Abstract
Patient: Male, 44-year-old
Final Diagnosis: Central pontine myelinolysis
Symptoms: Bulbar paralysis • locked-in syndrome • tetraplegia
Medication: —
Clinical Procedure: Brain MRI
Specialty: Neurology • Rehabilitation
Objective:
Unusual clinical course
Background:
Central pontine myelinolysis (CPM) includes symmetric demyelination of the central pons. CPM is a rare neurological disorder that generally develops after rapid correction of hyponatremia in individuals having underlying conditions, such as malnutrition, alcoholism, and severe burns. It can cause severe long-term disabilities. However, there is currently no pharmacotherapy capable of promoting remyelination, a process crucial for recovery from CPM. We present the case of a patient with alcoholism and malnutrition-related CPM, which developed following rapid correction of hyponatremia but then improved remarkably with supportive physical therapy.
Case Report:
A 44-year-old alcoholic and malnourished man was admitted to an emergency hospital for disorientation due to overdrinking, but later developed bulbar palsy after hyponatremia was unexpectedly, but rapidly, corrected. Axial scans of the diffusion-weighted brain MRI revealed a characteristic lesion known as a piglet sign in the central pons. Based on his underlying conditions, present episode of sodium correction, and MRI finding, the patient was diagnosed as having CPM, which progressively worsened, resulting in locked-in syndrome after 12 days. The patient was then transferred to a long-term care unit and received simple motion exercise daily, but no specific medication. His symptoms gradually improved, achieving discontinuation of tube feeding on day 21, independent walking on day 110, and discharge after 6 months.
Conclusions:
This report highlights the importance of physical therapy, the potential of which is often underestimated despite its broad benefits for human health, as a readily applicable intervention for patients with CPM. Further understanding of mechanisms underlying exercise-induced myelination should contribute to establishing novel therapies for a wide spectrum of brain disorders.
Keywords: Exercise; Myelinolysis, Central Pontine; Physical Therapy Modalities; Remyelination; Alcoholism; Hyponatremia; Malnutrition
Background
Central pontine myelinolysis (CPM), a demyelinating disorder of the central nervous system (CNS) caused by damage to myelin-forming oligodendrocytes (ODCs), typically develops after rapid correction of hyponatremia in patients having underlying conditions, such as chronic alcoholism, post-liver transplantation problems, poorly-controlled diabetes, malnutrition, and severe burns [1–5]. It is well recognized that a sudden rise in extracellular osmolarity due to sodium correction leads to rapid movement of water out of cells, causing cellular dysfunctions and, ultimately, cell death [1]. The underlying conditions preceding the onset of CPM are considered as a primary pathology producing the state of limited osmolyte response in the brain, whereas the rapid sodium correction is a disease-triggering event [6]. The tendency of lesions to occur in the central pons is thought to be due to its distinctive structure densified with ODCs, which is a cell type vulnerable to osmotic stress [1,7]. In fact, extrapontine myelinolysis (EPM) often occurs in other ODC-rich regions, such as the striatum and cerebellar white matter [1,7]. It has to be noted that there are also CPM cases with underlying illness, such as alcoholism and diabetes, but without hyponatremia and/or its acute correction [8–11]. This indicates that, for the development of CPM, brain damage caused by the underlying condition itself can be sufficient in some cases, whereas the sodium event is not always a requirement. Other osmolyte dysregulation, such as hypokalemia [12] and hypophosphatemia [13], are also reported to mediate CPM.
Symptoms of CPM appear depending on the spread of lesions within the brainstem. If the corticobulbar tracts are injured, patients present dysarthria and dysphagia. Depending on the extent of corticospinal pathway involvement, varying severity of muscle weakness appears, from mild hemiparesis to complete tetraplegia manifesting as locked-in syndrome [1]. Cooccurrence of EPM often makes the clinical picture complicated with added, or even preceding, extrapontine symptoms, such as involuntary movement and behavioral abnormalities [1]. One should thus bear in mind that the emergence of any new CNS symptom in individuals having known risks indicates possible occurrence of CPM plus/or EPM.
