Abstract
Objective:
The objective of this study was to compare the effects of liposomal bupivacaine (Lipo-B) and bupivacaine hydrochloride (B-HCl), in the presence of multimodal analgesia, on postoperative analgesia and opioids consumption in minimally invasive thoracic surgery (MITS) lobectomy.
Design:
Retrospective observational cohort study.
Setting:
Tertiary care cancer center.
Participants:
A total of 60 patients who underwent MITS lobectomy and received intercostal nerve blockade (ICNB) with either 0.66% Lipo-B (n=29) or 0.5% B-HCl (n=31).
Interventions:
All patients received intravenous patient-controlled analgesia for the first 12 h postoperatively, followed by opioids and nonsteroidal anti-inflammatory drugs as needed.
Measurements and Main Results:
Perioperative opioid and nonopioid consumption and pain scores were compared between groups at 12-h intervals for the first 72 h. Between the two groups, there were no statistically significant differences in demographic characteristics, intraoperative (p=0.46) and postoperative opioid consumption, Richmond Agitation-Sedation Scale scores and pain scores upon postanesthesia care unit arrival and 4 h, length of postanesthesia care unit stay (p=0.84), or length of hospital stay (p=0.55). Both groups received intra- and postoperative multimodal analgesia.
Conclusions:
In our cohort, we did not detect a difference in opioid consumption or pain scores in the immediate postoperative period following MITS lobectomy between patients given ICNB with Lipo-B and those given ICNB with B-HCl in the presence of multimodal analgesia.
Keywords: liposomal bupivacaine, minimally invasive thoracic surgery, intercostal nerve blockade, acute pain
INTRODUCTION
Multimodal analgesia has been recommended to potentiate the intended effects of opioids while decreasing their side effects in patients undergoing surgery. This recommendation has been incorporated in several recent guidelines on enhanced recovery after surgery pathways, including a multimodal approach, with nonnarcotic analgesics and local anesthetics playing an important role as first-line medications. 1
Several regional anesthetic techniques have been used to provide pain relief following lung resection. Although epidural analgesia remains the gold standard for open procedures, there is less consensus on first-line regional techniques for minimally invasive thoracic surgery (MITS). Paravertebral, fascial plane, and intercostal nerve blockade (ICNB) are used for these cases, but they may have a limited duration of action in the absence of catheter use. With the advent of longer-acting local anesthetics, peripheral nerve blockade has gained popularity for patients undergoing MITS.
As it is easy to perform and relatively safe, ICNB is commonly used for thoracic surgery.2 Typically, the surgeon infiltrates local anesthetic in several dermatomes, under direct thoracoscopic vision, at the end of the case. Long-acting local anesthetics increase the duration of the block and are therefore an ideal option. However, the potential systemic toxicity, related to the high rate of systemic absorption, has been identified as a limiting factor.
Liposomal bupivacaine (Lipo-B) was approved by the US Food and Drug Administration (FDA) in October 2011 as a slow-release formulation of bupivacaine (Exparel, Pacira Pharmaceuticals, Parsippany, NJ, USA) for single-dose infiltration. The FDA approval was extended to transabdominal plane and trigeminal nerve blocks in 2015 and to interscalene brachial plexus blocks in 2018.3 At present, Lipo-B is used off-label for ICNB. Its main advantage, in comparison with bupivacaine hydrochloride (B-HCl), is its prolonged duration of action. Both local anesthetics are amides that act by inhibiting the sodium influx in the neuronal axon. In Lipo-B, the slow release of the active drug after erosion of the liposomal layer seems to increase the half-life of the formulation from 3 h to 72 h, when compared with B-HCl.4
Several retrospective and observational studies have compared Lipo-B and B-HCl in thoracic noncardiac surgery, with mixed results.
The aim of this observational study was to compare the effects of ICNB with Lipo-B or B-HCl on immediate postoperative pain scores and narcotics consumption after MITS lung lobectomy. We hypothesized that the two local anesthetics had similar effects on analgesia in the immediate postoperative period, when used for ICNB after MITS lobectomy in the presence of a multimodal analgesic protocol.
