ABSTRACT
Enteroviruses initiate infection in the gastrointestinal tract, and sex is often a biological variable that impacts pathogenesis. Previous data suggest that sex hormones can influence the intestinal replication of Coxsackievirus B3 (CVB3), an enterovirus in the Picornaviridae family. However, the specific sex hormone(s) that regulates intestinal CVB3 replication is poorly understood. To determine if testosterone promotes intestinal CVB3 replication, we orally inoculated male and female Ifnar−/− mice that were treated with either placebo or testosterone-filled capsules. Following oral inoculation, we found that the testosterone-treated male and female mice shed significantly more CVB3 in their feces than did the placebo-treated mice, indicating that testosterone enhances intestinal replication. Similarly, testosterone enhanced viral dissemination in both sexes, as we observed higher viral loads in peripheral tissues following infection. Further, the testosterone-treated male mice also had a higher mortality rate than did the testosterone-depleted male mice. Finally, we observed that testosterone significantly affected the immune response to CVB3. We found that testosterone broadly increased proinflammatory cytokines and chemokines while decreasing the number of splenic B cells and dendritic cells following CVB3 infection. Moreover, while testosterone did not affect the early CD4 T cell response to CVB3, testosterone reduced the activation of CD8 T cells. These data indicate that testosterone can promote intestinal CVB3 replication and dissemination while also impacting the subsequent viral immune response.
IMPORTANCE Biological sex plays a significant role in the outcomes of various infections and diseases. The impact of sex hormones on the intestinal replication and dissemination of Coxsackievirus B3 remains poorly understood. Using an oral inoculation model, we found that testosterone enhances CVB3 shedding and dissemination in male and female mice. Further, testosterone can alter the immune response to CVB3. This work highlights the role of testosterone in CVB3 pathogenesis and suggests that sex hormones can impact the replication and dissemination of enteric viruses.
KEYWORDS: Coxsackievirus B3, sex bias, sex hormones, testosterone
INTRODUCTION
Sex plays a significant role in human disease. Males are often more susceptible to pathogens, while females are more predisposed to autoimmune diseases (1–4). This disparity is linked to the immune system, as sex hormones can significantly affect immune responses. Androgens, such as testosterone, can suppress immunity and delay the elimination of pathogens (5–9). On the other hand, estrogens can enhance both the cell-mediated and humoral immune responses, and there is evidence supporting the influence of estrogen on disease severity in cardiovascular diseases and traumatic brain injuries (10, 11). However, knowledge regarding the mechanisms and consequences of sex hormones on infectious diseases remains limited.
Coxsackievirus is a nonenveloped RNA virus in the Picornaviridae family and is a member of a group of viruses transmitted through the fecal-oral route, termed enteroviruses. Enteroviruses are some of the most common viruses infecting humans worldwide, with an estimated 10 to 15 million infections per year in the United States (12). Coxsackievirus is often the most isolated among enteroviruses, and it causes viral myocarditis, hand, foot, and mouth disease, and meningitis (13–15). Infants, children, and immunocompromised individuals are the most susceptible to these diseases, and Coxsackievirus is associated with an 11% fatality rate in neonates (16–18). To date, there are no vaccines or treatments available for Coxsackievirus infections.
Among Coxsackievirus serotypes, Coxsackievirus B3 (CVB3) is one of the leading causes of viral myocarditis, with nearly 40,000 symptomatic cases reported in the United States each year (19). There is a strong sex bias in viral myocarditis, as it affects more males than females, with a mortality rate of 2:1 in individuals infected under the age of 40 (20, 21). Animal models of CVB3 have provided further evidence of this sex bias. Our laboratory and others have shown that sex hormones contribute to CVB3 pathogenesis. The castration of male mice before infection reduces CVB3-induced myocarditis and mortality (22, 23). Further, these hormones contribute to the dysregulation of the immune response, which is hypothesized to contribute to mortality. Testosterone and estradiol influence the CD4+ T cell response to promote or protect against CVB3-induced myocarditis (24–26). The castration of male mice significantly reduces anti-inflammatory M2 macrophages in the heart, suggesting a possible role in CVB3-induced myocarditis (27). Along with T cells and macrophages, mast cells have also been implicated in heart disease, suggesting that multiple immune cells contribute to myocarditis (28). Finally, genes on the Y chromosome also likely contribute to CVB3-induced disease, indicating that viral myocarditis in males is multifactorial (29, 30). Therefore, many questions remain as to the mechanism of the sex bias in Coxsackievirus infections.
While animal models have provided vital information on CVB3 pathogenesis, many of these models mimic systemic infection through intraperitoneal injections of the virus. However, this is not representative of the natural route of infection, as Coxsackievirus is transmitted through the fecal-oral route. Few studies have shown the successful oral inoculation of mice with Coxsackievirus (31–35). Recently, we established an oral inoculation model by which to examine CVB3 replication in the intestine using C57BL/6 Ifnar−/− (deficient for the interferon α/β receptor) mice (36). Using this model, we observed a sex bias in the infection, in which the male mice supported robust intestinal replication and succumbed to the CVB3-induced disease. Female mice, however, are mainly resistant to CVB3 and display limited viral replication in the intestine (36). Gonadectomy before CVB3 infection impacted fecal shedding in male and female mice and protected the male mice from CVB3-induced lethality. These data suggest that sex hormones contribute to viral pathogenesis in our model; however, the roles of specific hormones are unclear. Here, we show that testosterone enhances CVB3 shedding and dissemination to peripheral tissues in male and female mice. Testosterone also enhanced lethality in male mice but not in female mice. Finally, we found that testosterone impacts the immune response, which may limit CVB3 pathogenesis. Overall, these data highlight the importance of examining the sex bias in CVB3-induced disease.
RESULTS
Testosterone enhances CVB3 lethality, fecal shedding, and dissemination in male Ifnar−/− mice following oral inoculation.
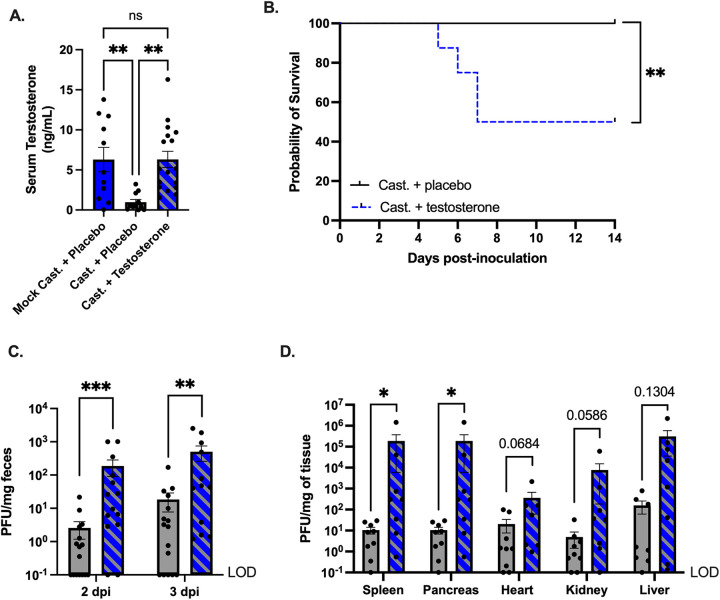
Previously, we found that the castration of male Ifnar−/− mice prior to oral infection with CVB3 protected against CVB3-induced lethality and significantly reduced CVB3 fecal shedding. These data implicated a role for testosterone, the primary sex hormone in males. To determine if testosterone could impact CVB3 replication and lethality, we surgically castrated male Ifnar−/− mice to deplete endogenous testosterone. We also performed mock-castrations on male mice as a control to confirm the testosterone treatments. One week after surgery, we implanted mice with placebo or exogenous testosterone capsules. To assess hormone replacement, we determined the serum concentrations of testosterone by enzyme-linked immunosorbent assay (ELISA). The castrated mice that received the placebo capsule had a significantly lower serum testosterone concentration than the mock-castrated and castrated male mice that received testosterone (Fig. 1A). Further, the mock-castrated and castrated mice receiving exogenous testosterone had similar amounts of serum testosterone, confirming a successful hormone treatment. One week following the hormone treatment, the male mice were orally inoculated with 5 × 107 PFU CVB3 and monitored for survival for 14 days postinoculation (dpi). We found that, as in our previous study (36), the testosterone-depleted mice (castrated + placebo) were protected from CVB3-induced lethality (Fig. 1B). However, the testosterone-treated (castrated + testosterone) mice were significantly more likely to die from the CVB3 inoculation than were the testosterone-depleted mice, with 50% of the mice succumbing to the disease. The survival rate and kinetics were similar to those of the oral inoculation of gonad-intact C57BL/6 Ifnar−/− and mock-castrated mice, as we previously demonstrated (36). Thus, these data indicate that testosterone contributes to CVB3-induced lethality in male mice.
FIG 1.
