Abstract
Tamoxifen is commonly used as a cancer treatment in humans and for inducing genetic alterations using cre-lox mouse models in the research setting. However, the extent of tamoxifen off-target effects in animal research is underappreciated. Here, we report significant changes in cellular infiltration in cre-recombinase-negative mice treated with tamoxifen intraperitoneally. These changes were noted in the lungs, which were characterized by the presence of alveolitis, vasculitis, and pleuritis. Despite significant immunological changes in response to tamoxifen-treatment, clinical symptoms were not observed. This study provides a cautionary note that tamoxifen treatment alone leads to histologic alterations that may obscure research interpretations and further highlights the need for the development of alternative mouse models for inducible cre-mediated deletion.
Keywords: tamoxifen, case report
Introduction
Tamoxifen is a selective estrogen receptor modulator (SERM), which outcompetes estradiol for estrogen receptor binding and forms a nuclear complex that inhibits downstream transcriptional activity1. The most prominent use of tamoxifen in human medicine is as a hormone therapy for breast cancer. The side effects for tamoxifen treatments in humans have been well-documented and include: hot flashes, increased risk for endometrial cancer, and uterine sarcomas1. However, due to the significant efficacy against cancer progression in humans, the data overwhelmingly support that the benefits of tamoxifen treatment outweigh the risks.
In animal research, tamoxifen is used to facilitate inducible, site-specific, gain- or loss-of-function for genes targeted by loxp sites2. A wide range of tamoxifen doses have been published with little uniformity. This is an issue, because tamoxifen treatment can cause a variety of effects across multiple organs. One study has shown that a single dose of tamoxifen can cause reproductive changes in male rodents that are detectable both transiently and long-term2. However, tamoxifen effects are not always detrimental. In SLE-like disease, tamoxifen-treated mice had increased survival rates3. More recent studies have established tamoxifen as an immune modulator3. Therefore, a greater understanding of its effects are needed to appropriately harness tamoxifen for cre-mediated genetic recombination.
Although the indisputable efficacy of tamoxifen in human patients outweighs its potential negative effects, animal researchers must be aware of potential biological consequences of tamoxifen usage, including reproductive2, intestinal4,5, and retinal impairment6, outside the tissue of intended gene ablation. In the current report, we observed that intraperitoneal tamoxifen administration induces distal histopathologic changes even in the absence of cre-recombinase activity. Abnormal histologic findings within the lungs of tamoxifen-treated mice included inflammation within alveoli, the vasculature, and in the pleura, as evidenced by significant leukocyte infiltration. Overall, this study demonstrates the confounding, unintended effects of tamoxifen treatment in mice and cautions that tamoxifen-induced histopathologic alterations may obscure research interpretations.
Materials and Methods
Mice
Tpl2flox/flox mice were offered by Dr. George Kollias7 and purchased from EMMA (EM:07150). Tpl2flox/flox mice were crossed with Sftpctm1(cre/ERT)Blh (Sftpc-CreERT2) mice. Because of poor deletion efficiency within this line, Sftpc-CreERT2+/−Tpl2flox/flox mice were further crossed with Tpl2−/− mice8 provided by Philip Tsichlis to enhance deletion efficiency and resulted in littermate Sftpc-CreERT2+Tpl2flox/− and Tpl2flox/− mice. Only cre-negative Tpl2flox/− littermates were used. C57BL/6 mice were supplied by Jackson Laboratories.
Mice were housed in specific pathogen-free conditions in microisolator cages in the Coverdell Rodent Vivarium within the University of Georgia (UGA, Athens, GA). Mice were maintained in accordance to the standards established by the Guide for the Care and Use of Laboratory Animals9, and all studies were approved by the UGA Institutional Animal Care and Use Committee (IACUC). The Coverdell Rodent Vivarium at UGA monitors all mouse cages daily and routinely tests for the presence of pathogenic infection in female sentinel cages, which tested negative for various endoparasites, ectoparasites, and viral infections, including mouse parvovirus, mouse hepatitis virus, Sendai virus, pneumonia virus of mice, Mycoplasma pulmonis, and lymphocytic choriomeningitis virus. Animals were confirmed Helicobacter-negative, and both male and female mice were used between six to nine weeks of age.
