Abstract
Background
Preterm birth is serious public health worldwide, and early prediction of preterm birth in pregnant women may provide assistance for timely intervention and reduction of preterm birth. This study aimed to develop a preterm birth prediction model that is readily available and convenient for clinical application.
Methods
Data used in this case-control study were extracted from the National Vital Statistics System (NVSS) database between 2018 and 2019. Univariate and multivariate logistic regression analyses were utilized to find factors associated with preterm birth. Odds ratio (OR) and 95% confidence interval (CI) were used as effect measures. The area under the curve (AUC), accuracy, sensitivity, and specificity were utilized as model performance evaluation metrics.
Results
Data from 3,006,989 pregnant women in 2019 and 3,039,922 pregnant women in 2018 were used for the model establishment and external validation, respectively. Of these 3,006,989 pregnant women, 324,700 (10.8%) had a preterm birth. Higher education level of pregnant women [bachelor (OR = 0.82; 95%CI, 0.81–0.84); master or above (OR = 0.82; 95%CI, 0.81–0.83)], pre-pregnancy overweight (OR = 0.96; 95%CI, 0.95–0.98) and obesity (OR = 0.94; 95%CI, 0.93–0.96), and prenatal care (OR = 0.48; 95%CI, 0.47–0.50) were associated with a reduced risk of preterm birth, while age ≥ 35 years (OR = 1.27; 95%CI, 1.26–1.29), black race (OR = 1.26; 95%CI, 1.23–1.29), pre-pregnancy underweight (OR = 1.26; 95%CI, 1.22–1.30), pregnancy smoking (OR = 1.27; 95%CI, 1.24–1.30), pre-pregnancy diabetes (OR = 2.08; 95%CI, 1.99–2.16), pre-pregnancy hypertension (OR = 2.22; 95%CI, 2.16–2.29), previous preterm birth (OR = 2.95; 95%CI, 2.88–3.01), and plurality (OR = 12.99; 95%CI, 12.73–13.24) were related to an increased risk of preterm birth. The AUC and accuracy of the prediction model in the testing set were 0.688 (95%CI, 0.686–0.689) and 0.762 (95%CI, 0.762–0.763), respectively. In addition, a nomogram based on information on pregnant women and their spouses was established to predict the risk of preterm birth in pregnant women.
Conclusions
The nomogram for predicting the risk of preterm birth in pregnant women had a good performance and the relevant predictors are readily available clinically, which may provide a simple tool for the prediction of preterm birth.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12887-022-03591-w.
Keywords: Preterm birth, Prediction, Nomogram, Area under the curve
Background
Preterm birth, defined as delivery of less than 37 completed gestational weeks [1, 2], may lead to high rates of maternal and perinatal morbidity and mortality worldwide. The global rate of preterm birth is approximately 11%, affecting 15 million newborns every year, and preterm birth is the leading cause of child mortality, accounting for 35% of all child deaths [3]. In the United State, the preterm delivery rate due to insufficient gestation age is approximately 9–10% [4]. The annual economic burden attributed to preterm birth is more than $5.8 billion and was about 47% of medical care costs for all infant hospitalizations [5]. Despite the survival rates of preterm birth infants having increased these years, the disability of the infants has increased [6]. Prediction of preterm birth is important due to the enormous personal, economic and health implications of preterm birth. These predictions could provide reassurance for women who are less likely to give birth early while providing interventions for women who are likely to deliver prematurely.
Previous studies have reported that the risk of preterm birth was associated with age, race, smoking, economic status, and previous preterm birth [7, 8]. A single predictor may be weak in predicting preterm birth, while a better prediction can be obtained by combining a predictive model of multiple predictors [9]. Kim et al. conducted a systematic review summarizing current predictive models for predicting the risk of preterm birth [10]. The area under the receiver operating characteristic curve (AUC) for predicting preterm birth in these studies varied from 62 to 80%, and the effect of prediction was related to the number of predictors, populations, and the period of data (the first trimester, second trimester, etc.) [11–14]. Predicting the risk of preterm birth based on pre-pregnancy or first trimester data is more meaningful for the implementation of interventions. However, models with good predictive performance in this regard are rarely reported.
In this study, we aimed to establish a model to predict maternal preterm birth using baseline information that can predict preterm birth with the information readily available in clinical trials. Furthermore, external validation was conducted to assess the prediction ability of the model.
Materials and methods
Data source and participants
Data used in this case-control study were extracted from the National Vital Statistics System (NVSS) database between 2018 and 2019 [15]. The NVSS compiles the information from birth certificates and makes data files for each year, which is open access. The National Center for Health Statistics (NCHS) receives these electronic information files from the registration offices of all regions through the Vital Statistics Cooperative Program. The NVSS database has detailed data on each of the nearly 4 million births and 2.5 million deaths in the United States each year, including age, sex, race and ethnicity, and detailed geographic information. In addition, key indicators available in vital statistics such as infant mortality, access to antenatal care, maternal risk factors and pregnancy history, adolescent birth rates, etc. are included. Women with complete gestational age information were included in the study. Exclusion criteria were as follows: (1) missing data on the number of fetuses; (2) pregnant women and their spouses with incomplete basic information, including age, race, education. A total of 3,006,989 pregnant women in 2019 and 3,039,922 pregnant women in 2018 were extracted for analysis. The data used in this study from the open access NVSS database, and the relevant information of participants was anonymized and did not involve human intervention. Therefore, this study was granted exemption ethics approval by the Ethics Committee of Beijing Haidian Maternal and Child Healthcare Hospital.
Data collection
Data of pregnant women were collected including age (< 35 years and ≥ 35 years), race (white, black, and others), education (high school or below, bachelor, and master or above), pre-pregnancy body mass index (BMI) (underweight, normal, overweight, and obesity), prenatal care (yes or no), pregnancy smoking (yes or no), pre-pregnancy diabetes (yes or no), gestation diabetes (yes or no), pre-pregnancy hypertension (yes or no), gestation hypertension (yes or no), hypertension eclampsia (yes or no), previous preterm birth (yes or no), infection (yes or no), plurality (yes or no), and preterm birth (yes or no). In addition, the age, race, and education of the pregnant spouse were also collected. The outcome of this study was preterm birth.
