Abstract
Purpose:
This prospective phase III randomized trial was designed to test whether ultra-high single-dose radiotherapy (24 Gy SDRT) improves local control of oligometastatic lesions over a standard hypofractionated stereotactic body radiotherapy regimen (3 × 9 Gy SBRT). The secondary endpoint was to assess the associated toxicity and the impact of ablation on clinical patterns of metastatic progression.
Methods and Materials:
Between November 2010 and September 2015, 117 patients with 154 oligometastatic lesions (≤ 5/patient) were randomized in a 1:1 ratio to receive 24 Gy SDRT or 3 × 9 Gy SBRT. Local control within the irradiated field and the state of metastatic spread were assessed by periodic whole-body PET/CT and/or MRI imaging. Median follow-up was 52 months.
Results:
59 patients with 77 lesions were randomized to 24 Gy SDRT and 58 patients with 77 lesions to 3 × 9 Gy SBRT. The cumulative incidence of local recurrence for SDRT lesions was 2.7% (95% CI 0–6.5%) and 5.8% (95% CI 0.2–11.5%) at years two and three, respectively, compared to 9.1% (95% CI 2.6–15.6%) and 22% (95% CI 11.9–32.1%) for SBRT lesions (P=.0048). The two- and three-years cumulative incidence of distant metastatic progression in the SDRT patients were 5.3% (95% CI 0–11.1%), compared to 10.7% (95% CI 2.5–18.8%) and 22.5% (95% CI 11.1–33.9%), respectively, for the SBRT patients (P=.010). No differences in toxicity were observed.
Conclusions:
The study confirms SDRT as a superior ablative treatment, indicating that effective ablation of oligometastatic lesions is associated with significant mitigation of distant metastatic progression.
INTRODUCTION
There is increasing recognition from prospective clinical trials that oligometastatic lesion-directed ablation using hypofractionated stereotactic body radiotherapy (SBRT; also termed stereotactic ablative radiotherapy, SABR) prolongs progression-free and overall survivals over standard of care in oligometastatic cancer.1–4 Yet, the SBRT dose to achieve optimal tumor ablation has not been established. The drive to use ablative radiotherapy emerged from the Hellman-Weichselbaum hypothesis on the biology of the oligometastatic phenotype,5 which defines oligometastasis as a transient phase in cancer progression from a primary localized cancer to widespread polymetastatic dissemination. A corollary of this paradigm posits that comprehensive lesion ablation occurring before polymetastatic conversion might lead to cancer cure.5 The Hellman-Weichselbaum hypothesis was derived from a compilation of retrospective data, which deployed empirical surgical ablation of limited oligometastatic disease (1–5 lesions) from the lung or liver, yielding approximately 20% sustained disease-free survival at 10–20 years.6–8
An early single-dose radiotherapy (SDRT), phase I, dose-escalation study9 showed that an image-guided, high-precision, and ultra-high single dose of 24 Gy SDRT was required to achieve a maximal tumor control. Further, phase II studies by the same group confirmed that 24 Gy SDRT was feasible and effective in multiple oligometastatic clinical settings, rendering 92% actuarial five-year local relapse-free survival.10–12 In addition to SDRT, ultra-high dose SBRT (i.e., total dose given in three fractions of 18–20 Gy) has achieved excellent tumor control rates without significant toxicity.13–16 However, cutting-edge technology alone does not account for the ultra-high dose therapeutic success. Recent experimental studies reported that ultra-high dose radiotherapy, beginning at a threshold of 12 Gy, operates a unique dual-target mechanism of action, which is fundamentally distinct from the classical fractionation model.17 This dual-target mechanism links a transient microvascular vasoactive dysfunction to the repression of high-fidelity homology-directed repair of SDRT-induced DNA damage within tumor cells, yielding synthetic lethality of tumor clonogens and tumor cure.17 Engagement of this dual-target mechanism was demonstrated in human oligometastatic disease treated with 24 Gy SDRT, but not with 3 × 9 Gy SBRT.17
We undertook the present prospective, randomized, phase III clinical trial to determine as its primary endpoint whether the long-term local control afforded by 24 Gy SDRT would be superior to 3 × 9 Gy SBRT. The SBRT regimen was selected as the study control arm, because this schedule was regarded at the time of the study as a standard treatment in bone and nodal oligometastatic disease.18–20 Our study’s secondary endpoints were toxicity and an assessment of whether a difference in local tumor control, if detected in the prospective randomised setting, would translate into a post-radiation difference in metastatic progression.
METHODS AND MATERIALS
Study design and participants
This study was a multicenter, open-labeled, randomized phase III trial. Eligible patients were randomized in a 1:1 ratio to receive either one fraction of 24 Gy SDRT to the planning target volume (PTV) or three fractions of 9 Gy SBRT to the PTV. While the original intent for accrual was for 200 patients over four years, the pace of accrual significantly declined during the last year of planned enrollment. As a result, the internal data and safety monitoring board recommended halting further accrual and analyzing the outcomes of accrued patients. It should be noted that the power analysis was re-calculated using the actual 154 accrued lesions and it was determined that this number would be sufficient for observing the hypothesized difference with a >90% probability. A total of 117 eligible patients with 154 metastatic lesions were enrolled and completed the study; 59 patients were randomized to the SDRT arm and 58 to the SBRT regimen. Local tumor control by the randomized radio-ablative protocol was assessed as the primary study endpoint, while treatment toxicity and patterns of metastatic progression constituted the secondary endpoints. Metastatic progression was registered when it was initially detected and was mandatorily confirmed at 12 and 24 months after radiotherapy.
