Abstract
Fear memories are important for survival and are implicated in the etiology of fear disorders such as Post Traumatic Stress Disorder (PTSD). Fear memories are well studied pre-clinically and sex differences in rodent fear expression have been reported: females tend to freeze less than males. Whether this is a difference in fear learning or expression is debated. We aimed to differentiate between these possibilities with a task that allowed female rats to express fear memory by moving, rather than freezing. We assessed fear extinction after contextual fear conditioning in the isolated Shock Arm of a Y-maze in female and male rats by either placing them back in the isolated Shock Arm (Fear Extinction in the Shock Context) or allowing them to move freely in the Y-maze during extinction training and enter/avoid the Shock Arm (Avoidance Extinction). We confirmed that female rats freeze less than males during fear extinction in both settings. During Avoidance Extinction, however, both sexes had similar avoidance of the Shock Context, showing comparable fear memory and extinction. Additionally, female rats made more entries into the non-shock arms. Thus, female and male rats have similar fear learning but females express it with an active motor response. Furthermore, female rats also exhibited an active motor response under other anxiogenic conditions (Elevated Plus Maze) and had higher reactivity (Acoustic Startle Response) but not when fear-eliciting stimuli were present: cat hair and foot-shock. In summary, female rats have an active motor response to anxiogenic stimuli which we termed ‘Anxioescapic’ behavior strategy.
Keywords: avoidance extinction, sex differences, rats, fear extinction, PTSD, anxiety
BACKGROUND
Fear memories play an important biological role in survival and have been the subject of intense investigation in humans and rodents (Fiorenza et al., 2011; Izquierdo et al., 2016; Maren, 2001; Myers and Davis, 2006; VanElzakker et al., 2014). During learning, the high emotional arousal makes fear memories long-lasting (Cahill & McGaugh, 1998; McGaugh, 2003, 2004; Quevedo et al., 2003). Dysregulation in the brain systems underlying fear memories can result in pathological conditions such as PTSD. In PTSD, a traumatic episodic memory is encoded while the individual is in a highly fearful state and results in long-lasting alterations in mood and behavior (Izquierdo et al., 2016; Milad et al., 2008; Rougemont-Bucking et al., 2011; VanElzakker et al., 2014). PTSD is often treatment-resistant when fear memories become so indelibly ingrained in the brain (James, 1890) that they impair the ability to form new memories of safety associated with the same contextual and sensory cues (Fiorenza et al., 2011; Myers and Davis, 2006).
To understand the mechanisms and behavioral implications of fear memories, researchers use fear conditioning and fear extinction in rodent models. Fear conditioning is the pairing of a neutral stimulus, such as a tone or light in cued fear conditioning or a spatial context in contextual fear conditioning (CFC), with an unconditioned aversive stimulus such as a foot-shock. The neutral stimulus then becomes associated with the unconditioned stimulus to signify the fearful experience (Maren, 2001; Pavlov, 1927). Fear extinction occurs when the rodent is presented with the cue or context in the absence of the aversive stimulus. The rodent learns, over repeated exposures, that the cue previously associated with the aversive stimulus no longer predicts the fearful experience (Myers and Davis, 2006). Fear extinction is typically demonstrated as a reduction in fear responses over time.
Fear responses in rodents are typically measured as freezing, which is an active suppression of movement except breathing (Bolles & Collier, 1976), or avoidance of a fear conditioned stimulus. Avoidance can be either ‘active avoidance,’ moving away from an expected fear-ful/aversive stimulus, or ‘inhibitory avoidance,’ choosing not to enter a zone known to present a fearful/aversive stimulus (Izquierdo et al., 2016).
An important consideration for measuring fear in rodents is the existence of sex differences in the expression of fear across multiple paradigms (Adamec et al., 2006; Blanchard & Blanchard, 1969; Cohen & Yehuda, 2011; Deslauriers et al., 2018; Faraday, 2002; Graham et al., 2009; Pineles et al., 2017). It is well documented that female rats tend to freeze less than their male counterparts (Gruene et al., 2015; Inslicht et al., 2013; Milad et al., 2006; Wilson et al., 2013). Low freezing can be interpreted to mean poor learning of the fearful event/fear conditioning (Glover et al., 2015; Jean-Richard-Dit-Bressel et al., 2018). An alternative explanation is that female rats are engaging in a different strategy. Others have shown that female rats engage in different strategies such as darting behavior in response to cued fear conditioning (Chowdhury et al., 2019; Gruene et al., 2015; Shansky, 2015). Even in cases where they are making choices about risk mitigation, female rats engage more in an active motor response to looming threats (Pellman et al., 2017). Therefore, freezing may not be the best measure to assess fear extinction and learning of safety in female rats (Alexander et al., 2020).
These differences are paralleled in the clinical presentation of PTSD as a sexually dimorphic condition with differences in incidence (women suffer with PTSD at twice the rate of men), presentation and treatment efficacy (Christiansen & Elklit, 2012; Dalla et al., 2008; Gamwell et al., 2015; Garza & Jovanovic, 2017; Haskell et al., 2010; Kessler et al., 1995; Meyer et al., 2018).
Navigating the differences in expression of fear behavior between female and male rats makes it difficult for researchers to investigate sex differences in the context of fear learning, memory, extinction, and PTSD. Therefore, new insights and tools to measure them are needed.
Here we tested the hypothesis that female rats have an active motor response during fear extinction. We suggested that a test that allows female rats to make choices with their movement, such as active avoidance, will be a suitable indicator of fear memory and extinction for female and male rats alike (Alexander et al., 2020). Because of documented influence of gonadal hormones on freezing (Milad et al., 2006), we allowed our female rats to cycle naturally and assessed estrous cycle phases during behavior.
EXPERIMENTAL PROCEDURES
Animals and handling
Young adult (2 months old) female (175–200 g) and male (250–300 g) Sprague-Dawley rats (Charles River Laboratories Inc, MA) were housed in pairs (females and males housed separately and in different rooms) on a 12 hr light/dark cycle (lights on at 7:00 am) with food and water freely available. Experiments were conducted during the light phase between 8 am and 5 pm. The total number of rats for Experiment 1 was: Females (n = 23), Males (n = 24), and for Experiment 2 Females (n = 18) and Males (n = 23). The number of rats per group is listed in each figure. All rodents were handled for 2–3 minutes for three consecutive days, starting three days after arrival. Behavioral testing began after handling. Female rats were habituated to vaginal lavage daily during handling (described below). All behavioral procedures were approved by the IACUC at the CNVAMC.
Behavioral tests:
All behavior was video recorded for later analysis. Freezing was scored from video, while the number of Entries into each arm, Crossings in the Shock Arm, and Time Spent in each arm were scored during the experiment. Scoring was done by observers “blinded” to the experimental assignment of each animal. The experimental design for Experiments 1 & 2 is illustrated in Fig. 1B.
Fig. 1.