Tamoxifen administration
100 mg tamoxifen citrate (USP-Grade, Spectrum Chemical, T1423) was dissolved in 5 ml of corn oil (Millipore Sigma, C8267) at a final concentration of 20 mg/mL, covered in aluminum foil and shaken overnight at 37°C. Mice were administered 75 mg/kg tamoxifen (or an equivalent volume of PBS or corn oil as negative controls) intraperitoneally for 5 days consecutively followed by a 9-day chase period. After the chase period, some mice were infected with 104 PFU influenza A/x31 intranasally.
Pathology Scoring
Lung, and in the second study, trachea, heart, liver, kidney, and spleen, were harvested and fixed in 10% neutral-buffered formalin for at least 24 hr at room temperature. Formalin-fixed lungs were placed in cassettes, embedded in paraffin, sectioned at 4 μm, mounted onto glass slides, and stained with hematoxylin and eosin (H&E) stains. Histologic sections were evaluated in a blinded manner by a board-certified, veterinary pathologist (K.S.) and scored according to the following criteria: (A) Percent of lung affected, (B) Alveolar score, Alveolar edema score, Pleuritis score: 1 = focal, 2 = multifocal, 3 = multifocal to coalescing, 4 = most of lobule affected. (C) PMN score: 1 = neutrophils compose up to 25% of cells in alveoli, 2 = 25–49%, 3 = 50–75%, 4 = 75%+ (D) Perivascular cuffing (PVC) score: 1 = vessel cuffed by 1 cellular layer, 2 = 2–5 cells thick, 3 = 6–9 cells thick, 4 = 10+ cells thick. (E) Vasculitis score: 1 = infiltration of vessel wall by leukocytes, 2 = infiltration and separation of smooth muscle cells, 3 = infiltration and fibrinoid change. (F) Interstitial pneumonia (IP) score: 1 = alveolar septa infiltrated and thickened by 1 leukocyte layer, 2 = thickened by 2 leukocyte layers, 3 = 3 leukocyte layers, 4 = 4 leukocyte layers.
Influenza viruses and infections
Mouse-adapted influenza virus A/HK-x31 (H3N2) stocks were provided by Dr. Mark Tompkins (UGA), expanded in embryonated chickens eggs and titered on Madin-Darby Canine Kidney cells (MDCK) as previously described10. Mice were sedated with 2.5% Avertin and intranasally infected with 50 μl of influenza A/x31 (104 PFU) in PBS. Body weights were recorded daily, and mice exhibiting severe signs of disease or more than 30% weight loss were euthanized.
Study Design
Littermate controls born of the appropriate genotype were randomly distributed into one of four treatment groups. Our previous study demonstrated a significant difference in viral titers from Tpl2−/− chimeric mice using 3 mice10. Therefore, we analyzed groups of at least four mice. There were no specific exclusion criteria for this study.
Statistics
P values were derived by Kruskal Wallis nonparametric test with Dunn’s multiple comparisons test as indicated using PRISM software unless otherwise noted. Differences were considered statistically significant if p ≤ 0.05. Data represent means ± SEM.