In addition to the underlying illness and the triggering episode (ie, rapid correction of lowered serum osmolarity), magnetic resonance imaging (MRI) of the brain provides key diagnostic information [14,15]. The first change in the central pons can usually be detected within 24 h of symptom onset as a symmetric high-signal area in a diffusion-weighted (DW) image. In the later stage, this lesion becomes clear and well-defined in T2-weighted (T2W) and T1-weighted (T1W) imaging. Particularly, the high-signal lesion recognized on axial T2W scans is described as being shaped like a pig snout (piglet sign) [16]. This characteristic appearance is attributed to relative sparing of the descending corticospinal and corticobulbar tracts (the nostrils of the pig snout) within the pontine lesion.
Despite increased understanding of the disease pathogenesis and accessibility of diagnostic MRI, evidence-based pharmacotherapy for CPM is lacking. We report the case of an alcoholic and malnourished man who developed CPM after his hyponatremia was rapidly corrected, but who then recovered remarkably with supportive physical therapy. We discuss the case with a particular focus on how motion exercises enhanced CNS remyelination, a process crucial for recovery from CPM.
Case Report
A 44-year-old man with a past history of chronic alcoholism presented to an emergency hospital for disorientation due to overdrinking, and an intravenous drip of standard maintenance solutions (100 mL/h) was initiated. However, his blood sodium level was unexpectedly, but rapidly, corrected from 107 mEq/L to 121 mEq/L within 18 h, and he developed dysarthria and dysphagia on the next day. Other abnormalities detected on admission blood tests included mild anemia, slight dehydration, and decreased protein levels. Together with his low body mass index, he was considered to have mild malnutrition (basic information summarized in Table 1). In axial MRI scans of the brain (1.5T) taken on the day of symptom onset (Figure 1), an abnormality was detected in the central pons in DW images as a relatively high-signal area resembling a piglet sign [16] but not clear in T2W and T1W images, suggesting that this was the lesion of recent onset, as described previously [14]. Based on his underlying illnesses (alcoholism and malnutrition), the present episode of sodium correction, and MRI findings, the patient was diagnosed as CPM. While nutritional and body water balance were maintained by tube feeding, intravenous drip of vitamin B1 (300 mg of thiamine per day) was given for 1 week to prevent development of Wernicke’s encephalopathy, which is another alcoholism-related CNS disorder caused by thiamine deficiency [17]. His vital signs, arterial oxygen saturation, and serum electrolytes, such as sodium and potassium, stayed within normal levels, but the palsy progressively worsened, resulting in locked-in syndrome after 12 days. After 1 month of being confined to bed and receiving tube feeding and range of motion (ROM) exercises to prevent limb-joint contracture, he was transferred to the long-term care unit of our hospital for further supportive care.
Table 1.
Summary of the patient’s past history and basic data on admission to the emergency hospital.
| Past history | |
|---|---|
| Education/Occupation | High school graduate/Factory worker since the age of 18 |
| Marriage/Child | Married at the age of early 20s/Three children |
| Alcohol drinking | More than 25 years since the age of 18 |
| Past illness | Chronic alcoholism diagnosed at the age of late 20s (details unknown) |
| Smoking/Drug abuse | None/None |
| Diet | Slightly unbalanced |
| Family history | Unremarkable |
| Basic data on admission to the emergency hospital | Normal range | |
|---|---|---|
| Body weight (kg)/height (cm) | 47.3/174 | |
| Body mass index | 15.6 | 18.5–25 |
| Blood pressure (mmHg) | 109/72 | |
| Heart rate (bpm) | 70 (Regular) | |
| Body temperature (°C) | 36.4 | |
| Blood test | ||
| Red blood cell (×1012/L) | 3.5 | 4.3–5.7 |
| White blood cell (×109/L) | 6.5 | 3.9–9.8 |
| Platelet (×109/L) | 333 | 130–362 |
| Sodium (mEq/L) | 107 | 135–147 |
| Potassium (mEq/L) | 4.0 | 3.5–5.0 |
| Glucose (mg/dL) | 83 | 70–110 |
| Blood urea nitrogen (mg/dL) | 28 | 8–20 |
| Creatinine (mg/dL) | 0.8 | 0.5–1.0 |
| Total protein (g/dL) | 6.0 | 6.7–8.3 |
| Albumin (g/dL) | 3.0 | 3.5–5.0 |
| AST (IU/L) | 29 | 12–33 |
| ALT (IU/L) | 32 | 5–35 |
| LDH (IU/L) | 148 | 115–254 |
| γ-GTP (IU/L) | 53 | 13–64 |
| Phosphate (mg/dL) | 3.8 | 2.7–4.6 |
| C-reactive protein (mg/dL) | 0.25 | <0.3 |
AST – aspartate aminotransferase; ALT – alanine aminotransferase; LDH – lactate dehydrogenase; γ-GTP – gamma-glutamyl transferase. Bold font indicates abnormal values.
