Abstract
Background
Malaria in human immunodeficiency virus (HIV)-positive patients is an ever-increasing global burden for human health. The present meta-analysis summarizes published literature on the prevalence of malaria infection in HIV-positive children, pregnant women and adults.
Methods
This study followed the PRISMA guideline. The PubMed, Science Direct, Google Scholar, Scopus and Cochrane databases were searched for relevant entries published between 1 January 1983 and 1 March 2020. All peer-reviewed original papers evaluating the prevalence of malaria among HIV-positive patients were included. Incoherence and heterogeneity between studies were quantified by the I2 index and Cochran’s Q test. Publication and population biases were assessed with funnel plots, and Egger’s regression asymmetry test.
Results
A total of 106 studies were included in this systematic review. The average prevalence of malaria among HIV-positive children, HIV-positive pregnant women and HIV-positive adults was 39.4% (95% confidence interval [CI]: 26.6–52.9), 32.3% (95% CI = 26.3–38.6) and 27.3% (95% CI = 20.1–35.1), respectively. In adult patients with HIV, CD4+ (cluster of differentiation 4) < 200 cells/µl and age < 40 years were associated with a significant increase in the odds of malaria infection (odds ratio [OR] = 1.5, 95% CI = 1.2–1.7 and OR = 1.1, 95% CI = 1–1.3, respectively). Antiretroviral therapy (ART) and being male were associated with a significant decrease in the chance of malaria infection in HIV-positive adults (OR = 0.8, 95% CI = 0.7–0.9 and OR = 0.2, 95% CI = 0.2–0.3, respectively). In pregnant women with HIV, CD4+ count < 200 cells/µl was related to a higher risk for malaria infection (OR = 1.5, 95% CI = 1.1–1.9).
Conclusions
This systematic review demonstrates that malaria infection is concerningly common among HIV-positive children, pregnant women and adults. Among HIV-positive adults, ART medication and being male were associated with a substantial decrease in infection with malaria. For pregnant women, CD4+ count of < 200 cells/µl was a considerable risk factor for malaria infection.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s13071-022-05432-2.
Keywords: AIDS, Anopheles, People living with HIV, Plasmodium, Protozoan parasite
Background
Infectious diseases pose a concerning threat to the health systems of both developed countries and countries with limited resources such as, for example, sub-Saharan countries [1, 2]. With 228 million malaria cases globally in 2018, future declines in the malaria burden caused by Plasmodium spp. infections are uncertain [3, 4]. Approximately 3.3 billion people are residing in malaria-endemic regions (parts of the Africa, Southeast Asia and Middle East) [5, 6].
The human immunodeficiency virus (HIV) is an emerging infectious disease agent defined by cellular immune system impairment [7]. HIV is a well-established global health burden, with > 36 million HIV-infected patients and > 1 million HIV-related deaths in 2017 [8]. While Plasmodium parasites causing human malaria are transmitted mainly by mosquitoes (Anopheles spp.) serving as biological vectors, malaria can also be transmitted directly via blood transfusion, needle sticks with contaminated needles and vertical transmission [9, 10]. The infection routes bypassing the biological vector are transmission routes shared by HIV and malaria [11]. Since HIV infection affects the immune system, the infected individuals are more susceptible to other infections [12–15]. Therefore, people living with HIV (including children, pregnant women and adults) are at risk for significant disease and may have fatal complications following infection [11, 16]. The vertical transmission option for both malaria and HIV facilitates co-transmission from infected pregnant women to their infants [17]. Since the co-infections of malaria and HIV can induce anemia, blood transfusion is often required, but blood transfusion can also contribute to the transmission of HIV and malaria [18, 19].
Although numerous studies have highlighted malaria prevalence in patients with HIV, there has been no comprehensive meta-analysis to demonstrate this prevalence in children, adults and pregnant women. Therefore, the aims of this systematic review and meta-analysis are to summarize malaria prevalence among HIV-positive children, pregnant women and adults, and to identify risk factors that increase the probability of HIV-positive patients being infected with malaria.
Methods
Search strategy
For inclusion in the present systematic review, the PubMed, Science Direct, Google Scholar, Scopus and Cochrane databases were searched for relevant English-language, full-text articles and abstracts published between 1 January 1983 (date of HIV discovery) and 1 March 2020. As the aim was to evaluate the prevalence of positive test results for malaria among HIV-positive and HIV-negative individuals, the following Medical Subject Headings (MeSH) terms were used: “Malaria” OR “Plasmodium” AND “prevalence” OR “epidemiology” OR “co-infection” AND “HIV” OR “AIDS” OR “acquired immune deficiency syndrome” OR “immunocompromised” OR “immunosuppressed” OR “immunodeficiency” AND “pregnancy women” OR “children” OR “adult” alone OR combined using “OR” and/or “AND”.
Study selection and data extraction
After an initial search of the databases, subject-related topics and their abstracts were double-checked, and then full texts of potentially eligible articles were selected for downloading. All potentially relevant full texts were reviewed by three independent reviewers (TM, HS, ASP). Discrepancies were resolved by discussion and consensus. The studies were assessed for quality using the Joanna Briggs Institute (JBI) checklist (Additional files 2, 3, 4, 5: Tables S1–S4). The required data were extracted by the reviewers and then re-checked. The criteria for inclusion in the review were: (i) peer-reviewed original research papers; (ii) cross-sectional and cohort studies that estimated the prevalence of malaria infection in HIV-positive and HIV-negative individuals; (iii) published papers in English; (iv) published online before 1 March 2020; and (v) sufficient sample size (n > 10). Any article that did not satisfy the above criteria were excluded. The reference lists of the eligible articles were also browsed manually to identify relevant papers that were not initially identified in the database search. Finally, details of each study were extracted using a data extraction form, including country, year of publication, first author, number of HIV+ and malaria-positive cases, education status of patients, alcohol consumption status, number of partners, marital status, level of CD4+ (cluster of differentiation 4) in HIV-positive patients, ART (antiretroviral therapy) status, sex protection status and diagnostic method (microscopy, serology or molecular). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were used to report the findings [20].
Meta-analysis
The point estimate and corresponding confidence interval (CI) for the prevalence of malaria in HIV-positive individuals for each study were calculated. Incoherence and heterogeneity among studies were assessed using the I2 index and Cochran’s Q test, respectively, and the random-effects model (DerSimonian-Laird) was used for analysis. The heterogeneity among subgroups was tested by meta-regression analysis. The relationship between prevalence, year of publication and sample size was estimated by meta-regression. Additionally, a funnel plot relying on the Egger’s regression asymmetry test was used to assess the small effects of the study and the population bias. For the meta-analysis, the included studies were evaluated as a random sample of each study population, and the analyses were performed using StatsDirect (version 2.7.2) statistical software (StatsDirect Ltd., Altrincham, UK).
Results
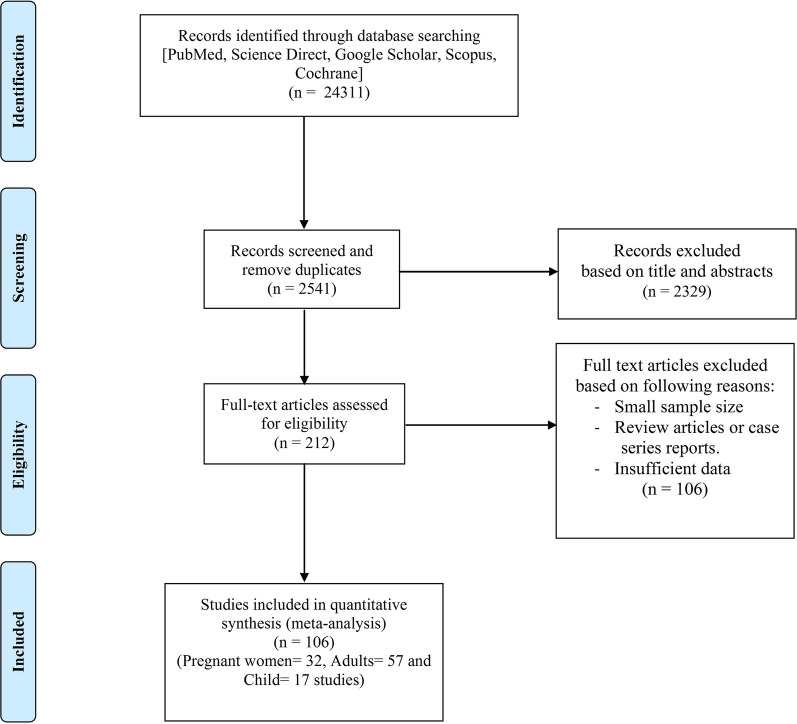
The systematic search of the electronic databases identified 24,311 potentially relevant papers. The full-text of 212 articles was assessed, resulting in exclusion from the study of 106 papers due to their small sample size, the review or case report nature of the report, duplication and insufficient data. The remaining 106 papers fulfilled the inclusion criteria and were included in the present systematic review and meta-analysis. All of these 106 articles were published between 1983 and 2020 and present data from malaria-endemic regions in Africa (n = 103) and Asia (n = 3). The inclusion/exclusion criteria at each step of screening and eligibility and the number of selected papers are shown in Fig. 1.
Fig. 1.
