Abstract
OBJECTIVE
Inhibiting sodium–glucose cotransporters (SGLTs) improves glycemic and cardiovascular outcomes in patients with type 2 diabetes (T2D). We investigated the differential impact of selective SGLT2 inhibition and dual inhibition of SGLT1 and SGLT2 on multiple parameters.
RESEARCH DESIGN AND METHODS
Using a double-blind, parallel-group design, we randomized 40 patients with T2D and hypertension to receive the dual SGLT1 and SGLT2 inhibitor sotagliflozin 400 mg or the selective SGLT2 inhibitor empagliflozin 25 mg, with preexisting antihypertensive treatment, for 8 weeks. In an in-house testing site, mixed-meal tolerance tests (MMTTs) and other laboratory and clinical evaluations were used to study metabolic, intestinal, cardiovascular, and urinary parameters over 24 h.
RESULTS
Changes from baseline in glycemic and blood pressure control; intestinal, urine, and metabolic parameters; and cardiovascular biomarkers were generally similar with sotagliflozin and empagliflozin. During the breakfast MMTT, sotagliflozin significantly reduced incremental area under the curve (AUC) values for postprandial glucose, insulin, and glucose-dependent insulinotropic polypeptide (GIP) and significantly increased incremental AUCs for postprandial glucagon-like peptide 1 (GLP-1) relative to empagliflozin, consistent with sotagliflozin-mediated inhibition of intestinal SGLT1. These changes waned during lunch and dinner MMTTs. Both treatments significantly lowered GIP incremental AUCs relative to baseline over the 14 h MMTT interval; the most vigorous effect was seen with sotagliflozin soon after start of the first meal of the day. No serious or severe adverse events were observed.
CONCLUSIONS
Changes from baseline in glycemic and blood pressure control, cardiovascular biomarkers, and other parameters were comparable between sotagliflozin and empagliflozin. However, sotagliflozin but not empagliflozin inhibited intestinal SGLT1 after breakfast as shown by larger changes in postprandial glucose, insulin, GIP, and GLP-1 AUCs, particularly after breakfast. Additional study is warranted to assess the clinical relevance of transient SGLT1 inhibition and differences in incretin responses (NCT03462069).
Introduction
Pharmacological inhibition of sodium–glucose cotransporter (SGLT) 2 is an established approach to reduce hyperglycemia in patients with type 2 diabetes (T2D). Intriguingly, this class of drugs also reduces major cardiovascular adverse events and heart failure in patients with and without T2D (1). Different mechanisms have been proposed to explain these beneficial effects, including better glucose control, weight loss, lowering of arterial blood pressure (BP), increased osmotic diuresis and natriuresis, recovery of tubuloglomerular feedback, improved vascular function, and inhibition of cardiac remodeling (2). While SGLT2 is predominantly expressed in the kidney—where its inhibition leads to marked glycosuria and natriuresis—SGLT1 is primarily present in the gastrointestinal tract, where it acts as the main effector of oral glucose absorption. Dual inhibition of SGLT1 and SGLT2 should therefore offer additional advantages, at least for glucose lowering; yet, our knowledge of the extent of any additional pharmacodynamic effects is incomplete. Sotagliflozin, a dual SGLT1 and SGLT2 inhibitor approved in the European Union for use as an adjunct to insulin therapy in patients with type 1 diabetes (T1D) and a BMI ≥27 kg/m2, improves glycemic control (HbA1c) in patients with T1D and T2D, accompanied by reductions in fasting plasma glucose, postprandial glucose (PPG), and increased concentrations of plasma glucagon-like peptide 1 (GLP-1) and peptide YY (PYY) (3–7). Recently, sotagliflozin was also shown to reduce deaths from cardiovascular causes and hospitalizations and urgent visits due to heart failure in patients with T2D and worsening heart failure (8) or chronic kidney disease (9).
Sotagliflozin delays and lowers PPG excursions in patients with T2D regardless of urinary glucose excretion (UGE) or estimated glomerular filtration rate (eGFR) (7,10). Preclinical time course studies showed that after an oral glucose challenge, plasma glucose, insulin, and glucose-dependent insulinotropic polypeptide (GIP) decreased, with a concomitant increase in cecal glucose, in mice treated with sotagliflozin and mice lacking SGLT1, but not in mice lacking SGLT2. In addition, plasma GLP-1 and PYY increased, while the pH of cecal contents decreased in the sotagliflozin-treated mice and those lacking SGLT1 but not SGLT2. This decreased pH likely reflects fermentation of cecal glucose to short-chain fatty acids (SCFAs) by the gut microbiome; SCFAs are a potent stimulus for the colon to release GLP-1 (11,12). These data indicate that the changes in plasma levels of glucose, insulin, GLP-1, PYY, and GIP result from inhibition of SGLT1-mediated absorption of intestinal glucose by sotagliflozin. This conclusion is supported by the observation that sotagliflozin-mediated inhibition of intestinal glucose absorption is associated with decreased area under the curve (AUC) for plasma glucose, insulin, and GIP, and increased AUC for plasma GLP-1 and PYY, in healthy humans (13).
The delayed and blunted PPG excursions and the characteristic changes in intestinal peptide levels associated with sotagliflozin might contribute to observed improvements in glycemic and cardiovascular outcomes seen with this agent (4–9). This is particularly true of the incretin peptides GLP-1 and GIP, which were first linked to glycemic control as intestine-derived peptides that stimulated insulin secretion after nutrient intake prompts their release into the circulation; more recent work now links increased GLP-1 activity and decreased GIP activity to improved diabetes and/or cardiovascular outcomes (14–19). In the present trial, we tested this hypothesis by comparing sotagliflozin with empagliflozin, the SGLT2 inhibitor most selective for SGLT2 (20), in patients with T2D and hypertension to assess changes in multiple intestinal, metabolic, and cardiovascular parameters.
