Abstract
OBJECTIVE
Lifestyle intervention is recommended as first-line treatment of diabetes at all ages; however, little is known about the efficacy of lifestyle intervention in older adults with diabetes. We aimed to determine whether lifestyle intervention would improve glycemic control and age-relevant outcomes in older adults with diabetes and comorbidities.
RESEARCH DESIGN AND METHODS
A total of 100 older adults with diabetes were randomly assigned to 1-year intensive lifestyle intervention (ILI) (diet and exercise at a facility transitioned into community-fitness centers and homes) or healthy lifestyle (HL) group. The primary outcome was change in HbA1c. Secondary outcomes included glucoregulation, body composition, physical function, and quality of life. Changes between groups were analyzed with mixed-model repeated-measures ANCOVA following the intention-to-treat principle.
RESULTS
HbA1c improved more in the ILI than the HL group (mean ± SE −0.8 ± 0.1 vs. 0.1 ± 0.1%), associated with improved insulin sensitivity (1.2 ± 0.2 vs. −0.4 ± 0.2) and disposition (26.0 ± 8.9 vs. −13.0 ± 8.4 109 min−1) indices (between-group P < 0.001 to 0.04). Body weight and visceral fat decreased more in the ILI than HL group (−8.4 ± 0.6 vs. −0.3 ± 0.6 kg, P < 0.001, and −261 ± 29 vs. −30 ± 27 cm3, P < 0.001, respectively). Physical Performance Test score increased more in the ILI than HL group (2.9 ± 0.6 vs. −0.1 ± 0.4, P < 0.001) as did VO2peak (2.2 ± 0.3 vs. −1.2 ± 0.2 mL/kg/min, P < 0.001). Strength, gait, and 36-Item Short Form Survey (SF-36) Physical Component Summary score also improved more in the ILI group (all P < 0.001). Total insulin dose decreased in the ILI group by 19.8 ± 4.4 units/day. Adverse events included increased episodes of mild hypoglycemia in the ILI group.
CONCLUSIONS
A lifestyle intervention strategy is highly successful in improving metabolic and functional health of older adults with diabetes.
Introduction
The highest prevalence of diabetes is among older adults (age ≥65 years), who constitute a rapidly expanding segment of the U.S. population (1). This high prevalence of diabetes is strongly linked to increasing adiposity and physical inactivity with aging (2) and is becoming a serious public health problem as more baby boomers become senior citizens. Obesity exacerbates the decline in metabolic and physical function that occurs with age and causes frailty (3). However, weight loss therapy is controversial for older adults because of concerns that weight loss could exacerbate underlying sarcopenia and frailty and that attempting to change ingrained, lifelong diet and activity habits might cause distress and anxiety (4,5). Losing weight is difficult, and interventions that work in younger adults cannot be assumed to translate to older adults with diabetes and comorbidities, low muscle mass, and frailty (5,6). Moreover, therapeutic approaches may differ between younger and older adults because of the increased importance of preventing loss of lean body mass (LBM) that occurs with weight loss in older persons (4). Conversely, we reported that older adults at risk for diabetes embraced lifestyle change and that the combination of weight loss and regular exercise provided the greatest improvement in physical function (7). Furthermore, we recently reported that in these at-risk older adults, lifestyle interventions associated with weight loss improved insulin sensitivity and other cardiometabolic risk factors, but continued improvement in insulin sensitivity was only achieved when regular exercise was added to weight loss (8). Thus, combined weight loss and exercise therapy may ameliorate the metabolic and functional complications in older adults at risk for diabetes. However, whether such lifestyle intervention is effective in the specific population of older adults with diabetes and associated comorbidities has not been established. Older adults have typically been excluded in previous studies (9,10), and the few studies with enrollment of older adults with diabetes have been limited to relatively healthy patients with reporting of data based on post hoc subgroup analyses of existing data sets (11,12). Because there are essentially little or no directly applicable clinical trial data on lifestyle interventions in older adults with diabetes, current treatment recommendations have mostly been based on expert opinion rather than high-level evidence (13,14).
To help provide level 1 evidence that could inform treatment recommendations in this older population, we conducted a randomized controlled trial (RCT) of lifestyle intervention in older adults with diabetes. We hypothesized that lifestyle intervention would be successful in this specific population of older adults with diabetes and comorbidities, resulting in improved glycemic control as accompanied by improved insulin action and secretion, as well as improved body composition, physical function, and quality of life.
Research Design and Methods
Study Design and Participants
The Lifestyle Intervention for Seniors with Diabetes (LISD) (clinical trial reg. no. NCT02348801, ClinicalTrials.gov) was an RCT conducted at Baylor College of Medicine and Michael E. DeBakey VA Medical Center from April 2015 to December 2020. The study was approved by the institutional review board and monitored by a data and safety monitoring committee. All participants provided written informed consent.
Volunteers were recruited through advertisements and underwent comprehensive medical screening. Persons of older age (65–85 years) and with type 2 diabetes were eligible for inclusion. Diabetes was determined on the basis of self-report with verification (medical records, current treatment, confirmation from health care provider, fasting plasma glucose ≥126 mg/dL, symptoms of hyperglycemia with plasma glucose ≥200 mg/dL, 2-h plasma glucose ≥200 mg/dL after a 75-g glucose load, or HbA1c ≥6.5%). Additional inclusion criteria were as follows: individuals with overweight or obesity (BMI ≥27 kg/m2), sedentary lifestyle (regular exercise <1 h/week), and stable body weight (±2 kg) and on stable medication use for 6 months before enrollment. Individuals with cardiopulmonary disease (e.g., recent myocardial infarction, unstable angina), musculoskeletal/neuromuscular impairments that precluded exercise training, or cognitive impairments (Mini-Mental State Examination score <24) or HbA1c >11% were excluded.
Intervention
For this 1-year study, participants were randomly assigned, with stratification according to sex, into one of two groups: 1) intensive lifestyle intervention (ILI) or 2) healthy lifestyle (HL). The randomization algorithm was generated and maintained by the study biostatistician (using random number generation from SAS software), who did not interact with the participants. Whenever a set of 10–15 participants completed the medical screening, the research coordinator contacted the biostatistician for randomization of each participant.
