Abstract
Fresh fishery products are highly perishable foods mainly due to their high-water content and high level of pH which act as promoters of spoilage processes. In these matrices, the deterioration phenomena are the result of the action of oxidative, and enzymatic processes due in part to the presence of specific microorganisms. Indeed, the microbial communities responsible for spoilage are a small fraction of the flora detectable in the fish and are known as specific spoilage organisms (SSOs). In the last decades, the scientific community has worked to achieve the ambitious goal of reducing the impact of microbial deterioration on food losses through innovative solutions, including antimicrobial packaging. The goal of this study was to evaluate the efficacy of an active polypropylene (PP)- based packaging functionalized with the antimicrobial peptide 1018K6 to extend the shelf life of dolphinfish burgers (Coryphaena hippurus) by evaluating its effect on sensorial and microbiological profile. The microbiological results showed an evident antimicrobial activity of the active packaging against hygiene indicator microorganisms and SSOs, recording a reduction of about 1 Log (CFU/g) of their concentrations compared to those of the control groups. Furthermore, a significant influence of functionalized packaging on the organoleptic characteristics was noted, accentuating the differences in freshness between the two experimental groups. This work confirmed the hypothesis of considering antimicrobial packaging as a potential tool capable of slowing down surface microbial replication and, therefore, extending the shelf-life and improving the health and hygiene aspect of fresh fish products.
Key words: Antimicrobial packaging, Spoilage microorganisms, Perishable foods
Introduction
Fish and fishery products are considered a rich source of high-quality proteins, n-polyunsaturated fatty acids (PUFAs), vitamins (A, B and D) and minerals (P, Mg, Se and I) (Gökoğlu and Yerlikaya, 2015), impacting their promotion as health foods. In fact, thanks to the dissemination among consumers of information on their health benefits (Thilsted et al., 2016), fish consumption has doubled from 9.9 kg in 1960 to 20 kg in 2015 (FAO, 2016), up to reach 24.36 kg in 2018 (EUMOFA, 2020). However, due to the composition (Chotphruethipong and Benjakul, 2019), seafood is very susceptible to deterioration phenomena which are the main cause of rejection by consumers. Decay processes cause the production of secondary products and microbiological metabolites that can lead to the formation of off-odour and offflavour, and texture and colour changes (Ambrosio et al., 2022). Although Pseudomonas putrefaciens and Pseudomonas fluorescens have been recognized as the major spoilage microorganisms in seafood (Ghaly et al., 2010), the total microbial count plays a crucial role in assessing the fish spoilage as it is inevitably influenced by the management of critical phases along the food chain (i.e. handling, storage and method of packaging). Over the years, synthetic antimicrobials have been used in the storage of fish products; however, consumers have associated these compounds with toxicity, increasing their interest toward natural bioactive compounds such as essential oils and plant extracts (Ambrosio et al., 2020; Marrone et al., 2021), bacteriocins, enzymes or antimicrobial peptides (AMP) (Festa et al., 2021).
Several agents with antimicrobial activity can be incorporated and coated onto the packaging materials, including antimicrobial peptides (Gogliettino et al., 2020; Panza et al., 2020; Rico et al., 2020; Ambrosio et al., 2022) that are immobilized on surface of polymers. AMPs represent a valid option to consider as innovative molecules able to replace the common antibiotics. They are short amino acid sequences that are part of the innate immune system of all multicellular organisms (De Smet and Contreras, 2005; Guaní- Guerra et al., 2010) and some of them have been isolated from a wide variety of animals, plants, bacteria, fungi, and viruses (Reddy et al., 2004). In a previous study, a cathelicidin-related antimicrobial peptide consisting of 12 residues and named 1018- K6 was in silico designed and characterized (Palmieri et al., 2018; Colagiorgi et al., 2020; Festa et al., 2021), showing high structural stability as well as powerful antimicrobial and antibiofilm activities against Gram-positive (Listeria monocytogenes, MRSA and MSSA Staphylococcus aureus) and Gram-negative (Salmonella enterica and Escherichia coli) food bacterial pathogens.
Since most of the time the spoilage in fish starts from the surface growth of microorganisms, the direct surface application of antimicrobial compounds is becoming the goal of many scientists and stakeholders (Gogliettino et al., 2020; Ambrosio et al., 2022). The antimicrobial packaging represents a good solution for microbial spoilage considering that the coating not only provides a physical barrier to environmental hazards but also, through the direct application of antimicrobial compounds, acts slowing down the replication of microbial communities (Khaneghah et al., 2018).
