Abstract
Background: Incidence of postoperative liver dysfunction continues to be high (ranging from 10 to 35%) in those who underwent cardiac surgeries using cardiopulmonary bypass (CPB) and is associated with considerable morbidity and mortality. Prolonged cardiopulmonary bypass time (CPBT) was found to be an independent predictor of postoperative liver dysfunction. So, the aim of this study was to evaluate the effect of prophylactic use of N-acetylcysteine (NAC) in patients undergoing on-pump cardiac surgery with expected prolonged CPBT in the prevention of liver dysfunction.
Methods: Sixty consenting adult patients undergoing cardiac surgeries using CPB with CPBT more than 120 minutes were included in this single-centre, randomised, parallel-group, double-blinded interventional study. Study group patients received NAC as per the protocol. Liver transferases, alkaline phosphatase, serum bilirubin, kidney function tests, and coagulation parameters were measured preoperatively, on the day of surgery and for 3 days postoperatively.
Results: Values for serum aminotransferase, alanine aminotransferase, and alkaline phosphatase were significantly raised in the control group compared to the study group, starting from the day of surgery till third postoperative day. Serum bilirubin levels (total and direct) were comparable till first postoperative day and were significantly raised on second and third postoperative days in the control group. Duration of mechanical ventilation, total chest tube drainage, the duration of ICU and hospital stay were significantly shorter in study group compared to control group.
Conclusion: Prophylactic intravenous NAC has a protective role in preventing postoperative hepatic dysfunction in patients undergoing cardiac surgery with CPB.
Keywords: N-Acetylcysteine, cardiopulmonary bypass, hepatic dysfunction, prolonged cardiopulmonary bypass time, prophylactic
Main Points
First study that evaluated the role of any specific medication in preventing hepatic dysfunction following cardiac surgery conducted using CPB.
Prophylactic administration of N-acetylcysteine protects against hepatic dysfunction in patients undergoing cardiac surgeries with prolonged cardiopulmonary bypass time.
The duration of mechanical ventilation and the duration of hospital and ICU stay were longer, and total chest tube drainage was higher in control group compared to N-acetylcysteine group.
Incidence of postoperative atrial fibrillation was much lower among N-acetylcysteine-treated group compared to control group.
Introduction
Increasing number of cardiac surgeries is being performed using cardiopulmonary bypass (CPB). In spite of refinement in techniques of CPB and surgery, incidence of postoperative liver dysfunction continues to be high in those who underwent cardiac surgeries using CPB. In late 60s, the incidence of postoperative jaundice (POJ) was reported to be 13%.1 Over the years, the incidence of hepatic dysfunction, defined as hyperbilirubinemia more than 3 mg dL-1, following cardiac surgery using CPB, was reported to be between 10 and 35% (20%,2 23.4%,3 35.1%,4 25.3%,5 26.5%,6 and 10.1%7). This hepatic dysfunction is also associated with increased morbidity (prolonged mechanical ventilation, longer ICU stay, and longer hospital stay)5,8 and mortality (10-25 times more).2,4,5,7–11
The liver is one of the most vital organs and is highly prone to damage while on CPB, and the possibility of liver damage increases owing to the non-pulsatile perfusion, low-flow state, free radicals formation, and increased levels of catecholamines.12 Among other factors, prolonged cardiopulmonary bypass time (CPBT) was found to be an independent predictor of postoperative liver dysfunction.2,10,11 Although several agents like glucocorticoids, serum protease inhibitors like aprotinin and antioxidants, and phosphodiesterase inhibitors were proposed to reduce generalised inflammatory reaction to CPB, none of them found favour with the clinicians owing to various drawbacks.13 No agent, specific to preventing hepatic dysfunction following CPB, is in practice as of now.
N-Acetyl cysteine (NAC) was first used as treatment of paracetamol overdose in 1979. Since then, it has been firmly established as an effective and safe treatment for this condition.14 Later, it was showed that NAC is safe for nonacetaminophen-induced acute liver failure too.15 Prophylactic use of NAC had been associated with reduction in the incidence of postoperative atrial fibrillation (POAF) and all-cause mortality in patients who underwent cardiac surgery.16 In a meta-analysis performed to evaluate the potential benefits of perioperative NAC administration in patients undergoing cardiac surgery in preventing atrial fibrillation (AF), myocardial infarction, stroke, acute kidney injury (AKI), need for renal replacement therapy (RRT), mortality, and total hospital length of stay, it was reported that NAC administration had a beneficial effect only on reducing the risk of AF among all the variables studied.17 Interestingly, the beneficial effects on preventing hepatic injury were not studied.
