Abstract
Background:
Insulin-like growth factor 1 (IGF-1) signaling has been implicated in Alzheimer’s disease pathogenesis, and further evidence suggests inflammation can be a moderator of this association. However, most research to date has been conducted on older adults.
Objective:
To investigate the association of serum IGF-1 and IGF binding protein 3 (IGFBP-3) concentrations with MRI markers of Alzheimer’s Disease in predominantly middle-aged adults, and further assess moderation by chronic inflammation.
Methods:
We included participants from the Framingham Heart Study (n=1852, mean age 46±8, 46% men) and the Study of Health in Pomerania (n=674, mean age 50±13, 42% men) with available serum IGF-1, IFGBP-3, as well as brain MRI. IGF-1 and IFGBP-3 were related to MRI outcomes (i.e., total brain, cortical gray matter, white matter, white matter hyperintensities (WMH), and hippocampal volumes) using multivariable regression models adjusting for potential confounders. Subgroup analyses by C-reactive protein (CRP) concentrations were also performed. Cohort-specific summary statistics were meta-analyzed using random-effects models and corrected for multiple comparisons.
Results:
Meta-analysis results revealed that higher IGF-1 concentrations were associated with lower WMH (estimate [β] [95% CI], −0.05 [−0.09, −0.02], p=0.006) and larger hippocampal volumes (0.07 [0.02, 0.12], p=0.01), independent of vascular risk factors. These associations occurred predominantly in individuals with CRP concentrations <75th percentile. We did not observe associations between IGFBP-3 and MRI outcomes.
Conclusion:
Our findings suggest that IGF-1-related signaling may be implicated in brain health as early as midlife.
INTRODUCTION
Impaired insulin-like growth factor (IGF-1) signaling has been proposed as a contributing factor to Alzheimer’s disease (AD) [1–4]. IGF-1 promotes cell survival and stimulates neurogenesis [5]. Furthermore, IGF-1 prevents abnormal oligomerization and aggregation of Aβ protein [1,6]. IGF-1 also regulates phosphorylation of tau [1]. Therefore, concentrations of bioavailable IGF-1, regulated by IGF binding proteins (IGFBP) [7], could be an important factor in the pathogenesis of AD. Prior studies [8–13] have related lower blood IGF-1 concentrations to an increased risk of cognitive impairment and brain atrophy. Other investigations suggest that once the disease has progressed, AD patients present with higher IGF-1 concentrations compared to non-demented controls [14,15] as the brains of individuals affected by AD may be resistant to IGF-1, particularly in the hippocampal region [16].
Prior studies relating blood IGF-1 concentrations to MRI have focused on older adults. However, studies investigating the relationship between IGF-1 concentrations and brain MRI measures in younger adults are lacking. Studies also suggest that inflammation disrupts IGF-1 signaling, leading to neurodegeneration [17], but associations between IGF-1 or IGFBP-3, and MRI have not been investigated in the context of inflammation. Elevated C-reactive protein (CRP), a marker of systemic inflammation, has been independently associated with neurodegeneration [18,19]. Therefore, our aim was to investigate the association of serum concentrations of IGF-1 and its binding protein, IGFBP-3, with MRI markers of early brain aging and Alzheimer’s Disease, and additionally to assess these associations by serum CRP concentrations in predominantly middle-aged adults from the Framingham Heart Study (FHS) and the Study of Health in Pomerania (SHIP)-TREND.
METHODS
Study Design and Participants
The FHS is a community-based, longitudinal cohort study that began in 1948 in Framingham, Massachusetts [20]. Since its inception, three generations of participants have been enrolled [21,22], and the present study focuses on the Third-Generation cohort. This cohort began in 2002 with the recruitment of 4095 children of the offspring and grandchildren of the original cohort. Participants have been evaluated at two examination cycles and a third examination is currently underway, where detailed information on cardiovascular risk factors, blood pressure, anthropometric measurements, and prevalent cardiovascular disease are being collected. Our study sample includes dementia-free participants who provided blood samples at exam 1 (2002–2005) and who underwent brain MRI close to exam 2 (2008–2011).
The SHIP-TREND cohort is a community-based, longitudinal cohort study comprised of participants aged 20 to 79 years who were randomly selected from population registries in Northeast Germany [23]. The baseline examinations (SHIP-TREND-0) were performed from September 2008 until September 2012 (n=4420). The study has collected extensive information on risk factors and health measures through medical examinations and self-administered questionnaires. As with the FHS sample, SHIP-TREND participants that were included were dementia-free, provided blood samples, and underwent brain MRI close to the time of blood sampling.
Both cohorts excluded participants who experienced stroke events, had large cerebral brain infarcts, or had other neurological disorders that could substantially influence the measurement of brain volumes. The final sample included 1,852 FHS and 674 SHIP-TREND participants with both brain MRI and IGF-related measurements available. Detailed insights on the sample selection within both cohorts can be found in the flowchart (Supplemental Figure 1).
All participants in the FHS provided written informed consent, and the study was approved by the Boston University Medical Center Institutional Review Board. SHIP-TREND was approved by the University of Greifswald ethics committee and meets the recommendations of the Declaration of Helsinki, and all participants gave written informed consent before taking part in the study.
Definition of independent variables and covariates
In FHS, a 3 mL blood sample was collected from each participant in the morning after overnight fasting. Samples were centrifuged, aliquoted, and stored at −80°C. Standard ELISA was used to measure both serum IGF-1 (R&D Systems Quantikine Human IGF-I Cat#DG100, SG100 and PDG100) and serum IGFBP-3 (R&D Systems Quantikine Human IGFBP-3 Cat#DGB300). The intra-assay coefficients of variation were 5.3% for the IGF-1 assay and 9.1% for the IGFBP-3 assay [24]. Diabetes mellitus was defined as fasting blood glucose of ≥126 mg/dL or use of an antidiabetic therapy. Smoking was defined as current smoker in the year preceding the FHS examination. Our definition of cardiovascular disease included TIA, coronary heart disease, congestive heart failure, and peripheral vascular disease. CRP was measured using a nephelometer (BN100; Dade Behring, Deerfield, IL).
In SHIP-TREND, a non-fasting blood sample was collected from each participant between 07:00 AM and 04:00 PM, and serum aliquots were prepared for immediate analysis and for storage at −80 °C in the Integrated Research Biobank (Liconic, Liechtenstein). Chemiluminescent immunometric assays were used to measure serum IGF-1 and IGFBP-3 (Immulite 2500, Siemens Healthcare Medical Diagnostics) and the inter-assay coefficients of variation were 3.5% or 8.3% at high level, 4.3% or 8.8% at median level, and 6.3% or 10% at low level in the IGF-1 or IGFBP-3 assay, respectively [25]. Diabetes mellitus was defined as Glycated hemoglobin HBA1c ≥6.1 % or treatment for diabetes. Smoking was defined as current or former smokers. The definition of cardiovascular disease included myocardial infarction and stroke. CRP was measured using a nephelometer (Behring Nephelometer II (Dade Behring)) [26].
CRP concentrations were dichotomized by the 75th percentile (FHS=2.91 mg/L, SHIP-TREND=2.33 mg/L) into top quartile vs. bottom three quartile concentrations in each cohort, where the top quartile may be indicative of low-grade inflammation.
In additional sensitivity analyses, we excluded individuals with CRP levels indicative of acute inflammation or infection (CRP > 10 mg/L) from this top quartile [27]. The number of participants with CRP > 10 mg/L in FHS was N=22 and in SHIP-TREND was N=18.
