 79.6 | 84 | 80.8 | ||
| Attended T6 | 321 | 0.52 | 0.77 | |||||||
| No | 76 | 23.7 | 27 | 24.8 | 23 | 21.3 | 26 | 25.0 | ||
| Yes | 245 | 76.3 | 82 | 75.2 | 85 | 78.7 | 78 | 75.0 | ||
| Attended T12 | 321 | 0.25 | 0.88 | |||||||
| No | 90 | 28.0 | 29 | 26.6 | 32 | 29.6 | 29 | 27.9 | ||
| Yes | 231 | 71.9 | 80 | 73.4 | 76 | 70.4 | 75 | 72.1 | ||
TAU: Training As Usual; ET: Extended Training; ETwSO: Extended Training with Significant Other; Tx: Treatment; OUD: Opioid Use Disorder.
3.2. Overdose events
Eight participants died while enrolled in the study (n = 3 were confirmed opioid overdoses, n = 3 were medical conditions unrelated to opioid overdose, n = 1 was unknown, and n = 1 was due to medical/surgical complications). Fourteen participants experienced a non-fatal opioid overdose. Of those, ten participants reported one overdose, two participants reported two overdoses and two participants experienced five overdoses while enrolled in the study. The frequency of overdose events (non-fatal and fatal overdoses combined) was similarly distributed across treatment groups (TAU: n = 4, ET: n = 8, ETwSO: n = 5).
3.3. Overdose intervention and naloxone utilization
Ten participants reported that their naloxone was used on them by someone else. The naloxone administrator in these instances included bystanders, friends/family, and the participant’s SO. Two participants reported having self-administered naloxone. For this outcome measure, these were not considered naloxone “uses” but instead were included in the participant non-fatal overdose count above. Additionally, kits that were lost (n = 25), taken by the police (n = 2), given away (n = 2), damaged (n = 1), or stolen (n = 1) were not included in the subsequent analyses.
Participants reported the use of their naloxone kit to reverse someone else’s overdose in 166 cases. Approximately 97% (161 of 166) of overdose reversals by study participants with naloxone were successful (i.e., to the participant’s knowledge the overdose victim survived), and only five reversals were unsuccessful (i.e., to the participant’s knowledge the overdose victim died). Given the small number, we refrained from statistically comparing the number of unsuccessful overdose reversals by treatment group.
Six naloxone kits were used after the basic NYSDOH opioid overdose education training, but before the T0 timepoint (i.e., before participants received randomized experimental training) and were not considered in further analyses. The 160 kits included in further analyses were used by 89 participants, who were defined as “unique kit users.” The majority of naloxone used was the intranasal formulation (89.3%), which did not significantly vary as a function of training group (p = 0.55). The majority of the 89 people who used a naloxone kit used only one, however, 23% (n = 20) used more than one and a small group (n = 4) used 5 or more kits throughout the one-year enrollment period.
The cumulative 12-month incidence of naloxone use did not significantly differ between groups. Additional analysis of the total count of naloxone kits used yielded no significant differences between TAU and the extended training groups in a negative binomial generalized linear model (combined ET and ETwSO vs TAU: β = 0.34, 95% CI [−0.17, 0.85], p = 0.189; ETwSO vs TAU: β = 0.39, 95% CI [−0.18, 0.96], p = 0.180; ET vs TAU: β = 0.20, 95% CI [−0.38, 0.77], p = 0.501).
The cumulative incidence of using a naloxone kit in the combined extended training arms (ET and ETwSO) was significantly higher than in the TAU arm during the first 90 days (p = 0.039) (Fig. 2). By 30 days 10.1% of participants in the combined extended training arms had already used a naloxone kit vs. 4.1% in the TAU arm. By 60 days the difference was 16.4% in the extended training versus 5.2% in TAU; and by 90 days, 17.9% vs 9.5%. The difference was no longer significant at 180 days (p = 0.131) or one year (p = 0.355). Adjusting for loss to follow-up or death, the cumulative incidence of using a kit at least once within the year was 30.0% (95% CI [21.4, 41.0]) for TAU (n = 26 participants used a kit) and 35.7% (95% CI [28.3%, 43.1%]) for the combined extended training arms (specifically 30.6% (95% CI [21.9, 41.7]) for ET (n = 28 used a kit), and 40.8% (95% CI [30.9, 52.5]) for ETwSO (n = 35 used a kit)). No statistically significant differences were found between the ET and ETwSO arms. Comprehensive comparisons among the three groups can be found in Table 3.
Fig. 2.