Research Design and Methods
Design Overview
This was an exploratory phase 2a, single-center, randomized, double-blind, double-dummy, active-control, parallel-group, 8 week study comparing sotagliflozin 400 mg to empagliflozin 25 mg in individuals with T2D and mild-to-moderate hypertension (ClinicalTrials.gov NCT03462069). Participants were confined at the phase 1 study unit of the Charité Research Organisation GmbH (Berlin, Germany) for 6 days at baseline and again for 6 days at the end of treatment, 8 weeks later. The study complies with the Declaration of Helsinki and was approved by the local ethics committee and regulatory authority. Written informed consent was obtained from all participants. The full clinical trial protocol is provided as a Supplementary Appendix.
Patients
We included male and female patients with T2D (diagnosed at least 1 year before the screening visit), 18 to 74 years of age, with a BMI between 18.0 and 38.0 kg/m2 inclusive, hypertension grades 1 or 2 as defined by the European Society of Hypertension/European Society of Cardiology (i.e., BP between 140/90 and 179/109 mmHg inclusive) (21), a screening HbA1c between 6.5% (48 mmol/mol) and 11.0% (97 mmol/mol), and an eGFR ≥60 mL/min/1.73 m2. Patients were required to be on stable treatment with metformin (no change in dose regimen in the 3 months prior to screening and until randomization) and either an ACE inhibitor or angiotensin II receptor blocker as monotherapy for hypertension. No other antihyperglycemic or antihypertensive agents were permitted nor were changes in the dose regimen during the 4 weeks prior to screening and until randomization. Key exclusion criteria were severe anemia, severe cardiovascular disease, stage 3 or higher chronic kidney disease, New York Heart Association stage III or IV heart failure, or a myocardial infarction within the 12 months prior to screening (see the Supplementary Material for the full list of inclusion and exclusion criteria).
Procedures
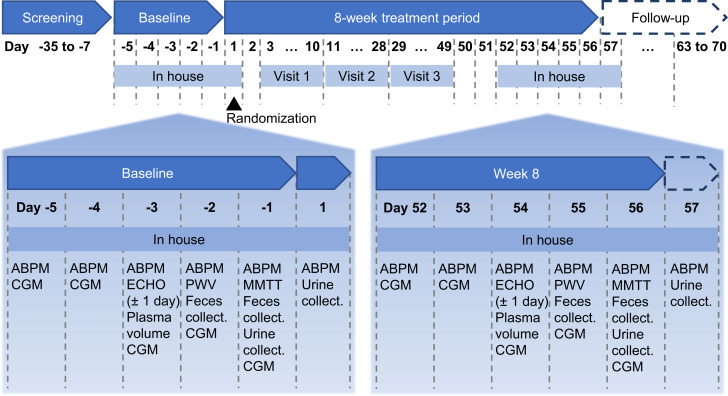
After the screening and washout period, all patients were confined at the in-house study unit for 6 days at baseline (day −5 until day 1) and at week 8 (day 52 until day 57) (Fig. 1) with identical meal schedules and constituents in both periods. Ambulatory BP monitoring (ABPM; OnTrak 90227; Spacelabs Healthcare, Snoqualmie, WA) and continuous glucose monitoring (CGM; Dexcom G4; Dexcom, Inc., San Diego, CA) were recorded throughout both in-house periods; 48 h feces and 24 h urine samples were collected twice at the end of each in-house period; and transthoracic echocardiograms (Vivid E9; GE Healthcare, Chicago, IL) and pulse wave velocity (SphygmoCor XCEL; ATCOR, Sydney, NSW, Australia) were evaluated once each during the in-house periods (see the Supplementary Material for detailed methods for all assessments). The plasma volume was estimated by means of the indocyanine method as described by Polidori and Rowley (22). On day 1, subjects were randomly assigned to sotagliflozin 400 mg (given as two 200-mg tablets) or empagliflozin 25 mg, taken once daily at 8:00 a.m. for 8 weeks. To ensure blinding, all patients took two tablets (either sotagliflozin 200 mg or placebo) plus one capsule (either empagliflozin 25 mg or placebo) in a double-blind, double-dummy design. On days −1 (baseline, before randomization) and 56 (last day of treatment), a mixed-meal tolerance test (MMTT) was performed. The MMTT consisted of a 14 h observation and blood sampling interval during which subjects ingested a standardized breakfast at 8:00 a.m., a high-calorie meal (∼1,000 kcal, 15% protein, 50% carbohydrate, and 35% fat) at 1:00 p.m. (5 h after dosing), and a standardized dinner at 6:00 p.m. Timed blood samples were obtained for the measurement of plasma glucose, insulin, intact proinsulin, C-peptide, glucagon, active GLP-1 (aGLP-1), total GLP-1 (tGLP-1), PYY, and GIP. All meals during the two in-house periods were standardized with exact matching of amount and composition.
Figure 1.
Study design, including timing of assessments taken during 6-day in-house periods at baseline and at week 8. collect., collection; ECHO, echocardiography; PWV, pulse wave velocity.
End Points
The three main pharmacodynamics end points included analyses of: 1) 24 h urine collections, including change from baseline in UGE, volume, electrolytes, calcium, magnesium, phosphate, pH, creatinine, uric acid, urea, albumin, proteins, and ketones; 2) 48 h fecal collections, including change from baseline in electrolyte, SCFA, and glucose excretion, fecal weight, fecal water, pH, bicarbonate, and Firmicutes:Bacteroidetes ratio; and 3) MMTT data, including change from baseline in absolute and incremental AUC for glucose, insulin, C-peptide, proinsulin, aGLP-1, tGLP-1, GIP, and glucagon over breakfast (0–5 h), lunch (5–10 h), dinner (10–14 h), and the entire 0–14 h interval.
Further pharmacodynamic end points included changes from baseline in 24 h systolic and diastolic BP (SBP and DBP) as measured per ABPM; seated SBP/DBP; echocardiography; pulse wave velocity; fasting blood for hematology panel and for levels of glucose, acetate, propionate, free fatty acids (FFA), β-hydroxybutyrate, total ketone bodies, plasma renin activity, angiotensin 1/2, aldosterone, N-terminal pro–B-type natriuretic peptide (NT-proBNP), copeptin, and erythropoietin; plasma volume; CGM; and self-monitored plasma glucose (SMPG).