Flowchart of study selection process
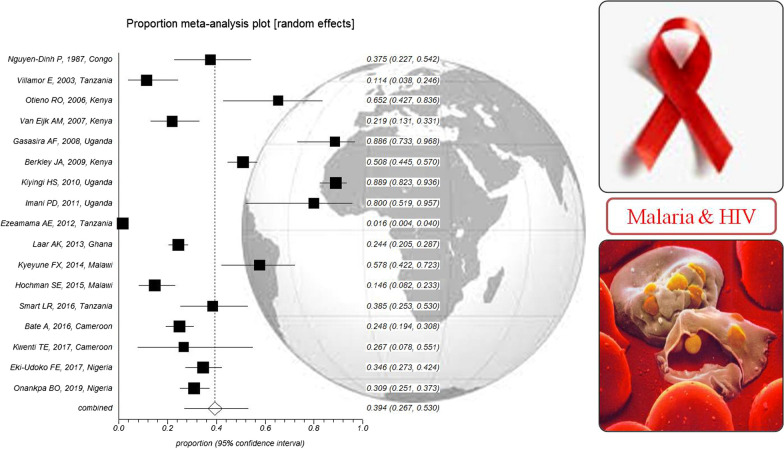
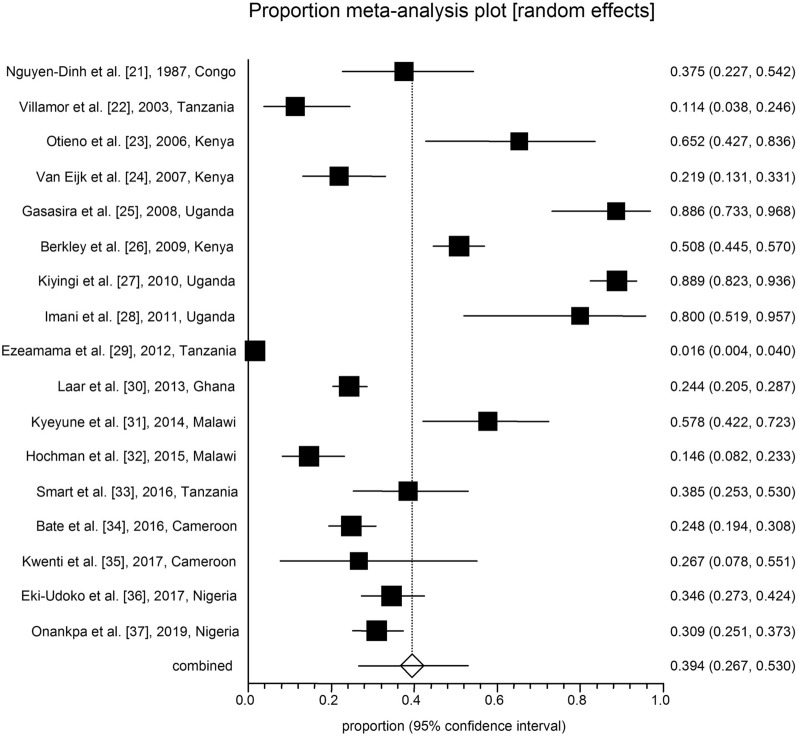
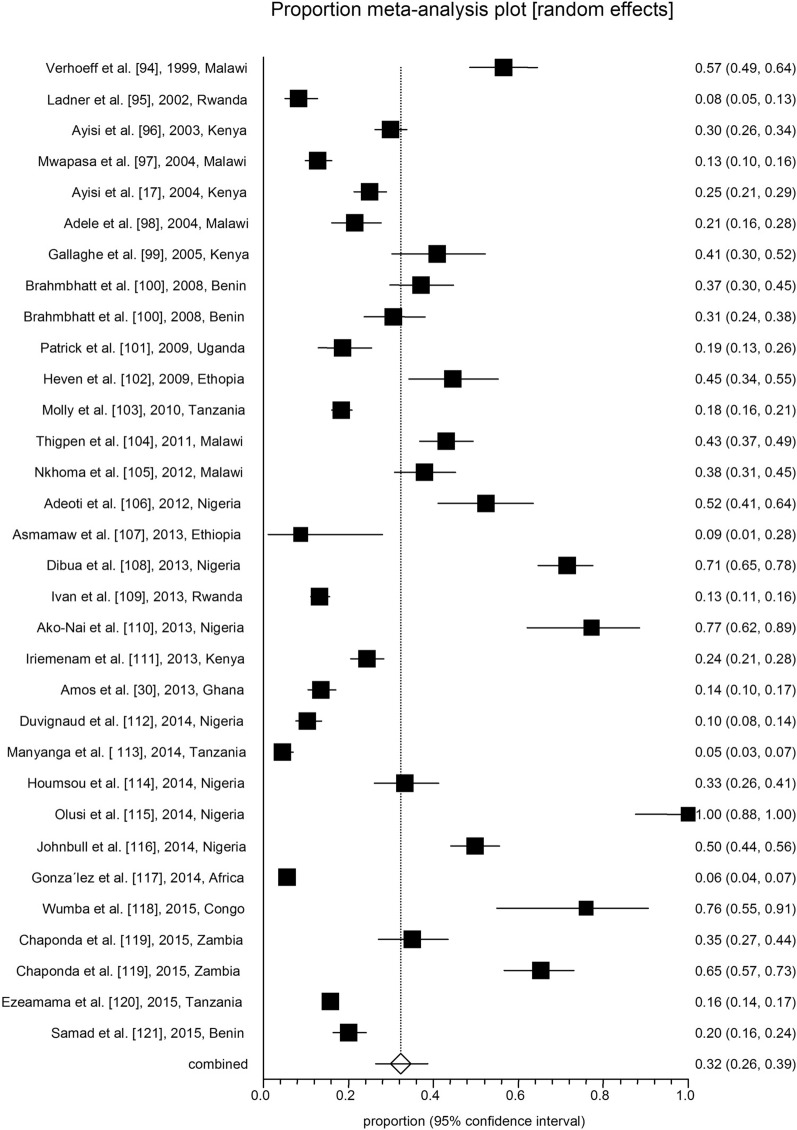
All analyses were conducted in three subgroups: children (n = 17; Table 1; Fig. 2), adults (n = 57; Table 2; Fig. 3) and pregnant women (n = 32; Table 3; Fig. 4). The pooled malaria prevalence among HIV-positive children was 39.4% (95% CI = 26.6–52.9). The combined prevalence of malaria in HIV-positive adults was 27.3% (95% CI = 20.1–35.1), and the collective malaria prevalence among HIV-positive pregnant women was 32.3% (95% CI = 26.3–38.6) (Figs. 2, 3, 4). The funnel plot showing a statistically significant Egger’s regression suggests the possibility of publication bias (Additional file 1: Figure S1). The published risk factors associated with HIV and malaria, namely CD4+ level, ART consumption, sex, education, gravidity and age, were analyzed (Table 4). In adult patients with HIV, CD4+ count < 200 cells/µl predisposes the patient to malaria infection (odds ratio [OR] = 1.5, 95% CI = 1.2–1.7). In adult HIV-positive patients, age < 40 years old was found to be associated with a significant increase in the odds of being infected with malaria (OR = 1.1, 95% CI = 1–1.3). Also, for adult HIV-positive patients, being male and being treated with ART medication have been associated with a significant decrease in the odds of being infected with malaria (OR = 0.8, 95% CI = 0.7–0.9 and OR = 0.2, 95% CI = 0.2–0.3, respectively). CD4+ count < 200 cells/µl was found to predispose pregnant women with HIV to malaria infection (OR = 1.5, 95% CI = 1.1–1.9) (Table 4).
Table 1.
Baseline characteristics of the included studies on malaria and human immunodeficiency virus co-infection in children
| No. | Year of publication | Country/region | Study design | No. of HIV-positive patients | No. of malaria-positive patients | Laboratory diagnostic method | Quality assessment | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 1987 | Zaire (Democratic Republic of Congo) | Case–control | 40 | 15 | Blood smear | 6/10 | [21] |
| 2 | 2003 | Tanzania | Cross-sectional | 44 | 5 | Blood smear | 6/8 | [22] |
| 3 | 2006 | Kenya | Cross-sectional | 23 | 15 | Blood smear | 7/8 | [23] |
| 4 | 2007 | Kenya | Cohort | 73 | 16 | Blood smear | 8/11 | [24] |
| 5 | 2008 | Uganda | Cohort | 35 | 31 | Blood smear | 8/11 | [25] |
| 6 | 2009 | Kenya | Case–control | 262 | 133 | Blood smear | 8/10 | [26] |
| 7 | 2010 | Uganda | Prospective cohort | 135 | 120 | Blood smear | 8/11 | [27] |
| 8 | 2011 | Uganda | Case–control | 15 | 12 | Blood smear | 9/10 | [28] |
| 9 | 2012 | Tanzania | Cohort | 255 | 4 | Blood smear | 7/11 | [29] |
| 10 | 2013 | Ghana | Cross-sectional | 443 | 108 | Rapid Test Kit | 6/8 | [30] |
| 11 | 2014 | Malawi | Cohort | 45 | 26 | Blood smear | 9/11 | [31] |
| 12 | 2015 | Malawi | Cohort | 19 | 15 | Autopsy | 8/11 | [32] |
| 13 | 2016 | Tanzania | Prospective cohort | 52 | 20 | Blood smear; rapid diagnostic test; PCR | 8/11 | [33] |
| 14 | 2016 | Cameroon | Cross-sectional | 234 | 58 | Blood smear | 8/8 | [34] |
| 15 | 2017 | Cameroon | Cross-sectional | 15 | 4 | Blood smear | 6/8 | [35] |
| 16 | 2017 | Nigeria | Cross-sectional | 162 | 56 | Blood smear | 7/8 | [36] |
| 17 | 2017 | Nigeria | Cross-sectional | 67 | 67 | Blood smear | 5/8 | [37] |
Fig. 2.
Forest plot diagram of malaria prevalence in human immunodeficiency virus-positive children (first author, year and country)
Table 2.