The ILI consisted of a weight-management program and exercise training. The weight-management program was achieved with group behavior therapy sessions designed to have older adults acquire positive weight-control skills. The curriculum addressed using food scales; reading nutrition labels; modifying meals to reduce carbohydrate intake with emphasis on nonstarchy vegetables, fruits, whole grains, and minimally processed foods; and using various behavioral strategies. Participants were prescribed a balanced diet that provided a deficit of 500–750 kcal/day from daily energy requirement. Because of the increased importance of minimizing weight loss–induced reduction of LBM in older adults, the diet contained ∼1 g/kg/day high-quality protein (4). Participants met weekly as a group with a study dietitian for dietary adjustments and behavioral therapy. They were instructed to set weekly behavioral goals and attend weekly weigh-in sessions. Food diaries were reviewed, and new goals were set based on diary reports. Individual behavior and dietary sessions were provided for those who were not compliant with dietary restrictions as judged by inadequate weight loss. The goal was to achieve a weight loss of ∼10% of baseline body weight at 6 months and maintain that weight loss for an additional 6 months. Visit frequency was decreased to every 2 weeks from 6 to 12 months. The exercise training involved aerobic and resistance exercises thrice weekly at our facility for 6 months. Combined aerobic and resistance exercise added to weight loss has been shown to be most effective in improving functional status and preserving LBM in older adults (15). The sessions lasted 90 min and began with 15-min warm-up flexibility exercises followed by 30-min aerobic exercises, 30-min resistance exercises, and 15-min balance exercises. The aerobic exercise consisted of treadmill walking, stationary cycling, and stair climbing. Participants exercised at ∼65% of their peak heart rate, which was gradually increased to 70–85%. The resistance exercises consisted of nine upper-body and lower-body exercises with use of weight-lifting machines. The initial sessions were one to two of 8–12 repetitions at 65% of one-repetition maximum (1RM), which increased progressively to two to three sets at ∼85% of 1RM. After 6 months of exercise training at our facility, participants transitioned their regular exercises to community-fitness centers of their choice and homes for the remaining 6 months of the study.
The HL consisted of group educational sessions about a healthful diet during monthly visits. Participants in this group were asked not to participate in external weight loss or exercise programs.
For protection against deficiencies during weight loss therapy of older adults, all participants received supplements to adjust calcium and vitamin D intake to ∼1,500 mg/day and ∼1,000 IU/day, respectively (4).
Outcomes
The primary outcome was the change in HbA1c from baseline to 1 year. Secondary outcomes were the changes in insulin sensitivity and secretion, glucose tolerance variables, body weight and composition, and physical function and quality of life.
Safety was assessed continuously through adverse event reports, physical examination, vital signs, and blood chemistries. To reduce the risk of severe hypoglycemia during intensive weight loss, the study physician adjusted the dose of insulin or insulin secretagogues using a prespecified algorithm (Supplementary Fig. 1); otherwise, all diabetes and medical care was conducted by the participant’s own clinician.
Baseline Assessments
HbA1c and Frequently Sampled Oral Glucose Tolerance Test
HbA1c was measured with Tosoh Automated Glycohemoglobin Analyzer (HLC-723G8; Tosoh Bioscience, San Francisco, CA). A 2-h frequently sampled oral glucose tolerance test (FSOGTT) was performed in the morning after a 12-h fast. Blood samples were taken before and at 10, 20, 30, 60, 90, and 120 min after administration of a 75-g oral glucose load. Plasma glucose was determined with the glucose oxidase method (YSI STAT PLUS; YSI, Yellow Springs, OH), insulin and C-peptide with chemiluminescent immunoassay (IMMULITE; Siemens, Malvern, PA), and glucagon with radioimmunoassay (LINCO Research, St. Louis, MO). Participants withheld their glucose-lowering medications in the morning prior to the FSOGTT and any intermediate-acting insulin the night prior to the FSOGTT. In addition, participants refrained from exercise for at least 48 h before the FSOGTT.
Composite insulin sensitivity index (SIComposite) was calculated according to the methodology of Matsuda and DeFronzo based on all measures of glucose and insulin made during the FSOGTT (16). HOMA of insulin resistance (HOMA-IR) was also calculated (17). Insulin secretion was estimated from glucose and C-peptide concentrations from the FSOGTT, using the oral minimal model of C-peptide secretion and kinetics (18) and incorporating parameters for C-peptide kinetics and volume of distribution (19). This model calculates several indexes of β-cell responsivity: 1) static responsivity index (ϕs [109 min−1]), an index of insulin secretion in response to a given glucose concentration; 2) dynamic responsivity index (ϕd [109]), an index of insulin secretion in response to the rate of change in glucose concentration; and 3) overall responsivity index (ϕo [109 min−1]), a global sensitivity-to-glucose index of postprandial insulin secretion. We calculated disposition indices (DIs) were calculated by multiplying each index by the SIComposite, in accordance with the methodology of Bergman et al. (20), to adjust insulin secretion for degree of insulin resistance. Total areas under the curve (AUCs) were calculated based on the trapezoid rule.
Body Composition
We evaluated fat mass and LBM of the whole body and visceral adipose tissue with DEXA using Horizon APEX software 5.5.2 (Hologic) as previously described (6).
Physical Function and Quality of Life
The 9-item modified Physical Performance Test (PPT) was used to assess degree of physical frailty as previously described (6,7,21). VO2peak was assessed during graded treadmill walking with indirect calorimetry (TrueMax 2400; Parvo Medics) as previously described (6). The 1-RMs (the maximal weight a person can lift at one repetition) for lateral pulldown, biceps curl, chest press, seated row, leg press, knee flexion, and knee extension were summed to calculate total 1-RM strength (7). Fast gait speed was assessed as the time needed to walk 25 feet (15). Physical activity presented as MET h/day above rest was determined with the Stanford 7-day Physical Activity Recall (22). Ability to perform activities of daily living was assessed with the Functional Status Questionnaire (FSQ) (23). We evaluated quality of life using The Physical Component Summary (PCS) score of the Medical Outcomes Study 36-Item Short Form Survey (SF-36) (modified SF-36v1) (24).
Follow-up Assessments
All baseline assessments were repeated at 6 months and 12 months. The personnel who conducted the assessments were not aware of the group assignments.
Statistical Analysis
We estimated that a sample size of 50 participants per group, which allowed for 20% dropout, would provide >90% power to detect a clinically important difference between groups in the change in HbA1c, assuming a mean between-group difference of 0.5%, with a pooled SD of 0.5 (based on preliminary data) at an α level of 5%.