CVB3-induced lethality, fecal shedding, and dissemination are enhanced by testosterone. (A) Serum testosterone concentrations in mock or castrated male C57BL/6 Ifnar−/− provided with either placebo or testosterone capsules. **, P < 0.01. one-way analysis of variance (ANOVA). (B) Survival of castrated + placebo (testosterone-depleted) and castrated + testosterone (testosterone-treated) mice after oral inoculation with 5 × 107 PFU of CVB3-Nancy. n = 8 to 9 mice per group. **, P < 0.01, ***, P < 0.001; log-rank test. (C) CVB3-Nancy fecal titers in testosterone-depleted (gray) and testosterone-treated (blue with diagonal lines) mice at days 2 and 3 postinoculation. n = 15 to 16 mice per group. **, P < 0.01, ***, P < 0.001; Mann-Whitney test. (D) CVB3-Nancy tissue titers. Mice were euthanized at 3 dpi, and tissues were collected from testosterone-depleted (gray) and testosterone-treated (blue with diagonal lines) Ifnar−/− mice. n = 8 to 9 mice per group. *, P < 0.05; Mann-Whitney test. Data are shown as mean ± the standard error of the mean (SEM). LOD = limit of detection.
CVB3 is spread through the fecal-oral route; therefore, we next examined CVB3 fecal shedding, which is indicative of viral replication in the intestine (36). Following oral inoculation, we collected feces at 1, 2, and 3 dpi and quantified fecal CVB3 using a standard plaque assay. We found that at 2 and 3 dpi, the testosterone-depleted mice shed significantly less CVB3 in their feces than did the testosterone-treated male mice (Fig. 1C). Since fecal shedding in testosterone-treated mice was higher, we hypothesized that testosterone would also increase the viral loads in the peripheral tissues following oral inoculation. To test this hypothesis, we harvested the heart, liver, kidney, spleen, and pancreas at 3 dpi and quantified the CVB3 tissue titers using a plaque assay. We observed significantly higher tissue CVB3 titers for the testosterone-treated mice compared to the testosterone-depleted mice in the spleen and pancreas (Fig. 1D). Further, we found higher titers in the heart and kidney of the testosterone-treated mice, and these results reached near statistical significance (P = 0.0684 and 0.0586, respectively). Taken together, these data suggest that testosterone promotes intestinal CVB3 replication and viral dissemination in male mice following oral inoculation.
Testosterone promotes proinflammatory cytokine and chemokine expression to CVB3.
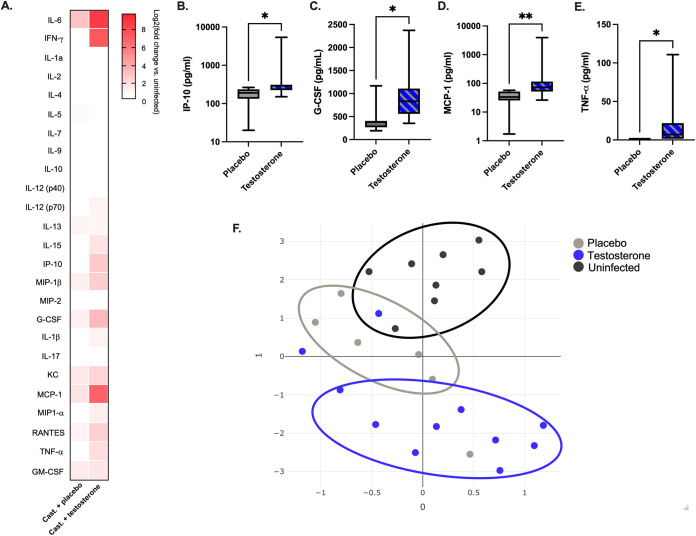
The immune response plays a pivotal role in tissue damage following CVB3 infection (24, 37–41). Previously, we observed an increase in serum cytokine and chemokine concentrations in CVB3-infected male, but not female, mice (36). To test whether testosterone altered the magnitude of the cytokine and chemokine responses, we measured the serum concentrations of a broad panel of 25 cytokines and chemokines at 3 dpi. In the mice with testosterone, we saw a general increase in the serum concentrations of proinflammatory cytokines and chemokines IL-6, IFN-γ, IL-15, IP-10, MIP-1β, G-CSF, KC, MCP-1, RANTES, and TNF-α compared to those of uninfected males (Fig. 2A). Further, there were significantly higher serum concentrations of IP-10, C-CSF, MCP-1, and TNF-α in the testosterone-treated mice than in the testosterone-depleted mice (Fig. 2B–E). Finally, we analyzed the cytokine and chemokine responses by uniform manifold approximation and projection (UMAP). This dimension reduction approach has been used to analyze complex data sets and to visualize relationships between experimental groups (42, 43). We found that UMAP could separate the cytokine and chemokine profiles into three clusters that correlated with CVB3 infection and the presence of testosterone (Fig. 2F). These data suggest that testosterone alters the cytokine and chemokine profile in CVB3-infected males. Overall, these data indicate that testosterone in CVB3 infected male Ifnar−/− mice promotes a proinflammatory response that is characterized by the induction of select cytokines and chemokines.
FIG 2.
Testosterone promotes proinflammatory cytokines and chemokines in response to CVB3. Infected mice were orally inoculated with 5 × 107 PFU of CVB3-Nancy, and serum was collected at 3 dpi. (A) Heat map of the serum levels of the indicated cytokines or chemokines at 3 dpi between CVB3-infected, testosterone-depleted and testosterone-treated male Ifnar−/− mice. The log2-fold change was normalized to uninfected mice. (B−E) The serum concentration of indicated cytokines and chemokines from infected mice. The serum concentration for each cytokine is represented by a box and whisker plot. The box represents the 25th through 75th percentiles of the data, and the center line in the box represents the median value. The black whiskers mark the 5th and 95th percentiles of the data. *, P < 0.05, **, P < 0.01, ***, P < 0.001; Kruskal-Wallis test. (F) Uniform manifold approximation and projection (UMAP) analysis of chemokine and cytokine serum concentrations in uninfected (black circles), testosterone-depleted (gray circles), and testosterone-treated (blue circles) male Ifnar−/− mice. Data are representative of two independent experiments with n = 7 to 11 mice per group.
Testosterone decreases the frequency of B cells and the number of dendritic cells in the spleen.
Since immune cells have been shown to greatly alter CVB3 pathogenesis, we next assessed the impact of testosterone on immune cells from the spleen. We harvested the spleen from infected testosterone-treated and testosterone-depleted male Ifnar−/− mice at 5 dpi and performed flow cytometry on splenocytes. We chose to investigate the immune cell response at 5 dpi because this represents the time when males begin to succumb to disease in our model (Fig. 1B). We found that testosterone decreased the total number of splenocytes in both uninfected and infected male mice (Table 1); however, this had no impact on the frequency and numbers of splenic macrophages, monocytes, and neutrophils between infected testosterone-treated and testosterone-depleted mice (Table 1). Contrary to the results observed with macrophages, monocytes, and neutrophils, testosterone significantly decreased the frequency and number of CD19+ B cells in the spleen following CVB3 infection (Table 1). However, the decrease in splenic B cells is testosterone-dependent, as a similar reduction in B cells was observed in uninfected mice. These data indicate that testosterone reduces the number of B cells in the spleen; however, these differences are not due to CVB3 infection.
TABLE 1.
The frequency and number of characterized splenocytes from uninfected and infected testosterone-depleted (placebo) or testosterone-treated (testosterone) micea,b
| Uninfected male mice |
CVB3-infected male mice |
|||||||
|---|---|---|---|---|---|---|---|---|
| Infection | Frequency of cells (%) per spleen |
Total no. of cells (×103) per spleen |
Frequency of cells (%) per spleen |
Total no. of cells (×103) per spleen |
||||
| Cell type | Placebo | Testosterone | Placebo | Testosterone | Placebo | Testosterone | Placebo | Testosterone |
| Total Splenocytes | 100 | 100 | 51375 ± 5134.57* | 29250 ± 2721.57* | 100 | 100 | 33166.667 ± 2216.10* | 21200 ± 2527.85* |
| Macrophages | 1.62 ± 0.10 | 2.55 ± 0.35 | 840.0 ± 114.8 | 653.8 ± 51.15 | 1.55 ± 0.15 | 2.08 ± 0.32 | 451.9 ± 90.6 | 521.8 ± 79.4 |
| Monocytes | 0.7538 ± 0.13 | 0.7111 ± 0.06 | 402.763 ± 102.02 | 197.672 ± 25.17 | 0.783 ± 0.08 | 1.07 ± 0.17 | 258.3 ± 27.4 | 218.1 ± 29.3 |
| Neutrophils | 0.435 ± 0.09 | 1.25 ± 0.36 | 205.025 ± 41.35 | 306.4 ± 74.07 | 0.2 ± 0.06 | 0.374 ± 0.08 | 66.4 ± 20.6 | 84.1 ± 21.5 |
| B cells | 58.86 ± 1.76* | 45.72 ± 3.95* | 30553.875 ± 3730.96* | 12776.25 ± 1380.84* | 58.18 ± 2.05* | 41.3 ± 2.73* | 19592 ± 1434.7* | 8 928.4 ± 1329.4* |
Data are presented as mean +/– SEM from two independent experiments (n = 5 to 6 per group).