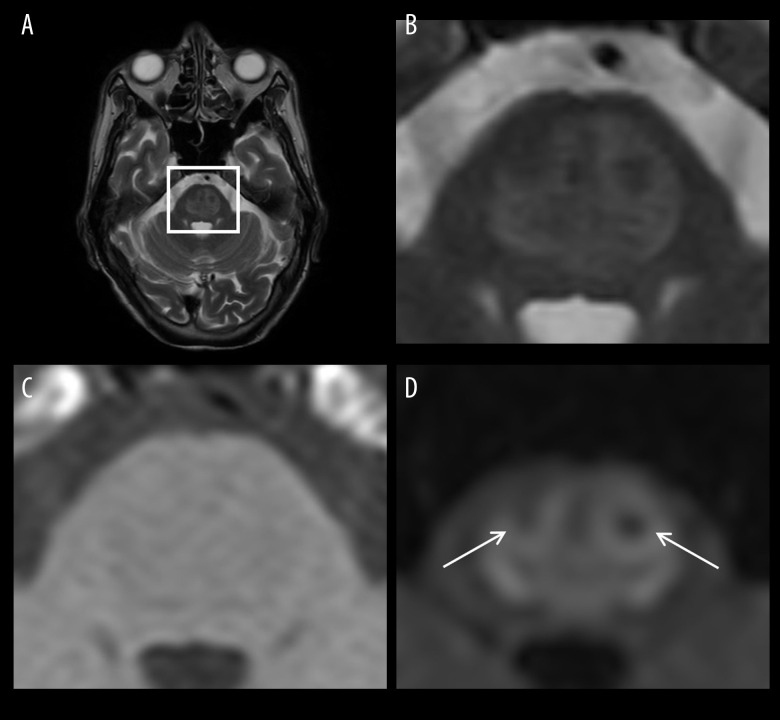
Figure 1.
Axial MRI scans taken on the day of disease onset. (A) T2-weighted (T2W) image at the level of the pons. (B) Enlarged T2W image of the square region indicated in (A) showing the central pontine lesion as a slightly high-signal area. (C) Enlarged T1-weighted (T1W) image of the square region indicated in (A) showing the central pontine lesion as a slightly low-signal area. (D) Enlarged diffusion-weighted (DW) image of the square region indicated in (A) showing the central pontine lesion as an apparently high-signal area resembling a piglet sign. Arrows indicate relatively spared descending corticospinal and corticobulbar tracts (the nostrils of the pig snout) within the lesion.
On admission (day 1), the patient was completely paraplegic but alert and could express “Yes” or “No” either by blinking or moving his eyes to communicate with medical staff. Deep-tendon reflexes in his extremities were normal, and neither sensory defect nor pathologic reflex was detected. The bedside ROM exercise was continued as done at the former hospital, but no medication was prescribed. From day 3 of hospitalization, the patient started to move his left hand, followed by gradual recovery of motor ability. The level of motion training was increased in a timely manner, with no specially-designed program but according to the patient’s motor recovery and motivation, achieving discontinuation of tube feeding on day 21, sit-to-stand movements on day 42, and independent walking on day 110. During the rehabilitation (about 1 h daily), no specialized equipment was used, and we only used commonly available aids such as a chair, parallel bars, walker, and cane. In the brain scans performed 11 weeks after disease onset (Figure 2), the central pontine piglet sign became apparent and well-defined in T2W and T1W images. There was no imaging evidence of EPM. The malnutrition, presumably a consequence of long-term alcoholism and unbalanced diet, was no longer detected in the examinations done after 5 months of hospitalization. The patient’s performance in activities of daily living as assessed by the Barthel index [18] had steadily improved, reaching a perfect score on day 170. Soon thereafter, the patient was discharged with only slight muscle weakness of his right hand and mildly slurred speech.