MRI outcomes
Brain MRI for FHS participants was acquired using a 1.5T Siemens Avanto scanner (version syngo MR B15). We used 3D T1-weighted coronal spoiled gradient-recalled echo images and fluid attenuated inversion recovery (FLAIR) sequences. The segmentation and quantification of white matter hyperintensities (WMH) was performed on a combination of FLAIR and 3D T1 images using a modified Bayesian probability structure based on a previously published method of histogram fitting [28]. The segmentation of gray and white matter volumes was based on an Expectation-Maximization algorithm that iteratively refines its segmentation estimates to produce outputs that are most consistent with the input intensities from the native-space T1 images along with a model of image smoothness. The segmentation was refined using a Markov Random Field model and an adaptive priors model [29]. Hippocampal volume was computed using a segmentation method that employs a standard atlas based diffeomorphic approach [30], with the minor modification of label refinement. We further modified this methodology to include the EADC-ADNI harmonized hippocampal masks as previously described [31]. Total intracranial volume was derived from 3D T1 after removal of non-brain tissues. The skull was removed using an atlas-based method [32] followed by human quality control to provide generally minor cleanup if needed.
Brain MRI in SHIP-TREND participants was acquired using a 1.5 T Siemens MRI scanner (Magnetom Avanto, Siemens Medical Systems). Axial MPRAGE and axial FLAIR sequences were used to derive WMH volumes [33] via a method based upon support vector machine [34]. The other brain phenotypes, including total intracranial volume, total brain volume, gray and white matter, and hippocampal volumes, were generated using FreeSurfer version 5.3.
Statistical Analysis
IGF-1 and WMH were natural-log transformed to normalize their skewed distributions. MRI volumetric measurements of total brain, cortical gray matter, white matter, WMH, and hippocampal volumes were separately regressed onto total intracranial volumes to account for differences in head size, and the residuals from each of these models were then standardized (mean=0, standard deviation=1) and used as the outcome measures. IGF-1 and IGFBP-3 concentrations were also standardized separately in each cohort (mean=0, standard deviation=1) to facilitate comparisons.
In each cohort, primary analyses consisted of assessing the association of IGF-1 or IGFBP-3 with each MRI outcome using multivariable linear regressions. Models were adjusted for age, age-squared, sex, time between blood draw and MRI, waist-to-hip ratio, systolic blood pressure, hypertension treatment, diabetes mellitus, current smoking, and cardiovascular disease.
In secondary analyses, separately within each cohort, two sample t-tests were used to compare mean IGF-1 and IGFBP-3 concentrations by CRP subgroups (bottom three quartiles vs. top quartile). Each cohort also conducted stratified regression analyses by CRP subgroup adjusting for the same set of confounders included in the primary analysis. Finally, both the primary and CRP-based subgroup analyses were meta-analyzed using random effects models. Heterogeneity of effect sizes was assessed using Cochrane’s Q tests and I2 statistics. In addition to the stratified regression analyses, we included meta-analyses of the interaction of IGF-1 or IGFBP-3 and dichotomized CRP levels on the MRI outcomes.
Linear regressions for the stratified sensitivity analyses (excluding individuals with CRP > 10 mg/L) were conducted using the same models as the stratified secondary analysis and followed by an additional meta-analysis of the interaction by CRP levels.
Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC) and R version 3.4.2. Significance was set at a 5% threshold for main effects and CRP subgroup analyses. Finally, meta-analysis results were corrected for multiple comparisons using False Discovery Rate (FDR) methods (reported as corrected p-values, pc); we did not correct for multiple comparisons in the cohort-specific results as our main findings are derived from meta-analyses.
RESULTS
Baseline characteristics are reported in Table 1 and the age distributions of both cohorts can be found in Supplemental Figure 2. The participants in both cohorts were predominantly middle-aged adults (FHS: age 46.02±8.48, 46.17% men; SHIP: age 49.89±13.43, 42.43% men) with a low burden of cardiovascular disease. Compared to FHS, the SHIP-TREND cohort had higher systolic blood pressure, as well as a higher proportion of participants under antihypertensive medication and current smokers.
Table 1.
Characteristics of study cohorts
| FHS Gen 3 (n=1852) | SHIP-TREND (n=674) | p-value* | |
|---|---|---|---|
|
| |||
| Men, n (%) | 855 (46.17) | 286 (42.43) | 0.105 |
| Age at blood draw, mean (SD) | 46.02 (8.48) | 49.89 (13.43) | <0.001 |
| Blood draw to MRI interval (days), mean (SD) | 2770.76 (327.55) | 28.69 (64.84) | <0.001 |
| Systolic blood pressure, mean (SD) | 116 (14) | 124 (17) | <0.001 |
| Antihypertensive medication, n (%) | 327 (17.69) | 186 (27.60) | <0.001 |
| Diabetes mellitus, n (%) | 82 (4.43) | 32 (4.75) | 0.815 |
| Current smoking, n (%) | 164 (8.86) | 136 (20.18) | <0.001 |
| Prevalent cardiovascular disease, n (%) | 33 (1.78) | 5 (0.74) | 0.086 |
| Waist/hip ratio, mean (SD) | 0.91 (0.08) | 0.87 (0.09) | <0.001 |
| CRP mg/L, median (Q1, Q3)) | 1.26 (0.59, 2.91) | 1.13 (0.60, 2.33) | 0.013 |
| IGF-1 ng/mL, median (Q1, Q3) | 125.57 (101.48, 153.15) | 140.2 (110.3, 170.7) | <0.001 |
| IGFBP-3 ng/mL, mean (SD) | 3038.93 (1075.67) | 4282 (1000.85) | <0.001 |
| MRI volumes, cm3 | |||
| Total Intracranial, mean (SD) | 1261.34 (125.09) | 1574.33 (154.80) | <0.001 |
| Total Brain, mean (SD) | 997.40 (102.06) | 1115.47 (113.12) | <0.001 |
| Cortical GM, mean (SD) | 479.96 (46.29) | 447.42 (49.27) | <0.001 |
| WM, mean (SD) | 472.31 (58.26) | 476.80 (59.29) | 0.088 |
| WMH, median (Q1,Q3) | 0.40 (0.21, 0.82) | 0.16 (0.06, 0.40) | <0.001 |
| Hippocampal, mean (SD) | 6.85 (0.72) | 4.00 (0.43) | <0.001 |
p-values are based on t-tests for continuous and chi-square-tests categorical variables. For CRP, IGF-1, and WMH the t-tests are based on the log-transform of these variables.
Abbreviations: CRP: C-reactive protein, IGF-1: Insulin-like growth factor 1, IGFBP-3: Insulin-like growth factor binding protein 3, GM: gray matter, Q: quartile; SD: standard deviation; WM: white matter, WMH: white matter hyperintensity
Cohort-specific and meta-analysis results adjusted for vascular risk factors are presented in Figure 1 for the main effects analyses. Meta-analysis results combining both cohorts showed that every SDU increase in IGF-1 concentrations was associated with reduced WMH burden (estimate [β] [95% CI], −0.06 [−0.09; −0.02], p=0.006). This association remained significant after correction for multiple testing (pc=0.048). We also observed a protective association between IGF-1 and hippocampal volumes in meta-analysis combining both cohorts—every SDU increase in IGF-1 concentrations was associated with larger hippocampal volumes (0.07 [0.02; 0.12], p=0.01], which persisted after correction for multiple testing (pc=0.048). Individually, these protective associations for WMH and hippocampal volume were nominally significant in FHS and had the same direction of effect in SHIP-TREND, although results did not reach nominal significance in this cohort alone.