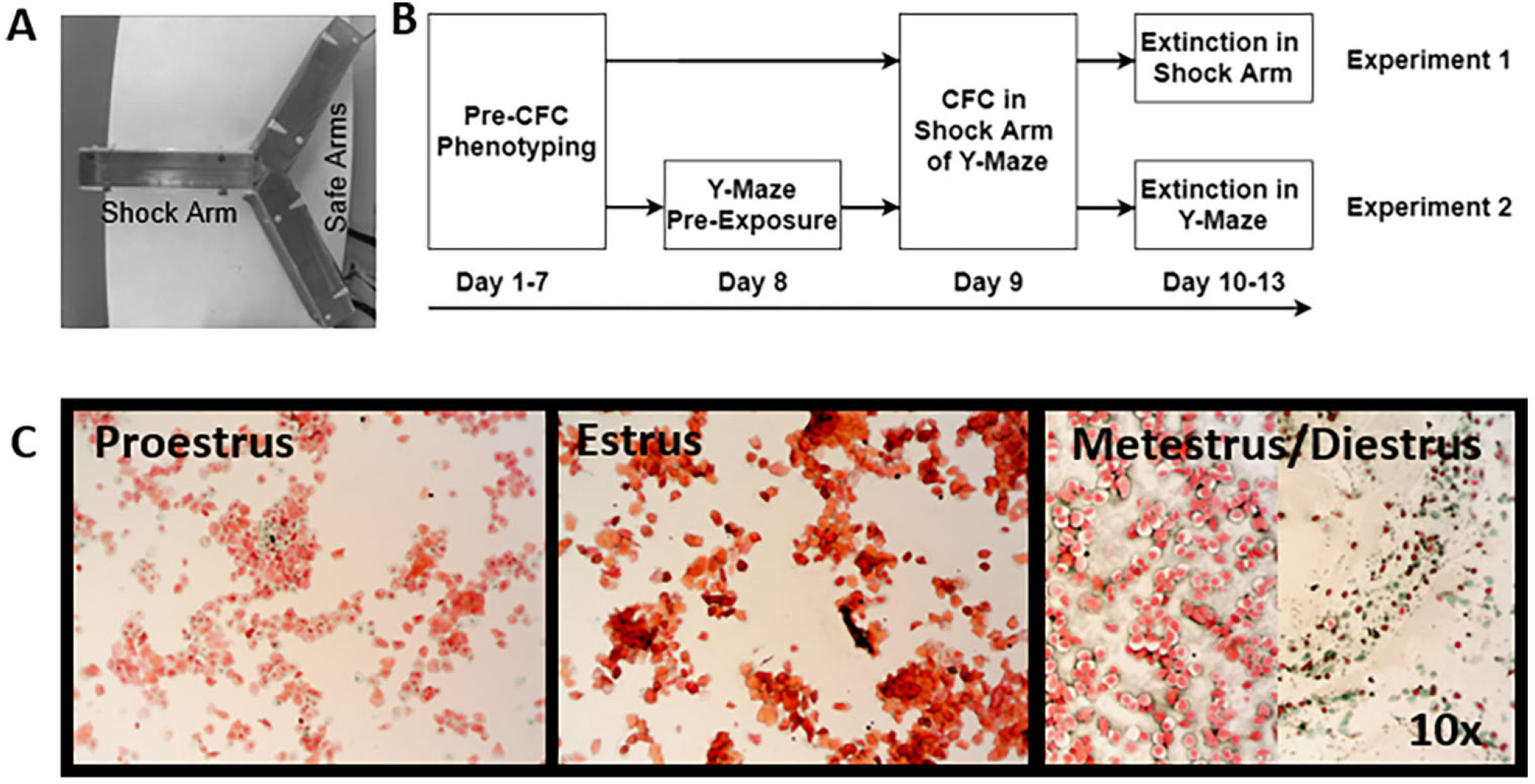
Experimental Design. (A) Picture of the Y-maze with Shock and Safe Arms. (B) Behavioral experimental design. (C) Representative images of estrous samples at 10× magnification.
Pre-contextual fear conditioning (pre-CFC) phenotyping
Cat hair exposure (CH).
Animals were exposed to a ball of cat hair (~10 cm) for 3 minutes obtained from a pathogen-free male cat and infused with ~150ul of cat urine in a 32 cm × 32 cm × 50 cm box. We have previously shown that such exposure elicits unconditioned fear responses but does not induce CFC (Nalloor et al., 2011, 2014; Vazdarjanova et al., 2001). Cat hair interactions were scored when the animal’s head was within 1 cm of, or front paws were in physical contact with, the ball of cat hair. The number of Entries into each quadrant of the box were recorded as an indicator of overall activity. The number of fecal boli and the presence of urine were recorded as an indicator of autonomic fear response.
Elevated plus maze (EPM).
We measured anxiety-like behavior on the EPM 5 days after Cat Hair exposure as we have seen from previous experiments that this is sufficient time for the stress response elicited by the cat hair exposure to subside (Nalloor et al., 2011). The EPM is plus-shaped with four 50 cm × 12 cm arms, elevated 84 cm above the floor. Two opposite arms are surrounded by 46 cm tall opaque black walls on three sides, and the other two are open, except for a 1 cm high ledge (Kinder Scientific, San Diego, CA). Each animal was introduced into the center area (10 cm × 10 cm) facing an Open Arm and allowed to explore freely for 5 min. Time spent in the Open arms, Closed arms, and the Center was scored. An Arm Entry was scored when all four paws and the base of the animal’s tail entered an arm. Because anxiolytics increase entries into the Open arms, more Entries into the Open arms indicates lower anxiety-like behavior (Hogg, 1996). Risk assessment into Open arms, another anxiety-related measure, was scored when the front paws and head of a rat were in an arm for more than 1 second, without the rat entering the arm.
Acoustic startle response (ASR).
The day after EPM, we tested their ASR in a sound-attenuated startle chamber (Kinder Scientific, San Diego, CA) equipped with a confining enclosure. Fifteen, 120db acoustic bursts, 40 ms each, were delivered at random intervals (between 30–45 s). ASR is measured in Newtons and indicates reactive force.
Contextual fear conditioning (CFC) training
CFC was done in the isolated Shock Arm of a Y-maze as previously described (Vazdarjanova & McGaugh, 1998). The apparatus consists of three identically shaped arms (50 cm × 20 cm × 12 cm), separated by 120° (Fig. 1A) and covered with translucent Plexiglass lids. One arm of the Y-maze (henceforth referred to as Shock Arm) was fitted with stainless steel walls and floor plates separated by a 1 cm gap in the floor through which a foot-shock can be administered and a removable wall that allows this arm to be isolated from the other arms. All arms had distinct visual cues. After 3 minutes of habituation, Shock groups received two foot-shocks (1 mA ac, 1 sec) delivered at 30-second intervals. Ninety seconds after the second foot-shock, the rats were returned to their home cages. The time spent Freezing, a measure of fear behavior, and the Number of Crossings defined as all four paws and the base of the tail crossing over the midline of the Shock Arm, a measure of locomotor activity, in the last minute were scored.
Fear extinction in the shock context (Experiment 1)
One day after CFC, fear extinction was performed by reintroducing each animal into the isolated Shock Arm for 5 min per day for four consecutive days, without foot-shocks. Freezing and the Number of Crossings were scored (Fig. 1B).
Avoidance fear extinction (Experiment 2)
Rats were allowed to explore the entire Y-maze for 5 minutes on the day before CFC training to habituate them to the maze and assess whether they have a natural aversion/preference to any of the arms. They were started in the Shock Arm and Freezing, Time Spent per arm, and the number of Arm Entries were scored. During the training day, the Shock groups received CFC, while those in the No-Shock groups underwent the same procedures, but no foot-shocks were delivered (Fig. 1B).
One day after CFC, fear extinction was performed in the Y-maze with access to all arms. Rats were reintroduced into one of the Safe Arms of the Y-maze (alternating arms each day) for 5 min per day for four consecutive days, without foot-shocks. Freezing, Time Spent in each arm, and the Number of Arm Entries were scored. Avoidance Extinction is defined as more Entries or more Time Spent in the Shock Arm over days of extinction.
Estrous sampling and analysis
To gain insight into the natural behavior of Female rats and the potential role of Estrous cycle, we allowed Female rats to cycle naturally in both experiments. Consequently, they were in different estrous phases on different days of testing, therefore sample numbers vary among tests.