We hypothesised that the prophylactic use of N-acetylcysteine in patients undergoing on-pump cardiac surgery with expected prolonged CPBs duration will prevent liver dysfunction.
Methods
This single-centre, randomised, parallel-group, double-blinded interventional study was approved by the Institutional Review Board of Dr Ram Manohar Lohia Institute of Medical Sciences, Lucknow, India (IEC No. 10/17), and a written informed consent was obtained from all the subjects participated for the trial. The trial was registered prior to patient enrolment at Clinical Trial Registry of India (www.ctri.nic.in) (CTRI/2018/05/014024).
Inclusion criteria
Age-18-65 years
Sex-both
Expected prolonged bypass duration (>2 hours)
Patient posted for valve surgery, coronary artery bypass graft, or combination of the two.
Exclusion criteria
Known allergy to N-acetylcysteine
Emergency cardiac surgery
Known blood borne infectious disease
Existing deranged liver function test or cirrhosis
End stage renal disease
Planned off-pump cardiac surgery
Sample size determination
It was based on the results of previous studies, wherein expected reduction in the hepatic dysfunction after prophylactic administration of NAC was reported to be 50%, and the overall prevalence of hepatic dysfunction in patients undergoing cardiac surgery with CPB to be about 20%. Using this, the sample size (N) was calculated by using the following formula, n = ; (pooled SD) = 5, d (difference in mean) = 8, P = anticipated population proportion= 20%, relative precision = 5%, Q = free of hepatic dysfunction = 50%, e = allowable error in the estimation = 0.05 (the type 1 error probability associated with this test of this null hypothesis is 0.05). Using the aforementioned formula, the calculated sample size was 24 patients in each group. In order to compensate for possible drop outs, we enrolled 67 consenting patients fulfilling the inclusion criteria. They were randomised into either of the two groups: 33 patients in the study group/group NAC who received prophylactic NAC, and 34 patients in the control group/group C (received equal volume of 5% dextrose). Patients received their routine cardiac medications until the morning of surgery, except ACE inhibitors and angiotensin-2 receptor antagonists. They were premedicated with Tab lorazepam 2 mg night before surgery and oral Tab ranitidine 150 mg night before surgery and on the morning of surgery. After attachment of all standard monitors including invasive blood pressure (radial and femoral) and central venous pressure, patients were induced with standard anaesthesia technique using fentanyl 2–5 mcg kg−1 and etomidate 0.2-0.4 mg kg−1 in titrated dose. Neuromuscular blockage was achieved with atracurium 0.5 mg kg−1. Anaesthesia was maintained with 1% propofol infusion (4-8 mg kg−1 h−1) and fentanyl infusion with intermittent doses of atracurium, supplemented with isoflurane to maintain depth of anaesthesia. Depth of anaesthesia was monitored with the help of bispectral index (BIS) and target BIS below 50. After induction of anaesthesia, median sternotomy was performed, followed by routine aortic and right atrial or bicaval (inferior and superior vena cavae) cannulations. CPB was established using membrane oxygenation, roller pump, a non-heparin-coated circuit, an arterial filter, and an open venous reservoir system. The CPB circuit was primed using acetated Ringer’s solution and 100 mL mannitol. Additional mannitol was given before opening of aortic cross-clamp. During CPB, hypothermia was induced, and flow rate was maintained at a level of 2.4 L min-1 m-2. Perfusion pressure was maintained between 60 and 90 mm Hg by intermittent boluses of phenylephrine. Myocardial protection was achieved by cold hyperkalemic blood cardioplegia and topical cooling with iced slush. Cardioplegia was administered in antegrade and retrograde fashion as per the need of surgery and patient. Transfusion of packed red blood cell concentrates was done when the haematocrit value dropped below 20% on CPB. Other blood products, such as random donor platelet (RDP) and fresh frozen plasma (FFP), were transfused as patient’s condition warranted.
For ICU sedation, midazolam and fentanyl infusion were used. Weaning from mechanical ventilator was done according to patient’s condition and hospital’s protocol. Routine echocardiography and chest skiagram were done to assess postoperative cardiac function as well as rule out any complication. Postoperative chest drain was recorded, and blood loss replaced as per need. Perioperative and postoperative urine output monitoring were done. If urine output remained under 0.5 mL kg-1 h-1 after CBP, intravenous (i.v.) furosemide was given as incremental boluses of 10-20 mg, after assessing adequate volume status. Norepinephrine infusion (0.01-0.1 mcg kg-1 min-1) was started whenever mean arterial blood pressure fell below 70 mm Hg despite adequate filling pressure. Epinephrine infusion (0.02-0.2 mcg kg-1 min-1) was started when cardiac index remained below 2.5 L min-1 m-2. Intra-aortic balloon pump was available for further cardiac support.