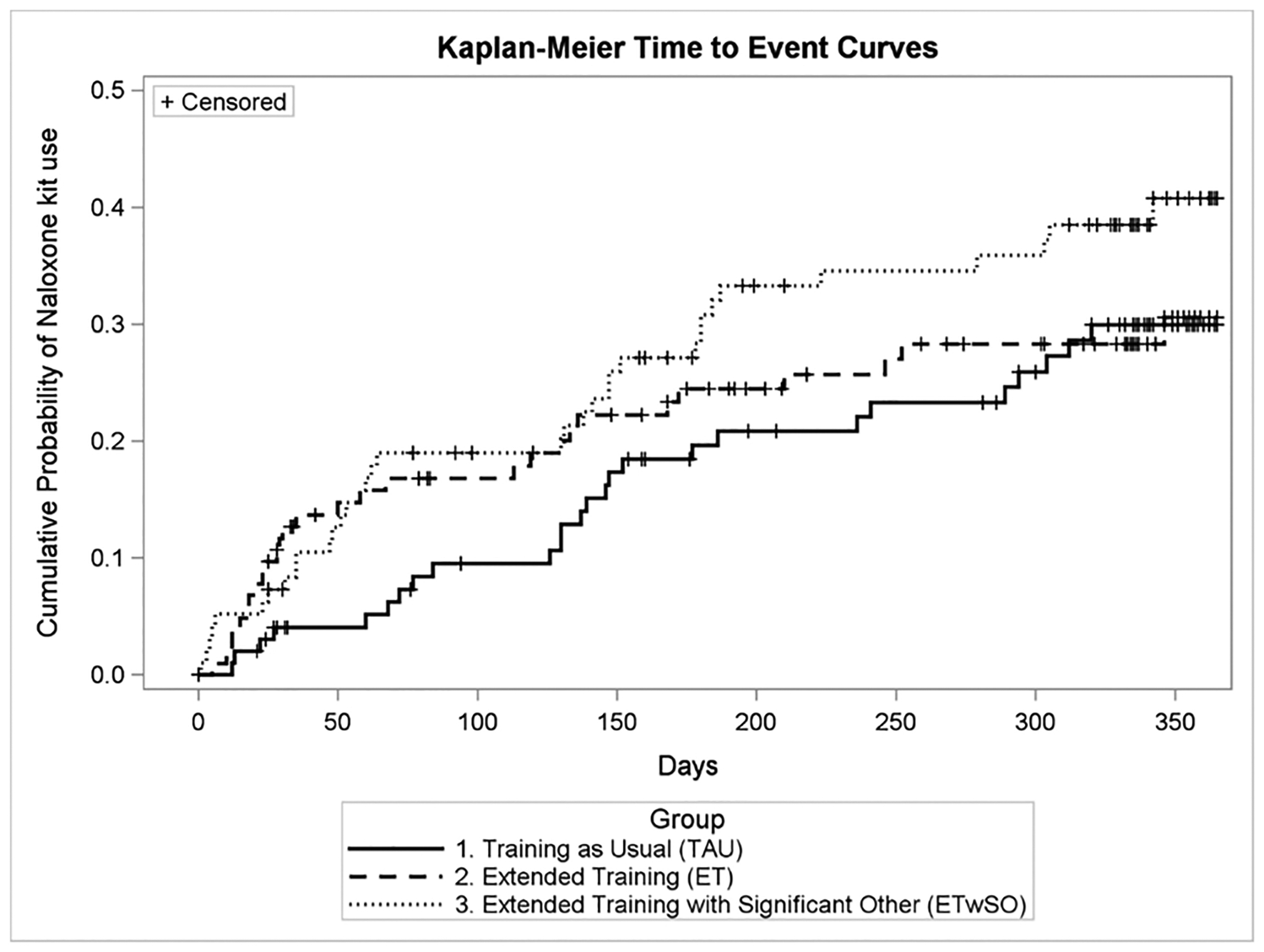
Cumulative incidence of naloxone kit use.
Table 3.
Time to first naloxone use and comparison to training as usual.