Figure 1. Associations between IGF-1 or IGFBP-3 with MRI outcomes.
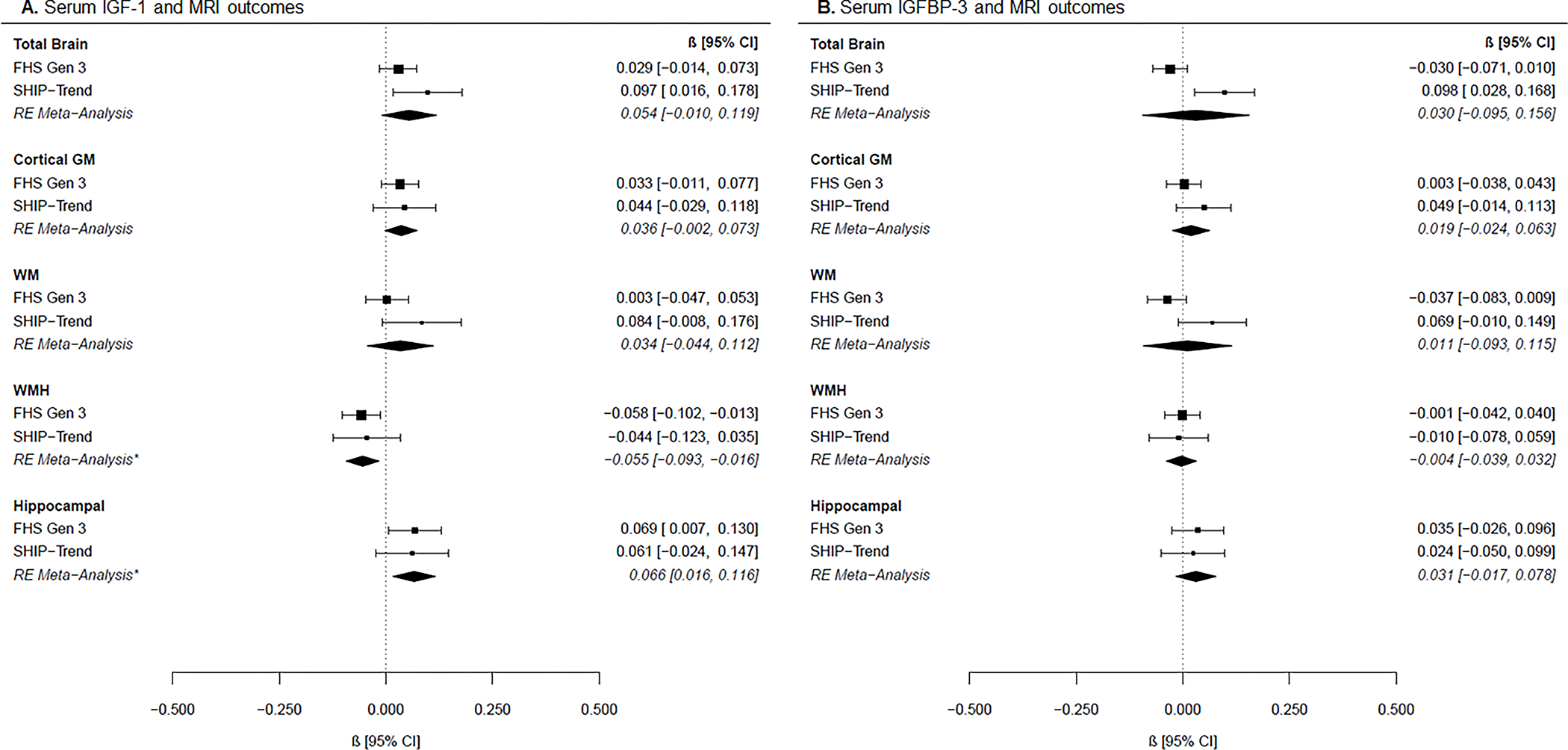
Forest plots of cohort-specific and meta-analysis results for the associations between (A) IGF-1 and MRI measures, and (B) IGFBP-3 and MRI measures. Beta estimates and 95% confidence intervals (CI) are presented graphically in the center, where the square represents beta coefficients and the error bars the 95% confidence intervals per cohort according to the text on the right column. Meta-analysis results derived from random-effects (RE) models are presented with diamonds and italicized text. Models are adjusted for age, age-squared, sex, time between blood draw and MRI, waist-to-hip ratio, systolic blood pressure, hypertension treatment, diabetes mellitus, current smoking, and prevalent cardiovascular disease. *p<0.05 after FDR correction.
Abbreviations: FHS: Framingham Heart Study Generation 3, SHIP: Study of Health in Pomerania Trend, RE Meta-Analysis: random effects meta-analysis, IGF-1: insulin-like growth factor 1, IGFBP-3: insulin-like growth factor binding protein-3, GM: gray matter, WM: white matter, WMH: white matter hyperintensity.
Table 2 shows heterogeneity measures. Associations between IGFBP-3 and MRI outcome measures were heterogeneous between cohorts, particularly for total brain volume and white matter volume. We did not observe significant associations for IGFBP-3 in the meta-analysis.
Table 2.
Heterogeneity measures for the associations between IGF-1 or IGFBP-3 and MRI outcomes
| Outcome | IGF-1 Heterogeneity | IGFBP-3 Heterogeneity | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Q | Q p-value | I^2 | Q | Q p-value | I^2 | |
|
| ||||||
| Total brain | 2.09 | 0.15 | 52.13 | 9.65 | <0.01 | 89.63 |
| CRP (Q1–3) | 2.08 | 0.15 | 51.91 | 6.58 | 0.01 | 84.80 |
| CRP (Q4) | 0.11 | 0.74 | 0.00 | 2.48 | 0.12 | 59.66 |
| Cortical GM | 0.07 | 0.79 | 0.00 | 1.46 | 0.23 | 31.48 |
| CRP (Q1–3) | 0.18 | 0.68 | 0.00 | 1.15 | 0.28 | 13.21 |
| CRP (Q4) | 0.14 | 0.70 | 0.00 | 0.09 | 0.77 | 0.00 |
| WM | 2.34 | 0.13 | 57.17 | 5.15 | 0.02 | 80.58 |
| CRP (Q1–3) | 1.35 | 0.25 | 25.82 | 3.15 | 0.08 | 68.29 |
| CRP (Q4) | 1.02 | 0.31 | 1.99 | 2.15 | 0.14 | 53.49 |
| WMH | 0.09 | 0.77 | 0.00 | 0.04 | 0.84 | 0.00 |
| CRP (Q1–3) | 0.10 | 0.76 | 0.00 | 0.77 | 0.38 | 0.00 |
| CRP (Q4) | 0.11 | 0.74 | 0.00 | 0.36 | 0.55 | 0.00 |
| Hippocampal | 0.02 | 0.89 | 0.00 | 0.05 | 0.83 | 0.00 |
| CRP (Q1–3) | 0.34 | 0.56 | 0.00 | 0.25 | 0.62 | 0.00 |
| CRP (Q4) | 0.02 | 0.90 | 0.00 | 1.83 | 0.18 | 45.24 |
Models are adjusted for age, age-squared, sex, time between blood draw and MRI, waist-to-hip ratio, systolic blood pressure, hypertension treatment, diabetes mellitus, current smoking, and prevalent cardiovascular disease. Fields in bold font show associations with high heterogeneity.