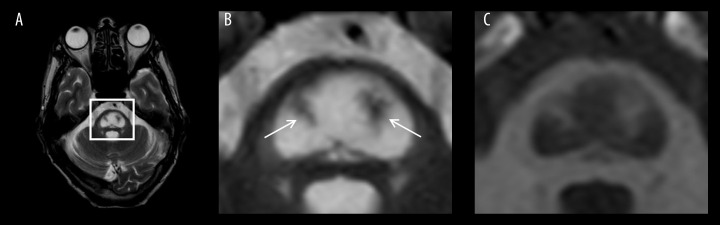
Figure 2.
Axial MRI scans taken 11 weeks after disease onset. (A) T2W image at the level of pons. (B) Enlarged T2W image of the square region indicated in (A) showing the clear central pontine piglet sign as a well-defined high-density area. Arrows indicate spared descending corticospinal and corticobulbar tracts (the nostrils of the pig snout) within the lesion. (C) Enlarged T1W image of the square region indicated in (A) showing the central pontine piglet sign as a low-density area.
Discussion
To the best of our knowledge, this is the first reported case of CPM that developed in a patient with co-occurrence of multiple disease risks: alcoholism, malnutrition, and hyponatremia and its rapid correction. As CPM, though, our case was quite typical in its underlying illness (alcoholism and malnutrition), triggering episode (rapid correction of hyponatremia), and brain MRI finding (piglet sign). Considering the patient’s severe symptoms manifested as locked-in syndrome, what is noteworthy in this case was his remarkable response to rehabilitation. Many cases of CPM with favorable prognosis have been reported [19,20]; however, few reports have emphasized the possible contribution of physical therapy to the patient’s recovery [21]. In CPM, the risk for poor prognosis is reported to be high in the patients with serum sodium of <115 mEq/L, hypokalemia, and low Glasgow coma scale (<11) during hospitalization [22]. Patients after liver transplantation were also reported to have worse outcomes as compared to those associated with other underlying conditions, including alcoholism and malnutrition [23]. Given his severe hyponatremia (107 mEq/L) and its subsequent rapid correction, our patient is categorized in the group of patients with poor prognosis. Our patient had an outstanding recovery, which we believe was achieved, at least in part, through daily performance of simple physical therapy. By a network meta-analysis of randomized controlled trials, recent studies have shown the positive effects of motion exercises on improving impaired motor functions of patients with multiple sclerosis (MS) [24,25], the most common demyelinating disorder of the CNS. In an animal model of CNS demyelination, Jensen et al demonstrated, at the histological level, that exercise actually accelerates oligodendrogenesis and the rate of remyelination [26]. Thus, physical exercise promotes the repair of demyelinated CNS axons, but the underlying mechanism has been unclear.
Neuronal activity-dependent myelination is the well-known process by which electrical activity of axons instructs ODCs to myelinate these active axons, thereby increasing their conduction velocity [27]. The mechanism underlying this phenomenon is not clearly understood; however, leakage of axonal components, such as potassium and glutamate, from unmyelinated sites of electrically active axons is considered to locally trigger a series of cellular responses towards myelination [27,28]. Even if a patient is completely paralyzed due to severe demyelination, action potentials generated by the one’s will to move his/her body might reach the unmyelinated sites and provoke the myelin repairing processes. Currently, passive brain stimulation (ie, transcranial magnetic stimulation), which presented encouraging results in preclinical studies, is attracting attention as a novel therapeutic strategy for MS [28].