Statistically significant differences between groups are bolded and denoted by asterisks (*, P < 0.05).
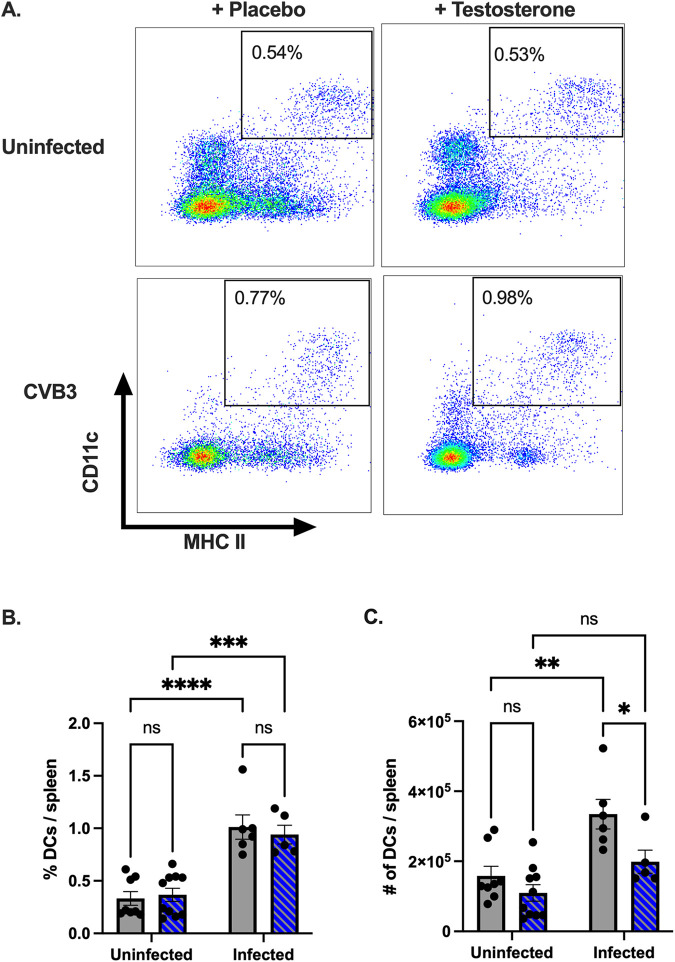
Next, we assessed the impact of testosterone on CD11c+, MHC II+ dendritic cells. In contrast to the results observed with B cells, no significant differences in the frequency or numbers of dendritic cells were observed between the uninfected mice, regardless of testosterone treatment (Fig. 3A and B). Following CVB3 infection, we observed a significant increase in the frequency of splenic dendritic cells in both the testosterone-depleted and the testosterone-treated mice compared to the uninfected mice. However, we only observed a significant increase in the numbers of splenic dendritic cells in the testosterone-depleted mice infected with CVB3 (Fig. 3C). Further, following infection, we found that the testosterone-depleted mice had significantly higher numbers of dendritic cells in the spleen compared to the testosterone-treated mice. Overall, these data indicate that while CVB3 infection increases the frequency of splenic dendritic cells, in the absence of testosterone, the number of dendritic cells in the spleen is significantly reduced following oral inoculation.
FIG 3.
The effect of testosterone on the frequency and number of dendritic cells following oral CVB3 inoculation. (A) Representative flow cytometry plots of CD11c+, MHC II splenic dendritic cells at 5 dpi in uninfected and CVB3-infected mice that were either testosterone-treated or testosterone-depleted (placebo). The frequency (B) and number (C) of splenic dendritic cells in testosterone-treated (blue with diagonal lines) and testosterone-depleted (gray) mice following infection. *, P < 0.05, **, P < 0.01, ****, P < 0.0001; two-way ANOVA. All data are from two independent experiments with n = 4 to 6 mice per group and are shown as mean ± SEM.
Testosterone limits the activation of CD8+ T cells following oral CVB3 infection.
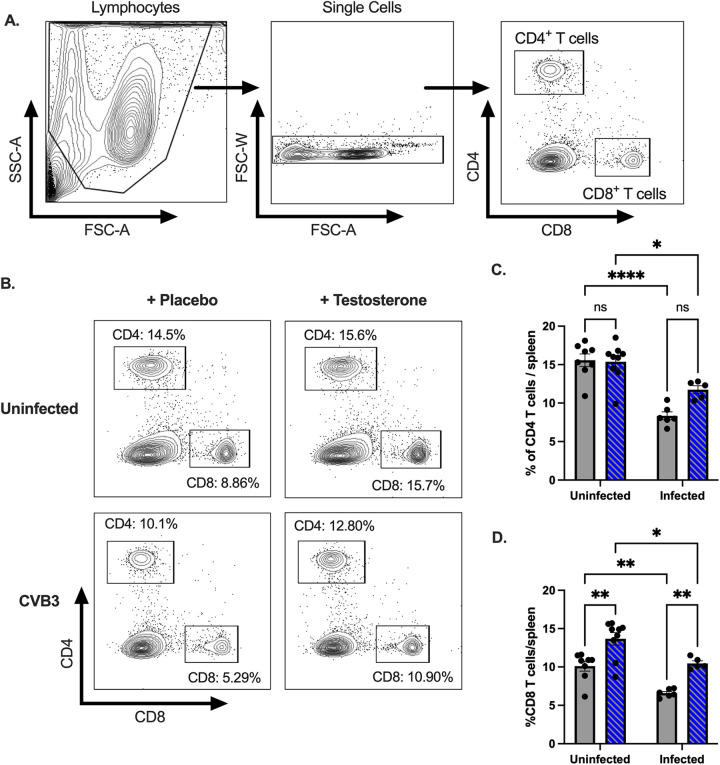
CD4+ and CD8+ T cells have been implicated in limiting CVB3 replication; however, they may also contribute to disease in myocarditis models (39). Since we observed a significant difference in the numbers of splenic dendritic cells between testosterone-treatment groups, we hypothesized that testosterone might alter the T cell response to CVB3 in our model. Following CVB3 inoculation, we harvested the spleen at 5 dpi and performed flow cytometry on splenocytes to evaluate our hypothesis. We found a significant decrease in the proportion of splenic CD4+ and CD8+ T cells in the infected mice compared to the uninfected mice from both the testosterone-depleted and the testosterone-treated groups (Fig. 4A–D). Testosterone did not appear to affect changes to the CD4+ T cell response, as no significant difference in frequency between testosterone groups was observed in the uninfected or the infected animals (Fig. 4C). In contrast, we found that the testosterone-depleted mice displayed a significant reduction in the frequency of CD8+ T cells in both the uninfected and the infected male mice compared to the testosterone-treated mice (Fig. 4D). These data indicate that oral inoculation with CVB3 leads to a contraction in splenic CD4+ and CD8+ T cells and that testosterone increases the frequency of CD8+, but not CD4+, T cells in the spleen.
FIG 4.
CVB3 and testosterone impact the proportions of splenic CD4+ and CD8+ T cells following oral inoculation. (A) Representative gating strategies by which to identify CD4+ and CD8+ T cells. (B) Representative flow cytometry plots of CD4+ and CD8+ T cells in uninfected and infected mice that are either testosterone-treated or testosterone-depleted (placebo). (C) The frequency of CD4+ T cells in the spleen in testosterone-treated (blue with diagonal lines) and testosterone-depleted (gray) mice. (D) The frequency of CD8+ T cells in the spleen in testosterone-treated (blue with diagonal lines) and testosterone-depleted (gray) mice. *, P < 0.05, **, P < 0.01, ****, P < 0.0001; two-way ANOVA. All data are from two independent experiments with n = 4 to 6 mice per group and are shown as mean ± SEM.
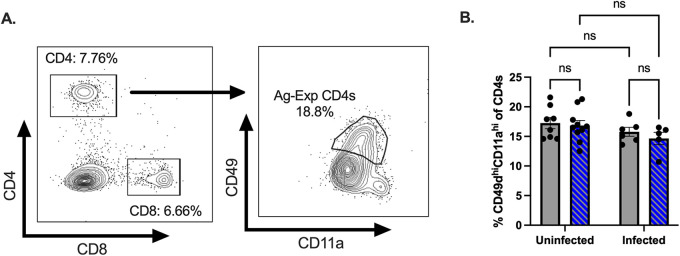
CVB3 infection and testosterone impacted the frequency of splenic T cells; however, these results do not distinguish between naive and activated T cells. Unfortunately, few CVB3-specific CD4+ and CD8+ T cell epitopes have been identified, which limits the ability to track virus-specific, activated T cells. However, previous studies have established that activated T cells can be followed, regardless of their specificity, by using surrogate markers (44–49). To investigate the impact of testosterone on activated T cells, we next examined the expression of CD11a and CD49d on CD4+ T cells as a measure of activated, antigen-experienced CD4+ T cells (Fig. 5A). Similar to the frequency of the total CD4+ T cells, we found no significant difference in the CD11ahiCD49dhi CD4+ T cells between the testosterone-treated and the testosterone-depleted mice (Fig. 5B). Further, in contrast to the total CD4+ T cells, CVB3 infection did not alter the frequency of splenic CD11ahiCD49dhi CD4+ T cells compared to uninfected mice. Taken together, these data indicate that testosterone does not impact the CD4+ T cell response at 5 dpi in male mice.