Additional post hoc end points, analyzed using MMTT data, included 0–2 h, 0–3 h, and 0–10 h AUCs based on sotagliflozin-mediated lowering of glucose, insulin, C-peptide, and GIP levels during MMTTs in healthy adults (13), and 2-h PPG levels based on data from subjects with SGLT1 haploinsufficiency (23). See the Supplementary Material for the full list of end points as described in the study protocol.
A discrepancy exists between the detailed list of main and further end points described in the clinical trial protocol and statistical analysis plan and an abbreviated list of primary and secondary end points cited at ClinicalTrials.gov. In this article, we analyze and present all data following the main and further end points outlined in the protocol, which is available online as a Supplementary Appendix.
Statistical Methods
No formal sample size calculation was performed because this was an exploratory phase 2a study. The main analyses compared the difference between sotagliflozin and empagliflozin for change from baseline (week −1) to week 8. Continuous pharmacodynamic end points were analyzed using an ANCOVA model with treatment groups as a fixed effect and baseline value of the corresponding dependent variable as a covariate. Baseline data are reported as means with SD. Descriptive statistics on raw data and change from baseline to week 8 are provided by treatment group and time point with mean values and SEM. Changes from baseline within treatment groups are presented as least-squares (LS) means with two-sided 95% CIs for AUC results and LS means ± SE for other parameters. Treatment group comparisons are summarized as LS mean differences and two-sided 95% CIs. A two-sided P value <0.05 was considered statistically significant, either taken as the observed P value estimated from the ANCOVA model or, for some parameters, inferred from the 95% CI computed on the difference in group LS means. Because all analyses on main pharmacodynamic parameters were performed on an exploratory basis, the use of any inferential statistics (i.e., 95% CI and P values) in this study should be considered descriptive.
Results
Of 137 patients screened, 41 entered the study and were randomly assigned to sotagliflozin (n = 21) and empagliflozin (n = 20). Most excluded patients did not meet the BP inclusion criteria (triplicate BP 140/90 to 179/109 mmHg in supine position at screening despite ACE inhibitor or angiotensin II receptor blocker as monotherapy; Supplementary Table 1); one patient in each group dropped out prior to the completion of the study (Supplementary Fig. 1). The baseline demographic and clinical characteristics of the completers were comparable between the two treatment groups (Supplementary Table 2).
Glucose, Insulin, and Intestinal Peptide Profiles During MMTTs
Over 0–14 h, both sotagliflozin and empagliflozin significantly decreased incremental glucose AUCs, with no significant difference between treatment groups (Supplementary Table 3). The pattern of incremental glucose lowering was similar during the entire 0–5 h breakfast interval, with no significant difference between treatment groups; however, the 0–2 h and 0–3 h incremental glucose AUCs after breakfast revealed significantly greater decreases with sotagliflozin compared with empagliflozin (Table 1). The mean changes in 2-h PPG concentration after breakfast were −1.4 ± 0.3 mmol/L (P = 0.0001) with sotagliflozin and −0.8 ± 0.3 mmol/L (P = 0.0213) with empagliflozin, with a nonsignificant treatment difference of −0.6 mmol/L (95% CI −1.6 to 0.3).
Table 1.
Effects on incremental changes in AUC during the 5 h interval after breakfast
| Parameter | Time interval | LS mean change from baseline (95% CI); P value | LS mean difference, sotagliflozin vs. empagliflozin (95% CI); P value | |
|---|---|---|---|---|
| Sotagliflozin | Empagliflozin | |||
| Glucose, h·mmol/L | 0–2 h | −2.0 (−2.6 to −1.3); <0.0001 | −1.0 (−1.6 to −0.3); 0.0036 | −1.0 (−1.9 to −0.1); 0.0283 |
| 0–3 h | −3.2 (−4.2 to −2.2); <0.0001 | −1.7 (−2.7 to −0.7); 0.0014 | −1.5 (−2.9 to −0.06); 0.0417 | |
| 0–5 h | −4.0 (−5.5 to −2.3); <0.0001 | −2.5 (−4.0 to −0.9); 0.0028 | −1.5 (−3.7 to 0.8); NS | |
| Insulin, h·pmol/L | 0–2 h | −205 (−277 to −132); <0.0001 | −115 (−187 to −42); 0.0029 | −90 (−194 to 13); NS |
| 0–3 h | −392 (−486 to −297); <0.0001 | −213 (−308 to −118); <0.0001 | −179 (−314 to −44); 0.0111 | |
| 0–5 h | −533 (−666 to −399); <0.0001 | −280 (−414 to −147); 0.0002 | −252 (−443 to −62); 0.0109 | |
| Proinsulin, h·pmol/L | 0–2 h | −4 (−6 to −2); 0.0003 | −1 (−3 to 1); NS | −3 (−5 to −0.01); 0.0495 |
| 0–3 h | −10 (−14 to −6); <0.0001 | −4 (−7 to −0.04); 0.0475 | −6 (−11 to −1); 0.0282 | |
| 0–5 h | −18 (−27 to −8); 0.0008 | −8 (−17 to 1); NS | −9 (−23 to 4); NS | |
| C-peptide, h·nmol/L | 0–2 h | −0.4 (−0.7 to −0.2); 0.0017 | −0.1 (−0.4 to 0.1); NS | −0.3 (−0.7 to 0.06); NS |
| 0–3 h | −1.0 (−1.3 to −0.6); <0.0001 | −0.3 (−0.6 to 0.1); NS | −0.7 (−1.2 to −0.2); 0.0120 | |
| 0–5 h | −1.5 (−2.1 to −0.9); <0.0001 | −0.5 (−1.1 to 0.1); NS | −1.0 (−1.9 to −0.2); 0.0220 | |
| aGLP-1, h·ng/L | 0–2 h | 7 (3–11); 0.0010 | −4 (−8 to 1); NS | 11 (5–17); 0.0007 |
| 0–3 h | 11 (6–17); 0.0002 | −4 (−10 to 1); NS | 16 (8–23); 0.0002 | |
| 0–5 h | 16 (9–23); <0.0001 | −6 (−13 to 1); NS | 22 (12–32); <0.0001 | |
| tGLP-1, h·ng/L | 0–2 h | 32 (16–47); 0.0002 | −8 (−24 to 7); NS | 40 (18–62); 0.0007 |
| 0–3 h | 52 (31–74); <0.0001 | −13 (−34 to 8); NS | 65 (35–96); 0.0001 | |
| 0–5 h | 87 (56–117); <0.0001 | −15 (−45 to 15); NS | 102 (59–145); <0.0001 | |
| PYY, h·ng/L | 0–2 h | 15 (−8 to 38); NS | −2 (−26 to 21); NS | 17 (−16 to 50); NS |
| 0–3 h | 44 (6–81); 0.0231 | 5 (−34 to 43); NS | 39 (−15 to 93); NS | |
| 0–5 h | 96 (30 to 163); 0.0057 | 33 (−35 to 102); NS | 63 (−32 to 158); NS | |
| GIP, h·ng/L | 0–2 h | −207 (−257 to −157); <0.0001 | −70 (−120 to −20); 0.0080 | −137 (−209 to −65); 0.0005 |
| 0–3 h | −278 (−350 to −206); <0.0001 | −102 (−174 to −29); 0.0073 | −176 (−280 to −73); 0.0015 | |
| 0–5 h | −336 (−444 to −229); <0.0001 | −173 (−280 to −65); 0.0025 | −164 (−317 to −11); 0.0368 | |
Boldface text highlights statistically significant differences. NS, not significant.
