Abstract
Objective:
We performed a systematic review of the epidemiology literature to identify the metabolic effects associated with phthalate exposure.
Data sources and study eligibility criteria:
Six phthalates were included in the review: di(2-ethylhexyl) phthalate (DEHP), diisononyl phthalate (DINP), dibutyl phthalate (DBP), diisobutyl phthalate (DIBP), butyl benzyl phthalate (BBP), and diethyl phthalate (DEP). The initial literature search (of PubMed, Web of Science, and Toxline) included all studies of metabolic effects in humans, and outcomes were selected for full systematic review based on data availability.
Study evaluation and synthesis methods:
Studies of diabetes and insulin resistance were evaluated using criteria defined a priori for risk of bias and sensitivity by two reviewers using a domain-based approach; studies identified with a pre-defined critical deficiency were excluded. Evidence was synthesized by outcome and phthalate and strength of evidence was summarized using a structured framework. Studies of obesity and renal effects received “screening level” reviews to determine whether full systematic review was warranted.
Results:
The primary outcomes reviewed here are (number of included/excluded studies in parentheses): type 2 diabetes (1/3), insulin resistance (13/3), and impaired glucose tolerance and blood glucose in pregnancy (4/2). For DEHP exposure, there was consistency among studies of insulin resistance and coherence with the single included study of diabetes, as well as an observed exposure-response gradient observed in a study of insulin resistance. This evidence is considered moderate. Similarly, for DBP and DIBP exposure, the evidence is considered moderate due to strong positive associations in the diabetes study and coherent results for insulin resistance. For DINP, BBP, and DEP, the evidence is considered slight. No association was reported in the single study of diabetes with BBP and DEP exposure (DINP was not investigated). The available evidence does indicate an association between exposure to these phthalates and insulin resistance, but the small number of studies and the lack of coherence with diabetes decreases confidence. The screening level reviews for obesity and renal effects determined that the currently available evidence is inadequate to assess the associations between these outcomes and phthalate exposure.
Conclusions and implications of key findings:
Overall, these results support that phthalate exposure at levels seen in human populations may have metabolic effects. Given the mechanistic support, the large effect sizes for incident diabetes in the single available study, and the coherence with insulin resistance, the association between phthalate exposure and diabetes risk should be considered when assessing the risks and costs of exposure to specific phthalates in humans.
The views expressed are those of the authors and do not necessarily represent the views or policies of the U.S. EPA.
1. Introduction
Human exposure to phthalates (manmade phthalic acid diesters) is ubiquitous because of the widespread use of these chemicals in consumer and industrial products. These compounds have a variety of different structures and properties that determine their uses. Phthalates are used in polyvinyl chloride products and in some medications and personal care products. In general, these diesters are rapidly metabolized to monoester metabolites and are excreted in the urine. Endocrine, and endocrine-disruption, effects on metabolism and on metabolic diseases is a growing area of research with significant public health implications (Heindel et al., 2017). This review focuses on studies of phthalate exposure and metabolic-related outcomes.
The phthalates evaluated in this paper are: di(2-ethylhexyl) phthalate (DEHP), diisononyl phthalate (DINP), dibutyl phthalate (DBP), diisobutyl phthalate (DIBP), butyl benzyl phthalate (BBP), and diethyl phthalate (DEP). Five of the selected phthalates (DEHP, DINP, DBP, DIBP, and BBP) were chosen because they are the most potent with respect to producing the “phthalate syndrome” of male reproductive effects in rats (NAS 2008) and their metabolites have been frequently observed in human populations studies; DEP is not one of the “phthalate syndrome” compounds but was included because it is often the phthalate to which humans have the highest exposure.
Past reviews examined the association between phthalates and diabetes and obesity and found the evidence inadequate to conclude whether an association existed (Kuo et al., 2013; Thayer et al., 2012). However, additional studies have been published in the interim, and no systematic review is available on insulin resistance, an emerging area of research in the phthalate literature. As part of a comprehensive evaluation of the epidemiological literature on phthalates, we performed a systematic review on the association between phthalate exposure and metabolic effects, specifically diabetes, insulin resistance, and obesity. We also examined renal effects because they can be a long-term complication of diabetes and because impaired renal function could affect interpretation of exposure measures based on urinary concentrations of phthalate metabolites (Table 1). We focus on Type 2 diabetes, which is characterized by insulin resistance and the resulting insulin deficiency due to the inability of the pancreas to produce enough insulin. Obesity is a strong risk factor for Type 2 diabetes, and the highest incidence of Type 2 diabetes is in middle- and older-aged adults. In contrast, Type 1 diabetes involves a different etiology, an autoimmune-mediated destruction of the pancreatic islets of Langerhans cells. Incidence of Type 1 diabetes is highest in children and teenagers.
Table 1.
Outcomes included in the review.
| Outcome | Background and relevance to human health |
|---|---|
|
| |
| Diabetes | • Diabetes is a group of metabolic diseases characterized by hyperglycemia due to β-cell dysfunction, insulin resistance, or both. Diabetes can result in long-term complications in different organs, in particular, microvascular and macrovascular complications. |
| • This review focuses on Type 2 (adult onset) diabetes. | |
| Insulin resistance | • Among study subjects without diabetes, fasting blood glucose, insulin, and a related measure based on the homeostatic model assessment of insulin resistance (HOMA-IR) are informative regarding future diabetes risk. |
| • Dysfunctions in insulin production and action in the peripheral tissues are the key pathways in diabetes etiology. | |
| Gestational diabetes | • Gestational diabetes is another form of diabetes that occurs during pregnancy. A history of gestational diabetes is a risk factor for subsequent development of diabetes. |
| • Definitions of gestational diabetes and impaired glucose tolerance are based on measures of blood glucose. All three of these outcomes in pregnant women are reviewed together, separate from the general population. | |
| Obesity | • Excessive body weight, or obesity, is associated with the leading causes of death worldwide, including diabetes, heart disease, stroke, and some types of cancer. |
| • Body mass index (BMI) is a screening tool calculated from a person’s weight and height that is moderately correlated with more direct measures of body fat (CDC 2016). | |
| Renal effects | • Kidney function can be assessed by measuring levels of urea, creatinine, and certain dissolved salts in blood and by analyzing protein levels in urine. |
| • Albuminuria is a pathological condition wherein the protein albumin is abnormally present in the urine. Albuminuria is considered a significant risk factor for the development of renal disease. | |
| • Renal disease is a long-term complication of diabetes, obesity, and possibly, insulin resistance independent of those two conditions (Whaley-Connell and Sowers, 2017). | |
| • Impaired renal function could affect urinary concentration of phthalate metabolites. | |
To make the systematic review more pragmatic, so that limited resources were not utilized to assess bodies of evidence that would likely be inadequate for reaching conclusions about the association, obesity and renal effects received less robust “screening level” reviews to determine the extent and nature of the literature for evidence synthesis. This approach is consistent with recommendations from the National Academies of Science encouraging the U.S. Environmental Protection Agency to explore ways to make systematic review more feasible, including a “rapid review in which components of the systematic review process are simplified or omitted (e.g., the need for two independent reviewers)” (National Academy of Sciences, 2017).
2. Methods
The full methods for this systematic review, including for literature search and screening, study evaluation, data extraction, and evidence synthesis are described in detail in the protocol (Supplemental materials). An abbreviated version is provided below, with references to specific sections of the protocol for ease of reference. In addition, the key tables from the protocol that are necessary to understand how conclusions were reached are included in a separate supplemental file (key methods supplement).
2.1. Literature search and screening
Epidemiology studies were identified by conducting a single broad literature search on all six phthalates of interest (DEHP, DINP, DBP, DIBP, BBP, DEP). The Population, Exposure, Comparators, and Outcome (PECO) criteria are available in the protocol (Section 2.2). The following databases were searched: PubMed, Web of Science, and Toxline, with the initial search in 2013, and updates every 6–12 months through August 2018 (protocol Section 3). Forward and backward searches were also performed. Title/abstract and full text screening was performed by two reviewers.
2.2. Study evaluation
Study evaluation (protocol Section 4.1) was conducted by two reviewers for those outcomes receiving full systematic review (i.e., diabetes, insulin resistance, gestational diabetes). Key concerns were risk of bias (factors that affect the magnitude or direction of effect) and insensitivity (factors that limit the ability of a study to detect a true effect). Evaluation was conducted for the following domains: exposure measurement, outcome ascertainment, participant selection, confounding, analysis, sensitivity, and selective reporting. These domains were based on the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool (Sterne et al., 2016), modified for use with environmental exposures. Phthalate and outcome-specific criteria were developed prior to evaluation and are described briefly below. Detailed exposure criteria are available in the key methods supplement and the protocol (Section 4.1.1). Detailed outcome-specific evaluation criteria are available in the supplement and summarized briefly below.
For evaluating exposure, most of the available studies relied on phthalate metabolite biomarkers (a list of metabolites for each phthalate is provided in the protocol, Section 1.3). Different criteria were developed for short-chain (DEP, DBP, DIBP, BBP) and long-chain (DEHP, DINP) phthalates due to greater reliability of single biomarker measures for short-chain phthalates. Measurement in urine was considered to be the best proxy of exposure (Johns et al., 2015). Biomarker measures based on samples other than urine (e.g., blood, amniotic fluid, breast milk) were considered to be critically deficient for all short-chain phthalates and for primary metabolites (e.g., MEHP, MINP) of long-chain phthalates (Johns et al., 2015). This critical deficiency was used as a basis for excluding studies from subsequent analyses.
Several outcome-specific criteria were applied across multiple evaluation domains (for more details, see outcome-specific criteria in the supplement). Timing of exposure measurement was an important consideration, particularly given the short half-life of phthalates (3–18 h for phthalate metabolites in a review by Johns et al. (2015)). For diabetes, it is important for studies to exclude individuals with diabetes at baseline and include only individuals with incident disease as cases, with exposure measured prior to development of diabetes in order to establish their temporality. Accordingly, studies of diabetes with exposure measured concurrent with the outcome were excluded, and prospective designs were generally needed for inclusion. For insulin resistance, it is appropriate to measure the exposure and outcome concurrently as the outcome can be a short-term response. Several criteria for outcome ascertainment were also applied. For diabetes, to identify undiagnosed cases, the use of the American Diabetes Association definition with information about required fasting, laboratory test requirements and assays, quality control procedures, and reliability or validity measures were preferred, while self-reported physician diagnosis or medical treatment for diabetes was appropriate for diagnosed cases. For insulin resistance, diabetics must be excluded as measures of insulin are not interpretable in the presence of diabetes, especially if diabetes is treated with hypoglycemic medication, as treatment will influence insulin production and secretion. In addition, fasting insulin and/or glucose measurements were required, and information on testing as described for diabetes was preferred. HOMA-IR was used as the primary measure of insulin resistance when available. In evaluating confounding, the following risk factors were considered possible confounders: age, gender, adiposity/body mass index, lifestyle (physical inactivity, poor diet, smoking, heavy alcohol consumption), and socioeconomic status. It was not required that a study adjusts for all of these variables; rather, the study evaluation considered whether omission of these covariates was likely to have introduced bias into the effect estimates. There is also potential for confounding across the phthalates, particularly when results are similar for two moderately (or higher) correlated phthalates in a study. Study evaluations were not downgraded due to this potential, but it was considered during evidence synthesis as another source of uncertainty in the results.
For each study, in each evaluation domain, reviewers reached a consensus rating regarding the utility of the study for hazard identification, with categories of Good, Adequate, Deficient, or Critically deficient. These ratings were then considered together to reach an overall study confidence classification of High, Medium, Low, or Uninformative. This overall classification was not based on pre-defined weights of the domains, but rather was based on reviewer judgments, and includes the likely impact the noted deficiencies in bias and sensitivity have on the results, which varies depending on the study and/or outcome. Studies were evaluated for their suitability for each outcome investigated and could receive different ratings for each outcome. Descriptions of each of the categories can be found in the protocol (Section 4) and the key methods supplement. Study evaluations were documented in Health Assessment Workspace (HAWC), and ratings and the rationales are available at links provided in the results.