We performed intention-to-treat analyses with SAS software, version 9.4 (SAS Institute, Cary, NC), by analyzing data from all participants originally randomized. Baseline characteristics were compared with independent t test or Fisher exact test. Longitudinal changes between groups were tested with mixed-model repeated-measures ANCOVA. Change from baseline was used as the dependent variable with group, time, and group × time as independent effects and baseline values and sex as covariates. The primary focus of the analyses was the 12-month change from baseline in outcomes in the two groups. When the overall P value for the interaction between group and time was <0.05, the specific contrasts were used to test the null hypothesis that changes in one group were equal to corresponding changes in the other group. P values for secondary outcomes were controlled for multiple testing with the Benjamini-Hochberg procedure (25). Analyses testing for within-group changes were also performed with mixed-model repeated-measures ANOVA. As exploratory analyses, changes in fat mass and VO2peak were included as covariates in the ANCOVA for assessment of whether the effects were largely driven by or independent of these changes.
Sensitivity analyses that supported the statistical results obtained included multiple imputation for missing fitness data (which confirmed the same pattern of results). Additional analyses included logistic regression, verifying that data were consistent with an assumption that data were missing at random. Data for change scores are presented as least squares–adjusted means ± SE. P values <0.05 were considered to indicate statistical significance.
Results
Study Population
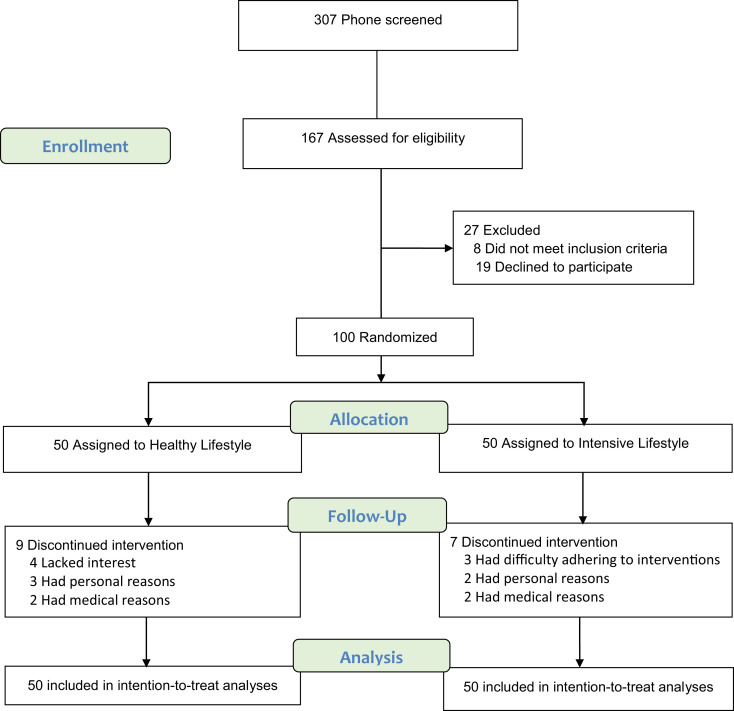
Of the 167 volunteers assessed for eligibility, 27 were excluded because they did not meet the inclusion criteria or declined to participate (Fig. 1). A total of 100 volunteers underwent randomization; 84 (84%) completed the study. Sixteen participants discontinued the intervention and were included in the treat analyses (14 participants provided follow-up data at 6 months and 2 at ∼12 months). Baseline characteristics are presented in Table 1. Approximately 40% of participants were of Black race. Participants were taking different types of diabetes medications, and almost half were on insulin therapy. There was a high prevalence of mild frailty (21) associated with coexisting conditions and use of chronic medications.
Figure 1.
Screening, randomization, and follow-up.
Table 1.
Baseline characteristics of participants
| HL (N = 50) | ILI (N = 50) | |
|---|---|---|
| Age (years) | 71.4 ± 3.7 | 72.3 ± 4.0 |
| BMI (kg/m2) | 34.5 ± 5.4 | 35.7 ± 5.1 |
| n male/n female | 34/16 | 31/19 |
| Race | ||
| White | 27 (54) | 31 (62) |
| Black | 21 (42) | 17 (34) |
| Other | 2 (4) | 2 (4) |
| Ethnic group | ||
| Hispanic or Latino | 9 (18) | 9 (18) |
| Not Hispanic or Latino | 41 (82) | 41 (82) |
| Education | ||
| Less than college degree | 16 (32) | 17 (34) |
| College degree | 20 (40) | 22 (44) |
| Graduate school | 14 (28) | 11 (22) |
| Marital status | ||
| Single | 7 (14) | 6 (12) |
| Married | 32 (64) | 31 (62) |
| Divorced | 6 (12) | 6 (12) |
| Widowed | 5 (10) | 7 (14) |
| Duration of diabetes (years) | 13.7 ± 8.7 | 13.8 ± 9.0 |
| HbA1c | ||
| % | 7.3 ± 1.2 | 7.5 ± 1.3 |
| mmol/mol | 55.7 ± 13.4 | 58.2 ± 14.3 |
| Diabetes medication use† | ||
| Oral medications | ||
| Metformin | 31 (62) | 35 (70) |
| Sulfonylureas | 15 (30) | 12 (24) |
| Other oral medications‡ | 13 (26) | 10 (20) |
| Injectable medications | ||
| Insulin | 19 (38) | 20 (40) |
| GLP-1 agonist | 4 (8) | 4 (8) |
| None | 6 (12) | 4 (8) |
| Frailty§ | 39 (78) | 35 (70) |
| Coexisting chronic conditionsǁ | ||
| Hypertension | 42 (84) | 38 (76) |
| Arthritis | 27 (54) | 34 (68) |
| Coronary artery disease/heart failure | 15 (30) | 21 (42) |
| Chronic kidney disease | 11 (22) | 14 (28) |
| Osteopenia/osteoporosis | 14 (28) | 16 (12) |
| Cancer | 10 (20) | 9 (18) |
| Chronic lung disease | 7 (14) | 13 (26) |
| Thyroid disease | 10 (20) | 10 (20) |
| Stroke | 3 (6) | 6 (12) |
| Chronic medications (other than antidiabetes)† | ||
| Antihypertensive | 42 (84) | 38 (76) |
| Antilipidemic | 40 (80) | 42 (84) |
| Antidepressant | 19 (38) | 16 (32) |
| Antidyspeptic | 15 (30) | 16 (32) |
| Diuretic | 11(22) | 14 (28) |
| Thyroid hormone | 10 (20) | 10 (20) |
| Antiplatelet/anticoagulant | 8 (16) | 12 (24) |
| Bronchodilator | 6 (12) | 8 (16) |
| Other medications | 29 (58) | 22 (44) |
Data are means ± SD or n (%) unless otherwise indicated. GLP-1, glucagon-like peptide 1.