The study was approved by the ethical review boards responsible for each participating center. All patients provided informed, written consent prior to enrollment, which was obtained at the participating institution.
Eligibility criteria for enrollment included ≤5 metastatic lesions documented on baseline MRI or PET-CT imaging studies, and no prior radiotherapy to the protocol-treated sites. Eligible lesions were limited to non-mobile metastatic target organs (osseous or lymph node metastases). The maximum tumor dimension was ≤6 cm in diameter as measured on imaging studies. Patients were required to have a Karnofsky performance status ≥80, as well as a normal bone marrow function with Hgb level ≥9.0 g/dl, absolute neutrophil count ≥1,500/ul, and platelet count ≥100,000/ul. Treatment with post-radiation adjuvant therapies (i.e., chemotherapy, immunotherapy, or biologic therapy) were permitted at the discretion of the treating physician. Patients with target lesions adjacent to critical normal organs at risk for radiation damage were excluded. Patients who died before the 24-month assessment were not evaluable for tumor control unless progression was previously demonstrated on imaging — so they were censored beyond the time of death.
Randomization was centralized at Memorial Sloan Kettering Cancer Center (MSKCC), and patients accrued at Champalimaud Centre for the Unknown, Lisbon, Portugal and University of California, San Francisco were assigned their designated treatment from the MSKCC Biostatistics Department. Once the participant’s eligibility was established, the registration was finalized and the participants from all participating sites were randomized using the Clinical Research Database (CRDB). Randomization was accomplished using the method of random permuted block. This study is registered with ClinicalTrials.gov (NCT01223248) and is closed.
Procedures
All patients were treated with linac-based stereotactic radiosurgery with on-line image-guided localization using three-dimensional kilovoltage cone beam CT (kV-CBCT). For each fraction, the patient position was adjusted corresponding to the designed and planned intensity-modulated radiation therapy (IMRT) treatment. For all lesions, the planning target volume applied a 2–3 mm circumferential margin around the clinical target volume (CTV) as defined on the baseline MRI or PET-CT scan, and it was insured that the PTV did not overlap an organ at risk as previously described.12 Because solid tumor metastases in bones and lymph nodes are discrete lesions, the radiographically defined gross tumor volume (GTV) was considered the same as the CTV for the purposes of target delineation.
Each eligible PTV was treated to the prescribed dose according to the randomization assignment. The dose was prescribed to the 100% isodose line, which completely encompassed the PTV. Radiation was delivered to predetermined eligible metastatic sites based on the randomized dose cohort: either 24 Gy in a single dose or 27 Gy in three fractions (treated every other day). If a patient had more than one lesion, all lesions required subsequent treatment with the assigned fractionation regimen.
Normal tissues were contoured to determine a dose volume histogram including: both lungs (as the total lung volume) minus gross target volume (GTV), spinal cord, liver, kidney, heart, trachea, esophagus, rectum, bladder, and bowel, if applicable. Dose volume constraints for normal tissue structures used for treatment planning have been previously described.12 For all spine lesions, a myelogram or MR fusion was used for treatment planning. During treatment delivery, intra-fraction motion was tracked with orthogonal kV imaging to ensure that significant motion >2 mm was not observed. If such target motion was noted, then the treatment was interrupted and the target position was corrected before further therapy was resumed.
Dexamethasone (4 mg twice daily) was given to patients who were treated for bone metastases, and in particular for single-fraction SDRT patients administered the day before and three hours before radio-ablation. Patients treated with three-fraction SBRT selectively received dexamethasone based on the volume of disease present and the severity of their pre-treatment pain. As part of the credentialing process, the first case for each participating center was submitted for review to the coordinating center and assessed for compliance to the radiation treatment standards stipulated in the protocol. Annual audits to verify data and source documentation were carried out at each participating site. Random sample audits were in general performed twice a year, at a minimum.
After randomized treatment was completed, patients were followed at 3- and 6-months post-therapy and every 6 months thereafter. Imaging assessment (bone scan, CT, or whole-body PET scan) of local relapse and metastatic spread was mandatorily required at 3, 6, 12, and 24 months after radiotherapy. Although the protocol required follow-up for only two years, patients continued to be followed as clinically appropriate.
Outcomes
The primary endpoint of the study was assessment of local control within the irradiated volume derived from imaging studies, which were repeated as described. Tumor control was defined as the lack of local disease progression or the lack of a recurrence after initial complete response within the treated field, based on standard RECIST criteria in cases where there was a soft tissue component of the disease to assess such a response. In cases where no soft tissue component was present in the treated lesion, local control was defined as no radiographic progression of disease in the gross tumor volume.
Assessed imaging responses were mandatorily confirmed centrally at MSKCC by the primary study radiologists. The secondary endpoints were the status of distant metastatic dissemination and the treatment-related toxicity, which were assessed repeatedly within the first 24 months as defined above. Metastatic dissemination was determined by whole-body CT and/or MRI performed at 6, 12, and 24 months from treatment. Time to local control and distant metastases were calculated from the end of radiotherapy to evaluate local tumor control. Toxicity incidence was reported as the maximum observed toxicity for the patient and assessed using the Common Toxicity Criteria for Adverse Events (CTCAE) version 4.0.
Statistical analysis
To determine the power of the clinical trial, a log rank test was used under the assumption that the two survival curves would follow exponential distributions, and with the understanding that competing risks analysis was applied instead if the data contained death without local recurrence. To examine whether the two treatment arms produced significant differences for local progression control outcomes, the hazard ratio between the two arms was assumed to be approximately two with the one-year local control rates of the single-fraction SDRT arm and the three-fraction SBDT arm being 85% and 70%, respectively, based on data available at the time of the design of this trial.21–22 After enrollment of the final patient, a minimum of two-year follow-up was performed. Under these assumptions and using the type I error rate of 0.05, a two-sided test was expected to have a power greater than 0.95 for detecting the difference between the two treatments. The O’Brien-Flemming boundary was applied for declaring statistical significance with three interim and one final analysis.