Definition
Preterm birth
Preterm birth means births occurring before 37 completed weeks of gestation are preterm for purposes of classification consistent with the ICD-9 (International Classification of Diseases, Ninth Revision) and ICD-10 (International Classification of Diseases, Tenth Revision) definitions.
Education
Educational status was divided into three categories, high school or below, bachelor, and master or above. 8th grade or less, 9th through 12th grade with no diploma, high school graduate or GED completed, some college credit, but not a degree, associate degree (AA, AS) combined into high school and below. Master’s degree (MA, MS, MEng, MEd, MSW, MBA) and doctorate (PhD, EdD) or professional degree (MD, DDS, DVM, LLB, JD) merged into master’s degree and above.
Pre-pregnancy BMI
Pre-pregnancy BMI was calculated as: [mother’s pre-pregnancy weight (lb) / [mother’s height (in)]2] * 703 [16]. Pre-pregnancy BMI: underweight (< 18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25–29.9 kg/m2), obesity (≥30 kg/m2). In this study Obesity I: 30.0–34.9, Obesity II: 35.0–39.9, Obesity III: ≥40.0 combined into obesity (≥30 kg/m2).
Prenatal care
Information on the timing and number of prenatal care visits was collected from the items “Date of first prenatal visit” (with a checkbox for “No prenatal care”) and “Total number of prenatal visits for this pregnancy.”
Smoking
All entries reporting packs of cigarettes are converted to the corresponding number of cigarettes (1 pack = 20 cigarette). If the mother reported smoking in any of the three trimesters of pregnancy she was classified as a smoker (smoked anytime during pregnancy).
Infections
Infections include gonorrhea, syphilis, chlamydia, hepatitis B and hepatitis C.
Plurality
Plurality was defined as twin, triplet, quadruplet, and quintuplet and higher-order births.
Model development and validation
The 2019 data were randomly divided into the training set and testing set with a ratio of 1:1. Univariate and multivariate logistic regression analyses were conducted. Variables that were statistically significant in univariate analysis were included in multivariate analysis using backward stepwise regression. The odds ratio (OR) and 95% confidence interval (CI) were used to assess the effect of the variable on preterm birth. Characteristics of the pregnant women and their spouses (age, race, education) and variables that were statistically significant in the multivariate regression analysis were included in the prediction model. The 2018 data were utilized for external validation of the predictive model. The performance of the predictive model was assessed by area under the curve (AUC), accuracy, sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV). A nomogram used to predict whether a pregnant woman had a preterm birth was drawn.
Sample size and model power
The sample size was calculated using the PASS 15.0.5 software (NCSS, LLC, Kaysville, UT, USA). Calculations of sample size and model power were shown in Supplement Fig. 1. The proportions of the general pregnant population who experienced age ≥ 35 (17.2%), pre-pregnancy underweight (6.0%), pregnancy smoking (5.2%), gestation diabetes (11.0%), gestation hypertension (11.6%), and previous preterm birth (3.9%) were used to determine the sample size for this study [17–19]. Having experience of pre-pregnancy underweight was chosen as the independent variable since it obtained a higher sample size among the other calculated explanatory variables. The sample size calculation was as follows: the proportion of pregnancy women having experiences of pre-pregnancy underweight was 6.0%, a detectable odds ratio of 1.17, confidence level of 95% (α = 0.05, two-sided test), and power of 95%. The minimum total sample size was calculated to be 31,156. After adding a 5% non-response rate, the total calculated sample size was 32,714. The sample size of the training set and the testing set in this study were 1,503,495 and 1,503,494, respectively, which fully met the needs of the analysis. In addition, the power of the model was calculated to be 1.000 based on the AUC of the model in the testing set of 0.688 and the preterm birth rate of 10.8% (162,269/1,503,494).
Statistical analysis
Categorical variables were described in numbers and percentages [n (%)] and the groups were compared using χ2 tests or Fisher’s exact tests. SAS 9.4 software (SAS Institute Inc., Cary, NC, USA) was used for analysis. R 4.02 software (Institute for Statistics and Mathematics, Vienna, Austria) was used to draw logistic prediction model nomogram. Python 3.7.3 software (Python Software Foundation, Delaware, USA) was utilized to calculate the AUC, accuracy, sensitivity, specificity, NPV, and PPV values. All statistical tests were used two-sided tests, and P < 0.05 was considered the difference to be statistically significant. Statistical power testing was performed using G*Power 3.1.9.7.
Results
Characteristics of participants
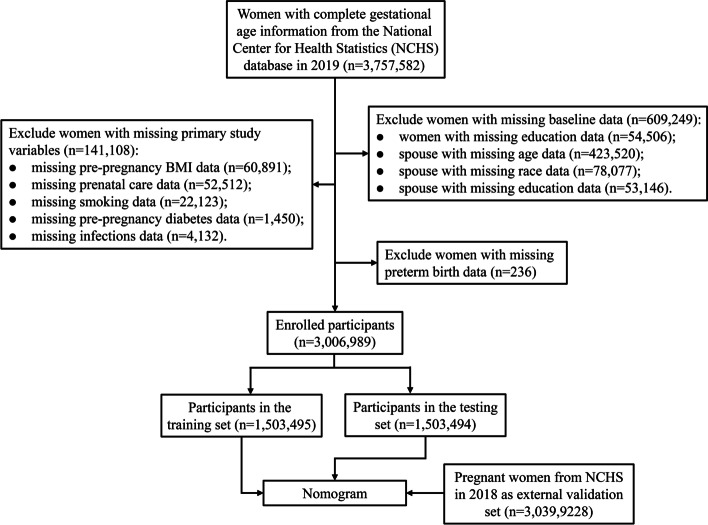
Data of 3,757,582 pregnant women were extracted in 2019, and after excluding 750,593 pregnant women with incomplete information, a total of 3,006,989 women’s information were used for analysis (Fig. 1). Of these participants, 324,700 (10.80%) had preterm birth, 2,415,844 (80.34%) were < 35 years, 2,306,813 (76.72%) were whites, 431,342 (14.34%) were master or above, 1,254,721 (41.73%) had normal pre-pregnancy BMI, 28,389 (0.94%) had pre-pregnancy diabetes, 62,970 (2.09%) had pre-pregnancy hypertension, 230,989 (7.68%) had gestation hypertension, 7630 (0.25%) had hypertension eclampsia, 100,366 (3.34%) had previous preterm birth, and 94,853 (3.15%) had plurality. In addition, a total of 3,039,922 pregnant women in 2018 were included for external validation. Detailed characteristics of pregnant women in 2019 and 2018 were displayed in Table 1.