Participants may use more than one medication.
Other oral medications include dipeptidyl peptidase 4 inhibitors, α-glucosidase inhibitors, sodium–glucose cotransporter 2 inhibitors, and thiazolidinediones.
Score of 18–31 on the modified PPT (score range 0–36) (21).
Participants may have more than one coexisting chronic condition.
Median attendance was at 87% (interquartile range 79–95) for diet therapy sessions and 91% (81–97) for exercise therapy sessions among participants in ILI group. Attendance was at 92% (75–100) for educational sessions among those in HL group. Mean ± SE baseline energy intake was 2,022 ± 88 and 2,015 ± 83 kcal/day in the ILI and HL group, respectively. Energy intake decreased more in the ILI group than HL group (−575 ± 60 and −541 ± 67 vs. −83 ± 57 and −88 ± 60 kcal/day at 6 and 12 months; between-group P < 0.001).
HbA1c and Glucoregulatory Control
Mean ± SE HbA1c (primary outcome) improved more in the ILI group than HL group (−0.8 ± 0.1% [−12.2 ± 1.9 mmol/mol] vs. 0.1 ± 0.1% [−0.8 ± 1.9 mmol/mol], respectively) (Table 2). Accordingly, the SIComposite increased more while the HOMA-IR decreased more in the ILI group than HL group (1.2 ± 0.2 vs. −0.4 ± 0.2 and −2.5 ± 2.4 vs. 4.1 ± 1.2). Moreover, both static DI and overall DI increased more in the ILI group than HL group (58.2 ± 15.2 vs. −17.2 ± 14.7 × 109 min−1 and 26.0 ± 8.9 vs. −13.0 ± 8.4 × 109 min−1). The fasting and 2-h glucose and AUCs for glucose, insulin, C-peptide, and glucagon in the FSOGTT decreased in the ILI group compared with HL group (Table 2 and Supplementary Fig. 3).
Table 2.
Effect of lifestyle intervention on primary and secondary outcomes
| HL (N = 50) | ILI (N = 50) | Difference (95% CI) | P † | |
|---|---|---|---|---|
| Primary outcome | ||||
| HbA1c (%) | ||||
| Baseline | 7.3 ± 0.2 | 7.5 ± 0.2 | ||
| Change at 6 months | −0.1 ± 0.1 | −0.7 ± 0.1‡ | ||
| Change at 1 year | 0.1 ± 0.1 | −0.8 ± 0.1‡ | 0.9 (0.5, 1.2) | <0.001 |
| HbA1c (mmol/mol) | ||||
| Baseline | 55.7 ± 1.9 | 58.2 ± 0.2 | ||
| Change at 6 months | −2.6 ± 1.8 | −9.2 ± 1.8‡ | ||
| Change at 1 year | −0.8 ± 1.9 | −12.2 ± 1.9‡ | 11.8 (5.0, 18.9) | <0.001 |
| Secondary outcomes | ||||
| Insulin sensitivity and secretion | ||||
| SIComposite | ||||
| Baseline | 2.4 ± 0.3 | 2.1 ± 0.3 | ||
| Change at 6 months | −0.0 ± 0.2 | 0.9 ± 0.2§ | ||
| Change at 1 year | −0.4 ± 0.2 | 1.2 ± 0.2‡ | 1.6 (−2.2, −0.9) | <0.001 |
| HOMA-IR | ||||
| Baseline | 11.3 ± 2.3 | 13.4 ± 2.8 | ||
| Change at 6 months | 1.9 ± 1.2 | −2.6 ± 1.2§ | ||
| Change at 1 year | 4.1 ± 1.2 | −2.5 ± 1.4§ | 6.8 (2.0, 11.7) | 0.03 |
| Static DI, Φs (109 min−1) | ||||
| Baseline | 91.7 ± 15.8 | 80.4 ± 19.8 | ||
| Change at 6 months | −6.9 ± 13.9 | 42.9 ± 13.5ǁ | ||
| Change at 1 year | −17.2 ± 14.7 | 58.2 ± 15.2§ | 76.5 (−127.8, −25.4) | 0.02 |
| Dynamic DI, Φd (109) | ||||
| Baseline | 1,595 ± 406 | 1,334 ± 347 | ||
| Change at 6 months | 286 ± 201 | 426 ± 192 | ||
| Change at 1 year | −162 ± 211 | 530 ± 215 | 757 (−1,529, 16) | 0.14 |
| Overall DI, Φo (109 min−1) | ||||
| Baseline | 55.6 ± 8.3 | 51.4 ± 10.2 | ||
| Change at 6 months | −4.9 ± 7.7 | 23.7 ± 7.9ǁ | ||
| Change at 1 year | −13.0 ± 8.4 | 26.0 ± 8.9ǁ | −39.2 (−70.2, −8.3) | 0.04 |
| Glucose tolerance variables | ||||
| Fasting glucose (mmol/L) | ||||
| Baseline | 7.1 ± 0.3 | 7.6 ± 0.3 | ||
| Change at 6 months | 0.3 ± 0.2 | −0.4 ± 0.2 | ||
| Change at 1 year | 0.8 ± 0.2ǁ | −0.6 ± 0.3§ | 1.7 (0.8, 2.6) | 0.004 |
| 2-h glucose (mmol/L) | ||||
| Baseline | 14.9 ± 0.8 | 16.4 ± 0.7 | ||
| Change at 6 months | 0.2 ± 0.4 | −1.7 ± 0.3‡ | ||
| Change at 1 year | 0.5 ± 0.4 | −1.6 ± 0.2‡ | 2.3 (1.0, 3.7) | 0.001 |
| Glucose AUC (mmol/L per 2 h) | ||||
| Baseline | 1,573 ± 65 | 1,644 ± 51 | ||
| Change at 6 months | 51 ± 43 | −102 ± 42 | ||
| Change at 1 year | 123 ± 44 | −135 ± 47ǁ | 237 (104, 421) | 0.009 |