FIG 5.
Testosterone does not affect the early CD4+ T cell response to CVB3. (A) Representative gating strategy for CD11ahiCD49dhi CD4+ T cells. (B) The frequency of CD49dhiCD11ahi CD4+ T cells in the spleen at 5 dpi. All data are from two independent experiments with n = 5 to 6 mice per group. A two-way ANOVA was performed.
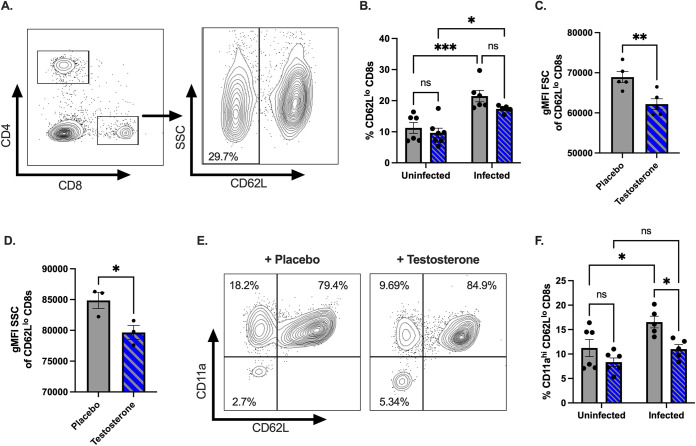
Next, we assessed the impact of testosterone on activated CD8+ T cells. Naive CD8+ T cells are CD62Lhi, and activated CD8+ T cells differentiate into effector subtypes during acute infections. During this effector phase, CD62L is downregulated (45, 50, 51). Therefore, we next assessed whether testosterone affects the activation of CD8+ T cells following CVB3 infection via the expression of CD62L. We found that the CVB3-infected mice had a significantly higher frequency of CD62Llo CD8+ T cells in the spleen than did the uninfected mice in both the testosterone-treated and the testosterone-depleted groups (Fig. 6A and B). Intriguingly, in the infected mice, we observed a trending reduction in the frequency of splenic CD62Llo CD8+ T cells in the testosterone-treated mice compared to the testosterone-depleted mice; however, this result did not reach statistical significance (P = 0.0754; unpaired t test) (Fig. 6B). Since testosterone nearly decreased the frequency of CD62Llo CD8+ T cells in infected mice, we next examined if CD62Llo CD8+ T cells from infected mice were undergoing a blast transformation. Within a few hours of antigen activation, CD8+ T cells can undergo a blast transformation, which results in larger lymphocytes, as they develop into mature effector cells. Since changes in the light scatter can reveal a blast transformation and reflect a measure of T cell activation (52), we assessed the mean fluorescence intensity (gMFI) for the forward scatter (FSC) and side scatter area (SSC) of the CD62Llo CD8+ T cell population. We found that CD62Llo CD8+ T cells from the testosterone-depleted mice had a significant increase in size and granularity, as measured by FSC and SSC, compared to CD62Llo CD8+ T cells from the testosterone-treated mice (Fig. 6C and D). These data suggest that testosterone reduces early blast transformation in CD8+ T cells in response to CVB3.
FIG 6.
Testosterone dampens the activation of CD8+ T cells following oral CVB3 infection. (A) Representative flow cytometry plot of the gating strategy by which to identify CD62Llo CD8+ T cells. (B) The frequency of CD62Llo CD8+ T cells in the spleen of testosterone-treated (blue with diagonal lines) and testosterone-depleted (gray) male mice. *, P < 0.05, **, P < 0.01, ***, P < 0.001, ns: not significant; two-way ANOVA. (C) The geometric mean fluorescence intensity (gMFI) forward scatter (FSC) of CD62Llo CD8+ T cells **, P < 0.01; unpaired t test. (D) Representative of the gMFI side scatter (SSC) of CD62Llo CD8+ T cells from two independent experiments. *, P < 0.05; unpaired t test. (E) Representative flow cytometry plots of the expression of CD11a and CD62L CD8+ T cells in the spleen of testosterone-treated (blue with diagonal lines) and testosterone-depleted (gray) male mice. (F) The frequency of CD11ahiCD62Llo CD8+ T cells in the spleen following CVB3 infection. *, P < 0.05; two-way ANOVA. All data are from two independent experiments with n = 5 to 6 mice per group and are shown as mean ± SEM.
To confirm our results, we next examined the expression of integrin molecule CD11a on CD8+ T cells. Following CD8+ T cell activation, the increased expression of CD11a can distinguish naive CD8+ T cells from antigen-experienced effector and memory CD8+ T cells (45). We observed no difference between the proportions of splenic CD11ahiCD62Llo CD8+ T cells in the infected and the uninfected male mice treated with testosterone (Fig. 6E and F). In contrast, we found a significant increase in the frequency of splenic CD11ahiCD62Llo CD8+ T cells between the infected and the uninfected male mice that were testosterone-depleted. Overall, these data suggest that testosterone limits the frequency of splenic CD11ahiCD62Llo CD8+ T cells following CVB3 infection, indicative of a dampening of the early activation of CD8+ T cells.
Testosterone does not enhance CVB3-induced lethality in female mice but does enhance fecal shedding and viral dissemination.
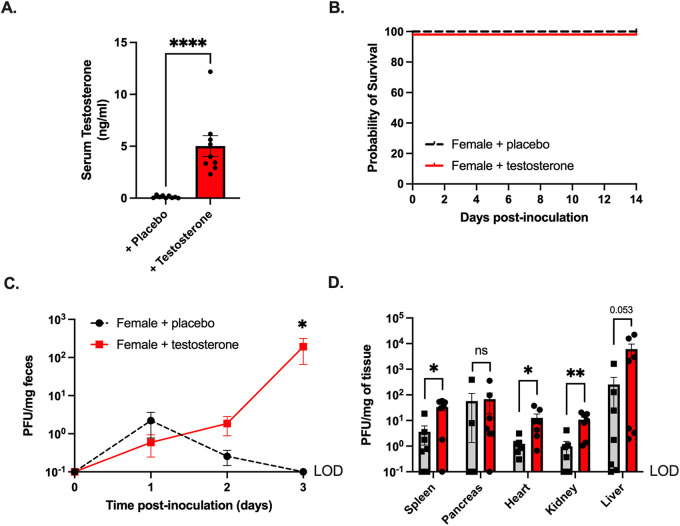
Previously, we found that CVB3 orally inoculated female Ifnar−/− mice were protected entirely from CVB3-induced lethality and had limited viral fecal shedding (36). Since exogenous testosterone treatment restored fecal shedding and lethality in male mice, we hypothesized that testosterone might enhance pathogenesis in female mice. To examine this hypothesis, we provided exogenous testosterone or placebo capsules to female mice before oral inoculation with CVB3. As in our castration studies, the female mice with testosterone had significantly higher serum testosterone concentrations than did the placebo controls (Fig. 7A). One week after the hormone treatment, the female mice were orally inoculated with 5 × 107 PFU of CVB3 and monitored for survival. In contrast to the males, both testosterone-treated and placebo-treated infected females were protected from CVB3-induced lethality (Fig. 7B). Next, to examine fecal CVB3 shedding, feces were collected from infected mice at 1, 2, and 3 dpi, processed, and quantified using a standard plaque assay. We observed significantly more CVB3 in the feces at 3 dpi in female mice that received testosterone than in female mice that received placebo capsules (Fig. 7C). Finally, we examined CVB3 titers in peripheral tissues at 3 dpi to test whether testosterone enhanced viral dissemination. We found a significant increase in the viral loads of the heart, kidney, and spleen in the testosterone-treated female mice (Fig. 7D). The viral load in the liver was higher in the testosterone-treated mice, but this result did not reach statistical significance. In contrast to the other organs, the viral load in the pancreas was similar between the placebo-treated and testosterone-treated female mice. Overall, these data indicate that testosterone can enhance fecal CVB3 shedding and viral dissemination to the heart, kidney, and spleen; however, testosterone is not sufficient to increase CVB3-induced lethality in female mice.
FIG 7.