Fig. 1.
Flowchart for patient inclusion
Table 1.
Characteristics of all participants
| Variables | 2019 (n = 3,006,989) | 2018 (n = 3,039,922) |
|---|---|---|
| Age (years), n (%) | ||
| < 35 | 2,415,844 (80.34) | 2,455,582 (80.78) |
| ≥ 35 | 591,145 (19.66) | 584,340 (19.22) |
| Race, n (%) | ||
| White | 2,306,813 (76.72) | 2,342,911 (77.07) |
| Black | 385,213 (12.81) | 381,387 (12.55) |
| Other | 314,963 (10.47) | 315,624 (10.38) |
| Education, n (%) | ||
| High school or below | 1,868,219 (62.13) | 1,902,319 (62.58) |
| Bachelor | 707,428 (23.53) | 708,539 (23.31) |
| Master or above | 431,342 (14.34) | 429,064 (14.11) |
| Age of spouse (years), n (%) | ||
| < 35 | 1,125,350 (37.42) | 1,149,463 (37.81) |
| ≥ 35 | 1,881,639 (62.58) | 1,890,459 (62.19) |
| Race of spouse, n (%) | ||
| White | 2,145,381 (71.35) | 2,190,206 (72.05) |
| Black | 437,487 (14.55) | 432,525 (14.23) |
| Other | 424,121 (14.10) | 417,191 (13.72) |
| Education of spouse, n (%) | ||
| High school or below | 2,049,096 (68.14) | 2,077,147 (68.33) |
| Bachelor | 611,655 (20.34) | 613,242 (20.17) |
| Master or above | 346,238 (11.51) | 349,533 (11.50) |
| Pre-pregnancy BMI (kg/m2), n (%) | ||
| Underweight | 86,346 (2.87) | 91,667 (3.02) |
| Normal | 1,254,721 (41.73) | 1,302,458 (42.85) |
| Overweight | 815,799 (27.13) | 814,083 (26.78) |
| Obesity | 850,123 (28.27) | 831,714 (27.36) |
| Prenatal care, n (%) | ||
| No | 35,112 (1.17) | 32,015 (1.05) |
| Yes | 2,971,877 (98.83) | 3,007,907 (98.95) |
| Pregnancy smoking, n (%) | ||
| No | 2,870,310 (95.45) | 2,888,128 (95.01) |
| Yes | 136,679 (4.55) | 151,794 (4.99) |
| Pre-pregnancy diabetes, n (%) | ||
| No | 2,978,600 (99.06) | 3,012,207 (99.09) |
| Yes | 28,389 (0.94) | 27,715 (0.91) |
| Gestation diabetes, n (%) | ||
| No | 2,791,240 (92.83) | 2,828,904 (93.06) |
| Yes | 215,749 (7.17) | 211,018 (6.94) |
| Pre-pregnancy hypertension, n (%) | ||
| No | 2,944,019 (97.91) | 2,980,984 (98.06) |
| Yes | 62,970 (2.09) | 58,938 (1.94) |
| Gestation hypertension, n (%) | ||
| No | 2,776,000 (92.32) | 2,825,319 (92.94) |
| Yes | 230,989 (7.68) | 214,603 (7.06) |
| Hypertension eclampsia, n (%) | ||
| No | 2,999,359 (99.75) | 3,032,983 (99.77) |
| Yes | 7630 (0.25) | 6939 (0.23) |
| Previous preterm birth, n (%) | ||
| No | 2,906,623 (96.66) | 2,940,996 (96.75) |
| Yes | 100,366 (3.34) | 98,926 (3.25) |
| Previous cesareans, n (%) | ||
| No | 2,536,384 (84.35) | 2,560,142 (84.22) |
| Yes | 470,605 (15.65) | 479,780 (15.78) |
| Infections, n (%) | ||
| No | 2,947,151 (98.01) | 2,980,523 (98.05) |
| Yes | 59,838 (1.99) | 59,399 (1.95) |
| Plurality, n (%) | ||
| No | 2,912,136 (96.85) | 2,942,193 (96.79) |
| Yes | 94,853 (3.15) | 97,729 (3.21) |
| Preterm birth, n (%) | ||
| No | 2,682,289 (89.20) | 2,725,371 (89.65) |
| Yes | 324,700 (10.80) | 314,551 (10.35) |
Differences in women with and without preterm birth
Table 2 shows the differences in women with and without preterm birth. The results indicated that there were differences in age (P < 0.001), race (P < 0.001), education (P < 0.001), age of spouse (P < 0.001), race of spouse (P < 0.001), education of spouse (P < 0.001), pre-pregnancy BMI (P < 0.001), prenatal care (P < 0.001), pregnancy smoking (P < 0.001), pre-pregnancy diabetes (P < 0.001), gestation diabetes (P < 0.001), pre-pregnancy hypertension (P < 0.001), gestation hypertension (P < 0.001), hypertension eclampsia (P < 0.001), previous preterm birth (P < 0.001), previous cesareans (P < 0.001), infections (P < 0.001), and plurality (P < 0.001) between women with and without preterm birth.
Table 2.