Baseline characteristics of the included studies on malaria and human immunodeficiency virus co-infection in adults
| No. | Year of publication | Country/region | Study design | No. of HIV-positive patients | No. of malaria-positive patients | Laboratory diagnostic method | Quality assessment | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 2001 | Uganda | Case–control | 65 | 14 | Blood smear and ELISA | 7/10 | [38] |
| 2 | 2002 | Nigeria | Cross-sectional | 91 | 23 | Blood smear | 6/8 | [39] |
| 3 | 2005 | Nigeria | Cross-sectional | 490 | 103 | Serology | 6/8 | [40] |
| 4 | 2005 | Malawi | Cross-sectional | 83 | 12 | Blood smear | 7/8 | [41] |
| 5 | 2006 | Malawi | Cross-sectional | 660 | 325 | Blood smear and serology | 7/8 | [42] |
| 6 | 2007 | Nigeria | Cross-Sectional | 81 | 72 | Blood smear | 6/8 | [43] |
| 7 | 2007 | Nigeria | Prospective study | 149 | 28 | RDT | 7/11 | [44] |
| 8 | 2008 | Cameron | Prospective cohort | 258 | 201 | Blood smear | 6/11 | [45] |
| 9 | 2009 | Nigeria | Cross-sectional | 560 | 476 | Blood smear | 7/8 | [46] |
| 10 | 2011 | Nigeria | Cross-sectional | 300 | 79 | RDT | 6/8 | [47] |
| 11 | 2012 | India | Cohort | 460 | 45 | PCR | 7/11 | [48] |
| 12 | 2012 | Cameroon | Cross-sectional | 312 | 7 | Blood smear | 8/8 | [49] |
| 13 | 2012 | Nigeria | Cross-sectional | 285 | 6 | Blood smear | 7/8 | [50] |
| 14 | 2012 | Nigeria | Cross-sectional | 2000 | 87 | Blood smear | 7/8 | [51] |
| 15 | 2012 | Nigeria | Cross-sectional | 1080 | 343 | Blood smear | 6/8 | [52] |
| 16 | 2012 | Nigeria | Cross-sectional | 97 | 24 | Blood smear | 8/8 | [53] |
| 17 | 2013 | Nigeria | Cross-sectional | 65 | 31 | Blood Smear and ELISA | 6/8 | [54] |
| 18 | 2013 | Nigeria | Cohort | 317 | 31 | Blood smear and PCR | 7/11 | [55] |
| 19 | 2013 | Ethiopia | Retrospective | 377 | 73 | Blood smear | 9/11 | [56] |
| 20 | 2013 | Nigeria | Cross-sectional | 342 | 254 | Blood smear | 7/8 | [57] |
| 21 | 2013 | Nigeria | Cross-sectional | 387 | 74 | RDT and blood smear | 8/8 | [58] |
| 22 | 2013 | Ghana | Cross-sectional | 933 | 15 | Blood smear | 7/8 | [59] |
| 23 | 2013 | Nigeria | Case–control | 68 | 17 | Blood smear | 8/10 | [60] |
| 24 | 2013 | Nigeria | Cross-sectional | 363 | 117 | Blood smear | 7/8 | [61] |
| 25 | 2014 | Mozambique | Cross-Sectional | 128 | 70 | Serology and PCR | 6/8 | [62] |
| 26 | 2014 | Nigeria | Cross-sectional | 200 | 37 | PCR | 7/8 | [63] |
| 27 | 2015 | Kenya | Cross-sectional | 46 | 27 | ELISA and blood Smear | 7/8 | [64] |
| 28 | 2015 | Ethiopia | Cross-Sectional | 1819 | 13 | Blood smear and serology | 6/8 | [65] |
| 29 | 2015 | Uganda | Cross-sectional | 160 | 30 | Blood smear | 6/8 | [66] |
| 30 | 2015 | Nigeria | Cross-sectional | 350 | 159 | Blood smear | 8/8 | [67] |
| 31 | 2015 | Ghana | Cross-sectional | 400 | 47 | Blood Smear and serology | 7/8 | [68] |
| 32 | 2016 | Niagara | Cross-sectional | 83 | 53 | Blood smear | 7/8 | [69] |
| 33 | 2016 | Uganda | Cross-sectional | 131 | 26 | LAMP and serology | 7/8 | [70] |
| 34 | 2016 | Cameroon | Cross-sectional | 35 | 6 | Blood smear | 7/8 | [71] |
| 35 | 2016 | Niagara | Cross-sectional | 226 | 56 | Blood smear | 6/8 | [72] |
| 36 | 2017 | Niagara | Case–control | 179 | 61 | PCR and serology | 8/10 | [73] |
| 37 | 2017 | Equatorial Guinea | Cross-sectional | 101 | 14 | Blood smear and ELISA | 8/8 | [74] |
| 38 | 2017 | Ethiopia | Cross-sectional | 528 | 92 | RDT | 8/8 | [75] |
| 39 | 2017 | India | Prospective cohort | 202 | 14 | Blood smear and PCR | 8/11 | [76] |
| 40 | 2017 | India | Prospective cohort | 131 | 8 | Blood smear and PCR | 8/11 | [76] |
| 41 | 2017 | Ethiopia | Cross-sectional | 172 | 86 | Blood smear | 7/8 | [77] |
| 42 | 2017 | Nigeria | Cross-sectional | 761 | 211 | RDT | 7/8 | [78] |
| 43 | 2017 | Gabon | Cross-sectional | 856 | 61 | Blood smear | 6/8 | [79] |
| 44 | 2018 | Nigeria | Case–control | 35 | 5 | PCR and serology | 6/8 | [80] |
| 45 | 2018 | Ethiopia | Cross-sectional | 53 | 12 | Blood smear | 7/8 | [81] |
| 46 | 2018 | Niagara | Cross-sectional | 324 | 254 | Blood smear | 7/8 | [82] |
| 47 | 2018 | Nigeria | Cross-sectional | 200 | 130 | Blood smear | 8/8 | [83] |
| 48 | 2018 | Mozambique | Retrospective | 701 | 232 | RDT | 8/11 | [84] |
| 49 | 2018 | Ghana | Cross-sectional | 466 | 64 | Blood smear | 8/8 | [85] |
| 50 | 2018 | Cameroon | Cross-sectional | 15 | 5 | Blood smear | 7/8 | [86] |
| 51 | 2019 | Nigeria | Cross-sectional | 262 | 60 | Blood smear | 8/8 | [87] |
| 52 | 2019 | Sudan | Cross-sectional | 70 | 1 | PCR | 6/8 | [88] |
| 53 | 2019 | Cameroon | Cross-sectional | 309 | 24 | Blood Smear | 8/8 | [89] |
| 54 | 2019 | Nigeria | Cross-sectional | 268 | 116 | Blood smear | 7/8 | [90] |
| 55 | 2020 | Niagara | Retrospective | 1472 | 1101 | n.a | 7/11 | [91] |
| 56 | 2020 | Nigeria | Cross sectional | 94 | 40 | Serology | 8/8 | [92] |
| 57 | 2020 | Malawi | Cohort | 30 | 11 | Blood smear | 8/11 | [93] |
ELISA enzyme-linked immunosorbent assay, LAMP loop-mediated isothermal amplification, n.a. information not available, RDT rapid diagnostic test
Fig. 3.
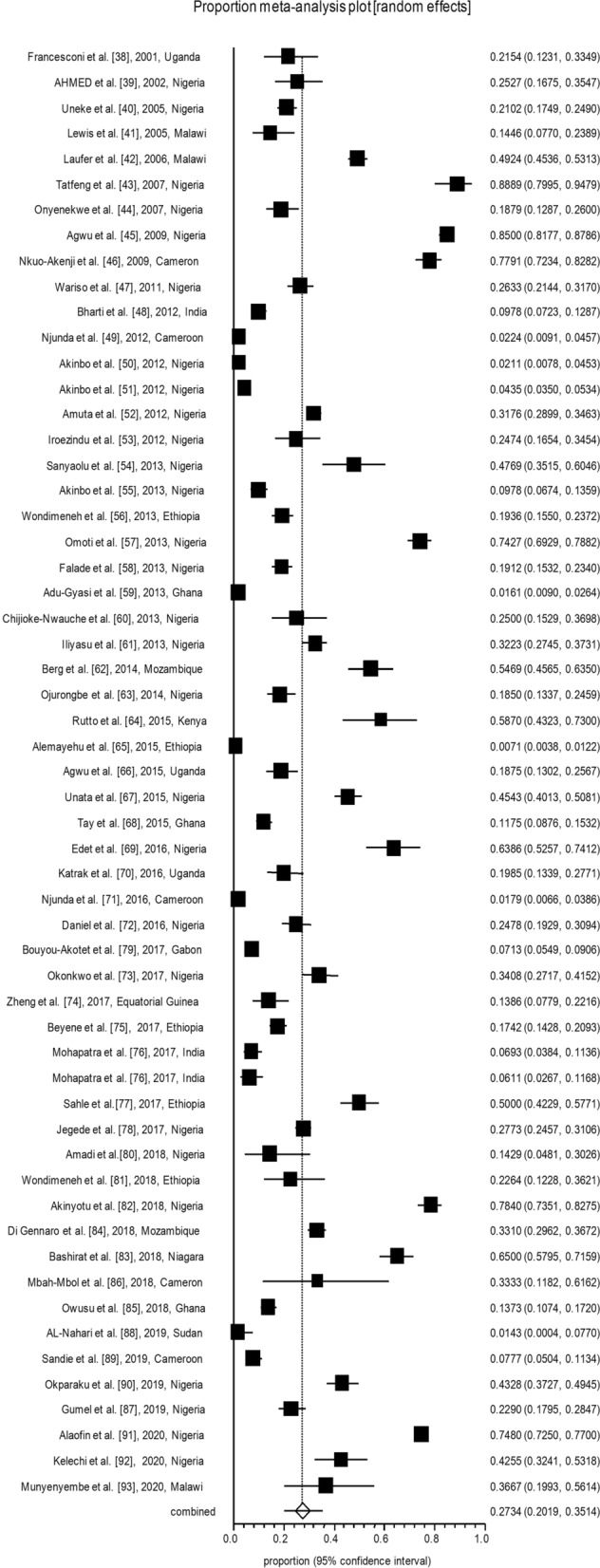
Forest plot diagram of malaria prevalence in human immunodeficiency virus-positive adults (first author, year, and country)
Table 3.
The baseline characteristics of the included studies on malaria and human immunodeficiency virus co-infection in pregnant women
| No. | Year of publication | Country/region | Study design | Number of HIV-positive patients | No. of malaria-positive patients | Laboratory diagnostic method | Quality assessment | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 1999 | Malawi | Cross-sectional | 159 | 90 | Blood smear | 8/8 | [94] |
| 2 | 2002 | Rwanda | Cohort | 228 | 19 | Blood smear | 7/11 | [95] |
| 3 | 2003 | Kenya | Cross-sectional | 599 | 179 | Blood smear | 7/8 | [96] |
| 4 | 2004 | Malawi | Cross-sectional | 480 | 61 | Blood smear | 7/8 | [97] |
| 5 | 2004 | Kenya | Cross-sectional | 512 | 128 | Blood smear | 7/8 | [17] |
| 6 | 2004 | Malawi | Cross-sectional | 205 | 44 | Blood smear | 8/8 | [98] |
| 7 | 2005 | Kenya | Cohort | 83 | 34 | Smear and/or PCR | 7/11 | [99] |
| 8 | 2008 | Uganda | Cohort | 170 | 63 | IHC | 8/11 | [100] |
| 9 | 2008 | Uganda | Cohort | 170 | 52 | ICT | 7/11 | [100] |
| 10 | 2009 | Uganda | Cross-sectional | 161 | 30 | Blood smear | 6/8 | [101] |
| 11 | 2009 | Ethiopia | Cross-sectional | 92 | 41 | RDT and smear | 6/8 | [102] |
| 12 | 2010 | Tanzania | Cross-sectional | 1006 | 185 | Blood smear | 8/8 | [103] |
| 13 | 2011 | Malawi | Clinical trial | 251 | 108 | Blood smear | 11/13 | [104] |
| 14 | 2012 | Malawi | Cross-sectional | 185 | 70 | Blood smear | 8/8 | [105] |
| 15 | 2012 | Nigeria | Cross-sectional | 82 | 43 | Blood smear | 6/8 | [106] |
| 16 | 2013 | Ethiopia | Cross-sectional | 23 | 2 | Blood smear | 7/8 | [107] |
| 17 | 2013 | Nigeria | Cohort | 203 | 145 | Blood smear | 8/10 | [108] |
| 18 | 2013 | Rwanda | Cross-sectional | 980 | 130 | Blood smear | 7/8 | [109] |
| 19 | 2013 | Nigeria | Cross-sectional | 44 | 34 | Blood smear | 7/8 | [110] |
| 20 | 2013 | Kenya | Cohort | 489 | 119 | Blood smear | 8/11 | [111] |
| 21 | 2013 | Ghana | Prospective | 443 | 60 | RDT | 7/11 | [30] |
| 22 | 2014 | Nigeria | Cohort | 432 | 45 | Smear or RDT | 8/11 | [112] |
| 23 | 2014 | Tanzania | Cross-sectional | 420 | 19 | RDT | 8/8 | [113] |
| 24 | 2014 | Nigeria | Cross-sectional | 159 | 53 | Blood smear | 7/8 | [114] |
| 25 | 2014 | Nigeria | Cross-sectional | 28 | 28 | Blood smear | 7/8 | [115] |
| 26 | 2014 | Nigeria | Cross-sectional | 301 | 150 | Blood smear | 6/8 | [116] |
| 27 | 2014 | Africa | Randomized controlled trial | 973 | 54 | Blood smear | 13/13 | [117] |
| 28 | 2015 | Congo | Cross-sectional | 25 | 19 | Smear and PCR | 8/8 | [118] |
| 29 | 2015 | Zambia | Cross-sectional | 140 | 49 | Blood smear | 8/8 | [119] |
| 30 | 2015 | Zambia | Cross-sectional | 138 | 90 | PCR | 7/8 | [119] |
| 31 | 2015 | Tanzania | Prospective | 2378 | 376 | Clinical | 8/11 | [120] |
| 32 | 2015 | Benin | Cross-sectional | 432 | 87 | Blood smear | 7/8 | [121] |
ICT Immunochromatography, IHC immunohistochemistry
Fig. 4.