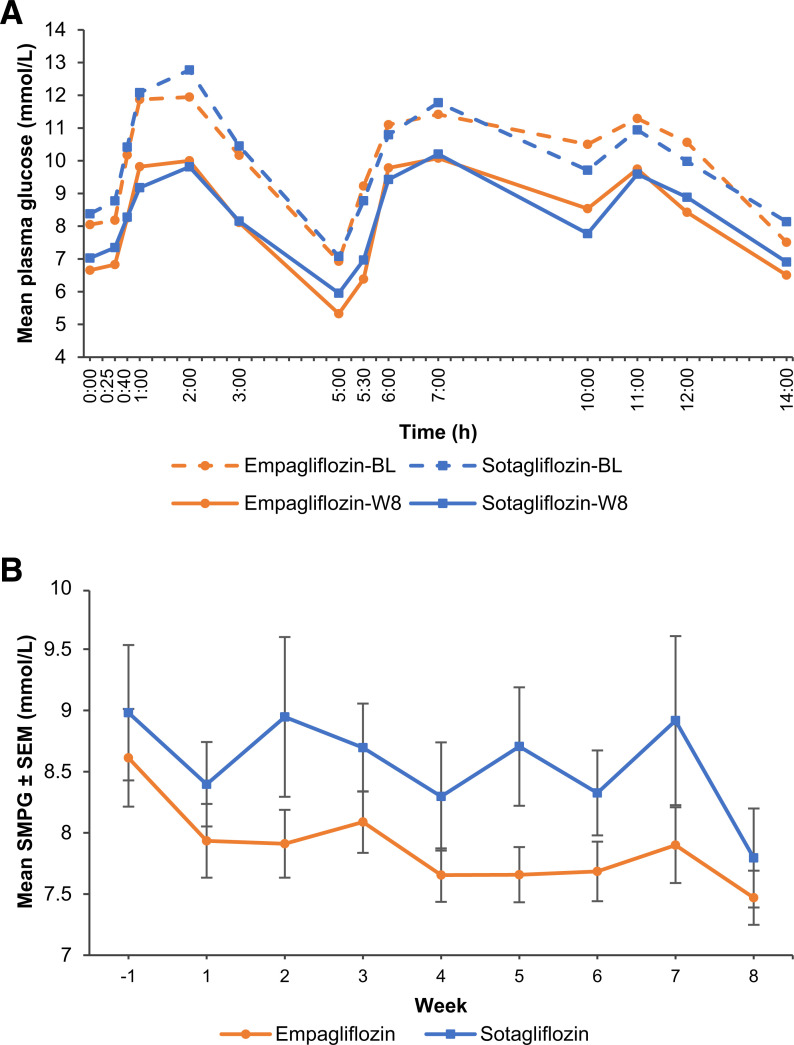
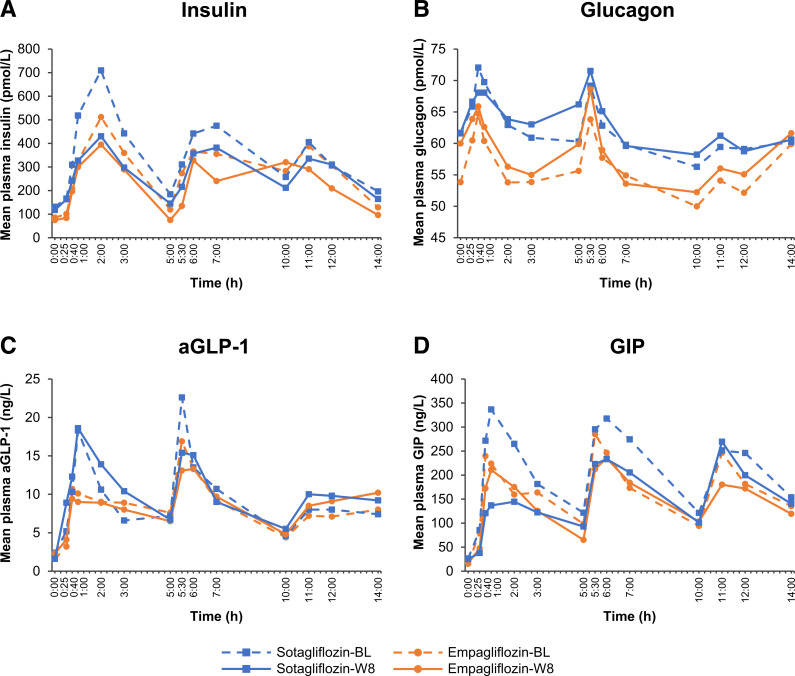
Levels of plasma glucose and insulin rose in phase over 0–14 h in the sotagliflozin and empagliflozin groups (Figs. 2A and 3A). The 14 h plasma insulin incremental and absolute AUCs decreased significantly in both groups (Supplementary Table 3), with C-peptide and proinsulin concentrations closely matching the 14-h plasma insulin pattern at baseline and week 8 and with no between-group differences in the 0–14-h AUC profiles (Supplementary Table 3). During the 0–5 h breakfast interval, incremental insulin, C-peptide, and proinsulin AUCs decreased with both treatments (Table 1 and Supplementary Table 4). For insulin and C-peptide, the decreases were significantly greater with sotagliflozin relative to empagliflozin, and additional analysis of 0–2 h and 0–3 h incremental AUC profiles revealed significantly greater decreases with sotagliflozin compared with empagliflozin for insulin, C-peptide, and proinsulin (Table 1). Plasma glucagon increased in response to all three meals, with modest increases in the glucagon responses after treatment (Fig. 3B).