| Estimated Cumulative Incidence | |||
|---|---|---|---|
| % | 95% Confidence Interval | Difference from TAU (p-value) | |
| 30 days | |||
| TAU | 4.1% | (0.002, 0.080) | REF |
| Extended Training Combined (ET + ETwSO) | 10.1% | (0.059, 0.143) | 0.041 |
| ET | 12.7% | (0.062, 0.191) | 0.029 |
| ETwSO | 7.3% | (0.021, 0.125) | 0.335 |
| 60 days | |||
| TAU | 5.2% | (0.008, 0.095) | REF |
| Extended Training Combined (ET + ETwSO) | 16.4% | (0.112, 0.216) | 0.001 |
| ET | 15.8% | (0.087, 0.229) | 0.016 |
| ETwSO | 16.9% | (0.093, 0.244) | 0.011 |
| 90 days | |||
| TAU | 9.5% | (0.036, 0.154) | REF |
| Extended Training Combined (ET + ETwSO) | 17.9% | (0.125, 0.233) | 0.039 |
| ET | 16.8% | (0.095, 0.241) | 0.132 |
| ETwSO | 19.0% | (0.111, 0.269) | 0.064 |
| 180 days | |||
| TAU | 19.6% | (0.115, 0.278) | REF |
| Extended Training Combined (ET + ETwSO) | 27.6% | (0.212, 0.340) | 0.131 |
| ET | 24.5% | (0.159, 0.330) | 0.426 |
| ETwSO | 30.8% | (0.213, 0.404) | 0.087 |
| 365 days | |||
| TAU | 30.0% | (0.202, 0.397) | REF |
| Extended Training Combined (ET + ETwSO) | 35.7% | (0.283, 0.431) | 0.355 |
| ET | 30.6% | (0.207, 0.405) | 0.929 |
| ETwSO | 40.8% | (0.300, 0.516) | 0.152 |
3.4. Overdose knowledge and retention
Knowledge of opioid overdose (OOKS sum score) showed a large increase from before compared to after basic OEND training (mean pre-training score = 31.9, mean post-training score = 39.2, standardized difference d = 1.34, p < 0.001). Similarly, attitudes towards helping in an overdose situation (OOAS sum score) improved greatly from pre-training (mean = 95.9) to post-basic training (mean = 111.4, d = 1.63, p < 0.001). The BORRA was not assessed prior to the basic NYSDOH training.
Following extended training, there was a small but statistically significant further increase in knowledge of opioid overdose (OOKS) in the combined extended training groups (ET and ETwSO) as compared to TAU group (difference in means = 1.11, d=0.20, p = 0.013). The difference between extended training and TAU was not sustained at any of the follow-up visits, nor was there any significant difference between ET with ETwSO. Across all three training conditions, there was no significant increase or decrease of the OOKS score trajectory throughout the year (Fig. 3).
Fig. 3.
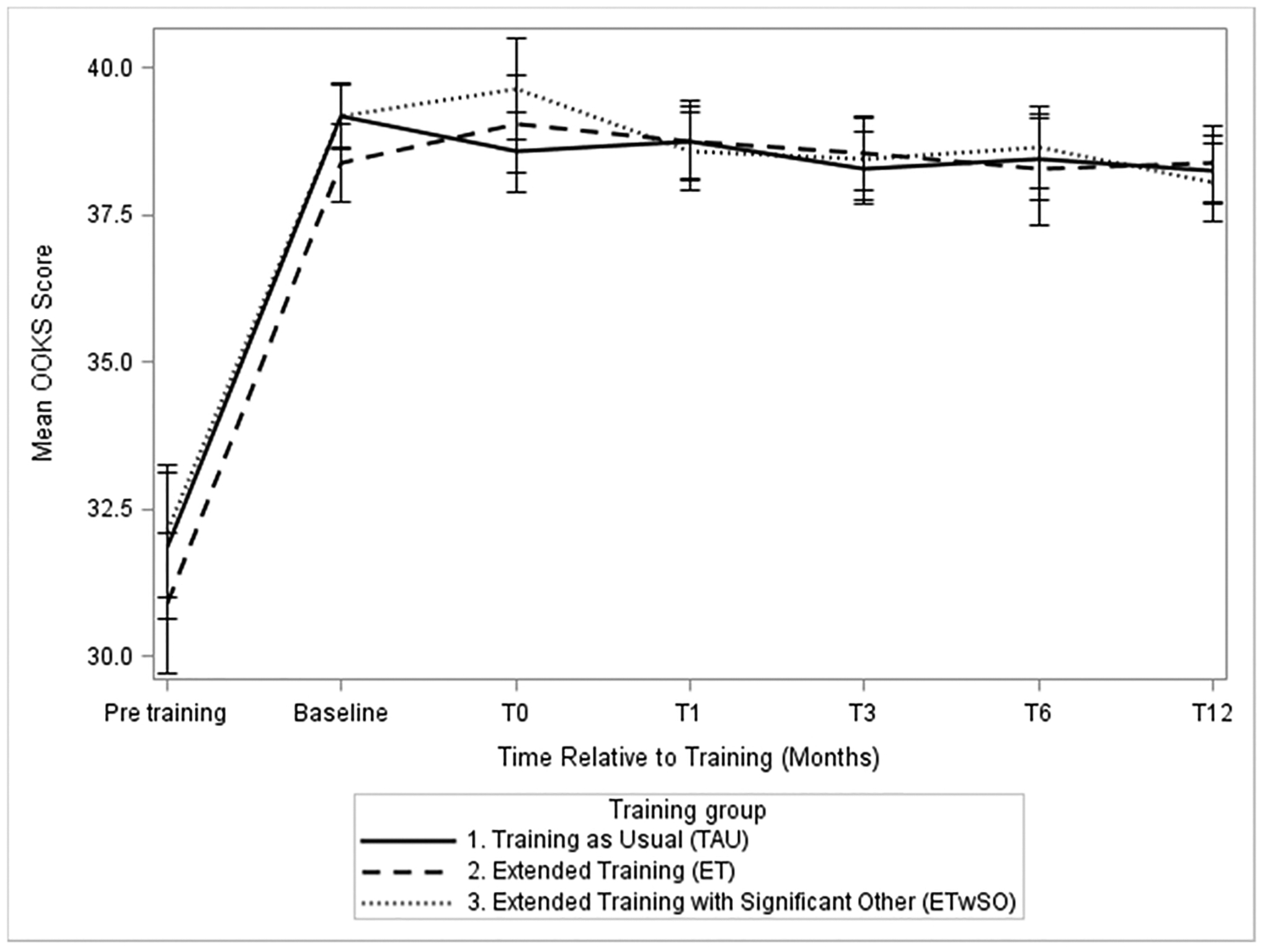
Mean Opioid Overdose Knowledge Scale (OOKS: Range 0–45) score for participants in each training condition measured immediately before (pre-training) and after basic training (baseline), immediately after their experimental training (T0), as well as 1, 3, 6, and 12 months post-T0 (T1, T3, T6, and T12).