| Insulin AUC (×103 pmol/L per 2 h) | ||||
| Baseline | 64.9 ± 9.1 | 66.2 ± 9.5 | ||
| Change at 6 months | 1.3 ± 3.2 | −12.2 ± 3.1§ | ||
| Change at 1 year | 3.8 ± 3.2 | −9.8 ± 3.5ǁ | 13.2 (1.4, 25.0) | 0.04 |
| C-peptide AUC (nmol/L per 2 h) | ||||
| Baseline | 340.9 ± 32.4 | 324.4 ± 31.8 | ||
| Change at 6 months | 9.6 ± 12.6 | −30.5 ± 12.6 | ||
| Change at 1 year | 17.2 ± 17.2 | −38.4 ± 13.9ǁ | 54.0 (8.6, 99.3) | 0.04 |
| Glucagon AUC (ng/L per 2 h) | ||||
| Baseline | 2,429 ± 178 | 2,416 ± 175 | ||
| Change at 6 months | 60 ± 76 | −225 ± 77ǁ | ||
| Change at 1 year | 141 ± 79 | −226 ± 78ǁ | 369 (82, 656) | 0.03 |
| Body weight and composition | ||||
| Weight (kg) | ||||
| Baseline | 100.5 ± 0.3 | 102.2 ± 0.2 | ||
| Change at 6 months | 0.9 ± 0.6 | −8.0 ± 0.6‡ | ||
| Change at 1 year | −0.3 ± 0.6 | −8.4 ± 0.6‡ | 8.1 (6.1, 10.2) | <0.001 |
| Fat mass (kg) | ||||
| Baseline | 41.3 ± 1.6 | 43.8 ± 1.5 | ||
| Change at 6 months | −0.3 ± 0.4 | −6.0 ± 0.4‡ | ||
| Change at 1 year | −0.4 ± 0.4 | −6.6 ± 0.5‡ | 6.2 (4.6, 7.8) | <0.001 |
| Visceral adipose tissue (cm3) | ||||
| Baseline | 1,196 ± 57 | 1,203 ± 50 | ||
| Change at 6 months | −33 ± 25 | −173 ± 26‡ | ||
| Change at 1 year | −30 ± 27 | −261 ± 29‡ | 234 (131, 337) | <0.001 |
| LBM (kg) | ||||
| Baseline | 59.3 ± 1.5 | 58.5 ± 1.6 | ||
| Change at 6 months | 0.3 ± 0.3 | −1.7 ± 0.3‡ | ||
| Change at 1 year | 0.2 ± 0.3 | −1.7 ± 0.3‡ | 1.8 (0.8, 2.9) | 0.001 |
| Physical function and quality of life | ||||
| PPT score | ||||
| Baseline | 28.1 ± 0.7 | 28.9 ± 0.6 | ||
| Change at 6 months | 0.6 ± 0.4 | 2.7 ± 0.4‡ | ||
| Change at 1 year | −0.1 ± 0.4 | 2.9 ± 0.4‡ | −2.9 (−4.3, −1.5) | <0.001 |
| VO2peak (mL/kg/min) | ||||
| Baseline | 16.5 ± 0.6 | 16.5 ± 0.4 | ||
| Change at 6 months | −0.9 ± 0.2‡ | 2.2 ± 0.2‡ | ||
| Change at 1 year | −1.2 ± 0.2‡ | 2.2 ± 0.3‡ | −3.4 (−4.4, −24) | <0.001 |
| Total 1-RM strength (kg) | ||||
| Baseline | 303 ± 15 | 304 ± 14 | ||
| Change at 6 months | 3 ± 4 | 54 ± 4‡ | ||
| Change at 1 year | −1 ± 4 | 46 ± 4‡ | −46 (−55, −39) | <0.001 |
| Gait speed (m/min) | ||||
| Baseline | 70.2 ± 2.8 | 76.2 ± 1.9 | ||
| Change at 6 months | −1.7 ± 1.2 | 4.9 ± 1.2‡ | ||
| Change at 1 year | −3.7 ± 1.2ǁ | 7.2 ± 1.3‡ | −10.1 (−14.4, −5.8) | <0.001 |
| Physical activity (MET h/day) | ||||
| Baseline | 9.3 ± 0.3 | 9.6 ± 0.3 | ||
| Change at 6 months | −0.3 ± 0.2 | 3.1 ± 0.2‡ | ||
| Change at 1 year | −0.4 ± 0.2 | 2.8 ± 0.2‡ | −3.1 (−3.9, −2.4) | <0.001 |
| FSQ score | ||||
| Baseline | 28.4 ± 0.7 | 29.7 ± 0.5 | ||
| Change at 6 months | −0.9 ± 0.3 | 1.4 ± 0.3‡ | ||
| Change at 1 year | −1.0 ± 0.3 | 1.9 ± 0.3‡ | −2.9 (−4.1, −1.7) | <0.001 |
| SF-36 PCS score | ||||
| Baseline | 43.1 ± 1.4 | 44.1 ± 1.3 | ||
| Change at 6 months | −2.0 ± 0.9 | 4.0 ± 0.9‡ | ||
| Change at 1 year | −4.9 ± 0.9‡ | 4.9 ± 1.0‡ | −9.6 (−12.9, −6.2) | <0.001 |
Baseline values are observed means ± SE, and change score values are least squares–adjusted means ± SE from repeated-measures ANCOVAs.
P values for comparison between the groups of changes from baseline to 1 year were calculated with use of mixed-model repeated-measures ANCOVA (with baseline values and sex as covariates) and are reported where the overall P value was <0.05 for the interaction among the groups over time. Benjamini-Hochberg correction for multiple comparisons was used to adjust the P values in the secondary outcomes.
P < 0.001 for comparison of the value at the follow-up time with the baseline value within the group, as calculated with use of mixed-model repeated-measures ANOVA.
P < 0.01 for comparison of the value at the follow-up time with the baseline value within the group, as calculated with use of mixed-model repeated-measures ANOVA.
P < 0.05 for comparison of the value at follow-up time with the baseline value within the group, as calculated with use of mixed-model repeated-measures ANOVA.