Figure 2.
A: Time course of mean plasma glucose concentrations during the 14 h in-house period before and after 8 weeks of treatment with sotagliflozin 400 mg or empagliflozin 25 mg. B: Weekly mean SMPG results from baseline (week −1) to week 8. Error bars represent SEM. BL, baseline (day −1); W8, end of treatment (day 56).
Figure 3.
Time course of mean plasma insulin (A), glucagon (B), aGLP-1 (C), and GIP (D) concentrations during the 14 h in-house period before and after 8 weeks of treatment with sotagliflozin 400 mg or empagliflozin 25 mg. BL, baseline (day −1); W8, end of treatment (day 56).
Sotagliflozin, but not empagliflozin, significantly increased incremental aGLP-1, tGLP-1, and PYY AUCs during the 0–5 h breakfast interval, with between-group differences significant for aGLP-1 and tGLP-1 (Table 1 and Supplementary Table 4). Sotagliflozin, but not empagliflozin, also significantly increased incremental aGLP-1 and tGLP-1 AUCs during the entire 0–14-h interval, with between-group differences significant for aGLP-1 (Fig. 3C and Supplementary Table 3). Both sotagliflozin and empagliflozin reduced incremental GIP AUCs during the 0–5-h breakfast interval, with a significantly greater decrease associated with sotagliflozin treatment (Table 1). Absolute changes in these parameters were similar to the incremental changes (Supplementary Table 4). Both drugs also reduced incremental GIP AUCs throughout the 0–14 h study interval (Fig. 3D). Significant between-group differences in incremental GIP AUCs were observed during the 0–10 h study interval; during the full 0–14-h interval, differences from baseline in incremental GIP AUCs remained significant within each group, but the between-group differences were no longer significant (Supplementary Table 3).
Glycemic Control
Baseline HbA1c was 7.7 ± 0.8% (61 ± 8.3 mmol/mol) and 7.4 ± 0.8% (57 ± 8.2 mmol/mol) in the sotagliflozin and empagliflozin groups, respectively (Supplementary Table 2) and at 8 weeks had changed by −0.51 ± 0.11% (5.6 ± 1.2 mmol/mol) with sotagliflozin and −0.57 ± 0.10% (6.2 ± 1.1 mmol/mol) with empagliflozin (P = 0.6518 for treatment difference). Baseline mean fasting plasma glucose concentrations were 8.4 ± 1.6 mmol/L (151 ± 30 mg/dL) and 8.0 ± 1.2 mmol/L (145 ± 22 mg/dL) with sotagliflozin and empagliflozin, respectively. CGM data confirmed the 0–14-h MMTT data for glucose, showing a mean decrease in daytime glycemia of 1.2 ± 0.2 mmol/L (21 ± 4 mg/dL) and 1.6 ± 0.2 mmol/L (28 ± 4 mg/dL) with sotagliflozin and empagliflozin, respectively, with no difference between treatments (P = 0.2730). Similar data were seen for nighttime and diurnal glycemia. Weekly SMPG yielded a downward trend in mean plasma glucose levels, with no significant difference between treatments at week 8 (Fig. 2B). Time in a glucose range of 3.9–10.0 mmol/L (70–180 mg/dL) increased by 12 ± 2% and 14 ± 2% and time with glycemia >10.0 mmol/L decreased by 13 ± 2% and 15 ± 2% with sotagliflozin and empagliflozin, respectively. Treatments differences were not significant.
Levels of Additional Metabolites
Over 8 weeks of treatment, fasting plasma concentrations of propionate did not differ between treatment groups, whereas acetate decreased with sotagliflozin and increased with empagliflozin, for a difference of −11.0 μmol/L (95% CI −21.3 to −0.6; P = 0.0385) (Supplementary Table 5). The treatments similarly increased FFA (by ∼ 0.04 mmol/L), total ketones (by ∼ 249 µmol/L), and β-hydroxybutyrate (by 178 [sotagliflozin] and 203 µmol/L [empagliflozin]).
Cardiovascular System
Baseline plasma volume averaged 3.9 ± 1.0 and 4.1 ± 1.1 L in the sotagliflozin and empagliflozin groups, respectively, with no significant changes. Hematocrit rose by 1.2 ± 0.4% and 2.1 ± 0.4% and hemoglobin by 0.8 ± 1.3 and 3.6 ± 1.3 g/L with sotagliflozin and empagliflozin, respectively, without significant treatment group differences. Red blood cells and erythropoietin showed a similar pattern of changes.
Baseline BP was 142/79 and 141/82 mmHg in the sotagliflozin and empagliflozin groups, respectively, and decreased by 4.6 ± 1.5/2.3 ± 0.9 mmHg (SBP/DBP) with sotagliflozin and 7.9 ± 1.4/3.7 ± 0.9 mmHg with empagliflozin as measured with ABPM. Decreases were similar during daytime and nighttime and without significant treatment-group differences. Changes in sitting SBP and DBP values over the course of the study appear in Supplementary Fig. 2.