Forest plot diagram of malaria prevalence in human immunodeficiency virus-positive pregnant women (first author, year, and country)
Table 4.
Risk factors associated with malaria infection in human immunodeficiency virus-positive patients
| Risk factors | Categories | No. study | Odds ratio (95% CI) | P-value | I2 (inconsistency), % | Cochran Q | Egger regression test (bias) | P-value |
|---|---|---|---|---|---|---|---|---|
| Children | ||||||||
| ART |
Yes No |
2 | 1.3 (0.2–6.6) | 0.7342 | - | 7.3 | - | 0.0069 |
| CD4+ |
< 200 cells/µl ≥ 200 cells/µl |
2 | 1.8 (0.8–3.8) | 0.1195 | - | 1.8 | - | 0.1681 |
| Adults | ||||||||
| Sex |
Male Female |
24 | 0.8 (0.7–0.9) | 0.1393 | 81.4 (72.9–86.3) | 123.4 | 0.6 | 0.007 |
| Age (years) |
< 40 ≥ 40 |
20 | 1.1 (1 -1.3) | 0.4716 | 53 (10.8–70.6) | 40.3 | 0.04 | 0.0148 |
| ART |
Yes No |
7 | 0.2 (0.2–0.3) | 0.0029* | 82.5 (49.5–90.8) | 92.9 | 1.09 | < 0.0001 |
| CD4+ |
< 200 cells/µl ≥ 200 cells/µl |
12 | 1.5 (1.2–1.7) | 0.0428* | 90.4 (85.7–93.1) | 114.9 | 1.1 | < 0.0001 |
| Education |
Primary level Higher-level |
3 | 0.9 (0.7–1.2) | 0.8935 | 0 (0–72.9) | 0.5 | – | 0.9389 |
| Pregnant women | ||||||||
| Gravidity |
Primigravida Multigravida |
9 | 0.96 (0.7–1.2) | 0.9758 | 38.2 (0–70.2) | 12.9 | 0.2 | 0.7916 |
| ART |
Yes No |
4 | 1.06 (0.7–1.5) | 0.96 | 51.8 (0–82.3) | 6.2 | 0.01 | 0.1012 |
| CD4+ |
< 200 cells/µl ≥ 200 cells/µl |
4 | 1.5 (1.1–1.9) | 0.7949 | 92.3 (83.2–95.4) | 38.7 | − 5.2 | 0.0012 |
ART Antiretroviral therapy, CD4 Cluster of differentiation 4, CI confidence interval
*Significant association (P = 0.05) with malaria infection
Discussion
Although extensive studies have been conducted on both HIV and Plasmodium spp. infections, a comprehensive meta-analysis aimed at precisely evaluating the prevalence of malaria infections among HIV-positive patients and related risk factors is lacking. Therefore, the aim of the present meta-analysis was to provide the pooled prevalence of malaria infection in HIV-positive children, pregnant women and adults and evaluate the related risk factors. The included studies represent African and Asian regions where both HIV and Plasmodium spp. are endemic. The pooled malaria prevalence in HIV-positive children, adults and pregnant women included in these studies was 39.4% (95% CI = 26.6–52.9), 27.3% (95% CI = 20.1–35.1) and 32.3% (95% CI = 26.3–38.6), respectively. In adult patients with HIV, receiving ART and having CD4+ count > 200 cells/µl were two factors significantly associated with malaria infection (P < 0.05).
Due to widespread ART coverage, mortality due to HIV as the main cause of death has decreased drastically over the years [8]. Notwithstanding the extensive efforts to end the acquired immunodeficiency syndrome (AIDS) epidemic by 2030 (set down in the Joint United Nations Program on HIV/AIDS), a lot of the work remains to be done [122]. The troublesome high prevalence of HIV, the increased life expectancy of affected patients, the common co-transmission of HIV and malaria and a remarkable geographical overlap between malaria and HIV high prevalence areas have paved the way for higher rates of co-infections in HIV-positive individuals [123].
Although the incidence of malaria and mortality due to malaria declined significantly by 62% and 41%, respectively, between 2000 and 2015, WHO reported that malaria remained an endemic disease in 76 countries at the beginning of 2016 [124], with approximately 216 million malaria cases in that year. Fifteen countries of the sub-Saharan African region alone were reported to be responsible for 80% of the total malaria burden [125]. Therefore, it is believed that many challenges remain to be overcome in order to eliminate malaria [126]. Regarding the burden of HIV and malaria and the immunosuppressive nature of HIV, there is an urgent need to clarify malaria prevalence in HIV-infected patients and the related risk factors.
According to the results of this systematic review and meta-analysis, the majority of published HIV/malaria studies to date have been in African countries. Socioeconomic conditions and a desirable climate for the biological vector, both of which can facilitate malaria transmission, may be the main reasons underlying this result [127]. Based on our findings, more than one-third of pregnant and HIV-positive women have been infected by malaria, which is worrisome because of the vertical transmission nature of malaria and HIV, which predisposes neonates to other infectious diseases [128, 129]. Indeed, pregnant women are among the most susceptible and vulnerable groups infected with malaria due to the altered immune system during pregnancy [3, 130]. The weakened immune response and HIV infection can lead to even deeper attenuation of the immune system. It is well-recognized that a decline in CD4+ cell numbers is associated with attenuation of the cell immune system and an increased vulnerability to being infected with other infections [131]. Our finding that CD4+ cell count < 200 cells/µl is linked to increased susceptibility to malaria infection (OR = 1.5, 95% CI = 1.1–1.9) confirms this association. In essence, AIDS and malaria are each controlled by adaptive and innate immune mechanisms, and declining immunity caused by HIV infection will cause an increase in malaria severity. CD4+ cells are depleted by the HIV virus, which leads to an impaired immune response to many pathogens, including Plasmodium spp. [43]. This pattern was corroborated by Grimwade et al. [132] who observed that malaria incidence in persons with CD4+ T cell count ≥ 500/µl, between 200 and 499/μl and < 200/μl was 57, 93 and 140 per 1000 person-year, respectively, in Uganda. It has been postulated that HIV increases malaria incidence in adults based on CD4+ cell count categories [133].
This meta-analysis also revealed the worrying situation of malaria infection among HIV-positive children. Approximately 39.4% of HIV-positive children in the analyzed studies were infected with malaria. This is a much higher prevalence than that observed in several studies investigating general children populations in African countries, with the prevalence in these studies ranging from 1% in Kenya to 22% in Uganda, with 14.5% prevalence in Tanzania and 20% in the Democratic Republic of Congo [134, 135]. The observation of increased malaria prevalence in HIV-positive children supports our assumption that susceptibility to co-infection is high in HIV-positive individuals. It is interesting to note that much of the pathogenesis of malaria during pregnancy is mediated by the accumulation of Plasmodium-infected red blood cells in the placental intervillous space, termed ‘placental malaria.’ The placenta is also the key interface in mother-to-child transmission of HIV, especially that involving in utero transfer [136]. No remarkable association between receiving ART and HIV infection status has been noted in HIV-positive children (OR = 1.3, 95% CI = 0.2–6.6). Moreover, there has been no significant association between the CD4+ cell count and the probability of malaria infection (P > 0.05), possibly due to the small number of studies that have considered this factor.
The present meta-analysis reveals that, on average, 27.3% of HIV-positive adults are infected with malaria in endemic countries. One of the consequences of this alarmingly high figure can be manifested in blood transfusion. With the ever-increasing need for a blood transfusion due to environmental and heredity diseases such as sickle cell anemia [137], the prevalence of transfusion-transmitted HIV/malaria can be expected to be high. A study conducted in the sub-African region has demonstrated that about 10–15% of HIV transmission is related to blood transfusion [138]. Ahmadpour et al. [19] reported that transfusion-transmitted malaria is a significant challenge in sub-Saharan African regions. In terms of risk factors, CD4+ cell count of < 200 cells/µl predisposes HIV-positive adults to Plasmodium spp. infection (OR = 1.5, 95% CI = 1.2–1.7). However, the association between malaria and HIV is more complex than expected. Some studies have corroborated that CD4+ T cells, as the prime targets for reproduction by HIV-1, play a vital role in immune responses to malaria [131, 139]. Malaria infection leads to upregulation of proinflammatory cytokines and stimulates CD4+ cell activation, thus providing the ideal microenvironment for the spread of the HIV virus among the CD4+ cells. On the other hand, the selective infection of CD4+ cells by HIV leads to the loss of these cells [140]. It is assumed that the increased susceptibility of HIV-seropositive individuals to malaria is related to some immune system-modulating mechanisms, such as depletion of CD4+ cells [131, 141].
Age < 40 years has also been associated with a significant increase in the chance of HIV-positive adults becoming infected with malaria (OR = 1.1, 95% CI = 1–1.3). In HIV-positive adults, being male and receiving ART have been associated with a significant decrease in the risk of being infected with Plasmodium spp. (OR = 0.8, 95% CI = 0.7–0.9 and OR = 0.2, 95% CI = 0.2–0.3, respectively). This is an interesting finding when compared to individual studies that described a higher risk of malaria infections in males compared to females in the general population in north-east Tanzania, irrespective of their HIV status [134]. Thus, it appears that HIV status may potentially alter malaria susceptibility differently in male patients than in female patients. It is worth emphasizing that the reported figures may not reflect the current status of this co-infection because these endemic areas are limited in terms of healthcare resources, and testing may not be conducted on all people unless they show clinical symptoms. Furthermore, there is insufficient evidence to determine whether or not malaria-induced changes in CD4+ T cell counts or viral loads translate to accelerated HIV disease progression or death in areas of stable malaria transmission.
This is the first meta-analysis on malaria prevalence among HIV-positive patients. We broke down the data into three categories, namely infancy, pregnancy and adulthood, and identified the available risk factors for each group. Since there has been little research on the prevalence of malaria in HIV patients in malaria endemic areas, further studies are needed in this regard. Also, due to the incomplete data in the studies included in our meta-analysis, we were unable to evaluate some risk factors, including duration of illness, time of diagnosis and response to treatment. Unfortunately, no data on the health status of individuals having both malaria and HIV infection were provided in these studies. On the other hand, publication bias is one of the main concerns in systematic review studies. As expected, publication bias was observed in the analyzed studies. The main limitation of this systematic review and meta-analysis is related to the different study designs and varying laboratory methods used to determine infection status. Diagnostic methods have varying sensitivity and specificity and, therefore, the heterogeneous prevalence data reported may partially be caused by flaws in methodology. The use of an accurate, reliable and uniform diagnostic techniques would support the correct interpretation of results.