Cumulative incidence functions (CIFs) and Kaplan-Meier survival curves were generated to examine the incidence and survival experiences of the sample level with respect to local recurrence (LR) and distant metastases (DM). Death was a competing risk for LR and DM. Curves and associated estimates were lesion-level (i.e., generated using data from all evaluable lesions). To correct for immortal time bias when correlating the post-radiation receipt of systemic adjuvant therapy (chemotherapy, hormonal therapy, or immunotherapy) with LR and/or distant metastatic spread, we used a landmark analysis. This analysis was selected because the receipt of post-ablative systemic therapy was not a baseline factor nor was it known at the end of radiotherapy. To this end, a 12-month landmark time was used; patients were excluded from this analysis if they were not followed for at least 12 months after radiotherapy or if the information about systemic adjuvant treatment was not available. CIFs were generated to examine the incidence rates with respect to LR or DM by regarding death as a competing risk. Curves and associated estimates were calculated at lesion-level for LR and at patient-level for DM. Gray’s test was used to assess whether receipt of post-radiotherapy systemic treatment was associated with incidence of LR and/or DM.
Fisher’s exact test was used to compare grade 3+ toxicity between the treatment arms at the lesion level. Additionally, grade 3+ pain, peripheral sensory neuropathy, and fracture were reported descriptively for each treatment arm at the lesion level. This analysis and summarization were repeated for grade 2+ toxicity.
All statistical computations were performed, and all output were generated, using SAS Software Version 9.4.
RESULTS
Patients were recruited for the study between November 2010 and September 2015. A total of 59 patients with 77 lesions were randomized to the 24 Gy SDRT arm and 58 patients with 77 lesions to the 3 × 9 Gy SBRT arm. The baseline characteristics for the entire cohort of 117 patients and for each treatment arm are shown in Table 1. The median follow-up time of treated lesions from surviving patients was 52 months (range: 10.7–102.2 months).
Table 1:
Patient characteristics grouped by treatment regimen.
| All patients | SDRT | SBRT | ||
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Sample size | 117 | 59 (50.4) | 58 (49.6) | |
| Age | Median (range) | 64.0 (32.0–89.0) | 63.0 (32.0–82.0) | 65.5 (42.0–89.0) |
| Sex | Male | 83 (70.9) | 44 (74.6) | 39 (67.2) |
| Female | 32 (27.4) | 15 (25.4) | 17 (29.3) | |
| Missing | 2 (1.7) | 0 (0) | 2 (3.4) | |
| Number of lesions | 1 | 93 (79.5) | 46 (78) | 47 (81) |
| 2 | 18 (15.4) | 9 (15.3) | 9 (15.5) | |
| ≥3 | 6 (5.1) | 4 (6.7) | 2 (3.4) | |
| Primary cancer | Prostate | 55 (47) | 27 (45.8) | 28 (48.3) |
| Lung | 11 (9.4) | 5 (8.5) | 6 (10.3) | |
| Colorectal | 10 (8.5) | 4 (6.8) | 6 (10.3) | |
| Renal | 8 (6.8) | 3 (5.1) | 5 (8.6) | |
| Breast | 7 (6) | 5 (8.5) | 2 (3.4) | |