2.3. Evidence synthesis
After study evaluation, the evidence for each outcome was synthesized separately for each phthalate using a structured framework, using the following aspects of an association that may support causation: consistency, exposure-response relationship, strength of association, temporal relationship, biological plausibility, and coherence. In evaluating the evidence for each of these considerations, syntheses also consider study evaluation decisions, with high confidence studies carrying the most weight and consideration of specific strengths and limitations of individual studies described. Based on this synthesis, the evidence was assigned a strength of evidence conclusion of Robust, Moderate, Slight, Indeterminate, or Compelling evidence of no effect. Robust and Moderate describe evidence that supports a hazard, differentiated by the quantity and quality of information available to rule out alternative explanations for the results. Slight and Indeterminate describe evidence for which uncertainties prevent drawing a causal conclusion in either direction. These categories are generally limited in terms of quantity or confidence level of studies, and serve to encourage additional research across the exposure range experienced by humans. Compelling evidence of no effect requires several high confidence studies with consistent null results. The ratings for the individual outcomes were then summarized into an overall conclusion for diabetes risk (encompassing incident diabetes and insulin resistance) for each phthalate, using a structured framework available in the key methods supplement and the protocol (Section 6). This structured framework is conceptually similar to and is informed by the well-established Grading of Recommendations, Assessment, Development and Evaluations (GRADE) approach (Schünemann et al., 2013), but is designed to address the challenges specific to the analysis of environmental health data rather than clinical evidence. No statistical quantitative meta-analysis was performed due to substantial differences across studies.
For obesity and renal disease, screening-level reviews were conducted to determine whether new literature suggest full systematic reviews would be warranted and to identify key research gaps. One reviewer identified studies that should be excluded based on exposure assessment and temporality. The remaining studies were then each evaluated by a single reviewer and were assessed for consistency, while considering whether any observed inconsistency could potentially be attributed to differences in study confidence or exposure level. If this screening level review indicated little consistency among studies, with no clear explanation based on study confidence, then full data extraction and additional evidence analysis and synthesis was not pursued, and the evidence was considered inadequate. The screening level reviews should not be considered as substitutes for systematic review.
Reporting of this systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2009). The work described herein was federally funded.
3. Results
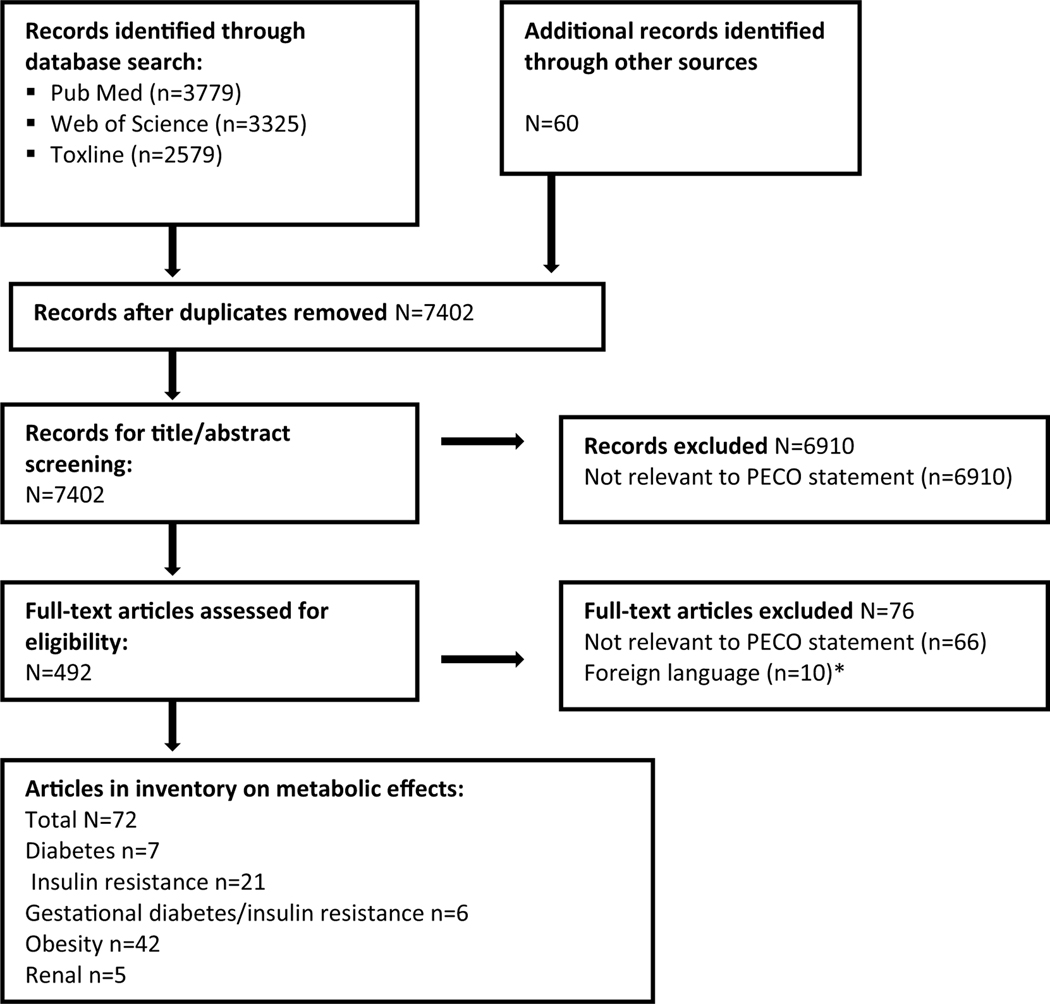
The literature flow diagram, depicting the identification and disposition of the literature search records, is shown in Fig. 1. A list of all the studies identified in the search that met the PECO criteria is available in the supplement. For each outcome, the included and excluded studies are described in the respective section within the text.
Fig. 1.
Literature flow diagram for metabolic effects of phthalates.
*Included one study on insulin resistance and one on obesity. Based on the abstracts, the former would be excluded due to exposure measurement in blood and the latter would be excluded due to temporality issues.
Number of articles in inventory does not account for multiple publications on the same study.
3.1. Type 2 diabetes
3.1.1. Study selection and evaluation
Based on evaluation of temporality, six of the seven epidemiology studies identified in the search with data on type 2 diabetes were excluded because exposure was measured at the same time as ascertainment of diabetes status. One study (Sun et al., 2014), a nested case-control study within two cohorts (Nurses’ Health Study (NHS) and NHSII), was identified in the search as having prospective data on diabetes risk. The specific phthalates examined in that study and the evaluation are summarized in Table 2; this study was classified as medium confidence and rationales are available in HAWC at https://hawcprd.epa.gov/rob/study/100000200/.
Table 2.
Epidemiology studies of diabetes.
| Reference | Study description | Includes metabolites of: | Study evaluation | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||||
| Population | Exposure | Outcome | DEHP | DINP | DBP | DIBP | BBP | DEP | Exposure | Outcome | Selection | Confounding | Analysis | Overall | confidence | ||
| Included | Sun et al. (2014) | Case-control nested in two cohorts in U.S. (N=971 case-control pairs) | Single urine sample at least one year prior to diagnosis | Self-report of physician diagnosis (validated in cohort) | ✓ | ✓ | ✓ | ✓ | ✓ | A/D | G | A | A | G | Medium | ||
Excluded studies (6): exposure measured after development of outcome: James-Todd et al. (2012), Lind et al. (2012), Svensson et al. (2011), Castro-Correia et al. (2018), Dong et al. (2017), Piecha et al. (2016).
G =good; A =adequate; A/D =adequate for short-chain phthalates, deficient for long chain phthalates.
3.1.2. Results
In Sun et al. (2014), there were statistically significant strong positive associations between increased exposure to DBP and DIBP and diabetes risk (Table 3), however pooled results were not available and the positive associations were observed only in participants from NHSII, which is a more homogenous and younger cohort (mean age = 46 vs. 66) with higher exposure levels. In addition, the association was stronger when adjusting for BMI. It is possible that adiposity could lie on the causal pathway between phthalate exposure and diabetes risk, and this should be considered when interpreting the effect estimates. Results were similar for DEHP, but the positive association was smaller and not statistically significant. For BBP and DEP, no association was reported, and no results were available for DINP, so the evidence for these three phthalates is considered indeterminate.
Table 3.
Association between phthalate metabolites and type 2 diabetes in Sun et al. (2014).
| Phthalate (metabolite) | NHS OR (95% CI) | NHSII OR (95% CI) | Pooled OR (95% CI) for Q4 vs. Q1 |
|---|---|---|---|
|
| |||
| DEHP (summed metabolites) | ∑DEHP | ∑DEHP | MEOHP |
| Q2: 0.88 (0.52,1.50) | Q2: 1.80 (1.07,3.04) | 1.42 (0.95,2.11) | |
| Q3: 1.02 (0.61,1.71) | Q3: 1.62 (0.95,2.76) | ||
| Q4: 1.34 (0.77,2.30) | Q4: 1.91 (1.04,3.49) | ||
| Ptrend=0.1 | Ptrend=0.2 | ||
|
| |||
| DBP+DIBP (MBP+MIBP) | Q2: 1.26 (0.75,2.12) | Q2: 1.38 (0.81,2.35) | n/a |
| Q3: 1.01 (0.59,1.73) | Q3: 1.17 (0.66,2.10) | ||
| Q4: 0.91 (0.50,1.68) | Q4: 3.16 (1.68,5.95) | ||
| Ptrend=0.5 | Ptrend=0.0002 | ||
|
| |||
| DBP (MBP) | n/a | Q2: 1.53 (0.90,2.61) | n/a |
| Q3: 1.18 (0.67,2.09) | |||
| Q4: 3.16 (1.69,5.92) | |||
| Ptrend =0.0003 | |||
|
| |||
| DIBP (MIBP) | n/a | Q2: 1.28 (0.76,2.13) | n/a |
| Q3: 1.12 (0.62,2.02) | |||
| Q4: 2.67 (1.44,4.95) | |||
| Ptrend =0.001 | |||
|
| |||
| BBP (MBzP) | Q2: 0.91 (0.55,1.51) | Q2: 0.85 (0.50,1.44) | 0.96 (0.65,1.43) |
| Q3: 0.85 (0.51,1.40) | Q3: 1.08 (0.62,1.86) | ||
| Q4: 0.82 (0.48,1.43) | Q4: 1.14 (0.65,2.01) | ||
| Ptrend=0.5 | Ptrend=0.4 | ||
|
| |||
| DEP (MEP) | Q2: 1.13 (0.69,1.84) | Q2: 1.00 (0.60,1.67) | 0.80 (0.56,1.16) |
| Q3: 0.98 (0.60,1.61) | Q3: 0.46 (0.26,0.82) | ||
| Q4: 0.72 (0.43,1.20) | Q4: 0.91 (0.54,1.54) | ||
| Ptrend=0.09 | Ptrend=0.97 | ||
Exposure levels presented in paper as medians of each quartile.
Results that support an association are shaded. Dark grey represents one or more of the following: p < 0.05, large effect size (e.g., OR ≥1.5), or exposure-response trend. Light grey represents other supportive results.
3.2. Insulin resistance
3.2.1. Study selection and evaluation
Three of the 21 publications identified in the search with data on insulin resistance were excluded due to exposure measurement in tissue other than urine or other critical deficiencies. Of the remaining 18 publications, six were based on data from the National Health and Nutrition Examination Survey (NHANES), with different samples but overlapping populations in many. Among the NHANES analyses in adults, one was selected as the primary study (Huang et al., 2014) and the results of the others were reviewed for discrepancies in methods or results. Two NHANES analyses in adolescents did not have overlapping years and were included separately in the review of data in adolescents (Trasande et al., 2013; Attina and Trasande, 2015). Thus, there were a total of 13 studies in the review. The specific phthalates examined in these included studies and the evaluations are summarized in Table 4. Rationales for ratings are available at: https://hawcprd.epa.gov/summary/visual/100000076/.
Table 4.