Abbreviations: IGF-1: insulin-like growth factor 1, IGFBP-3: insulin-like growth factor binding protein-3, Q: quartile; WMH: white matter hyperintensity.
In both FHS and SHIP-TREND, there were significant group differences between mean IGF-1 concentrations by CRP subgroups (p <0.001), but there were no group differences for IGFBP-3 concentrations (FHS p=0.933, SHIP-TREND p=0.073). Stratified association results by CRP subgroups are shown in Figure 2. Although we observed nominal associations for higher IGF-1 concentrations with lower WMH volumes (−0.05 [−0.09, −0.001], p=0.044) and larger hippocampal volumes (0.09 [0.03, 0.15], p=0.003) in participants with CRP concentrations <75th percentile, these results did not survive correction for multiple testing (pc=0.07 and pc=0.24, respectively). Another association at the nominal level was observed between higher IGF-1 concentrations and larger total brain volumes in participants with CRP concentrations ≥75th percentile (0.08 [0.01, 0.16], p=0.03, pc=0.24). For the interaction analyses, we did not reveal any results that passed significance after correction for multiple testing. However, there was one nominal significant interaction of IGFBP-3 and CRP levels on cortical GM (−0.09 [−0.17, −0.01], p=0.02, pc=0.20) (Figure 3).
Figure 2. Associations between IGF-1 or IGFBP-3 with MRI outcomes by CRP subgroups.
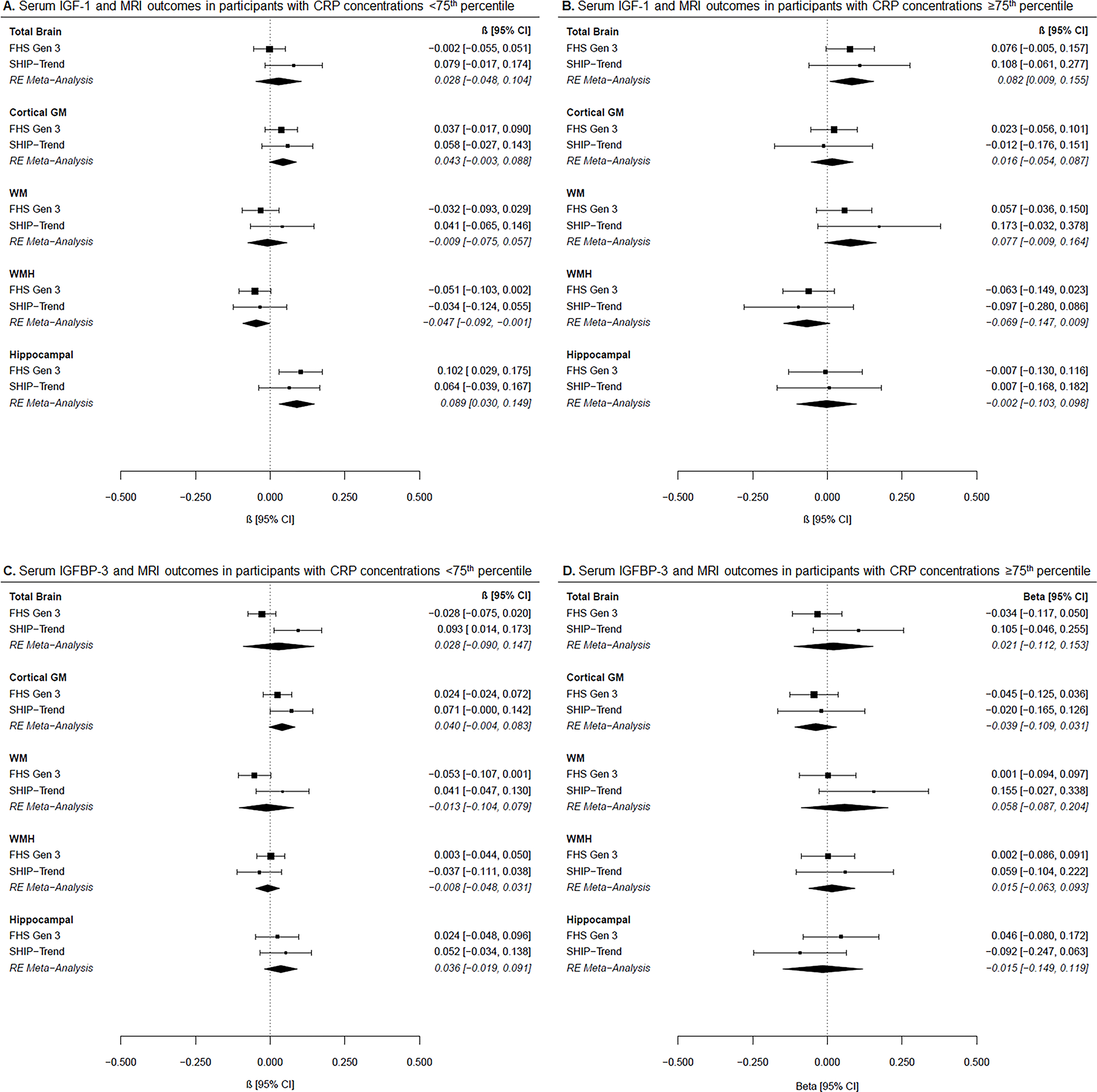
Forest plots of cohort-specific and meta-analysis results for the associations between (A) IGF-1 and MRI measures in participants with CRP concentrations <75th percentile, (B) IGF-1 and MRI measures in participants with CRP concentrations ≥75th percentile, (C) IGFBP-3 and MRI measures in participants with CRP concentrations <75th percentile, and (D) IGFBP-3 and MRI measures in participants with CRP concentrations ≥75th percentile. Beta estimates and 95% confidence intervals (CI) are presented graphically in the center, where the square represents beta coefficients and the error bars the 95% confidence intervals per cohort according to the text on the right column. Meta-analysis results derived from random-effects (RE) models are presented with diamonds and italicized text. Models are adjusted for age, age-squared, sex, time between blood draw and MRI, waist-to-hip ratio, systolic blood pressure, hypertension treatment, diabetes mellitus, current smoking, and prevalent cardiovascular disease. *p<0.05 after FDR correction.
Meta-analysis results are derived from random-effects models and presented in italic. </P>Abbreviations: FHS Gen 3: Framingham Heart Study Generation 3, SHIP-TREND: Study of Health in Pomerania TREND, IGF-1: insulin-like growth factor 1, IGFBP-3: insulin-like growth factor binding protein-3, GM: gray matter, WM: white matter, WMH: white matter hyperintensity, CRP: C-reactive protein
Figure 3: Interaction of IGF-1 or IGFBP-3 and CRP on MRI outcomes.