METHODS
After obtaining approval from our institutional review board (IRB#16–442; approved 5/09/2016) for this historical cohort study, we retrospectively identified consecutive patients who underwent MITS lung lobectomy via either video-assisted thoracoscopy (VATS) or robotic-assisted thoracic surgery (RATS) at our institution between 2015 and 2017. Patients received either 0.66% Lipo-B or 0.5% B-HCl.
Inclusion criteria were age ≥18 years, MITS lobectomy, and ICNB with either Lipo-B or B-HCl for postoperative analgesia. Exclusion criteria were thoracotomy procedures, postoperative epidural analgesia, chronic pain, use of two local anesthetics for one procedure, or intraoperative use of a continuous infusion of nonopioid analgesics, such as ketamine, dexmedetomidine, or lidocaine. Patients enrolled in alternate research protocols were also excluded.
General anesthesia was induced using propofol, fentanyl, and a nondepolarizing neuromuscular blocker, at the discretion of the anesthesiologist. Single-lung ventilation was facilitated by endotracheal intubation with a left-sided double-lumen endotracheal tube. Anesthesia was maintained with sevoflurane in an oxygen and air mixture; analgesia was achieved with intravenous fentanyl and hydromorphone in intermittent boluses. All patients received intravenous ketorolac and acetaminophen before extubation unless contraindicated (creatinine >1.5 mg/dL and ALT >3 times the upper limit of normal, respectively). ICNB was performed by the surgeon at the end of the procedure, under direct thoracoscopic vision, with either 0.66% Lipo-B (1.33% diluted in normal saline) or 0.5% B-HCl, depending on personal preference. The number of dermatomes blocked, and the volume of local anesthetic used in each dermatome were also at the surgeon’s discretion, in accordance with their individual standard of practice. The same local anesthetic was used to infiltrate the wound at the site of the thoracoscopic ports in variable aliquots, at the surgeon’s discretion.
At the end of the surgical case, residual neuromuscular blockade was reversed with a combination of neostigmine and glycopyrrolate at a dose calculated according to the train-of-four ratio. Patients were extubated in the operating room once criteria were met and were then transferred to the recovery room, where they stayed until meeting the floor’s discharge criteria.
Data were retrospectively collected for each patient from the medical record. Postoperative analgesic requirements were assessed at 12-h intervals for a total of 72 h and included patient-controlled analgesia (PCA), intermittent boluses of opioids, and nonopioid medications.
All patients were admitted to the postanesthesia care unit (PACU) at the end of their operation and were given either fentanyl or hydromorphone PCA. Richmond Agitation-Sedation Scale (RASS) scores and number of clinician-activated boluses were recorded immediately upon arrival in the PACU and at 4 h.
A total postoperative opioid dosage was calculated for each patient as the sum of the PCA dose and the oral intake. All opioid doses were converted to intravenous morphine equivalent doses (milligrams) to allow for comparison between groups. No adjustments were used.
Statistical Methods
Data are presented as mean (SD), number (%), or median (25th-75th percentile). Student’s t test was used to analyze continuous variables, and the χ2 test was used to compare categorical data. P<0.05 was considered to indicate statistical significance. Statistical analysis was performed using the appropriate functions in IBM SPSS software.
RESULTS
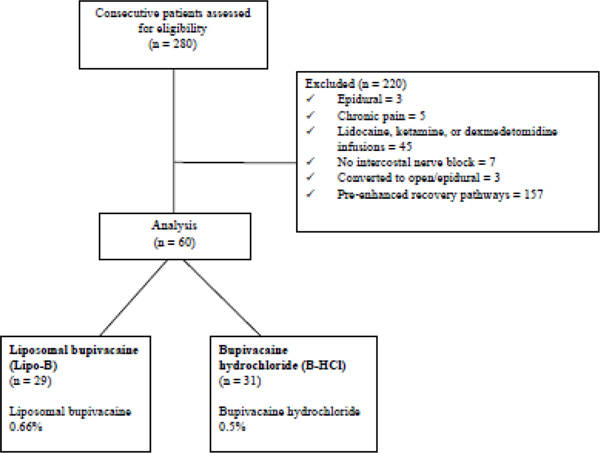
We identified 60 eligible patients: 29 in the Lipo-B group and 31 in the B-HCl group (Figure 1). Patient demographic characteristics and comorbidities (Table 1) were similar between groups, except for a higher incidence of hypertension (p=0.007) and more frequent use of angiotensin-converting enzyme inhibitors (p=0.02) in the Lipo-B group. Most patients in the Lipo-B group underwent RATS, whereas most patients in the in the B-HCl group underwent VATS (Table 2). Operative duration, blood loss, use of fluids, and pressor requirements were similar between the two groups (Table 2). The Lipo-B group received a higher volume of local anesthetic in the ICNB (Table 2), whereas the volume used for skin infiltration at the port sites was not statistically significantly different between the groups.