Female rats were habituated to lavage procedures during handling. Briefly, a pipette with 200ul of dH20 (~37 °C) was placed at the opening of the rat’s vaginal canal, and the water was pipetted in and out a few times while the rat was lightly restrained by swaddling in a towel. Great care was taken to ensure that estrous sampling was minimally stressful to the rats. Estrous samples were acquired from female rats daily during habituation and testing. Samples were placed on slides and stained with Shorr stain (MilliporeSigma, Burlington, MA). Briefly, slides were washed in 100 % MeOH for 3 minutes, then 1× PBS for 1 minute, followed by hematoxylin stain (Ricca, Arlington, TX) for 30 seconds. Slides were then washed in dH20 then 100 % EtOH for 1 minute. They were then placed in Shorr stain for 3 minutes, washed in 100 % EtOH for 1 minute, and finally in Histo-Clear Il (National Diagnostics, Atlanta, GA) for 30 seconds. Classification of different estrous cycle stages was done by examining the slides under a standard light microscope (10×) by two independent observers using a cytological guide (Paccola et al., 2013). Representative images taken at 10× magnification are shown in Fig. 1C. Any samples of poor quality with too few cells for agreement between observers were excluded from the analysis. Samples from Experiment 1 could not be reliably classified and were excluded. Therefore, estrous data is present only for animals in Experiment 2.
The estrous cycle in rats is documented to be a 3 to 5 day cycle consisting of 4 phases: Proestrus, Estrus, Metestrus, and Diestrus (Ajayi & Akhigbe, 2020). To account for known effects of estrous cycle (Milad et al., 2009), we grouped them as Metestrus and Diestrus (M/D), Proestrus (P) and Estrus (E).
Statistics
Unpaired t-tests were used to compare Female and Male rats on Cat Hair and EPM parameters and comparisons at single time points during Extinction. Two-Way ANOVA with factors Sex and Shock condition was used for comparisons in Experiment 2 for single time points. Mixed design RM-ANOVA with factor Sex, Estrous phase, or Shock condition and repeated factor Days of Extinction or ASR trials was used to compare differences in ASR and Extinction parameters (Freezing, Crossings, Time in Shock Arm, Total Arm Entries, and Entries in Shock Arm.) One-Way repeated measures ANOVA were used when significant overall effects in the mixed design RM-ANOVA were observed to evaluate habituation or extinction for each Sex, Estrous phase, or Shock condition. Fisher’s post hoc test was used for individual group comparisons after overall effects were significant. All data sets passed Kolmogorov-Smirnov Test of Normality.
Differences were considered statistically significant at p < 0.05 (StatView software, SAS Institute, Cary, NC), data was visualized with PRISM (GraphPad Prism version 8.0.0 for Windows, GraphPad Software, San Diego, CA).
RESULTS
Female rats show unconditioned fear responses similar to Male rats but have an active motor response in anxiogenic situations
During cat hair exposure, a measure of unconditioned fear responses, Female and Male rats show no difference in activity as measured by the total number of entries (Total Entries) into all quadrants of the small box (p = 0.7424) (Fig. 2A). There were also no differences in the Number of Interactions with the cat hair (p = 0.6088), indicating similar unconditioned fear responses between Female and Male rats. No difference in fecal boli (Mean Female = 2.5, Male = 2.8, p = 0.4827), an indicator of the autonomic stress response (Bailey & Crawley, 2009; Hall, 1934), suggests that the cat hair exposure was equally stressful to both Female and Male rats (Fig. 2A).
Fig. 2.
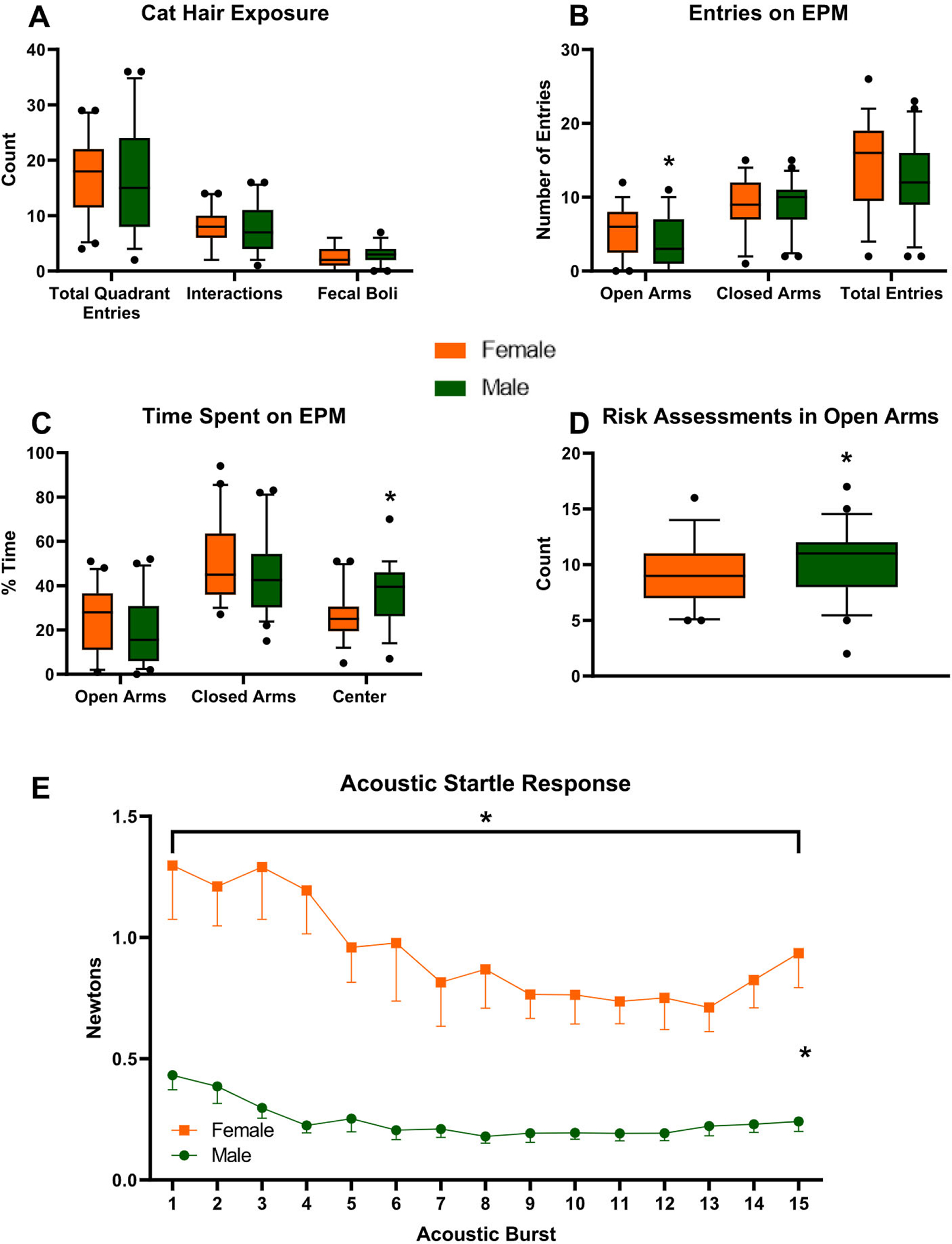
Phenotypical differences between Female and Male rats pre-CFC. (Female n = 41, Male n = 47). (A) Unconditioned fear behavior during Cat Hair Exposure. Number of Total Quadrant Entries in the Cat Hair box, number of Interactions with the cat hair ball, and the number of fecal boli present after exploration. (B) Number of Entries in the Open and Closed Arms of the Elevated Plus Maze. (C) Percent Time Spent in the Open and Closed arm and the Center of the Elevated Plus Maze. (D) Risk Assessments on the Elevated Plus Maze. (E) Acoustic Startle Response (Female n = 23, Male n = 24 for ASR only). All box plots include the median (line), 25–75 quartiles (box), and 5–95 percentiles (whisker), data points outside of the 5–95 percentile are represented with dots. *p < 0.05.