Although the antimicrobial efficiency of a novel class of active packaging, functionalized with the bactericidal peptide 1018K6, has already been demonstrated (Ambrosio et al., 2022), the present study aimed to evaluate its activity also against bacteria responsible for the food spoilage in fish burgers of dolphinfish (Coryphaena hippurus), validating the potential extension of shelf-life of more food categories.
Materials and methods
Coating formulation
One of the most common supports in the food industry is polypropylene (PP). It has been used in the production of 1018K6- PP or non-functionalized PP disks, according to the protocol of Ambrosio et al. (2022). The peptide was covalently immobilized onto the PP surface, previously activated by plasma treatment (Openair- Plasmaâ Technology). The derivative 12- mer peptide 1018-K6 was purchased from GenScript Biotech (Leiden, Netherlands).
Samples preparation
Fish burgers of dolphinfish (Coryphaena hippurus, Linnaeus 1758) were purchased from a local fishery industry in Naples (Italy) and transported in refrigerated boxes to the microbiological laboratory. A total of 21 samples (burger weighed 200 g each) were included in the experimental trial. Specifically, 3 burgers were immediately analysed to fix the initial levels of the microbial communities; while the other samples (18) were randomly assigned into two different groups: a control group (HL CTR) and a functionalized films group (HL 1018K6). Burgers were coated aseptically with 1018K6-PP or non-functionalized PP disks (4x4 cm), which were placed on the upper and lower surfaces of each sample. Subsequently, all burgers were stored in polystyrene trays cover with film paper at 4±1°C for 7 days. Microbiological, physicochemical, and sensorial analyses were performed at four sampling intervals: day 0 (beginning of the experimental study), day 3, day 5 and day 7.
pH and aw measurements
The pH measurements were carried out with a digital pH meter (Crison-Micro TT 2022, Crison Instruments, Barcelona). Water activity (aw) was measured with Aqualab 4 TE (Decagon Devices Inc., USA).
Microbiological analyses
Ten grams of each sample were aseptically transferred in a sterile stomacher bag with 90 mL (1:10 (w/v)) of sterilized Peptone Water (PW, Oxoid) and homogenized for three minutes at 230 rpm using a peristaltic homogenizer (BagMixer®400 P, Interscience, Saint Nom, France). Appropriate serial decimal dilutions of each homogenate were prepared for the following microorganism counts: Total Viable Count (TVC) and Psychotropic bacteria Count (PTC) were performed on Plate Count Agar (PCA, Oxoid, Madrid, Spain) incubated at 30°C for 48/72 h and 7°C for 10 days, respectively (ISO 4833-1:2013 and ISO 17410:2019); Total Coliforms on Violet Red Bile Lactose Agar (VRBL, Oxoid, Madrid, Spain) incubated at 37°C for 48 h (ISO 4831:2006); Enterobacteriaceae on Violet Red Bile Glucose Agar (VRBG, Oxoid, Madrid, Spain) incubated at 37°C for 48 h (ISO 21528-2:2017); Lactic Acid Bacteria (LAB) on MRS agar with Tween 80 (Oxoid, Madrid, Spain), incubated at 30°C for 72 h (ISO 15214:2015); Pseudomonas spp. on Pseudomonas Agar Base with CFC supplement (Oxoid, Madrid, Spain) incubated at 25°C for 48 h (ISO 13720:2010); β-glucuronidase- positive Escherichia coli (ISO 16649-1:2018) on Triptone Bile X-glucoronide Agar (TBX, Oxoid, Madrid, Spain) at 44°C for 24 h; Enterococcus spp. on KAA (Kanamycin Aesculin Azide, Oxoid, Madrid, Spain) at 37°C for 48 h; coagulase positive staphylococci on Baird-Parker Agar (Oxoid, Madrid, Spain) at 37°C for 24/48 h (ISO 6888-1:1999). After counting, the data were expressed as logarithms of the number of colony-forming units (CFU/g) and means and standard error were calculated.