Body Weight and Composition
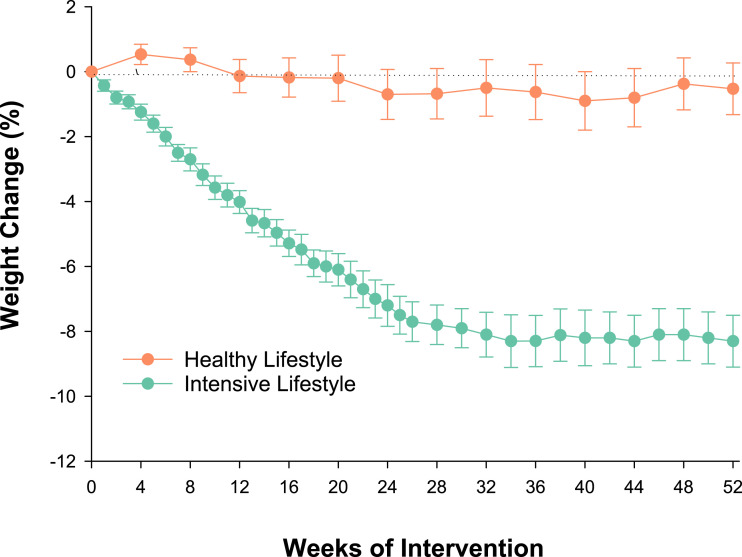
There was a substantial mean ± SE decrease in body weight in the ILI group (−8.4 ± 0.6 kg) but not HL group (−0.3 ± 0.6 kg). The time course of weight loss is shown in Fig. 2. In accordance with the weight loss, fat mass and visceral adipose tissue decreased in the ILI group but did not change in HL group (−6.6 ± 0.5 vs. −0.4 kg and −261 ± 29 vs. −30 ± 27 cm3, respectively) (Table 2). Likewise, LBM decreased in the ILI group but was maintained in the HL group (−1.7 ± 0.3 vs. 0.2 ± 0.3 kg).
Figure 2.
Mean percentage changes in body weight during the interventions. I bars indicate SEs.
Physical Performance, Endurance, Strength, Gait, and Quality of Life
Mean ± SE score on the PPT increased in the ILI group (2.9 ± 0.4) but did not change in the HL group (−0.1 ± 0.4) (Table 2). VO2peak also increased in the ILI group (2.2 ± 0.3 mL/kg/min), whereas it decreased in the HL group (−1.2 ± 0.2 mL/kg/min). Moreover, total 1-RM strength and gait speed increased more in the ILI group than HL group (46 ± 4 vs. −1 ± 4 kg and 7.2 ± 1.3 vs. −3.7 ± 1.2 m/min, respectively). Physical activity increased in the ILI group but did not change in the HL group (2.8 ± 0.2 vs. −0.4 ± 0.2 MET h/day). Similar between-group differences were observed for changes in FSQ and SF-36 PCS scores of subjective ability to function and quality of life.
Adverse Events
There were 30 events of hypoglycemia in the ILI group as compared with 20 events of hypoglycemia in the HL group. All except two were classified as level 1 hypoglycemia—sufficiently low for treatment with fast-acting carbohydrate (26). A summary of adverse events is provided in Supplementary Table 1.
Diabetes Medications
There were no significant differences between groups in the number of participants whose medications changed during the study period (Supplementary Table 2). For participants taking insulin, mean ± SE total daily dose at baseline was 93.8 ± 12.7 units in the ILI group and 86.4 ± 12.4 units in the HL group. Total daily insulin dose decreased in the ILI group compared with HL group (−19.8 ± 4.4 vs. 8.0 ± 5.8 units, respectively; between-group P = 0.002).
Controlling Treatment Effects for Changes in Fat Mass and VO2peak
Including changes in fat mass as covariates in ANCOVA eliminated the between-group differences in changes in HbA1c and FSOGTT variables (Supplementary Table 3). Likewise, including changes in VO2peak as covariates eliminated the between-group differences in changes in HbA1c and FSOGTT variables (Supplementary Table 4). These analyses indicate that the changes in fat mass and/or VO2peak were involved in these between-group differences. The relationship of biomarkers to HbA1c and weight outcomes is depicted in Supplementary Fig. 2.
Conclusions
Our 1-year RCT indicated that a lifestyle intervention program can be highly successful in older adults with diabetes and chronic comorbidities. In this specific population, lifestyle intervention not only improved glycemic control associated with improved insulin action and secretion but also improved age-relevant outcomes such as body composition, physical function, and quality of life.
Currently, evidence-based data to guide treatment of older adults with diabetes are still limited. Although lifestyle intervention is recommended as first-line treatment of diabetes at all ages, older adults were often excluded or underrepresented in studies that led to this evidence (27,28). In the few studies of lifestyle intervention, older adults with diabetes were not specifically enrolled and data reported on older adults with or without diabetes were primarily based on secondary subgroup analyses of existing data sets (11,12). Moreover, most prior studies were conducted in healthy older adults with diabetes or at risk for diabetes (9,10). The results of our RCT in older adults with diabetes and chronic comorbidities showed that a lifestyle intervention of behavioral diet and exercise therapy started at a facility and transitioned into community-fitness centers and homes can be associated with sustained glucometabolic and functional improvements. Our findings suggest that in the specific population of older adults with diabetes, it may not be too late in life (mean age 72 years) to start lifestyle intervention, which can complement or reduce the need for medical therapy.
Indeed, lifestyle intervention could directly counter the increasing adiposity and physical inactivity that are primarily responsible for the age-related increase in insulin resistance (2). Accordingly, the lifestyle-induced decrease in body fat and increase in physical fitness underlie the improvement in insulin sensitivity that occurred in our participants (2,29). Current evidence indicates that diabetes in older adults is caused by insulin resistance in conjunction with decreased pancreatic β-cell function (30). Importantly, data from our study also demonstrated that lifestyle intervention improved β-cell responsivity to insulin resistance–induced hyperglycemia. The mechanisms responsible for the improvement in β-cell function in our participants could involve metabolic processes that reduce β-cell glucotoxicity and lipotoxicity in response to the lifestyle intervention (31). Aging and obesity are also associated with increased pancreatic α-cell glucagon production, which can contribute to hyperglycemia by increasing hepatic glucose production (32). Therefore, the lifestyle-induced reduction in hyperglucagonemia in our participants may have additionally contributed to the observed improvement in glucose homeostasis. In fact, controlling for changes in fat mass and VO2peak using ANCOVA suggested that the improvements in glucometabolic control were mostly driven by the decrease in body fat and increase in physical fitness in our participants.