There was no effect of Estrous on Quadrant Entries in the cat hair exposure box [F(2,15) = 0.264, p = 0.6088], cat hair Interactions [F(2,15) = 1.228, p = 0.3208], or fecal boli [F(2,15) = 0.439, p = 0.6527] (data not shown). (n per group: P = 5, E = 4, M/D = 9).
On the Elevated Plus Maze, Female rats had a higher number of Open Arms Entries compared to Males (p = 0.0306) which was not due to increased overall locomotion as there were no differences in the number of Closed Arm Entries (p = 0.9006) or Total Entries (p = 0.1828) (Fig. 2B). In addition, Female rats performed significantly fewer Risk Assessments into the Open arms (Fig. 2D) (p = 0.0207). These findings are most readily interpreted as Female rats having lower anxiety. Female rats, however, were similar to Male rats in Time Spent in the Open Arms (p = 0.0758) or Closed Arms (p = 0.1478).
This apparent discrepancy between two measures of anxiety in the same task suggests an alternative interpretation of these findings: specifically, Female rats engage in an active motor response to anxiogenic stimuli. In other words, they are engaging a different strategy compared to Males. Consistent with this interpretation are the findings that Female rats spent significantly less time in the Center of the EPM compared to Male rats (p < 0.0001) (Fig. 2C).
On the EPM, there was an overall effect of Estrous on Open Arm Entries [F(2,15) = 7.374, p = 0.0059] with significantly fewer Entries during Metestrus/Diestrus compared to Estrus (p = 0.0019). Likewise, there was a significant effect of Estrous on Total Arm Entries [F(2,15) = 11.224, p = 0.0010] and significantly fewer Total Entries during Metestrus/Diestrus compared to Proestrus (p = 0.0062) and Estrus (p = 0.0005) (data not shown). There was no effect of Estrous on Time spent in the open Arms [F(2,15) = 0.793, p = 0.4705], in the Closed Arms [F(2,15) = 2.942, p = 0.0836], or the Center [F(2,15) = 2.565, p = 0.1101], and Risk Assessments [F(2, 15) = 1.595, p = 0.2356] (data not shown). (n per group: P = 6, E = 4, M/D = 8).
In addition to differences in the EPM, there were differences in ASR. Female rats had a significantly higher startle response compared to Males [F(1,45) = 34.179, p < 0.0001] (Fig. 2E). There was an overall habituation effect [F(14,630) = 5.681, p < 0.0001] which was true for both Female [F(14,308) = 3.430, p < 0.0001] and Male rats [F(14,322) = 7.572, p < 0.0001]. Male rats had a stronger habituation as revealed by a significant interaction effect of sex [F(14,630) = 1.912, p = 0.0226]. The data for the ASR is from the groups run on one of the startle boxes, as we discovered technical concerns with the output of the second one. All animals, however, had the same startle experience.
EXPERIMENT 1: FEAR EXTINCTION IN THE SHOCK CONTEXT
Female rats freeze less than Male rats during fear extinction in the shock context and have an active motor response in the presence of conditioned cues
During CFC, Female and Male rats show no difference in Freezing in the last minute of CFC (p = 0.2421) (Fig. 3A). Concurrent with this, there was no difference between Female and Male rats in the number of Crossings during the last minute of CFC (Female mean = 0.348, Male mean = 0.130, p = 0.1953) (Fig. 3B). This again indicates that Female and Male rats have similar unconditioned fear responses.
Fig. 3.
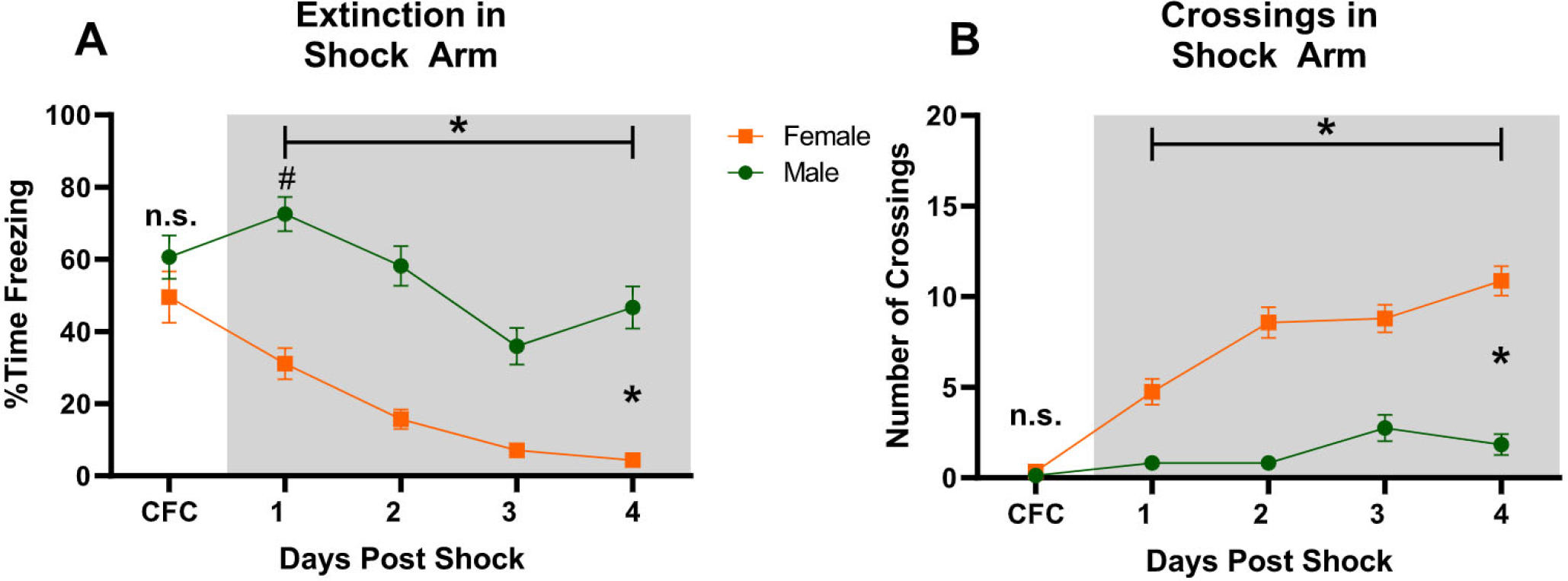
Fear Extinction in the Shock Context. (A) Freezing during CFC and extinction training (Female n = 23, Male n = 24). (B) Number of Crossings in the Shock Arm during CFC (Female n = 23, Male n = 23) and extinction training (Female n = 23, Male n = 24). One video from the Male group during CFC was corrupted, and we could not report crossings for that animal. *p < 0.05, #p < 0.05 for Females versus Males on day 1 of Extinction in the Shock Context.
During Extinction in the Shock Context, both Female and Male rats show reduction in Freezing [Overall Freezing: F(3, 135) = 55.264, p < 0.0001; Female Freezing: F(3,66) = 22.582, p < 0.0001; Male Freezing: F(3,69) = 35.605, p < 0.0001] with a significant interaction between groups [F(3,135) = 3.272, p = 0.0232]. Additionally, Female rats started Day 1 of extinction training with lower Freezing than Males (p < 0.0001) (Fig. 3A). At the same time, Female rats engaged in more activity as measured by Crossings in the Shock Arm on Day 1 of extinction (p < 0.0001), which remained higher than Males throughout extinction as demonstrated by a significant effect of Sex [F(1,43) = 67.361, p < 0.0001] (Fig. 3B). Although both Female and Male rats increased their Crossings over extinction days [F(3,129) = 24.243, p < 0.0001 note: the video from 1 animal was corrupted and therefore not included in this analysis], Female rats increased their Crossings over time more than Males shown by a significant Crossings*Sex interaction [F(3,129) = 11.942, p < 0.0001].