| Head & neck | 7 (6) | 5 (8.5) | 2 (3.4) | |
| Sarcoma | 7 (6) | 3 (5.1) | 4 (6.9) | |
| Thyroid | 6 (5.1) | 3 (5.1) | 3 (5.2) | |
| Skin | 4 (3.4) | 2 (3.4) | 2 (3.4) | |
| Bladder | 1 (0.9) | 1 (1.7) | 0 (0) | |
| Gyn | 1 (0.9) | 1 (1.7) | 0 (0) | |
| Systemic or Hormonal Therapy During RT | Yes | 71 | 37 | 34 |
| No | 46 | 22 | 24 | |
| Bone vs. node lesions | Bone | 103 (88) | 50 (84.7) | 53 (91.4) |
| Node | 10 (8.5) | 6 (10.2) | 4 (6.9) | |
| Bone & Node | 4 (3.4) | 3 (5.1) | 1 (1.7) | |
| Spine vs. non-spine lesions | Spine | 66 (56.4) | 32 (54.2) | 34 (58.6) |
| Non-spine | 44 (37.6) | 23 (39) | 21 (36.2) | |
| Spine & non-spine | 7 (6.1) | 4 (7) | 3 (5.2) | |
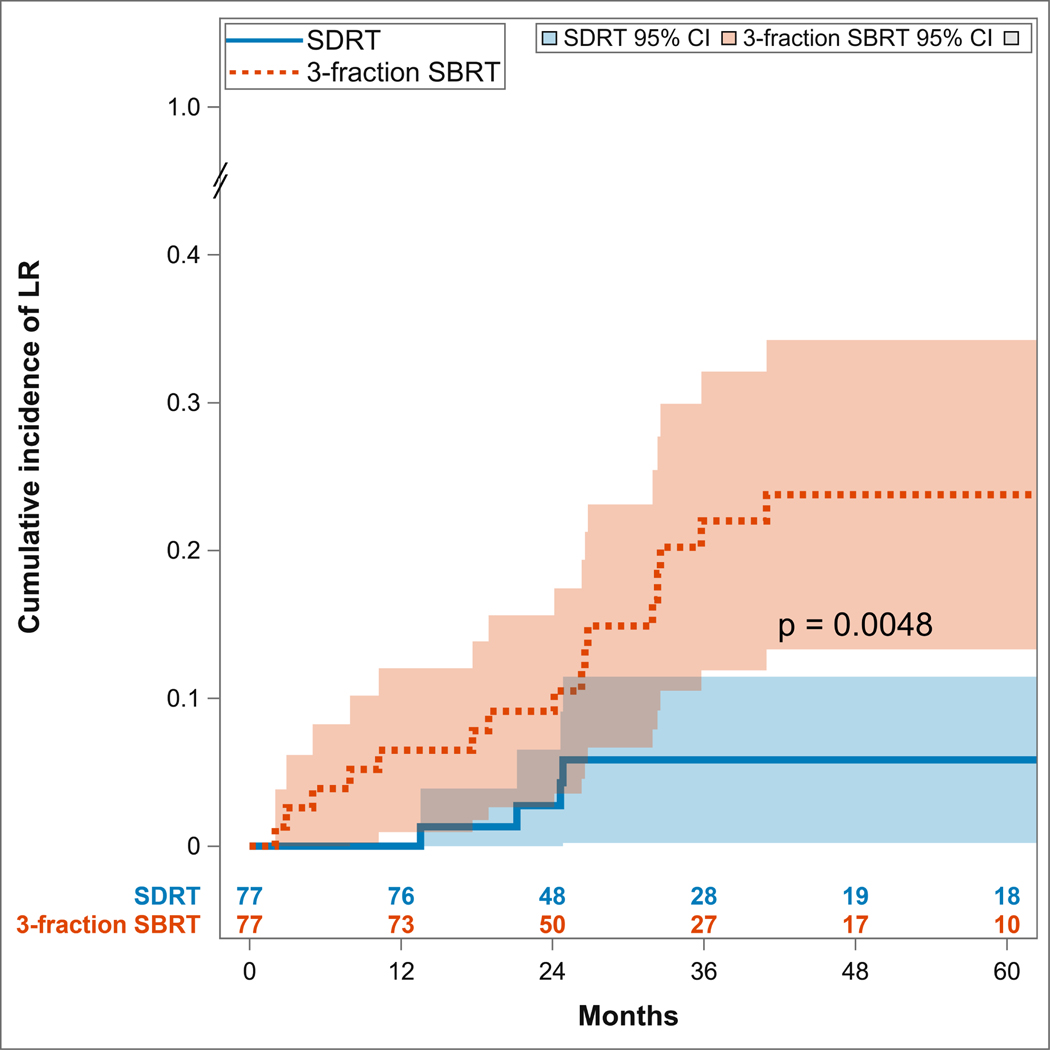
As shown in Figure 1, lesions treated with SDRT had a significantly lower cumulative incidence of local recurrences compared to those treated with SBRT (two-sided Gray test p=0.0048). The cumulative incidence of local recurrence at years one, two, and three for SDRT was 0% (95% CI: limits not reached), 2.7% (95% CI: 0–6.5%), and 5.8% (95% CI: 0.2–11.5%), respectively, compared to 6.5% (95% CI: 1–12%), 9.1% (95% CI: 2.6–15.6%), and 22% (95% CI: 11.9–32.1%) for the lesions treated with 3 × 9 Gy SBRT.. Interestingly, we observed that among the 18 patients who had LR within their respective SBRT or SDRT fields, 9 (50%) exhibited distant metastatic progression, while for the 99 patients who were locally controlled at the end of the study, 7 (7.1%) exhibited distant metastases.
Figure 1:
Cumulative incidence of local recurrence (LR) and progression of disease within the irradiated region, demonstrating superior outcomes of ablative SDRT compared to fractionated SBRT.
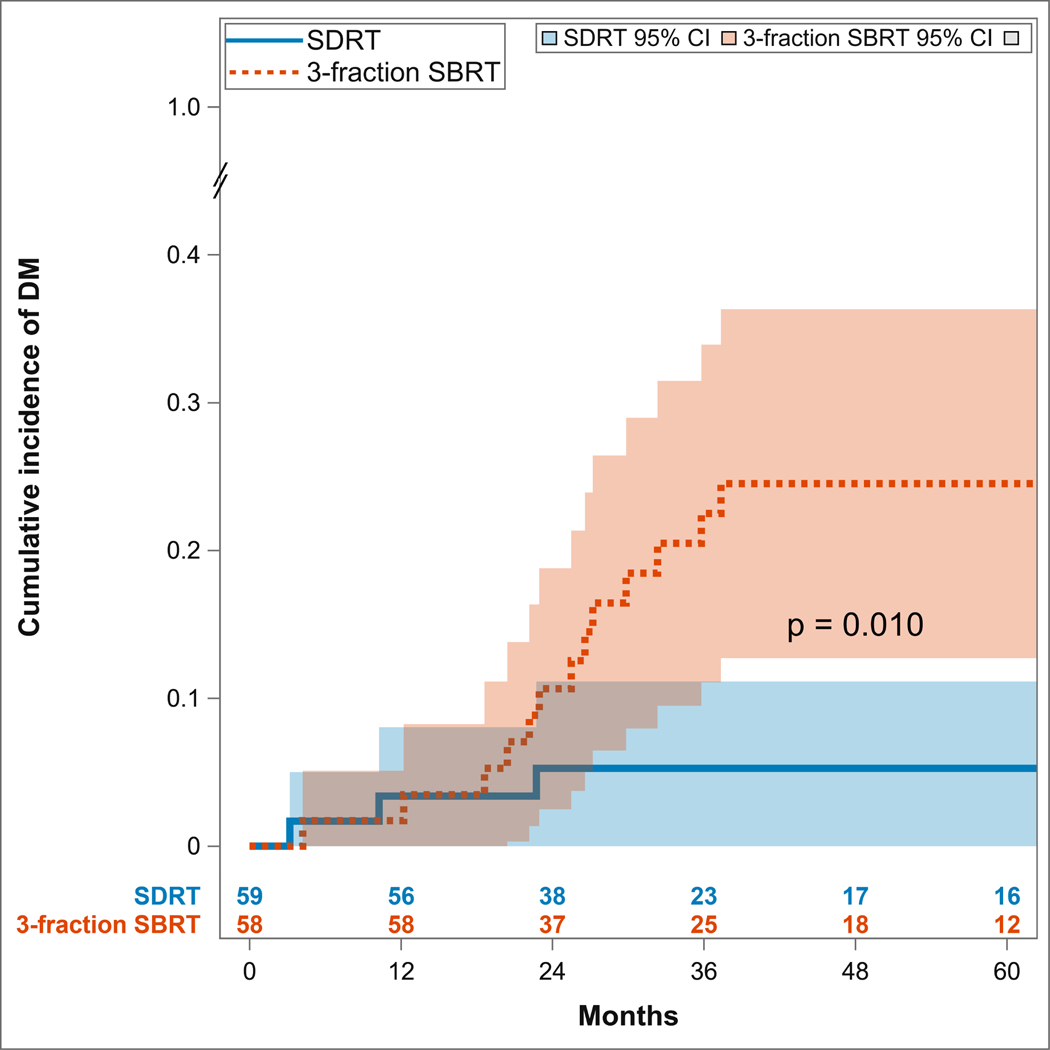
To explore whether there might be an association between LR and metastatic progression, we compared the cumulative incidence of new metastatic lesions in the patients randomized to the two treatment arms. Figure 2 shows that patients treated with SDRT had a significantly lower cumulative incidence of distant metastatic spread as compared to those treated with SBRT (two-sided Gray test p=0.010). The cumulative incidence of distant metastases at years one, two, and three for SDRT was 3.4% (95% CI: 0–8%), 5.3% (95%CI: 0–11.1%), and 5.3% (95% CI: 0–11.1%), respectively, compared to 1.7% (95% CI: 0–5.1%), 10.7% (95% CI: 2.5–18.8%), and 22.5% (95% CI: (11.1–33.9%) for the SBRT regimen. No significant differences in LR or metastatic progression was observed among prostate or breast primary tumors compared with other histologies.
Figure 2:
Cumulative incidence of distant metastases (DM) and progression of disease outside of the irradiated field, showing superior outcomes of ablative SDRT compared with fractionated SBRT.