Sensory testing
Sensory testing of dolphinfish burgers was performed by 5 trained panel members, who assessed odour, colour, and general acceptability. To minimize individual differences and ensure repeatability of results, the panellists were trained (2 sessions of 2 h) to fix the appropriate attributes. In this regard, the analyses were carried out with the aim of collecting useful information on changes in the appearance of burgers over time; indeed, despite the low number of people employed in the study, the evaluation of the general appearance could suggest and orient the data interpretation, reinforcing the chemical and microbiological results. Sensory evaluations were performed under controlled humidity, light, and temperature conditions and the Likert scale (9-point) was used to assess each attribute. According to Angiolillo et al. (2018), in the scale each number corresponds to the following adjective: 9 corresponded to excellent, 8 to very good, 7 to good, 6 to reasonable, 5 to not good (acceptable limit), 4 to disliked, 3 to bad, 2 to very bad, and 1 to completely unacceptable. The coded samples were randomly and simultaneously distributed to each panel member.
Statistical analyses
Microbiological and sensory data were statistically analysed using SPSS version 26 (IBM Analytics, Armonk, NY, US). Generalized linear mixed model was adopted to study parameters of dolphinfish burgers at each sampling time, including the fixed effect of packaging used at different sampling times (0, 3, 5 and 7 days).
Results and discussion
pH and aw
The pH of fish burgers increased over the whole shelf-life, from the initial value of 6.23±0.01 to final values of 6.39±0.02 and 6.31±0.03 in HL CTR and HL 1018K6 groups, respectively. The detected pH values were found to be almost neutral and in agreement with those reported by Messina et al. (2018) in fresh fillets of dolphinfish. The rise in pH could be linked to the protein degradation and the subsequent production of alkaline compounds which affected negatively sensorial characteristics such as odour, colour and texture (Gonzalez- Rodriguez et al., 2001). Furthermore, pH values relatively high (pH>6) could facilitate the replication of common spoilage microorganisms, creating an environment suitable for their survival. The trends highlighted a value overlapping among the two experimental groups (data not showed), pointing out the absence of interferences between the active packaging and the phenomena underlying the modification of the pH. This finding is consistent with the results reported by Ambrosio et al. (2022) for salmon fillets and bonito fish burgers.
Water activity influences significantly the perishability of fish and fish products, being one of the main factors that affect the microbial replications. The aw values related to each experimental group along the storage time did not show differences connectable to the coating typologies. Indeed, the aw homogeneously decreased both in HL CTR as HL 1018K6 groups, ranged from 0.973±0.001 to 0.967±0.003 and 0.965±0.008, respectively.
Microbiological results
Microbiological results (Table 1) revealed that the application of antimicrobial films slowed down the growth trend of many bacterial classes investigated. TVC and PTC represent important keys to investigate the contamination status of foods. In the absence of mandatory regulatory references regarding the microbiological aspect of fish and fishery products, the maximum acceptable limit has been set at 7 Log (CFU/g) by the International Commission on Microbiological Specifications for Foods (ICMSF). As shown in Table 1, the initial levels of TVC and PTC in fish burgers were found to be lower than the aforementioned microbiological limit. Nevertheless, the bacterial counts on day 1 were higher and not comparable with those reported in other studies, recording a gap of more than 3-4 Log (CFU/g) (Albertos et al., 2019; Ehsani et al., 2020) in fish burgers. Clearly, the shredding process underlying the manufacture of these food typologies could affect their overall hygiene quality, making them more sensitive to microbiological contamination (Roohinejad et al., 2017). The differences in concentration between TVC and PTC at the beginning of the experimental trial agree with the literature (Hoel et al., 2017), recording a higher value of mesophilic bacteria. Overall, total aerobic counts, both mesophilic and psychrophilic, increased over the storage period and reached their highest levels over the 5 days of storage in both experimental groups. Substantial differences in TVC levels (p<0.01) were found between the two packaging systems, highlighting the potential role of the active coating in suppressing the growth of this bacterial population.
It is well known that specific microbial groups are responsible for the off-flavours and the unpleasant odours typical of deteriorated fish products (Geeroms et al., 2008); certainly, the microorganisms belonging to the Pseudomonas species are predominant among fish spoilage bacteria and are recognized as one of the most common sources of some opportunistic and food-borne illnesses (Zhou et al., 2018). The counts of these bacteria increased over the storage period, albeit differently among the two groups. In the control group, the concentration of Pseudomonas spp. reached the highest value on the 5th day, followed by a plateau phase up to the 7th day; this trend was found to be very similar to that of TCV. In this regard, Total Bacteria Count and Pseudomonas spp. levels could justify the observed progressive decay of organoleptic characteristics of control fish burgers. On the other hand, the groups packed with the films containing antimicrobial peptides were characterized by a slow and progressive increase of Pseudomonas spoilage bacteria, recording significative lower values than the control group on 3rd and 5th days (p<0.01). These results highlighted the potential of the coating to retard their growth.