Results
Tamoxifen-treatment induces histopathologic changes within the lungs
We initially generated Sftpc-creERT2 Tpl2fl/− mice to study the role of Tpl2 specifically within type II alveolar epithelial cells in a murine model of influenza infection. A regimen of five days of intraperitoneal tamoxifen-treatment followed by a nine-day chase period was used to induce cre-mediated gene ablation prior to influenza injection. Unexpectedly, uninfected cre-negative mice displayed bilateral white nodules on the lungs that resembled inducible bronchus-associated lymphoid tissue (iBALT) (Fig. 1A), suggesting an ongoing immune response. We, therefore, further investigated the tamoxifen-dependent changes to the lungs of cre-negative mice. Tamoxifen treatment induced histopathologic evidence of inflammation within the alveoli, vasculature, and pleura in uninfected mice (Fig. 1B). Uninfected, tamoxifen-treated mice (UI/Tmx) displayed inflammation scores that trended higher than uninfected, non-treated mice (UI/NT). Notably, perivascular inflammation scores from (UI/Tmx) mice were not significantly different from mice that were tamoxifen-treated and infected with influenza at the peak of infection (day 7, 7 dpi/Tmx) or following influenza resolution (28 dpi/Tmx) (Fig. 1B, 1E). As expected, influenza-infected mice displayed more widespread histopathologic effects and more alveolar edema than UI/Tmx mice (Fig. 1C, 1E). In influenza-infected mice, lung samples exhibited significant changes within the alveoli, including type 2 pneumocyte hyperplasia, the presence of foamy macrophages, and cellular debris (Fig. 1E). The extent of neutrophil recruitment and interstitial pneumonia were significantly increased in the influenza-infected tamoxifen-treated mice (Fig. 1C). Despite signs of mild inflammation throughout their lungs, UI/Tmx mice displayed normal body weights comparable to UI/NT mice (Fig. 1B–D), and no clinical signs were induced by tamoxifen treatment. These data demonstrate that tamoxifen treatment alone induces inflammation in the lungs of uninfected mice that partially mimics the inflammatory phenotype of an antiviral response.
Figure 1.
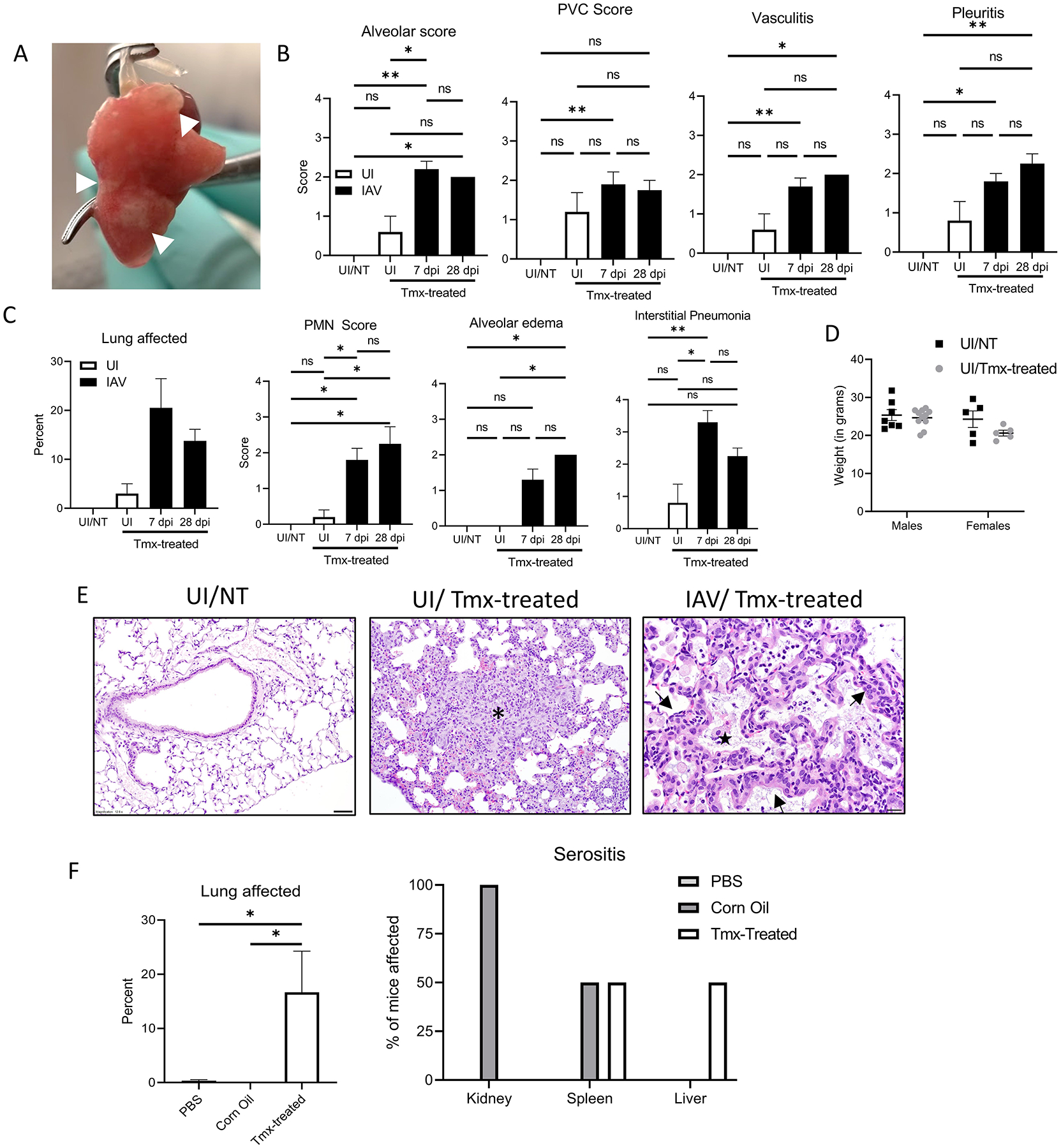