Conclusions
The current systematic review has revealed concerning prevalence data for malaria among HIV-positive persons, including children, adults and pregnant women. In view of the fact that malaria can quickly become a life-threatening condition in risk groups (e.g. people living with HIV), prevention, chemoprophylaxis, early diagnosis and treatment of clinical malaria are recommended. Recent information also indicates that malaria is associated with the availability of ART and CD4+ cell count numbers in adults. Therefore, the related risk factors should be given appropriate attention in HIV/malaria co-infected patients. As HIV infection affects the host immune response, future studies are needed to elucidate the pathogenesis aspects of this co-infection, as well as the severity of its complications, and to investigate possible drugs and drug effectiveness.
Supplementary Information
Additional file 1: Figure S1. Funnel plot of standard error by logit event rate to assess publication or other types of bias across prevalence studies. Studies based on the prevalence of malaria in HIV patients: children (A), adults (B), and pregnant women (C).
Additional file 2: Table S1. Summary score for methodological quality of analytic cross-sectional studies.
Additional file 3: Table S2. Summary score for methodological quality of analytic case–control studies.
Additional file 4: Table S3. Summary score for methodological quality of analytic cohort studies.
Additional file 5: Table S4. Summary score for methodological quality of analytic RCT studies.
Acknowledgements
Not applicable.
Abbreviations
- AIDS
Acquired immunodeficiency syndrome
- ART
Antiretroviral therapy
- CD4
Cluster of differentiation 4
- HIV
Human immunodeficiency virus
Author contributions
TM, EA and AB designed the study. TM, HS, MAS and ASP were involved in searching the databases. TM, HS, MAS, ASP and BB screened the papers and extracted the data. AB and EA performed the statistical analysis. MAS, ASP and BB wrote the manuscript, with revision by EA and AB. All authors read and approved the final manuscript.
Funding
The authors received no financial support for the research.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Seyedeh-Tarlan Mirzohreh, Email: tarlan_mirzohre@yahoo.com.
Hanieh Safarpour, Email: hanie.safarpour@yahoo.com.
Abdol Sattar Pagheh, Email: satar2011@gmail.com.
Berit Bangoura, Email: bbangour@uwyo.edu.
Aleksandra Barac, Email: aleksandrabarac85@gmail.com.
Ehsan Ahmadpour, Email: ehsanahmadpour@gmail.com, Email: ahmadpoure@tbzmed.ac.ir.
References
- 1.Huerga H, Lopez-Velez R. Infectious diseases in sub-Saharan African immigrant children in Madrid, Spain. Pediatr Infect Dis J. 2002;21:830–834. doi: 10.1097/00006454-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Stäger K, Legros F, Krause G, Low N, Bradley D, Desai M, et al. Imported malaria in children in industrialized countries, 1992–2002. Emerg Infec Dis. 2009;15:185–191. doi: 10.3201/eid1502.080712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nkumama IN, O’Meara WP, Osier FH. Changes in malaria epidemiology in Africa and new challenges for elimination. Trends Parasitol. 2017;33:128–140. doi: 10.1016/j.pt.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qureshi NA, Fatima H, Afzal M, Khattak AA, Nawaz MA. Occurrence and seasonal variation of human Plasmodium infection in Punjab Province, Pakistan. BMC Infect Dis. 2019;19:935. doi: 10.1186/s12879-019-4590-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379:413–431. doi: 10.1016/S0140-6736(12)60034-8. [DOI] [PubMed] [Google Scholar]
- 6.Alencar Filho AC, Lacerda MVG, Okoshi K, Okoshi MP. Malaria and vascular endothelium. Arq Bras Cardiol. 2014;103:165–169. doi: 10.5935/abc.20140088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss RA. How does HIV cause AIDS? Science. 1993;260:1273–1279. doi: 10.1126/science.8493571. [DOI] [PubMed] [Google Scholar]
- 8.Frank TD, Carter A, Jahagirdar D, Biehl MH, Douwes-Schultz D, Larson SL, et al. Global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2017, and forecasts to 2030, for 195 countries and territories: a systematic analysis for the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Lancet HIV. 2019;6:e831–e859. doi: 10.1016/S2352-3018(19)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guelbéogo WM, Gonçalves BP, Grignard L, Bradley J, Serme SS, Hellewell J, et al. Variation in natural exposure to anopheles mosquitoes and its effects on malaria transmission. elife. 2018;7:e32625. doi: 10.7554/eLife.32625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ashley EA, Phyo AP, Woodrow CJ. Malaria. Lancet. 2018;391:1608–1621. doi: 10.1016/S0140-6736(18)30324-6. [DOI] [PubMed] [Google Scholar]
- 11.Chen Q, Zeng D, She Y, Lyu Y, Gong X, Feinstein MJ, et al. Different transmission routes and the risk of advanced HIV disease: a systematic review and network meta-analysis of observational studies. eClinicalMedicine. 2019;16:121–128. doi: 10.1016/j.eclinm.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmadpour E, Safarpour H, Xiao L, Zarean M, Hatam-Nahavandi K, Barac A, et al. Cryptosporidiosis in HIV-positive patients and related risk factors: a systematic review and meta-analysis. Parasite. 2020;27:27. doi: 10.1051/parasite/2020025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghate M, Deshpande S, Tripathy S, Nene M, Gedam P, Godbole S, et al. Incidence of common opportunistic infections in HIV-infected individuals in Pune, India: analysis by stages of immunosuppression represented by CD4 counts. Int J Infect Dis. 2009;13:e1–8. doi: 10.1016/j.ijid.2008.03.029. [DOI] [PubMed] [Google Scholar]
- 14.Safarpour H, Cevik M, Zarean M, Barac A, Hatam-Nahavandi K, Rahimi MT, et al. Global status of Toxoplasma gondii infection and associated risk factors in people living with HIV. AIDS. 2020;34:469–474. doi: 10.1097/QAD.0000000000002424. [DOI] [PubMed] [Google Scholar]
- 15.Ahmadpour E, Ghanizadegan MA, Razavi A, Kangari M, Seyfi R, Shahdust M, et al. Strongyloides stercoralis infection in human immunodeficiency virus-infected patients and related risk factors: a systematic review and meta-analysis. Transbound Emerg Dis. 2019;66:2233–2243. doi: 10.1111/tbed.13310. [DOI] [PubMed] [Google Scholar]
- 16.Alemu A, Shiferaw Y, Addis Z, Mathewos B, Birhan W. Effect of malaria on HIV/AIDS transmission and progression. Parasit Vectors. 2013;6:18. doi: 10.1186/1756-3305-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayisi JG, Van Eijk AM, Newman RD, Ter Kuile FO, Shi YP, Yang C, et al. Maternal malaria and perinatal HIV transmission, western Kenya. Emerg Infect Dis. 2004;10:643–652. doi: 10.3201/eid1004.030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naing C, Sandhu NK, Wai VN. The effect of malaria and HIV co-infection on anemia: a meta-analysis. Medicine. 2016;95:e3205. doi: 10.1097/MD.0000000000003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmadpour E, Foroutan-Rad M, Majidiani H, Moghaddam SM, Hatam-Nahavandi K, Hosseini S-A, et al. Transfusion–transmitted malaria: a systematic review and meta-analysis. Open Forum Infect Dis. 2019;11:ofz283. doi: 10.1093/ofid/ofz283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen-Dinh P, Greenberg A, Mann JM, Kabote N, Francis H, Colebunders R, et al. Absence of association between Plasmodium falciparum malaria and human immuno-deficiency virus infection in children in Kinshasa, Zaire. Bull World Health Organ. 1987;65:607–613. [PMC free article] [PubMed] [Google Scholar]
- 22.Villamor E, Fataki MR, Mbise RL, Fawzi WW. Malaria parasitaemia in relation to HIV status and vitamin A supplementation among pre-school children. Trop Med Int Health. 2003;8:1051–1061. doi: 10.1046/j.1360-2276.2003.01134.x. [DOI] [PubMed] [Google Scholar]
- 23.Otieno RO, Ouma C, Ong'echa JM, Keller CC, Were T, Waindi EN, et al. Increased severe anemia in HIV-1-exposed and HIV-1-positive infants and children during acute malaria. AIDS. 2006;20:275–280. doi: 10.1097/01.aids.0000200533.56490.b7. [DOI] [PubMed] [Google Scholar]
- 24.van Eijk AM, Ayisi JG, Ter Kuile FO, Slutsker L, Shi YP, Udhayakumar V, et al. HIV, malaria, and infant anemia as risk factors for postneonatal infant mortality among HIV-seropositive women in Kisumu, Kenya. J Infect Dis. 2007;196:30–37. doi: 10.1086/518441. [DOI] [PubMed] [Google Scholar]
- 25.Gasasira AF, Kamya MR, Achan J, Mebrahtu T, Kalyango JN, Ruel T, et al. High risk of neutropenia in HIV-infected children following treatment with artesunate plus amodiaquine for uncomplicated malaria in Uganda. Clin Infect Dis. 2008;46:985–991. doi: 10.1086/529192. [DOI] [PubMed] [Google Scholar]
- 26.Berkley JA, Bejon P, Mwangi T, Gwer S, Maitland K, Williams TN, et al. HIV infection, malnutrition, and invasive bacterial infection among children with severe malaria. Clin Infect Dis. 2009;49:336–343. doi: 10.1086/600299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiyingi HS, Egwang TG, Nannyonga M. Prolonged elevation of viral loads in HIV-1-infected children in a region of intense malaria transmission in Northern Uganda: a prospective cohort study. Pan Afr Med J. 2010;7:11. [PMC free article] [PubMed] [Google Scholar]
- 28.Imani PD, Musoke P, Byarugaba J, Tumwine JK. Human immunodeficiency virus infection and cerebral malaria in children in Uganda: a case–control study. BMC Pediatr. 2011;11:5. doi: 10.1186/1471-2431-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ezeamama AE, Spiegelman D, Hertzmark E, Bosch RJ, Manji KP, Duggan C, et al. HIV infection and the incidence of malaria among HIV-exposed children from Tanzania. J Infect Dis. 2012;205:1486–1494. doi: 10.1093/infdis/jis234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laar AK, Grant FE, Addo Y, Soyiri I, Nkansah B, Abugri J, et al. Predictors of fetal anemia and cord blood malaria parasitemia among newborns of HIV-positive mothers. BMC Res Notes. 2013;6:350. doi: 10.1186/1756-0500-6-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kyeyune FX, Calis JC, Phiri KS, Faragher B, Kachala D, Brabin BJ, et al. The interaction between malaria and human immunodeficiency virus infection in severely anaemic Malawian children: a prospective longitudinal study. Trop Med Int Health. 2014;19:698–705. doi: 10.1111/tmi.12295. [DOI] [PubMed] [Google Scholar]