Figure 1.
CONSORT diagram for the study population.
Table 1.
Patient demographic and clinical characteristics
| Variable | Lipo-B (N=29) | B-HCl (N=31) | p |
|---|---|---|---|
|
| |||
| Age, years | 69.5 (8.7) | 65.6 (8.1) | 0.337 |
| Sex | |||
| Male | 12 (41) | 8 (26) | 0.201 |
| Female | 17 (59) | 23 (74) | |
| BMI, kg/m2 | 28 (5.3) | 25.5 (4.9) | 0.339 |
| Comorbidity | |||
| Coronary artery disease | 6 (21) | 6 (19) | 0.897 |
| Hypertension | 23 (79) | 14 (45) | 0.007 |
| Chronic obstructive pulmonary disease | 13 (45) | 9 (29) | 0.25 |
| Diabetes mellitus | 7 (24) | 3 (10) | 0.133 |
| Atrial fibrillation | 5 (17) | 1 (3) | 0.07 |
| FEV1, % predicted | 93.9 (22.1) | 92.3 (17.2) | 0.10 |
| DLCO, % predicted | 80.8 (20.5) | 80.0 (17) | 0.17 |
| Albumin, g/dL | 4.3 (0.2) | 4.2 (0.3) | 0.758 |
| Preoperative medication | |||
| Beta blockers | 12 (41) | 12 (39) | 0.83 |
| Calcium channel blockers | 7 (24) | 6 (19) | 0.65 |
| Statins | 11 (38) | 14 (45) | 0.57 |
| ACE inhibitors | 17 (59) | 9 (29) | 0.02 |
Data are no. (%) or mean (SD). The p value compares ICNB with Lipo-B and ICNB with B-HCl. The t test was used to compare means, and the χ2 test was used to compare proportions. BMI, body mass index; DLCO, diffusion capacity of the lung for carbon monoxide; FEV1, forced expiratory volume in 1 second; ACE, angiotensin-converting enzyme.
Table 2.
Intraoperative and postoperative data
| Variable | Lipo-B (N=29) | B-HCl (N=31) | p |
|---|---|---|---|
|
| |||
| Intraoperative analgesia | |||
| MSO4, mga | 29 (25 to 38) | 26 (23 to 34) | 0.46 |
| Ketorolacb | 22 (76) | 24 (77) | 0.80 |
| Acetaminophenb | 27 (93) | 30 (97) | 0.54 |
| Dexamethasoneb | 12 (41) | 25 (81) | 0.02 |
| Volume of LA in the wound, mLa | 0 (0 to 10) | 0 (0 to 10) | 0.76 |
| Volume of LA in the ICNB, mLa | 20 (15 to 33) | 19 (10 to 20) | 0.01 |
| Pain score at arrivala | 0 (0 to 0) | 0 (0 to 0.5) | 0.98 |
| Pain score at 4 ha | 1 (0 to 3) | 0 (0 to 2) | 0.06 |
| RASS score on arrivala | −1 (−2 to −1) | −1 (−1 to −1) | 0.68 |
| RASS score at 4 ha | 0 (0 to 0) | 0 (−1 to 0) | 0.99 |
| Length of PACU stay, h:mina | 5:13 (4:04 to 9:00) | 6:33 (4:51 to 10:53) | 0.84 |
| Length of hospital stay, daysa | 3 (2 to 4) | 3 (2.5 to 4) | 0.55 |
| Intensive care unit admissionb | 0 | 1 (3) | |
| Postoperative nonopioidsb | |||
| Ketorolac | 20 (69) | 25 (81) | 0.3 |
| Acetaminophen | 16 (55) | 22 (71) | 0.21 |
| Diclofenac | 17 (59) | 15 (48) | 0.43 |
| Magnesium | 8 (28) | 15 (48) | 0.1 |
| Lidocaine patch | 11 (38) | 8 (26) | 0.31 |