Differences in women with and without preterm birth in the training set
| Variables | No preterm birth (n = 1,341,064) | Preterm birth (n = 162,431) | Statistic | P-value |
|---|---|---|---|---|
| Age (years), n (%) | χ2 = 1762.893 | < 0.001 | ||
| < 35 | 1,083,492 (80.79) | 124,110 (76.41) | ||
| ≥ 35 | 257,572 (19.21) | 38,321 (23.59) | ||
| Race, n (%) | χ2 = 4117.058 | < 0.001 | ||
| White | 1,036,752 (77.31) | 116,955 (72.00) | ||
| Black | 163,447 (12.19) | 28,920 (17.80) | ||
| Other | 140,865 (10.50) | 16,556 (10.19) | ||
| Education, n (%) | χ2 = 2515.445 | < 0.001 | ||
| High school or below | 823,393 (61.40) | 110,114 (67.79) | ||
| Bachelor | 321,473 (23.97) | 32,480 (20.00) | ||
| Master or above | 196,198 (14.63) | 19,837 (12.21) | ||
| Age of spouse (years), n (%) | χ2 = 27.163 | < 0.001 | ||
| < 35 | 502,507 (37.47) | 59,788 (36.81) | ||
| ≥ 35 | 838,557 (62.53) | 102,643 (63.19) | ||
| Race of spouse, n (%) | χ2 = 3841.278 | < 0.001 | ||
| White | 965,494 (71.99) | 107,583 (66.23) | ||
| Black | 186,516 (13.91) | 31,739 (19.54) | ||
| Other | 189,054 (14.10) | 23,109 (14.23) | ||
| Education of spouse, n (%) | χ2 = 2421.738 | < 0.001 | ||
| High school or below | 905,120 (67.49) | 119,379 (73.50) | ||
| Bachelor | 278,139 (20.74) | 27,937 (17.20) | ||
| Master or above | 157,805 (11.77) | 15,115 (9.31) | ||
| Pre-Pregnancy BMI (kg/m2), n (%) | χ2 = 1860.765 | < 0.001 | ||
| Underweight | 37,969 (2.83) | 5123 (3.15) | ||
| Normal | 566,124 (42.21) | 61,369 (37.78) | ||
| Overweight | 364,452 (27.18) | 43,258 (26.63) | ||
| Obesity | 372,519 (27.78) | 52,681 (32.43) | ||
| Prenatal care, n (%) | χ2 = 1499.057 | < 0.001 | ||
| No | 14,127 (1.05) | 3489 (2.15) | ||
| Yes | 1,326,937 (98.95) | 158,942 (97.85) | ||
| Pregnancy smoking, n (%) | χ2 = 1000.187 | < 0.001 | ||
| No | 1,282,591 (95.64) | 152,537 (93.91) | ||
| Yes | 58,473 (4.36) | 9894 (6.09) | ||
| Pre-pregnancy diabetes, n (%) | χ2 = 2960.522 | < 0.001 | ||
| No | 1,330,375 (99.20) | 158,888 (97.82) | ||
| Yes | 10,689 (0.80) | 3543 (2.18) | ||
| Gestation diabetes, n (%) | χ2 = 1822.489 | < 0.001 | ||
| No | 1,248,946 (93.13) | 146,570 (90.24) | ||
| Yes | 92,118 (6.87) | 15,861 (9.76) | ||
| Pre-pregnancy hypertension, n (%) | χ2 = 4859.660 | < 0.001 | ||
| No | 1,316,914 (98.20) | 155,257 (95.58) | ||
| Yes | 24,150 (1.80) | 7174 (4.42) | ||
| Gestation hypertension, n (%) | χ2 = 16,894.51 | < 0.001 | ||
| No | 1,251,215 (93.30) | 136,777 (84.21) | ||
| Yes | 89,849 (6.70) | 25,654 (15.79) | ||
| Hypertension eclampsia, n (%) | χ2 = 2871.288 | < 0.001 | ||
| No | 1,338,663 (99.82) | 160,985 (99.11) | ||
| Yes | 2401 (0.18) | 1446 (0.89) | ||
| Previous preterm birth, n (%) | χ2 = 15,999.98 | < 0.001 | ||
| No | 1,304,996 (97.31) | 148,372 (91.34) | ||
| Yes | 36,068 (2.69) | 14,059 (8.66) | ||
| Previous cesareans, n (%) | χ2 = 1441.546 | < 0.001 | ||
| No | 1,136,372 (84.74) | 131,751 (81.11) | ||
| Yes | 204,692 (15.26) | 30,680 (18.89) | ||
| Infections, n (%) | χ2 = 240.924 | < 0.001 | ||
| No | 1,315,097 (98.06) | 158,359 (97.49) | ||
| Yes | 25,967 (1.94) | 4072 (2.51) | ||
| Plurality, n (%) | χ2 = 107,024.1 | < 0.001 | ||
| No | 1,320,388 (98.46) | 135,475 (83.40) | ||
| Yes | 20,676 (1.54) | 26,956 (16.60) |
Factors associated with preterm birth
Table 3 demonstrates the univariate and multivariate analyses of factors associated with preterm birth. The univariate analysis found that age ≥ 35 years, black race of pregnant women and their spouses, pre-pregnancy overweight, underweight and obesity, pregnancy smoking, pre-pregnancy diabetes, gestation diabetes, pre-pregnancy hypertension, gestation hypertension, hypertension eclampsia, previous preterm birth, previous cesareans, infections, and plurality may be linked to a higher risk of preterm birth (all P < 0.05), and higher education level of pregnant women and their spouses and prenatal care may have a lower risk of preterm birth (P < 0.05). The multivariate analysis presented that higher education level of pregnant women [bachelor (OR = 0.82; 95%CI, 0.81–0.84); master or above (OR = 0.82; 95%CI, 0.81–0.83)] and their spouses [bachelor (OR = 0.86; 95%CI, 0.84–0.87); master or above (OR = 0.82; 95%CI, 0.80–0.84)], pre-pregnancy overweight (OR = 0.96; 95%CI, 0.95–0.98) and obesity (OR = 0.94; 95%CI, 0.93–0.96), and prenatal care (OR = 0.48; 95%CI, 0.47–0.50) were associated with a decreased risk of preterm birth, while age ≥ 35 years (OR = 1.27; 95%CI, 1.26–1.29), the black race of pregnant women (OR = 1.26; 95%CI, 1.23–1.29) and their spouses (OR = 1.15; 95%CI, 1.12–1.18), pre-pregnancy underweight (OR = 1.26; 95%CI, 1.22–1.30), pregnancy smoking (OR = 1.27; 95%CI, 1.24–1.30), pre-pregnancy diabetes (OR = 2.08; 95%CI, 1.99–2.16), gestation diabetes (OR = 1.27; 95%CI, 1.24–1.29), pre-pregnancy hypertension (OR = 2.22; 95%CI, 2.16–2.29), gestation hypertension (OR = 2.49; 95%CI, 2.45–2.53), hypertension eclampsia (OR = 4.12; 95%CI, 3.83–4.42), previous preterm birth (OR = 2.95; 95%CI, 2.88–3.01), previous cesareans (OR = 1.13; 95%CI, 1.11–1.14), infections (OR = 1.12; 95%CI, 1.08–1.16), and plurality (OR = 12.99; 95%CI, 12.73–13.24) were related to an increased risk of preterm birth.