To determine whether post-radiation systemic adjuvant therapy had an impact on LR and and/or metastatic progression, a landmark competing risk analysis was performed. For LR, 145 lesions were included in the analysis — with 63 exposed to systemic adjuvant therapy (6 patients with LRs observed) and 82 without systemic adjuvant therapy (8 patients with LRs observed). The Gray’s test p-value was 0.95. For distant metastases, 110 patients were included in the landmark competing risks analysis — 66 patients with systemic adjuvant therapy (7 distant metastatic spreads observed) and 44 without systemic adjuvant therapy (6 distant metastatic spreads observed). The Gray’s test p-value was 0.58. Thus, this study observed no significant association between post-radiation systemic adjuvant therapy and the incidence of either LR or metastatic spread. However, a multivariate model was not possible due to the relatively small number of events observed.
Toxicity outcomes were relatively low and similar in each of the treatment arms as summarized in Table 2. There was no statistically significant difference in the incidence of grade 2+ or 3+ lesions between the two treatment arms with an incidence of 9/77 [11.7%] grade 2+ and 6/77 [7.8%] grade 3+ for SDRT, and 5/77 [6.5%] grade 2+ and 3/77 (3.9%) grade 3+ lesions for SBRT (Fisher’s p=0.40 grade 2+ and 0.49 grade 3+). While the incidence of fracture post-treatment is clinically relevant, the incidence was low — with 2.6% (4/154) grade 3 fractures in both the SDRT and SBRT regimens (Fisher’s p>0.99).
Table 2:
Grade 2+ pain, peripheral sensory neuropathy, and fracture rates by treatment regimen
| All lesions | SDRT | SBRT | ||
|---|---|---|---|---|
| N (%) | N (%) | N (%) | ||
| Total lesions | 154 | 77 (50) | 77 (50) | |
| Grade 2+ Pain | Yes | 10 (6.5) | 7 (9.1) | 3 (3.9) |
| No | 144 (93.5) | 70 (90.9) | 74 (96.1) | |
| Grade 2+ Neuropathy | Yes | 2 (1.3) | 2 (2.6) | 0 (0) |
| No | 152 (98.7) | 75 (97.4) | 77 (100) | |
| Grade 2+ Fracture | Yes | 4 (2.6) | 2 (2.6) | 2 (2.6) |
| No | 150 (97.4) | 75 (97.4) | 75 (97.4) | |
DISCUSSION
The present study provides evidence that SDRT is associated with superior tumor control of the irradiated lesion, and this in turn is associated with a reduction in further clinical metastatic progression. The data show that patients treated with the 3 × 9 Gy SBRT exhibit at year three a 4-fold increase of radiation in-field local recurrences and a 4-fold increase in distant metastatic spread when compared with the corresponding rates observed in patients randomized to 24 Gy SDRT. The cumulative rate of LR in lesions treated with SDRT was 5.8% at year three, which is consistent with previous studies reporting that 24 Gy SDRT targeting metastatic tumors in non-mobile organs, such as bone and lymph nodes, renders a five-year local relapse-free survival rate of 95%.11 The improved local control observed with SDRT is likely related to the enhanced biologic equivalent dose that is delivered to the tumor volume compared to what is received with lower dose fractionated regimens. The impressive mitigation of metastatic progression upon ablative consolidation, as observed with 24 Gy SDRT, has never been substantiated before in a randomized trial setting.
