Abstract
OBJECTIVE
Remnant cholesterol (remnant-C) predicts atherosclerotic cardiovascular disease, regardless of LDL-cholesterol (LDL-C) levels. This study assessed the associations between remnant-C and cardiovascular outcomes in type 2 diabetes.
RESEARCH DESIGN AND METHODS
This post hoc analysis of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial used patient (type 2 diabetes >3 months) remnant-C and major adverse cardiovascular event (MACE) data from the study database. The associations between remnant-C and MACEs were evaluated using Cox proportional hazards regression analyses. We examined the relative MACE risk in remnant-C versus LDL-C discordant/concordant groups using clinically relevant LDL-C targets by discordance analyses.
RESULTS
The baseline analysis included 10,196 participants, with further visit-to-visit variability analysis including 9,650 participants. During follow-up (median, 8.8 years), 1,815 patients (17.8%) developed MACEs. After adjusting for traditional cardiovascular risk factors, each 1-SD increase in remnant-C was associated with a 7% higher MACE risk (hazard ratio [HR] 1.07, 95% CI 1.02–1.12, P = 0.004). In the fully adjusted model, the visit-to-visit remnant-C variability calculated using logSD (HR 1.41, 95% CI 1.18–1.69, P < 0.001) and logARV (HR 1.45, 95% CI 1.22–1.73, P < 0.001) was associated with MACEs. Residual lipid risk (remnant-C ≥31 mg/dL) recognized individuals at a higher MACE risk, regardless of LDL-C concentrations. Within each LDL-C subgroup (>100 or ≤100 mg/dL), high baseline remnant-C was associated with a higher MACE risk (HR 1.37, 95% CI 1.09–1.73, P = 0.007; HR 1.22, 95% CI 1.04–1.41, P = 0.015, respectively).
CONCLUSIONS
Remnant-C levels were associated with MACEs in patients with type 2 diabetes independent of LDL-C, and visit-to-visit remnant-C variability helped identify those with higher cardiovascular risk.
Introduction
Type 2 diabetes is associated with an increased incidence of atherosclerotic cardiovascular disease (CVD) (1,2), which is partially attributable to dyslipidemia and is characterized by elevated plasma triglyceride (TG) levels, low levels of HDL-cholesterol (HDL-C), and high levels of small, dense LDL particles (3,4). Current guidelines for CVD prevention include lowering plasma LDL-cholesterol (LDL-C) levels (3,5); however, patients with a substantial reduction in LDL-C levels continue to have considerable residual cardiovascular risk (6). Efforts have been made to identify strategies to tackle this residual risk (7,8). Since HDL-C–raising therapies have failed to reduce atherosclerotic cardiovascular events (9,10), the research focus has shifted to TG-rich lipoproteins (TRLs), which are composed of chylomicron remnants, VLDL-C, and intermediate-density lipoprotein. TG-rich lipoproteins are associated with the development of CVD (11–15). Notably, TG, but not cholesterol, can be easily metabolized in most cells. Therefore, it has been hypothesized that cholesterol, not TG, is the harmful component of TRLs (16).
Remnant cholesterol (remnant-C) is the cholesterol content of TRLs (6). High serum levels of remnant-C contribute to increased penetration into the arterial wall (17), ultimately leading to major adverse cardiovascular events (MACEs). Multiple studies have shown that circulatory remnant-C levels correlate with MACEs in the primary and secondary prevention of CVD (18–21). More specifically, one study showed that the median remnant-C level was 20 mg/dL in patients without known CVD and that elevated remnant-C levels were associated with CVD, independent of traditional risk factors, LDL-C, and apolipoprotein B levels, suggesting that remnant-C might be important in primary prevention (22). In overweight or obese individuals at high cardiovascular risk, every 10-mg/dL increase in the remnant-C (but not in LDL-C or HDL-C) level was associated with a 21% higher MACE risk after full adjustment for potential confounders (21). However, limited data are available in patients with type 2 diabetes. Notably, TG-lowering therapy in type 2 diabetes failed to reduce the rate of coronary heart disease events in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) (2) and Fenofibrate Intervention and Event Lowering in Diabetes (23) trials. This suggests that remnant-C might play an important role in the occurrence of MACEs in type 2 diabetes. Data also suggest that remnant-C levels are elevated in type 2 diabetes and might predict myocardial function and future coronary outcomes (24,25). Additionally, evidence indicates that individuals with prediabetes with high remnant-C levels have a higher tendency to develop diabetes than those with normoglycemia (26,27).
Previous studies relied on a single time point or baseline remnant-C sample for relationship analysis and did not address changes in remnant-C levels derived from multiple measurements over time and the discordance between remnant-C and LDL-C levels. Bangalore et al. (28) showed that visit-to-visit variability in LDL-C levels was an independent predictor of cardiovascular events in the Treating to New Targets (TNT) trial, but whether dynamic changes in remnant-C over time are also associated with adverse cardiovascular outcomes is unknown. In the current study, we evaluated the relationship between MACEs and visit-to-visit remnant-C variability during follow-up in patients with type 2 diabetes. To further identify whether the risk associated with remnant-C is independent of LDL-C levels, discordance analysis was performed in patients with type 2 diabetes using data from the ACCORD study and its follow-up study (ACCORDION).
Research Design and Methods
Study Design and Participants
This post hoc analysis of a prospective study used data from the ACCORD study (ClinicalTrials.gov number, NCT00000620). Participants in the ACCORD trial were recruited between June 2001 and October 2005 at 77 sites across the U.S. and Canada. The trial enrolled 10,251 people whose mean age was 62 years, who had type 2 diabetes for a median duration of 10 years, with a mean glycated hemoglobin (HbA1c) level of 8.3%, and who had previous CVD or CVD risk factors. All surviving ACCORD participants from participating sites who could be contacted were subsequently offered the opportunity to participate in the ACCORDION study, during which data on cardiovascular and other health-related outcomes and measurements were collected and analyzed between May 2011 and October 2014.