Testosterone promotes CVB3 shedding and dissemination, but not lethality, in female Ifnar−/− mice. (A) Serum testosterone concentrations in female C57BL/6 Ifnar−/− mice provided with either placebo or testosterone capsules. (B) Survival of female C57BL/6 Ifnar−/− mice provided with either placebo or testosterone capsules after oral inoculation with 5 × 107 PFU of CVB3-Nancy. (C) CVB3-Nancy fecal titers in female C57BL/6 Ifnar−/− mice provided with either placebo or testosterone. *, P < 0.05; Mann-Whitney test. (D) CVB3-Nancy tissue titers in female C57BL/6 Ifnar−/− mice provided with either placebo or testosterone. All data are from two to three independent experiments with n = 5 to 8 mice per group. *, P < 0.05, **, P < 0.01; Mann-Whitney test. LOD = limit of detection.
DISCUSSION
Sexual dimorphism is commonly observed in various infectious, autoimmune, and cardiovascular diseases. Males are often more susceptible to diseases caused by bacteria, viruses, fungi, or parasites. Females, however, are more predisposed to autoimmune diseases, such as systemic lupus erythematosus, multiple sclerosis, and rheumatoid arthritis. Additionally, clinical and epidemiological studies have highlighted the disparity in cardiovascular diseases, with an increased mortality rate in men. CVB3 also displays a sex bias in human infections, in which males are twice as likely to have severe sequelae. Previously, we established an oral inoculation model for CVB3 using male and female Ifnar−/− mice. Ifnar−/− mice were chosen for this model based on previous work showing that the lack of a type I IFN response enhances the susceptibility to human enteric viruses via the oral route (53–55). Using this model, we observed a sex bias in CVB3 orally inoculated male and female Ifnar−/− mice, consistent with the results observed in human infections (36). Here, we show that testosterone, the primary sex hormone in males, impacts CVB3 pathogenesis following oral infection.
Our data indicate that testosterone enhances the intestinal replication and dissemination of CVB3. We observed that testosterone could promote fecal shedding and higher viral loads of CVB3 in the tissues of both male and female Ifnar−/− mice following infection (Fig. 1C, 1D, 7C, and 7D). To our knowledge, this is the first time that testosterone has been demonstrated to influence the intestinal replication and dissemination of an enteric virus. These data differ from previous studies examining the impact of sex hormones on CVB3 and other viruses, such as influenza. In these studies, testosterone modulates the immune response rather than directly impacting viral replication to influence disease (56–58). Similarly, in CVB3 models, the castration of wild-type, immunocompetent male mice did not directly affect viral replication in the heart (27). Further, testosterone treatment in males only led to a modest increase in the CVB3 load in the heart (24). The discrepancy between these data and ours is likely due to the different inoculation routes. However, in our oral inoculation model, our data suggest that sex differences in intestinal CVB3 replication and viral dissemination are likely a significant contributor to the sex bias in mortality.
The mechanisms for the sex hormone modulation of intestinal replication and dissemination are currently unclear. Previous data indicate that sex hormones, including testosterone, can enhance CVB3 attachment to cardiomyocytes (23); therefore, testosterone may alter the expression of viral receptors on intestinal cells. It is also intriguing to speculate that sex hormones may change the overall intestinal ecosystem to directly or indirectly impact CVB3 replication. Recent data show that intestinal bacteria can influence the intestinal replication of enteric viruses, including CVB3 (59–61). Our lab recently demonstrated that specific intestinal bacteria in male mice promote CVB3 infectivity and viral stability (62). While the mechanism is unclear for CVB3, a similar enterovirus, poliovirus, has been found to use bacteria to enhance attachment to the poliovirus receptor (63). Interestingly, sex-specific differences in intestinal bacteria can account for the sex bias in mouse models of type 1 diabetes (64, 65). Since bacteria can also metabolize hormones that alter bacterial growth, future studies are required to examine the link between bacteria and sex hormones as enhancers of CVB3 attachment and replication in the intestine.
With a systemic infection model of CVB3, previous studies have found that testosterone enhances lethality in male mice (22), consistent with our data. Further, systemic models of CVB3 infection have shown that while testosterone treatment promoted cardiac inflammation in female mice following CVB3 infection, testosterone did not increase mortality (22, 24). In agreement, we also found that testosterone was insufficient to enhance lethality in female mice following oral inoculation. However, we found that testosterone enhances fecal CVB3 shedding and CVB3 dissemination, suggesting that other unknown immune correlates of protection may help limit mortality in females. Further, it is possible that the predominant female hormones, estrogen and progesterone, also offer protection for females in our model. This current study focused on gonad-intact females; therefore, a limitation in our current study is that we cannot rule out the potential protective effects of estrogen and progesterone. Experiments examining the role of these hormones are underway in our laboratory and may reveal additional sex-dependent factors that protect female mice from CVB3-induced mortality.
In contrast to directly enhancing viral replication, testosterone may indirectly enhance viral replication and pathogenesis by modulating the immune response following infection. Previous studies have shown that testosterone acts as an anti-inflammatory hormone (66); however, our data indicate that testosterone broadly increases the proinflammatory cytokine and chemokine responses to CVB3 (Fig. 2A–C). In the testosterone-treated mice, we saw an overall increase in the serum concentrations for various cytokines and chemokines, including IL-6, Il-15, IP-10, MIP-1β, G-CSF, KC, MCP-1, RANTES, and TNF-α. Interestingly, we only found that IP-10, G-CSF, MCP-1, and TNF-α were significantly upregulated in the presence of testosterone. Using a UMAP analysis to recognize significant trends in the cytokine/chemokine data, we found that the mice clustered into three distinct groups. From our data, we discovered that the testosterone-treated mice responded differently than did the testosterone-depleted mice and the uninfected mice. Interestingly, the testosterone-depleted mice grouped closest to the uninfected mice (Fig. 2F). However, whether this reduction is due to a loss of testosterone or the limited replication and dissemination of CVB3 in the testosterone-depleted mice is unclear. These data, intriguingly, mirror our previous findings, in which the serum concentrations of proinflammatory cytokines and chemokines did not significantly differ between infected and uninfected female mice (36). Taken together, these data suggest that the improved outcome of CVB3 pathogenesis may be associated with limited cytokine and chemokine expression. However, we recognize that a limitation of our data is that the time at which we analyzed the inflammatory response may not be representative of all of the cytokines and chemokines investigated. Further, our model's variability in viral dissemination may account for cytokine and chemokine differences in mice that fell outside the groups tested in our UMAP analysis. Additionally, the lack of a type I IFN response in certain cell types may impair the ability to produce specific proinflammatory cytokines. The kinetics of the cytokine and chemokine responses and their induction in orally inoculated wild-type C57BL/6 mice warrant further investigation.
Along with the cytokine and chemokine response, testosterone can also influence the functions and phenotypes of many immune cells (9). We observed a significant increase in total splenocytes in the testosterone-depleted male mice in uninfected and infected groups (Table 1). We found that B cells likely accounted for this increase in overall splenic cells (Table 1). These data confirm previous reports that show that testosterone dampens the number of B cells in the spleen (67). However, it is unclear whether the difference in B cell dynamics impacts antibody production and maturation, as we observed no significant difference between the infected and the uninfected mice, regardless of testosterone treatment. We previously demonstrated that surviving male mice mount a neutralizing antibody response to CVB3 (36); therefore, it is possible that testosterone could limit neutralizing antibodies that provide protection. Future work will be required to examine the effect of testosterone on B cell function and antibody production during infection.
Previous studies have shown that other immune cells, including T cells, can contribute to not only CVB3 clearance but also CVB3-induced disease (39). In the current study, we observed that CVB3 infection decreased CD4+ and CD8+ T cell frequency in the spleen (Fig. 4C and D). However, testosterone only affected the frequency of CD8+ T cells. Interestingly, when we further investigated the T cell response, we found no difference in the frequency and number of antigen-experienced CD49dhiCD11ahi CD4+ T cells (Fig. 5). This contrasts with previous studies of systemic models of CVB3 infection. In those models, testosterone can affect the CD4 T helper (Th) cell phenotype to promote myocarditis (24, 26). Differences between our findings and those of others may be due to the limited time point selected for our study, as CD4+ T cells may be activated later during infection. However, given that male mice succumb to disease starting at 5 dpi, even if CD4+ T cells are induced later during infection, our data indicate that CD4+ T cells do not contribute to the mortality associated with our model.