| Ibuprofen | 3 (10) | 2 (6) | 0.59 |
| Gabapentin | 2 (7) | 8 (26) | 0.05 |
| Aspirin | 8 (28) | 5 (16) | 0.28 |
| RATSb | 20 (69) | 7 (23) | 0.001 |
| VATSb | 9 (31) | 24 (77) | 0.001 |
| Operative duration, mina | 166 (126 to 202) | 162 (123 to 188) | 0.46 |
| Estimated blood loss, La | 20 (25 to 75) | 50 (30 to 100) | 0.39 |
| Crystalloids, La | 0.9 (0.7 to 1.0) | 0.9 (0.7 to 1.0) | 0.45 |
| Colloids, La | 0.3 (0.3 to 0.4) | 0.4 (0.2 to 0.5) | 0.37 |
| Phenylephrine, mcga | 100 (0 to 200) | 120 (0 to 340) | 0.41 |
| Ephedrine, mga | 0 (0 to 5) | 0 (0 to 9) | 0.24 |
The p value compares ICNB with Lipo-B and ICNB with B-HCl. The t test was used to compare means, and the χ2 test was used to compare proportions. MSO4, morphine; LA, local anesthetic; ICNB, intercostal nerve block; RASS, Richmond Agitation Sedation Score; PACU, Post Anesthesia Care Unit; RATS, Robotic Assisted Thoracic Surgery; VATS, Video Assisted Thoracic Surgery.
Median (interquartile range)
No. (%).
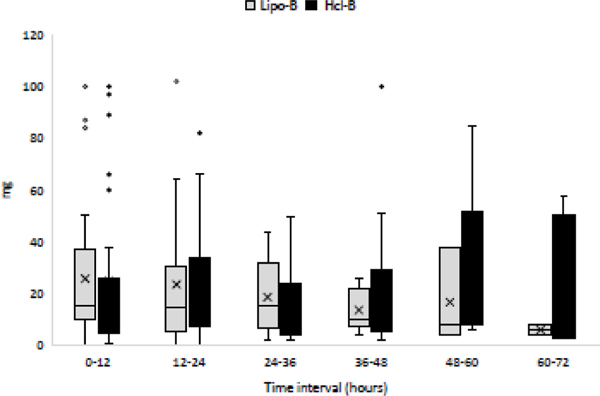
Multimodal intraoperative analgesia and opioid requirements were comparable between the groups (Table 2). Postoperative pain scores and RASS scores at arrival in the PACU and at 4 h (Table 2), opioid requirements (Figure 2), multimodal analgesic use (Table 2), length of PACU stay, and length of hospital stay (Table 2) were not significantly different between the groups. Only 1 patient, in the B-HCl group, required ICU admission. There were no deaths during the study period. All patients received intravenous PCA, in addition to oral opioid and nonopioid medications, in the postoperative period.
Figure 2.
Postoperative total morphine equivalents (intravenous plus oral) use at 12-h intervals for 72 h. B-HCl, bupivacaine hydrochloride; Lipo-B, liposomal bupivacaine.
DISCUSSION
The results of this retrospective study seem to confirm our primary hypothesis of non-superiority in the quality or duration of postoperative analgesia between ICNB with Lipo-B and ICNB with B-HCl in patients undergoing MITS lobectomy in the presence of multimodal analgesia. These findings are consistent with those from recent reports on MITS and are not surprising, considering the high level of systemic absorption of local anesthetic when deposited in the intercostal space. 5, 6,7, 8 When an adequate dose of local anesthetic is used in a dermatome and when enough dermatomes are injected, the quality and duration of postoperative analgesia appears to be similar between formulations in this patient population.