These findings could be interpreted as female rats having impaired memory of CFC. However, considering their behavior in the EPM and ASR, it is also possible that female rats froze less because they expressed an active motor response in an anxiogenic situation (being placed back in the fear conditioned context in the absence of the stimulus but with the expectation of the stimulus). Therefore, we explicitly tested this hypothesis in Experiment 2, where both Female and Male rats were allowed to express their memory of the CFC training with either freezing or an active motor response.
EXPERIMENT 2: AVOIDANCE EXTINCTION IN THE Y-MAZE
During Avoidance Extinction, Female rats avoid the Shock Arm similarly to Males but show low freezing and more active motor responses
We tested animals for avoidance of the Shock Context after CFC in the Y-maze. During pre-exposure, both Female and Male rats explored the Y-maze equally [Total Arm Entries: F(1,32) = 0.415, p = 0.5241, Time in Shock Arm: F(1,32) = 2.505, p = 0.1233, Entries in Shock Arm: F(1,32) = 0.222, p = 0.6406] (Fig. 4 B–D). Similarly, to Experiment 1, there was no Sex difference in Freezing during the 1 minute after foot-shocks [F(1,32) = 0.130, p = 0.7207] (Fig. 4A).
Fig. 4.
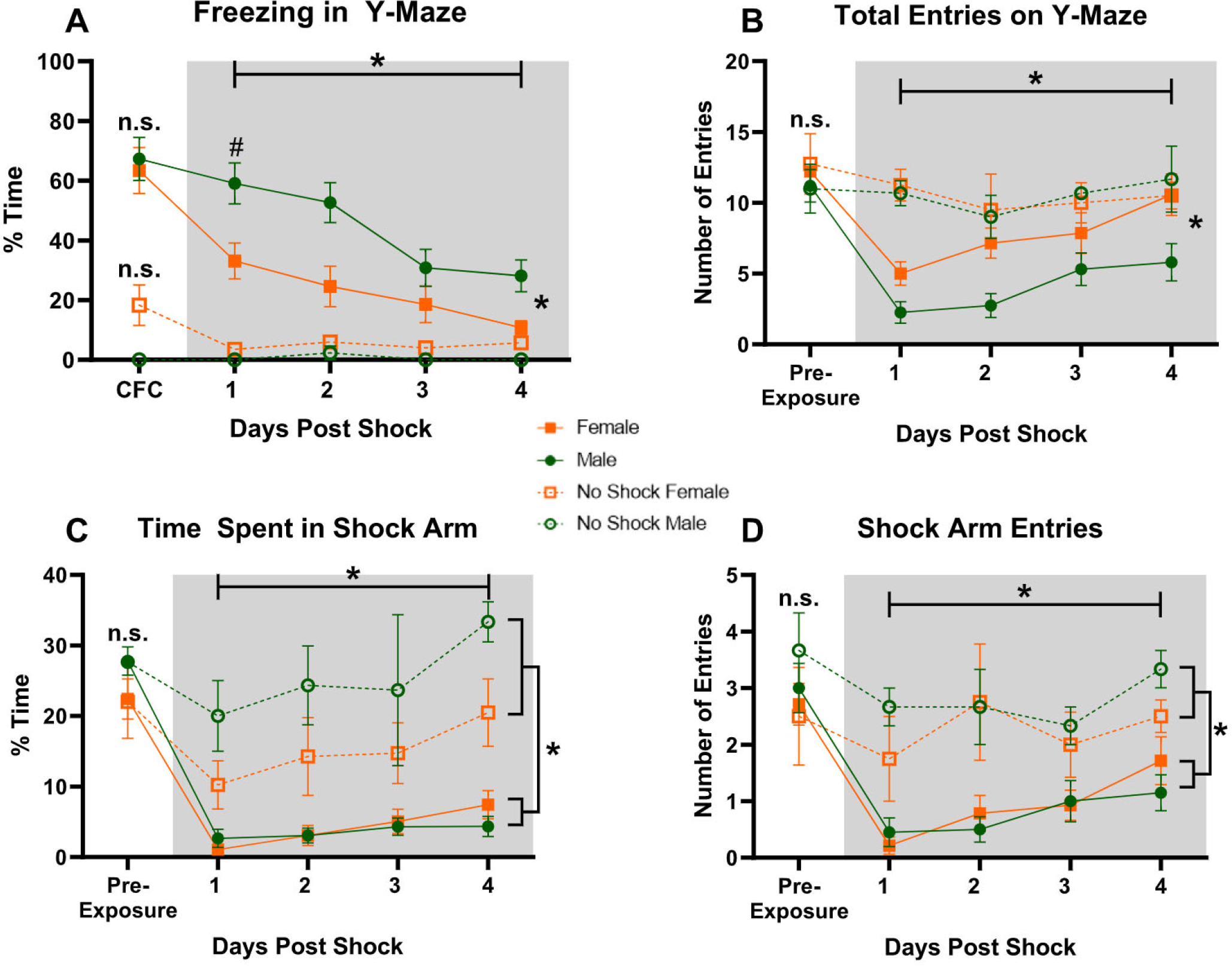
Avoidance Extinction after CFC in the Y-maze. (A) Freezing in the last minute of CFC and Avoidance Extinction Training. (B) Total Arm Entries on the Y-maze during Pre-exposure and Avoidance Extinction. (C) Time in Shock Arm of the Y-maze during Pre-exposure and Avoidance Extinction. (D) Entries in Shock Arm of the Y-maze during Pre-exposure and Avoidance Extinction. (Female Shock n = 14, Female No Shock n = 4, Male Shock n = 20, Male No Shock n = 3) however, during CFC in panel A, two videos: one from the Female No Shock group and one from the Male No Shock group, were corrupted and could not be scored. *p < 0.05, #p < 0.05 for Shocked Females versus Males on day 1 of Avoidance Extinction
During extinction training, there was a significant effect of Freezing [F(3,96) = 16.286, p < 0.0001] and Sex [F(3,96) = 8.590, p = 0.0062] with no Sex*Freezing interaction [F(3,96) = 1.480, p = 0.2249] suggesting that both Female and Male rats decreased freezing over time but Males froze more than Females (Fig. 4A). This replicates the findings on sex differences in freezing from Experiment 1, regardless of the fact that they were allowed to move between arms. Additionally, Female rats engaged in more activity than Males as measured by the Total Number of Arm Entries into the three arms of the Y-maze [F(1,32) = 7.017, p = 0.0124]. Both Female and Male rats increased their number of total entries over time as revealed by Total Arm Entries effect [F(3,96) = 15.496, p < 0.0001] and no Total Number of Arm Entries*Sex interaction [F(3,96) = 1.256, p = 0.2940] (Fig. 4B).
Despite these differences, Female and Male rats had similar avoidance of the Shock Arm (Fig. 4C–D). There was no difference between Female and Male rats in Time Spent in the Shock Arm [F(1,32) 0.128, p = 0.7228] (Fig. 4C) or Shock Arm entries [F(1,32) = 0.132, p = 0.7185] (Fig. 4D). Despite well-documented and replicated differences in freezing behavior between Female and Male rats, these data show that after fear conditioning both sexes express fear equally by avoiding a fearful place when given a choice.
Avoidance extinction results from fear conditioning
To examine whether Avoidance Extinction results from fear conditioning, the behavior of the described animals was compared to female and male rats that were put through the same procedures without foot-shock.