Obesity and diabetes additively predispose to frailty in older adults because of low muscle mass relative to body weight (relative sarcopenia) (3,6) and accelerated age-related loss of muscle mass that involves nutritional, inflammatory, and neurological pathways (33). Therefore, there has been some concern that lifestyle intervention that includes weight loss could worsen frailty by further reducing muscle mass (5). However, lifestyle intervention in our participants improved physical function, which is likely due to the larger reduction in fat mass relative to LBM (7) and improved muscle quality via reduction in muscle inflammation (34). We combined weight loss with aerobic and resistance training, which we have shown to additively improve cardiovascular fitness and muscle strength, thereby translating into the greatest improvement in physical function and quality of life (7,15). We have also shown that this specific lifestyle approach is the most effective in reducing ectopic fat deposition (35). Accordingly, data from the current study extend our findings of positive effects of lifestyle intervention on body composition and physical function to older adults with diabetes. The LBM loss (mean 1.7 kg) in the ILI group is less than the LBM loss (3.2 kg) we previously reported in older adults randomized to weight loss alone (without exercise) (7), suggesting that exercise (particularly resistance training) with adequate protein intake attenuated the weight loss–induced reduction of LBM in our current participants. Moreover, this modest LBM loss is likely outweighed by the improved muscle quality and physical function that occurred in response to ILI. Our results are in line with those of a recent RCT that showed that a multimodal intervention program improved functional performance in older adults with diabetes and frailty (36). However, that trial differed from our current RCT in that the nutritional intervention did not involve weight loss for obesity and the exercise intervention was limited to 12 weeks of resistance training at lower intensity.
An adverse effect of our lifestyle intervention was increase in hypoglycemic episodes in those on insulin or insulin secretagogues. However, nearly all episodes were mild (level 1) (26), promptly corrected with ingestion of easily absorbable carbohydrate. Nevertheless, this points to the importance of regular self-monitoring of blood glucose and periodic review of blood glucose records to assess the need for medication adjustments during intensive lifestyle change. Accordingly, total insulin requirements were reduced in those on insulin in the intervention group from concomitant improvement in glycemic control. Our participants had several age-related comorbidities, but they were cognitively intact and remained functionally independent with improved health status after lifestyle intervention. Therefore, the mean reduction of ∼1% in HbA1c in our participants may be consistent with a reasonable HbA1c goal of <7.0–7.5%, recommended for those with relatively preserved life expectancy and better health status (14).
Strengths of our study include the RCT design, the unique lifestyle intervention strategy of behavioral diet and exercise started at a facility and transitioned into community-fitness centers and homes, the high rate of adherence to the lifestyle intervention, and the comprehensive appraisals of glucose homeostasis and age-relevant outcomes (e.g., body composition, physical function, quality of life) that enabled the evaluation of treatment effects on overall health. Findings from our study may have practical implications because Medicare currently covers behavioral therapy for weight loss (37) and a growing number of Medicare plans now offer health club memberships (38). Our lifestyle intervention program has the key characteristics of medical nutrition therapy (MNT) that are covered by Medicare Part B: intensive, focused, and comprehensive nutrition therapy provided by a nutritional professional, in-depth individualized nutrition assessment, setting of personal goals and care plans, and emphasis on follow-up counseling to provide reinforcement in changing behavior (37,39). However, we also adapted our MNT to the special challenges of lifestyle intervention in older adults with diabetes. These included ensuring adequate protein intake to minimize weight loss induced reduction of LBM that could lead to sarcopenia, emphasis on group behavioral therapy to provide social support and enhance adherence in older adults, and use of multicomponent exercise to optimally improve physical function, an important age-related outcome (4). In the clinical setting, our MNT for older adults may be covered by Medicare through referral from the treating physician (37). Participation of older adults in fitness centers may also be covered by Medicare through Medicare plans, such as Medicare Part C (Medicare Advantage) or Medicare Supplement Insurance (Medigap). An example of a successful fitness program covered by Medicare Part C is SilverSneakers (40). We enrolled older adults with diabetes typically associated with comorbidities and functional impairments, though all were still living independently in the community. A limitation of our study is that in accordance with the exclusion criteria, the participants were physically able to participate in a lifestyle program and thus may not be fully representative of the general population of older adults with diabetes. Our participants had a higher educational level, which may have contributed to attaining intervention goals more easily. Despite our recruitment efforts, there was a lower proportion of Hispanics (18%) compared with that in the greater Houston area (37%). Although Medicare coverage underscores the potential for translation, we did not test implementation and associated challenges. Our study was limited to 1 year’s duration, so additional studies are needed to determine longer-term adherence and whether the beneficial effects of lifestyle intervention therapy can reduce diabetes complications and associated medical costs or prevent the institutionalization of older adults with diabetes.
In conclusion, our RCT provides evidence that a lifestyle intervention strategy may be effective in improving glycemic control and functional status in older adults with diabetes. Therefore, lifestyle intervention may have an important role in this older population in complementing medical therapy of diabetes and improving quality of life.
Article Information
Acknowledgments. The authors thank the participants for their cooperation and John Wade, Stephen Decker, Uma Phadnis, and Arjun Paudyal from Baylor College of Medicine for technical assistance. The authors also thank the members of the Alkek Foundation for their support.
Funding. This study was supported by grants from the American Diabetes Association (1-14-LLY-38) and National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (P30-DK020579), and resources at the Michael E. DeBakey VA Medical Center.
The contents do not represent the views of the U.S. Department of Veterans Affairs or the U.S. government.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. R.A.-V., C.Q., and D.T.V. designed the research. A.C., Y.B., B.J., D.B., G.C., S.M., R.A.-V., and D.T.V. conducted the study. C.Q. and D.T.V. performed the statistical analyses. C.Q. and D.T.V. drafted the manuscript and had primary responsibility for the final content. All authors contributed to the interpretation of the findings and critically reviewed, commented on, read, and approved the final manuscript. D.T.V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 98th Annual Meeting of the Endocrine Society (ENDO 2016), Boston, MA, 1–4 April 2016.
Footnotes
Clinical trial reg. no. NCT02348801, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.20198705.