Tamoxifen-treatment induces gross and histopathologic changes within the lungs. (A) Representative photograph of white nodules (white arrows) seen in tamoxifen-treated mouse lungs. (B-C) Histology scoring of H&E-stained lungs performed by a board-certified, veterinary pathologist blinded to sample identity. UI/NT (N=5), UI/Tmx (N=5), 7 dpi/Tmx (N=10), 28 dpi/Tmx (N=4). (D) Weights recorded from UI/NT males (N=7), UI/NT females (N=5), UI/Tmx males (N=11), and UI/Tmx females (N=5). (E) Representative photomicrographs from UI and IAV (7 dpi)-infected lungs with or without prior tamoxifen treatment. Panel 1, 12.5x; Panel 2, 20x, Panel 3, 40x. Asterisk, focal area of loss of alveolar air spaces due to expansion of alveolar septa by macrophages, lymphocytes, neutrophils, and rare eosinophils. Black arrow, mild perivascular cuffing. Star, alveoli filled with foamy macrophages, neutrophils, and cell debris. Black arrowhead, type 2 pneumocyte hyperplasia. (F) C57BL/6 mice were administered PBS (N=6), Corn Oil (N=6), or tamoxifen dissolved in corn oil (Tmx-treated, N=6) i.p. Organs were collected, sections were stained with H&E and scored as in B. Percent lung affected (left) and percent of mice displaying serositis for the indicated organs (right) are shown. Error bars represent means ± sem. *p < 0.05, **p < 0.01; Kruskal-Wallis Test with Dunn’s multiple comparison post-test used for histological scoring and one-way ANOVA with Tukey’s multiple comparison post-test for percent of lung affected.
Previous studies have shown that corn oil may induce peritonitis11. To investigate the possible contribution of the corn oil vehicle to lesion development, a follow-up study was performed in which PBS, corn oil alone, or tamoxifen dissolved in corn oil were administered to mice using the same 5-day treatment regimen followed by 9-day chase period, and multiple organs (including lung, trachea, liver, spleen, and kidney) were examined histologically. All pulmonary lesions occurred within the tamoxifen-treated groups (Fig. 1F, left), and both male and female mice were similarly affected (data not shown). Serositis was variably observed in the tamoxifen and corn oil groups across multiple organs (Fig. 1F), suggesting that the corn oil vehicle induces mild peritonitis, consistent with a previous study11.