Recently, Palma et al2 reported the results of a randomized phase II trial where patients with limited oligometastases were assigned using a 2:1 randomization to receive SBRT versus a palliative conventional fractionated (CF) radiotherapy regimen. The fractionation schemes for the SBRT arm included dose levels ranging from 30–60 Gy in 3–8 fractions as well as single fraction doses ranging from 16 Gy–24 Gy. The CF regimen utilized doses ranging from 8 Gy in a single fraction to 30 Gy in 10 fractions. With a median follow-up of two years, the local recurrence rates were 25% and 51% in the SBRT and CF arms, respectively, and the distant progression rates were 59% and 85% for the SBRT and CF, respectively. In a single arm prospective study using 20 Gy delivered in a single fraction for 33 patients with oligometastases (50 lesions treated), the two-year local recurrence rate was 7% and the incidence of distant progression was 42%.23 The significantly lower rates of distant progression (5.3% at three years) observed in our current experience could possibly be related to the more effective ablative potential of 24 Gy in a single fraction compared to the SBRT regimens used in these aforementioned studies. Indeed, in a dose escalation study, we observed significantly improved local control rates for SBRT when dose levels of 24 Gy were used compared to lower doses.9
While the present study was designed as a prospective, randomized, phase III trial, several study limitations should be highlighted. These include the relatively small sample size of the study arms; the restriction of metastatic deposits to non-mobile bone and lymph nodes; the realization that nearly 50% of the patient population had prostate cancer histology; the lack of strict protocol guidelines regarding the use of adjuvant systemic therapy; and the relatively short-term follow-up in recording distant metastatic spread. In addition, while significant attrition of follow-up after two to three years was noted, patients were not required to return for routine follow-up after two years because the primary and secondary endpoints of this study mandated outcome evaluation only at one and two years after therapy. This was, in particular, the case for patients who lived far from the institution and were treated by local medical oncologists for their metastatic disease. Notwithstanding these limitations, the observation that consolidated ablation of macroscopic oligometastatic lesions hypothetically impacts the natural history of the residual and microscopic disease cannot be ignored. This observation adds a new dimension to the oligometastatic model,5,24 and is consistent with multiple reports with long-term follow-up on the outcomes of non-randomized trials of ablative surgery in oligometastatic patients.6,7
The present outcomes are also consistent with the recent phase II ORIOLE trial, which randomized observation only versus radio-ablation of oligometastatic prostate cancer lesions.3 Baseline PSMA-avid PET imaging data were blinded during radiation treatment planning of the SBRT-randomized arm, which resulted in at least one PSMA-avid lesion failing to co-register with the treatment planning CT or MRI in 45% of the SBRT patients, and thus remained untreated, as realized in retrospect. Distant metastatic lesions developed by six months in 63% of the patients with radiation-untreated PSMA-avid lesions — compared with 16% in the remaining patients who did receive comprehensive oligometastases-directed radio-ablation (P = 0.006) — but long-term follow-up was not provided.3
The mechanism by which ablation of macroscopic tumor lesions might drive repression or dissolution of residual microscopic disease remains unknown. Because of the lack of animal models of human oligometastatic disease, progress in the field has largely been derived from comparative genomic, transcriptomic, and somatic driver studies of archived human oligometastatic tumor tissues resected from the liver or lung.25–27 While such studies yielded signatures and identified metabolic drivers differentiating oligometastases from the respective primary or from poly-metastatic disease,25,26 an operational mechanistic pathway that drives the oligo- to poly-metastatic conversion has not been defined. Nonetheless, there is substantial evidence that the oligometastatic syndrome is a biologically dynamic process, which is associated with the evolution of a propensity for distant metastatic spread along a linear continuum that gradually modifies the metastatogenic equilibrium towards polymetastatic conversion.27,28,29 Employing integrative molecular analysis of pro-metastatogenic virulence in hepatic colorectal oligometastatic lesions, combined with clinical risk stratification, Pitroda and Weichselbaum26 reported tumor categorization into low, intermediate, and high pro-metastatic virulence, yielding overall 10-year survivals of 94%, 45%, and 19%. respectively.26 Similarly, a recent phase II oligometastatic SDRT study reported a three-tiered categorization of oligometastatic lesions, defined by pre-SDRT PET/CT metrics, rendering 89%, 58%, and 17% actuarial five-year metastases-free survival in low, intermediate, and high-risk patients, respectively.11 Taken together, these observations suggest a hypothesis that mitigation of post-SDRT distant metastatic spread depends not only on the ability to ablate the macroscopic tumor pool, but also on the propensity virulence state of the tumor towards metastatic spread. Hence, it might be preferred to ablate oligometastatic lesions at the earliest phase post-diagnosis, in order to target each macrometastatic lesion at its least virulent risk setting, thereby potentially maximizing repression of metastatic dissemination.