The inclusion criteria of this study were identical to those of the original ACCORD study: 1) type 2 diabetes defined according to the 1997 American Diabetes Association guidelines; 2) HbA1c 7.5–11% or 7.5–9% (depending on insulin dosage and administration of oral hypoglycemic agents); 3) type 2 diabetes duration >3 months; 4) stable type 2 diabetes for >3 months; and 5) an age of 40–79 years with CVD or an age of 55–79 years with anatomical evidence of significant atherosclerosis, albuminuria, left ventricular hypertrophy, or at least two additional risk factors for CVD (dyslipidemia, hypertension, current smoking status, or obesity). Individuals meeting the inclusion criteria of the original ACCORD study were screened. Patients were excluded from the study if their baseline remnant-C values were missing or if they had fewer than three valid remnant-C measurements during follow-up.
Data Collection and Outcomes
Data collected included demographics (age, sex, education, and living status), fasting plasma lipid profiles, and cardiovascular risk factors. The following equation was used to calculate remnant-C levels: remnant-C(mg/dL) = HDL-C − LDL-C. The blood lipid and clinical follow-up time points were 0, 4, 8, 12, 24, 36, 48, 60, 72, 84, and 96 months. Non–HDL-C levels were calculated using the following equation: non–HDL-C(mg/dL) = TC − HDL-C (22). Visit-to-visit remnant-C variability was defined as variability in remnant-C values between visits. For patients with missing remnant-C values at any visit, any other available remnant-C data were used to calculate remnant-C variability.
The primary outcome was MACE occurrence, including nonfatal myocardial infarction (MI), nonfatal stroke, and death from cardiovascular causes.
Statistical Analysis
Normally distributed continuous data are expressed as mean ± SD, nonnormally distributed continuous data as median (interquartile range), and categorical data as numbers (percentage). Differences among groups were evaluated using the ANOVA or Kruskal-Wallis H test for continuous variables and the χ2 test for categorical variables. The Bonferroni test was used to evaluate differences among the groups. Correlation analysis was conducted using the Pearson test.
The participants were classified into three remnant-C groups (low, middle, and high) based on the remnant-C tertiles within the entire cohort, as previously described (20). Kaplan-Meier estimates were used to compute the cumulative incidence of incident MACEs according to remnant-C tertiles. Differences in the estimates were compared using the log-rank test. Unadjusted and adjusted Cox proportional hazard models were used to assess the associations between remnant-C and incident MACEs and the components of MACEs. Univariate analyses were conducted to evaluate relationships between all variables and MACEs before the multivariate Cox regression analyses. Variables with P < 0.10 in the univariate analyses were included in the multivariable analyses. Additionally, variables that were clinically closely related to MACEs were included in the multivariable analyses (even if P > 0.10 in univariable analyses) to avoid missing important conventional cardiovascular risk factors (e.g., age, sex, smoking). Three multivariable models with progressive degrees of adjustment were used to adjust for potential confounders of incident MACEs and components of MACEs. Model 1 was adjusted for sex, age, education, years of hypertension diagnosis, depression, smoking habit, alcohol consumption, systolic blood pressure, diastolic blood pressure, heart rate, BMI, and cardiovascular history; model 2 was adjusted for model 1 covariables plus plasma glucose, estimated glomerular filtration rate (eGFR), HbA1c, and LDL-C; and model 3 was adjusted for model 2 covariables plus treatment with biguanide, statins, and insulin.
To explore the effect of changes in remnant-C on MACEs during follow-up, visit-to-visit remnant-C level variability was calculated. Various measurements of variability were used, as described in previous studies (28,29): 1) the SD of remnant-C levels and logSD; 2) the average real variability (ARV) and logARV, defined as the average absolute difference between successive values; and 3) the coefficient of variation (CV) and logCV.
Discordance analysis was used to identify whether the risk associated with remnant-C was independent of LDL-C levels. We used a clinically relevant LDL-C cutoff (100 mg/dL) based on worldwide guideline recommendations (5,30). The remnant-C cutoff point was identified using equivalent population percentiles from the cohort corresponding to the clinically relevant LDL-C target. In addition, previous studies used the aforementioned methods for determining remnant-C cutoffs corresponding to three different LDL-C values (70, 100, and 130 mg/dL) for discordance analyses (22,31). Furthermore, according to the 2019 European Society of Cardiology guidelines on diabetes, prediabetes, and CVDs developed in collaboration with the European Association for the Study of Diabetes (3), LDL-C target levels for the management of patients with diabetes were summarized as <1.4 mmol/L (<55 mg/dL) for patients with a very high cardiovascular risk, <1.8 mmol/L (<70 mg/dL) for patients with a high cardiovascular risk, and <2.6 mmol/L (<100 mg/dL) for patients with a moderate cardiovascular risk. A target LDL-C level of <3.0 mmol/L (<116 mg/dL) may be considered for patients without diabetes but with a low cardiovascular risk. Because only 13 patients in the ACCORD database had an LDL-C level <55 mg/dL, the corresponding remnant-C cutoff was not analyzed. Therefore, we determined the corresponding remnant-C cutoff values according to the four different LDL-C cutoff values (70, 100, 116, and 130 mg/dL) and conducted a Cox regression correction analysis. The discordant groups were defined as low remnant-C/high LDL-C and high remnant-C/low LDL-C levels. Concordant groups included those with low remnant-C/low LDL-C and high remnant-C/high LDL-C levels. Baseline characteristics were compared across the four concordance/discordance groups using the χ2 test for categorical variables or ANOVA (or Kruskal-Wallis when appropriate) for continuous variables. A Cox proportional hazards regression model was used to assess the association between remnant-C and LDL-C concordant/discordant groups and incident MACEs. A receiver operating characteristic (ROC) curve was constructed to determine the predictive value of remnant-C.
All analyses were conducted using SPSS 23.0 (IBM, Armonk, NY) and Stata 15.1 (StataCorp LLC, College Station, TX) software. Statistical significance was defined as a two-sided P value <0.05.