Characterization of the pulmonary cellular infiltrates induced by tamoxifen treatment
We next characterized the cellular infiltrates in the lungs of the mice represented in Fig. 1A–E. Forty percent of UI/Tmx mice showed cellular infiltration within the alveoli, lung vasculature, interstitium, and pleura, and 60% showed perivascular cuffing compared to UI/NT mice (Fig. 2A). The cellular populations recruited to the lungs by tamoxifen included macrophages, plasma cells, neutrophils, lymphocytes and to a lesser extent, eosinophils. These cells were likewise observed in influenza-infected tamoxifen-treated mice and persisted well beyond the normal 14-day resolution period for influenza (Fig. 2B). Additionally, we noted type 2 pneumocyte hyperplasia and multinucleated giant cells (Fig. 2C). Collectively, these data suggest that tamoxifen treatment induces abnormal cellular recruitment in lungs independently of cre-recombinase.
Figure 2.
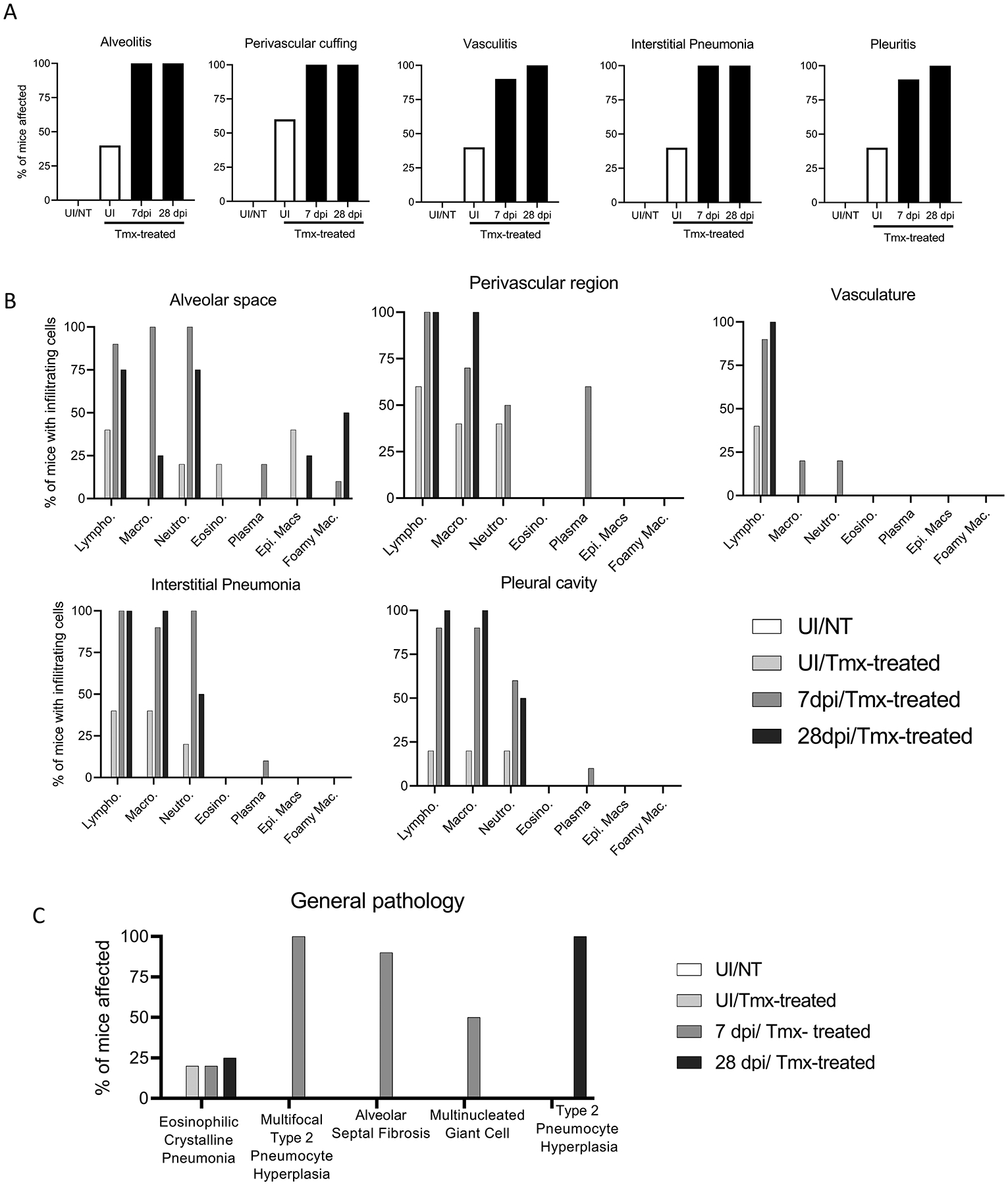
Characterization of the pulmonary cellular infiltrates induced by tamoxifen treatment. (A) H&E-stained lung sections from Fig. 1A–E were further analyzed by a veterinary pathologist blinded to sample identity to determine the percent of mice displaying regional pulmonary inflammation. (B) Percentage of mouse lungs characterized by a given infiltrating cell type as assessed by the pathologist. (C) Other histologic findings of H&E-stained lungs from UI/NT mice, UI/Tmx, 7 or 28 dpi/Tmx mice are presented as average number of mice displaying each lesion.
Discussion
This study shows that intraperitoneal tamoxifen administration induces distal histopathological alterations in the lungs of mice. Excessive recruitment of various immune cell types (most notably macrophages, neutrophils, and lymphocytes) was observed throughout the lungs even in the absence of cre-recombinase activity, suggesting global inflammation of the lung rather than targeted alteration of a single pathway. Furthermore, the presence of foamy macrophages and multinucleated giant cells in tamoxifen-treated mice infected with influenza (Fig. 1E and 2B, C), suggests an abnormal microenvironment driven by chronic inflammation. It should be noted that all mice were separated by group and housed in individual microisolator cages with no chance of cross-infection between uninfected and infected mice.