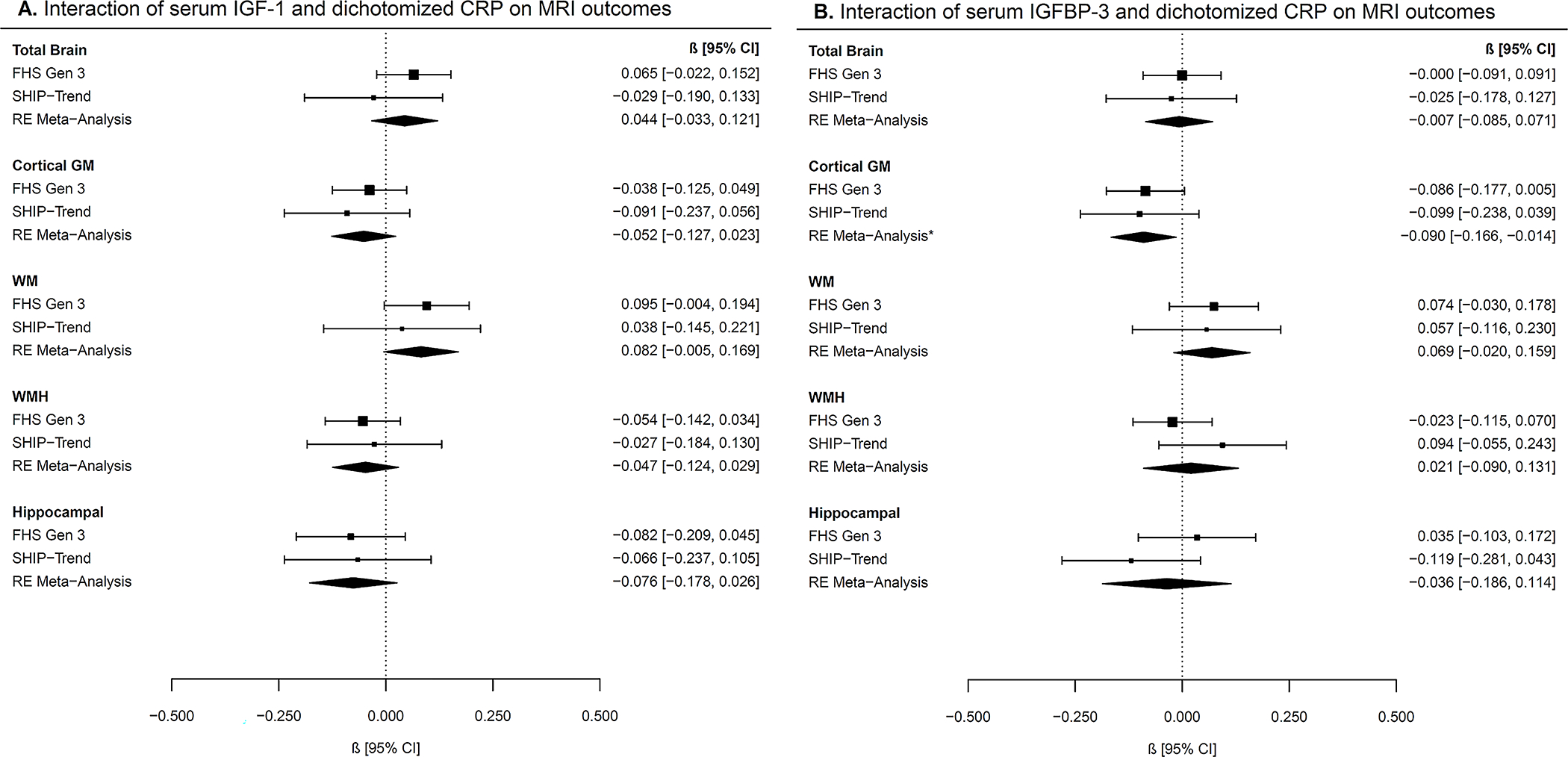
Forest plots of cohort-specific and meta-analysis results for the interactions of (A) IGF-1 and dichotomized CRP on MRI measures and (B) IGFBP-3 and dichotomized CRP on MRI measures. CRP was dichotomized according to the 75th percentile (<75th percentile, ≥75th percentile).
Beta estimates and 95% confidence intervals (CI) are presented graphically in the center, where the square represents beta coefficients and the error bars the 95% confidence intervals per cohort according to the text on the right column. Meta-analysis results derived from random-effects (RE) models are presented with diamonds.
Models are adjusted for age, age-squared, sex, time between blood draw and MRI, waist-to-hip ratio, systolic blood pressure, hypertension treatment, diabetes mellitus, current smoking, and prevalent cardiovascular disease.
Abbreviations: FHS Gen 3: Framingham Heart Study Generation 3, SHIP-TREND: Study of Health in Pomerania TREND, IGF-1: insulin-like growth factor 1, IGFBP-3: insulin-like growth factor binding protein-3, GM: gray matter, WM: white matter, WMH: white matter hyperintensity, CRP: C-reactive protein
The results of the sensitivity analyses (see Methods) by excluding individuals with CRP >10 mg/L from the ≥75th percentile were virtually unchanged (Supplementary Figure 3), for both the CRP subgroup and interaction analyses. Additional nominal significant interactions were observed for IGF-1 and CRP on WM (0.09 [0.00, 0.18], p=0.05, pc=0.25) and for the interaction of IGFBP-3 and CRP on cortical GM (−0.10 [−0.18, −0.02], p=0.02, pc=0.17) (Supplementary Figure 4).
DISCUSSION
Our findings suggest that circulating IGF-1 is associated with a lower burden of WMH and with larger hippocampal volumes, two important MRI predictors of early brain aging and AD, in predominantly middle-aged adults. Previous findings in older adults from FHS have shown that lower IGF-1 concentrations are associated with an increased risk of dementia and lower brain volumes [8]. The present work expands on those findings by showing that higher IGF-1 concentrations are associated with larger hippocampal volumes as early as the fifth decade across two population-based studies—twenty years before the usual age at which clinical dementia manifests. Additionally, we show that IGF-1 concentrations were related to reduced WMH burden, indicating not only a potential protective role in neurodegeneration, but also against cerebrovascular disease. Previous findings in adults with traumatic brain injury have shown that higher IGF-1 concentrations are associated with a greater improvement in white matter recovery over time [35]. Other studies have also reported that increases in IGF-1 concentrations are associated with decreased white matter damage after stroke in mice [36]. Together, our findings showing protective associations for IGF-1 with reduced WMH and preserved hippocampal volume supports existing reports of lower IGF-1 concentrations seen in AD patients [37–39]. IGF-1 is a neurotrophic factor that promotes cell differentiation, proliferation and myelination during brain development and maturation. In adults, IGF-1 modulates hippocampal neurogenesis and angiogenesis [40], which could explain part of the protective role to preserve the hippocampus and protect from vascular insults leading to WMH.
A recent Mendelian randomization study [41] suggested that epidemiologic studies have put undue emphasis on a relationship between circulating IGF-1 and IGFBP-3 concentrations and AD, as they found only one single nucleotide polymorphism in the FOXO3 gene, that impacts IGF-1 and IGFBP-3 concentrations, to be associated with AD risk. However, other reports [42–44] have found associations between IGF-1 related polymorphisms and dementia. These conflicting results might indicate that AD risk associated with IGF-1 and IGFBP-3 polymorphisms is dependent on other factors, such as age, environmental exposures (such as inflammation), or comorbidities.