- 32.Hochman SE, Madaline TF, Wassmer SC, Mbale E, Choi N, Seydel KB, et al. Fatal pediatric cerebral malaria is associated with intravascular monocytes and platelets that are increased with HIV coinfection. MBio. 2015;6:e01390–e1415. doi: 10.1128/mBio.01390-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smart LR, Orgenes N, Mazigo HD, Minde M, Hokororo A, Shakir M, et al. Malaria and HIV among pediatric inpatients in two Tanzanian referral hospitals: a prospective study. Acta Trop. 2016;159:36–43. doi: 10.1016/j.actatropica.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bate A, Kimbi HK, Lum E, Lehman LG, Onyoh EF, Ndip LM, et al. Malaria infection and anaemia in HIV-infected children in Mutengene, Southwest Cameroon: a cross sectional study. BMC Infect Dis. 2016;16:523. doi: 10.1186/s12879-016-1853-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwenti TE, Edo E, Ayuk BS, Kwenti TD. Prevalence of coinfection with malaria and HIV among children in Yaoundé, Cameroon: a cross-sectional survey performed in three communities in Yaoundé. Yangtze Med. 2017;1:178–188. doi: 10.4236/ym.2017.13018. [DOI] [Google Scholar]
- 36.Eki-Udoko FE, Sadoh AE, Ibadin MO, Omoigberale AI. Prevalence of congenital malaria in newborns of mothers co-infected with HIV and malaria in Benin city. Infect Dis (Lond) 2017;49:609–616. doi: 10.1080/23744235.2017.1312667. [DOI] [PubMed] [Google Scholar]
- 37.Onankpa BO, Jiya NM, Yusuf T. Malaria parasitemia in HIV-infected children attending antiretroviral therapy clinic in a teaching hospital. Sahel Med J. 2017;20:30–32. [Google Scholar]
- 38.Francesconi P, Fabiani M, Dente MG, Lukwiya M, Okwey R, Ouma J, et al. HIV, malaria parasites, and acute febrile episodes in Ugandan adults: a case–control study. AIDS. 2001;15:2445–2450. doi: 10.1097/00002030-200112070-00013. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed S, Ibrahim U. Malaria parasitemia in patients with acquired immune deficiency syndrome and opportunistic infections. Niger J Exp Appl Biol. 2002;3:339–343. [Google Scholar]
- 40.Uneke C, Ogbu O, Inyama P, Anyanwu G. Malaria infection in HIV-seropositive and HIV-seronegative individuals in Jos-Nigeria. J Vector Borne Dis. 2005;42:151–154. [PubMed] [Google Scholar]
- 41.Lewis DK, Whitty CJ, Walsh AL, Epino H, Broek NRVD, Letsky EA, et al. Treatable factors associated with severe anaemia in adults admitted to medical wards in Blantyre, Malawi, an area of high HIV seroprevalence. Trans R Soc Trop Med Hyg. 2005;99:561–567. doi: 10.1016/j.trstmh.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Laufer MK, van Oosterhout JJ, Thesing PC, Thumba F, Zijlstra EE, Graham SM, et al. Impact of HIV-associated immunosuppression on malaria infection and disease in Malawi. J Infect Dis. 2006;193:872–878. doi: 10.1086/500245. [DOI] [PubMed] [Google Scholar]
- 43.Tatfeng Y, Ihongbe J, Okodua M, Oviasogie F, Isibor J, Tchougang S, et al. CD4 count, viral load and parasite density of HIV positive individuals undergoing malaria treatment with dihydroartemisinin in Benin City, Edo State, Nigeria. J Vector Borne Dis. 2007;44:111–115. [PubMed] [Google Scholar]
- 44.Onyenekwe C, Ukibe N, Meludu S, Ilika A, Aboh N, Ofiaeli N, et al. Prevalence of malaria as co-infection in HIV-infected individuals in a malaria endemic area of southeastern Nigeria. J Vector Borne Dis. 2007;44:250–254. [PubMed] [Google Scholar]
- 45.Nkuo-Akenji T, Tevoufouet EE, Nzang F, Ngufor N, Fon E. High prevalence of HIV and malaria co-infection in urban Douala, Cameroon. Afr J AIDS Res. 2008;7:229–235. doi: 10.2989/AJAR.2008.7.2.8.525. [DOI] [PubMed] [Google Scholar]
- 46.Agwu E, Ihongbe J, Okogun G, Inyang N. High incidence of co-infection with malaria and typhoid in febrile HIV infected and AIDS patients in Ekpoma, Edo State, Nigeria. Braz J Microbiol. 2009;40:329–332. doi: 10.1590/S1517-83822009000200022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wariso KT, Nwauche CA. The prevalence of malaria antigen in the serum of HIV seropositive patients in port harcourt. Niger Health J. 2011;11:120–122. [Google Scholar]
- 48.Bharti AR, Saravanan S, Madhavan V, Smith DM, Sharma J, Balakrishnan P, et al. Correlates of HIV and malaria co-infection in Southern India. Malar J. 2012;1:306. doi: 10.1186/1475-2875-11-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Njunda LA, Kamga H-LF, Nsagha DS, Assob J-CN, Kwenti TE. Low malaria prevalence in HIV-positive patients in Bamenda, Cameroon. J Microbiol Res. 2012;2:56–59. doi: 10.5923/j.microbiology.20120203.03. [DOI] [Google Scholar]
- 50.Akinbo FO, Omoregie R. Plasmodium falciparum infection in HIV-infected patients on highly active antiretroviral therapy (HAART) in Benin City, Nigeria. J Res Health Sci. 2012;12:8–15. [PubMed] [Google Scholar]
- 51.Akinbo FO, Okaka CE, Omoregie R. Plasmodium falciparum and intestinal parasitic co-infections in HIV-infected patients in Benin City, Edo State, Nigeria. J Infect Dev Ctries. 2012;6:430–435. doi: 10.3855/jidc.1889. [DOI] [PubMed] [Google Scholar]
- 52.AkyalaIshaku A, Amuta E, Abiodun P, Agieni A. Malaria parasitaemia among seropositive drug naïve and drug experience HIV patient attending Federal Medical Center, Keffi, Nasarawa State-Nigeria. J Microbiol Immunol Res. 2012;1:6–9. [Google Scholar]
- 53.Iroezindu MO, Agaba EI, Okeke EN, Daniyam CA, Obaseki DO, Isa SE, et al. Prevalence of malaria parasitaemia in adult HIV-infected patients in Jos, north-central Nigeria. Niger J Med. 2012;21:209–213. [PubMed] [Google Scholar]
- 54.Sanyaolu AO, Fagbenro-Beyioku A, Oyibo W, Badaru O, Onyeabor O, Nnaemeka C. Malaria and HIV co-infection and their effect on haemoglobin levels from three healthcare institutions in Lagos, southwest Nigeria. Afr Health Sci. 2013;13:295–300. doi: 10.4314/ahs.v13i2.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akinbo FO, Anate PJ, Akinbo DB, Omoregie R, Okoosi S, Abdulsalami A, et al. Prevalence of malaria among HIV patients on highly active antiretroviral therapy in Kogi State, North Central Nigeria. Ann Niger Med. 2016;10:11. doi: 10.4103/0331-3131.189802. [DOI] [Google Scholar]
- 56.Wondimeneh Y, Ferede G, Atnafu A, Muluye D. HIV-malaria co-infection and their immunohematological profiles. Eur J Exp Biol. 2013;3:497–502. [Google Scholar]
- 57.Omoti CE, Ojide CK, Lofor PV, Eze E, Eze JC. Prevalence of parasitemia and associated immunodeficiency among HIV-malaria co-infected adult patients with highly active antiretroviral therapy. Asian Pac J Trop Med. 2013;6:126–130. doi: 10.1016/S1995-7645(13)60007-3. [DOI] [PubMed] [Google Scholar]
- 58.Falade C, Adesina-Adewole B, Dada-Adegbola H, Ajayi I, Akinyemi J, Ademowo O, et al. Evaluation of Paracheck-Pf TM rapid malaria diagnostic test for the diagnosis of malaria among HIV-positive patients in Ibadan, south-western Nigeria. Pathog Glob Health. 2013;107:69–77. doi: 10.1179/2047773213Y.0000000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Adu-Gyasi D, Fanello CI, Baiden F, Porter JD, Korbel D, Adjei G, et al. Prevalence of clinically captured and confirmed malaria among HIV seropositve clinic attendants in five hospitals in Ghana. Malar J. 2013;12:382. doi: 10.1186/1475-2875-12-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chijioke-Nwauche I, van Wyk A, Nwauche C, Beshir KB, Kaur H, Sutherland CJ. HIV-positive Nigerian adults harbor significantly higher serum lumefantrine levels than HIV-negative individuals seven days after treatment for Plasmodium falciparum infection. Antimicrob Agents Chemother. 2013;57:4146–4150. doi: 10.1128/AAC.02508-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Iliyasu Z, Babashani M, Abubakar IS, Salahudeen AA, Aliyu MH. Clinical burden and correlates of HIV and malaria co-infection, in northwest Nigeria. Acta Trop. 2013;128:630–635. doi: 10.1016/j.actatropica.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 62.Berg A, Patel S, Aukrust P, David C, Gonca M, Berg ES, et al. Increased severity and mortality in adults co-infected with malaria and HIV in Maputo, Mozambique: a prospective cross-sectional study. PLoS ONE. 2014;9:e88257. doi: 10.1371/journal.pone.0088257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ojurongbe O, Oyeniran OA, Alli OAT, Taiwo SS, Ojurongbe TA, Olowe AO, et al. Prevalence of Plasmodium falciparum parasitaemia and its correlation with haematological parameters among HIV-positive individuals in Nigeria. J Trop Med. 2014;2014:1–6. doi: 10.1155/2014/161284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rutto EK, Nyagol J, Oyugi J, Ndege S, Onyango N, Obala A, et al. Effects of HIV-1 infection on malaria parasitemia in milo sub-location, western Kenya. BMC Res Notes. 2015;8:303. doi: 10.1186/s13104-015-1270-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alemayehu G, Melaku Z, Abreha T, Alemayehu B, Girma S, Tadesse Y, et al. Burden of malaria among adult patients attending general medical outpatient department and HIV care and treatment clinics in Oromia, Ethiopia: a comparative cross-sectional study. Malar J. 2015;14:501. doi: 10.1186/s12936-015-1029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Agwu E, Nyakerario E, Moazzam M. Updates on Malaria parasites distribution among HIV infected and AIDS patients in Comboni Hospital, Uganda. Specif Parasites Pathog J (SPPJ) 2015;1:29–35. [Google Scholar]