Table 3.
Univariate and multivariate analyses of factors associated with preterm birth
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | |
| Age | ||||
| < 35 years | Ref | Ref | ||
| ≥ 35 years | 1.30 (1.28–1.32) | < 0.001 | 1.27 (1.26–1.29) | < 0.001 |
| Race | ||||
| White | Ref | Ref | ||
| Black | 1.57 (1.55–1.59) | < 0.001 | 1.26 (1.23–1.29) | < 0.001 |
| Others | 1.04 (1.02–1.06) | < 0.001 | 1.05 (1.03–1.07) | < 0.001 |
| Education | ||||
| High school or below | Ref | Ref | ||
| Bachelor | 0.76 (0.75–0.77) | < 0.001 | 0.82 (0.81–0.84) | < 0.001 |
| Master or above | 0.76 (0.74–0.77) | < 0.001 | 0.82 (0.81–0.83) | < 0.001 |
| Age of spouse | ||||
| < 35 years | Ref | Ref | ||
| ≥ 35 years | 1.03 (1.02–1.04) | < 0.001 | 0.98 (0.97–0.99) | < 0.001 |
| Race of spouse | ||||
| White | Ref | Ref | ||
| Black | 1.53 (1.51–1.55) | < 0.001 | 1.15 (1.12–1.18) | < 0.001 |
| Others | 1.10 (1.08–1.11) | < 0.001 | 1.12 (1.10–1.14) | < 0.001 |
| Education of spouse | ||||
| High school or below | Ref | Ref | ||
| Bachelor | 0.76 (0.75–0.77) | < 0.001 | 0.86 (0.84–0.87) | < 0.001 |
| Master or above | 0.73 (0.71–0.74) | < 0.001 | 0.82 (0.80–0.84) | < 0.001 |
| Pre-pregnancy BMI | ||||
| Normal | Ref | Ref | ||
| Underweight | 1.24 (1.21–1.28) | < 0.001 | 1.26 (1.22–1.30) | < 0.001 |
| Overweight | 1.09 (1.08–1.11) | < 0.001 | 0.96 (0.95–0.98) | < 0.001 |
| Obesity | 1.30 (1.29–1.32) | < 0.001 | 0.94 (0.93–0.96) | < 0.001 |
| Prenatal care | ||||
| No | Ref | Ref | ||
| Yes | 0.48 (0.47–0.50) | < 0.001 | 0.48 (0.47–0.50) | < 0.001 |
| Pregnancy smoking | ||||
| No | Ref | Ref | ||
| Yes | 1.42 (1.39–1.46) | < 0.001 | 1.27 (1.24–1.30) | < 0.001 |
| Pre-pregnancy diabetes | ||||
| No | Ref | Ref | ||
| Yes | 2.78 (2.67–2.88) | < 0.001 | 2.08 (1.99–2.16) | < 0.001 |
| Gestation diabetes | ||||
| No | Ref | Ref | ||
| Yes | 1.47 (1.44–1.49) | < 0.001 | 1.27 (1.24–1.29) | < 0.001 |
| Pre-pregnancy hypertension | ||||
| No | Ref | Ref | ||
| Yes | 2.52 (2.45–2.59) | < 0.001 | 2.22 (2.16–2.29) | < 0.001 |
| Gestation hypertension | ||||
| No | Ref | Ref | ||
| Yes | 2.61 (2.57–2.65) | < 0.001 | 2.49 (2.45–2.53) | < 0.001 |
| Hypertension eclampsia | ||||
| No | Ref | Ref | ||
| Yes | 5.01 (4.70–5.35) | < 0.001 | 4.12 (3.83–4.42) | < 0.001 |
| Previous preterm birth | ||||
| No | Ref | Ref | ||
| Yes | 3.43 (3.36–3.50) | < 0.001 | 2.95 (2.88–3.01) | < 0.001 |
| Previous cesareans | ||||
| No | Ref | Ref | ||
| Yes | 1.29 (1.28–1.31) | < 0.001 | 1.13 (1.11–1.14) | < 0.001 |
| Infections | ||||
| No | Ref | Ref | ||
| Yes | 1.30 (1.26–1.35) | < 0.001 | 1.12 (1.08–1.16) | < 0.001 |
| Plurality | ||||
| No | Ref | Ref | ||
| Yes | 12.71 (12.47–12.95) | < 0.001 | 12.99 (12.73–13.24) | < 0.001 |
Model performance and validation
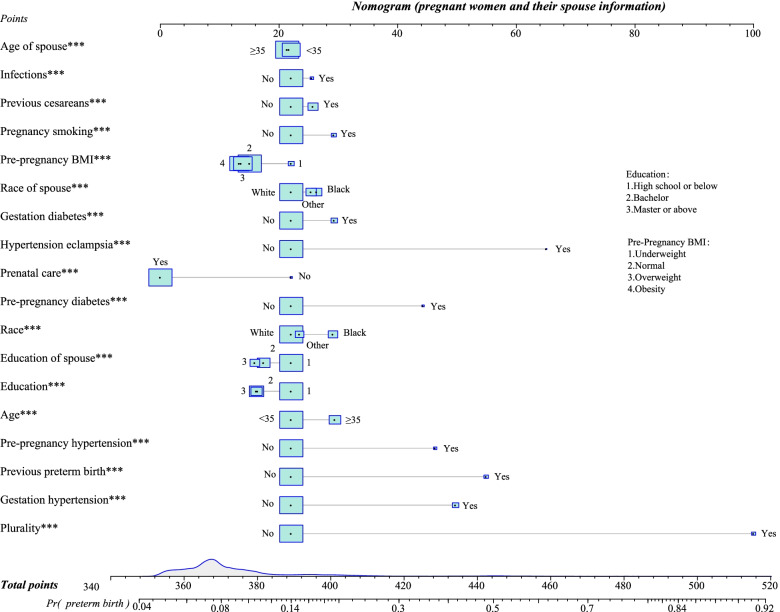
Variables such as age, race, education of pregnant women and their spouses, pre-pregnancy BMI, prenatal care, pregnancy smoking, pre-pregnancy diabetes, gestation diabetes, pre-pregnancy hypertension, gestation hypertension, hypertension eclampsia, previous preterm birth, previous cesareans, infection, and plurality were included to develop a prediction model. Table 4 presents the model performance in the training set, testing set, and external validation set. According to the Yoden index, the cut-off point was 0.099. The AUC of the model in the training set, testing set, and external validation set was 0.689 (95%CI, 0.687–0.690), 0.688 (95%CI, 0.686–0.689), and 0.694 (95%CI, 0.693–0.695), respectively. The accuracy of the model in the training set, testing set, and external validation set was 0.763 (95%CI, 0.762–0.764), 0.762 (95%CI, 0.762–0.763), and 0.771 (95%CI, 0.770–0.771), respectively. Furthermore, the nomogram used to predict the occurrence of preterm birth in the pregnant woman was shown in Fig. 2.