Increasing focus is being given to the involvement of neuroinflammatory agents in the progression of AD [45]. While the relationship between systemic and CNS inflammation has yet to be established in this context, it has been shown that midlife systemic inflammation is associated with smaller current [46] and late-life brain volume on MRI [47]. Additionally, pro-inflammatory cytokines are able to cross the blood brain barrier and alter IGF-1 signaling [17]. IGF-1 can be complexed to IGFBP-3 in peripheral circulation, but unbound IGF-1 can freely cross the blood-brain barrier and modulate an effect in the CNS [48,49]. CNS and peripheral concentrations of IGF-1 and inflammatory markers often drive each other and are closely related, as shown in a rat model of sporadic AD [50]. Additionally, a positive association between concentrations of IGF-1 in the circulation and cerebrospinal fluid has been described in rats [51]. Our results of the CRP subgroup analyses showing that IGF-1 levels are related to larger hippocampal volumes predominantly in individuals with CRP concentrations <75th percentile compared to the upper percentile suggests, that CRP levels towards a low-grade systemic inflammation might alter IGF-1 signaling and its potential beneficial impact on brain health as indicated in other studies [52]. However, the lack of results in participants with CRP concentrations ≥75th percentile could simply reflect decreased power as this group is smaller. Considering that both the CRP-stratified results and the interaction test did not pass correction for multiple testing, these results should be interpreted with caution, and validation in a larger sample is needed to shed light on these findings. The lack of associations between IGFBP-3 and MRI outcomes, in contrast to IGF-1, may suggest that the regulation of IGF-1 bioavailability by IGF proteins may be less relevant for neuroimaging markers. We have recently shown that another binding protein member, IGFBP-2, although related to Alzheimer’s Disease and all-cause dementia, was not associated with MRI measures in the Framingham Offspring cohort [31]. Additional research is needed to expand on these findings.
Strengths of this study comprise the inclusion of two community-based samples with large numbers of predominantly middle-aged adults with both blood biomarker and MRI data, and the adjustment for additional common vascular risk factors to control for potential confounding. A limitation of this study is the use of CRP, which is a non-specific marker of low-grade inflammation [53] and does not provide insight into which pathologies might be underlying chronic inflammation. Additionally, FHS and SHIP-TREND participants are largely of white European ancestry; therefore, our results might not be readily applicable to ethnically different populations until tested in separate, diverse samples. As indicated in Table 1, most of the cohort characteristics showed a statistically significant difference between FHS and SHIP-TREND which is not unexpected taking the underlying populations and large sample sizes into account where already small differences pass significance. However, the analyses were performed cohort-stratified correcting for these differences leaving only residual heterogeneity. Further, the algorithms to derive MRI measures were different in both cohorts and this might have introduced some heterogeneity, notably for total brain and white matter volume. Even though our analysis used standardized MRI measures, some heterogeneity may have remained. Finally, it is important to note that whereas SHIP performed MRI close to blood draw (about a month on average), in FHS, MRI was acquired 7.6 years on average after blood draw. This could potentially be another source of heterogeneity in our analysis. To address this limitation, cohort-specific analyses were corrected for the time interval between blood draw and MRI to obtain effect estimates independently of this time gap within cohorts, and have further performed random-effects meta-analysis to account for potential heterogeneity between cohorts. However, despite this discrepancy, we observed significant results, which indicate a robust association of IGF-1 with WMH and hippocampus volume. Our association of higher IGF-1 levels with higher hippocampus volume – a known marker for dementia, could at least partially explain the association of higher IGF-1 with increased cognitive performance that was observed in other studies [54].
Thus, our study adds to the body of work investigating the role of IGF-1 in the progression of AD and its potential as a blood biomarker associated with early brain changes seen in predominantly middle-aged adults before the onset of dementia symptoms. IGF-1 has been put forth as a promising therapeutic target for AD given the evidence of its implications in the disease [50] and investigations into the link between the epidemiologic findings and possible molecular signaling mechanisms for the role of IGF-1 in AD are ongoing [55]. Animal studies have shown improvements of symptoms in AD models with administration of IGF-1. However, human trials administering IGF-1 or its homolog, insulin, to subjects with cognitive impairment have had limited success in achieving beneficial outcomes [6,56]. The continually growing evidence of a relationship between IGF-1 and Alzheimer’s disease suggests that further human trials may still be worthwhile pursuits.
Our results suggest associations between IGF-1 and brain health in predominantly middle-aged adults. In the future, designing interventions targeted at individuals at high risk of AD (i.e. based on genetic, fluid, or neuroimaging biomarkers of AD) before the full manifestation of clinical symptoms could perhaps offer the most benefit from IGF-1 or insulin-based therapeutic strategies.
In summary, our findings suggest that IGF-1 signaling pathways could be implicated in abnormal brain aging as early as midlife. Further studies relating IGF-1 to brain aging and elucidating the potential role of inflammatory pathways are needed in other populations and experimental settings.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by grants from the National Heart, Lung, and Blood Institute contract for the Framingham Heart Study (contract no. N01-HC-25195, no. HHSN268201500001I, and no. 75N92019D00031), the National Institute on Aging (R01 AG054076, R01 AG049607, R01 AG033193, U01 AG049505, U01 AG052409) and the National Institute of Neurological Disorders and Stroke (NS017950 and UH2 NS100605). The measurement of growth factors was funded by R01 HL077477 and R01 AG031287. Dr. Raman received funding from NHLBI (T32HL125232–01A1). Ms. Conner reports funding from the National Institute of General Medical Sciences (NIGMS) Interdisciplinary Training Grant for Biostatisticians (T32GM74905–14). Dr. DeCarli reports funding from P30 AG0101029. Dr. Seshadri reports funding from P30 AG066546. Dr. Satizabal was supported by a New Investigator Research Grant to promote Diversity from the Alzheimer’s Association (AARGD-16–443384).
SHIP is part of the Community Medicine Research net of the University of Greifswald, Germany, which is funded by the Federal Ministry of Education and Research (grants no. 01ZZ9603, 01ZZ0103, and 01ZZ0403), the Ministry of Cultural Affairs and the Social Ministry of the Federal State of Mecklenburg-West Pomerania. MRI scans in SHIP-TREND have been supported by a joint grant from Siemens Healthineers, Erlangen, Germany and the Federal State of Mecklenburg-West Pomerania. Dr. Habes was supported in part by NIH (grant 1RF1AG054409 and R01 HL127659–04S1) and Allen H. and Selma W. Berkman Charitable Trust (Accelerating Research on Vascular Dementia).
Footnotes
CONFLICTS OF INTEREST
HJG has received travel grants and speakers honoraria from Fresenius Medical Care, Neuraxpharm, Servier and Janssen Cilag as well as research funding from Fresenius Medical Care.