No group differences in change from baseline over time between ET, ETwSO, and TAU were found for OOAS sum score (η2 = 0.005, 95% CI [0, 0.007], p = 0.710), BORRA overdose knowledge score (η2 = 0.004, 95% CI [0, 0.004], p = 0.865), or BORRA naloxone indication knowledge score (η2 = 0.004, 95% CI [0, 0.004], p = 0.856). Similar to the OOKS, the OOAS also showed no waning of the initial effect of basic training on attitude over time (T0 OOAS mean = 113.1, T12 OOAS mean = 113.5, p = 0.390).
4. Discussion
Provision of naloxone to non-medical persons likely to encounter an opioid overdose is a growing global strategy to address opioid overdose mortality (Davis and Carr, 2020; United Nations General Assembly, 2016). The results of the current trial reiterate the efficacy of these programs by showing that people who use opioids can effectively intervene in cases of opioid overdose (McDonald and Strang, 2016; Piper et al., 2008). As in other trials, naloxone interventions were over-whelmingly successful (Mueller et al., 2015; Strang et al., 2008; Walley et al., 2013). The current study also highlights the importance of the educational component found in most OEND programs (Davis et al., 2017; Naumann et al., 2019). In the current study, all forms of overdose education led to substantial knowledge gains, which were retained significantly longer than previous studies would suggest (Gaston et al., 2009).
This study is the first prospective, randomized, controlled trial to compare the effects of comprehensive and basic OEND training components on overdose intervention outcomes. A more comprehensive training curriculum resulted in greater uptake of naloxone use in suspected overdose situations with more than double the use by 30 days (10.1% vs 4.1%), triple the use by 60 days (16.4% vs 5.2%), and still significantly higher use by 90 days (17.9% vs 9.5%). However, training a significant other did not produce a significant increase in naloxone kit use. While the overall kit use by the end of the year did not differ between groups, it is noteworthy that the cumulative incidence was high in each group: 30.0% for TAU, 30.6% for ET, and 40.8% for ETwSO. Comprehensive opioid training together with a SO led to the most naloxone use by approximately 10%, though this difference was not statistically significant. However, extensive training may have increased the likelihood that SOs obtained their own naloxone kit, and/or the probability they would utilize naloxone in an overdose scenario. Unfortunately, due to practical limitations, these outcomes among SOs were not measured as part of the study.
The increased use by those who received extended training could not be explained by differential likelihood of encountering or experiencing an overdose (i.e., stratified randomization), overdose knowledge or self-perceived competence (i.e., OOKS, OOAS, BORRA scores). Only a small additional increase in OOKS just after the comprehensive training was observed that did not last by the 1-month follow-up visit. It may be that the study’s design hampered our ability to detect an effect of the comprehensive training on these measures. All participants received basic overdose education training before randomization. Basic training resulted in a significant increase in knowledge and competency gains. Little additional benefit of comprehensive training was found suggesting that the more intensive training does not improve knowledge/attitudes, and/or indicating a ceiling effect in the scales used.
Naloxone utilization in this trial was high (~33% at 12 months), even greater than the rates the investigators used to estimate the upper approximation of naloxone use (Piper et al., 2008). This is most likely attributable to the fact that studies published at the time of initiation of the current trial (2014) were conducted before the exponential growth phase of the opioid overdose curve (Clark et al., 2014; Hedegaard et al., 2018). A somewhat more recent systematic review of the international literature on take-home naloxone calculated a ‘proportion of use’ relative to the amount of naloxone supplied. The authors estimated that approximately 9% of naloxone kits are used within 3 months (McAuley et al., 2015). These data are close to the 3-month naloxone utilization rate seen in the current trial (~14%).