Table 4.
Performances of the model in the training set, testing set, and external validation set
| Parameters | Training set | Testing set | Validation set |
|---|---|---|---|
| AUC (95%CI) | 0.689 (0.687–0.690) | 0.688 (0.686–0.689) | 0.694 (0.693–0.695) |
| Accuracy (95%CI) | 0.763 (0.762–0.764) | 0.762 (0.762–0.763) | 0.771 (0.770–0.771) |
| Specificity (95%CI) | 0.796 (0.795–0.797) | 0.796 (0.795–0.796) | 0.803 (0.802–0.803) |
| Sensitivity (95%CI) | 0.489 (0.487–0.492) | 0.487 (0.484–0.489) | 0.490 (0.488–0.492) |
| PPV (95%CI) | 0.225 (0.224–0.226) | 0.224 (0.222–0.225) | 0.223 (0.222–0.224) |
| NPV (95%CI) | 0.928 (0.927–0.928) | 0.928 (0.927–0.928) | 0.932 (0.931–0.932) |
AUC Area under the curve, PPV Positive predictive value, NPV Negative predictive value, CI Confidence interval
Fig. 2.
Nomogram for predicting the occurrence of preterm birth in pregnant women
Discussion
In this study, we established a prediction model based on a large-sample database to predict preterm birth. Our results demonstrated that bachelor or above education level of pregnant women and their spouses, pre-pregnancy overweight and obesity, and prenatal care were linked to a reduced risk of preterm birth, while age ≥ 35 years, the black race of pregnant women and their spouses, pre-pregnancy underweight, pregnancy smoking, pre-pregnancy diabetes, gestation diabetes, pre-pregnancy hypertension, gestation hypertension, hypertension eclampsia, previous preterm birth, previous cesareans, infections, and plurality were associated with an increased risk of preterm birth. In the preterm birth prediction model constructed by these variables, the AUC of the model was 0.688 in the testing set. In addition, the model still performed well in the external validation set.
Black ethnicity and advanced maternal age may be indicators of preterm birth in some studies [2, 20]. In a meta-analysis examining racial differences among United States residents, black ethnicity had a higher rate of preterm birth than whites [20]. Our results showed that pregnant women aged ≥35 years and of the black race were associated with an increased risk of preterm birth. The relationship between pre-pregnancy BMI and preterm birth may influence by many factors [21, 22]. A large sample study indicated that pre-pregnancy obesity and preterm birth risk vary by age and race of pregnant women [21]. Our results found that pre-pregnancy overweight and obesity were linked to a decreased risk of preterm birth. This may be due to other confounders affecting our results. Furthermore, previous studies have reported that hypertension [23, 24] and diabetes [25] were associated with the risk of preterm birth. The risk of preterm birth increased with a plurality. Hiersch et al. found a higher rate of preterm birth in triplet pregnancies compared with twin pregnancies [26]. Smoking was also a risk factor which was a key necessary risk factor for fetal death or disability [27]. Our results showed that pregnancy smoking was related to an increased risk of preterm birth. In addition, our results found that prenatal care and higher education level were associated with a decreased risk of preterm birth. Previous studies have also shown that education was associated with a reduced risk of preterm birth in pregnant women, but the relationship is not linear [28, 29].
There were some studies focused on prediction models related to preterm birth, such as preterm birth prevention, cesarean delivery during the preterm period, or nulliparous women with a short cervix [11–14]. Many predictors were related to preterm birth such as general risk factors (maternal characteristics), pregnancy complication status (hypertension, diabetes), current pregnancy status, environmental complications, and medical intake [7]. During the pregnancy, the baseline information was much easier to collect. However, there were few studies to research the pregnant spouse’s information as the predictor. In our preterm birth prediction model, baseline information such as age, race, and education level of pregnant women and their spouses were included. The AUC of our prediction model in the testing set was 0.688. The prediction performance of our model may not be significantly improved compared to previous studies (0.688 vs. 0.63–0.74) [11–13]. However, our model was based on clinically readily available baseline information, which may increase the applicability of our model. In addition, compared with the baseline information, clinical indicators may also have an important role in predicting preterm birth. Some studies used clinical indicators as the risk factors to predict preterm birth [30–32], such as cervix length which was the strongest clinical predictor of preterm birth, ultrasound, and blood test, which were expensive or complicated to use in normal maternal people. Furthermore, a blood test can be selected as a biomarker to show whether the pregnant has inflammation or oxidative stress [33]. The general biological markers C-reactive protein (CRP), cytokines, 8-isoprostane, and 8-hydroxydeoxyguanosine could be tested through a blood test. Moreover, from a study, the measurement of nine cell-free RNA (cf-RNA) transcripts in maternal blood predicted gestational age with comparable accuracy to ultrasound [32]. However, in the low-resource area, the source of medical personnel and medical resource for blood tests were limited and unsupported to examine preterm birth. Therefore, convenient indicators or inexpensive access to get information is also necessary.