Dr. Seshadri is a consultant for Biogen
Dr. Charles DeCarli is a consultant to Novartis on a safety trial for heart failure
Other authors have nothing to report
References
- [1].Bedse G, Di Domenico F, Serviddio G, Cassano T (2015) Aberrant insulin signaling in Alzheimer’s disease: current knowledge. Front Neurosci 9, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM (2005) Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease - is this type 3 diabetes? J Alzheimer’s Dis 7, 63–80. [DOI] [PubMed] [Google Scholar]
- [3].Hoyer S, Muller D, Plaschke K (1994) Desensitization of brain insulin receptor. Effect on glucose/energy and related metabolism. J Neural Transm Suppl 44, 259–268. [DOI] [PubMed] [Google Scholar]
- [4].Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, De La Monte SM (2005) Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J Alzheimer’s Dis 8, 247–268. [DOI] [PubMed] [Google Scholar]
- [5].Lee E, Son H (2009) Adult hippocampal neurogenesis and related neurotrophic factors. BMB Rep 42, 239–244. [DOI] [PubMed] [Google Scholar]
- [6].Claxton A, Baker LD, Hanson A, Trittschuh EH, Cholerton B, Morgan A, Callaghan M, Arbuckle M, Behl C, Craft S (2015) Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s Disease dementia. J Alzheimer’s Dis 44, 897–906. [DOI] [PubMed] [Google Scholar]
- [7].Benarroch EE (2012) Insulin-like growth factors in the brain and their potential clinical implications. Neurology 79, 2148–2153. [DOI] [PubMed] [Google Scholar]
- [8].Westwood AJ, Beiser A, DeCarli C, Harris TB, Chen TC, He XM, Roubenoff R, Pikula A, Au R, Braverman LE, Wolf PA, Vasan RS, Seshadri S (2014) Insulin-like growth factor-1 and risk of Alzheimer dementia and brain atrophy. Neurology 82, 1613–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kalmijn S, Janssen JAMJL, Pols HAP, Lamberts SWJ, Breteler MMB (2000) A prospective study on circulating insulin-like growth factor I (IGF-I), IGF-binding proteins, and cognitive function in the elderly. J Clin Endocrinol Metab 85, 4551–4555. [DOI] [PubMed] [Google Scholar]
- [10].Rollero A, Murialdo G, Fonzi S, Garrone S, Gianelli MV., Gazzerro E, Barreca A, Polleri A (1998) Relationship between cognitive function, growth hormone and insulin-like growth factor I plasma levels in aged subjects. Neuropsychobiology 38, 73–79. [DOI] [PubMed] [Google Scholar]
- [11].Al-Delaimy WK, von Muhlen D, Barrett-Connor E (2009) Insulinlike growth factor-1, insulinlike growth factor binding protein-1, and cognitive function in older men and women. J Am Geriatr Soc 57, 1441–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Angelini A, Bendini C, Neviani F, Bergamini L, Manni B, Trenti T, Rovati R, Neri M (2009) Insulin-like growth factor-1 (IGF-1): relation with cognitive functioning and neuroimaging marker of brain damage in a sample of hypertensive elderly subjects. Arch Gerontol Geriatr 49 Suppl 1, 5–12. [DOI] [PubMed] [Google Scholar]
- [13].Dik MG, Pluijm SMF, Jonker C, Deeg DJH, Lomecky MZ, Lips P (2003) Insulin-like growth factor I (IGF-I) and cognitive decline in older persons. Neurobiol Aging 24, 573–581. [DOI] [PubMed] [Google Scholar]
- [14].Johansson P, Åberg D, Johansson JO, Mattsson N, Hansson O, Ahrén B, Isgaard J, Åberg ND, Blennow K, Zetterberg H, Wallin A, Svensson J (2013) Serum but not cerebrospinal fluid levels of insulin-like growth factor-I (IGF-I) and IGF-binding protein-3 (IGFBP-3) are increased in alzheimer’s disease. Psychoneuroendocrinology 38, 1729–1737. [DOI] [PubMed] [Google Scholar]
- [15].Vardy ERLC Rice PJ, Bowie PCW Holmes JD, Grant PJ Hooper NM (2007) Increased circulating insulin-like growth factor-1 in late-onset Alzheimer’s disease. J Alzheimer’s Dis 12, 285–290. [DOI] [PubMed] [Google Scholar]
- [16].Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE (2012) Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 122, 1316–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Spielman LJ, Little JP, Klegeris A (2014) Inflammation and insulin/IGF-1 resistance as the possible link between obesity and neurodegeneration. J Neuroimmunol 273, 8–21. [DOI] [PubMed] [Google Scholar]
- [18].Bettcher BM, Wilheim R, Rigby T, Green R, Miller JW, Racine CA, Yaffe K, Miller BL, Kramer JH (2012) C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain Behav Immun 26, 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C (2012) Circulating IL-6 and CRP are associated with MRI findings in the elderly: The 3C-Dijon Study. Neurology 78, 720–727. [DOI] [PubMed] [Google Scholar]
- [20].Dawber TR, Meadors GF, Moore FE (1951) Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health 41, 279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP (1975) The Framingham Offspring Study. Design and preliminary data. Prev Med (Baltim) 4, 518–525. [DOI] [PubMed] [Google Scholar]
- [22].Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D’Agostino RB, Fox CS, Larson MG, Murabito JM, O’Donnell CJ, Vasan RS, Wolf PA, Levy D (2007) The Third Generation Cohort of the National Heart, Lung, and Blood Institute’s Framingham Heart Study: Design, recruitment, and initial examination. Am J Epidemiol 165, 1328–1335. [DOI] [PubMed] [Google Scholar]
- [23].Völzke H, Alte D, Schmidt CO, Radke D, Lorbeer R, Friedrich N, Aumann N, Lau K, Piontek M, Born G, Havemann C, Ittermann T, Schipf S, Haring R, Baumeister SE, Wallaschofski H, Nauck M, Frick S, Arnold A, Jünger M, Mayerle J, Kraft M, Lerch MM, Dörr M, Reffelmann T, Empen K, Felix SB, Obst A, Koch B, Gläser S, Ewert R, Fietze I, Penzel T, Dören M, Rathmann W, Haerting J, Hannemann M, Röpcke J, Schminke U, Jürgens C, Tost F, Rettig R, Kors JA, Ungerer S, Hegenscheid K, Kühn JPJ, Kühn JPJ, Hosten N, Puls R, Henke J, Gloger O, Teumer A, Homuth G, Völker U, Schwahn C, Holtfreter B, Polzer I, Kohlmann T, Grabe HJ, Rosskopf D, Kroemer HK, Kocher T, Biffar R, John U, Hoffmann W (2011) Cohort profile: The study of health in Pomerania. Int J Epidemiol 40, 294–307. [DOI] [PubMed] [Google Scholar]
- [24].Lam CSP, Chen MH, Lacey SM, Yang Q, Sullivan LM, Xanthakis V, Safa R, Smith HM, Peng X, Sawyer DB, Vasan RS (2010) Circulating insulin-like growth factor-1 and its binding protein-3: Metabolic and genetic correlates in the community. Arterioscler Thromb Vasc Biol 30, 1479–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Knacke H, Pietzner M, Do KT, Römisch-Margl W, Kastenmüller G, Völker U, Völzke H, Krumsiek J, Artati A, Wallaschofski H, Nauck M, Suhre K, Adamski J, Friedrich N (2016) Metabolic fingerprints of circulating IGF-1 and the IGF-1/IGFBP-3 Ratio: A Multifluid Metabolomics Study. J Clin Endocrinol Metab 101, 4730–4742. [DOI] [PubMed] [Google Scholar]
- [26].Gocke C, Holtfreter B, Meisel P, Grotevendt A, Jablonowski L, Nauck M, Markus MRP, Kocher T (2014) Abdominal obesity modifies long-term associations between periodontitis and markers of systemic inflammation. Atherosclerosis 235, 351–357. [DOI] [PubMed] [Google Scholar]
- [27].Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Taubert K, Tracy RP, Vinicor F (2003) Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the centers for disease control and prevention and the American Heart Association. Circulation 107, 499–511. [DOI] [PubMed] [Google Scholar]