References
- 1. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 2015;314:1021–1029 [DOI] [PubMed] [Google Scholar]
- 2. Amati F, Dubé JJ, Coen PM, Stefanovic-Racic M, Toledo FG, Goodpaster BH. Physical inactivity and obesity underlie the insulin resistance of aging. Diabetes Care 2009;32:1547–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol 2018;14:513–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Villareal DT, Apovian CM, Kushner RF; American Society for Nutrition; NAASO, The Obesity Society . Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr 2005;82:923–934 [DOI] [PubMed] [Google Scholar]
- 5. Waters DL, Ward AL, Villareal DT. Weight loss in obese adults 65years and older: a review of the controversy. Exp Gerontol 2013;48:1054–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical frailty and body composition in obese elderly men and women. Obes Res 2004;12:913–920 [DOI] [PubMed] [Google Scholar]
- 7. Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med 2011;364:1218–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bouchonville M, Armamento-Villareal R, Shah K, et al. Weight loss, exercise or both and cardiometabolic risk factors in obese older adults: results of a randomized controlled trial. Int J Obes 2014;38:423–431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ryan DH, Espeland MA, Foster GD, et al.; Look AHEAD Research Group . Look AHEAD (Action for Health in Diabetes): design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Control Clin Trials 2003;24:610–628 [DOI] [PubMed] [Google Scholar]
- 10. The Diabetes Prevention Program . The Diabetes Prevention Program. Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 1999;22:623–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Espeland MA, Rejeski WJ, West DS, et al.; Action for Health in Diabetes Research Group . Intensive weight loss intervention in older individuals: results from the Action for Health in Diabetes Type 2 diabetes mellitus trial. J Am Geriatr Soc 2013;61:912–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crandall J, Schade D, Ma Y, et al.; Diabetes Prevention Program Research Group . The influence of age on the effects of lifestyle modification and metformin in prevention of diabetes. J Gerontol A Biol Sci Med Sci 2006;61:1075–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. LeRoith D, Biessels GJ, Braithwaite SS, et al. Treatment of diabetes in older adults: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab 2019;104:1520–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. American Diabetes Association Professional Practice Committee . 13. Older adults: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022;45:S195–S207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Villareal DT, Aguirre L, Gurney AB, et al. Aerobic or resistance exercise, or both, in dieting obese older adults. N Engl J Med 2017;376:1943–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 1999;22:1462–1470 [DOI] [PubMed] [Google Scholar]
- 17. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–419 [DOI] [PubMed] [Google Scholar]
- 18. Cobelli C, Dalla Man C, Toffolo G, Basu R, Vella A, Rizza R. The oral minimal model method. Diabetes 2014;63:1203–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Comparison of individual and standard kinetic parameters for C-peptide clearance. Diabetes 1992;41:368–377 [DOI] [PubMed] [Google Scholar]
- 20. Bergman RN, Ader M, Huecking K, Van Citters G. Accurate assessment of beta-cell function: the hyperbolic correction. Diabetes 2002;51(Suppl. 1):S212–S220 [DOI] [PubMed] [Google Scholar]
- 21. Brown M, Sinacore DR, Binder EF, Kohrt WM. Physical and performance measures for the identification of mild to moderate frailty. J Gerontol A Biol Sci Med Sci 2000;55:M350–M355 [DOI] [PubMed] [Google Scholar]
- 22. Blair SN, Haskell WL, Ho P, et al. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol 1985;122:794–804 [DOI] [PubMed] [Google Scholar]
- 23. Jette AM, Cleary PD. Functional disability assessment. Phys Ther 1987;67:1854–1859 [DOI] [PubMed] [Google Scholar]
- 24. Lyons RA, Perry HM, Littlepage BN. Evidence for the validity of the Short-form 36 Questionnaire (SF-36) in an elderly population. Age Ageing 1994;23:182–184 [DOI] [PubMed] [Google Scholar]
- 25. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 1995;57:289–300 [Google Scholar]
- 26. International Hypoglycaemia Study Group . Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2017;40:155–157 [DOI] [PubMed] [Google Scholar]
- 27. Saunders C, Byrne CD, Guthrie B, et al.; Scottish Diabetes Research Network Epidemiology Group . External validity of randomized controlled trials of glycaemic control and vascular disease: how representative are participants? Diabet Med 2013;30:300–308 [DOI] [PubMed] [Google Scholar]
- 28. Kirkman MS, Briscoe VJ, Clark N, et al. Diabetes in older adults. Diabetes Care 2012;35:2650–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cefalu WT, Wang ZQ, Werbel S, et al. Contribution of visceral fat mass to the insulin resistance of aging. Metabolism 1995;44:954–959 [DOI] [PubMed] [Google Scholar]
- 30. Lee PG, Halter JB. The pathophysiology of hyperglycemia in older adults: clinical considerations. Diabetes Care 2017;40:444–452 [DOI] [PubMed] [Google Scholar]
- 31. Robertson RP, Harmon J, Tran PO, Poitout V. β-Cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes 2004;53(Suppl. 1):S119–S124 [DOI] [PubMed] [Google Scholar]
- 32. Basu R, Breda E, Oberg AL, et al. Mechanisms of the age-associated deterioration in glucose tolerance: contribution of alterations in insulin secretion, action, and clearance. Diabetes 2003;52:1738–1748 [DOI] [PubMed] [Google Scholar]
- 33. Bellary S, Kyrou I, Brown JE, Bailey CJ. Type 2 diabetes mellitus in older adults: clinical considerations and management. Nat Rev Endocrinol 2021;17:534–548 [DOI] [PubMed] [Google Scholar]
- 34. Colleluori G, Aguirre L, Phadnis U, et al. Aerobic plus resistance exercise in obese older adults improves muscle protein synthesis and preserves myocellular quality despite weight loss. Cell Metab 2019;30:261–273.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Waters DL, Aguirre L, Gurney B, et al. Effect of aerobic or resistance exercise, or both, on intermuscular and visceral fat and physical and metabolic function in older adults with obesity while dieting. J Gerontol A Biol Sci Med Sci 2022;77:131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rodriguez-Mañas L, Laosa O, Vellas B, et al.; European MID-Frail Consortium . Effectiveness of a multimodal intervention in functionally impaired older people with type 2 diabetes mellitus. J Cachexia Sarcopenia Muscle 2019;10:721–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Centers for Medicare and Medicaid Services . Decision Memo for Intensive Behavioral Therapy for Obesity (CAG-00423N). Baltimore, MD, Centers for Medicare and Medicaid Services, 2011 [Google Scholar]
- 38. Nguyen HQ, Maciejewski ML, Gao S, Lin E, Williams B, Logerfo JP. Health care use and costs associated with use of a health club membership benefit in older adults with diabetes. Diabetes Care 2008;31:1562–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care 2019;42:731–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kell KP, Rula EY. Increasing exercise frequency is associated with health and quality-of-life benefits for older adults. Qual Life Res 2019;28:3267–3272 [DOI] [PMC free article] [PubMed] [Google Scholar]