The study’s reliance on self-report data could be seen as a limitation of its design. An additional design feature of the study (the requirement for a SO) should be considered when assessing the generalizability of our findings. As shown in Fig. 1, 17% of participants, who met all other study criteria during the baseline visit, did not move forward in the study because they could not find a SO to attend T0. This finding suggests a level of social isolation that raises concerns for OEND as a global harm-reduction strategy. Due to the nature of opioid overdose, people experiencing an overdose are not usually able to administer naloxone to themselves. If people overdose alone without anyone else present to offer assistance, the likelihood of an overdose becoming fatal is increased (Rudolph et al., 2011; White and Irvine, 1999). Social isolation appears to be common among individuals with OUD, possibly aggravated among those with more severe diagnoses (Hasin et al., 2013; De Maeyer et al., 2010), and a lack of “social capital” is associated with the risk of dying due to overdose (Zoorob and Salemi, 2017). Thus, the field should focus on how overdose harm reduction can better target socially isolated people who use drugs. This finding also demonstrates the need for additional OD harm reduction services such as safe consumption sites.
To summarize, previous research has shown that OEND programs are feasible and cost-effective, with no evidence of compensatory drug use risk behavior (Albert et al., 2011; Bennett et al., 2011; Coffin and Sullivan, 2013; Doe-Simkins et al., 2009, 2014; Enteen et al., 2010; Evans et al., 2012; Green at al, 2008; Jones et al., 2014, 2017; Maldjian et al., 2016; Piper et al., 2008; Seal et al., 2005; Williams et al., 2014). The current study uniquely adds to this literature by empirically demonstrating that all intensities of OEND training (i.e., basic and more comprehensive) produced robust and sustained increases in overdose knowledge and competency among opioid users. Furthermore, more comprehensive training appears to facilitate naloxone use in suspected overdose events. Although the total amount of naloxone used over 1 year did not significantly differ among our groups, extended training increased naloxone utilization by 2–3 times during the first 3 months. Yet, it is important to note that the benefits of providing more comprehensive training should be balanced against concerns about feasibility. The additional burden of more detailed training on harm reduction providers (e.g., more staffing needs) and clients (e.g., less willingness to tolerate a longer training) could hamper the implementation of this service. Thus, when developing their OEND training curricula, the authors recommend that service providers consider the needs of their clientele (e.g., time constraints), along with their own limitations (e.g., personnel resources).
Acknowledgements
This study was supported by the National Institute on Drug Abuse grant R01DA035207 to Dr. Sandra Comer. Dr. Laura Brandt’s involvement in this trial was supported by the Erwin Schroedinger Fellowship from the Austrian Science Fund (ASF). Dr. Neale is part-funded by, and Dr. Strang is supported by, the NIHR (National Institute for Health Research) Maudsley Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London.
Role of the Funder/Sponsor
The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Trial Registration: Clinicaltrials.gov Identifier: NCT02535494
References
- Akers RL, 2009. Social Learning and Social Structure: A General Theory of Crime and Deviance. Transaction Publishers, New Brunswick, NJ. [Google Scholar]
- Albert S, Brason FW 2nd, Sanford CK, Dasgupta N, Graham J, Lovette B, 2011. Project Lazarus: community-based overdose prevention in rural North Carolina. Pain Med. 12 (Suppl. 2), S77–S85. 10.1111/j.1526-4637.2011.01128. [DOI] [PubMed] [Google Scholar]
- Bennett AS, Bell A, Tomedi L, Hulsey EG, Kral AH, 2011. Characteristics of an overdose prevention, response, and naloxone distribution program in Pittsburgh and Allegheny County, Pennsylvania. J. Urban Health 88 (6), 1020–1030. 10.1007/s11524-011-9600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert AS, Bonar EE, Cunningham R, Greenwald MK, Thomas L, Chermack S, Blow FC, Walton M, 2016. A pilot randomized clinical trial of an intervention to reduce overdose risk behaviors among emergency department patients at risk for prescription opioid overdose. Drug Alcohol Depend. 163, 40–47. [DOI] [PubMed] [Google Scholar]
- Boyer EW, 2012. Management of opioid analgesic overdose. N. Engl. J. Med 367 (2), 146–155. 10.1056/NEJMra1202561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AK, Wilder CM, Winstanley EL, 2014. A systematic review of community opioid overdose prevention and naloxone distribution programs. J. Addict. Med 8 (3), 153–163. 10.1097/ADM.0000000000000034. [DOI] [PubMed] [Google Scholar]
- Coffin PO, Sullivan SD, 2013. Cost-effectiveness of distributing naloxone to heroin users for lay overdose reversal. Ann. Intern. Med 158 (1), 1–9. 10.7326/0003-4819-158-1-201301010-00003. [DOI] [PubMed] [Google Scholar]
- Coffin PO, Santos GM, Matheson T, Behar E, Rowe C, Rubin T, Silvis J, Vittinghoff E, 2017. Behavioral intervention to reduce opioid overdose among high-risk persons with opioid use disorder: a pilot randomized controlled trial. PLoS One 12 (10), e0183354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S, Mills KL, Ross J, Teesson M, 2011. Rates and correlates of mortality amongst heroin users: findings from the Australian Treatment Outcome Study (ATOS), 2001–2009. Drug Alcohol Depend. 115 (3), 190–195. 10.1016/j.drugalcdep.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Davis CS, Carr D, 2020. Over the counter naloxone needed to save lives in the United States. Prev. Med 130. [DOI] [PubMed] [Google Scholar]
- Davis C, Chang S, Carr D, Hernandez-Delgado H, 2017. Legal Interventions to Reduce Overdose Mortality: Naloxone Access and Overdose Good Samaritan laws: The Network for Public Health Law, pp. 1–13. 〈https://www.networkforphl.org/resources_collection/2017/06/08/396/resource_legal_interventions_to_reduce_overdose_mortality/?utm_source=Network+Report+6-22-17&utm_campaign=network+report+6-22-17&utm_medium=email&utm_content=308〉. (Accessed 3 March 2020).