- 67.Unata IM, Bunza NM, Ashcroft OF, Abubakar A, Faruk N. Prevalence of malaria parasites among HIV/AIDS patients attending HIV clinic in Usmanu Danfodiyo university teaching hospital and Sokoto State specialist hospital Sokoto, Nigeria. Int J Nov Res Life Sci. 2015;2:39–43. [Google Scholar]
- 68.Tay SC, Badu K, Mensah AA, Gbedema SY. The prevalence of malaria among HIV seropositive individuals and the impact of the co-infection on their hemoglobin levels. Ann Clin Microbiol Antimicrob. 2015;14:10. doi: 10.1186/s12941-015-0064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Edet U, Ebana R, Etok C, Nwamuo L. Prevalence of human immunodeficiency virus and Plasmodium falciparum dual infection amongst residents of Kaduna South in north western Nigeria. Int J Trop Dis Health. 2016;17:1–7. [Google Scholar]
- 70.Katrak S, Day N, Ssemmondo E, Kwarisiima D, Midekisa A, Greenhouse B, et al. Community-wide prevalence of malaria parasitemia in HIV-infected and uninfected populations in a high-transmission setting in Uganda. J Infect Dis. 2016;213:1971–1978. doi: 10.1093/infdis/jiw057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Njunda AL, Njumkeng C, Nsagha SD, Assob JCN, Kwenti TE. The prevalence of malaria in people living with HIV in Yaounde, Cameroon. BMC Public Health. 2016;16:964. doi: 10.1186/s12889-016-3647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Daniel L, Chessed G, Joseph R, Haruna Y, Yako A, Atinga A. Malaria and HIV co-infection among HIV patients attending hospitals in Yola, Adamawa State Nigeria. J Med Lab Sci. 2016;1:215–220. [Google Scholar]
- 73.Okonkwo I, Ibadin M, Sadoh W, Omoigberale A. A study of malaria parasite density in HIV-1 positive under-fives in Benin City, Nigeria. J Trop Pediatr. 2018;64:289–296. doi: 10.1093/tropej/fmx065. [DOI] [PubMed] [Google Scholar]
- 74.Zheng X, Lin M, Xie DD, Li J, Chen JT, Eyi UM, et al. Prevalence of HIV and malaria: a cross-sectional study on Bioko Island, Equatorial Guinea. Afr J AIDS Res. 2017;16:65–70. doi: 10.2989/16085906.2016.1257495. [DOI] [PubMed] [Google Scholar]
- 75.Beyene HB, Tadesse M, Disassa H, Beyene MB. Concurrent Plasmodium infection, anemia and their correlates among newly diagnosed people living with HIV/AIDS in Northern Ethiopia. Acta Trop. 2017;169:8–13. doi: 10.1016/j.actatropica.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 76.Mohapatra PK, Pachuau E, Kumar C, Borkakoty B, Zomawia E, Singh A, et al. HIV-malaria interactions in North-East India: a prospective cohort study. Indian J Med Res. 2017;145:387–394. doi: 10.4103/ijmr.IJMR_1427_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sahle T, Yemane T, Gedefaw L. Effect of malaria infection on hematological profiles of people living with human immunodeficiency virus in Gambella, southwest Ethiopia. BMC Hematol. 2017;17:2. doi: 10.1186/s12878-017-0072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jegede FE, Oyeyi TI, Abdulrahman SA, Mbah HA, Badru T, Agbakwuru C, et al. Effect of HIV and malaria parasites co-infection on immune-hematological profiles among patients attending anti-retroviral treatment (ART) clinic in Infectious Disease Hospital Kano, Nigeria. PLoS ONE. 2017;12:e0174233. doi: 10.1371/journal.pone.0174233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bouyou-Akotet MK, Lengongo JVK, Mboumba DPM, Ondounda M, Kendjo E, Nkoumou MO. Comparison of asymptomatic and clinical malaria frequencies between HIV positive and HIV negative individuals living in Gabon. Am J Trop Med Hyg. 2017;95:546. [Google Scholar]
- 80.Amadi CP, Ikon GM, Inyang UC. Current prevalence of falciparum malarial infection among HIV patients on highly active antiretroviral therapy in University of Uyo teaching Hospital, Uyo, Nigeria. Int J Res Med Sci. 2018;6:2916–2922. doi: 10.18203/2320-6012.ijrms20183627. [DOI] [Google Scholar]
- 81.Wondimeneh Y, Gebrecherkos T, Muluye D, Damtie D, Ferede G. HIV and malaria infections and associated risk factors among febrile illness patients in Northwest Ethiopia. Turkiye Parazitol Derg. 2018;42:180–186. doi: 10.5152/tpd.2018.5878. [DOI] [PubMed] [Google Scholar]
- 82.Akinyotu O, Bello F, Abdus-Salam R, Arowojolu A. Comparative study of mefloquine and sulphadoxine–pyrimethamine for malaria prevention among pregnant women with HIV in southwest Nigeria. Int J Gynaecol Obstet. 2018;142:194–200. doi: 10.1002/ijgo.12516. [DOI] [PubMed] [Google Scholar]
- 83.Bello B, Ishaleku D. Prevalence of malaria infection among people living with HIV/AIDS at Federal Medica l Center Keffi (Nasarawa State), Nigeria. J Adv Microbiol. 2018;11:1–6. [Google Scholar]
- 84.Di Gennaro F, Marotta C, Pizzol D, Chhaganlal K, Monno L, Putoto G, et al. Prevalence and predictors of malaria in human immunodeficiency virus infected patients in Beira, Mozambique. Int J Environ Res Public Health. 2018;15:2032. doi: 10.3390/ijerph15092032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Owusu ED, Djonor SK, Brown CA, Grobusch MP, Mens PF. Plasmodium falciparum diagnostic tools in HIV-positive under-5-year-olds in two ART clinics in Ghana: are there missed infections? Malar J. 2018;17:17–92. doi: 10.1186/s12936-018-2231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mbah-Mbole FG, Tufon KA, Meriki DH, Enow-Orock G, Mbah-Mbole P, Njunda LA, et al. Malaria and human immunodeficiency virus coinfection in febrile patients attending the Regional Hospital of Buea, Southwest region, Cameroon. Int J Adv Med Health Res. 2019;6:46–51. doi: 10.4103/IJAMR.IJAMR_24_19. [DOI] [Google Scholar]
- 87.Gumel S, Ibrahim A, Olayinka A, Ibrahim M, Balogun M, Dahiru A, et al. HIV-malaria co-infection and its determinants among patients attending antiretroviral treatment clinic in Zaria, Kaduna state, Nigeria. J Interval Epidemiol Public Health. 2021;4:2. [Google Scholar]
- 88.AL-Nahari W, Abdelkreem E, Abdueghni S, Omer A, Estimation of Plasmodium falciparum among HIV patients in Khartoum-Sudan. Int J Sci Res. 2019;8:15–17. [Google Scholar]
- 89.Sandie SM, Sumbele IUN, Tasah MM, Kimbi HK. Malaria parasite prevalence and Haematological parameters in HIV seropositive patients attending the regional hospital Limbe, Cameroon: a hospital-based cross-sectional study. BMC Infect Dis. 2019;19:988. doi: 10.1186/s12879-019-4629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Okparaku S, Emelike O, Dic-Ijiewere E, Idehen C, Airhomwanbor K, Ene I. Malaria parasites burden at various stages of human immunodeficiency virus (HIV) infection. J Med Lab Sci. 2019;29:61–71. [Google Scholar]
- 91.Alaofin OS, Naidoo K, Sibanda W. Socio-economic determinants of HIV-malaria co-infection among adults in the North Central Zone, Nigeria. Glob J Health Sci. 2020;12:1–9. [Google Scholar]
- 92.Kelechi NC, Ositadinma I, Isaac N. Selected immunoglobulins (IgA, IgG, IgM) and lambda free light chain levels in persons with HIV-malaria co-infection. Int J Health Sci Res. 2020;10:134–144. [Google Scholar]
- 93.Munyenyembe AU, Gausi K, Hiestand J, Mallewa J, Mandala W. The effect of frequent exposure to P. falciparum, HIV-infection and other co-morbidities on development of severe malaria in Malawian Adults. Infect Drug Resist. 2020;13:63–68. doi: 10.2147/IDR.S230112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Verhoeff FH, Brabin BJ, Hart CA, Chimsuku L, Kazembe P, Broadhead RL. Increased prevalence of malaria in HIV-infected pregnant women and its implications for malaria control. Trop Med Int Health. 1999;4:5–12. doi: 10.1046/j.1365-3156.1999.00349.x. [DOI] [PubMed] [Google Scholar]
- 95.Ladner J, Leroy V, Simonon A, Karita E, Bogaerts J, De Clercq A, et al. HIV infection, malaria, and pregnancy: a prospective cohort study in Kigali, Rwanda. Am J Trop Med Hyg. 2002;66:56–60. doi: 10.4269/ajtmh.2002.66.56. [DOI] [PubMed] [Google Scholar]
- 96.Ayisi JG, Van Eijk AM, Ter Kuile FO, Kolczak MS, Otieno JA, Misore AO, et al. The effect of dual infection with HIV and malaria on pregnancy outcome in western Kenya. AIDS. 2003;17:585–594. doi: 10.1097/00002030-200303070-00014. [DOI] [PubMed] [Google Scholar]
- 97.Mwapasa V, Rogerson SJ, Molyneux ME, Abrams ET, Kamwendo DD, Lema VM, et al. The effect of Plasmodium falciparum malaria on peripheral and placental HIV-1 RNA concentrations in pregnant Malawian women. AIDS. 2004;18:1051–1059. doi: 10.1097/00002030-200404300-00014. [DOI] [PubMed] [Google Scholar]
- 98.Mount AM, Mwapasa V, Elliott SR, Beeson JG, Tadesse E, Lema VM, et al. Impairment of humoral immunity to Plasmodium falciparum malaria in pregnancy by HIV infection. Lancet. 2004;363:1860–1867. doi: 10.1016/S0140-6736(04)16354-X. [DOI] [PubMed] [Google Scholar]
- 99.Gallagher M, Malhotra I, Mungai PL, Wamachi AN, Kioko JM, Ouma JH, et al. The effects of maternal helminth and malaria infections on mother-to-child HIV transmission. AIDS. 2005;19:1849–1855. doi: 10.1097/01.aids.0000189846.90946.5d. [DOI] [PubMed] [Google Scholar]
- 100.Brahmbhatt H, Sullivan D, Kigozi G, Askin F, Wabwire-Mangenm F, Serwadda D, et al. Association of HIV and malaria with mother-to-child transmission, birth outcomes, and child mortality. J Acquir Immune Defic Syndr. 2008;47:472–476. doi: 10.1097/QAI.0b013e318162afe0. [DOI] [PubMed] [Google Scholar]
- 101.Newman PM, Wanzira H, Tumwine G, Arinaitwe E, Waldman S, Achan J, et al. Placental malaria among HIV-infected and uninfected women receiving anti-folates in a high transmission area of Uganda. Malaria J. 2009;8:254. doi: 10.1186/1475-2875-8-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Heven S. The Prevalence of HIV/Malaria co-infection during pregnancy in Adama Hospital and ‘Awash Sebat Kilo’ Health Center, Ethiopia. PhD thesis. Addis Ababa: Addis Ababa University. 2009. http://etd.aau.edu.et/handle/123456789/5521.