In group N patients, 150 mg kg-1 of NAC mixed in 200 mL of 5% dextrose was administered over 15 minutes as i.v. infusion, immediately after induction of anaesthesia and completed prior to surgical incision. This was followed by 50 mg kg−1 of NAC mixed in 500 mL of 5% dextrose administered i.v. over 4 hours. Finally, 100 mg kg−1 of NAC mixed in 1,000 mL of 5% dextrose was administered i.v. over 20 hours.14 In patients of group C, equivalent volume of 5% dextrose was infused over same time period.
Five liver function parameters were measured-serum total and indirect bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and alkaline phosphatase (ALP). International normalised ratio (INR) was also measured to assess early liver dysfunction. Renal function parameters including serum creatinine, blood urea, and 24 hours urine output were measured. All these parameters were measured preoperatively, on the day of surgery after shifting to ICU (POD0) and on first to third postoperative days (POD1, POD2, and POD3). Preoperative heart rhythm and postoperative incidence of atrial fibrillation were also recorded. Hyperbilirubinemia was considered when serum bilirubin level was more than 3 mg dL−1 (51 µmol L−1). Acute liver dysfunction is defined as increase in liver enzymes AST and ALT ≥ 10 times of baseline. Acute renal dysfunction is defined as any of the following-increase in SCr by ≥0.3 mg dL−1 within 48 hours or increase in SCr to ≥1.5 time of baseline or urine volume < 0.5 mL kg−1 h−1 for 6 hours.
Primary outcome measures-absolute values of LFT preoperatively, on day of surgery and first 3 postoperative days.
Secondary outcome measures-acute renal dysfunction and incidence of postoperative AF.
Statistical Package for the Social Sciences (SPSS) version 21.0 (IBM SPSS Corp.; Armonk, NY, USA) was used for data analysing. P ≤ .05 was considered with 95% CI (confidence interval) in this study. The difference in mean values and Chi-square test were utilised for categorical, and paired t-test and independent student t test were applied to compare the preoperative and postoperative liver function and renal function analysis and other parametric data. Significant difference was accepted at P ≤ .05.
Results
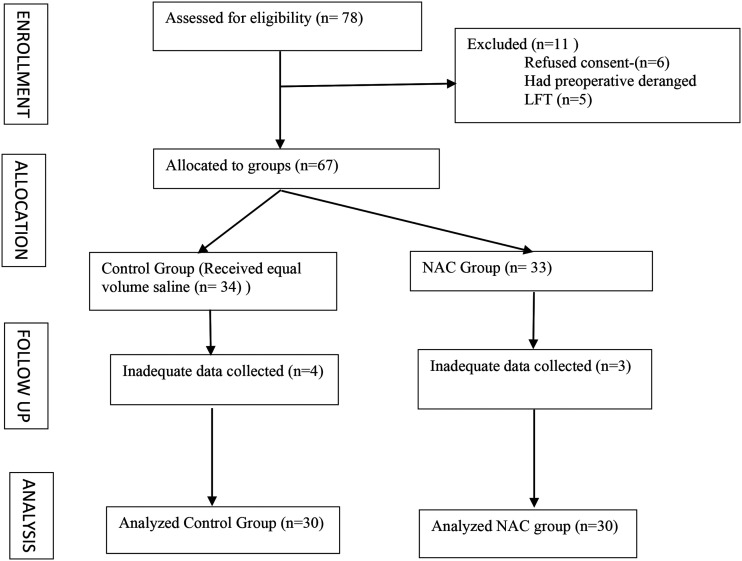
Of the 67 patients included, complete data could not be collected in four patients of control groups and three patients of study group. Hence, the data of 30 patients of each group were analysed.
Table 1 shows the comparison of demographic and preoperative parameters between the group which received prophylactic NAC and the control group. All the parameters were comparable, which shows that the study population selected is homogeneous and comparable between the two groups.
Table 1.