Exercise-induced myelination is also achieved by various trophic factors released into the blood from different organs. Among them is insulin-like growth factor (IGF-1), a liver-derived molecule that increases in peripheral circulation in response to physical activity, then crosses the blood–brain barrier and turns on multiple cellular pathways involved in myelinogenesis [29]. IGF-1 is also known for its ability to activate CNS vascularization and neurogenesis, and thus is a potential agent for treating various CNS disorders [29]. Exercise also stimulates skeletal muscles to secrete molecules [30], such as brain-derived neurotrophic factor (BDNF) [31], which are capable of driving CNS myelination. For factor release, however, a certain extent of physical activity is needed, making it impractical for some patients with limited mobility; therefore, researchers are now studying electrical muscle stimulation for possible clinical application in the future [32].
The present study shows that physical exercise can promote CNS myelination by electrically stimulating unmyelinated axon segments and by inducing production and release of neurotrophic factors from organs into the blood (Figure 3). Exercise improves modulation of immune, metabolic, and mental functions [33], helping patients to recover earlier. While we could not quantify the extent to which exercise contributed to the significant recovery seen in our patient, we believe that early and continuous application of physical therapy is important as an easily used and readily available intervention for patients with CPM [34]. Clinical trials with appropriate controls are needed to verify this hypothesis; however, offering careful physical therapy within a reasonable extent, not provoking any physical or mental stress, may be useful.
Figure 3.
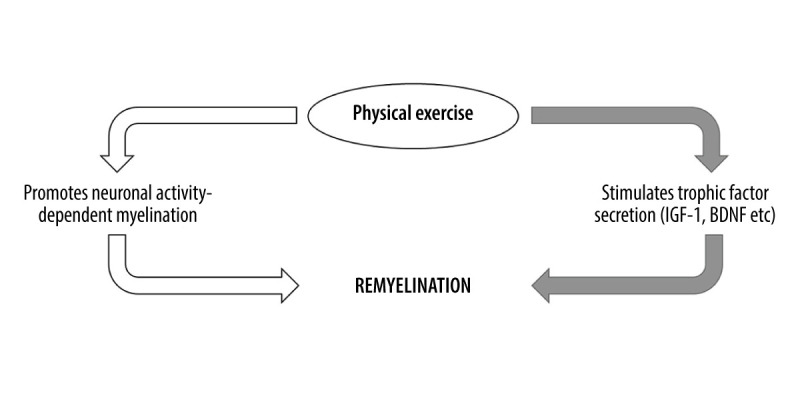
Schematic summarizing 2 major pathways involved in exercise-induced CNS remyelination. Physical exercise excites related neurons in the brain and promotes their myelination via axonal action potentials (white arrows). Physical exercise also stimulates secretion and increases serum levels of myelination-promoting factors that subsequently enter the CNS via blood circulation (gray arrows).
Conclusions
This report highlights that physical therapy, the pivotal roles of which are already established in various neurological disorders, including stroke [35], Parkinson’s disease [36], and MS [24,25], is also quite important for improving the prognosis of CPM. Considering the broad spectrum of the effects of exercise on human health, this subject warrants further clinical and basic research. Better understanding of the mechanisms underlying exercise-induced myelination should contribute to establishing novel therapies for a wide spectrum of CNS disorders.