Epidemiology studies of insulin resistance (James-Todd et al., 2016a; Lin et al., 2016; Stahlhut et al., 2007).
| Reference | Study description | Includes metabolites of: | Study evaluation | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||||
| Population | Exposure | Outcome | DEHP | DINP | DBP | DIBP | BBP | DEP | Exposure | Outcome | Selection | Confounding | Analysis | Overall | confidence | ||
| Included | Studies in Adults | ||||||||||||||||
| Chen et al. (2017); Lin et al. (2016)a | Cross-sectional analysis of cohort in China of adults and adolescents, follow-up from mass urine screening of children (N=786) | Single urine sample collected in AM | Fasting blood glucose and insulin in serum. HOMA-IR calculated. | ✓ | ✓ | ✓ | ✓ | A/D | G | D | A | A | Medium | ||||
|
| |||||||||||||||||
| Dales et al. (2018) | Cross-sectional in Canada (Canadian Health Measures Survey 2009–11) (N=2119 adults) | Single urine sample | Fasting blood glucose and insulin. HOMA-IR calculated | ✓ | ✓ | ✓ | ✓ | ✓ | A/D | G | G | A | A | Medium | |||
|
| |||||||||||||||||
| Dirinck et al. (2015) | Cross-sectional in Belgium (N=123) of obese patients at weight clinic | 24-hour urine collected day prior to visit | Fasting blood glucose and insulin collected in AM. HOMA-IR calculated. | ✓ | ✓ | ✓ | ✓ | ✓ | A/D | A | D | A | A | Low | |||
|
| |||||||||||||||||
|
Attina and Trasande (2015); Huang et al. (2014); James-Todd et al. (2016a); James-Todd et al. (2012); Stahlhut et al. (2007) |
Cross-sectional in U.S. (NHANES). Overlapping samples: 2001–08, 12–80 yrs 2001–10, 20–80 yrs 2001–08, Women 20–79 yrs 1999–2002, 18+ yrs (N=3,083 in Huang et al. (2014)) | Single urine sample | Fasting blood glucose and insulin collected in AM. HOMA-IR calculated. | ✓ | ✓ | ✓ | ✓ | ✓ | A/D | G | G | A | A | Medium | |||
|
| |||||||||||||||||
| Hong et al. (2009) | Cross-sectional in Korea (N=1131 adults) | Single urine sample collected in AM | Fasting glucose and insulin in serum. HOMA-IR calculated. | ✓ | ✓ | A/D | A | D | A | D | Low | ||||||
|
| |||||||||||||||||
| Hong et al. (2016) | Cross-sectional in Korea (N=296 women aged 30–49 yrs) | Single first AM urine sample | Fasting blood glucose and insulin collected in AM. HOMA-IR calculated | ✓ | ✓ | A/D | G | A | D | A | Medium | ||||||
|
| |||||||||||||||||
|
Kim and Hong (2014)
Kim et al. (2013) |
Cohort in Korea (N=560) of older adults (aged 60+) enrolled through community center | Urine samples at up to 5 visits | Fasting glucose and insulin in serum. HOMA-IR calculated. | ✓ | ✓ | G | A | A | G | A | Medium | ||||||
|
| |||||||||||||||||
| Studies in Adolescents | |||||||||||||||||
|
| |||||||||||||||||
| Attina and Trasande (2015) | Cross-sectional in U.S. (NHANES). 2009–12, 12–19 yrs (N=356) | Single urine sample | Fasting blood glucose and insulin collected in AM. HOMA-IR calculated. | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | A/D | G | G | A | A | Medium | ||
|
| |||||||||||||||||
| Trasande et al. (2013) a | Cross-sectional in U.S. (NHANES). 2003–08, 12–19 yrs (N=766) | Single urine sample | Fasting blood glucose and insulin collected in AM. HOMA-IR calculated. | ✓ | ✓ | ✓ | ✓ | ✓ | A/D | G | G | A | A | Medium | |||
|
| |||||||||||||||||
| Studies in Children | |||||||||||||||||
|
| |||||||||||||||||
| Carlsson et al. (2018) | Cross-sectional in Thailand (N=107 healthy children) | Single first AM urine sample | Oral glucose tolerance test; insulin sensitivity index calculated | ✓ | ✓ | ✓ | ✓ | ✓ | A/D | A | D | D | D | Low | |||
|
| |||||||||||||||||
| Kataria et al. (2017) | Cross-sectional pilot in U.S. (N=43 healthy children) | Single first AM urine sample | Fasting blood glucose and insulin collected in AM. HOMA-IR calculated | ✓ | ✓ | D | G | A | A | D | Low | ||||||
|
| |||||||||||||||||
| Saengkaew et al. (2017) | Cross-sectional of overweight/obese children and healthy controls (N=155) | Single AM urine sample | Fasting blood glucose and insulin collected in AM. HOMA-IR calculated | ✓ | A | G | D | D | D | Low | |||||||
|
| |||||||||||||||||
| Watkins et al. (2016) | Birth cohort in Mexico. Subset of offspring (n=250/2,095) had follow-up at 8–14 yrs. | Single maternal sample in 3rd trimester; Single child sample at follow-up | Fasting glucose and insulin in serum. HOMA-IR calculated. | ✓ | ✓b | ✓b | ✓ | ✓ | A/D | A | A | D | A | Medium | |||
Excluded studies (3): exposure measurement based on tissue other than urine: Olsén et al. (2012). Approaches for confounding and analysis unclear, incomplete reporting of results: Smerieri et al. (2015), Milošević et al. (2017).
G = good; A= adequate; D = deficient; A/D= adequate for short chain phthalates, deficient for long chain phthalates.
Included estimates in adolescents.
Study summed DBP and DIBP metabolites, and results discussed with DBP because it contributed a larger amount to the total.
In adults, there were seven studies, including six cross-sectional (Chen et al., 2017; Dales et al., 2018; Dirinck et al., 2015; Huang et al., 2014; Hong et al., 2009; Hong et al., 2016) and one cohort (Kim and Hong, 2014). Three studies reported on adolescents (Chen et al., 2017; Trasande et al., 2013; Attina and Trasande, 2015) and four studies reported on children (Carlsson et al., 2018; Kataria et al., 2017; Saengkaew et al., 2017; Watkins et al., 2016). All used a single urine sample to measure exposure, with the exception of Kim et al. (2013), which collected samples at up to five visits. Eight studies (Chen et al., 2017; Dales et al., 2018; Huang et al., 2014; Hong et al., 2016; Kim and Hong, 2014; Trasande et al., 2013; Attina and Trasande, 2015; Watkins et al., 2016) were classified as medium confidence and the remaining five were classified as low confidence. This set of studies as a whole would be improved with additional studies with repeated exposure measures, and with prospective rather than cross-sectional data collection. However, the strengths of these studies include the wide variety of populations and the availability of data from multiple nationally representative samples, which have large sample sizes and are expected to represent the general population.
3.2.2. Results and synthesis
The majority of available studies examined adult and/or adolescent exposure with concurrent measures of insulin resistance (or repeated measures in the case of Kim et al. (2013)), so these are the focus of this review. Three of the four studies in children were classified as low confidence and the fourth study (Watkins et al., 2016) used an in-utero exposure measure which may not be comparable to the other studies, so results in children are briefly summarized in text only.
Evaluation of the evidence of an association between exposure to DEHP and insulin resistance in adults is based on seven studies (Table 5). Five studies, including four of the five medium confidence studies, reported higher glucose, insulin, and HOMA-IR with higher exposure levels. Results were statistically significant for at least one outcome in four studies and exposure-response gradients were observed for all three outcomes in Huang et al., 2014. However, there is some uncertainty resulting from study limitations. The positive result in Kim et al. (2013) is difficult to interpret because the analysis included subjects with a history of diabetes diagnosis, though this is ameliorated to some extent by an analysis in women adjusting for history of DM and showing consistent results. Additionally, the results in Dirinck et al. (2015) should be interpreted with caution due to the study evaluation concerns. The fact that the latter study had the highest effect estimates could possibly be explained by selection bias, by the specific population (obese patients), or some other factor. In addition, there is potential for residual confounding by diet, but the impact of this is uncertain. While two studies (Huang et al. (2014); Kim et al. (2013)) adjusted for caloric intake, this may not be adequate to adjust for differing exposure levels by food type (e.g., packaged foods). This issue is considered further in the discussion. Despite these limitations, the overall consistency in the direction of the association across studies and the exposure-response gradient observed in a well-conducted and sensitive study increase confidence in the association. In adolescents, only one of the three studies reported higher insulin resistance with higher exposure. The three studies in children found no clear association (Watkins et al., 2016; Carlsson et al., 2018; Kataria et al., 2017).
Table 5.
Association between DEHP metabolites and insulin resistance in adults and adolescents.
| Reference; Study Confidence Rating; N | Median exposure | Exposure IQR | Effect estimate | Fasting glucose | Insulin | HOMA-IR |
|---|---|---|---|---|---|---|
|
| ||||||
| ∑DEHP in Adults | ||||||
|
| ||||||
| Huang et al. (2014); medium; 3,083 | 11.5 μmol/100 g creatinine (women) | 6.5–23 | Median change (95% CI) | Q2: 1.47 (0.3,2.63) | Q2: 1.74 (1.04,2.45) | Q2: 0.49 (0.31,0.66) |
| Q3: 1.75 (0.66,2.84) | Q3: 1.99 (1.31,2.66) | Q3: 0.51 (0.34,0.69) | ||||
| Q4: 2.45 (1.29,3.60) | Q4: 2.60 (1.82,3.38) | Q4: 0.68 (0.47,0.88) | ||||
| Ptrend=0.0016* | Ptrend<0.0001* | Ptrend<0.0001* | ||||
|
| ||||||
| Dales et al. (2016); medium, 2,119 | 47 ng/mL | β (95% CI) for IQR increase | 0.04 (0.00,0.08)* | 0.63 (0.21,1.05)* | 0.15 (0.04,0.26)* | |
|
| ||||||
| MEOHP in Adults | ||||||
|
| ||||||
| Dirinck et al. (2015);low; 123 | 6.2 μg/g creatinine | 0.8–42 (range) | β (95% CI) | 3.88 (−6.19,13.95) | 26.62 (0.69,52.5)* | 0.31 (−0.72,0.69) |
|
| ||||||
| Hong et al. (2016); medium; 296 | 6.5μg/g creatìnine | 3.9–10 | β (95% CI) | 0.12 (−1.11,1.36) | 0.14 (−0.08,0.36) | 0.12 (−0.08,0.33) |
|
| ||||||
| Chen et al. (2017); medium; 552 | 17μg/g creatinine | 16 | β (95% CI) | −0.001 (−0.01,0.01) | 0.003 (−0.07,0.07) | 0.001 (−0.07,0.07) |
|
| ||||||
| Kim et al. (2013) medium; 560 | 19 ng/mL | 9.5–33 | β (95% CI) | 0.11 (0.01,0.22)*Ŧ | 0.70 (0.01,1.40)* Ŧ | 0.26 (0.01,0.51) *Ŧ |
|
| ||||||
| MEOHP in Adolescents | ||||||
|
| ||||||
| Chen et al. (2017); medium; 234 | 17μg/g creatinine | 16 | β (95% CI) | 0.001 (−0.01,0.01) | 0.04 (−0.05,0.12) | 0.04 (−0.05,0.13) |
|
| ||||||
| Attina and Trasande (2015); medium; 356 | 0.14 μM | β (95% CI) | NR | NR | 0.06 (−0.001,0.12) | |
|
| ||||||
| Trasande et al. (2013); medium; 766 | NR | β (95% CI) | NR | NR | 0.27 (0.14,0.40)* | |
Results that support an association are shaded based on effect size and precision. Dark grey represents one or more of the following: p < 0.05, large effect size (e.g., OR ≥1.5, β≥ 0.5), or exposure-response trend. Light grey represents other supportive results. Strength of the evidence (i.e., contribution to the synthesis conclusions) from an individual study is influenced by other factors, such as study confidence.
p < 0.05.
Estimates for total population, including those with history of diabetes. Association for the strata without history of diabetes was not reported.
Hong et al. (2009) reported that no significant association was observed but did not provide quantitative estimates.
One medium confidence study included results on the association between DINP and insulin resistance. Attina and Trasande (2015), an NHANES sample of adolescents, reported increased HOMA-IR levels with increasing exposure, with the third tertile being statistically significant (β for tertile 2: 0.07 (−0.09, 0.22), tertile 3: 0.23 (0.08, 0.38)). In addition, one low confidence study in children (Kataria et al., 2017) reported no significant association, but did not report quantitative estimates.