- [28].DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Garner J, Jack L, Carmelli D (1999) Predictors of brain morphology for the men of the NHLBI twin study. Stroke 30, 529–536. [DOI] [PubMed] [Google Scholar]
- [29].Fletcher E, Singh B, Harvey D, Carmichael O, Decarli C (2012) Adaptive image segmentation for robust measurement of longitudinal brain tissue change. Annu Int Conf IEEE Eng Med Biol Soc 2012, 5319–5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Vercauteren T, Pennec X, Perchant A, Ayache N (2007) Non-parametric diffeomorphic image registration with the demons algorithm. Med Image Comput Comput Assist Interv 10, 319–326. [DOI] [PubMed] [Google Scholar]
- [31].McGrath ER, Himali JJ, Levy D, Conner SC, DeCarli CS, Pase MP, Courchesne P, Satizabal CL, Vasan RS, Beiser AS, Seshadri S (2019) Circulating IGFBP-2: a novel biomarker for incident dementia. Ann Clin Transl Neurol 6, 1659–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Aljabar P, Heckemann RA, Hammers A, Hajnal JV, Rueckert D (2009) Multi-atlas based segmentation of brain images: Atlas selection and its effect on accuracy. Neuroimage 46, 726–738. [DOI] [PubMed] [Google Scholar]
- [33].Habes M, Erus G, Toledo JB, Zhang T, Bryan N, Launer LJ, Rosseel Y, Janowitz D, Doshi J, Van der Auwera S, von Sarnowski B, Hegenscheid K, Hosten N, Homuth G, Völzke H, Schminke U, Hoffmann W, Grabe HJ, Davatzikos C (2016) White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain 139, 1164–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Lao Z, Shen D, Liu D, Jawad AF, Melhem ER, Launer LJ, Bryan RN, Davatzikos C (2008) Computer-Assisted Segmentation of White Matter Lesions in 3D MR Images Using Support Vector Machine. Acad Radiol 15, 300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Feeney C, Sharp DJ, Hellyer PJ, Jolly AE, Cole JH, Scott G, Baxter D, Jilka S, Ross E, Ham TE, Jenkins PO, Li LM, Gorgoraptis N, Midwinter M, Goldstone AP (2017) Serum insulin-like growth factor-I levels are associated with improved white matter recovery after traumatic brain injury. Ann Neurol 82, 30–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cui X, Chopp M, Zacharek A, Cui C, Yan T, Ning R, Chen J (2016) D-4F Decreases White Matter Damage After Stroke in Mice. Stroke 47, 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Morel GR, León ML, Uriarte M, Reggiani PC, Goya RG (2017) Therapeutic potential of IGF-I on hippocampal neurogenesis and function during aging. Neurogenes 4, e1259709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ostrowski PP, Barszczyk A, Forstenpointner J, Zheng W, Feng ZP (2016) Meta-analysis of serum insulin-like growth factor 1 in Alzheimer’s disease. PLoS One 11, e0155733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hu X, Yang Y, Gong D (2016) Circulating insulin-like growth factor 1 and insulin-like growth factor binding protein-3 level in Alzheimer’s disease: a meta-analysis. Neurol Sci 37, 1671–1677. [DOI] [PubMed] [Google Scholar]
- [40].Åberg ND, Brywe KG, Isgaard J (2006) Aspects of growth hormone and insulin-like growth factor-I related to neuroprotection, regeneration, and functional plasticity in the adult brain. ScientificWorldJournal 6, 53–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Williams DM, Karlsson IK, Pedersen NL, Hägg S (2018) Circulating insulin-like growth factors and Alzheimer disease: A mendelian randomization study. Neurology 90, e291–e297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang W, Yu JT, Tan L, Liu QY, Wang HF, Ma XY (2012) Insulin-like growth factor 1 (IGF1) polymorphism is associated with Alzheimer’s disease in Han Chinese. Neurosci Lett 531, 20–23. [DOI] [PubMed] [Google Scholar]
- [43].Garcia J, Ahmadi A, Wonnacott A, Sutcliffe W, Nägga K, Söderkvist P, Marcusson J (2006) Association of insulin-like growth factor-1 receptor polymorphism in dementia. Dement Geriatr Cogn Disord 22, 439–444. [DOI] [PubMed] [Google Scholar]
- [44].Vargas T, Martinez-Garcia A, Antequera D, Vilella E, Clarimon J, Mateo I, Sanchez-Juan P, Rodriguez-Rodriguez E, Frank A, Rosich-Estrago M, Lleo A, Molina-Porcel L, Blesa R, Gomez-Isla T, Combarros O, Bermejo-Pareja F, Valdivieso F, Bullido MJ, Carro E (2011) IGF-I gene variability is associated with an increased risk for AD. Neurobiol Aging 32, 556.e3–11. [DOI] [PubMed] [Google Scholar]
- [45].Wyss-Coray T, Rogers J (2012) Inflammation in Alzheimer disease-A Brief Review of the Basic Science and Clinical Literature. Cold Spring Harb Perspect Med 2, a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Janowitz D, Habes M, Toledo JB, Hannemann A, Frenzel S, Terock J, Davatzikos C, Hoffmann W, Grabe HJ (2020) Inflammatory markers and imaging patterns of advanced brain aging in the general population. Brain Imaging Behav 14, 1108–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Walker KA, Hoogeveen RC, Folsom AR, Ballantyne CM, Knopman DS, Windham BG, Jack CR, Gottesman RF (2017) Midlife systemic inflammatory markers are associated with late-life brain volume: The ARIC study. Neurology 89, 2262–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wennberg AM V, Hagen CE, Machulda MM, Hollman JH, Roberts RO, Knopman DS, Petersen RC, Mielke MM (2018) The association between peripheral total IGF-1, IGFBP-3, and IGF-1/IGFBP-3 and functional and cognitive outcomes in the Mayo Clinic Study of Aging. Neurobiol Aging 66, 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Reinhardt RR, Bondy CA (1994) Insulin-like growth factors cross the blood-brain barrier. Endocrinology 135, 1753–61. [DOI] [PubMed] [Google Scholar]
- [50].de la Monte SM, Tong M, Schiano I, Didsbury J (2017) Improved Brain Insulin/IGF Signaling and Reduced Neuroinflammation with T3D-959 in an Experimental Model of Sporadic Alzheimer’s Disease. J Alzheimer’s Dis 55, 849–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ashpole NM, Sanders JE, Hodges EL, Yan H, Sonntag WE (2015) Growth hormone, insulin-like growth factor-1 and the aging brain. Exp Gerontol 68, 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Andersson KME, Wasén C, Juzokaite L, Leifsdottir L, Erlandsson MC, Silfverswärd ST, Stokowska A, Pekna M, Pekny M, Olmarker K, Heckemann RA, Kalm M, Bokarewa MI (2018) Inflammation in the hippocampus affects IGF1 receptor signaling and contributes to neurological sequelae in rheumatoid arthritis. Proc Natl Acad Sci U S A 115, E12063–E12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lloyd-Jones DM, Liu K, Tian L, Greenland P (2006) Narrative review: Assessment of C-reactive protein in risk prediction for cardiovascular disease. Ann Intern Med 145, 35–42. [DOI] [PubMed] [Google Scholar]
- [54].Okereke OI, Kang JH, Ma J, Gaziano JM, Grodstein F (2006) Midlife Plasma Insulin-Like Growth Factor I and Cognitive Function in Older Men. J Clin Endocrinol Metab 91, 4306–4312. [DOI] [PubMed] [Google Scholar]
- [55].Ribe EM, Lovestone S (2016) Insulin signalling in Alzheimer′s disease and diabetes: from epidemiology to molecular links. J Intern Med 280, 430–442. [DOI] [PubMed] [Google Scholar]
- [56].Sevigny JJ, Ryan JM, Van Dyck CH, Peng Y, Lines CR, Nessly ML (2008) Growth hormone secretagogue MK-677: No clinical effect on AD progression in a randomized trial. Neurology 71, 1702–1708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