Footnotes
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Lambeck J, Hieber M, Dreßing A, Niesen WD. Central pontine myelinolysis and osmotic demyelination syndrome. Dtsch Arztebl Int. 2019;116(35–36):600–6. doi: 10.3238/arztebl.2019.0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh TD, Fugate JE, Rabinstein AA. Central pontine and extrapontine myelinolysis: A systematic review. Eur J Neurol. 2014;21(12):1443–50. doi: 10.1111/ene.12571. [DOI] [PubMed] [Google Scholar]
- 3.Kinoshita H, Grant L, Xoinis K, Purohit PJ. Central pontine myelinolysis in pediatric diabetic ketoacidosis. Case Rep Crit Care. 2018;2018:4273971. doi: 10.1155/2018/4273971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amann B, Schäfer M, Sterr A, et al. Central pontine myelinolysis in a patient with anorexia nervosa. Int J Eat Disord. 2001;30(4):462–66. doi: 10.1002/eat.1109. [DOI] [PubMed] [Google Scholar]
- 5.Cohen BJ, Jordan MH, Chapin SD, et al. Pontine myelinolysis after correction of hyponatremia during burn resuscitation. J Burn Care Rehabil. 1991;12(2):153–56. doi: 10.1097/00004630-199103000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Shah MK, Mandayam S, Adrogué HJ. Osmotic demyelination unrelated to hyponatremia. Am J Kidney Dis. 2018;71(3):436–40. doi: 10.1053/j.ajkd.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Rebedew DL. Is central pontine myelinolysis reversible? WMJ. 2016;115(6):326–28. [PubMed] [Google Scholar]
- 8.Mochizuki H, Masaki T, Miyakawa T, et al. Benign type of central pontine myelinolysis in alcoholism – clinical, neuroradiological and electrophysio-logical findings. J Neurol. 2003;250(9):1077–83. doi: 10.1007/s00415-003-0157-6. [DOI] [PubMed] [Google Scholar]
- 9.Feng XM, Zhao T, Zhou CK, Liu JY. Psychiatric symptoms and limb tremors associated with central pontine myelinolysis: A case of alcoholism without hyponatremia. Exp Ther Med. 2016;12(5):3485–87. doi: 10.3892/etm.2016.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNamara PH, Williams J, McCabe DJ, Walsh RA. Striking central pontine myelinolysis in a patient with alcohol dependence syndrome without hyponatremia. JAMA Neurol. 2016;73(2):234–35. doi: 10.1001/jamaneurol.2015.2201. [DOI] [PubMed] [Google Scholar]
- 11.Mir WAY, Shrestha DB, Aryal BB, et al. Central pontine myelinolysis secondary to hyperglycemia in a young patient. Cureus. 2021;13(10):e18495. doi: 10.7759/cureus.18495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bähr M, Sommer N, Petersen D, Wiethölter H, Dichgans J. Central pontine myelinolysis associated with low potassium levels in alcoholism. J Neurol. 1990;237(4):275–76. doi: 10.1007/BF00314635. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita C, Shigeto H, Maeda N, et al. A case of central pontine myelinolysis caused by hypophosphatemia secondary to refeeding syndrome. Case Rep Neurol. 2015;7(3):196–203. doi: 10.1159/000440711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruzek KA, Campeau NG, Miller GM. Early diagnosis of central pontine myelinolysis with diffusion-weighted imaging. AJNR Am J Neuroradiol. 2004;25(2):210–13. [PMC free article] [PubMed] [Google Scholar]
- 15.Kusel K, Azzam O, Youssef A, Prentice D. Alcoholic pontine myelinolysis: Beware the stroke mimic. BJR Case Rep. 2021;7(4):20210005. doi: 10.1259/bjrcr.20210005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beh SC. Temporal evolution of the trident and piglet signs of osmotic demyelination syndrome. J Neurol Sci. 2017;373:268–73. doi: 10.1016/j.jns.2017.01.024. [DOI] [PubMed] [Google Scholar]
- 17.Smith H, McCoy M, Varughese K, Reinert J. Thiamine dosing for the treatment of alcohol-induced Wernicke’s encephalopathy: A review of the literature. J Pharm Technol. 2021;37(2):107–13. doi: 10.1177/8755122520962859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohura T, Hase K, Nakajima Y, Nakayama T. Validity and reliability of a performance evaluation tool based on the modified Barthel Index for stroke patients. BMC Med Res Methodol. 2017;17(1):131. doi: 10.1186/s12874-017-0409-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lv X, Hong Q, Lin X, et al. Osmotic demyelination syndrome: clinical, neuroimaging characteristics, and outcomes in a series of 18 cases. Biomed Res Int. 2021;2021:9944632. doi: 10.1155/2021/9944632. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Musana AK, Yale SH. Central pontine myelinolysis: Case series and review. WMJ. 2005;104(6):56–60. [PubMed] [Google Scholar]