As regards Enterobacteriaceae, Total Coliforms and Enterococcus faecalis, the growth curves were similar to each other, with a gradual decrease occurring subsequently up to the 5th day of storage and counts lower than the start time (significant differences were found for Enterobacteriaceae and Enterococcus faecalis, p<0.01). It is interesting to note the differences in concentration of these bacterial communities between the two experimental groups on exactly the 5th day, when the highest values of these microorganisms were detected. The novel storage system caused a slowdown in the replication of these bacteria with a strong reduction of microbial load in the samples belonging to the HL 1018K6 group. The sensitivity of these bacterial genera and species, recognized as indicators of hygiene, to the antimicrobial activities of the innovative packaging enhances this system and promotes its applicability as a “controller tool” for Escherichia coli. Indeed, as displayed in Table 1, a very low proliferation of this bacterium was recorded in the samples kept in contact with the active disks. In this regard, the authors have already proved the bactericidal efficacy of the free peptide 1018-K6 against a specific genus belonging to the Enterobacteriaceae, Salmonella spp. (Festa et al. 2021). In particular, several serotyped wild strains were selected on the basis of their resistance to common antibiotics and used to validate and demonstrate the peptide antimicrobial efficacy even against microorganisms which have developed defence mechanisms.
Table 1.
Microbiological results of fish burgers.
| Day | 0 | 3 | 5 | 7 | |
|---|---|---|---|---|---|
| M ± sE | M ± sE | M ± sE | M ± sE | ||
| TVC 30°C | HL CTR | 5.66±0.05A | 6.77±0.06B, X | 7.04±0.00aC,X | 6.67±0.14BCb,x |
| HL 1018K6 | 5.66±0.05A | 5.81±0.07a, Y | 6.44±0.00B,Y | 6.22±0.14bB,y | |
| PTC 7°C | HL CTR | 4.89±0.06A | 6.35±0.09B,x | 7.04±0.14aC | 6.67±0.05bC,X |
| HL 1018K6 | 4.89±0.06A | 6.03±0.12B,y | 7.43±0.13C | 6.17±0.15B,Y | |
| Total Coliforms | HL CTR | 3.53±0.13A | 3.80±0.00A,x | 4.49±0.10B,X | 3.81±0.08A,X |
| HL 1018K6 | 3.53±0.13a | 3.19±0.23y | 3.11±0.09b,Y | 3.22±0.14Y | |
| Enterobacteriaceae | HL CTR | 3.55±0.15A | 3.61±0.19aA | 4.27±0.13ABb,X | 2.65±0.03B |
| HL 1018K6 | 3.55±0.15aA | 3.61±0.19aA | 3.12±0.09bA,Y | 2.46±0.09B | |
| Pseudomonas spp. | HL CTR | 3.72±0.06A | 5.89±0.03B,X | 6.54±0.05C,X | 5.33±0.14D |
| HL 1018K6 | 3.72±0.06A | 4.19±0.23A,Y | 5.07±0.12Ba,Y | 5.57±0.16Bb | |
| E. coli | HL CTR | ni A | 2.85±0.04B,X | 3.79±0.13C,X | 3.32±0.06D,X |
| HL 1018K6 | ni A | 2.22±0.09B,Y | 2.45±0.08ab,Y | 2.20±0.08bB,Y | |
| Enterococcus faecalis | HL CTR | 3.46±0.08A | 3.33±0.07Aa,x | 4.19±0.23B,X | 3.89±0.24b,X |
| HL 1018K6 | 3.46±0.08A | 2.52±0.28BC,y | 2.06±0.10B,Y | 2.73±0.04C,Y | |
| Lactic Acid Bacteria 30°C | HL CTR | 3.48±0.08A | 3.98±0.06B,x | 4.76±0.10C,X | 4.98±0.04C |
| HL 1018K6 | 3.48±0.08A | 3.81±0.03B,y | 3.94±0.02C,Y | 4.94±0.08D | |
| Staphylococcus coagulase positive | HL CTR | 4.82±0.05A | 4.98±0.28 | 5.37±0.13B,X | 4.63±0.11A,X |
| HL 1018K6 | 4.82±0.05AA | 4.36±0.29 | 4.21±0.14B,Y | 4.04±0.11B,Y |
*ni: not isolated. In each sampling day, three samples by experimental group were analyzed. Statistical analysis was performed comparing experimental groups at each sampling time and within each experimental group along the ripening period. All data were presented as the mean (M) ± standard error (sE). Different superscript uppercase letters indicate a significant difference at p<0.01. Different superscript lowercase letters indicate a significant difference at p<0.05. a–dMean values in the same row (same group in different days) with different letters presented significant differences. x–yMean values in the same column (different groups on the same sampling time) with different letters presented significant differences.