Results
Baseline Characteristics According to Tertiles of Remnant-C
The baseline analysis included 10,196 participants (Supplementary Fig. 1); 61.48% were men, and the average age was 62.8 ± 6.6 years. Baseline blood tests revealed mean levels of TC, 183.3 ± 41.9 mg/dL; HDL-C, 41.9 ± 11.6 mg/dL; LDL-C, 104.9 ± 33.9 mg/dL; non–HDL-C, 141.4 ± 41.4 mg/dL; and TG, 190.1 ± 148.4 mg/dL. The mean remnant-C level was 36.5 ± 24.4 mg/dL. The baseline characteristics were used to stratify participants according to tertiles of remnant-C levels (Table 1). The mean age differed slightly across the groups. Individuals with high tertile remnant-C levels (Table 1) were more likely to be men, smokers, to have had previous cardiovascular events, and to have a high BMI, diastolic blood pressure, heart rate, HbA1c, and fasting blood glucose levels. Insulin and statin use was lower in individuals with remnant-C levels in the high tertile, whereas metformin use was higher in the low tertile. Correlation testing confirmed expected direct associations between remnant-C, TC, TG, and non–HDL-C levels. Interestingly, there was a clear inverse association between HDL-C and remnant-C levels (Supplementary Table 1).
Table 1.
Subject baseline characteristics by tertiles of remnant-C level
| Total | Low | Middle | High | ||
|---|---|---|---|---|---|
| N = 10,196 | n = 3,448 | n = 3,485 | n = 3,263 | P value | |
| Age, years | 62.8 ± 6.6 | 63.4 ± 6.8 | 62.9 ± 6.6 | 61.9 ± 6.4 | <0.001 |
| Sex | 0.681 | ||||
| Female | 3,928 (38.5) | 1,339 (38.8) | 1,352 (38.8) | 1237 (37.9) | |
| Male | 6,268 (61.5) | 2,109 (61.2) | 2,133 (61.2) | 2026 (62.1) | |
| Education | |||||
| Less than high school | 1,502 (14.7) | 583 (16.9) | 527 (15.1) | 392 (12.0) | <0.001 |
| High school graduate or GED | 2,692 (26.4) | 926 (26.9) | 898 (25.8) | 868 (26.6) | 0.561 |
| Some college | 3,343 (32.8) | 1,052 (30.5) | 1,144 (32.8) | 1,147 (35.2) | <0.001 |
| College degree or higher | 2,652 (26.0) | 885 (25.7) | 913 (26.2) | 854 (26.2) | 0.852 |
| Living alone | 2,065 (20.7) | 722 (20.9) | 710 (20.4) | 633 (19.4) | 0.284 |
| Depression | 2,412 (23.7) | 650 (18.9) | 836 (24.0) | 926 (28.4) | <0.001 |
| History of CVD | 3,586 (35.2) | 1,171 (34.0) | 1,193 (34.2) | 1,222 (37.5) | 0.004 |
| Duration of hypertension, years | 10.2 ± 9.6 | 9.9 ± 9.4 | 10.4 ± 9.6 | 10.4 ± 9.7 | 0.109 |
| Cigarette-smoking | <0.001 | ||||
| Yes | 5,925 (58.1) | 1,920 (55.7) | 2,024 (58.1) | 1,981 (60.7) | |
| No | 4,271 (41.9) | 1,528 (44.3) | 1,461 (41.9) | 1,282 (39.3) | |
| Alcohol use | 0.006 | ||||
| Yes | 2,434 (23.9) | 814 (23.6) | 892 (25.6) | 728 (22.3) | |
| No | 7,757 (76.1) | 2,633 (76.4) | 2,590 (74.3) | 2,534 (77.7) | |
| BMI, kg/m2 | 32.2 ± 5.4 | 31.2 ± 5.5 | 32.5 ± 5.4 | 32.9 ± 5.2 | <0.001 |
| Blood pressure, mmHg | |||||
| Systolic | 136.4 ± 17.1 | 136.8 ± 17.1 | 135.9 ± 17.3 | 136.3 ± 16.9 | 0.082 |
| Diastolic | 74.9 ± 10.7 | 73.9 ± 10.5 | 75.1 ± 10.7 | 75.7 ± 10.7 | <0.001 |
| Heart rate, bpm | 72.7 ± 11.8 | 72.0 ± 11.7 | 72.2 ± 11.8 | 73.8 ± 11.9 | <0.001 |
| Medication use | |||||
| Insulin | 3,565 (35.0) | 1,458 (42.3) | 1,162 (33.3) | 945 (29.0) | <0.001 |
| Metformin | 6,519 (63.9) | 2,070 (60.0) | 2,320 (66.6) | 2,129 (65.3) | <0.001 |
| Statin | 6,468 (63.4) | 2,317 (67.2) | 2,238 (64.2) | 1,913 (58.6) | <0.001 |
| Cholesterol absorption inhibitors | 207 (2.0) | 60 (1.7) | 79 (2.3) | 68 (2.1) | 0.288 |
| HbA1c, % | 8.3 ± 1.1 | 8.3 ± 1.0 | 8.3 ± 1.1 | 8.4 ± 1.1 | <0.001 |
| Fasting plasma glucose, mg/dL | 175.2 ± 56.2 | 162.6 ± 53.8 | 173.7 ± 53.6 | 190.2 ± 57.8 | <0.001 |
| eGFR, mL/min/1.73 m2 | 91.1 ± 27.2 | 92.2 ± 23.6 | 89.7 ± 25.5 | 91.3 ± 32.0 | <0.001 |
| Remnant-C, mg/dL | 36.5 ± 24.4 | 17.8 ± 4.3 | 31.8 ± 4.5 | 61.5 ± 28.1 | <0.001 |
| Plasma concentration | |||||
| TG, mg/dL | 190.1 ± 148.4 | 88.8 ± 21.5 | 158.9 ± 23.4 | 330.6 ± 190.1 | <0.001 |
| Total cholesterol, mg/dL | 183.3 ± 41.9 | 168.2 ± 34.9 | 180.4 ± 37.0 | 202.4 ± 46.0 | <0.001 |
| LDL-C, mg/dL | 104.9 ± 33.9 | 102.6 ± 30.3 | 107.1 ± 33.7 | 105.0 ± 37.5 | <0.001 |
| HDL-C, mg/dL | 41.9 ± 11.6 | 47.8 ± 12.8 | 41.5 ± 1.0 | 35.9 ± 8.4 | <0.001 |
| non–HDL-C, mg/dL | 141.4 ± 41.4 | 120.3 ± 31.1 | 138.9 ± 34.0 | 166.5 ± 44.7 | <0.001 |
| Incident MACEs | 1,815 (17.8) | 552 (16.0) | 599 (17.2) | 664 (20.4) | <0.001 |
| CVD death | 663 (6.5) | 192 (5.6) | 221 (6.3) | 250 (7.7) | 0.002 |
| Nonfatal MI | 932 (9.1) | 267 (7.7) | 302 (8.7) | 363 (11.1) | <0.001 |
| Nonfatal stroke | 485 (4.8) | 160 (4.6) | 160 (4.6) | 165 (5.1) | 0.618 |
Data are presented as mean ± SD or as n (%). The tertile ranges were low (3–24 mg/dL), middle (25–40 mg/dL), and high (41–474 mg/dL). P value for the test of the difference across tertiles of remnant-C were obtained by using the χ2 test (categorical variables), ANOVA (continuous variables), or Kruskal-Wallis test (nonparametric comparisons). GED, General Education Development.