During pre-exposure to the Y-maze, all animals explored all arms and there were no group differences in the Total Arm Entries [Shock condition: F(1,37) = 0.008, p = 0.9286; Sex: F(1,37) = 0.552, p = 0.4621 or Shock condition*Sex interaction F(1,37) = 0.039, p = 0.8443] (Fig. 4B), Time in Shock Arm [Shock condition: F(1,37) = 0.010, p = 0.9198; Sex: F(1,37) = 2.069, p = 0.1588 or Shock condition*Sex interaction F(1,37) = 0.009, p = 0.9242] (Fig. 4C), or Entries in Shock Arm [Shock condition: F(1,37) = 0.099, p = 0.7548; Sex: F(1,37) = 1.020, p = 0.3190 or Shock condition*Sex interaction F(1,37) = 0.375, p = 0.5438] (Fig. 4D).
As expected, the rats in the Shock groups showed significantly higher Freezing compared to the No Shock groups in the last minute of CFC or the equivalent time point in the No Shock groups [F(1,35) = 15.035, p = 0.0004 note: the videos from 2 animals were corrupted and therefore not included in this analysis]. There were no Sex differences [F(1,35) = 0.252, p = 0.6186] and no Shock condition*Sex interaction [F(1,35) = 0.593, p = 0.4464] (Fig. 4A).
During extinction training, there was a significant effect of Freezing [F(3,111) = 3.248, p = 0.0246] with a significant Shock condition effect [F(1,37) = 13.631, p = 0.0007] and Freezing*Shock condition interaction [F(3,111) = 3.270, p = 0.0239] indicating that shocked animals froze more than controls especially during the first days of extinction. There was no overall effect of Sex on Freezing [F(1,37) = 1.087, p = 0.3038] or Sex*Shock condition interaction [F(1,37) = 2.470, p = 0.1245] due to the fact that both Females and Males in the No Shock group showed freezing close to zero (Fig. 4A).
Importantly, there was a significant effect of Shock condition in Time in Shock Arm [F(1,37) = 56.355, p < 0.0001] with a significant Time in Shock Arm *Shock condition interaction [F(3,111) = 3.050, p = 0.0316] and this was not influenced by sex as there was no Time in Shock Arm*Sex interaction [F(3,111) = 0.131, p = 0.9416] (Fig. 4C). Furthermore, there was a significant effect of Shock condition on Entries in Shock Arm [F(1,37) = 14.350, p = 0.0005] with no Entries in Shock Arm*Sex interaction [F(3,111) = 0.885, p = 0.4515] (Fig. 4D). Combined, all findings from Experiment 2 indicate that Avoidance Extinction results from the CFC training and that the sex differences reported above emerge after CFC training.
As mentioned in the Introduction, the effect of estrous cycle during foot-shock has been documented to show an effect on freezing during fear extinction (Milad et al., 2009). We attempted to evaluate the effect of estrous cycle on Avoidance Extinction. Unfortunately, only one animal was in Estrus phase during CFC. Therefore, we compared only Proestrus (n = 4) and Metestrus/Diestrus (n = 9) There was no effect of Estrous on Total Arm Entries [F(1,11) = 0.075, p = 0.7899] or Estrous*Total Entries interaction over days of extinction [F(3,33) = 0.388, p = 0.7621]. There was also no effect of Estrous on Time in Shock Arm [F(1,11) = 0.154, p = 0.7027] or Estrous*-Time in Shock Arm interaction [F(3,33) = 1.616, p = 0.2043] nor Entries in Shock Arm [F(1,11) = 0.079, p = 0.7838] and Estrous*Entries in Shock Arm interaction [F(3,33) = 0.833, p = 0.4852]. Finally, there was no effect of Estrous on Freezing during Avoidance Extinction [F(1,11) = 0.578, p = 0.4631] and Estrous*Freezing interaction F(3,33) = 0.523, p = 0.6696].
DISCUSSION
Fear memories are indelibly ingrained in the brain and are essential for survival. Pathological alterations in these processes contribute to the etiology of conditions such as PTSD which is sexually dimorphic. Here we attempt to address the sex differences in fear extinction by testing the hypothesis that female rats engage in an active motor response during fear extinction. To this end, we allowed female and male rats to choose either freezing or an active motor response to avoid a context previously associated with foot shock.
We report that: 1) Female and Male rats display similar unconditioned fear responses. 2) Female rats display an active motor response compared to Males, especially under anxiogenic conditions; and 3) Avoidance Extinction can be used to assess fear memory in both Female and Male rats while still accommodating both strategies.
Female and Male rats display similar unconditioned fear responses using multiple measures under both cat hair exposure (Fig. 2A) and CFC. In the last minute of CFC after foot-shock (Figs. 2, 3A), female and male rats freeze to the same extent and show the same level of activity measured by Crossings in the Shock Arm.
However, they differ under anxiogenic conditions which involves the anticipation of a threat that can occur in the absence of a threat as opposed to situations eliciting fear which requires the presence of a threat (Izquierdo et al., 2016). Female rats tended to have an active motor response whereas Male rats tended to move less in the EPM, where they are faced with height, open space, and bright light. This is also true in situations where both fear and anxiety are present such as fear extinction when the conditioned fearful stimulus is no longer present but the association of that stimulus with the shock context is presumably present. Consistent with other findings (Frye et al., 2000; Maeng & Milad, 2015), female rats make more entries into the Open arms of the EPM (Fig. 2B) and behave differently in other tests of anxiety such as the Light-Dark Open Field (Shanazz et al., 2021). Female rats also perform fewer risk assessments into the Open arms of the EPM indicating that they are adopting a different strategy and committing to enter the arms (Fig. 2B–D). This is also substantiated by Female rats having higher reactivity when tested for acoustic startle response compared to males (Fig. 2E).
The active motor response strategy in Female rats was also present during Fear Extinction in the Shock Context. We show that Female rats freeze less and made more crossings in the Shock Arm than Male rats (Fig. 3A–B) which is consistent with reports in the literature (Gruene et al., 2015; Inslicht et al., 2013; Milad et al., 2006; Shansky, 2015; Wilson et al., 2013). More crossings and lower freezing in Female rats were accompanied by observed pushing on the Plexiglass lid with more force and frequency compared to Male rats, although this was not quantified, suggesting they were actively looked for way to escape the Shock Context. The sexually dimorphic choice of strategy was also present during Avoidance Extinction on the Y-Maze (Fig. 4A–B).
Given the clear difference in strategies between Female and Male rats under anxiogenic conditions, we propose a term to describe this behavior that allows us to acknowledge and discuss this behavior as a community. We term this behavior ‘anxioescapic’ to mean an active motor response to anxiogenic situations. This term encompasses and applies to behaviors such as darting in response to a conditioned cue but in the absence of the fear-eliciting stimulus (Gruene et al., 2015) and escape tendencies during foraging (Pellman et al., 2017). This can also be applied to behaviors in other strains of rats such as Wistar female rats that show increased locomotion in novel situations and increased escape behavior when given the opportunity to escape a fearful stimulus (Jolles et al., 2015). We believe other specific behaviors that encompass an active motor response under anxiogenic conditions will also fit under this descriptor. It is worth noting that sex differences in defensive behaviors have been known for some time (Blanchard et al., 1991) but to the best of our knowledge there is no specific term for these behaviors.
It is difficult to speculate an evolutionary relevance to why female rats tend to engage in anxioescapic behavior. Perhaps female rats in general have more biological imperative to escape because their innate behavior is designed for protection of their offspring. Moving may then be a tactic to distract predators or get back to the nest to defend it. On the other hand, although female rats have more involvement in parenting than males (Schultz & Lore, 1993) anxioescapic behavior may be in pursuit of self-preservation. Because rats reproduce often with relatively large litters and are sometimes known to eat their pups, it is not inconceivable that female rats might be willing to abandon their nest and escape so that they may reproduce again. Future studies can elucidate the ethological significance of this strategy. However, it remains relevant that research should consider these sex differences in strategy preference when rats are faced with fear and/or anxiety.