The results on the active packaging also highlighted the surprising ability of the bound peptide to reduce the growth of bacteria belonging to Staphylococcus species. Indeed, the antimicrobial coating appears to successfully act against the survival and replicative capacity of these microorganisms, showing a significant difference (p<0.01) between the HL CTR and HL 1018K6 groups on the 5th and 7th days. This outcome is supported by the good results obtained during the studies in vitro with the free antimicrobial peptide 1018-K6 against the Gram-positive bacterium Staphylococcus aureus (Colagiorgi et al., 2020). Specifically, the authors demonstrated that 20 μM of AMP are enough to kill S. aureus at a concentration of approximately 105 CFU/mL.
Sensory evaluation
In Figure 1 are reported the sensory evaluations on fish burgers packed with two different types of films. As can be seen from the graphs and Figure 2, the coating slightly interferes with the appearance of the samples. In particular, for seven days of storage the colour values were acceptable in all samples with no significant differences between the two experimental groups. As regards the odour, only the HL CTR group achieved an unacceptable mean score (less than five) on day 7, suggesting a potential role for active packaging in slowing down the production of off-odours. It is worth noting that the overall quality score of CTR samples was characterized by a gradual decrease, more pronounced after the first 5 days of storage. Specifically, the sample without active films exceeded the limit (score=5) after one week. The off-odour affected the overall acceptability of the HL CTR samples which are judged by the panel members as unpleasant foods. Consumer acceptability depends on the appearance of marine foods, influenced by organoleptic characteristics such as colour, odour and overall appearance. Some authors (Wang et al., 2007) have attributed great weight to the odour, considering it the most critical sensory characteristic for fish products.
Figure 1.
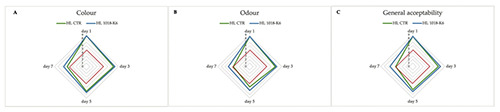
Changes in colour (A), odour (B), and overall appearance (C) of fish burgers during storage at refrigerator.
Figure 2.
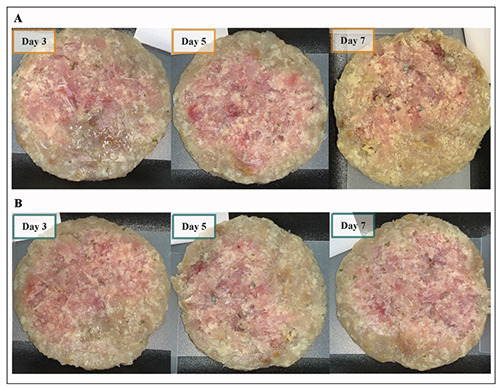
Fish burgers coated with non-functionalized PP (A) or 1018-K6 -PP disks (B) at each sampling time.
Conclusions
The microbiological contamination plays an important role in the food spoilage and foodborne diseases, causing public health and economic damage. To the best of the authors knowledge, one of the most common strategies for extending the shelflife of perishable foods or/and reducing the risk of foodborne disease is the use of packaging that incorporates natural antimicrobial compounds. To this purpose, this work represents one of the first studies to introduce the potential application of AMP-PPs as antimicrobial packaging in the agri-food industry. The offering of this novel strategy could arouse the interest of Food Business Operators, who could meet the consumer’s requests by using natural antimicrobial compounds and, at same way, take part in the research of antibacterial molecules capable to escape the common mechanisms of bacterial resistance. This study validated the antibacterial and anti-adhesion properties of 1018K6-PP, and its ability to control the alteration processes in food matrices, probably by reducing the formations of microbiological metabolites that affect the odour and the general appearance of fish burgers. Based on these results, natural antimicrobial systems demonstrate to have a great potential for extending shelf-life, being able to control and reduce the microbial replication. In conclusion, it is possible to hypothesize that antimicrobial packaging can open new horizon in the field of preservation methods for perishable foods, suppling a valid and natural solution.