Baseline Remnant-C Levels and MACEs
During a mean follow-up period of 7.7 years (median 8.8 years), 1,815 patients (17.8%) developed MACEs. As shown in Table 1, the risk of MACEs increased with increasing tertiles of remnant-C. Similarly, the risk of CVD death and nonfatal MI also increased with increasing tertiles of remnant-C. In tertile analyses, the remnant-C level showed a graded association with MACEs (P for trends <0.001), but a significant difference was only observed between tertiles 1 and 3 (hazard ratio [HR] 1.38, 95% CI 1.20–1.58, P < 0.001) (Table 2). In model 3, when the remnant-C level was measured as a continuous variable, a 1-SD increase in the remnant-C level was associated with a 7% higher risk of MACEs after full adjustment for potential confounders (HR 1.07, 95% CI 1.02–1.12, P = 0.004) (Table 2). These findings suggest that the baseline remnant-C level could be used as a predictor of MACEs. Furthermore, when evaluating associations between the baseline remnant-C level and individual outcomes, we found that there was also a graded association of the remnant-C level with CVD death and nonfatal MI (P for trends = 0.002 and <0.001, respectively), but not for nonfatal stroke. After adjusting for potential confounders, significant differences in CVD death and nonfatal MI were still observed between tertiles 1 and 3. However, when the remnant-C level was measured as a continuous variable, a 1-SD increase in the remnant-C level was only associated with a higher risk of nonfatal MI. These findings indicate that the baseline remnant-C level was correlated with major coronary events. Kaplan-Meier curves were used to determine the probability of MACEs and individual outcomes (Supplementary Fig. 2). Compared with patients with a low remnant-C level, the probability of poor patient outcomes was significantly higher in patients with a high remnant-C level (P < 0.05), further indicating that the baseline remnant-C level could be used as a prognostic marker for patients with type 2 diabetes.
Table 2.
Risk of incident MACEs for baseline remnant-C level
| Remnant-C | Events/n at risk | Unadjusted | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||
| MACEs | |||||||||
| Tertile 1 | 552/3,448 | Ref | Ref | Ref | Ref | ||||
| Tertile 2 | 599/3,485 | 1.07 (0.96–1.21) | 0.228 | 1.11 (0.97–1.26) | 0.144 | 1.08 (0.94–1.23) | 0.285 | 1.11 (0.97–1.27) | 0.137 |
| Tertile 3 | 664/3,263 | 1.32 (1.18–1.47) | <0.001 | 1.36 (1.19–1.55) | <0.001 | 1.31 (1.14–1.50) | <0.001 | 1.38 (1.20–1.58) | <0.001 |
| Per 1 SD | 1.07 (1.04–1.12) | <0.001 | 1.08 (1.03–1.13) | <0.001 | 1.06 (1.01–1.11) | 0.010 | 1.07 (1.02–1.12) | 0.004 | |
| P for trend | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| CVD death | |||||||||
| Tertile 1 | 192/3,448 | Ref | Ref | Ref | Ref | ||||
| Tertile 2 | 221/3,485 | 1.15 (0.95–1.40) | 0.152 | 1.21 (0.96–1.52) | 0.099 | 1.17 (0.93–1.47) | 0.192 | 1.20 (0.95–1.51) | 0.129 |
| Tertile 3 | 250/3,263 | 1.41 (1.17–1.70) | <0.001 | 1.51 (1.21–1.88) | <0.001 | 1.35 (1.08–1.70) | 0.009 | 1.38 (1.10–1.74) | 0.006 |
| Per 1 SD | 1.06 (0.99–1.13) | 0.081 | 1.07 (0.99–1.16) | 0.058 | 1.05 (0.97–1.14) | 0.249 | 1.05 (0.97–1.13) | 0.270 | |
| P for trend | <0.001 | <0.001 | 0.003 | 0.002 | |||||
| Nonfatal MI | |||||||||
| Tertile 1 | 267/3,448 | Ref | Ref | Ref | Ref | ||||
| Tertile 2 | 302/3,485 | 1.11 (0.92–1.34) | 0.181 | 1.09 (0.90–1.32) | 0.386 | 1.06 (0.88–1.29) | 0.534 | 1.10 (0.91–1.34) | 0.329 |
| Tertile 3 | 363/3,263 | 1.48 (1.26–173) | <0.001 | 1.38 (1.14–1.67) | 0.001 | 1.37 (1.13–1.65) | 0.001 | 1.53 (1.26–1.85) | <0.001 |
| Per 1 SD | 1.10 (1.06–1.16) | <0.001 | 1.10 (1.05–1.17) | <0.001 | 1.09 (1.03–1.16) | 0.004 | 1.10 (1.04–1.17) | <0.001 | |
| P for trend | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| Nonfatal stroke | |||||||||
| Tertile 1 | 160/3,448 | Ref | Ref | Ref | Ref | ||||
| Tertile 2 | 160/3,485 | 0.99 (0.79–1.23) | 0.917 | 1.16 (0.90–1.49) | 0.240 | 1.13 (0.88–1.45) | 0.348 | 1.14 (0.88–1.47) | 0.311 |
| Tertile 3 | 165/3,263 | 1.12 (0.90–1.39) | 0.313 | 1.23 (0.96–1.59) | 0.105 | 1.18 (0.91–1.53) | 0.223 | 1.20 (0.92–1.57) | 0.177 |
| Per 1 SD | 1.05 (0.97–1.14) | 0.201 | 1.05 (0.96–1.15) | 0.304 | 1.03 (0.94–1.14) | 0.489 | 1.04 (0.95–1.14) | 0.414 | |
| P for trend | 0.318 | 0.091 | 0.200 | 0.138 | |||||
Model 1: Adjusted for sex, age, education, years of hypertension diagnosis, depression, smoking habit, alcohol consumption, systolic blood pressure, diastolic blood pressure, heart rate, BMI, and CVD history. Model 2: Adjusted for model 1 covariables plus levels of plasma glucose, eGFR, HbA1c, and LDL-C. Model 3: Adjusted for model 2 covariables plus treatment with biguanide, statins, and insulin.