Evaluation of the evidence of an association between exposure to DBP and insulin resistance in adults is based on seven studies (Table 6). Four studies, including three of the five medium confidence studies (Table 6) reported increased glucose, insulin, and/or HOMA-IR. Results in two of these studies were statistically significant, and an exposure-response gradient was observed for glucose and HOMA-IR in Huang et al. (2014). However, in Dales et al. (2018), a weak association was only observed for insulin. Further, as with DEHP, the positive results in Kim et al. (2013) and Dirinck et al. (2015) are difficult to interpret, and there is potential for residual confounding by diet. The other studies reported no association, resulting in some unexplained inconsistency, including between NHANES and CHMS, the nationally representative surveys for fasting glucose and HOMA-IR. This lack of consistency decreases confidence in the association since there is no clear explanation such as study sensitivity (e.g., exposure levels or range) for the differing results. In adolescents, one of the three studies reported higher insulin resistance with higher exposure (Trasande et al., 2013). The three studies in children (Watkins et al., 2016) found no clear association, though there was a statistically significant increase in fasting glucose among pubertal girls based on peripubertal exposure in Watkins et al. (2016) for summed MBP + MIBP.
Table 6.
Association between DBP metabolites and insulin resistance in adults and adolescents.
| Reference | Median MBP | Exposure IQR | Effect estimate | Fasting glucose | Insulin | HOMA-IR |
|---|---|---|---|---|---|---|
|
| ||||||
| Adults | ||||||
|
| ||||||
| Huang et al. (2014); medium; 3,083 | 22 μg/g creatinine | 13–36 | Median change (95% CI) | Q2: 0.95 (−0.22,2.13) | Q2: 1.15 (0.52,1.78) | Q2: 0.28 (0.11,0.44) |
| Q3: 1.70 (0.51,2.89) | Q3: 1.41 (0.72,2.09) | Q3: 0.28 (0.11,0.46) | ||||
| Q4: 1.91 (0.51,3.31) | Q4: 1.11 (0.31,1.92) | Q4: 0.34 (0.15,0.54) | ||||
| ptrend=0.019* | ptrend<0.09 | Ptrend<0.006* | ||||
|
| ||||||
| Hong et al. (2016); medium; 296 | 30 μg/g creatinine | 21–42 | β (95% CI) | −0.37 (−1.38,0.65) | 0.06 (−0.13,0.24) | 0.04 (−0.13,0.21) |
|
| ||||||
| Dales et al. (2016); medium, 2,119 | 31 ng/mL | β (95% CI) for IQR increase | 0.00 (−0.06,0.06) | 0.47 (−0.02,0.96) | 0.08 (−0.09,0.24) | |
|
| ||||||
| Chen et al. (2017); medium; 552 | 38 μg/g creatinine | 41 | β (95% CI) | 0.01 (−0.002,0.01) | 0.01 (−0.06,0.08) | 0.02 (−0.05,0.09) |
|
| ||||||
| Dirinck et al. (2015); low; 123 | 38 μg/g creatinine | 3.9–370 (range) | β (95% CI) | 7.27 (−1.29,15.83) | 22.52 (3.13,41.90)* | 0.29 (−0.05,0.63) |
|
| ||||||
| Kim et al. (2013); medium; 560 | 57 ng/mL | 29–158 | β (95% CI) | 0.06 (−0.04,0.17)Ŧ | 0.38 (−0.30,1.07)Ŧ | 0.16 (−0.09,0.40)Ŧ |
|
| ||||||
| Adolescents | ||||||
|
| ||||||
| Chen et al. (2017); medium; 234 | 38 μg/g creatinine | 41 | β (95% CI) | 0.01 (−0.001,0.02) | 0.002 (−0.08,0.08) | 0.01 (−0.07,0.10) |
|
| ||||||
| Attina and Trasande (2015); medium; 356 | NR | β (95% CI) | NR | NR | 0.05 (−0.02,0.12) | |
|
| ||||||
| Trasande et al. (2013); medium; 766 | NR | β (95% CI) | NR | NR | 0.13 (0.01,0.26)* | |
Results that support an association are shaded based on effect size and precision. Dark grey represents one or more of the following: p < 0.05, large effect size (e.g., OR≥ 1.5, β ≥ 0.5), or exposure-response trend. Light grey represents other supportive results. Strength of the evidence (i.e., contribution to the synthesis conclusions) from an individual study is influenced by other factors, such as study confidence.
p < 0.05.
Estimates for total population, including those with history of diabetes. Association for the strata without history of diabetes was not reported.
Hong et al. (2009) reported that no significant association was observed, but did not provide quantitative estimates.
Three studies reported results on the association between DIBP exposure and insulin resistance in adults (Table 7). One of two medium confidence studies and one low confidence study reported higher glucose, insulin, and HOMA-IR with higher exposure, with Huang et al. (2014) reporting statistical significance for HOMA-IR and exposure-response gradients for glucose and HOMA-IR. However, as with DBP, there was inconsistency between the nationally representative surveys with no clear explanation and the results from Dirinck et al. (2015) have low confidence, and the small number of studies makes evaluating consistency a challenge. As with the other phthalates, there is potential for residual confounding by diet. In adolescents, one of the two studies reported higher insulin resistance with higher exposure (Trasande et al., 2013). Results for BBP (Table 8) and DEP (Table 9) were similar to those of DIBP, though there was no association in adolescents for these phthalates. The studies in children (Watkins et al., 2016) found no clear association for BBP and DEP, though there was a statistically significant increase in fasting glucose among pubertal girls based on peripubertal exposure for DEP.
Table 7.
Association between DIBP metabolites and insulin resistance in adults and adolescents.
| Reference | Median MIBP | Exposure IQR | Effect estimate | Fasting glucose | Insulin | HOMA-IR |
|---|---|---|---|---|---|---|
|
| ||||||
| Adults | ||||||
|
| ||||||
| Huang et al. (2014); medium; 3,083 | 4.9 μg/g creatinine (women) | 2.6–8.9 | Median change (95% CI) | Q2: 1.87 (0.83,2.92) | Q2: 1.45 (0.85,2.04) | Q2: 0.38 (0.23,0.52) |
| Q3: 2.77 (1.75,3.80) | Q3: 1.23 (0.57,1.89) | Q3: 0.35 (0.19,0.51) | ||||
| Q4: 3.69 (2.60,4.78) | Q4: 1.73 (0.92,2.54) | Q4: 0.53 (0.33,0.72) | ||||
| Ptrend<0.0001 | Ptrend=0.003 | ptrend=0.0002* | ||||
|
| ||||||
| Dales et al. (2016); medium, 2,119 | 13 ng/mL | β (95% CI) for IQR increase | 0.00 (−0.02,0.03) | 0.00 (−0.19,0.19) | 0.00 (−0.05,0.05) | |
|
| ||||||
| Dirinck et al. (2015); low; 123 | 57 μg/g creatinine | 7.5–55 (range) | β (95% CI) | 5.44 (−3.57,14.5) | 7.69 (−15.9,31.3) | 0.15 (−0.18,0.47) |
|
| ||||||
| Adolescents | ||||||
|
| ||||||
| Attina and Trasande (2015); medium; 356 | NR | β (95% CI) | NR | NR | 0.03 (−0.05,0.11) | |
|
| ||||||
| Trasande et al. (2013); medium; 766 | NR | β (95% CI) | NR | NR | 0.15 (0.04, 0.26)* | |
Results that support an association are shaded based on effect size and precision. Dark grey represents one or more of the following: p < 0.05, large effect size (e.g., OR≥ 1.5, β≥ 0.5), or exposure-response trend. Light grey represents other supportive results. Strength of the evidence (i.e., contribution to the synthesis conclusions) from an individual study is influenced by other factors, such as study confidence.
p < 0.05.
Estimates for toal population, including those with history of diabetes. Association for the strata without history of diabetes was not reported.
Table 8.
Association between BBP metabolites and insulin resistance in adults and adolescents.
| Reference | Median MBzP | Exposure IQR | Effect estimate | Fasting glucose | Insulin | HOMA-IR |
|---|---|---|---|---|---|---|
|
| ||||||
| Adults | ||||||
|
| ||||||
| Chen et al. (2017); medium; 552 | 1.9 μg/g creatinine | 2.4 | β (95% CI) | −0.002 (−0.01,0.004) | −0.05 (−0.12,0.01) | −0.06 (−0.12,0.01) |
|
| ||||||
| Dales et al. (2016); medium, 2,119 | 8.4 ng/mL | β (95% CI) for IQR increase | 0.00 (−0.02,0.02) | 0.03 (−0.20,0.26) | 0.00 (−0.06,0.06) | |
|
| ||||||
| Huang et al. (2014); medium; 3,083 | 13 μg/g creatinine (women) | 7.1–24 | Median change (95% CI) | Q2: −0.30 (−1.48,0.87) | Q2: 0.77 (0.16,1.39) | Q2: 0.21 (0.06,0.37) |
| Q3: −0.06 (−1.25,1.13) | Q3: 1.09 (0.39,1.79) | Q3: 0.26 (0.09,0.44) | ||||
| Q4: −0.24 (−1.49,1.02) | Q4: 1.44 (0.50,2.38) | Q4: 0.37 (0.15,0.59) | ||||
| ptrend=0.7 | ptrend=0.007* | ptrend<0.003* | ||||
|
| ||||||
| Dirinck et al. (2015); low; 123 | 8.7 μg/g creatinine | 0.7–90 (range) | β (95% CI) | 8.74 (1.42,16.06)* | 14.90 (4.38,34.18) | 0.15 (−0.16,0.46) |
|
| ||||||
| Adolescents | ||||||
|
| ||||||
| Chen et al. (2017); medium; 234 | 1.9 μg/g creatinine | 2.4 | β (95% CI) | −0.002 (−0.01,0.01) | 0.03 (−0.06,0.13) | 0.03 (−0.07,0.13) |
|
| ||||||
| Attina and Trasande (2015); medium; 356 | NR | β (95% CI) | NR | NR | 0.03 (−0.03,0.09) | |
|
| ||||||
| Trasande et al. (2013); medium; 766 | NR | β (95% CI) | NR | NR | 0.02 (−0.08,0.13) | |
Results that support an association are shaded based on effect size and precision. Dark grey represents one or more of the following: p < 0.05, large effect size (e.g., OR≥ 1.5, β≥ 0.5), or exposure-response trend. Light grey represents other supportive results. Strength of the evidence (i.e., contribution to the synthesis conclusions) from an individual study is influenced by other factors, such as study confidence.
p < 0.05.
Estimates for total population, including those with history of diabetes. Association for the strata without history of diabetes was not reported.
Table 9.
Association between DEP metabolites and insulin resistance in adults and adolescents.
| Reference | Median MEP | Exposure IQR | Effect estimate | Fasting glucose | Insulin | HOMA-IR |
|---|---|---|---|---|---|---|
|
| ||||||
| Adults | ||||||
|
| ||||||
| Dales et al. (2016); medium, 2,119 | 8.4 ng/mL | β (95% CI) for IQR increase | 0.00 (−0.02,0.03) | 0.00 (−0.27,0.27) | −0.01 (−0.08,0.06) | |
|
| ||||||
| Chen et al. (2017); medium; 552 | 32 μg/g creatinine | 63 | β (95% CI) | 0.00 (−0.01,0.01) | −0.04 (−0.10,0.01) | −0.04 (−0.10,0.01) |
|
| ||||||
| Huang et al. (2014); medium; 3,083 | 182 μg/g creatinine (women) | 89–426 | Median change (95% CI) | Q2: 1.30 (0.15,2.46) | Q2: 0.25 (−0.50,0.99) | Q2: 0.11 (−0.06,0.27) |
| Q3: 0.38 (−0.75,1.51) | Q3: 0.34 (−0.35,1.02) | Q3: 0.10 (−0.07,0.28) | ||||
| Q4: 0.49 (−0.80,1.77) | Q4: 0.60 (−0.13,1.34) | Q4: 0.20 (0.03,0.38) | ||||
| ptrend=0.8 | ptrend=0.15 | ptrend<0.006* | ||||
|
| ||||||
| Dirinck et al. (2015); low; 123 | 81 μg/g creatinine | 3.4–6865 (range) | β (95% CI) | 1.31 (−3.23,5.85) | 9.81 (−1.88,21.5) | 0.15 (−0.022,0.31) |
|
| ||||||
| Adolescents | ||||||
|
| ||||||
| Chen et al. (2017); medium; 234 | 32 μg/g crea”nine | 63 | β (95% CI) | 0.001 (−0.01,0.01) | −0.02 (−0.08,0.05) | −0.02 (−0.08,0.05) |
|
| ||||||
| Attina and Trasande (2015); medium; 356 | NR | β (95% CI) | NR | NR | 0.02 (−0.05,0.05) | |
|
| ||||||
| Trasande et al. (2013); medium; 766 | NR | β (95% CI) | NR | NR | −0.07 (−0.16,0.03) | |
Results that support an association are shaded based on effect size and precision. Dark grey represents one or more of the following: p < 0.05, large effect size (e.g., OR≥1.5, β≥0.5), or exposure-response trend. Light grey represents other supportive results. Strength of the evidence (i.e., contribution to the synthesis conclusions) from an individual study is influenced by other factors, such as study confidence.
p < 0.05.