Funding Statement
Funding: This research was supported by the “Packaging innovativi a base di pEptidi antimicRobici per la SIcurezza Alimentare” (PERSIA) project, grant number POR FESR CAMPANIA 2014/2020 n. B63D18000530007; the “Attività battericida ed anti-biofilm di nano-sistemi ibridi coniugati con peptidi antimicrobici: una nuova strategia per la formulazione di bio-sanitizzanti contro ceppi patogeni resistenti”-Ricerca Corrente 2017 project, grant number IZS ME 06/18 RC.
References
- Albertos I, Martin-Diana AB, Burón M, Rico D, 2019. Development of functional bio-based seaweed (Himanthalia elongata and Palmaria palmata) edible films for extending the shelflife of fresh fish burgers. Food Packag Shelf Life 22:100382. [Google Scholar]
- Ambrosio RL, Gogliettino M, Agrillo B, Proroga YTR, Balestrieri M, Gratino L, Cristiano D, Palmieri G, Anastasio A, 2022. An Active Peptide-Based Packaging System to Improve the Freshness and Safety of Fish Products: A Case Study. Foods 11:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio RL, Gratino L, Mirino S, Cocca E, Pollio A, Anastasio A, Palmieri G, Balestrieri M, Genovese A, Gogliettino M, 2020. The bactericidal activity of protein extracts from Loranthus europaeus berries: a natural resource of bioactive compounds. Antibiotics 9:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angiolillo L, Conte A, Del Nobile MA, 2018. A new method to bio-preserve sea bass fillets. Int J Food Microbiol 271:60-6. [DOI] [PubMed] [Google Scholar]
- Chotphruethipong L, Benjakul S, 2019. Use of cashew (Anacardium occidentale L.) leaf extract for prevention of lipidoxidation in Mayonnaise enriched with fish oil. Turkish J Fish Aquat Sci 19:825-36. [Google Scholar]
- Colagiorgi A, Festa R, Di Ciccio PA, Gogliettino M, Balestrieri M, Palmieri G, Anastasio A, Ianieri A, 2020. Rapid biofilm eradication of the antimicrobial peptide 1018-K6 against Staphylococcus aureus: A new potential tool to fight bacterial biofilms. Food Control 107:106815. [Google Scholar]
- De Smet K, Contreras R, 2005. Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol Lett 27:1337-47. [DOI] [PubMed] [Google Scholar]
- Ehsani A, Hashemi M, Afshari A, Aminzare M, Raeisi M, Zeinali T, 2020. Effect of different types of active biodegradable films containing lactoperoxidase system or sage essential oil on the shelf life of fish burger during refrigerated storage. LWT–Food Sci Technol 117:108633. [Google Scholar]
- EUMOFA, 2020. The EU Fish Market. Luxembourg: EUMOFA (European Market Observatory for Fisheries and Aquaculture Products). [Google Scholar]
- FAO, 2016. The state of world fisheries and aquaculture. Contributing to food security and nutrition for all. Food and Agriculture Organization of the United Nations. [Google Scholar]
- Festa R, Ambrosio RL, Lamas A, Gratino L, Palmieri G, Franco CM, Cepeda A, Anastasio A, 2021. A Study on the Antimicrobial and Antibiofilm Peptide 1018-K6 as Potential Alternative to Antibiotics against Food- Pathogen Salmonella enterica. Foods 10:1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geeroms N, Verbeke W, Van Kenhove P, 2008. Consumers’ health-related motive orientations and ready meal consumption behaviour. Appetite 51:704-12. [DOI] [PubMed] [Google Scholar]
- Ghaly A, Dave D, Budge S, Brooks M, 2010. Fish spoilage mechanisms and preservation techniques: Review. Am J Appl Sci 7:859-77. [Google Scholar]
- Gogliettino M, Balestrieri M, Ambrosio RL, Anastasio A, Smaldone G, Proroga YTR, Moretta R, Rea I, De Stefano L, Agrillo B, Palmieri G, 2020. Extending the Shelf-Life of Meat and Dairy Products via PET-Modified Packaging Activated With the Antimicrobial Peptide MTP1. Front Microbiol 10:2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gökoglu N, Yerlikaya P, 2015. Seafood chilling, refrigeration and freezing: Science and technology (1st ed.). Chichester, UK: John Wiley and Sons, Ltd. [Google Scholar]