Associations Between Visit-to-Visit Remnant-C Level Variability and MACEs
Further visit-to-visit variability analysis including 9,650 participants was performed to explore the effects of changes in the remnant-C level on MACEs over time. Table 3 shows that the visit-to-visit remnant-C level variability, calculated using logSD and logARV, was associated with MACEs in the fully adjusted model. For CVD death, an association with visit-to-visit remnant-C level variability was observed for logARV. The visit-to-visit remnant-C level variability calculated using all three calculation methods was associated with nonfatal MI. None of the visit-to-visit remnant-C level variability values were associated with nonfatal stroke (all P > 0.05). These results indicate that visit-to-visit remnant-C level variability is a predictor of MACEs, CVD death, and nonfatal MI in patients with type 2 diabetes.
Table 3.
Risk of incident MACEs for visit-to-visit variability of remnant-C level
| Remnant-C | Unadjusted | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| MACEs | ||||||||
| logSD | 1.52 (1.31–1.76) | <0.001 | 1.45 (1.22–1.73) | <0.001 | 1.39 (1.16–1.65) | <0.001 | 1.41 (1.18–1.69) | <0.001 |
| logARV | 1.57 (1.35–1.82) | <0.001 | 1.49 (1.25–1.77) | <0.001 | 1.43 (1.20–1.71) | <0.001 | 1.45 (1.22–1.73) | <0.001 |
| logCV | 1.31 (1.02–1.69) | 0.035 | 1.11 (0.84–1.49) | 0.461 | 1.04 (0.78–1.39) | 0.770 | 1.06 (0.79–1.41) | 0.700 |
| CVD death | ||||||||
| logSD | 1.31 (1.02–1.69) | 0.037 | 1.39 (1.03–1.87) | 0.029 | 1.28 (0.94–1.73) | 0.111 | 1.27 (0.94–1.72) | 0.120 |
| logARV | 1.42 (1.10–1.83) | 0.007 | 1.57 (1.17–2.12) | 0.003 | 1.47 (1.08–1.99) | 0.013 | 1.46 (1.08–1.97) | 0.014 |
| logCV | 1.03 (0.67–1.57) | 0.901 | 1.03 (0.63–1.68) | 0.910 | 0.93 (0.57–1.53) | 0.786 | 0.92 (0.56–1.51) | 0.743 |
| Nonfatal MI | ||||||||
| logSD | 2.07 (1.69–2.52) | <0.001 | 1.91 (1.51–2.41) | <0.001 | 1.86 (1.46–2.36) | <0.001 | 1.92 (1.51–2.43) | <0.001 |
| logARV | 2.04 (1.67–2.50) | <0.001 | 1.90 (1.50–2.40) | <0.001 | 1.86 (1.47–2.36) | <0.001 | 1.90 (1.50–2.40) | <0.001 |
| logCV | 2.09 (1.48–2.95) | 0.000 | 1.63 (1.10–2.43) | 0.016 | 1.67 (1.05–2.33) | 0.027 | 1.62 (1.09–2.42) | 0.017 |
| Nonfatal stroke | ||||||||
| logSD | 1.22 (0.92–1.62) | 0.176 | 1.28 (0.92–1.78) | 0.136 | 1.23 (0.88–1.72) | 0.218 | 1.26 (0.90–1.76) | 0.175 |
| logARV | 1.25 (0.94–1.67) | 0.126 | 1.28 (0.92–1.78) | 0.142 | 1.24 (0.89–1.73) | 0.207 | 1.26 (0.90–1.76) | 0.171 |
| logCV | 0.92 (0.57–1.48) | 0.728 | 0.89 (0.52–1.52) | 0.665 | 0.83 (0.48–1.43) | 0.502 | 0.84 (0.49–1.45) | 0.535 |
Model 1: Adjusted for sex, age, education, years of hypertension diagnosis, depression, smoking habit, alcohol consumption, systolic blood pressure, diastolic blood pressure, heart rate, BMI, and CVD history. Model 2: Adjusted for model 1 covariables plus levels of plasma glucose, eGFR, HbA1c, and LDL-C. Model 3: Adjusted for model 2 covariables plus treatment with biguanide, statins, and insulin.
Contribution of Remnant-C to Residual Lipid Risk by LDL-C Level
To examine the relative risk of MACEs in the remnant-C versus LDL-C discordant/concordant groups using clinically relevant LDL-C target levels, we performed discordance analyses. A high LDL-C level was defined as >100 mg/dL for the primary prevention cohort. Abnormally high remnant-C values used for the cutoff were defined as ≥31 mg/dL. The baseline characteristics of the participants stratified by the four remnant-C/LDL-C concordance/discordance groups are shown in Supplementary Table 2. The subgroups with higher LDL-C levels had fewer men and a lower frequency of insulin, metformin, and statin use, whereas those with high remnant-C levels had lower HDL-C and higher TG levels, a higher BMI, and a greater frequency of depression.