- 103.Franke MF, Spiegelman D, Ezeamama A, Aboud S, Msamanga GI, Mehta S, et al. Malaria parasitemia and CD4 T cell count, viral load, and adverse HIV outcomes among HIV-infected pregnant women in Tanzania. Am J Trop Med Hyg. 2010;82:556–562. doi: 10.4269/ajtmh.2010.09-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thigpen MC, Filler SJ, Kazembe PN, Parise ME, Macheso A, Campbell CH, et al. Associations between peripheral Plasmodium falciparum malaria parasitemia, human immunodeficiency virus, and concurrent helminthic infection among pregnant women in Malawi. Am J Trop Med Hyg. 2011;84:379–385. doi: 10.4269/ajtmh.2011.10-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nkhoma ET, Kalilani-Phiri L, Mwapasa V, Rogerson SJ, Meshnick SR. Effect of HIV infection and Plasmodium falciparum parasitemia on pregnancy outcomes in Malawi. Am J Trop Med Hyg. 2012;87:29–34. doi: 10.4269/ajtmh.2012.11-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Adeoti O, Anumudu C, Nwuba R, Awobode H, Olaniyan M, Olayiwola O, et al. Prevalence of HIV and malaria parasites co-infection in pregnant mothers and their babies post delivery. J Biol Agric Healthcare. 2012;2:59–64. [Google Scholar]
- 107.Asmamaw T, Alemu A, Alemu A, Unakal C. Prevalence of malaria and HIV among pregnant women attending antenatal clinics at Felege Hiwot referral hospital and Addis Zemen health center. Int J Life Sci Biotechnol Pharma Res. 2013;2:81–91. [Google Scholar]
- 108.Uju MD, Lorina B-E, Joseph AU. HIV and malaria co-infection: their combined effects on pregnancy outcomes in Anambra State, Southeast Nigeria. Int J Med Med Sci. 2013;5:438–449. [Google Scholar]
- 109.Ivan E, Crowther NJ, Mutimura E, Osuwat LO, Janssen S, Grobusch MP. Helminthic infections rates and malaria in HIV-infected pregnant women on anti-retroviral therapy in Rwanda. PLoS Negl Trop Dis. 2013;7:e2380. doi: 10.1371/journal.pntd.0002380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ako-Nai KA, Ebhodaghe BI, Osho P, Adejuyigbe E, Adeyemi FM, Kassim O. Preponderance of bacterial isolates in urine of HIV-positive malaria-infected pregnant women with urinary tract infection. J Infect Dev Ctries. 2014;8:1591–1600. doi: 10.3855/jidc.4854. [DOI] [PubMed] [Google Scholar]
- 111.Iriemenam NC, Pandey JP, Williamson J, Blackstock AJ, Yesupriya A, Namboodiri AM, et al. Association between immunoglobulin GM and KM genotypes and placental malaria in HIV-1 negative and positive women in western Kenya. PLoS ONE. 2013;8:e53948. doi: 10.1371/journal.pone.0053948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Duvignaud A, Denoeud-Ndam L, Akakpo J, Agossou KV, Afangnihoun A, Komongui DG, et al. Incidence of malaria-related fever and morbidity due to Plasmodium falciparum among HIV1-infected pregnant women: a prospective cohort study in South Benin. Malar J. 2014;13:255. doi: 10.1186/1475-2875-13-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Manyanga VP, Minzi O, Ngasala B. Prevalence of malaria and anaemia among HIV infected pregnant women receiving co-trimoxazole prophylaxis in Tanzania: a cross sectional study in Kinondoni Municipality. BMC Pharmacol Toxicol. 2014;15:24. doi: 10.1186/2050-6511-15-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Houmsou R, Wama B, Elkanah S, Garba L, Hile T, Bingbeng J, et al. Malarial infection in HIV infected pregnant women attending a rural antenatal clinic in Nigeria. Adv Epidemiol. 2014;2014:1–6. doi: 10.1155/2014/694213. [DOI] [Google Scholar]
- 115.Olusi T, Abe A. Co-infection of HIV and malaria parasites in pregnant women attending major ante-natal health facilities in Akure, Ondo State, Nigeria. J Parasitol Vector Biol. 2014;6:124–130. [Google Scholar]
- 116.Johnbull OS, Uche AP, Kesiena AJ, Francis FA, Oyemocho A, Obianwu I, et al. Prevalence and risk factors of malaria in HIV-infected pregnant women on anti-retroviral therapy in Enugu, South East Nigeria. J AIDS Clin Res. 2014;5:321–326. [Google Scholar]
- 117.González R, Mombo-Ngoma G, Ouédraogo S, Kakolwa MA, Abdulla S, Accrombessi M, et al. Intermittent preventive treatment of malaria in pregnancy with mefloquine in HIV-negative women: a multicentre randomized controlled trial. PLoS Med. 2014;11:e1001733. doi: 10.1371/journal.pmed.1001733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wumba RD, Zanga J, Aloni MN, Mbanzulu K, Kahindo A, Mandina MN, et al. Interactions between malaria and HIV infections in pregnant women: a first report of the magnitude, clinical and laboratory features, and predictive factors in Kinshasa, the Democratic Republic of Congo. Malar J. 2015;14:82. doi: 10.1186/s12936-015-0598-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chaponda EB, Chandramohan D, Michelo C, Mharakurwa S, Chipeta J, Chico RM. High burden of malaria infection in pregnant women in a rural district of Zambia: a cross-sectional study. Malar J. 2015;14:380. doi: 10.1186/s12936-015-0866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ezeamama A, Duggan C, Manji K, Spiegelman D, Hertzmark E, Bosch R, et al. Clinical malaria diagnosis in pregnancy in relation to early perinatal mother-to-child transmission of HIV: a prospective cohort study. HIV Med. 2014;15:276–285. doi: 10.1111/hiv.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ibitokou SA, Denoeud-Ndam L, Ezinmegnon S, Ladékpo R, Zannou D-M, Massougbodji A, et al. Insights into circulating cytokine dynamics during pregnancy in HIV-infected Beninese exposed to Plasmodium falciparum malaria. Am J Trop Med Hyg. 2015;93:287–292. doi: 10.4269/ajtmh.14-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stover J, Bollinger L, Izazola JA, Loures L, DeLay P, Ghys PD, et al. What is required to end the AIDS epidemic as a public health threat by 2030? The cost and impact of the fast-track approach. PLoS ONE. 2016;11:e0154893. doi: 10.1371/journal.pone.0154893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hochman S, Kim K. The impact of HIV and malaria coinfection: what is known and suggested venues for further study. Interdiscip Perspect Infect Dis. 2009;2009:617954. doi: 10.1155/2009/617954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.WHO. World malaria report 2018. 2018. https://www.who.int/malaria/publications/world-malaria-report-2018/report/en/. Accessed 3 Feb 2019.
- 125.Dhiman S. Are malaria elimination efforts on right track? An analysis of gains achieved and challenges ahead. Infect Dis Poverty. 2019;8:14. doi: 10.1186/s40249-019-0524-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Karunamoorthi K. Vector control: a cornerstone in the malaria elimination campaign. Clin Microbiol Infect. 2011;17:1608–1616. doi: 10.1111/j.1469-0691.2011.03664.x. [DOI] [PubMed] [Google Scholar]
- 127.Wang X, Zhao X-Q. A climate-based malaria model with the use of bed nets. J Math Biol. 2018;77:1–25. doi: 10.1007/s00285-017-1183-9. [DOI] [PubMed] [Google Scholar]
- 128.Malhotra I, Dent A, Mungai P, Wamachi A, Ouma JH, Narum DL, et al. Can prenatal malaria exposure produce an immune tolerant phenotype?: A prospective birth cohort study in Kenya. PLoS Med. 2009;6:e1000116. doi: 10.1371/journal.pmed.1000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rubio EV, Gahona RG. Vertical transmission of HIV—medical diagnosis, therapeutic options and prevention strategy. In: Okechukwu IB, editor. Trends in basic and therapeutic options in HIV infection: towards a functional cure. London: IntechOpen; 2015. 10.5772/61202.
- 130.Morelli SS, Mandal M, Goldsmith LT, Kashani BN, Ponzio NM. The maternal immune system during pregnancy and its influence on fetal development. Res Rep Biol. 2015;6:171–189. [Google Scholar]
- 131.Van Geertruyden JP. Interactions between malaria and human immunodeficiency virus anno 2014. Clin Microbiol Infect. 2014;20:278–285. doi: 10.1111/1469-0691.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Grimwade K, French N, Mbatha DD, Zungu DD, Dedicoat M, Gilks CF. Childhood malaria in a region of unstable transmission and high human immunodeficiency virus prevalence. Pediatr Infect Dis J. 2003;22:1057–1063. doi: 10.1097/01.inf.0000101188.95433.60. [DOI] [PubMed] [Google Scholar]
- 133.Kimbi HK, Njoh D, Ndamukong K, Lehman L. Malaria in HIV/AIDS patients at different CD4+ T cell levels in Limbe, Cameroon. J Bacteriol Parasitol. 2013;4:164. [Google Scholar]
- 134.Winskill P, Rowland M, Mtove G, Malima RC, Kirby MJ. Malaria risk factors in north-east Tanzania. Malar J. 2011;10:98. doi: 10.1186/1475-2875-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mfueni E, Devleesschauwer B, Rosas-Aguirre A, Van Malderen C, Brandt PT, Ogutu B, et al. True malaria prevalence in children under five: Bayesian estimation using data of malaria household surveys from three sub-Saharan countries. Malar J. 2018;17:65. doi: 10.1186/s12936-018-2211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ned RM, Moore JM, Chaisavaneeyakorn S, Udhayakumar V. Modulation of immune responses during HIV–malaria co-infection in pregnancy. Trends Parasitol. 2005;21:284–291. doi: 10.1016/j.pt.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 137.Fortin PM, Hopewell S, Estcourt LJ. Red blood cell transfusion to treat or prevent complications in sickle cell disease: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2018;8:CD012082. doi: 10.1002/14651858.CD012082.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fleming AF. HIV and blood transfusion in sub-Saharan Africa. Transfus Sci. 1997;18:167–179. doi: 10.1016/S0955-3886(97)00006-4. [DOI] [PubMed] [Google Scholar]
- 139.Artavanis-Tsakonas K, Tongren J, Riley E. The war between the malaria parasite and the immune system: immunity, immunoregulation and immunopathology. Clin Exp Immunol. 2003;133:145–152. doi: 10.1046/j.1365-2249.2003.02174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lawn S. AIDS in Africa: the impact of coinfections on the pathogenesis of HIV-1 infection. J Infect. 2004;48:1–12. doi: 10.1016/j.jinf.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 141.Patnaik P, Jere CS, Miller WC, Hoffman IF, Wirima J, Pendame R, et al. Effects of HIV-1 serostatus, HIV-1 RNA concentration, and CD4 cell count on the incidence of malaria infection in a cohort of adults in rural Malawi. J Infect Dis. 2005;192:984–991. doi: 10.1086/432730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Funnel plot of standard error by logit event rate to assess publication or other types of bias across prevalence studies. Studies based on the prevalence of malaria in HIV patients: children (A), adults (B), and pregnant women (C).
Additional file 2: Table S1. Summary score for methodological quality of analytic cross-sectional studies.
Additional file 3: Table S2. Summary score for methodological quality of analytic case–control studies.
Additional file 4: Table S3. Summary score for methodological quality of analytic cohort studies.
Additional file 5: Table S4. Summary score for methodological quality of analytic RCT studies.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.