To overcome the limitations of behavioral differences in Female and Male strategies and provide a unifying measure for both sexes, we used Avoidance Extinction. When given the opportunity to avoid the Shock Arm, both female and male rats spent the same time and made similar number of entries in the Shock Arm which was not seen in the non-shocked rats (Fig. 4C–D). This suggests that both female and male rats had associated the foot-shock with the shock context and had similar fear extinction/learning of safety. Thus, Avoidance Extinction is a useful measure of fear extinction that accommodates different behavioral strategies in situations of fear and/or anxiety.
Avoidance Extinction in rodents has utility because avoidance is a relevant symptom of PTSD and is observed more frequently in women (Dalla et al., 2008). Additionally, Avoidance Extinction has utility when experimental manipulations have impaired the expression of freezing behavior, and it is difficult to infer if they have learned fear after foot-shock as in the case with basolateral amygdala (BLA) lesioned animals (Vazdarjanova, 2000; Vazdarjanova et al., 2001; Vazdarjanova & McGaugh, 1998). When tested for avoidance on the Y-maze, the BLA lesioned male rats demonstrated learning by avoiding the Shock Arm. Therefore, avoidance can test fear memory retrieval when freezing behavior is impaired or, as in the case of female rats, is not the preferred behavioral response.
A potential driver for the differences in fear expression between female and male rats is the effects of gonadal hormones on behavior. We were unable to determine the effect of estrous phase on Avoidance Extinction due to small sample size. Replication is necessary to elucidate the true impact of estrous cycle on Avoidance Extinction. However, we did find an effect of estrous cycle on anxiety-like behavior on the EPM such that rats in the M/D phase displayed higher anxiety-like behavior indicated by lower number of Open Arm Entries. The effects of the estrous cycle are documented in rodent models of PTSD and estrogen has been shown to be neuroprotective in the context of anxiety and trauma. Female rats in low estrogen phases exhibit similar number of entries in the EPM compared to males (Frye et al., 2000; Frye & Walf, 2002; Scholl et al., 2019). Other findings have been reported with foot-shock such that female rats shocked during low estrogen and progesterone phases display similar levels of freezing during fear extinction as male rats (Barha et al., 2010; Milad et al., 2009).
In summary, this paper presents evidence that female and male rats have different behavioral responses in anxiogenic situations, but not at baseline or when directly faced with a fear-eliciting stimulus. We term the active motor response in anxiogenic conditions anxioescapic behavior. We further show that Avoidance Extinction can be successfully used to examine fear memory in female and male rats and avoid ‘floor effects’ caused by low freezing.
ACKNOWLEDGEMENTS
We want to thank Danielle Crethers and Kristopher Bunting for their technical support.
FUNDING
This research was supported by funding from the Department of Veterans Affairs Merit.
Review Awards 1I01BX001978 and I01BX00389.
The content presented here does not represent the views of the Department of Veterans.
Affairs or the United States Government.
Abbreviation:
- PTSD
post traumatic stress disorder
Footnotes
DECLARATION OF COMPETING INTEREST
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Adamec R, Head D, Blundell J, Burton P, Berton O (2006) Lasting anxiogenic effects of feline predator stress in mice: Sex differences in vulnerability to stress and predicting severity of anxiogenic response from the stress experience. Physiol Behav 88(1–2):12–29. [DOI] [PubMed] [Google Scholar]
- Ajayi AF, Akhigbe RE (2020) Staging of the estrous cycle and induction of estrus in experimental rodents: An update. Fert Res Pract 6(1):5. 10.1186/s40738-020-00074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander KS, Nalloor R, Bunting KM, Vazdarjanova A (2020) Investigating individual pre-trauma susceptibility to a PTSD-like phenotype in animals. Front Syst Neurosci 13. 10.3389/fnsys.2019.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KR, Crawley JN (2009) Anxiety-related behaviors in mice. In: Buccafusco JJ, editor. Methods of behavior analysis in neuroscience. CRC Press/Taylor & Francis. [PubMed] [Google Scholar]
- Barha CK, Dalton GL, Galea LA (2010) Low doses of 17α-estradiol and 17β-estradiol facilitate, whereas higher doses of estrone and 17α- and 17β-estradiol impair, contextual fear conditioning in adult female rats. Neuropsychopharmacology 35(2):547–559. 10.1038/npp.2009.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard DC, Shepherd JK, Carobrez ADP, Blanchard RJ (1991) Sex effects in defensive behavior: Baseline differences and drug interactions. Neurosci Biobehav Rev 15(4):461–468. 10.1016/S0149-7634(05)80132-0. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC (1969) Passive and active reactions to fear-eliciting stimuli. J Comp Physiol Psychol 68(1, Pt. 1):129–135. [DOI] [PubMed] [Google Scholar]
- Bolles RC, Collier AC (1976) The effect of predictive cues on freezing in rats. Anim Learn Behav 4(1-A):6–8. 10.3758/BF03211975. [DOI] [Google Scholar]
- Cahill L, McGaugh JL (1998) Mechanisms of emotional arousal and lasting declarative memory. Trends Neurosci 21(7):294–299. [DOI] [PubMed] [Google Scholar]
- Chowdhury TG, Wallin-Miller KG, Rear AA, Park J, Diaz V, Simon NW, Moghaddam B (2019) Sex differences in reward- and punishment-guided actions. Cogn Affect Behav Neurosci 19(6):1404–1417. 10.3758/s13415-019-00736-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen D, Elklit A (2012) Sex differences in PTSD. Post traumatic stress disorders in a global context. lnTech. http://www.intechopen.com/books/post-traumatic-stress-disorders-in-a-global-context/sex-differences-in-ptsd. [Google Scholar]
- Cohen H, Yehuda R (2011) Gender differences in animal models of posttraumatic stress disorder. Dis Markers 30(2–3):141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla C, Edgecomb C, Whetstone AS, Shors TJ (2008) Females do not express learned helplessness like males do. Neuropsychopharmacology 33(7):1559–1569. 10.1038/sj.npp.1301533. [DOI] [PubMed] [Google Scholar]
- Deslauriers J, Toth M, Der-Avakian A, Risbrough VB (2018) Current status of animal models of posttraumatic stress disorder: behavioral and biological phenotypes, and future challenges in improving translation. Biol Psychiatry 83(10):895–907. 10.1016/j.biopsych.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraday MM (2002) Rat sex and strain differences in responses to stress. Physiol Behav 75(4):507–522. 10.1016/S0031-9384(02)00645-5. [DOI] [PubMed] [Google Scholar]
- Fiorenza NG, Sartor D, Myskiw JC, Izquierdo I (2011) Treatment of fear memories: Interactions between extinction and reconsolidation. Anais Da Academia Brasileira de Ciências 83:1363–1372. 10.1590/S0001-37652011000400023. [DOI] [PubMed] [Google Scholar]
- Frye CA, Petralia SM, Rhodes ME (2000) Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3alpha,5alpha-THP. Pharmacol Biochem Behav 67(3):587–596. 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Frye CA, Walf AA (2002) Changes in progesterone metabolites in the hippocampus can modulate open field and forced swim test behavior of proestrous rats. Horm Behav 41(3):306–315. 10.1006/hbeh.2002.1763. [DOI] [PubMed] [Google Scholar]
- Gamwell K, Nylocks M, Cross D, Bradley B, Norrholm SD, Jovanovic T (2015) Fear conditioned responses and PTSD symptoms in children: Sex differences in fear-related symptoms. Dev Psychobiol 57(7):799–808. 10.1002/dev.21313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza K, Jovanovic T (2017) Impact of gender on child and adolescent PTSD. Current Psychiatry Reports 19(11):87. 10.1007/s11920-017-0830-6. [DOI] [PubMed] [Google Scholar]