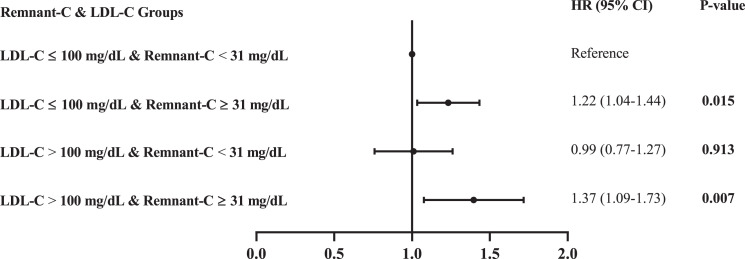
Residual lipid risk assessed by a remnant-C level ≥31 mg/dL recognized individuals at higher risk of MACEs, regardless of LDL-C concentrations (Fig. 1). Within each LDL-C subgroup (>100 or ≤100 mg/dL), in model 3, high baseline remnant-C concentrations identified individuals at a higher risk of MACEs compared with those with lower remnant-C concentrations (LDL-C ≤100 mg/dL and remnant-C ≥31 mg/dL: HR 1.22, 95% CI 1.04–1.44, P = 0.015; LDL-C >100 mg/dL and remnant-C ≥31 mg/dL: HR 1.37, 95% CI 1.09–1.73, P = 0.007) (Fig. 1). Additional comparisons were made between various groups, including LDL-C >100 mg/dL and remnant-C ≥31 mg/dL vs. LDL-C ≤100 mg/dL and remnant-C ≥31 mg/dL (HR 1.18, 95% CI 0.95–1.47, P = 0.139), LDL-C >100 mg/dL and remnant-C <31 mg/dL vs. LDL-C ≤100 mg/dL and remnant-C ≥31 mg/dL (HR 0.86, 95% CI 0.67–1.10, P = 0.241), and LDL-C >100 mg/dL and remnant-C ≥31 mg/dL vs. LDL-C >100 mg/dL and remnant-C <31 mg/dL (HR 1.30, 95% CI 1.12–1.52, P = 0.001). These results further demonstrated that a remnant-C level ≥31 mg/dL was a predictor of MACEs in patients with type 2 diabetes, regardless of LDL-C levels. Using the 70 mg/dL LDL-C and 17 mg/dL remnant-C level cutoffs did not yield significant associations in model 3, whereas using a 130 mg/dL cutoff showed a significant association only for an LDL-C level ≤130 mg/dL and remnant-C level ≥49 mg/dL (Supplementary Table 3). Although cutoffs of 116 mg/dL for LDL-C and 100 mg/dL for remnant-C levels both demonstrated significant associations in model 3, a target LDL-C level of 100 mg/dL is desirable for patients with type 2 diabetes according to clinical guidelines. Thus, in the current study, we considered a remnant-C cutoff value (31 mg/dL) corresponding to an LDL-C level of 100 mg/dL.
Figure 1.
Discordance analyses of remnant-C and LDL-C levels. In order to assess the risk of MACEs by categories of low and high LDL-C and remnant-C levels, HRs were estimated relative to the lowest risk category (LDL-C ≤100 mg/dL and remnant-C <31 mg/dL). Data were adjusted for sex, age, years of hypertension diagnosis, depression, smoking habit, alcohol consumption, systolic blood pressure, diastolic blood pressure, heart rate, BMI, previous cardiovascular history, plasma glucose level, eGFR level, HbA1c level, LDL-C level, biguanide treatment, statin treatment, and insulin treatment. A high remnant-C level was associated with MACEs, regardless of LDL-C values.
To explore associations between the remnant-C level and MACEs in more detail, we performed subgroup analyses stratified by age, sex, BMI, and CVD history. There were no differences in the relationship between the remnant-C level and MACEs in patients <65 vs. ≥65 years of age, with a BMI ≥25 vs. <25 kg/m2, and a CVD history versus no CVD history (all P > 0.05), but the association was stronger in women (HR 1.43, 95% CI 1.11–1.83) than in men (HR 1.28, 95% CI 1.08–1.51, P = 0.02) (Supplementary Fig. 3). To assess the predictive value of the outcomes, we further analyzed the ROC curves. Combined models with and without remnant-C were established using MACEs as the outcome. After increasing remnant-C levels, the area under the ROC curve increased significantly, indicating that the combination with remnant-C improved prediction efficiency (Supplementary Fig. 4).
Conclusions
Our study sought to examine correlations between adverse cardiovascular outcomes and remnant-C levels (including visit-to-visit variability in the remnant-C level) in patients with type 2 diabetes while performing an analysis designed to identify the impact of changes in the remnant-C level on MACEs. We demonstrated that for patients with type 2 diabetes at high cardiovascular risk, baseline remnant-C levels are associated with MACEs. During long-term follow-up, visit-to-visit variability in the remnant-C level is an independent predictor of adverse cardiovascular events, suggesting that a more uniform/less variable remnant-C level is desirable. Discordance analyses indicated that elevated remnant-C levels are associated with MACEs, independent of traditional risk factors and LDL-C levels.
With elevated plasma remnant-C levels, patients with type 2 diabetes represent a special population that deserves more attention regarding residual risk (24). Our primary findings indicated that baseline estimated remnant-C levels were associated with MACEs, regardless of clinical phenotypes, lifestyle confounders related to cardiovascular risk, and lipid-lowering treatment. Similarly, an observational study showed that the remnant-C level was associated with a higher risk of death from CVD in patients with type 2 diabetes and incidental diabetic nephropathy (32). In addition, post hoc data from the TNT trial showed that increased remnant-C levels are associated with increased cardiovascular risk (19). Furthermore, genetic studies strongly suggest that a higher remnant-C level is a causal risk factor for coronary artery disease (33,34). These observational, clinical intervention, and genetic studies not only confirmed a strong association between elevated remnant-C levels and a high risk of developing cardiovascular events (26,27,32) but also implied that the atherogenic effects of remnant-C may explain associations with an increased incidence of MACEs.
Notably, previous studies on remnant-C were based on single time point measurements (21,22,25,32,35), which may not reflect long-term exposure given that remnant-C levels often vary over time. In patients with coronary artery disease and an LDL-C level <130 mg/dL who were enrolled in the TNT, visit-to-visit variability in the LDL-C level was predictive of adverse long-term cardiovascular outcomes, including coronary events, cardiovascular events, death, MI, and stroke, independent of the treatment effect and achieved LDL-C levels (28). This suggests that a more uniform and less variable LDL-C level is desirable. However, it is not known whether the same is applicable to remnant-C. In the current study, patients were followed over a mean period of 7.7 years, multiple remnant-C tests were conducted on individual participants, and variability in the remnant-C levels was calculated as indicated by logSD, logASV, and logCV. Our results demonstrate that visit-to-visit remnant-C variability is a powerful predictor of MACEs, CVD death, and nonfatal MI in patients with type 2 diabetes who are at a high risk of CVDs. Therefore, a more uniform, less variable visit-to-visit remnant-C level is more important than the remnant-C level itself.