Estimates for total population, including those with history of diabetes. Association for the strata without history of diabetes was not reported.
Overall, across the phthalates, there is generally a stronger association in adults than adolescents and children. Because there was a smaller number of studies of the younger age groups, and there were limitations in the studies that are available, including a lack of exposure information, it is unclear whether this is a true difference and whether it can be explained by changing exposure levels, biological factors, or some other explanation. In addition to the main effects described above, some of the analyses of NHANES data examined differences in the association by sub-population. In Huang et al. (2014), these factors did not generally change the direction of association, but there were differences in strength of association by race/ethnicity and sex. For some phthalates, the association was stronger in black and Hispanic people than in whites. The sex with the stronger association differed by phthalate. Since there were no additional studies that examined these differences, there was limited ability to draw conclusions about the interactions.
3.3. Gestational diabetes
3.3.1. Study selection and evaluation
Two of the six epidemiology studies identified in the search with data on diabetes and/or insulin resistance during pregnancy were excluded due to exposure measurement in tissue other than urine or unavailability in full text. The specific phthalates examined in the remaining four included studies and the evaluations are summarized in Table 10. Rationales for ratings are available at: https://hawcprd.epa.gov/summary/visual/100500026/. All four studies were pregnancy cohorts, with exposure measurement during pregnancy. Two studies collected more than one urine sample (James-Todd et al., 2016b; James-Todd et al., 2018). One study was considered high confidence (James-Todd et al., 2018), two medium confidence (James-Todd et al., 2016b; Shapiro et al., 2015), and one low confidence (Robledo et al., 2015). Looking across the studies, this set of cohorts is generally well-conducted with low risk of bias, but the exposure levels trend slightly lower than in other studies, so sensitivity to observe an effect may be lower.
Table 10.
Epidemiology studies of gestational diabetes and blood glucose in pregnancy.
| Reference | Study description | Includes metabolites of: | Study evaluation | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||||
| Population | Exposure | Outcome | DEHP | DINP | DBP | DIBP | BBP | DEP | Exposure | Outcome | Selection | Confounding | Analysis | Overall | confidence | ||
| Included | Studies in Adults | ||||||||||||||||
|
| |||||||||||||||||
| James-Todd et al. (2016b) | Sample of women from pregnancy cohort in U.S. (N=352) | Urine samples collected at up to 4 prenatal visits | Impaired glucose tolerance and blood glucose during oral glucose tolerance test | ✓ | ✓ | ✓ | ✓ | G/A | G | A | A | A | Medium | ||||
|
| |||||||||||||||||
| James-Todd et al. (2018) | Cohort of women seeking infertility evaluation/ treatment in U.S. (N=245) | Urine samples collected in each trimester | Impaired glucose tolerance and blood glucose during oral glucose tolerance test | ✓ | ✓ | ✓ | ✓ | ✓ | G/A | G | G | G | G | High | |||
|
| |||||||||||||||||
| Robledo et al. (2015) | Pilot cohort of pregnant women in U.S. (N=72) enrolled at first prenatal visit | Single urine sample | Blood glucose during oral glucose tolerance test. | ✓ | ✓ | ✓ | ✓ | ✓ | A/D | D | A | A | A | Low | |||
|
| |||||||||||||||||
| Shapiro et al. (2015) | Cohort of pregnant women in U.S. (N=1,885) enrolled in first trimester | Single urine sample | Gestational diabetes and impaired glucose tolerance diagnosed from glucose challenge test | ✓ | ✓ | ✓ | ✓ | A/D | A | A | A | A | Medium | ||||
Excluded studies (2): exposure measurement based on tissue other than urine and high percent non-detects for secondary metabolites of DEHP: Fisher et al. (2018); unable to obtain full text: Zhang et al. (2017).
G = good; A= adequate; D = deficient; A/D= adequate for short chain phthalates, deficient for long chain phthalates.
Included estimates in adolescents.
3.3.2. Results and synthesis
Among the four studies that examined blood glucose and/or impaired glucose tolerance in pregnant women, there was no clear indication of an increase in blood glucose with increased phthalate exposure for DEHP (Table 11), DBP, and DIBP (Table 12). For BBP (Table 13), there were two studies indicating higher blood glucose or impaired glucose tolerance with higher exposure, but neither was statistically significant (James-Todd et al., 2016b; Shapiro et al., 2015). For DEP (Table 13), two studies reported statistically significant associations (James-Todd et al. (2018) for blood glucose and James-Todd et al. (2016b) for impaired glucose tolerance), but the other two studies reported no association. Across the phthalates, with the exception of DEP, the high confidence study (James-Todd et al., 2018) reported no association. This could be due to the lower exposure levels in the study population, which may have reduced study sensitivity.
Table 11.
Association between DEHP and blood glucose in pregnant women.
| Reference | Median Exposure | Exposure IQR | Blood glucose 1st trimester Mean (95% CI) | Blood glucose 2nd trimester Mean (95% CI) | Impaired glucose tolerance* OR (95%) | |
|---|---|---|---|---|---|---|
| DEHP | James-Todd et al., (2016b) medium; 352 | 0.4 nmol/l (GM) | 0.2–0.8 | Q1: 110 (100,120) | Q1: 117 (106,129) | Q2: 0.46 (0.15,1.40) |
| Q2: 115 (105, 127) | Q2: 110 (100,121) | Q3: 0.74 (0.27,2.04) | ||||
| Q3: 108 (98,118) | Q3: 109 (99,120) | Q4: 0.25 (0.08,0.85) | ||||
| Q4: 114 (104, 126) | Q4: 110 (100,121) | p-trend: 0.06 | ||||
| p-trend: 0.16 | ||||||
|
| ||||||
| James-Todd et al. (2018) high; 245 | 0.2 nmol/l | 0.1–0.4 | Q1: 116 (109,123) | Q1: 111 (105,118) | no association | |
| Q2: 114 (107,121) | Q2: 111 (105,118) | |||||
| Q3: 114 (107,121) | Q3: 115 (109,122) | |||||
| Q4: 113 (107,120) | Q4: 116 (109, 123) | |||||
|
| ||||||
| Shapiro et al. (2015) medium; 1,885 | 7.4 (MEOHP) | NR | NR | NR | Q2: 1.0 (0.5,2.0) | |
| 11 (MEHHP) | Q3: 0.6 (0.3,1.5) | |||||
| 2.6 (MEHP) ng/mL | Q4: 0.9 (0.4,2.3) | |||||
|
| ||||||
| Robledo et al. (2015) low; 72 | 66 ng/mL | 37–101 | NR | β (95% CI) | NR | |
| T2: 3.82 (−10.22,17.86) | ||||||
| T3: −9.97 (−27.11,7.17) | ||||||
Results that support an association are shaded based on effect size and precision.
Shapiro includes impaired glucose tolerance and gestational diabetes.
p-Values >0.20 are not presented. NR= not reported.
Table 12.
Association between DBP and DIBP and blood glucose in pregnant women.
| Reference | Median Exposure (ng/mL) | Exposure IQR | Blood glucose 1st trimester Mean (95% CI) | Blood glucose 2nd trimester Mean (95% CI) | Impaired glucose tolerance* OR (95%) | |
|---|---|---|---|---|---|---|
| DBP | James-Todd et al., (2016b) medium; 352 | 18 (GM) | 11–27 | Q1: 115 (104,126) | Q1: 111 (100,123) | Q2: 0.95 (0.31,2.91) |
| Q2: 113 (103,124) | Q2: 113 (103,125) | Q3: 1.26 (0.43,3.68) | ||||
| Q3: 114 (103,125) | Q3: 114 (104,125) | Q4: 1.14 (0.37,3.51) | ||||
| Q4: 109 (99,119) | Q4: 108 (98,119) | |||||
| p-trend: 0.13 | ||||||
|
| ||||||
| James-Todd et al. (2018) high; 245 | 12 | 6.8–21 | Q1: 116 (109,123) | Q1: 112 (106,119) | no association | |
| Q2: 112 (105,118) | Q2: 113 (106,120) | |||||
| Q3: 116 (109, 123) | Q3: 114 (107,121) | |||||
| Q4: 113 (107,120) | Q4: 115 (109,122) | |||||
|
| ||||||
| Shapiro et al. (2015) medium; 1,885 | 13 | NR | NR | NR | Q2: 1.8 (0.9,3.6) | |
| Q3: 1.3 (0.6,3.0) | ||||||
| Q4: 0.8 (0.3,2.2) | ||||||
| p-trend: 0.51 | ||||||
|
| ||||||
| Robledo et al. (2015) low; 72 | 29 | 16–57 | NR | β (95% CI) | NR | |
| T2: 0.11 (−14.71,14.93) | ||||||
| T3: −15.61 (−32.99,1.76) | ||||||
|
| ||||||
| DIBP | James-Todd et al., (2016b) medium; 352 | 7.3 (GM) | 4.5–12 | Q1: 114 (103,125) | Q1: 111 (100,122) | Q2: 0.47 (0.14,1.58) |
| Q2: 111 (101,123) | Q2: 109 (99,120) | Q3: 1.16 (0.38,3.54) | ||||
| Q3: 114 (103,125) | Q3: 110 (100,121) | Q4: 1.79 (0.62,5.16) | ||||
| Q4: 110 (100,121) | Q4: 114 (104,126) | p-trend: 0.18 | ||||
| p-trend: 0.17 | ||||||
|
| ||||||
| James-Todd et al. (2018) high; 245 | 6.2 | 3.3–10 | Q1: 118 (111,125) | Q1: 119 (113,126) | 0.37 (0.12,1.16) | |
| Q2: 114 (107,121) | Q2: 115 (109,122) | |||||
| Q3: 114 (107,121) | Q3: 115 (109, 122) | |||||
| Q4: 111 (104,117) | Q4: 105 (99,111) | |||||
| p-trend: 0.15 | p-trend: 0.003 | |||||
|
| ||||||
| Robledo et al. (2015) low; 72 | 11 | 6.7–18 | NR | β (95% CI) | NR | |
| T2: −2.01 (−16.18,12.17) | ||||||
| T3: −18.30 (−35.41,−1.19) | ||||||
Results that support an association are shaded based on effect size and precision.
Shapiro includes impaired glucose tolerance and gestational diabetes.
p-Values >0.20 are not presented. NR= not reported.
Table 13.