In contrast to CD4+ T cells, we observed that testosterone might dampen the activation of CD8+ T cells following oral inoculation. CD62L is a cell-adhesion molecule that is downregulated during the activation of CD8+ T cells. Interestingly, we observed a decrease in the frequency of CD62Llo CD8+s in the testosterone-treated mice, and this result trended toward statistical significance. To further investigate potential CD8+ T cell activation, we measured the sizes of CD62Llo CD8+ T cells from both testosterone-depleted and testosterone-treated mice. As measured by FSC and SSC, we found that the CD62Llo CD8+ T cells from the testosterone-depleted mice differed in shape and were larger than the CD62Llo CD8+ T cells in the testosterone-treated mice. These data suggest that the CD62Llo CD8 T cells from testosterone-depleted mice are activated and in the early stages of a blast transformation (Fig. 6C and D). In support of this finding, we found a significant increase in the frequency of CD11ahiCD62Llo CD8 cells in the testosterone-depleted mice (Fig. 6F). Since the expression of CD11a is upregulated in activated CD8+ T cells, these data indicate that testosterone may dampen the CD8+ T cell response to CVB3. These data are surprising, considering that previous studies have shown a limited in vivo CD8+ T cell response to CVB3 (68–70). Our data confirm that CVB3 fails to elicit a robust CD8+ T cell response in male mice with testosterone; however, our findings suggest that in the absence of testosterone, male mice may mount a CD8 T cell response to CVB3 that could be protective. The size and breadth of this response in testosterone-depleted mice require further investigation. Additionally, identifying CVB3-specific epitopes is necessary to determine whether the CD8+ T cell response is virus-specific or due to bystander activation.
In conclusion, we found that testosterone promotes intestinal CVB3 replication and viral dissemination in orally inoculated male and female Ifnar−/− mice. Further, testosterone enhances virus-induced lethality in a sex-dependent manner. The exact mechanism of how testosterone aggravates disease is currently unclear, but our data indicate that alterations to the host immune response may play an important role. Future studies are necessary to determine how testosterone directly or indirectly promotes intestinal CVB3 infection. Overall, these data reinforce the importance of sex as a biological variable in enteric viral infections.
MATERIALS AND METHODS
Cells and virus.
HeLa cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% calf serum and 1% penicillin-streptomycin at 37°C with 5% CO2. The CVB3-Nancy infectious clone was obtained from Marco Vignuzzi (Pasteur Institute, Paris, France) and propagated in HeLa cells as described previously (36). CVB3 was quantified using a standard plaque assay with HeLa cells.
Mouse experiments.
All animals were handled according to the Guide for the Care of Laboratory Animals of the National Institutes of Health. All mouse studies were performed at the Indiana University School of Medicine, using protocols approved by the local Institutional Animal Care and Use Committee in a manner designated to minimalize pain. Any animals that exhibited severe disease were euthanized immediately by CO2 inhalation. C57BL/6 PVR+/+ Ifnar−/− mice were obtained from S. Koike (Tokyo, Japan) (54). One week after the hormone treatment, mice were orally infected with 5 × 107 PFU of CVB3 IC Nancy. All adult experimental mice were 10 to 15 weeks old at the time of infection. Feces from infected mice were collected 1, 2, and 3 days postinfection and processed as previously described, and the fecal virus was quantified using a standard plaque assay (36).
Castration and hormone manipulation.
8-week-old to 10-week-old male mice were put under anesthesia, and their testes were surgically removed or mock-castrated as a surgical control. Testosterone implants were constructed using silastic tubing (inner diameter: 0.078 inches, outer diameter: 0.125 inches; Dow Chemical Company). After placing 7.5 mm of Crystalline Testosterone (Sigma-Aldrich catalog number T1500) in the tubing, the ends of the tubing were sealed with 2.5 mm of medical adhesive (732 Multi-Purpose Sealant, Dowsil). After the medical adhesive dried, the implants were incubated overnight at 37°C in sterile phosphate-buffered saline. To ensure osmoregulation, implants that were found to be floating were discarded. Due to the light sensitivity of the sex steroid hormones, the testosterone implants were concealed from light. 1 week after castration, the castrated mice were administered either testosterone or placebo capsules subcutaneously under the right shoulder. Mock-castrated mice were given placebo capsules. The testosterone levels of mouse serum were quantified using a rat/mouse testosterone ELISA, following the manufacturer's instructions (MP Biomedical).
Tissue collection and processing.
The heart, liver, spleen, kidneys, and pancreas were aseptically collected at 3 dpi and homogenized in phosphate-buffered saline using 0.9 to 2.0 mm stainless steel beads in a Bullet Blender (Next Advance). Cellular debris were removed by centrifugation at 12,000 × g for 10 min at 4°C, supernatants were collected, and CVB3 was quantified using a plaque assay with HeLa cells.
Flow cytometry analysis.
The spleen was collected at 5 dpi from testosterone-depleted (placebo) or testosterone-treated mice. The spleen was mechanically disrupted to generate a single-cell suspension, and erythrocytes were lysed using RBC lysis buffer as previously described (49). The cells were washed and stained with antibodies for indicated immune cells and then fixed using IC fixation buffer (eBioscience). Samples were analyzed on a BD LSRFortessa flow cytometer with FlowJo software (BD Biosciences). The following mouse antibodies were used in an appropriate combination of fluorochromes: CD4 (clone GK1.5, BioLegend, catalog number 100412), CD8α (clone 53-6.7, BioLegend, catalog number 100725), MHC II (clone M5/114.15.2, BioLegend, catalog number 107619), CD11a (clone N418, BioLegend, catalog number 117327), CD19 (clone 6D5, BioLegend, catalog number 115507), CD62L (clone MEL-14, BioLegend, catalog number 104438), and CD49d (clone R1-2, BioLegend, catalog number 103607).
Serum collection and analysis.
Blood was collected from the inferior vena cava of infected and uninfected male and female mice at 3 dpi and incubated at room temperature for 30 min to initiate coagulation. Samples were centrifuged at 2,000 rpm for 15 min, and separated serum was collected and stored at −20 C for downstream analysis. Alanine transaminase (EN0207Mu-1, CusaBio Technologies) and pancreatic lipase (E91453Mu-1, Cusabio Technologies) were measured via ELISA. Serum cytokine levels were measured by the Indiana University Multiplex Analysis Core using a Millipore Milliplex MAP Mouse Cytokine/Chemokine Magnetic Kit (Millipore Sigma, Burlington, MA). Undiluted serum was provided to the Indiana University Multiplex Analysis Core and was run according to the manufacturer’s protocol. A heat map was generated using the log2-fold change of the average serum concentration of each cytokine and chemokine from the infected groups compared to the uninfected control mice. A UMAP analysis on the serum cytokine and chemokine levels across the three groups was carried out using the R UMAP 0.2.8.0 package. UMAP is a method by which to reduce high-dimensional data in order to recognize significant trends in similarity. This technique assumes that all of the data points are connected and that these data points approximate the total data points. The data set was z score normalized prior to UMAP analysis. The UMAP default parameters, as well as a minimum Euclidean distance of 0.1, were used.
Statistical analysis.
Comparisons between the control and study groups were analyzed using either an unpaired t test, a Mann-Whitney U test, or a one-way analysis of variance (ANOVA). A log-rank test was used for survival curve analysis. The error bars in the figures represent the standard errors of the means. A P-value of <0.05 was considered to be indicative of a statistically significant result. All analyses were performed using GraphPad Prism 9 (GraphPad Software, La Jolla, CA).
ACKNOWLEDGMENTS
We thank Samantha Scholz and Keely Szilagyi for their help and input with the mouse castration and testosterone experiments. We also thank the members of the Indiana University Melvin and Bren Simon Cancer Center Flow Cytometry Resource Facility for their outstanding technical support. This work was funded by a K01 DK110216, an R03 DK124749, a Showalter Trust Award, and a Biomedical Research Grant from the Indiana Clinical and Translational Sciences Institute to C.M.R. The Indiana University Melvin and Bren Simon Comprehensive Cancer Center Flow Cytometry Resource Facility (FCRF) is funded in part by the National Institutes of Health (NIH), National Cancer Institute (NCI) grant P30 CA082709 and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant U54 DK106846. The FCRF is supported in part by the NIH instrumentation grant 1S10D012270.
Contributor Information
Christopher M. Robinson, Email: cmrobin@iu.edu.
Stacey Schultz-Cherry, St. Jude Children's Research Hospital.