Tamoxifen-induced lung injury has been noted in human patients in response to treatment demonstrating that tamoxifen can lead to lung histopathologic changes in humans12. In animal models, tamoxifen toxicity has been observed anecdotally in a variety of organs. For example, dramatic changes occur within the gastric mucosa in response to tamoxifen administration via intraperitoneal injection or oral gavage4,5. This toxicity was found to be independent of mouse strain or sex and observed even in the absence of estrogen4. In some studies, this may be attributed to excess tamoxifen administration; however, long-term alterations to spermatogenesis have been noted in mice given a single dose of either 250 μg or 1 mg tamoxifen2.
There is no general consensus on the appropriate dosage or routes of administration for tamoxifen. In a meta-analysis considering 50 studies from 1998 onwards, 85 tamoxifen regimens were published2. Uniformity within the field is challenging because tamoxifen doses must be determined empirically for each cell type and tissue-specific cre-system to ensure appropriate deletion efficiency13. The tamoxifen dose of 75 mg/kg was chosen based upon recommendations from JAX from which our cre-strain was purchased and upon consideration of several relevant studies14–16. Notably, airway epithelial cells were previously shown to undergo recombination using conditions approximating JAX recommendations14. However, this dose, which caused significant confounding effects within the lungs, only achieved 30% deletion efficiency of the floxed allele in Sftpc-creERT2 Tpl2fl/− mice. Given the known immunomodulatory effects of tamoxifen3, it may be difficult to successfully use tamoxifen for gene ablation without triggering unintended consequences.
These data highlight the confounding effects of tamoxifen treatment in mouse lungs that make it difficult to distinguish bona fide infection-driven phenotypes versus the lasting side effects of tamoxifen-treatment. In addition to inclusion of tamoxifen treatment “baseline” controls, a more comprehensive understanding of tamoxifen-induced effects in mice will help to inform researchers considering tamoxifen-inducible models. Overall, this study describes distal, tamoxifen-induced, histopathologic alterations of the lungs that may obscure research interpretations and highlights the need for the development of optimized mouse models for inducible cre-mediated deletion.