Lipo-B is currently used off-label for ICNB. Several case reports and retrospective studies on patients undergoing thoracic surgery have claimed that Lipo-B is superior to B-HCl.9–14 However, these conclusions have been drawn from small surgical studies. Rice et al. and Khalil et al. were the first to report that ICNB with Lipo-B carries an advantage over thoracic epidural analgesia. 11,13 Both of these studies share similar limitations, including small sample sizes, the comparison of two different regional techniques using two different drugs, the inability to measure the primary endpoint until the end of the study (owing to early discharge), and the gradual change in clinical practice toward enhanced recovery protocols (which strongly recommend the use of multimodal analgesia).1 Despite both authors concurring that the use of Lipo-B is safe, there is still some theoretical concern of systemic toxicity secondary to the high vascularity of the intercostal space and the potential of high systemic absorption. In vivo and cadaveric studies have respectively shown a migration of both radiotracer and colored latex soon after injection in the intercostal space. This has been confirmed by increased plasma levels after placement of ICNB with bupivacaine hydrochloride, either plain or with epinephrine, as well as after using liposomal bupivacaine 7, 15–17. In the two original studies that demonstrated a prolonged duration of liposomal bupivacaine, the drug was injected in the wound, an area with more adipose content than the intercostal space. 18, 19 The conclusion that the drug lasts for 72 h after injection is based on the use of cumulative times rather than time intervals and may have masked the true duration of the medication. When the data were reanalyzed at 12-h intervals for 72 h, there was a statistically significant difference in pain intensity and opioid consumption in just the first 12 h.20 The difference in the analgesic rescue modality between the groups and the nonblinded status of the primary investigator are limiting factors that may have affected data interpretation.
Two small studies that investigated patients who underwent a variety of minimally invasive lung resections found that the two local anesthetics had similar effects on postoperative analgesia when used for ICNB, in the presence or absence of multimodal analgesia. Our findings are consistent with these results.5, 6 When included in a broad multimodal analgesic approach, ICNB with Lipo-B and ICNB with B-HCl after MITS lobectomy had similar effects. Intraoperative and postoperative analgesic use, sedation, and pain scores were similar during each 12-h interval of the postoperative period. There was no statistically significant effect on length of PACU stay or length of hospital stay. Weksler et al in their prospective randomized study demonstrated that the similarities in postoperative analgesia extend up to three months post surgery.6 Currently, the higher cost of Lipo-B is the true limiting factor for its use, because of limited hospital availability or strict criteria for use.
Our study has several limitations, starting with its retrospective nature. Even though the two groups were comparable, there is still the possibility that inherent selection bias may have introduced hidden differences. In addition, despite the standardization of the intraoperative and postoperative analgesic regimens, in accordance with enhanced recovery protocols, there were likely variations and protocol departures during the study period. For instance, the choice of surgical technique (VATS vs RATS), the local anesthetic used for ICNB, the volume and number of dermatomes were at the surgeon’s discretion, leading to an imbalance between the groups. While the quality and the duration of postoperative analgesia are affected by the volume and the concentration of the local anesthetic, and by the number of dermatomes blocked, there is still some controversy in the literature on the role of the surgical technique 21–24. The small sample size of the study may have limited our ability to discern a true difference between groups. Last, the use of pain scores and morphine equivalents in pain studies carries limitations. Pain scores are extremely subjective and therefore may not be comparable between patients. The scores available were documented at rest, with incomplete information about analgesia with movement, cough, or incentive spirometry. Care providers may administer pain medication because they assume patients have pain, rather than after a formal request from the patients themselves.
Despite the above limitations, our results are consistent with previous findings on Lipo-B for peripheral nerve block in thoracic and nonthoracic procedures. 5, 6, 25 At present, the high cost of Lipo-B and the lack of extensive prospective data on its use limit our ability to definitively state whether Lipo-B or B-HCl is better for ICNB after MITS lobectomy. One certainty is that the use of Lipo-B for peripheral nerve blocks precludes the use of local anesthetics in rescue epidural catheters, due to concerns over bupivacaine toxicity. Furthermore, with the development of newer blocks, such as serratus plane blocks, erector spinae plane blocks and mid transverse process to pleural blocks, ICNB may become less popular in the future. 26, 27,28, 29,30–32 These fascial blocks have several advantages, including the ability to place catheters to facilitate the spread of local anesthetics to cover several dermatomes, the lack of major vasculature at the site of injection, and low complication rates.
Conclusions
In this single-center study, we did not observe significant opioid sparing effects of lipoB in comparison to B-HCL in patients undergoing MITS lobectomy. Future randomized studies which are currently investigating the postoperative analgesic effects of Lipo-B and B-HCl for ICNB after MITS will help clarify the indications for Lipo-B.
Acknowledgments
We acknowledge the assistance of David B. Sewell, senior editor in the Department of Surgery, for his help with editing this paper.