- Glover EM, Jovanovic T, Norrholm SD (2015) Estrogen and extinction of fear memories:implications for posttraumatic stress disorder treatment. Biol Psychiatry 78(3):178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham LK, Yoon T, Lee HJ, Kim JJ (2009) Strain and sex differences in fear conditioning: 22 kHz ultrasonic vocalizations and freezing in rats. Psychol Neurosci 2(2):219–225. 10.3922/j.psns.2009.2.015. [DOI] [Google Scholar]
- Gruene TM, Flick K, Stefano A, Shea SD, Shansky RM (2015) Sexually divergent expression of active and passive conditioned fear responses in rats. ELife 4:e11352. 10.7554/eLife.11352. PMC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall CS (1934) Emotional behavior in the rat. I. Defecation and urination as measures of individual differences in emotionality. J comp Psychol 18(3):385. 10.1037/h0071444. [DOI] [Google Scholar]
- Haskell SG, Gordon KS, Mattocks K, Duggal M, Erdos J, Justice A, Brandt CA (2010) Gender differences in rates of depression, PTSD, pain, obesity, and military sexual trauma among connecticut war veterans of Iraq and Afghanistan. J Women’s Health 19(2):267–271. 10.1089/jwh.2008.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg S (1996) A review of the validity and variability of the Elevated Plus-Maze as an animal model of anxiety. Pharmacol Biochem Behav 54(1):21–30. 10.1016/0091-3057(95)02126-4. [DOI] [PubMed] [Google Scholar]
- Inslicht SS, Metzler TJ, Garcia NM, Pineles SL, Milad MR, Orr sp, Marmar CR, Neylan TC (2013) Sex differences in fear conditioning in posttraumatic stress disorder. J Psychiatr Res 47(1):64–71. 10.1016/j.jpsychires.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo l, Furini CRG, Myskiw JC (2016) Fear memory. Physiol Rev 96(2):695–750. 10.1152/physrev.00018.2015. [DOI] [PubMed] [Google Scholar]
- James W (1890) The principles of pyschology. New York: Henry Holt and Company, New York. [Google Scholar]
- Jean-Richard-Dit-Bressel P, Killcross S, McNally GP (2018) Behavioral and neurobiological mechanisms of punishment: Implications for psychiatric disorders. Neuropsychopharmacology 43(8):1639–1650. 10.1038/s41386-018-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolles JW, Boogert NJ, van den Bos R (2015) Sex differences in risk-taking and associative learning in rats. R Soc Open Sci 2(11). 10.1098/rsos.150485150485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB (1995) Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry 52(12):1048–1060. [DOI] [PubMed] [Google Scholar]
- Maeng LY, Milad MR (2015) Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Horm Behav 76:106–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S (2001) Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci 24:897–931. 10.1146/annurev.neuro.24.1.89724/1/897 pii. [DOI] [PubMed] [Google Scholar]
- McGaugh JL (2003) Memory and emotion: the making of lasting memories. Columbia University Press. [Google Scholar]
- McGaugh JL (2004) The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci 27:1–28. 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Meyer EC, Konecky B, Kimbrel NA, DeBeer BB, Marx BP, Schumm J, Penk WE, Gulliver SB, Morissette SB (2018) Gender differences in associations between DSM–5 posttraumatic stress disorder symptom clusters and functional impairment in war veterans. Psychol Serv 15(2):230–237. 10.1037/ser0000171. pdh. [DOI] [PubMed] [Google Scholar]
- Milad MR, Goldstein JM, Orr SP, Wedig MM, Klibanski A, Pitman RK, Rauch SL (2006) Fear conditioning and extinction: Influence of sex and menstrual cycle in healthy humans. Behav Neurosci 120:1196–1203. https://doi.org/2006-22387-002 pii 10.1037/0735-7044.120.5.1196. [DOI] [PubMed] [Google Scholar]
- Milad MR, Igoe SA, Lebron-Milad K, Novales JE (2009) Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience 164(3):887–895. 10.1016/j.neuroscience.2009.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Orrsp, Lasko NB, Chang Y, Rauch SL, Pitman RK(2008) Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res 42:515–520. 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M (2006) Mechanisms of fear extinction. Mol Psychiatry 12(2):120–150. [DOI] [PubMed] [Google Scholar]
- Nalloor R, Bunting KM, Vazdarjanova A (2014) Altered hippocampal function before emotional trauma in rats susceptible to PTSD-like behaviors. Neurobiol Learn Mem 112:158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalloor R, Bunting K, Vazdarjanova A, Harris J (2011) Predicting impaired extinction of traumatic memory and elevated startle. PLoS ONE 6(5):e19760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paccola CC, Resende CG, Stumpp T, Miraglia SM, & Cipriano I (2013). The rat estrous cycle revisited: A quantitative and qualitative analysis. 7. [Google Scholar]
- Pavlov IP (1927) Conditioned reflexes: an investigation of the physiological activity of the cerebral cortex. Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellman BA, Schuessler BP, Tellakat M, Kim JJ (2017) Sexually dimorphic risk mitigation strategies in rats. ENeuro 4(1). 10.1523/ENEURO.0288-16.2017. ENEURO.0288-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineles SL, Brownley KA, Rasmusson AM (2017) Gender and PTSD: Different pathways to a similar phenotype. Curr Opin Psychol 14:44–48. 10.1016/j.copsyc.2016.11.002. [DOI] [PubMed] [Google Scholar]
- Quevedo J, Sant’ Anna MK, Madruga M, Lovato I, de-Paris F, Kapczinski F, Izquierdo I, Cahill L (2003) Differential effects of emotional arousal in short- and long-term memory in healthy adults. Neurobiol Learn Mem 79(2):132–135. [DOI] [PubMed] [Google Scholar]
- Rougemont-Bucking A, Linnman C, Zeffiro TA, Zeidan MA, Lebron-Milad K, Rodriguez-Romaguera J, Rauch SL, Pitman RK, Milad MR (2011) Altered processing of contextual information during fear extinction in PTSD: an fMRI study. CNS Neurosci Ther 17:227–236. https://doi.org/CNS152 pii 10.1111/j.1755-5949.2010.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl JL, Afzal A, Fox LC, Watt MJ, Forster GL (2019) Sex differences in anxiety-like behaviors in rats. Physiol Behav 211. 10.1016/j.physbeh.2019.112670112670. [DOI] [PubMed] [Google Scholar]
- Schultz LA, Lore RK (1993) Communal reproductive success in rats (Rattus norvegicus): Effects of group composition and prior social experience. J Comp Psychol 107(2):216–222. 10.1037/0735-7036.107.2.216. [DOI] [PubMed] [Google Scholar]
- Shanazz K, Dixon-Melvin R, Bunting KM, Nalloor R, Vazdarjanova Al (2021) Light-Dark Open Field (LDOF): A novel task for sensitive assessment of anxiety. J Neurosci Methods 363. 10.1016/j.jneumeth.2021.109325109325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM (2015) Sex differences in PTSD resilience and susceptibility: Challenges for animal models of fear learning. Neurobiol Stress 1:60–65. 10.1016/j.ynstr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanElzakker MB, Dahlgren MK, Davis FC, Dubois S, Shin LM (2014) From Pavlov to PTSD: The extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem 113:3–18. 10.1016/j.nlm.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A (2000) Does the basolateral amygdala store memories for emotional events? Trends Neurosci 23:345–346. https://doi.org/S0166-2236(00)01599-X pii. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, Cahill L, McGaugh JL (2001) Disrupting basolateral amygdala function impairs unconditioned freezing and avoidance in rats. Eur J Neurosci 14(4):709–718. 10.1046/j.0953-816x.2001.01696.x. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, McGaugh JL (1998) Basolateral amygdala is not critical for cognitive memory of contextual fear conditioning. PNAS 95(25):15003–15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CA, Vazdarjanova A, Terry AV (2013) Exposure to variable prenatal stress in rats: Effects on anxiety-related behaviors, innate and contextual fear, and fear extinction. Behav Brain Res 238:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]