REFERENCES
- 1.Fuller AC, Kang B, Kang HK, Yahikozowa H, Dal Canto MC, Kim BS. 2005. Gender bias in Theiler's virus-induced demyelinating disease correlates with the level of antiviral immune responses. J Immunol 175:3955–3963. 10.4049/jimmunol.175.6.3955. [DOI] [PubMed] [Google Scholar]
- 2.Shah VA, Chong CY, Chan KP, Ng W, Ling AE. 2003. Clinical characteristics of an outbreak of hand, foot and mouth disease in Singapore. Ann Acad Med Singap 32:381–387. [PubMed] [Google Scholar]
- 3.Aminu M, Ahmad AA, Umoh JU, de Beer MC, Esona MD, Steele AD. 2007. Adenovirus infection in children with diarrhea disease in Northwestern Nigeria. Ann Afr Med 6:168–173. 10.4103/1596-3519.55702. [DOI] [PubMed] [Google Scholar]
- 4.Curiel RE, Miller MH, Ishikawa R, Thomas DC, Bigley NJ. 1993. Does the gender difference in interferon production seen in picornavirus-infected spleen cell cultures from ICR Swiss mice have any in vivo significance? J Interferon Res 13:387–395. 10.1089/jir.1993.13.387. [DOI] [PubMed] [Google Scholar]
- 5.Hou J, Zheng WF. 1988. Effect of sex hormones on NK and ADCC activity of mice. Int J Immunopharmacol 10:15–22. 10.1016/0192-0561(88)90145-2. [DOI] [PubMed] [Google Scholar]
- 6.Rettew JA, Huet-Hudson YM, Marriott I. 2008. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod 78:432–437. 10.1095/biolreprod.107.063545. [DOI] [PubMed] [Google Scholar]
- 7.McKay LI, Cidlowski JA. 1999. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev 20:435–459. 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- 8.Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiebaut R, Tibshirani RJ, Davis MM. 2014. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci USA 111:869–874. 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trigunaite A, Dimo J, Jorgensen TN. 2015. Suppressive effects of androgens on the immune system. Cell Immunol 294:87–94. 10.1016/j.cellimm.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Brotfain E, Gruenbaum SE, Boyko M, Kutz R, Zlotnik A, Klein M. 2016. Neuroprotection by estrogen and progesterone in traumatic brain injury and spinal cord injury. Curr Neuropharmacol 14:641–653. 10.2174/1570159x14666160309123554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy E. 2011. Estrogen signaling and cardiovascular disease. Circ Res 109:687–696. 10.1161/CIRCRESAHA.110.236687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strikas RA, Anderson LJ, Parker RA. 1986. Temporal and geographic patterns of isolates of nonpolio enterovirus in the United States, 1970–1983. J Infect Dis 153:346–351. 10.1093/infdis/153.2.346. [DOI] [PubMed] [Google Scholar]
- 13.Kosrirukvongs P, Kanyok R, Sitritantikorn S, Wasi C. 1996. Acute hemorrhagic conjunctivitis outbreak in Thailand, 1992. Southeast Asian J Trop Med Public Health 27:244–249. [PubMed] [Google Scholar]
- 14.He SJ, Han JF, Ding XX, Wang YD, Qin CF. 2013. Characterization of enterovirus 71 and coxsackievirus A16 isolated in hand, foot, and mouth disease patients in Guangdong, 2010. Int J Infect Dis 17:E1025–E1030. 10.1016/j.ijid.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Andreoletti L, Leveque N, Boulagnon C, Brasselet C, Fornes P. 2009. Viral causes of human myocarditis. Arch Cardiovasc Dis 102:559–568. 10.1016/j.acvd.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 16.Khetsuriani N, Lamonte A, Oberste MS, Pallansch M. 2006. Neonatal enterovirus infections reported to the national enterovirus surveillance system in the United States, 1983–2003. Pediatr Infect Dis J 25:889–893. 10.1097/01.inf.0000237798.07462.32. [DOI] [PubMed] [Google Scholar]
- 17.Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA, Centers for Disease C, Prevention . 2006. Enterovirus surveillance–United States, 1970–2005. MMWR Surveill Summ 55:1–20. [PubMed] [Google Scholar]
- 18.Abedi GR, Watson JT, Nix WA, Oberste MS, Gerber SI. 2018. Enterovirus and Parechovirus Surveillance - United States, 2014–2016. MMWR Morb Mortal Wkly Rep 67:515–518. 10.15585/mmwr.mm6718a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gebhard JR, Perry CM, Harkins S, Lane T, Mena I, Asensio VC, Campbell IL, Whitton JL. 1998. Coxsackievirus B3-induced myocarditis: perforin exacerbates disease, but plays no detectable role in virus clearance. Am J Pathol 153:417–428. 10.1016/S0002-9440(10)65585-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fairweather D, Cooper LT, Jr, Blauwet LA. 2013. Sex and gender differences in myocarditis and dilated cardiomyopathy. Curr Probl Cardiol 38:7–46. 10.1016/j.cpcardiol.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodruff JF. 1980. Viral myocarditis. A review. Am J Pathol 101:425–484. [PMC free article] [PubMed] [Google Scholar]
- 22.Huber SA, Job LP, Auld KR. 1982. Influence of sex hormones on Coxsackie B-3 virus infection in Balb/c mice. Cell Immunol 67:173–179. 10.1016/0008-8749(82)90210-6. [DOI] [PubMed] [Google Scholar]
- 23.Lyden DC, Olszewski J, Feran M, Job LP, Huber SA. 1987. Coxsackievirus B-3-induced myocarditis. Effect of sex steroids on viremia and infectivity of cardiocytes. Am J Pathol 126:432–438. [PMC free article] [PubMed] [Google Scholar]
- 24.Huber SA, Kupperman J, Newell MK. 1999. Hormonal regulation of CD4(+) T-cell responses in coxsackievirus B3-induced myocarditis in mice. J Virol 73:4689–4695. 10.1128/JVI.73.6.4689-4695.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huber SA. 2008. Coxsackievirus B3-induced myocarditis: infection of females during the estrus phase of the ovarian cycle leads to activation of T regulatory cells. Virology 378:292–298. 10.1016/j.virol.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huber SA, Pfaeffle B. 1994. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J Virol 68:5126–5132. 10.1128/JVI.68.8.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frisancho-Kiss S, Coronado MJ, Frisancho JA, Lau VM, Rose NR, Klein SL, Fairweather D. 2009. Gonadectomy of male BALB/c mice increases Tim-3(+) alternatively activated M2 macrophages, Tim-3(+) T cells, Th2 cells and Treg in the heart during acute coxsackievirus-induced myocarditis. Brain Behav Immun 23:649–657. 10.1016/j.bbi.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fairweather D, Frisancho-Kiss S, Gatewood S, Njoku D, Steele R, Barrett M, Rose NR. 2004. Mast cells and innate cytokines are associated with susceptibility to autoimmune heart disease following coxsackievirus B3 infection. Autoimmunity 37:131–145. 10.1080/0891693042000196200. [DOI] [PubMed] [Google Scholar]
- 29.Robinson DP, Huber SA, Moussawi M, Roberts B, Teuscher C, Watkins R, Arnold AP, Klein SL. 2011. Sex chromosome complement contributes to sex differences in coxsackievirus B3 but not influenza A virus pathogenesis. Biol Sex Differ 2:8. 10.1186/2042-6410-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Case LK, Toussaint L, Moussawi M, Roberts B, Saligrama N, Brossay L, Huber SA, Teuscher C. 2012. Chromosome y regulates survival following murine coxsackievirus b3 infection. G3 (Bethesda) 2:115–121. 10.1534/g3.111.001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bopegamage S, Kovacova J, Vargova A, Motusova J, Petrovicova A, Benkovicova M, Gomolcak P, Bakkers J, van Kuppeveld F, Melchers WJ, Galama JM. 2005. Coxsackie B virus infection of mice: inoculation by the oral route protects the pancreas from damage, but not from infection. J Gen Virol 86:3271–3280. 10.1099/vir.0.81249-0. [DOI] [PubMed] [Google Scholar]
- 32.Bopegamage S, Borsanyiova M, Vargova A, Petrovicova A, Benkovicova M, Gomolcak P. 2003. Coxsackievirus infection of mice. I. Viral kinetics and histopathological changes in mice experimentally infected with coxsackieviruses B3 and B4 by oral route. Acta Virol 47:245–251. [PubMed] [Google Scholar]
- 33.Harrath R, Bourlet T, Delezay O, Douche-Aourik F, Omar S, Aouni M, Pozzetto B. 2004. Coxsackievirus B3 replication and persistence in intestinal cells from mice infected orally and in the human CaCo-2 cell line. J Med Virol 74:283–290. 10.1002/jmv.20179. [DOI] [PubMed] [Google Scholar]
- 34.Loria RM, Kibrick S, Broitman SA. 1974. Peroral infection with group B coxsackievirus in the adult mouse: protective functions of the gut. J Infect Dis 130:539–543. 10.1093/infdis/130.5.539. [DOI] [PubMed] [Google Scholar]
- 35.Loria RM, Kibrick S, Broitman SA. 1974. Peroral infection with group B coxsackievirus in the newborn mouse: a model for human infection. J Infect Dis 130:225–230. 10.1093/infdis/130.3.225. [DOI] [PubMed] [Google Scholar]
- 36.Robinson CM, Wang Y, Pfeiffer JK. 2017. Sex-dependent intestinal replication of an enteric virus. J Virol 91 10.1128/JVI.02101-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huber SA, Job LP, Woodruff JF. 1981. Sex-related differences in the pattern of coxsackievirus B-3-induced immune spleen cell cytotoxicity against virus-infected myofibers. Infect Immun 32:68–73. 10.1128/iai.32.1.68-73.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Estrin M, Huber SA. 1987. Coxsackievirus B3-induced myocarditis. Autoimmunity is L3T4+ T helper cell and IL-2 independent in BALB/c mice. Am J Pathol 127:335–341. [PMC free article] [PubMed] [Google Scholar]