Another noteworthy observation is that remnant-C was the major cholesterol fraction contributor to MACEs in patients with type 2 diabetes at high cardiovascular risk. Participants with remnant-C levels ≥31 mg/dL had a higher risk of MACEs in the current study, regardless of whether the LDL-C level was optimal (≤100 mg/dL [2.59 mmol/L]). The discordance analysis showed that remnant-C levels predicted cardiovascular outcomes independent of LDL-C levels and support remnant-C as both a clinical predictor and a clinical intervention target. Interestingly, recent reports showed that lowering remnant-C levels by 32 mg/dL (0.83 mmol/L) reduced recurrent MACEs by 20% in secondary prevention (36). Additionally, a large observational study showed that high levels of remnant-C, but not LDL-C, are associated with cardiovascular outcomes in overweight or obese individuals (21) and in atherosclerotic CVD-free individuals (22). From these data, we could postulate that treatment of residual risk, measured as remnant-C, was likely more beneficial than further reduction of LDL-C levels in high-risk individuals already treated with moderate- or high-dose statins (19,36,37). In context, while LDL-C and non–HDL-C were reduced to levels below guideline-recommended targets, the remnant-C level was only mildly reduced, suggesting that residual risk could possibly be targeted with remnant-C–lowering medications such as apolipoprotein C3 and angiopoietin-like 3 (ANGPTL3) (38). To explore the association between remnant-C level and MACEs in more detail, we categorized the study population based on patient demographics and medical history. After adjusting for potential confounders, the association between higher remnant-C levels and an increased risk of study outcomes remained significant. In addition, we found an interaction between sex and MACEs, which could be explained by the theory that female patients who already had a certain level of risk for CVD may have a higher incidence of MACEs. Furthermore, conventional risk factors combined with remnant-C could improve prediction efficiency. Taken together, these data suggest that remnant-C may be both a prognostic marker and a potential target for future therapeutic interventions.
The strengths of this study include its large population with complete follow-up of MACEs and repeat assessment of remnant-C measurements, allowing us to model long-term remnant-C level changes and assess the associations between visit-to-visit remnant-C level variability and MACEs. These advantages not only allowed us to more reliably estimate remnant-C-related risk beyond LDL-C level in patients with type 2 diabetes with high cardiovascular risk but also demonstrated that patients with type 2 diabetes with high visit-to-visit remnant-C level variability had greater risks of MACEs, suggesting this population may benefit from earlier and more frequent screening for adverse cardiovascular events, aggressive risk factor management, and maintenance of metabolic health.
This study had some limitations. First, indirect calculations of remnant-C levels might have overestimated values compared with direct measurements. Second, our findings were observational, and the causal role of remnant-C level on cardiovascular event risk should be verified in further prospective intervention studies. Third, despite adjustments for potential known confounding variables in multivariable Cox regression analysis, we cannot exclude a possible residual bias because of the post hoc nature of this study or assess all metabolic factors and parameters related to insulin resistance. Finally, the population of the ACCORD trial included only high-risk patients with type 2 diabetes. Additional studies are necessary to increase the generalizability of the results.
In type 2 diabetes, remnant-C levels are associated with MACEs regardless of LDL-C. Visit-to-visit changes in remnant-C levels can help identify patients at higher risk of CVD and may allow for the development of specific preventive and therapeutic approaches.
Article Information
Acknowledgments. The authors gratefully acknowledge the ACCORD/ACCORDION study group and the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center. The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the ACCORD/ACCORDION study authors or the National Heart, Lung, and Blood Institute.
Funding. This research was supported by the National Natural Science Foundation of China (81801394 to S.T.) and the Natural Science Foundation of Hunan Province (2019JJ50878 to S.T.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. L.F. wrote the paper. L.F. and J.S. analyzed the data. S.T., N.Z., Y.Z., Z.X., and Y.W. edited the manuscript. S.T. and S.Z. defined the study themes and methods. All authors read and approved the final manuscript and approved the manuscript for publication. S.T. and Z.X. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT00000620, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.20092712.
References
- 1. American Diabetes Association . 10. Cardiovascular disease and risk management: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021;44(Suppl. 1):S125–S150 [DOI] [PubMed] [Google Scholar]
- 2. Accord Study Group; Ginsberg HN, Elam MB, Lovato LC, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362:1563–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cosentino F, Grant PJ, Aboyans V, et al.; ESC Scientific Document Group . 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020;41:255–323 [DOI] [PubMed] [Google Scholar]
- 4. Chahil TJ, Ginsberg HN. Diabetic dyslipidemia. Endocrinol Metab Clin North Am 2006;35:491–510, vii–viii [DOI] [PubMed] [Google Scholar]
- 5. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation 2019;139:e1082–e1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Twickler TB, Dallinga-Thie GM, Cohn JS, Chapman MJ. Elevated remnant-like particle cholesterol concentration: a characteristic feature of the atherogenic lipoprotein phenotype. Circulation 2004;109:1918–1925 [DOI] [PubMed] [Google Scholar]
- 7. Fruchart JC, Sacks FM, Hermans MP, et al.; Residual Risk Reduction Initiative (R3I) . The Residual Risk Reduction Initiative: a call to action to reduce residual vascular risk in dyslipidaemic patients. Diab Vasc Dis Res 2008;5:319–335 [DOI] [PubMed] [Google Scholar]