- De Maeyer J, Vanderplasschen W, Broekaert E, 2010. Quality of life among opiate-dependent individuals: a review of the literature. Int. J. Drug Policy 21 (5), 364–380. 10.1016/j.drugpo.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Whiteford HA, Ferrari AJ, et al. , 2013. Global burden of disease attributable to illicit drug use and dependence: findings from the Global Burden of Disease Study 2010. Lancet 382 (9904), 1564–1574. 10.1016/S0140-6736(13)61530-5. [DOI] [PubMed] [Google Scholar]
- Doe-Simkins M, Walley AY, Epstein A, Moyer P, 2009. Saved by the nose: bystander-administered intranasal naloxone hydrochloride for opioid overdose. Am. J. Public Health 99 (5), 788–791. 10.2105/AJPH.2008.146647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe-Simkins M, Quinn E, Xuan Z, et al. , 2014. Overdose rescues by trained and untrained participants and change in opioid use among substance-using participants in overdose education and naloxone distribution programs: a retrospective cohort study. BMC Public Health 14, 297. 10.1186/1471-2458-14-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne RB, 2018. Prescribing naloxone for opioid overdose intervention. Pain Manag. 8 (3), 197–208. 10.2217/pmt-2017-0065. [DOI] [PubMed] [Google Scholar]
- Enteen L, Bauer J, McLean R, et al. , 2010. Overdose prevention and naloxone prescription for opioid users in San Francisco. J. Urban Health 87 (6), 931–941. 10.1007/s11524-010-9495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JL, Tsui JI, Hahn JA, Davidson PJ, Lum PJ, Page K, 2012. Mortality among young injection drug users in San Francisco: a 10-year follow-up of the UFO study. Am. J. Epidemiol 175 (4), 302–308. 10.1093/aje/kwr318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston RL, Best D, Manning V, Day E, 2009. Can we prevent drug related deaths by training opioid users to recognise and manage overdoses? Harm Reduct. J 6, 26. 10.1186/1477-7517-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TC, Heimer R, Grau LE, 2008. Distinguishing signs of opioid overdose and indication for naloxone: an evaluation of six overdose training and naloxone distribution programs in the United States. Addiction 103 (6), 979–989. 10.1111/j.1360-0443.2008.02182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, O’Brien CP, Auriacombe M, et al. , 2013. DSM-5 criteria for substance use disorders: recommendations and rationale. Am. J. Psychiatry 170 (8), 834–851. 10.1176/appi.ajp.2013.12060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedegaard H, Miniño AM, Warner M, 2018. Drug Overdose Deaths in the United States, 1999–2017. NCHS Data Brief, No. 329. Hyattsville, MD: National Center for Health Statistics. [Google Scholar]
- Jones JD, Roux P, Stancliff S, Matthews W, Comer SD, 2014. Brief overdose education can significantly increase accurate recognition of opioid overdose among heroin users. Int. J. Drug Policy. 25 (1), 166–170. 10.1016/j.drugpo.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Campbell A, Metz VE, Comer SD, 2017. No evidence of compensatory drug use risk behavior among heroin users after receiving take-home naloxone. Addict. Behav 71, 104–106. 10.1016/j.addbeh.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Campbell A, Brandt L, Castillo F, Abbott R, Comer SD, 2020. Intervention in an opioid overdose event increases interest in treatment among individuals with opioid use. Subst. Abus 19, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane C, Egan JE, Hawk M, 2018. Effects of naloxone distribution to likely bystanders: results of an agent-based model. Int. J. Drug Policy 55, 61–69. 10.1016/j.drugpo.2018.02.008. [DOI] [PubMed] [Google Scholar]
- Kim D, Irwin KS, Khoshnood K, 2009. Expanded access to naloxone: options for critical response to the epidemic of opioid overdose mortality. Am. J. Public Health 99 (3), 402–407. 10.2105/AJPH.2008.136937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JP, Logan B, Harhoff M, et al. , 2007. Analyzing survival curves at a fixed point in time. Stat. Med 26 (24), 4505–4519. [DOI] [PubMed] [Google Scholar]