Association between BBP and DEP and blood glucose in pregnant women.
| Reference | Median Exposure (ng/mL or as specified) | Exposure IQR | Blood glucose 1st trimester Mean (95% CI) | Blood glucose 2nd trimester Mean (95% CI) | Impaired glucose tolerance* OR (95%) | |
|---|---|---|---|---|---|---|
| BBP | James-Todd et al. (2016b) medium; 352 | 7.4 (GM) | 3.3–15 | Q1: 108 (98,119) | Q1: 106 (96,118) | Q2: 1.17 (0.40,3.48) |
| Q2: 118 (108,130) | Q2: 113 (102,126) | Q3: 1.16 (0.39,3.49) | ||||
| Q3: 112 (102, 122) | Q3: 114 (103,125) | Q4: 1.28 (0.40,4.07) | ||||
| Q4: 111 (102,122) | Q4: 110 (101,121) | |||||
|
| ||||||
| James-Todd et al. (2018) high; 245 | 2.8 | 1.4–5.4 | Q1: 113 (107,120) | Q1: 111 (105,118) | no association | |
| Q2: 117 (110,124) | Q2: 117 (110,124) | |||||
| Q3: 110 (103,116) | Q3: 110 (104,117) | |||||
| Q4: 117 (110,124) | Q4: 116 (109,123) | |||||
|
| ||||||
| Shapiro et al. (2015) medium; 1,885 | 5.8 | NR | NR | NR | Q2: 1.3 (0.6,2.8) | |
| Q3: 2.0 (0.9, 4.4) | ||||||
| Q4: 2.0 (0.9,4.8) | ||||||
| p-trend: 0.07 | ||||||
|
| ||||||
| Robledo et al. (2015) low; 72 | 16 | 8.5–37 | NR | β (95% CI) | NR | |
| T2: −0.82 (−14.37,12.73) | ||||||
| T3: −17.26 (−34.12,−0.40) | ||||||
|
| ||||||
| DEP | James-Todd et al., (2016b) medium; 352 | 133 (GM) | 49–325 | Q1: 113 (103,125) | Q1: 109 (100,120) | Q2: 2.14 (0.57,8.10) |
| Q2: 114 (103,125) | Q2: 112 (102,124) | Q3: 2.53 (0.71,8.97) | ||||
| Q3: 115 (105,126) | Q3: 110 (99,121) | Q4: 7.18 (1.97,26.15) | ||||
| Q4: 107 (98,118) | Q4: 116 (105,128) | p-trend: <0.01 | ||||
| p-trend: 0.19 | ||||||
|
| ||||||
| James-Todd et al. (2018) high; 245 | 48 | 22–144 | Q1: 114 (108,121) | Q1: 109 (103,116) | 2.48 (0.86,7.20) | |
| Q2: 115 (108,122) | Q2: 113 (107,120) | |||||
| Q3: 109 (103,116) | Q3: 111 (105,117) | |||||
| Q4: 118 (111,125) | Q4: 121 (114,128) | |||||
| p-trend: 0.02 | ||||||
|
| ||||||
| Shapiro et al. (2015) medium; 1,885 | 39 | NR | NR | NR | Q2: 1.0 (0.5,2.0) | |
| Q3: 0.8 (0.4,1.7) | ||||||
| Q4: 0.7 (0.3,1.5) | ||||||
| p-trend: 0.29 | ||||||
|
| ||||||
| Robledo et al. (2015) low; 72 | 206 | 92–525 | NR | β (95% CI) | NR | |
| T2: 4.45 (−9.30,18.19) | ||||||
| T3: 0.18 (−14.57,14.94) | ||||||
Results that support an association are shaded based on effect size and precision.
Shapiro includes impaired glucose tolerance and gestational diabetes.
p-Values >0.20 are not presented. NR= not reported.
3.4. Diabetes risk summary
For each phthalate, Table 14 presents a summary of the key factors that increased or decreased confidence in the evidence, a summary of the findings, and an overall judgment of the strength of evidence that considers coherence across the two main outcomes (i.e., type 2 diabetes and insulin resistance), focusing on the studies in adults. For DEHP exposure, there was consistency and an exposure-response gradient in one study for insulin resistance and coherence across diabetes and insulin resistance, though the results for diabetes were not statistically significant. Given the overall consistency, but some lingering uncertainty in the evidence, this evidence is considered moderate.
Table 14.
Summary of evidence for diabetes risk in adults.
| Phthalate | Outcome | Studies | Factors that increase confidence | Factors that decrease confidence | Summary of findings | Evidence judgment |
|---|---|---|---|---|---|---|
|
| ||||||
| DEHP | Diabetes | Medium confidence Sun et al. (2014) (nested CC in two C) | • Study well conducted | • Potential for residual confounding by diet • Single study • Imprecision |
Positive association between DEHP exposure and type 2 diabetes diagnosis (not statistically significant, Table 3). | ⊕⊕⚪ MODERATE for diabetes risk |
| Insulin resistance in adults | Medium confidence Chen et al. (2017) (CS) Dales et al. (2018) (CS) Hong et al. (2016) (CS) Huang et al. (2014) (CS) Kim et al. (2013) (C) Low confidence Dirinck et al. (2015) (CS) Hong et al. (2009) (CS) |
Among medium confidence studies: • Exposure-response gradient observed in one study • Generally consistent results across populations • Minimal concerns for bias |
• Potential for residual confounding by diet | Positive association between DEHP exposure and glucose, insulin and/or HOMA-IR in 5 studies (Dales et al., 2018; Dirinck et al., 2015; Huang et al., 2014; Kim et al., 2013; Hong et al., 2016), with statistical significance in four (Table 5). Exposure-response gradients were reported in Huang et al. (2014) for all endpoints (not evaluated in other studies) | Supported by coherence across outcomes and plausible mechanism from animal and in vitro studies | |
| DINP | Insulin resistance in adolescents | Medium confidence Attina and Trasande (2015) (CS) |
• Minimal concerns for bias • Exposure-response gradient |
• Potential for residual confounding by diet • Single study |
Positive association between DINP exposure and HOMA-IR, with statistical significance in the third tertile. | ⊕⚪⚪ SLIGHT |
| DBP | Diabetes | Medium confidence Sun et al. (2014) (nested CC in two C) |
• Strong association • Study well conducted |
• Potential for residual confounding by diet • Single study |
Strong positive (OR=3.2 for Q4) statistically significant association between DBP exposure and type 2 diabetes diagnosis (Table 3). | ⊕⊕⚪ MODERATE |
| Insulin resistance in adults | Medium confidence Chen et al. (2017) (CS) Dales et al. (2018) (CS) Hong et al. (2016) (CS) Huang et al. (2014) (CS) Kim et al. (2013) (C) Low confidence Dirinck et al. (2015) (CS) Hong et al. (2009) (CS) |
Among medium confidence studies: • Exposure-response gradient observed in one study • Minimal concerns for bias |
• Potential for residual confounding by diet | Positive association between DBP exposure and at least one measure of insulin resistance (i.e., glucose, insulin and/or HOMA-IR) in 4 studies (Huang et al., 2014, Dirinck et al., 2015, Dales et al., 2018, Kim et al., 2013), with statistical significance in 2 (Table 6). An exposure-response gradient was reported in Huang et al. (2014) for glucose (not evaluated in other studies). | Supported by coherence across outcomes and plausible mechanism from animal and in vitro studies | |
| DIBP | Diabetes | Medium confidence Sun et al. (2014) (nested CC in two C) |
• Strong association • Study well conducted |
• Potential for residual confounding by diet • Single study |
Strong positive (OR=2.7 for Q4) statistically significant association between DIBP exposure and type 2 diabetes diagnosis (Table 3). | ⊕⊕⚪ MODERATE |
| Insulin resistance in adults | Medium confidence Dales et al. (2018) (CS) Huang et al. (2014) (CS) Low confidence Dirinck et al. (2015) (CS) |
• Exposure-response gradient observed in one study | • Potential for residual confounding by diet • Few studies |
Positive association between DIBP exposure and glucose, insulin, and/or HOMA-IR in 2 studies (Huang et al., 2014) (Dirinck et al., 2015) (Table 7). Huang et al. (2014) reported statistical significance for all endpoints and an exposure-response gradient for glucose (not evaluated in other study) | Supported by coherence across outcomes and plausible mechanism from animal and in vitro studies | |
| BBP | Diabetes | Medium confidence Sun et al. (2014) (nested CC in two C) |
• Study well conducted | • Potential for residual confounding by diet • Single study |
No association reported between type 2 diabetes risk and BBP exposure. | ⊕⚪⚪ SLIGHT |
| Insulin resistance in adults | Medium confidence Chen et al. (2017) (CS) Dales et al. (2018) (CS) Huang et al. (2014) (CS) Low confidence Dirinck et al. (2015) (CS) |
• Exposure-response gradient observed in one study | • Potential for residual confounding by diet • Few studies • Inconsistency among medium confidence studies |
Positive association between BBP exposure and glucose, insulin, and/or HOMA-IR in 2 studies (Dirinck et al., 2015; Huang et al., 2014) (Table 8). Exposure-response gradients were observed for insulin and HOMA-IR in Huang et al. (2014) (not evaluated in other study). | ||
| DEP | Diabetes | Medium confidence Sun et al. (2014) (nested CC in two C) |
• Study well conducted | • Potential for residual confounding by diet • Single study |
No association reported between type 2 diabetes risk and DEP exposure. | ⊕⚪⚪ SLIGHT |
| Insulin resistance in adults | Medium confidence Chen et al. (2017) (CS) Dales et al. (2018) (CS) Huang et al. (2014) (CS) Low confidence Dirinck et al. (2015) (CS) |
• Exposure-response gradient observed in one study • Consistent results across populations |
• Potential for residual confounding by diet • Few studies |
Positive association between DEP exposure and glucose, insulin and/or HOMA-IR in 2 studies (Huang et al, 2014) (Dirinck et al., 2015) (Table 9). Exposure-response gradients were observed for insulin and HOMA-IR in (Huang et al., 2014) (not evaluated in other study). | ||
C=cohort; CC=case-control; CS=cross-sectional.
For DBP and DIBP exposure, in contrast, the overall evidence is considered moderate primarily due to the strong positive associations in the single diabetes study. There is substantial unexplained inconsistency in the results for insulin resistance, but some of the results, including exposure-response gradients in one well-conducted study, provide coherence with the incident diabetes results.
For DINP, BBP, and DEP, the evidence is considered slight. No association was reported in the single study of diabetes with BBP and DEP exposure (DINP was not investigated). The available evidence does indicate an association between exposure to these phthalates and insulin resistance, including in pregnant women, but the small number of studies and the lack of coherence with diabetes decreases confidence.
3.5. Obesity
A prior review concluded that the data on obesity and phthalate exposure is inconsistent (Thayer et al., 2012). We conducted a screening level review encompassing these and more recent studies. Twenty-five of the 32 epidemiology studies (described in 35 publications) identified in the search with data on obesity or BMI were excluded (Supplementary Table). The majority were excluded due to issues with temporality (i.e., exposure was measured at the same time as BMI), or due to measurement of exposure in tissues other than urine. Six of the remaining studies focused on obesity in children or infants based on prenatal or childhood exposure. The screening level review of these studies indicated little consistency with no clear explanation based on a preliminary evaluation of study confidence or exposure levels (Supplementary Table). Therefore, a full systematic review was not performed and the evidence is considered inadequate.
Along among the included studies was a single prospective study of adult weight gain (Song et al., 2014), which used the controls from the nested case-control study on type 2 diabetes described previously (Sun et al., 2014) and measured weight gain over ten years. For summed DBP + DIBP and BBP, there were statistically significant associations between increased exposure and weight gain, with exposure-response gradients observed. For DEHP and DEP, no association was observed, and no results were available for DINP. Since the effect sizes for the positive associations were small (approximately 0.3 kg per year) and there is potential for residual confounding by diet (as described for diabetes), the evidence from a single study is considered inadequate to draw a conclusion about the association with obesity.
3.6. Renal effects
Five epidemiology studies examined the association between phthalate exposure and renal effects. Three studies were excluded due to critical deficiencies in exposure measurement (Supplementary Table). Both of the remaining studies were considered to be low confidence by a single reviewer due to selection bias (Tsai et al. (2016) or possible reverse causation (Trasande et al. (2014). Because of inconsistency in the results and the availability of only low confidence studies, a full systematic review was not performed, and the evidence is considered inadequate.