Funding:
This work was supported, in part, by the National Institutes of Health/National Cancer Institute (Cancer Center Support Grant P30CA008748). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funder played no role in any aspect of the study.
Footnotes
Declarations of Interest: The authors declare no conflicts of interest.
Declaration of interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. : Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS(R)) Society and the European Society of Thoracic Surgeons (ESTS). European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 55:91–115, 2019. [DOI] [PubMed] [Google Scholar]
- 2.Goto T: What is the best pain control after thoracic surgery? J Thorac Dis. 10:1335–1338, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Namdari S, Nicholson T, Abboud J, et al. : Interscalene Block with and without Intraoperative Local Infiltration with Liposomal Bupivacaine in Shoulder Arthroplasty: A Randomized Controlled Trial. J Bone Joint Surg Am. 100:1373–1378, 2018. [DOI] [PubMed] [Google Scholar]
- 4.Prabhakar A, Ward CT, Watson M, et al. : Liposomal bupivacaine and novel local anesthetic formulations. Best Practice & Research Clinical Anaesthesiology. 33:425–432, 2019. [DOI] [PubMed] [Google Scholar]
- 5.Rincavage M, Hammond L, Reddy S, et al. : Pain control using liposomal bupivacaine versus bupivacaine for robotic assisted thoracic surgery. International Journal of Clinical Pharmacy. 41:258–263, 2019. [DOI] [PubMed] [Google Scholar]
- 6.Weksler B, Sullivan JL, Schumacher LY: Randomized trial of bupivacaine with epinephrine versus bupivacaine liposome suspension in patients undergoing minimally invasive lung resection. The Journal of thoracic and cardiovascular surgery.Online ahead of print, 2020. [DOI] [PubMed] [Google Scholar]
- 7.Moore DC, Bush WH, Scurlock JE: Intercostal nerve block: a roentgenographic anatomic study of technique and absorption in humans. Anesth Analg. 59:815–825, 1980. [PubMed] [Google Scholar]
- 8.Perttunen K, Nilsson E, Heinonen J, et al. : Extradural, paravertebral and intercostal nerve blocks for post-thoracotomy pain. Br J Anaesth. 75:541–547, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Balkhy HH, Arnsdorf S, Krienbring D, et al. : Liposome Bupivacaine for Postsurgical Analgesia in Patients Undergoing Robotically Assisted Cardiac Surgery. Innovations. 10:416–419, 2015. [DOI] [PubMed] [Google Scholar]
- 10.Bergese SD, Ramamoorthy S, Patou G, et al. : Efficacy profile of liposome bupivacaine, a novel formulation of bupivacaine for postsurgical analgesia. Journal of pain research. 5:107–116, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khalil KG, Boutrous ML, Irani AD, et al. : Operative Intercostal Nerve Blocks With Long-Acting Bupivacaine Liposome for Pain Control After Thoracotomy. The Annals of thoracic surgery. 100:2013–2018, 2015. [DOI] [PubMed] [Google Scholar]
- 12.King NM, Quiko AS, Slotto JG, et al. : Retrospective analysis of quality improvement when using liposome bupivacaine for postoperative pain control. Journal of pain research. 9:233–240, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rice DC, Cata JP, Mena GE, et al. : Posterior Intercostal Nerve Block With Liposomal Bupivacaine: An Alternative to Thoracic Epidural Analgesia. The Annals of thoracic surgery. 99:1953–1960, 2015. [DOI] [PubMed] [Google Scholar]
- 14.Parascandola SA, Ibañez J, Keir G, et al. : Liposomal bupivacaine versus bupivacaine/epinephrine after video-assisted thoracoscopic wedge resection†. Interactive cardiovascular and thoracic surgery. 24:925–930, 2017. [DOI] [PubMed] [Google Scholar]
- 15.Johnson MD, Mickler T, Arthur GR, et al. : Bupivacaine with and without epinephrine for intercostal nerve block. Journal of Cardiothoracic Anesthesia. 4:200–203, 1990. [DOI] [PubMed] [Google Scholar]
- 16.Moore DC, Mather LE, Bridenbaugh PO, et al. : Arterial and venous plasma levels of bupivacaine following epidural and intercostal nerve blocks. Anesthesiology. 45:39–45, 1976. [DOI] [PubMed] [Google Scholar]