- Gonzalez-Rodriguez MN, Sanz JJ, Santos JA, Otero A, Garcia-Lopez ML, 2001. Bacteriological quality of aquacultured freshwater fish portions in prepackaged trays stored at 3 C. J Food Prot 64:1399-404. [DOI] [PubMed] [Google Scholar]
- Guaní-Guerra E, Santos-Mendoza T, Lugo-Reyes SO, Terán LM, 2010. Antimicrobial peptides: general overview and clinical implications in human health and disease. Clin Immunol 135:1-11. [DOI] [PubMed] [Google Scholar]
- Hoel S, Jakobsen AN, Vadstein O, 2017. Effects of storage temperature on bacterial growth rates and community structure in fresh retail sushi. J Appl Microbiol 123:698-709. [DOI] [PubMed] [Google Scholar]
- ICMSF. (1978). International Commission of Microbiological Specifications for Foods. Microorganisms in Foods. 2. Sampling for Microbial Analysis. Principles and Specific Applications. [Google Scholar]
- Khaneghah AM, Hashemi SMB, Limbo S, 2018. Antimicrobial agents and packaging systems in antimicrobial active food packaging: an overview of approaches and inter- actions. Food Bioprod Process 111:1-19. [Google Scholar]
- Marrone R, Smaldone G, Ambrosio RL, Festa R, Ceruso M, Chianese A, Anastasio A, 2021. Effect of beetroot (Beta vulgaris) extract on Black Angus burgers shelf life. Ital J Food Saf 10:9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina CM, Bono G, Arena R, Randazzo M, Morghese M, Manuguerra S, La Barbera L, Ozogul F, Sadok S, Santulli A, 2019. The combined impact of cold smoking and natural antioxidants on quality and shelf life of dolphinfish (Coryphaena hippurus) fillets. Nutr Food Sci 7:1239-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmieri G, Balestrieri M, Capuano F, Proroga YT, Pomilio F, Centorame P, Riccio A, Marrone R, Anastasio A, 2018. Bactericidal and antibiofilm activity of bactenecin-derivative peptides against the food-pathogen Listeria monocytogenes: New perspectives for food processing industry. Int J Food Microbiol 279: 33-42. [DOI] [PubMed] [Google Scholar]
- Panza O, Lacivita V, Palermo C, Conte A, Del Nobile MA, 2020. Food By- Products to Extend Shelf Life: The Case of Cod Sticks Breaded with Dried Olive Paste. Foods 9:1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy KV, Yedery RD, Aranha C, 2004. Antimicrobial peptides: premises and promises. Int J Antimicrob Agents 24:536-47. [DOI] [PubMed] [Google Scholar]
- Rico D, Albertos I, Martinez-Alvarez O, Lopez-Caballero ME, Martin-Diana AB, 2020. Use of Sea Fennel as a Natural Ingredient of Edible Films for Extending the Shelf Life of Fresh Fish Burgers. Molecules 25: 5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roohinejad S, Koubaa M, Barba FJ, Saljoughian S, Amid M, Greiner R, 2017. Application of seaweeds to develop new food products with enhanced shelf-life, quality and health-related beneficial properties. Int Food Res 99:1066–83. [DOI] [PubMed] [Google Scholar]
- Thilsted SH, Thorne-Lyman A, Webb P, Bogard JR, Subasinghe R, Phillips MJ, Allison EH, 2016. Sustaining healthy diets: The role of capture fisheries and aquaculture for improving nutrition in the post-2015 era. Food Policy 61:126–31. [Google Scholar]
- Wang T, Sveinsdóttir K, Magnússon H, Martinsdóttir E, 2007. Combined Application of Modified Atmosphere Packaging and Superchilled Storage to Extend the Shelf Life of Fresh Cod (Gadus morhua) Loins. J Food Sci 73:S11–9. [DOI] [PubMed] [Google Scholar]
- Zhou JW, Luo HZ, Jiang H, Jian TK, Chen ZQ, Jia AQ, 2018. Hordenine: a novel quorum sensing inhibitor and antibiofilm agent against Pseudomonas aeruginosa. J Agr Food Chem 66:1620-8. [DOI] [PubMed] [Google Scholar]