- 21.Sohn MK, Nam JH. Locked-in syndrome due to central pontine myelinolysis: Case report. Ann Rehabil Med. 2014;38(5):702–6. doi: 10.5535/arm.2014.38.5.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kallakatta RN, Radhakrishnan A, Fayaz RK, et al. Clinical and functional outcome and factors predicting prognosis in osmotic demyelination syndrome (central pontine and/or extrapontine myelinolysis) in 25 patients. J Neurol Neurosurg Psychiatry. 2011;82(3):326–31. doi: 10.1136/jnnp.2009.201764. [DOI] [PubMed] [Google Scholar]
- 23.Morard I, Gasche Y, Kneteman M, et al. Identifying risk factors for central pontine and extrapontine myelinolysis after liver transplantation: A case-control study. Neurocrit Care. 2014;20(2):287–95. doi: 10.1007/s12028-013-9928-9. [DOI] [PubMed] [Google Scholar]
- 24.Lyons J, Campese S, Learmonth YC, et al. Comparing the effectiveness, safety and tolerability of interventions for depressive symptoms in people with multiple sclerosis: A systematic review and network meta-analysis protocol. BMJ Open. 2022;12:e055796. doi: 10.1136/bmjopen-2021-055796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hao Z, Zhang X, Chen P. Effects of different exercise therapies on balance function and functional walking ability in multiple sclerosis disease patients – a network meta-analysis of randomized controlled trials. Int J Environ Res Public Health. 2022;19:7175. doi: 10.3390/ijerph19127175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen SK, Michaels NJ, Ilyntskyy S, et al. Multimodal enhancement of remyelination by exercise with a pivotal role for oligodendroglial PGC1alpha. Cell Rep. 2018;24(12):3167–79. doi: 10.1016/j.celrep.2018.08.060. [DOI] [PubMed] [Google Scholar]
- 27.Foster AY, Bujalka H, Emery B. Axoglial interactions in myelin plasticity: Evaluating the relationship between neuronal activity and oligodendrocyte dynamics. Glia. 2019;67(11):2038–49. doi: 10.1002/glia.23629. [DOI] [PubMed] [Google Scholar]
- 28.Maas DA, Angulo MC. Can enhancing neuronal activity improve myelin repair in multiple sclerosis? Front Cell Neurosci. 2021;15:645240. doi: 10.3389/fncel.2021.645240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Åberg D. Role of the growth hormone/insulin-like growth factor 1 axis in neurogenesis. Endocr Dev. 2010;17:63–76. doi: 10.1159/000262529. [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Wang L, You W, Shan T. Myokines mediate the cross talk between skeletal muscle and other organs. Cell Physiol. 2021;236(4):2393–412. doi: 10.1002/jcp.30033. [DOI] [PubMed] [Google Scholar]
- 31.Fletcher JL, Murray SS, Xiao J. Brain-derived neurotrophic Factor in central nervous system myelination: A new mechanism to promote myelin plasticity and repair. Int J Mol Sci. 2018;19(12):4131. doi: 10.3390/ijms19124131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrie MA, Sharma A, Taylor EB, et al. Impact of short- and long-term electrically induced muscle exercise on gene signaling pathways, gene expression, and PGC1a methylation in men with spinal cord injury. Physiol Genomics. 2020;52(2):71–80. doi: 10.1152/physiolgenomics.00064.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sui SX, Williams LJ, Holloway-Kew KL, Hyde NK, Pasco JA. Skeletal muscle health and cognitive function: A narrative review. Int J Mol Sci. 2020;22(1):255. doi: 10.3390/ijms22010255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuller K, Guerrero C, Kyin M, et al. The role of the interdisciplinary team in subacute rehabilitation for central pontine myelinolysis. Disabil Rehabil. 2020;42(21):3112–18. doi: 10.1080/09638288.2019.1579261. [DOI] [PubMed] [Google Scholar]
- 35.Han P, Zhang W, Kang L, et al. Clinical evidence of exercise benefits for stroke. Adv Exp Med Biol. 2017;1000:131–51. doi: 10.1007/978-981-10-4304-8_9. [DOI] [PubMed] [Google Scholar]
- 36.Feng YS, Yang SD, Tan ZX, et al. The benefits and mechanisms of exercise training for Parkinson’s disease. Life Sci. 2020;245:117345. doi: 10.1016/j.lfs.2020.117345. [DOI] [PubMed] [Google Scholar]