From the perspective of genetics, the neonatal father and mother were responsible for fetuses. Many parents were associated with maternal outcomes directly and indirectly [34]. Sparse researches were on parents’ demographic information, especially father’s baseline information. There were knowledge gaps between preterm birth and the father’s information. In Portuguese association had research on the mothers and fathers to support premature babies [35]. The research collected the father’s demographic information. However, it was not for the prediction model. In that cohort study, the author explored the consequences of premature delivery with information about mother’s and father’s low socioeconomic status. Thus, our study elucidates the prediction model with the father’s baseline information as a predicting factor.
This study used a lot of demographic information which can obtain easily from clinical data. Because there were many restrictions on pregnancy and medical resources, such as in rural areas or low-income families. Using this prediction model can filter some pregnant who may have a preterm birth. Then the doctor can provide some suggestions or give preventions for pregnant to prevent them from preterm birth. This prediction model can save humans, material resources, and time. There were some limitations in this study. Firstly, the external validation group was still from the United States. Preterm birth was a global public health problem. This external validation may ignore the different countries pregnant. Because in different countries, pregnancy may have various characteristics, this prediction model may have different effects to predict preterm birth. Secondly, although our data source was easy to obtain, the effect of prediction model performance was not accurate because there was no gold standard in this prediction model. If there were some other information such as blood test information, and mid-term cervical length value, the predictive ability may increase. Third, 98.8% of the included population had prenatal care, and the predictive effect of the model on preterm birth in the population without prenatal care may need further verification.
Conclusion
A nomogram for predicting the risk of preterm birth in pregnant women was established. The prediction model had good performance in the testing set and external validation set. In addition, the relevant predictors of the prediction model are readily available clinically, and the nomogram may provide a simple tool for the prediction of preterm birth.
Supplementary Information
Additional file 1: Supplement Fig. 1. Calculations of sample size and model power.
Acknowledgements
Not applicable.
Abbreviations
- NVSS
National Vital Statistics System
- NCHS
National Center for Health Statistics
- BMI
Body mass index
- OR
Odds ratio
- CI
Confidence interval
- AUC
Area under the curve
- NPV
Negative predictive value
- PPV
Positive predictive value
Authors’ contributions
YL and JS designed the study. YL wrote the manuscript. XF, XG, HL and DC collected, analyzed and interpreted the data. JS critically reviewed, edited and approved the manuscript. All authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the NVSS repository, https://www.cdc.gov/nchs/nvss/index.htm.
Declarations
Ethics approval and consent to participate
All procedures performed in this study in accordance with the Declaration of Helsinki. This study was granted exemption ethics approval by the Ethics Committee of Beijing Haidian Maternal and Child Healthcare Hospital due to the data used in this study from the open access NVSS database, and the relevant information of participants was anonymized and did not involve human intervention.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet (London, England) 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogel JP, Chawanpaiboon S, Moller AB, Watananirun K, Bonet M, Lumbiganon P. The global epidemiology of preterm birth. Best Pract Res Clin Obstet Gynaecol. 2018;52:3–12. doi: 10.1016/j.bpobgyn.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 3.Walani SR. Global burden of preterm birth. Int J Gynaecol Obstetr. 2020;150:31–33. doi: 10.1002/ijgo.13195. [DOI] [PubMed] [Google Scholar]
- 4.Purisch SE, Gyamfi-Bannerman C. Epidemiology of preterm birth. Semin Perinatol. 2017;41:387–391. doi: 10.1053/j.semperi.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Russell RB, Green NS, Steiner CA, Meikle S, Howse JL, Poschman K, et al. Cost of hospitalization for preterm and low birth weight infants in the United States. Pediatrics. 2007;120:e1–e9. doi: 10.1542/peds.2006-2386. [DOI] [PubMed] [Google Scholar]
- 6.Ion R, Bernal AL. Smoking and preterm birth. Reprod Sci (Thousand Oaks, Calif) 2015;22:918–926. doi: 10.1177/1933719114556486. [DOI] [PubMed] [Google Scholar]
- 7.Koullali B, Oudijk MA, Nijman TA, Mol BW, Pajkrt E. Risk assessment and management to prevent preterm birth. Semin Fetal Neonatal Med. 2016;21:80–88. doi: 10.1016/j.siny.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Martin JN, Jr, D’Alton M, Jacobsson B, Norman JE. In pursuit of Progress toward effective preterm birth reduction. Obstet Gynecol. 2017;129:715–719. doi: 10.1097/AOG.0000000000001923. [DOI] [PubMed] [Google Scholar]
- 9.Moons KG, Royston P, Vergouwe Y, Grobbee DE, Altman DG. Prognosis and prognostic research: what, why, and how? BMJ (Clinical research ed) 2009;338:b375. doi: 10.1136/bmj.b375. [DOI] [PubMed] [Google Scholar]
- 10.Kim JI, Lee JY. Systematic review of prediction models for preterm birth using CHARMS. Biol Res Nurs. 2021;23:708–722. doi: 10.1177/10998004211025641. [DOI] [PubMed] [Google Scholar]
- 11.Weber A, Darmstadt GL, Gruber S, Foeller ME, Carmichael SL, Stevenson DK, et al. Application of machine-learning to predict early spontaneous preterm birth among nulliparous non-Hispanic black and white women. Ann Epidemiol. 2018;28:783–9.e1. doi: 10.1016/j.annepidem.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Grobman WA, Lai Y, Iams JD, Reddy UM, Mercer BM, Saade G, et al. Prediction of spontaneous preterm birth among nulliparous women with a short cervix. J Ultrasound Med. 2016;35:1293–1297. doi: 10.7863/ultra.15.08035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morken NH, Källen K, Jacobsson B. Predicting risk of spontaneous preterm delivery in women with a singleton pregnancy. Paediatr Perinat Epidemiol. 2014;28:11–22. doi: 10.1111/ppe.12087. [DOI] [PubMed] [Google Scholar]