- 39.Henke A, Huber S, Stelzner A, Whitton JL. 1995. The role of CD8+ T lymphocytes in coxsackievirus B3-induced myocarditis. J Virol 69:6720–6728. 10.1128/JVI.69.11.6720-6728.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frisancho-Kiss S, Davis SE, Nyland JF, Frisancho JA, Cihakova D, Barrett MA, Rose NR, Fairweather D. 2007. Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J Immunol 178:6710–6714. 10.4049/jimmunol.178.11.6710. [DOI] [PubMed] [Google Scholar]
- 41.Fairweather D, Rose NR. 2007. Coxsackievirus-induced myocarditis in mice: a model of autoimmune disease for studying immunotoxicity. Methods 41:118–122. 10.1016/j.ymeth.2006.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Becht E, McInnes L, Healy J, Dutertre CA, Kwok IWH, Ng LG, Ginhoux F, Newell EW. 2019. Dimensionality reduction for visualizing single-cell data using UMAP. Nat Biotechnol 37:38–44. 10.1038/nbt.4314. [DOI] [PubMed] [Google Scholar]
- 43.Herr C, Mang S, Mozafari B, Guenther K, Speer T, Seibert M, Srikakulam SK, Beisswenger C, Ritzmann F, Keller A, Mueller R, Smola S, Eisinger D, Zemlin M, Danziger G, Volk T, Hoersch S, Krawczyk M, Lammert F, Adams T, Wagenpfeil G, Kindermann M, Marcu C, Ataya ZWD, Mittag M, Schwarzkopf K, Custodis F, Grandt D, Schaefer H, Eltges K, Lepper PM, Bals R, Group CS, CORSAAR Study Group . 2021. Distinct patterns of blood cytokines beyond a cytokine storm predict mortality in COVID-19. J Inflamm Res 14:4651–4667. 10.2147/JIR.S320685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDermott DS, Varga SM. 2011. Quantifying antigen-specific CD4 T cells during a viral infection: CD4 T cell responses are larger than we think. J Immunol 187:5568–5576. 10.4049/jimmunol.1102104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rai D, Pham NL, Harty JT, Badovinac VP. 2009. Tracking the total CD8 T cell response to infection reveals substantial discordance in magnitude and kinetics between inbred and outbred hosts. J Immunol 183:7672–7681. 10.4049/jimmunol.0902874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Butler NS, Schmidt NW, Vaughan AM, Aly AS, Kappe SH, Harty JT. 2011. Superior antimalarial immunity after vaccination with late liver stage-arresting genetically attenuated parasites. Cell Host Microbe 9:451–462. 10.1016/j.chom.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. 2011. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol 13:188–195. 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kimura D, Miyakoda M, Kimura K, Honma K, Hara H, Yoshida H, Yui K. 2016. Interleukin-27-producing CD4(+) T cells regulate protective immunity during malaria parasite infection. Immunity 44:672–682. 10.1016/j.immuni.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 49.Pardy RD, Rajah MM, Condotta SA, Taylor NG, Sagan SM, Richer MJ. 2017. Analysis of the T cell response to Zika virus and identification of a novel CD8+ T cell epitope in immunocompetent mice. PLoS Pathog 13:e1006184. 10.1371/journal.ppat.1006184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schlub TE, Badovinac VP, Sabel JT, Harty JT, Davenport MP. 2010. Predicting CD62L expression during the CD8+ T-cell response in vivo. Immunol Cell Biol 88:157–164. 10.1038/icb.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nolz JC, Starbeck-Miller GR, Harty JT. 2011. Naive, effector and memory CD8 T-cell trafficking: parallels and distinctions. Immunotherapy 3:1223–1233. 10.2217/imt.11.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bohmer RM, Bandala-Sanchez E, Harrison LC. 2011. Forward light scatter is a simple measure of T-cell activation and proliferation but is not universally suited for doublet discrimination. Cytometry A 79:646–652. 10.1002/cyto.a.21096. [DOI] [PubMed] [Google Scholar]
- 53.Ohka S, Igarashi H, Nagata N, Sakai M, Koike S, Nochi T, Kiyono H, Nomoto A. 2007. Establishment of a poliovirus oral infection system in human poliovirus receptor-expressing transgenic mice that are deficient in alpha/beta interferon receptor. J Virol 81:7902–7912. 10.1128/JVI.02675-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ida-Hosonuma M, Iwasaki T, Yoshikawa T, Nagata N, Sato Y, Sata T, Yoneyama M, Fujita T, Taya C, Yonekawa H, Koike S. 2005. The alpha/beta interferon response controls tissue tropism and pathogenicity of poliovirus. J Virol 79:4460–4469. 10.1128/JVI.79.7.4460-4469.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wells AI, Grimes KA, Coyne CB. 2022. Enterovirus replication and dissemination are differentially controlled by type I and III interferons in the gastrointestinal tract. mBio 13:e00443–22. 10.1128/mbio.00443-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.vom Steeg LG, Klein SL. 2016. SeXX matters in infectious disease pathogenesis. PLoS Pathog 12:e1005374. 10.1371/journal.ppat.1005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klein SL, Flanagan KL. 2016. Sex differences in immune responses. Nat Rev Immunol 16:626–638. 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 58.Fairweather D, Stafford KA, Sung YK. 2012. Update on coxsackievirus B3 myocarditis. Curr Opin Rheumatol 24:401–407. 10.1097/BOR.0b013e328353372d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robinson CM, Woods Acevedo MA, McCune BT, Pfeiffer JK. 2019. Related enteric viruses have different requirements for host microbiota in mice. J Virol 93 10.1128/JVI.01339-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuss SK, Best GT, Etheredge CA, Pruijssers AJ, Frierson JM, Hooper LV, Dermody TS, Pfeiffer JK. 2011. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science 334:249–252. 10.1126/science.1211057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baldridge MT, Nice TJ, McCune BT, Yokoyama CC, Kambal A, Wheadon M, Diamond MS, Ivanova Y, Artyomov M, Virgin HW. 2015. Commensal microbes and interferon-lambda determine persistence of enteric murine norovirus infection. Science 347:266–269. 10.1126/science.1258025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dhalech AH, Fuller TD, Robinson CM. 2021. Specific bacterial cell wall components influence the stability of coxsackievirus B3. J Virol 95:e0142421. 10.1128/JVI.01424-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robinson CM, Jesudhasan PR, Pfeiffer JK. 2014. Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell Host Microbe 15:36–46. 10.1016/j.chom.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Markle JG, Frank DN, Mortin-Toth S, Robertson CE, Feazel LM, Rolle-Kampczyk U, von Bergen M, McCoy KD, Macpherson AJ, Danska JS. 2013. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339:1084–1088. 10.1126/science.1233521. [DOI] [PubMed] [Google Scholar]
- 65.Yurkovetskiy L, Burrows M, Khan AA, Graham L, Volchkov P, Becker L, Antonopoulos D, Umesaki Y, Chervonsky AV. 2013. Gender bias in autoimmunity is influenced by microbiota. Immunity 39:400–412. 10.1016/j.immuni.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maggio M, Blackford A, Taub D, Carducci M, Ble A, Metter EJ, Braga-Basaria M, Dobs A, Basaria S. 2006. Circulating inflammatory cytokine expression in men with prostate cancer undergoing androgen deprivation therapy. J Androl 27:725–728. 10.2164/jandrol.106.000141. [DOI] [PubMed] [Google Scholar]
- 67.Wilhelmson AS, Lantero Rodriguez M, Stubelius A, Fogelstrand P, Johansson I, Buechler MB, Lianoglou S, Kapoor VN, Johansson ME, Fagman JB, Duhlin A, Tripathi P, Camponeschi A, Porse BT, Rolink AG, Nissbrandt H, Turley SJ, Carlsten H, Martensson IL, Karlsson MCI, Tivesten A. 2018. Testosterone is an endogenous regulator of BAFF and splenic B cell number. Nat Commun 9:2067. 10.1038/s41467-018-04408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slifka MK, Pagarigan R, Mena I, Feuer R, Whitton JL. 2001. Using recombinant coxsackievirus B3 to evaluate the induction and protective efficacy of CD8+ T cells during picornavirus infection. J Virol 75:2377–2387. 10.1128/JVI.75.5.2377-2387.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cornell CT, Kiosses WB, Harkins S, Whitton JL. 2007. Coxsackievirus B3 proteins directionally complement each other to downregulate surface major histocompatibility complex class I. J Virol 81:6785–6797. 10.1128/JVI.00198-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kemball CC, Harkins S, Whitmire JK, Flynn CT, Feuer R, Whitton JL. 2009. Coxsackievirus B3 inhibits antigen presentation in vivo, exerting a profound and selective effect on the MHC class I pathway. PLoS Pathog 5:e1000618. 10.1371/journal.ppat.1000618. [DOI] [PMC free article] [PubMed] [Google Scholar]