4. Discussion
Based on screening-level reviews, the currently available evidence was determined to be inadequate to assess associations between obesity and renal effects and phthalate exposure. It is unlikely that a full systematic review of the existing literature would indicate a clear association, either due to a small number of low confidence studies available or disparate results. The use of screening-level reviews allowed us to avoid using additional resources required for a full systematic review for these outcomes at this time. It is important to note, however, that we do not mean to imply that systematic reviews are not useful in situations where the evidence is inconsistent. To the contrary, systematic review may be particularly beneficial for evidence bases with conflicting results because it provides a process for identifying factors that may explain the discrepancies (e.g., study quality, exposure levels). The benefit gained from the review will depend on the context of the review. In this case, the focus was on identifying key hazards of phthalate exposure with a robust database, and there was a need to be pragmatic in the use of resources to conduct the review.
Evidence for an association between phthalate exposure and diabetes risk is more convincing. The single prospective study that examined incident diabetes as an outcome reported strong associations for multiple phthalates, and several studies reported associations with insulin resistance. When coherence across these outcomes was considered, DEHP, DBP, and DIBP had moderate evidence of an association, and DINP, BBP, and DEP had slight evidence of an association (Table 14).
Diet was considered a possible confounder for the observed association with diabetes and obesity. Only a portion of the studies controlled for diet, and those that did used only caloric intake as a proxy. While this would account for increased exposure simply from eating more in general, there may be other diet-related factors associated with phthalate exposure, such as frequent consumption of packaged/processed foods or fatty foods, which may not be fully accounted for with adjustment for caloric intake, and which may carry an additional risk with respect to diabetes (Micha et al., 2010; Franz et al., 2002; Hu et al., 2001). Phthalates, particularly DEHP and DBP, are present in most food-packaging materials (Balafas et al., 1999) and plastic-packaged food products (Cirillo et al., 2013) and can migrate into food (Serrano et al., 2014a; Castle et al., 1994). This migration is increased with microwave heating (Chen et al., 2008). Phthalate levels in different food types have been reviewed previously and meats (particularly poultry) and fats/oils have been consistently associated with increased phthalate exposure (Serrano et al., 2014a). Since the 2014 review, participants in a pre-defined “fatty, sweet, and ready meal” group had higher exposure to DEHP and DBP in one study (Serrano et al., 2014b). Other foods may vary in phthalate levels, based partly on region (Serrano et al., 2014a). A change in diet to fresh, mostly organic meals prepared without plastics in procurement, cooking, and serving has been shown to reduce DEHP exposure (Rudel et al., 2011). Taken as a whole, it is conceivable that individuals who eat a diet higher in packaged, microwaved, and fatty foods may be at higher risk for diabetes and obesity, and this diet may also increase phthalate exposure, though the influence of diet may differ by phthalate. The concern would be highest for DEHP, which is commonly used in packaging and is more associated with fatty foods due to its more lipophilic nature (Cao, 2010). DBP, DIBP, BBP are all used in packaging, though perhaps less so than DEHP (Van Holderbeke et al., 2014; Fierens et al., 2012), while DEP is not frequently used in packaging and is less likely to contaminate food (Schettler, 2006). The fact that a similar association with insulin resistance was observed for DEP as the other phthalates reduces the concern about residual confounding overall, but given the small evidence base, it is worth further, more detailed exploration of diet as a potential confounder.
As mentioned in the methods, another important source of potential confounding is across phthalates. When drawing conclusions about associations between individual phthalates and health effects, it can be difficult to conclusively determine that the observed associations are not due to confounding across the phthalates. Correlations between some of the phthalates included in this review are moderately high (e.g., DBP, DIBP, BBP metabolites r = 0.5 to 0.7 (Johns et al., 2015)), which increases this concern. While some phthalate studies present multi-pollutant models, this is far from universal, and there are also concerns that bias can be amplified in multipollutant regression models (Weisskopf et al., 2018). Thus, this is an area of uncertainty for reviews of phthalates and other chemical mixtures, and is discussed in more detail in a forthcoming special issue editorial. Our ability to assess this type of confounding is somewhat limited by the different sensitivity of the studies for different phthalates (e.g., due to exposure levels), which reduced the ability to assess coherence across phthalates, and by the fact that we might expect more similar phthalates to be both highly correlated with each other and have similar effects (e.g., DBP and DIBP). However, it is unlikely that this issue would significantly alter the conclusions of this review. None of the associations in this review approached the level of “Robust” evidence, where uncertainty is largely eliminated and alternative explanations for the association such as chance, bias, and confounding are ruled out. Still, this is certainly an important issue for phthalate research moving forward. Methods development to address this concern is a rapidly developing field, and researchers should consider the potential for confounding across phthalates in designing their studies and analyzing data. In addition, given that people are exposed to mixtures of chemicals like phthalates, there may be a benefit in some cases to drawing conclusions for mixtures (such as all phthalates or subclasses of phthalates) rather than for individual chemicals, though this is not without its own complications.
An established mechanism for the association between phthalate exposure and diabetes and insulin resistance would also increase confidence in the association despite the possibility of residual confounding. Plausible mechanisms for the association have been described elsewhere. Phthalates can alter peroxisome proliferator-activated receptors (PPARs), which contribute to adipogenesis, lipid metabolism, and metabolic homeostasis (Benjamin et al., 2017a; Watkins et al., 2016; Attina and Trasande, 2015; Sun et al., 2014; Thayer et al., n.d.). Oxidative stress is another possible mechanism (Watkins et al., 2016).
Given the mechanistic plausibility, the large effect sizes for incident diabetes in the single available study for some phthalates, and the coherence with insulin resistance, the association between phthalate exposure and diabetes risk should be considered when assessing the risks and costs of exposure to specific phthalates in humans. This is consistent with findings by Legler et al. (2015) and Benjamin et al. (2017b) in narrative reviews. Additional prospective research with repeated measures of exposure would further clarify the relationship, as would additional high-quality research on the related metabolic effects (obesity, renal effects) to better understand coherence across outcomes. Discussion of some additional issues that apply to all phthalate studies, is presented in the special issue editorial (forthcoming).
Supplementary Material
Acknowledgements
We would like to thank Carolyn Gigot, Susan Rieth, Michelle Angrish, Tom Bateson, Erin Yost, Ana Navas-Ascien, Robin Puett, Mike Wright, and Leonid Kopylev for their contributions to this work.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2019.04.040.
References
- Attina TM, Trasande L, 2015. Association of exposure to di-2-ethylhexylphthalate replacements with increased insulin resistance in adolescents from NHANES 2009–2012. J. Clin. Endocrinol. Metab 100, 2640–2650. 10.1210/jc.2015-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balafas D, Shaw KJ, Whitfield FB, 1999. Phthalate and adipate esters in Australian packaging materials. Food Chem. 65, 279–287. [Google Scholar]
- Benjamin JS, Culpepper CB, Brown LD, Wesolowski SR, Jonker SS, Davis MA, Limesand SW, Wilkening RB, Hay WW, Rozance PJ, 2017a. Chronic anemic hypoxemia attenuates glucose-stimulated insulin secretion in fetal sheep. Am J Physiol Regul Integr Comp Physiol 312, R492–R500. 10.1152/ajpregu.00484.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin S, Masai E, Kamimura N, Takahashi K, Anderson RC, Faisal PA, 2017b. Phthalates impact human health: epidemiological evidences and plausible mechanism of action [review]. J. Hazard. Mater 340, 360–383. 10.1016/j.jhazmat.2017.06.036. [DOI] [PubMed] [Google Scholar]
- Cao X,. uL, 2010. Phthalate esters in foods: sources, occurrence, and analytical methods. Compr. Rev. Food Sci. Food Saf 9, 21–43. 10.1111/j.1541-4337.2009.00093.x. [DOI] [PubMed] [Google Scholar]
- Carlsson A, Sørensen K, Andersson AM, Frederiksen H, Juul A, 2018. Bisphenol a, Phthalate Metabolites and Glucose Homeostasis in Healthy Normal-Weight Children. vol. 7. pp. 232–238. 10.1530/EC-17-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle L, Nichol J, Gilbert J, 1994. Migration of mineral hydrocarbons into foods. 4. Waxed paper for packaging dry goods including bread, confectionery and for domestic use including microwave cooking. Food Addit. Contam 11, 79–89. 10.1080/02652039409374204. [DOI] [PubMed] [Google Scholar]
- Castro-Correia C, Correia-Sá L, Norberto S, Delerue-Matos C, Domingues V, Costa-Santos C, Fontoura M, Calhau C, 2018. Phthalates and type 1 diabetes: is there any link? Environ. Sci. Pollut. Res. Int 25, 17915–17919. 10.1007/s11356-018-1997-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ML, Chen JS, Tang CL, Mao IF, 2008. The internal exposure of Taiwanese to phthalate–an evidence of intensive use of plastic materials. Environ. Int 34, 79–85. 10.1016/j.envint.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Chen SY, Hwang JS, Sung FC, Lin CY, Hsieh CJ, Chen PC, Su TC, 2017. Mono-2-ethylhexyl phthalate associated with insulin resistance and lower testosterone levels in a young population. Environ. Pollut 225, 112–117. 10.1016/j.envpol.2017.03.037. [DOI] [PubMed] [Google Scholar]
- Cirillo T, Fasano E, Esposito F, Prete ED, Cocchieri RA, 2013. Study on the influence of temperature, storage time and packaging type on di-n-butylphthalate and di(2-ethylhexyl)phthalate release into packed meals. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 30, 403–411. 10.1080/19440049.2012.745198. [DOI] [PubMed] [Google Scholar]
- Dales RE, Kauri LM, Cakmak S, 2018. The associations between phthalate exposure and insulin resistance, β-cell function and blood glucose control in a population-based sample. Sci. Total Environ 612, 1287–1292. 10.1016/j.scitotenv.2017.09.009. [DOI] [PubMed] [Google Scholar]
- Dirinck E, Dirtu AC, Geens T, Covaci A, Van Gaal L, Jorens PG, 2015. Urinary phthalate metabolites are associated with insulin resistance in obese subjects. Environ. Res 137, 419–423. 10.1016/j.envres.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Dong R, Zhou T, Chen J, Zhang M, Zhang H, Wu M, Li S, Zhang L, Chen B, 2017. Gender- and age-specific relationships between phthalate exposures and obesity in Shanghai adults. Arch. Environ. Contam. Toxicol 73, 431–441. 10.1007/s00244-017-0441-6. [DOI] [PubMed] [Google Scholar]
- Fierens T, Servaes K, Van Holderbeke M, Geerts L, De Henauw S, Sioen I, Vanermen G, 2012. Analysis of phthalates in food products and packaging materials sold on the Belgian market. Food Chem. Toxicol 50, 2575–2583. 10.1016/j.fct.2012.04.029. [DOI] [PubMed] [Google Scholar]
- Fisher BG, Frederiksen H, Andersson AM, Juul A, Thankamony A, Ong KK, Dunger DB, Hughes IA, Acerini CL, 2018. Serum phthalate and triclosan levels have opposing associations with risk factors for gestational diabetes mellitus. Front Endocrinol (Lausanne) 9, 99. 10.3389/fendo.2018.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz MJ, Bantle JP, Beebe CA, Brunzell JD, Chiasson JL, Garg A, Holzmeister LA, Hoogwerf B, Mayer-Davis E, Mooradian AD, Purnell JQ, Wheeler M, 2002. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications [review]. Diabetes Care 25, 148–198. 10.2337/diacare.25.1.148. [DOI] [PubMed] [Google Scholar]
- Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, Nadal A, Palanza P, Panzica G, Sargis R, Vandenberg LN, Vom Saal F, 2017. Metabolism disrupting chemicals and metabolic disorders. Reprod. Toxicol 68, 3–33. 10.1016/j.reprotox.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YC, Park EY, Park MS, Ko JA, Oh SY, Kim H, Lee KH, Leem JH, Ha EH, 2009. Community level exposure to chemicals and oxidative stress in adult population. Toxicol. Lett 184, 139–144. 10.1016/j.toxlet.2008.11.001. [DOI] [PubMed] [Google Scholar]
- Hong SH, Sung YA, Hong YS, Ha E, Jeong K, Chung H, Lee H, 2016. Urinary bisphenol A is associated with insulin resistance and obesity in reproductive-aged women. Clin. Endocrinol 86, 506–512. 10.1111/cen.13270. [DOI] [PubMed] [Google Scholar]
- Hu FB, van Dam RM, Liu S, 2001. Diet and risk of type II diabetes: the role of types of fat and carbohydrate. Diabetologia 44, 805–817. 10.1007/s001250100. [DOI] [PubMed] [Google Scholar]
- Huang T, Saxena AR, Isganaitis E, James-Todd T, 2014. Gender and racial/ethnic differences in the associations of urinary phthalate metabolites with markers of diabetes risk: national health and nutrition examination survey 2001–2008. Environ. Health 13, 6. 10.1186/1476-069X-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Todd T, Huang T, Isganaitis E, Saxena A, 2012. Urinary phthalate metabolite concentrations and biomarkers of diabetes risk by race/ethnicity and sex, the National Health and nutrition examination survey 2001–2008 [abstract]. J. Women’s Health 21, 992. 10.1089/jwh.2012.ab02. [DOI] [Google Scholar]
- James-Todd TM, Huang T, Seely EW, Saxena AR, 2016a. The association between phthalates and metabolic syndrome: the National Health and Nutrition Examination Survey 2001–2010. Environ. Health 15, 52. 10.1186/s12940-016-0136-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Todd TM, Meeker JD, Huang T, Hauser R, Ferguson KK, Rich-Edwards JW, Mcelrath TF, Seely EW, 2016b. Pregnancy urinary phthalate metabolite concentrations and gestational diabetes risk factors. Environ. Int 96, 118–126. 10.1016/j.envint.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James-Todd TM, Chiu YH, Messerlian C, Mínguez-Alarcón L, Ford JB, Keller M, Petrozza J, Williams PL, Ye X, Calafat AM, Hauser R, Team ES, 2018. Trimester-specific phthalate concentrations and glucose levels among women from a fertility clinic. Environ. Health 17, 55. 10.1186/s12940-018-0399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns LE, Cooper GS, Galizia A, Meeker JD, 2015. Exposure assessment issues in epidemiology studies of phthalates [review]. Environ. Int 85, 27–39. 10.1016/j.envint.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataria A, Levine D, Wertenteil S, Vento S, Xue J, Rajendiran K, Kannan K, Thurman JM, Morrison D, Brody R, Urbina E, Attina T, Trasande L, Trachtman H, 2017. Exposure to bisphenols and phthalates and association with oxidant stress, insulin resistance, and endothelial dysfunction in children. Pediatr. Res 81, 857–864. 10.1038/pr.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Hong YC, 2014. HSP70-hom gene polymorphisms modify the association of diethylhexyl phthalates with insulin resistance. Environ. Mol. Mutagen 55, 727–734. 10.1002/em.21884. [DOI] [PubMed] [Google Scholar]
- Kim JH, Park HY, Bae S, Lim YH, Hong YC, 2013. Diethylhexyl phthalates is associated with insulin resistance via oxidative stress in the elderly: a panel study. PLoS One 8, e71392. 10.1371/journal.pone.0071392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CC, Moon K, Thayer KA, Navas-Acien A, 2013. Environmental chemicals and type 2 diabetes: an updated systematic review of the epidemiologic evidence [review]. Curr Diab Rep 13, 831–849. 10.1007/s11892-013-0432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legler J, Fletcher T, Govarts E, Porta M, Blumberg B, Heindel JJ, Trasande L, 2015. Obesity, diabetes, and associated costs of exposure to endocrine-disrupting chemicals in the European Union. J. Clin. Endocrinol. Metab 100, 1278–1288. 10.1210/jc.2014-4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CY, Hsieh CJ, Lo SC, Chen PC, Torng PL, Hu A, Sung FC, Su TC, 2016. Positive association between concentration of phthalate metabolites in urine and microparticles in adolescents and young adults. Environ. Int 92–93, 157–164. 10.1016/j.envint.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Lind PM, Zethelius B, Lind L, 2012. Circulating levels of phthalate metabolites are associated with prevalent diabetes in the elderly. Diabetes Care 35, 1519–1524. 10.2337/dc11-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micha R, Wallace SK, Mozaffarian D, 2010. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis [review]. Circulation 121, 2271–2283. 10.1161/CIRCULATIONAHA.109.924977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milošević N, Milić N, Živanović Bosić D, Bajkin I, Perčić I, Abenavoli L, Medić Stojanoska M, 2017. Potential influence of the phthalates on normal liver function and cardiometabolic risk in males. Environ. Monit. Assess 190, 17. 10.1007/s10661-017-6398-0. [DOI] [PubMed] [Google Scholar]
- National Academy of Sciences, National Academy of Engineering, Institute of Medicine, 2017. Application of systematic review methods in an overall strategy for evaluating low-dose toxicity from endocrine active chemicals. In: Application of Systematic Review Methods in an Overall Strategy for Evaluating Low-Dose Toxicity from Endocrine Active Chemicals. The National Academies Press, Washington, D.C.. 10.17226/24758. [DOI] [PubMed] [Google Scholar]
- Olsén L, Lampa E, Birkholz DA, Lind L, Lind PM, 2012. Circulating levels of bisphenol A (BPA) and phthalates in an elderly population in Sweden, based on the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS). Ecotoxicol. Environ. Saf 75, 242–248. 10.1016/j.ecoenv.2011.09.004. [DOI] [PubMed] [Google Scholar]
- Piecha R, Svačina Š, Malý M, Vrbík K, Lacinová Z, Haluzík M, Pavloušková J, Vavrouš A, Matějková D, Müllerová D, Mráz M, Matoulek M, 2016. Urine levels of phthalate metabolites and bisphenol A in relation to main metabolic syndrome components: dyslipidemia, hypertension and type 2 diabetes. A pilot study. Cent. Eur. J. Public Health 24, 297–301. 10.21101/cejph.a4704. [DOI] [PubMed] [Google Scholar]
- Robledo CA, Peck JD, Stoner J, Calafat AM, Carabin H, Cowan L, Goodman JR, 2015. Urinary phthalate metabolite concentrations and blood glucose levels during pregnancy. Int. J. Hyg. Environ. Health 218, 324–330. 10.1016/j.ijheh.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudel RA, Gray JM, Engel CL, Rawsthorne TW, Dodson RE, Ackerman JM, Rizzo J, Nudelman JL, Brody JG, 2011. Food packaging and bisphenol A and bis (2-ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ. Health Perspect 119, 914–920. 10.1289/ehp.1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saengkaew T, Jantarat C, Nosoognoen W, Supornsilchai V, 2017. Association between Urinary Phthalates and Metabolic Abnormalities in Obese Thai Children and Adolescents. vol. 30. pp. 931–938. 10.1515/jpem-2017-0172. [DOI] [PubMed] [Google Scholar]
- Schettler T, 2006. Human exposure to phthalates via consumer products [review]. Int. J. Androl 29, 134–139. discussion 181–135. 10.1111/j.1365-2605.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- Updated October 2013 Schünemann H, Brożek J, Guyatt G, Oxman A. (Eds.), 2013. GRADE handbook for grading quality of evidence and strength of recommendations. The GRADE Working Group. Available from guidelinedevelopment.org/handbook. [Google Scholar]
- Serrano SE, Braun J, Trasande L, Dills R, Sathyanarayana S, 2014a. Phthalates and diet: a review of the food monitoring and epidemiology data [review]. Environ. Health 13, 43. 10.1186/1476-069X-13-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano SE, Karr CJ, Seixas NS, Nguyen RH, Barrett ES, Janssen S, Redmon B, Swan SH, Sathyanarayana S, 2014b. Dietary phthalate exposure in pregnant women and the impact of consumer practices. Int. J. Environ. Res. Public Health 11, 6193–6215. 10.3390/ijerph110606193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro GD, Dodds L, Arbuckle TE, Ashley-Martin J, Fraser W, Fisher M, Taback S, Keely E, Bouchard MF, Monnier P, Dallaire R, Morisset AS, Ettinger AS, 2015. Exposure to phthalates, bisphenol A and metals in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: the MIREC study. Environ. Int 83, 63–71. 10.1016/j.envint.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Smerieri A, Testa C, Lazzeroni P, Nuti F, Grossi E, Cesari S, Montanini L, Latini G, Bernasconi S, Papini AM, Street ME, 2015. Di-(2-ethylhexyl) phthalate metabolites in urine show age-related changes and associations with adiposity and parameters of insulin sensitivity in childhood. PLoS One 10, e0117831. 10.1371/journal.pone.0117831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Hauser R, Hu FB, Franke AA, Liu S, Sun Q, 2014. Urinary concentrations of bisphenol A and phthalate metabolites and weight change: a prospective investigation in US women. Int. J. Obes 38, 1532–1537. 10.1038/ijo.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlhut RW, van Wijngaarden E, Dye TD, Cook S, Swan SH, 2007. Concentrations of urinary phthalate metabolites are associated with increased waist circumference and insulin resistance in adult U.S. males. Environ. Health Perspect 115, 876–882. 10.1289/ehp.9882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne J, Higgins J, Reeves B, 2016. ROBINS-I: a tool for assessing risk of bias in non-randomized studies of interventions, version 7 March 2016. Br. Med. J 355, i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Cornelis MC, Townsend MK, Tobias DK, Eliassen AH, Franke AA, Hauser R, Hu FB, 2014. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: a prospective investigation in the Nurses’ Health Study (NHS) and NHSII cohorts. Environ. Health Perspect 122, 616–623. 10.1289/ehp.1307201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson K, Hernández-Ramírez RU, Burguete-García A, Cebrián ME, Calafat AM, Needham LL, Claudio L, López-Carrillo L, 2011. Phthalate exposure associated with self-reported diabetes among Mexican women. Environ. Res 111, 792–796. 10.1016/j.envres.2011.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer KA, Heindel JJ, Bucher JR, Gallo MA, 2012. Role of environmental chemicals in diabetes and obesity: a national toxicology program workshop report [review]. Environ. Health Perspect 120, 779–789. 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer KA; Heindel JJ; Bucher JR; Gallo MA. (In Press) Role of environmental chemicals in diabetes and obesity: a national toxicology program workshop report [review]. Environ. Health Perspect 120: 779–789. doi: 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Attina TM, Sathyanarayana S, Spanier AJ, Blustein J, 2013. Race/ ethnicity-specific associations of urinary phthalates with childhood body mass in a nationally representative sample. Environ. Health Perspect 121, 501–506. 10.1289/ehp.1205526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Sathyanarayana S, Trachtman H, 2014. Dietary phthalates and low-grade albuminuria in US children and adolescents. Clin. J. Am. Soc. Nephrol 9, 100–109. 10.2215/CJN.04570413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai HJ, Chen BH, Wu CF, Wang SL, Huang PC, Tsai YC, Chen ML, Ho CK, Hsiung CA, Wu MT, 2016. Intake of phthalate-tainted foods and microalbuminuria in children: the 2011 Taiwan food scandal. Environ. Int 89–90, 129–137. 10.1016/j.envint.2016.01.015. [DOI] [PubMed] [Google Scholar]
- Van Holderbeke M, Geerts L, Vanermen G, Servaes K, Sioen I, De Henauw S, Fierens T, 2014. Determination of contamination pathways of phthalates in food products sold on the Belgian market. Environ. Res 134, 345–352. 10.1016/j.envres.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Watkins DJ, Peterson KE, Ferguson KK, Mercado-García A, Ortiz MT, Cantoral A, Meeker JD, Téllez-Rojo MM, 2016. Relating phthalate and BPA exposure to metabolism in peripubescence: the role of exposure timing, sex, and puberty. J. Clin.Endocrinol. Metab 101, jc20152706. 10.1210/jc.2015-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, Seals RM, Webster TF, 2018. Bias amplification in epidemiologic analysis of exposure to mixtures. Environ. Health Perspect 126 (4), 047003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whaley-Connell A, Sowers JR, 2017. Insulin resistance in kidney disease: is there a distinct role separate from that of diabetes or obesity? [review]. Cardiorenal Med 8, 41–49. 10.1159/000479801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YW, Gao H, Huang K, Xu YY, Sheng J, Tao FB, 2017. A cohort study on association between the first trimester phthalates exposure and fasting blood glucose level in the third trimester. Zhonghua Liuxingbingxue Zazhi 38, 388–392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.