- 14.Arabi Belaghi R, Beyene J, McDonald SD. Prediction of preterm birth in nulliparous women using logistic regression and machine learning. PLoS One. 2021;16:e0252025. doi: 10.1371/journal.pone.0252025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ventura SJ. The U.S. National Vital Statistics System: Transitioning Into the 21st Century, 1990-2017. Vital and health statistics Ser 1, Programs and collection procedures. 2018. pp. 1–84. [PubMed] [Google Scholar]
- 16.Latib A, Ruparelia N, Menozzi A, Castriota F, Micari A, Cremonesi A, et al. 3-year follow-up of the balloon elution and late loss optimization study (BELLO) JACC Cardiovasc Interv. 2015;8:1132–1134. doi: 10.1016/j.jcin.2015.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Ju AC, Heyman MB, Garber AK, Wojcicki JM. Maternal obesity and risk of preterm birth and low birthweight in Hawaii PRAMS, 2000-2011. Matern Child Health J. 2018;22:893–902. doi: 10.1007/s10995-018-2464-7. [DOI] [PubMed] [Google Scholar]
- 18.Miller ES, Tita AT, Grobman WA. Second-trimester cervical length screening among asymptomatic women: an evaluation of risk-based strategies. Obstet Gynecol. 2015;126:61–66. doi: 10.1097/AOG.0000000000000864. [DOI] [PubMed] [Google Scholar]
- 19.Kondracki AJ, Hofferth SL. A gestational vulnerability window for smoking exposure and the increased risk of preterm birth: how timing and intensity of maternal smoking matter. Reprod Health. 2019;16:43. doi: 10.1186/s12978-019-0705-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehra R, Boyd LM, Ickovics JR. Racial residential segregation and adverse birth outcomes: a systematic review and meta-analysis. Soc Sci Med. 2017;191:237–250. doi: 10.1016/j.socscimed.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 21.Liu B, Xu G, Sun Y, Du Y, Gao R, Snetselaar LG, et al. Association between maternal pre-pregnancy obesity and preterm birth according to maternal age and race or ethnicity: a population-based study. Lancet Diabetes Endocrinol. 2019;7:707–714. doi: 10.1016/S2213-8587(19)30193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gete DG, Waller M, Mishra GD. Prepregnancy dietary patterns and risk of preterm birth and low birth weight: findings from the Australian longitudinal study on Women's health. Am J Clin Nutr. 2020;111:1048–1058. doi: 10.1093/ajcn/nqaa057. [DOI] [PubMed] [Google Scholar]
- 23.Premkumar A, Henry DE, Moghadassi M, Nakagawa S, Norton ME. The interaction between maternal race/ethnicity and chronic hypertension on preterm birth. Am J Obstet Gynecol. 2016;215(787):e1–e8. doi: 10.1016/j.ajog.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 24.Bertagnolli M, Luu TM, Lewandowski AJ, Leeson P, Nuyt AM. Preterm birth and hypertension: is there a link? Curr Hypertens Rep. 2016;18:28. doi: 10.1007/s11906-016-0637-6. [DOI] [PubMed] [Google Scholar]
- 25.Wei Y, Xu Q, Yang H, Yang Y, Wang L, Chen H, et al. Preconception diabetes mellitus and adverse pregnancy outcomes in over 6.4 million women: a population-based cohort study in China. PLoS Med. 2019;16:e1002926. doi: 10.1371/journal.pmed.1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiersch L, Rosen H, Okby R, Freeman H, Barrett J, Melamed N. The greater risk of preterm birth in triplets is mirrored by a more rapid cervical shortening along gestation. Am J Obstet Gynecol. 2016;215(357):e1–e6. doi: 10.1016/j.ajog.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Stock SJ, Bauld L. Maternal smoking and preterm birth: an unresolved health challenge. PLoS Med. 2020;17:e1003386. doi: 10.1371/journal.pmed.1003386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Auger N, Abrahamowicz M, Park AL, Wynant W. Extreme maternal education and preterm birth: time-to-event analysis of age and nativity-dependent risks. Ann Epidemiol. 2013;23:1–6. doi: 10.1016/j.annepidem.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Auger N, Roncarolo F, Harper S. Increasing educational inequality in preterm birth in Quebec, Canada, 1981-2006. J Epidemiol Community Health. 2011;65:1091–1096. doi: 10.1136/jech.2009.102350. [DOI] [PubMed] [Google Scholar]
- 30.Son M, Miller ES. Predicting preterm birth: cervical length and fetal fibronectin. Semin Perinatol. 2017;41:445–451. doi: 10.1053/j.semperi.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park HS, Kwon H, McElrath TF. Assisted reproductive technology and the risk of unplanned peripartum hysterectomy: analysis using propensity score matching. Human Reprod (Oxford, England) 2018;33:1466–1473. doi: 10.1093/humrep/dey228. [DOI] [PubMed] [Google Scholar]
- 32.Ngo TTM, Moufarrej MN, Rasmussen MH, Camunas-Soler J, Pan W, Okamoto J, et al. Noninvasive blood tests for fetal development predict gestational age and preterm delivery. Science (New York, NY) 2018;360:1133–1136. doi: 10.1126/science.aar3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aung MT, Yu Y, Ferguson KK, Cantonwine DE, Zeng L, McElrath TF, et al. Prediction and associations of preterm birth and its subtypes with eicosanoid enzymatic pathways and inflammatory markers. Sci Rep. 2019;9:17049. doi: 10.1038/s41598-019-53448-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith LK, Dickens J, Bender Atik R, Bevan C, Fisher J, Hinton L. Parents’ experiences of care following the loss of a baby at the margins between miscarriage, stillbirth and neonatal death: a UK qualitative study. BJOG. 2020;127:868–874. doi: 10.1111/1471-0528.16113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matos J, Amorim M, Silva S, Nogueira C, Alves E. Prematurity-related knowledge among mothers and fathers of very preterm infants. J Clin Nurs. 2020;29:2886–2896. doi: 10.1111/jocn.15361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplement Fig. 1. Calculations of sample size and model power.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the NVSS repository, https://www.cdc.gov/nchs/nvss/index.htm.