- Lagu T, Anderson BJ, Stein M, 2006. Overdoses among friends: drug users are willing to administer naloxone to others. J. Subst. Abus. Treat 30 (2), 129–133. 10.1016/j.jsat.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Lyu J, Chen Z, Li H, Chen J, Huang X, 2020. Comparison of Survival Curves between Two Groups. R Package ComparisonSurv. CRAN Repository. [Google Scholar]
- Maldjian L, Siegler A, Kunins HV, 2016. Evaluation of overdose prevention trainings in New York City: knowledge and self-efficacy among participants 12 months after training. Subst. Abus 37 (3), 459–465. 10.1080/08897077.2015.1135850. [DOI] [PubMed] [Google Scholar]
- Martinez S, Jones JD, Brandt L, Campbell AN, Abbott R, Comer SD, 2020. The increasing prevalence of fentanyl: a urinalysis-based study among individuals with opioid use disorder in New York City. Am. J. Addict 30 (1), 65–71. 10.1111/ajad.13092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez S, Jones JD, Brandt L, Hien D, Campbell ANC, Batchelder S, Comer SD, 2021. Factor structure and psychometric properties of the connor–davidson resilience scale (CD-RISC) in individuals with opioid use disorder. Drug Alcohol Depend. 10.1016/j.drugalcdep.2021.108632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley A, Lindsay G, Woods M, Louttit D, 2010. Responsible management and use of a personal take-home naloxone supply: a pilot project. Drugs Educ. Prev. Policy 17 (4), 388–399. 10.3109/09687630802530712. [DOI] [Google Scholar]
- McAuley A, Aucott L, Matheson C, 2015. Exploring the life-saving potential of naloxone: a systematic review and descriptive meta-analysis of take home naloxone (THN) programmes for opioid users. Int. J. Drug Policy 26 (12), 1183–1188. 10.1016/j.drugpo.2015.09.011. [DOI] [PubMed] [Google Scholar]
- McDonald R, Strang J, 2016. Are take-home naloxone programmes effective? Systematic review utilizing application of the Bradford Hill criteria. Addiction 111 (7), 1177–1187. 10.1111/add.13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, 1992a. The fifth edition of the addiction severity index. J. Subst. Abus. Treat 9 (3), 199–213. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Alterman AI, Cacciola J, Metzger D, O’Brien CP, 1992b. A new measure of substance abuse treatment. Initial studies of the treatment services review. J. Nerv. Ment. Dis 180 (2), 101–110. 10.1097/00005053-199202000-00007. [DOI] [PubMed] [Google Scholar]
- Mueller SR, Walley AY, Calcaterra SL, Glanz JM, Binswanger IA, 2015. A review of opioid overdose prevention and naloxone prescribing: implications for translating community programming into clinical practice. Subst. Abus 36 (2), 240–253. 10.1080/08897077.2015.1010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann RB, Durrance CP, Ranapurwala SI, et al. , 2019. Impact of a community-based naloxone distribution program on opioid overdose death rates. Drug Alcohol Depend. 204, 107536 10.1016/j.drugalcdep.2019.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navaline HA, Snider EC, Petro CJ, Tobin D, Metzger D, Alterman AI, et al. , 1994. An automated version of the risk assessment battery (RAB): enhancing the assessment of risk behaviors. AIDS Res. Hum. Retrovir 10, S281–S283. [PubMed] [Google Scholar]
- Pietrusza LM, Puskar KR, Ren D, Mitchell AM, 2018. Evaluation of an opiate overdose educational intervention and naloxone prescribing program in homeless adults who use opiates. J. Addict. Nurs 29 (3), 188–195. 10.1097/JAN.0000000000000235. [DOI] [PubMed] [Google Scholar]
- Piper TM, Stancliff S, Rudenstine S, et al. , 2008. Evaluation of a naloxone distribution and administration program in New York City. Subst. Use Misuse 43 (7), 858–870. 10.1080/10826080701801261. [DOI] [PubMed] [Google Scholar]
- Prescription Drug Abuse Policy System, 2019. Naloxone Overdose Prevention Laws. 〈http://pdaps.org/datasets/laws-regulating-administration-of-naloxone-1501695139〉. (Accessed 3 March 2020).