Circulating levels of angiotensin I (but not angiotensin II), renin activity, aldosterone, and copeptin increased similarly in the treatment groups at 8 weeks, whereas plasma NT-proBNP showed some decrease, with no difference between treatments (Supplementary Table 6). Echocardiography and pulse wave velocity assessments did not show any substantial between-group differences at baseline or end of treatment.
Urine
From a baseline of ∼ 1.7 L, 24 h urine volume changed by 200 ± 144 and 593 ± 140 mL with sotagliflozin and empagliflozin, respectively, at week 8; the between-group difference was not significant (Supplementary Table 7). The urinary glucose concentrations could not be analyzed in a significant number of samples due to a technical problem during laboratory processing, and therefore, descriptive statistics for the UGE could not be calculated.
There were no significant between-group differences in 24 h excretion of sodium, potassium, uric acid, or β-hydroxybutyrate. Daily excretion of calcium and magnesium increased with sotagliflozin, and excretion of albumin decreased with empagliflozin (Supplementary Table 7). Phosphate, chloride, pH, creatinine, total ketones, and urea excretion did not change with either drug.
eGFR
At baseline, eGFR was similar with a mean of 88 mL/min/1.73 m2 (Supplementary Table 2). As expected, there was an initial decrease in eGFR with both treatments followed by a return to baseline values after study completion (Supplementary Fig. 3).
Forty-Eight–Hour Feces
Feces were slightly acidic and ∼ 50% aqueous at baseline. At 8 weeks, fecal glucose, sodium, potassium, butyrate, propionate, and acetate values were not increased above baseline values (Supplementary Table 8). Fecal calprotectin, a protein marker of bowel inflammation, was not significantly changed by either treatment. The Firmicutes/Bacteroidetes ratio decreased significantly with both treatments.
Safety
Both drugs were generally well tolerated, with similar overall safety profiles (Supplementary Table 9). No deaths, serious or severe adverse events, or adverse events leading to treatment discontinuation occurred during the study. Gastrointestinal disorders occurred at similar rates in the sotagliflozin and empagliflozin groups, and no episodes of diarrhea or abdominal pain were reported in patients receiving sotagliflozin (Supplementary Table 9).
Conclusions
This analysis of SGLT inhibition was conducted under rigorous, standardized conditions during confinement in a designated phase 1 unit and demonstrated that sotagliflozin and empagliflozin had similar effects on most end points, including overall glycemic and BP control; selected metabolic, urinary, and intestinal parameters; and cardiovascular biomarkers. Differences were observed, however, in the glucose, insulin, and intestinal peptide responses during MMTTs, particularly after breakfast. Past studies in mice and humans showed that, after a glucose-containing meal, sotagliflozin inhibition of SGLT1-mediated intestinal glucose absorption was accompanied by decreased PPG, insulin, C-peptide, and GIP AUCs and by increased postprandial GLP-1 and PYY AUCs (7,13,24). Each of these findings is reproduced in this study by sotagliflozin during the 5 h postbreakfast interval (Table 1), indicating that sotagliflozin inhibited SGLT1-mediated intestinal glucose absorption after breakfast. In contrast, empagliflozin had no effect on incremental C-peptide, GLP-1, or PYY AUCs after breakfast, and its effects on incremental glucose, insulin, C-peptide, GLP-1, and GIP AUCs were significantly smaller than those of sotagliflozin, confirming the mechanistic differences between the dual SGLT1 and SGLT2 inhibitory effects of sotagliflozin and the action of empagliflozin as a highly selective SGLT2 inhibitor.
This study recapitulates many of the previously reported short-term responses to SGLT inhibition (25). Thus, SGLT inhibition caused marked glycosuria with the attendant osmotic diuresis, natriuresis (probably transient) (7,26), a small contraction of plasma volume with a concomitant increase in hematocrit and hemoglobin—presumably the result of a rise in erythropoietin (26)—a decrease in BP (accompanied by the typical initial, temporary decrease in eGFR), a decrease in glycemia and HbA1c, a decrement in plasma insulin (and proinsulin), small increments in glucagon and GLP-1, and a decrease in GIP. From the metabolic angle, the drug-induced deficit in glucose availability was balanced by a rise in circulating FFA, in turn causing increased ketonemia and ketonuria. On the vascular side, the increased transfer of sodium beyond the proximal nephron following SGLT inhibition triggered some activation of the renin-angiotensin-aldosterone axis, and circulating NT-proBNP declined (27). These hemodynamic and hormonal changes had no detectable influence on a range of indices of cardiac function at week 8, very likely because baseline cardiac function was normal in our participants, and treatment duration was short.
Recent evidence suggests that, in humans, SGLT1 haploinsufficiency may be beneficial; 2 h PPG concentration was used as a biomarker of SGLT1 inhibition in these individuals to show that, over 25 years, a 1.1 mmol/L (20 mg/dL) decrease significantly reduced the prevalence of a number of adverse outcomes, including heart failure (23). In individuals with T2D studied in this article and previously (7), sotagliflozin reached this 1.1 mmol/L 2 h PPG benchmark, with a decrease of 1.4 mmol/L (25.2 mg/dL) in this study. For comparison, empagliflozin lowered PPG by 0.8 mmol/L (14.4 mg/dL) 2 h after breakfast in this study. Although the effect of sotagliflozin on PPG and many of the other outcomes that result from inhibiting SGLT1-mediated intestinal glucose absorption may be less pronounced after meals later in the day, sotagliflozin-mediated lowering of GIP levels was strong and prolonged through the lunch meal in a previous study (13), consistent with the finding in this study that GIP levels continued to fall during the 5–10-h interval after lunch and remained significantly lower over the 0–14 h study interval. A smaller but still significant GIP decrease over 0–14 h was also observed for empagliflozin. Recent studies suggest that decreased GIP receptor (GIPR) activity, either by inactivating the GIPR in mice or through decreased GIP levels in humans, is cardioprotective, possibly mediated by a decrease in inflammation that was not evaluated in the current study (14–16). Although this hypothesis seems incompatible with the development of GLP-1/GIP co-agonists to treat T2D and obesity, recognition that GIPR antagonism may also be metabolically beneficial suggests the unifying mechanism that pharmacologic doses of GIPR agonists work by desensitizing the GIPR. Thus, it is a decrease, not an increase, in GIPR signaling that is beneficial (17–19). Additional studies are required to determine whether, and to what extent, sotagliflozin-mediated SGLT1 inhibition, and the associated lowering of GIP levels, contribute to the improved cardiovascular outcomes in individuals with worsening heart failure and to the decrease in major adverse cardiovascular events in individuals with T2D and chronic kidney disease (8,9), and whether lower GIP levels contribute to the improved cardiovascular outcomes associated with empagliflozin treatment (1).