- 8. Joshi PH, Martin SS, Blumenthal RS. The remnants of residual risk. J Am Coll Cardiol 2015;65:2276–2278 [DOI] [PubMed] [Google Scholar]
- 9. HPS2-THRIVE Collaborative Group; Landray MJ, Haynes R, Hopewell JC, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med 2014;371:203–212 [DOI] [PubMed] [Google Scholar]
- 10. AIM-HIGH Investigators; Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011;365:2255–2267 [DOI] [PubMed] [Google Scholar]
- 11. Nordestgaard BG, Benn M, Schnohr P, Tybjaerg-Hansen A. Nonfasting triglycerides and risk of myocardial infarction, ischemic heart disease, and death in men and women. JAMA 2007;298:299–308 [DOI] [PubMed] [Google Scholar]
- 12. Langsted A, Freiberg JJ, Tybjaerg-Hansen A, Schnohr P, Jensen GB, Nordestgaard BG. Nonfasting cholesterol and triglycerides and association with risk of myocardial infarction and total mortality: the Copenhagen City Heart Study with 31 years of follow-up. J Intern Med 2011;270:65–75 [DOI] [PubMed] [Google Scholar]
- 13. Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation 2009;119:931–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aday AW, Lawler PR, Cook NR, Ridker PM, Mora S, Pradhan AD. Lipoprotein particle profiles, standard lipids, and peripheral artery disease incidence. Circulation 2018;138:2330–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lawler PR, Akinkuolie AO, Chu AY, et al. Atherogenic lipoprotein determinants of cardiovascular disease and residual risk among individuals with low low-density lipoprotein cholesterol. J Am Heart Assoc 2017;6:e005549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet 2014;384:626–635 [DOI] [PubMed] [Google Scholar]
- 17. Miller YI, Choi SH, Fang L, Tsimikas S. Lipoprotein modification and macrophage uptake: role of pathologic cholesterol transport in atherogenesis. Subcell Biochem 2010;51:229–251 [DOI] [PubMed] [Google Scholar]
- 18. Fukushima H, Sugiyama S, Honda O, et al. Prognostic value of remnant-like lipoprotein particle levels in patients with coronary artery disease and type II diabetes mellitus. J Am Coll Cardiol 2004;43:2219–2224 [DOI] [PubMed] [Google Scholar]
- 19. Vallejo-Vaz AJ, Fayyad R, Boekholdt SM, et al. Triglyceride-rich lipoprotein cholesterol and risk of cardiovascular events among patients receiving statin therapy in the TNT trial. Circulation 2018;138:770–781 [DOI] [PubMed] [Google Scholar]
- 20. Jepsen AM, Langsted A, Varbo A, Bang LE, Kamstrup PR, Nordestgaard BG. Increased remnant cholesterol explains part of residual risk of all-cause mortality in 5414 patients with ischemic heart disease. Clin Chem 2016;62:593–604 [DOI] [PubMed] [Google Scholar]
- 21. Castañer O, Pintó X, Subirana I, et al. Remnant cholesterol, not LDL cholesterol, is associated with incident cardiovascular disease. J Am Coll Cardiol 2020;76:2712–2724 [DOI] [PubMed] [Google Scholar]
- 22. Quispe R, Martin SS, Michos ED, et al. Remnant cholesterol predicts cardiovascular disease beyond LDL and ApoB: a primary prevention study. Eur Heart J 2021;42:4324–4332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Keech A, Simes RJ, Barter P, et al.; FIELD study investigators . Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet 2005;366:1849–1861 [DOI] [PubMed] [Google Scholar]
- 24. Schaefer EJ, McNamara JR, Shah PK, et al.; Framingham Offspring Study . Elevated remnant-like particle cholesterol and triglyceride levels in diabetic men and women in the Framingham Offspring Study. Diabetes Care 2002;25:989–994 [DOI] [PubMed] [Google Scholar]
- 25. Jørgensen PG, Jensen MT, Biering-Sørensen T, et al. Cholesterol remnants and triglycerides are associated with decreased myocardial function in patients with type 2 diabetes. Cardiovasc Diabetol 2016;15:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 1999;22:233–240 [DOI] [PubMed] [Google Scholar]
- 27. Xu Y, Wang L, He J, et al.; 2010 China Noncommunicable Disease Surveillance Group . Prevalence and control of diabetes in Chinese adults. JAMA 2013;310:948–959 [DOI] [PubMed] [Google Scholar]
- 28. Bangalore S, Breazna A, DeMicco DA, Wun CC; TNT Steering Committee and Investigators . Visit-to-visit low-density lipoprotein cholesterol variability and risk of cardiovascular outcomes: insights from the TNT trial. J Am Coll Cardiol 2015;65:1539–1548 [DOI] [PubMed] [Google Scholar]
- 29. Barzegar N, Ramezankhani A, Tohidi M, Azizi F, Hadaegh F. Long-term glucose variability and incident cardiovascular diseases and all-cause mortality events in subjects with and without diabetes: Tehran Lipid and Glucose Study. Diabetes Res Clin Pract 2021;178:108942. [DOI] [PubMed] [Google Scholar]
- 30. Catapano AL, Graham I, De Backer G, et al.; ESC Scientific Document Group . 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999–3058 [DOI] [PubMed] [Google Scholar]
- 31. Lawler PR, Akinkuolie AO, Ridker PM, et al. Discordance between circulating atherogenic cholesterol mass and lipoprotein particle concentration in relation to future coronary events in women. Clin Chem 2017;63:870–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Després JP. Body fat distribution and risk of cardiovascular disease: an update. Circulation 2012;126:1301–1313 [DOI] [PubMed] [Google Scholar]
- 33. Donahoe SM, Stewart GC, McCabe CH, et al. Diabetes and mortality following acute coronary syndromes. JAMA 2007;298:765–775 [DOI] [PubMed] [Google Scholar]
- 34. Taskinen MR, Borén J. New insights into the pathophysiology of dyslipidemia in type 2 diabetes. Atherosclerosis 2015;239:483–495 [DOI] [PubMed] [Google Scholar]
- 35. Goldberg IJ, Eckel RH, McPherson R. Triglycerides and heart disease: still a hypothesis? Arterioscler Thromb Vasc Biol 2011;31:1716–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schwartz GG, Abt M, Bao W, et al. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol 2015;65:2267–2275 [DOI] [PubMed] [Google Scholar]
- 37. Jørgensen AB, Frikke-Schmidt R, West AS, Grande P, Nordestgaard BG, Tybjærg-Hansen A. Genetically elevated non-fasting triglycerides and calculated remnant cholesterol as causal risk factors for myocardial infarction. Eur Heart J 2013;34:1826–1833 [DOI] [PubMed] [Google Scholar]
- 38. Lévesque V, Poirier P, Després JP, Alméras N. Relation between a simple lifestyle risk score and established biological risk factors for cardiovascular disease. Am J Cardiol 2017;120:1939–1946 [DOI] [PubMed] [Google Scholar]