- Rudolph SS, Jehu G, Nielsen SL, Nielsen K, Siersma V, Rasmussen LS, 2011. Prehospital treatment of opioid overdose in Copenhagen-is it safe to discharge on-scene? Resuscetation 82 (11), 1414–1418. 10.1016/j.resuscitation.2011.06.027. [DOI] [PubMed] [Google Scholar]
- Seal KH, Thawley R, Gee L, et al. , 2005. Naloxone distribution and cardiopulmonary resuscitation training for injection drug users to prevent heroin overdose death: a pilot intervention study. J. Urban Health 82 (2), 303–311. 10.1093/jurban/jti053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SG, Gann DS, Tobin KE, Latkin CA, Welsh C, Bielenson P, 2009. “The life they save may be mine”: diffusion of overdose prevention information from a city sponsored programme. Int. J. Drug Policy 20 (2), 137–142. 10.1016/j.drugpo.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Smyth B, Hoffman V, Fan J, Hser Y-I, 2007. Years of potential life lost among heroin addicts 33 years after treatment. Prev. Med 44 (4), 369–374. 10.1016/j.ypmed.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang J, Best D, Man L, Noble A, Gossop M, 2000. Peer-initiated overdose resuscitation: fellow drug users could be mobilized to implement resuscitation. Int. J. Drug Policy 11, 437–445. 10.1016/s0955-3959(00)00070-0. [DOI] [PubMed] [Google Scholar]
- Strang J, Manning V, Mayet S, et al. , 2008. Overdose training and take-home naloxone for opiate users: prospective cohort study of impact on knowledge and attitudes and subsequent management of overdoses. Addiction 103 (10), 1648–1657. 10.1111/j.1360-0443.2008.02314.x. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, 2013. SAMHSA Opioid Overdose Prevention Toolkit. HHS Publication No. (SMA) 18–4742. Rockville, MD: Substance Abuse and Mental Health Services Administration. [Google Scholar]
- Tobin KE, Sherman SG, Beilenson P, Welsh C, Latkin CA, 2009. Evaluation of the Staying Alive programme: training injection drug users to properly administer naloxone and save lives. Int. J. Drug Policy 20 (2), 131–136. 10.1016/j.drugpo.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Tracy M, Piper TM, Ompad D, et al. , 2005. Circumstances of witnessed drug overdose in New York City: implications for intervention. Drug Alcohol Depend. 79 (2), 181–190. 10.1016/j.drugalcdep.2005.01.010. [DOI] [PubMed] [Google Scholar]
- United Nations General Assembly, 2016. Special Session on the World Drug Problem (General Assembly Resolution S-30–1, annex) New York, 19–21 April 2016. [Google Scholar]
- Wagner KD, Liu L, Davidson PJ, Cuevas-Mota J, Armenta RF, Garfein RS, 2015. Association between non-fatal opioid overdose and encounters with healthcare and criminal justice systems: identifying opportunities for intervention. Drug Alcohol Depend. 153, 215–220. 10.1016/j.drugalcdep.2015.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walley AY, Xuan Z, Hackman HH, Quinn E, Doe-Simkins M, Sorensen-Alawad A, Ruiz S, Ozonoff A, 2013. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ 346, f174. 10.1136/bmj.f174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JM, Irvine RJ, 1999. Mechanisms of fatal opioid overdose. Addiction 94 (7), 961–972. 10.1046/j.1360-0443.1999.9479612.x. [DOI] [PubMed] [Google Scholar]
- Williams AV, Strang J, Marsden J, 2013. Development of opioid overdose knowledge (OOKS) and attitudes (OOAS) scales for take-home naloxone training evaluation. Drug Alcohol Depend. 132 (1–2), 383–386. 10.1016/j.drugalcdep.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Williams AV, Marsden J, Strang J, 2014. Training family members to manage heroin overdose and administer naloxone: randomized trial of effects on knowledge and attitudes. Addiction 109 (2), 250–259. 10.1111/add.12360. [DOI] [PubMed] [Google Scholar]
- Winhusen T, Wilder C, Lyons MS, Theobald J, Kropp F, Lewis D, 2020. Evaluation of a personally-tailored opioid overdose prevention education and naloxone distribution intervention to promote harm reduction and treatment readiness in individuals actively using illicit opioids. Drug Alcohol Depend. 216, 108265 10.1016/jdrugalcdep.2020.108265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, 2018. Information Sheet on Opioid Overdose. 〈https://www.who.int/substance_abuse/information-sheet/en/〉. (Accessed 23 February 2020).
- Zoorob MJ, Salemi JL, 2017. Bowling alone, dying together: the role of social capital in mitigating the drug overdose epidemic in the United States. Drug Alcohol Depend. 173, 1–9. 10.1016/jdrugalcdep.2016.12.011. [DOI] [PubMed] [Google Scholar]