Sotagliflozin treatment did not increase fecal levels of either glucose or the SCFAs propionate, butyrate, or acetate, which are terminal products of glucose fermentation in the colon, and also did not increase fasting plasma SCFA levels. Thus, the hypothesis that dual SGLT1 and SGLT2 inhibition would offer an advantage over selective SGLT2 inhibition by retarding intestinal glucose absorption in addition to causing glycosuria is not supported by these results. However, after a glucose-containing meal, analysis of intestinal contents in sotagliflozin-treated mice showed that increased intestinal glucose and SCFA levels were transient postprandial events that likely modulated release of intestinal peptides (11,12), and analysis of intestinal glucose absorption in humans showed that an initial significant delay in glucose absorption was followed by complete absorption of ingested glucose by 5 h after the meal (13). These findings suggest that analyzing glucose or SCFAs in 48 h stool samples and SCFA in fasting plasma samples may not be sensitive enough to detect sotagliflozin-mediated inhibition of intestinal glucose absorption. In previous clinical trials, sotagliflozin treatment has been associated with a modest but significant increase in diarrhea (9), which may be due to enhanced carbohydrate fermentation by the gut microbiome, but none of the sotagliflozin-treated patients examined in this study reported diarrhea or abdominal pain, precluding closer examination of their data for more exaggerated changes in stool glucose or SCFA levels.
When contrasting sotagliflozin with empagliflozin, some differences did emerge, although the small sample size in this study was not powered to show statistical significance. Changes from baseline in urine volume, plasma volume, and hematocrit were numerically larger with empagliflozin, but only the change in hematocrit achieved statistical significance. In contrast, urinary excretion of albumin, calcium, and magnesium were significantly higher with sotagliflozin, but these results are not consistent with past data showing that sotagliflozin had no effect relative to placebo on urinary excretion of these analytes in patients with T2D and normal kidney function (7). Overall, the above differences were small and of uncertain clinical significance. As mentioned above, possible differences in UGE could not be elucidated due to laboratory failure. Previous clinical data indicate that sotagliflozin overall shows a lower UGE than selective SGLT2 inhibitors but reaches the same level of improved glycemic control by being founded on more than one mechanistic pillar (13).
A key strength of this phase 2a study was that clinical end points were assessed under rigorously controlled and standardized conditions. However, it is possible that diverse patient phenotypes, longer treatment duration, or combinations thereof might amplify the small drug response differences observed in this study. However, this seems unlikely because: 1) the patients enrolled in this study were fairly typical—by age, duration of diabetes, degree of glycemic control, background antidiabetic therapy, and complications—of the population with T2D at large; 2) an appropriate comparator, empagliflozin, the most selective SGLT2 inhibitor, was randomized into the experiment; 3) the study duration was sufficient to induce the typical decrement in HbA1c (1); and 4) several key tests were performed both at baseline and study end during in-house periods.
In summary, sotagliflozin and empagliflozin did not show any major differences in overall glycemic and BP control or in selected metabolic, urinary, and intestinal parameters. In contrast, mechanistic differences were confirmed. Inhibition of SGLT1 by sotagliflozin was accompanied by prolonged lowering of plasma GIP levels as well as a postprandial increase in aGLP-1. For the most part, the current study was unable to identify obvious features that would convincingly relate to a different clinical impact of dual versus single SGLT inhibition in patients with T2D, but the potential benefit of lower GIP levels on cardiovascular outcomes suggests the need for additional studies to determine if prolonged sotagliflozin-mediated lowering of plasma GIP levels contributes to improved cardiovascular outcomes observed with this drug.
Article Information
Acknowledgments. The authors thank the study participants and study staff at Charité Research Organisation GmbH. The authors also thank Michael Davies (Lexicon Pharmaceuticals, Inc.) for critically reviewing the manuscript and Amanda M. Justice (independent consultant, Brooklyn, NY) for editorial support, which was funded by Lexicon Pharmaceuticals, Inc.
Funding. This study was cosponsored by Lexicon Pharmaceuticals, Inc., and Sanofi.
Duality of Interest. M.G.P., and N.W. were employed by Charité Research Organisation GmbH at the time the study was conducted. M.G.P. is now employed at SCIRENT Clinical Research and Science. E.F. has served as an advisory board member/consultant for Boehringer Ingelheim/Eli Lilly and Company and Sanofi and has received research grants from AstraZeneca, Boehringer Ingelheim, and Janssen. D.R.P., P.B., and S.W. are employed by Lexicon Pharmaceuticals, Inc., and R.D. was employed by Sanofi at the time the study was conducted and is now an employee at Bayer AG.
Author Contributions. E.F., S.W., and R.D. were involved in the study conception, design, and/or protocol review. The statistical analysis was designed by P.B., and R.D. M.G.P., N.W., and R.D. conducted the study and collected the data. Results were interpreted by E.F., D.R.P., P.B., S.W., and R.D. M.G.P., E.F., and R.D. wrote and significantly revised the manuscript. All authors critically reviewed the manuscript and provided substantive comments. S.W. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT03462069, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.20069063.