- 17.Manson WC, Blank RS, Martin LW, et al. : An Observational Study of the Pharmacokinetics of Surgeon-Performed Intercostal Nerve Blockade With Liposomal Bupivacaine for Posterior-Lateral Thoracotomy Analgesia. Anesth Analg. 131:1843–1849, 2020. [DOI] [PubMed] [Google Scholar]
- 18.Golf M, Daniels SE, Onel E: A phase 3, randomized, placebo-controlled trial of DepoFoam(R) bupivacaine (extended-release bupivacaine local analgesic) in bunionectomy. Advances in therapy. 28:776–788, 2011. [DOI] [PubMed] [Google Scholar]
- 19.Gorfine SR, Onel E, Patou G, et al. : Bupivacaine extended-release liposome injection for prolonged postsurgical analgesia in patients undergoing hemorrhoidectomy: a multicenter, randomized, double-blind, placebo-controlled trial. Diseases of the colon and rectum. 54:1552–1559, 2011. [DOI] [PubMed] [Google Scholar]
- 20.Hadley RM, Dine AP: Where is the evidence? A critical review of bias in the reporting of clinical data for exparel: a liposomal bupivacaine formulation. Clin Res Bioeth. 5, 2014. [Google Scholar]
- 21.Louie BE, Farivar AS, Aye RW, et al. : Early Experience With Robotic Lung Resection Results in Similar Operative Outcomes and Morbidity When Compared With Matched Video-Assisted Thoracoscopic Surgery Cases. The Annals of thoracic surgery. 93:1598–1605, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Duclos G, Charvet A, Resseguier N, et al. : Postoperative morphine consumption and anaesthetic management of patients undergoing video-assisted or robotic-assisted lung resection: a prospective, propensity score-matched study. J Thorac Dis. 10:3558–3567, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon ST, Zhao L, Reddy RM, et al. : Evaluation of acute and chronic pain outcomes after robotic, video-assisted thoracoscopic surgery, or open anatomic pulmonary resection. The Journal of thoracic and cardiovascular surgery. 154:652–659 e651, 2017. [DOI] [PubMed] [Google Scholar]
- 24.Duclos G, Charvet A, Resseguier N, et al. : Postoperative morphine consumption and anaesthetic management of patients undergoing video-assisted or robotic-assisted lung resection: a prospective, propensity score-matched study. Journal of thoracic disease. 10:3558–3567, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh PM, Borle A, Trikha A, et al. : Role of Periarticular Liposomal Bupivacaine Infiltration in Patients Undergoing Total Knee Arthroplasty—A Meta-analysis of Comparative Trials. The Journal of Arthroplasty. 32:675-688.e671, 2017. [DOI] [PubMed] [Google Scholar]
- 26.Blanco R, Parras T, McDonnell JG, et al. : Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia. 68:1107–1113, 2013. [DOI] [PubMed] [Google Scholar]
- 27.Park MH, Kim JA, Ahn HJ, et al. : A randomised trial of serratus anterior plane block for analgesia after thoracoscopic surgery. Anaesthesia. 73:1260–1264, 2018. [DOI] [PubMed] [Google Scholar]
- 28.Adhikary S, Pruett A, Forero M, et al. : Erector spinae plane block as an alternative to epidural analgesia for post-operative analgesia following video-assisted thoracoscopic surgery: A case study and a literature review on the spread of local anaesthetic in the erector spinae plane. Indian J Anaesth. 62:75–78, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsui BCH, Fonseca A, Munshey F, et al. : The erector spinae plane (ESP) block: A pooled review of 242 cases. J Clin Anaesth. 53:29–34, 2019. [DOI] [PubMed] [Google Scholar]
- 30.Costache I: Mid-point transverse process to pleura block for surgical anaesthesia. 7:1–3, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma RS, Kumar R, Kamal M, et al. : Use of the mid-transverse process to pleura block in a patient undergoing intercostal drain placement and rib resection. Indian J Anaesth. 63:245–246, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pedoto A, Kalchiem-Dekel O, Baselice S, et al. : Ultrasound-Guided Midpoint Transverse Process to Pleura Nerve Block for Medical Thoracoscopy: A Case Report. A A Pract. 14:e01240, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]