Acknowledgements
The authors thank the UGA Coverdell Rodent Vivarium for animal care, our laboratory animal veterinarian, Dr Stephen Harvey, the Athens Veterinary Diagnostic Laboratory, and the UGA Histology Laboratory. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding Acknowledgements
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health [R21AI147003–01] to WTW.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors declare that there is no conflict of interest.
Research Data availability statement
The original data is accessible on request from WTW who can be contacted at: watfordw@uga.edu
References
- 1.Heery M, Corbett P, Zelkowitz R. Precautions for Patients Taking Tamoxifen. J Adv Pract Oncol 2018; 9: 78–83. [PMC free article] [PubMed] [Google Scholar]
- 2.Patel SH, O’hara L, Atanassova N, et al. Low-dose tamoxifen treatment in juvenile males has long-term adverse effects on the reproductive system: Implications for inducible transgenics. Sci Rep; 7. Epub ahead of print 1 December 2017. DOI: 10.1038/s41598-017-09016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behjati S, Frank M. The Effects of Tamoxifen on Immunity. Curr Med Chem 2009; 16: 3076–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huh WJ, Khurana SS, Geahlen JH, et al. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology 2012; 142: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keeley TM, Horita N, Samuelson LC. Tamoxifen-Induced Gastric Injury: Effects of Dose and Method of Administration. CMGH 2019; 8: 365–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brash JT, Bolton RL, Rashbrook VS, et al. Tamoxifen-Activated CreERT Impairs Retinal Angiogenesis Independently of Gene Deletion. Circulation Research 2020; 127: 849–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koliaraki V, Roulis M, Kollias G. Tpl2 regulates intestinal myofibroblast HGF release to suppress colitis-associated tumorigenesis. J Clin Invest 2012; 122: 4231–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dumitru CD, Ceci JD, Tsatsanis C, et al. TNF-α Induction by LPS Is Regulated Posttranscriptionally via a Tpl2/ERK-Dependent Pathway. Cell 2000; 103: 1071–1083. [DOI] [PubMed] [Google Scholar]
- 9.Animals NRC (US) C for the U of the G for the, Laboratory C and U of. Guide for the Care and Use of Laboratory Animals. National Academies Press. Epub ahead of print 27 December 2011. DOI: 10.17226/12910. [DOI] [Google Scholar]
- 10.Kuriakose T, Tripp RA, Watford WT. Tumor Progression Locus 2 Promotes Induction of IFNλ, Interferon Stimulated Genes and Antigen-Specific CD8+ T Cell Responses and Protects against Influenza Virus. PLOS Pathog 2015; 11: e1005038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hubbard JS, Chen PH, Boyd KL. Effects of repeated intraperitoneal injection of pharmaceutical-grade and nonpharmaceuticalgrade corn oil in female C57BL/6J mice. J Am Assoc Lab Anim Sci 2017; 56: 779–785. [PMC free article] [PubMed] [Google Scholar]
- 12.Etori S, Nakano R, Kamada H, et al. Tamoxifen-induced lung injury. Intern Med 2017; 56: 2903–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rawlins EL, Perl AK. The a”MAZE”ing world of lung-specific transgenic mice. American Journal of Respiratory Cell and Molecular Biology 2012; 46: 269–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon H-G, Kim S-J, Jeong JJ, et al. Airway Epithelial Cell-Derived Colony Stimulating Factor-1 Promotes Allergen Sensitization. Immunity 2018; 49: 275–287.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye R, Wang QA, Tao C, et al. Impact of tamoxifen on adipocyte lineage tracing: Inducer of adipogenesis and prolonged nuclear translocation of Cre recombinase. Mol Metab 2015; 4: 771–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madisen L, Zwingman TA, Sunkin SM, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 2010; 13: 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original data is accessible on request from WTW who can be contacted at: watfordw@uga.edu