References
- 1. Seferović PM, Fragasso G, Petrie M, et al. Sodium-glucose co-transporter 2 inhibitors in heart failure: beyond glycaemic control. A position paper of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020;22:1495–1503 [DOI] [PubMed] [Google Scholar]
- 2. Lopaschuk GD, Verma S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (SGLT2) inhibitors: a state-of-the-art review. JACC Basic Transl Sci 2020;5:632–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cariou B, Charbonnel B. Sotagliflozin as a potential treatment for type 2 diabetes mellitus. Expert Opin Investig Drugs 2015;24:1647–1656 [DOI] [PubMed] [Google Scholar]
- 4. Buse JB, Garg SK, Rosenstock J, et al. Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the North American inTandem1 Study. Diabetes Care 2018;41:1970–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Danne T, Cariou B, Banks P, et al. HbA1c and hypoglycemia reduction at 24 and 52 weeks with sotagliflozin in combination with insulin in adults with type 1 diabetes: the European inTandem2 study. Diabetes Care 2018;41:1981–1990 [DOI] [PubMed] [Google Scholar]
- 6. Garg SK, Henry RR, Banks P, et al. Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med 2017;377:2337–2348 [DOI] [PubMed] [Google Scholar]
- 7. Zambrowicz B, Freiman J, Brown PM, et al. LX4211, a dual SGLT1/SGLT2 inhibitor, improved glycemic control in patients with type 2 diabetes in a randomized, placebo-controlled trial. Clin Pharmacol Ther 2012;92:158–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bhatt DL, Szarek M, Steg PG, et al.; SOLOIST-WHF Trial Investigators . Sotagliflozin in patients with diabetes and recent worsening heart failure. N Engl J Med 2021;384:117–128 [DOI] [PubMed] [Google Scholar]
- 9. Bhatt DL, Szarek M, Pitt B, et al.; SCORED Investigators . Sotagliflozin in patients with diabetes and chronic kidney disease. N Engl J Med 2021;384:129–139 [DOI] [PubMed] [Google Scholar]
- 10. Zambrowicz B, Lapuerta P, Strumph P, et al. LX4211 therapy reduces postprandial glucose levels in patients with type 2 diabetes mellitus and renal impairment despite low urinary glucose excretion. Clin Ther 2015;37:71–82.e12 [DOI] [PubMed] [Google Scholar]
- 11. Powell DR, Smith M, Greer J, et al. LX4211 increases serum glucagon-like peptide 1 and peptide YY levels by reducing sodium/glucose cotransporter 1 (SGLT1)-mediated absorption of intestinal glucose. J Pharmacol Exp Ther 2013;345:250–259 [DOI] [PubMed] [Google Scholar]
- 12. Powell DR, DaCosta CM, Smith M, et al. Effect of LX4211 on glucose homeostasis and body composition in preclinical models. J Pharmacol Exp Ther 2014;350:232–242 [DOI] [PubMed] [Google Scholar]
- 13. Powell DR, Zambrowicz B, Morrow L, et al. Sotagliflozin decreases postprandial glucose and insulin concentrations by delaying intestinal glucose absorption. J Clin Endocrinol Metab 2020;105:e1235–e1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jujić A, Atabaki-Pasdar N, Nilsson PM, et al. Glucose-dependent insulinotropic peptide and risk of cardiovascular events and mortality: a prospective study. Diabetologia 2020;63:1043–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berglund LM, Lyssenko V, Ladenvall C, et al. Glucose-dependent insulinotropic polypeptide stimulates osteopontin expression in the vasculature via endothelin-1 and CREB. Diabetes 2016;65:239–254 [DOI] [PubMed] [Google Scholar]
- 16. Ussher JR, Campbell JE, Mulvihill EE, et al. Inactivation of the glucose-dependent insulinotropic polypeptide receptor improves outcomes following experimental myocardial infarction. Cell Metab 2018;27:450–460.e6 [DOI] [PubMed] [Google Scholar]
- 17. Baggio LL, Drucker DJ. Glucagon-like peptide-1 receptor co-agonists for treating metabolic disease. Mol Metab 2021;46:101090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Holst JJ, Rosenkilde MM. GIP as a therapeutic target in diabetes and obesity: insight from incretin co-agonists. J Clin Endocrinol Metab 2020;105:e2710–e2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Killion EA, Lu SC, Fort M, Yamada Y, Véniant MM, Lloyd DJ. Glucose-dependent insulinotropic polypeptide receptor therapies for the treatment of obesity, do agonists = antagonists? Endocr Rev 2020;41:bnz002. [DOI] [PubMed] [Google Scholar]
- 20. Mudaliar S, Polidori D, Zambrowicz B, Henry RR. Sodium-glucose cotransporter inhibitors: effects on renal and intestinal glucose transport: from bench to bedside. Diabetes Care 2015;38:2344–2353 [DOI] [PubMed] [Google Scholar]
- 21. Williams B, Mancia G, Spiering W, et al.; ESC Scientific Document Group . 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J 2018;39:3021–3104 [DOI] [PubMed] [Google Scholar]
- 22. Polidori D, Rowley C. Optimal back-extrapolation method for estimating plasma volume in humans using the indocyanine green dilution method. Theor Biol Med Model 2014;11:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seidelmann SB, Feofanova E, Yu B, et al. Genetic variants in SGLT1, glucose tolerance, and cardiometabolic risk. J Am Coll Cardiol 2018;72:1763–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Powell DR, DaCosta CM, Gay J, et al. Improved glycemic control in mice lacking Sglt1 and Sglt2. Am J Physiol Endocrinol Metab 2013;304:E117–E130 [DOI] [PubMed] [Google Scholar]
- 25. Ferrannini E. Sodium-glucose co-transporters and their inhibition: clinical physiology. Cell Metab 2017;26:27–38 [DOI] [PubMed] [Google Scholar]
- 26. Ferrannini E, Baldi S, Frascerra S, et al. Renal handling of ketones in response to sodium-glucose cotransporter 2 inhibition in patients with type 2 diabetes. Diabetes Care 2017;40:771–776 [DOI] [PubMed] [Google Scholar]
- 27. Thomas MC, Cherney DZI. The actions of SGLT2 inhibitors on metabolism, renal function and blood pressure. Diabetologia 2018;61:2098–2107 [DOI] [PubMed] [Google Scholar]