Currently, the predominant strategy for primary therapy of metastatic disease, be it oligo- or poly-metastatic disease, is with systemic therapy. Local radio-ablative therapy is usually not incorporated into initial treatment regimens, as it has been long assumed that even the earliest clinical-phase metastatic disease may already be sub-clinically poly-metastatic. The present study provides a hypothetical alternative to this approach. Accepting, for the sake of discussion, the basic concept of polymetastatic conversion, there is no current knowledge on whether, how, or when polymetastatic conversion might occur relative to the initial diagnosis of metastatic disease. Whereas the effect of systemic therapies on the virulence of metastatic progression is presently unknown, we posit that there may be an indication for a comprehensive SDRT ablation to be used as a first line therapy after initial diagnosis of oligometastatic disease, regardless of lesion number. While the present study suggests that comprehensive radio-ablation mitigates metastatic progression, we posit that a radio-ablated state in early metastatic cancer may represent a baseline for testing the potential of tumor type-specific adjuvant systemic therapies to increase the rate and/or duration of metastasis-free survival. We also posit that this approach may render cure in specific phenotypes of early metastatic human cancer.
CONCLUSION
Treatment with single-dose 24 Gy was associated with superior local control of irradiated oligometastases compared to a 3-fraction SBRT regimen. In addition, we observed significantly reduced metastatic progression in the SDRT arm compared to the SBRT arm. Yet, we did not observe increased toxicity for those who were treated with SDRT despite its higher biologic dose delivered to the PTV compared to SBRT. It is interesting to note that although there is an increased rate radiographic compression fracture after 24 Gy in a single fraction, the cumulative symptomatic fracture rate in the spine (requiring an intervention) was reported to be 7.2%.30 The lower rates of <3% fractures in the. current trial may be related to less advanced tumors in the bone without significant cortical destruction present. It is also plausible that given the relatively small sample size, the incidence of grade 2+ fracture could have been higher in a larger cohort of patients. We do agree, however, that 24 Gy in a single fraction should be given judiciously in the setting of metastases in long bone/weight bearing bones.
The intent of the current trial was primarily to address oligometastases in bone and soft tissue where these dose regimens are appropriate. However, our conclusions cannot be applied to liver or lung metastases, for instance, as the treated populations in the current trial were exclusively limited to bone, nodal metastases, and soft tissue metastases. Our data indicate that SDRT is an effective treatment for oligometastases and suggest that effective ablation of oligometastatic lesions is associated with significant mitigation of distant metastatic progression.
CONFLICTS OF INTEREST
Dr. Yamada reports personal fees from University of Wollongong, Vision RT, BrainLAB, and Varian Medical Systems for work outside this submitted work; and serves on the medical advisory board of Chordoma Foundation. Dr Bilsky reports royalties from Globus Medical and Depuy Synthes, and payment for lectures from Stryker Spine. Dr Fuks and Dr Greco have activities outside the submitted work as founders of Ceramedix Holding and stock owners. In addition, Dr. Fuks and Dr. Greco have a patent 62880797 pending. Dr. Powell reports grants from NIH/NCI, during the conduct of the study. The remaining authors have no relevant disclosures.
Footnotes
CLINICAL TRIAL INFORMATION
This study is registered with ClinicalTrials.gov (NCT01223248) and is closed.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA SHARING STATEMENT
Individual participant data that underlie the results reported in this article will be shared after de-identification. The study protocol, statistical analysis plan, and analytic code will also be shared. This data will be available — beginning three months and ending five years following publication of the article — upon request on a case-by-case basis to researchers who provide a methodologically sound proposal. Requests should be made to the corresponding author, which will then be evaluated by the participating study institutions.
REFERENCES
- 1.Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018; 36(5): 446–453. doi: 10.1200/JCO.2017.75.4853 [DOI] [PubMed] [Google Scholar]
- 2.Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019; 393(10185): 2051–2058. doi: 10.1016/S0140-6736(18)32487-5 [DOI] [PubMed] [Google Scholar]
- 3.Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020; 6(5): 650–659. doi: 10.1001/jamaoncol.2020.0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez DR, Tang C, Zhang J, et al. Local consolidative therapy vs. Maintenance therapy or observation for patients with oligometastatic non–small-cell lung cancer: Long-term results of a multi-institutional, phase II, randomized study. J Clin Oncol. 2019; 37(18): 1558–1565. doi: 10.1200/JCO.19.00201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995; 13(1): 8–10. doi: 10.1200/jco.1995.13.1.8 [DOI] [PubMed] [Google Scholar]
- 6.Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: Prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997; 113(1): 37–49. doi: 10.1016/S0022-5223(97)70397-0 [DOI] [PubMed] [Google Scholar]
- 7.Fortner JG, Fong Y. Twenty-Five-Year Follow-up for Liver Resection. Ann Surg. 2009; 250(6): 908–913. doi: 10.1097/SLA.0b013e3181b59491 [DOI] [PubMed] [Google Scholar]
- 8.Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol. 2007; 25(29): 4575–4580. doi: 10.1200/JCO.2007.11.0833 [DOI] [PubMed] [Google Scholar]
- 9.Greco C, Zelefsky MJ, Lovelock M, et al. Predictors of local control after single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases. Int J Radiat Oncol Biol Phys. 2011; 79(4): 1151–1157. doi: 10.1016/j.ijrobp.2009.12.038 [DOI] [PubMed] [Google Scholar]
- 10.Zelefsky MJ, Greco C, Motzer R, et al. Tumor control outcomes after hypofractionated and single-dose stereotactic image-guided intensity-modulated radiotherapy for extracranial metastases from renal cell carcinoma. Int J Radiat Oncol Biol Phys. 2012; 82(5): 1744–1748. doi: 10.1016/j.ijrobp.2011.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greco C, Pares O, Pimentel N, et al. Phenotype-Oriented Ablation of Oligometastatic Cancer with Single Dose Radiation Therapy. Int J Radiat Oncol Biol Phys. 2019; 104(3): 593–603. doi: 10.1016/j.ijrobp.2019.02.033 [DOI] [PubMed] [Google Scholar]
- 12.Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys 2008;71:484–490. [DOI] [PubMed] [Google Scholar]
- 13.McCammon R, Schefter TE, Gaspar LE, Zaemisch R, Gravdahl D, Kavanagh B. Observation of a Dose-Control Relationship for Lung and Liver Tumors After Stereotactic Body Radiation Therapy. Int J Radiat Oncol Biol Phys. 2009; 73(1): 112–118. doi: 10.1016/j.ijrobp.2008.03.062 [DOI] [PubMed] [Google Scholar]
- 14.Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-Institutional Phase I/II Trial of Stereotactic Body Radiation Therapy for Lung Metastases. J Clin Oncol. 2009; 27(10): 1579–1584. doi: 10.1200/jco.2008.19.6386 [DOI] [PubMed] [Google Scholar]
- 15.Schefter TE, Rusthoven KE, Kavanagh BD, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009; 27(10): 1572–1578. doi: 10.1200/JCO.2008.19.6329 [DOI] [PubMed] [Google Scholar]
- 16.Hong JC, Lee J, et al. Classification for long-term survival in oligometastatic patients treated with ablative radiotherapy: A multi-institutional pooled analysis. PLoS One. 2018; 13(4): 1–17. doi: 10.1371/journal.pone.0195149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodo S, Campagne C, Thin TH, et al. Single-dose radiotherapy disables tumor cell homologous recombination via ischemia/reperfusion injury. J Clin Invest. 2019; 129(2): 786–801. doi: 10.1172/JCI97631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg AK, Wang X-S, Shiu AS, et al. Prospective evaluation of spinal reirradiation by using stereotactic body radiation therapy. Cancer. 2011; 117(15): 3509–3516. doi: 10.1002/cncr.25918 [DOI] [PubMed] [Google Scholar]
- 19.Sahgal A, Ma L, Weinberg V, et al. Reirradiation human spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2012; 82(1): 107–116. doi: 10.1016/j.ijrobp.2010.08.021 [DOI] [PubMed] [Google Scholar]
- 20.Wang XS, Rhines LD, Shiu AS, et al. Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: A phase 1–2 trial. Lancet Oncol. 2012; 13(4): 395–402. doi: 10.1016/S1470-2045(11)70384-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salama JK, Chmura SJ, Mehta N, et al. An initial report of a radiation dose-escalation trial in patients with one to five sites of metastatic disease. Clin Cancer Res. 2008; 14(16): 5255–9. doi: 10.1158/1078-0432 [DOI] [PubMed] [Google Scholar]
- 22.Chang EL, Shiu AS, Mendel E, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J. Neurosurg Spine; 2007, 7(20): 151–160. [DOI] [PubMed] [Google Scholar]
- 23.Siva SM, Bressel M, Murphy DG, et al. Stereotactic Ablative Body Radiotherapy (SABR) for Oligometastastatic Prostate Cancer: A Prospective Clinical Trial. Eur Urol 2018; 74(4): 455–462. doi: 10.1016/j.eururo.2018.06.004 [DOI] [PubMed] [Google Scholar]
- 24.Weichselbaum RR, Hellman S. Oligometastases revisited. Nat Rev Clin Oncol. 2011; 8(6): 378–382. doi: 10.1038/nrclinonc.2011.44 [DOI] [PubMed] [Google Scholar]
- 25.Lussier YA, Khodarev NN, Regan K, et al. Oligo- and polymetastatic progression in lung metastasis(es) patients is associated with specific microRNAs [published correction appears in PLoS One. 2013;8(6). doi: 10.1371/annotation/2489ae5e-3650-4897-8df6-3e974ca585c4. Gnerlich, Jennifer [corrected to Gnerlich, Jennifer L]]. PLoS One. 2012; 7(12): e50141. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pitroda SP, Khodarev NN, Huang L. et al. Integrated molecular subtyping defines a curable oligometastatic state in colorectal liver metastasis. Nat Commun. 2018; 9(1): 1793. 10.1038/s41467-018-04278-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turajlic S, Xu H, Litchfield K, et al. Tracking Cancer Evolution Reveals Constrained Routes to Metastases: TRACERx Renal. Cell. 2018; 173(3): 581–594.e12. doi: 10.1016/j.cell.2018.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bakhoum SF, Cantley LC. The multifaceted role of chromosomal instability in cancer and its microenvironment. Cell. 2018;174(6):1347–1360. doi: 10.1016/j.cell.2018.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pitroda SP, Weichselbaum RR. Integrated molecular and clinical staging defines the spectrum of metastatic cancer. Nat Rev Clin Oncol. 2019; 16(9): 581–588. doi: 10.1038/s41571-019-0220-6 [DOI] [PubMed] [Google Scholar]
- 30.Virk MS, Han JE, Reiner AS, et al. Frequency of symptomatic vertebral body compression fractures requiring intervention following single-fraction stereotactic radiosurgery for spinal metastases. Neurosurg Focus 2017; 42(1): E8. doi: 10.3171/2016.10 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Individual participant data that underlie the results reported in this article will be shared after de-identification. The study protocol, statistical analysis plan, and analytic code will also be shared. This data will be available — beginning three months and ending five years following publication of the article — upon request on a case-by-case basis to researchers who provide a methodologically sound proposal. Requests should be made to the corresponding author, which will then be evaluated by the participating study institutions.