Comparison of Demographic Data and Preoperative Parameters
| NAC Group (n = 30) | Control Group (n = 30) | P | ||
|---|---|---|---|---|
| Age (mean±SD) (years) | 37±15.36 | 44.77±17.16 | .871 | |
| Number of males (%) | 22 (36.7) | 16 (26.7) | .34 | |
| Height (cm) | 161.90±8.26 | 158.93±9.15 | .12 | |
| Weight (kg) | 53.87±9.7 | 50.20±10.05 | .261 | |
| Body surface area (BSA) (m2) | 1.54±0.170 | 1.48±0.163 | .067 | |
| Preoperative rhythm | Sinus rhythm | 15 (25%) | 15 (25%) | .33 |
| Atrial fibrillation | 15 (25%) | 15 (25%) | .33 | |
| Mild to moderate pulmonary hypertension, n (%) | 15 (25) | 15 (25) | .33 | |
| Diabetes mellitus, n (%) | 1 (3.33) | 1 (3.33) | ||
| Systolic blood pressure (SBP) | 115.2±8.98 | 115.47±10.84 | .432 | |
| Diastolic blood pressure (DBP) | 66.50±12.48 | 61.10±12.80 | .213 | |
SD, standard deviation.
Table 2 demonstrates the intraoperative parameters (CPBT, aortic cross clamp time, total duration of anaesthesia, and minimum temperature during CBP), type of surgeries, and intraoperative use of blood products [packed red blood cells (PRBC), fresh frozen plasma (FFP), random donor platelets (RDP)] between the groups.
Table 2.
Comparison of Intraoperative Parameters
| NAC Group (mean±SD) | Control Group (mean±SD) | P | |||
|---|---|---|---|---|---|
| Cardiopulmonary bypass time (minutes) | 217.37±30.96 | 216±38.72 | .12 | ||
| Aortic cross clamp time (minutes) | 189.63±31.64 | 186±40.14 | .14 | ||
| Duration of anaesthesia (hours) | 3.86±50.01 | 3.87±59.43 | .16 | ||
| Minimum temperature of CPB (°C) | 29.10±0.332 | 29.03±0.26 | .23 | ||
| Type of surgeries | AVR, n | 1 (1.7%) | 2 (3.3%) | .11 | |
| MVR, n | 1 (1.7%) | 1 (1.7%) | |||
| MVR + AVR, n | 24 (40%) | 23 (38.3%) | .13 | ||
| MVR + TA, n | 1 (1.7%) | 2 (3.3%) | .16 | ||
| Redo MV, n | 2 (3.3%) | 0 | NA | ||
| VSD + PDA, n | 1 (1.7%) | 0 | NA | ||
| CABG, n | 1 (1.7%) | 2 (3.3%) | .15 | ||
| Intraoperative blood product transfusion (number of units) | PRBC | 0.33±0.54 | 0.40±0.498 | .06 | |
| FFP | 3.3±0.65 | 3.17±0.699 | .17 | ||
| RDP | 3.40±0.62 | 3.30±0.65 | .058 | ||
SD, standard deviation.
Table 3 shows the comparison of liver function test (serum AST, ALT, alkaline phosphatase, and total and direct bilirubin) between the two groups, at preoperative, POD0, POD1, POD2, and POD3 of surgery. The values were comparable preoperatively for all the values. Values for serum ALT, AST, and ALP were significantly raised in the control group compared to the study group, starting from the day of surgery till third postoperative day. Serum bilirubin levels (total and direct) were comparable till first postoperative day and were significantly raised on second and third postoperative days.
Table 3.
Comparison of Absolute Changes in Liver Function Test Between the Groups
| NAC Group (Mean±SD) | Control Group (Mean±SD) | P | ||
|---|---|---|---|---|
| Preoperative | Serum AST (units L−1) | 35.77±19.39 | 37.97±19.05 | .382 |
| POD0 | 75.53±44.84 | 304.6±31.65 | .001 * | |
| POD1 | 108.1±22.2 | 810.23±14.6 | .007 * | |
| POD2 | 124.4±27.49 | 1,163.96±14.3 | .014 * | |
| POD3 | 46.7±70.72 | 622.3±36.84 | .005 * | |
| Preoperative | Serum ALT (units L−1) | 27.6±15.22 | 28.73±16.26 | .12 |
| POD0 | 36.29±18.9 | 174.47±88.16 | .021 * | |
| POD1 | 53.66±91.9 | 482.66±61.08 | .001 * | |
| POD2 | 68.2±17.16 | 779.2±10.31 | .011 * | |
| POD3 | 61.6±14.9 | 850.6±10.32 | .014 * | |
| Preoperative | Serum alkaline phosphatase (units L−1) | 100.67±35.80 | 94.87±30.64 | .056 |
| POD0 | 73.17±19.6 | 99.67±39.23 | .013 * | |
| POD1 | 70.06±19.79 | 98.96±46.78 | .016 * | |
| POD2 | 71.96±18.49 | 124.4±43.54 | .018 * | |
| POD3 | 71.3±24.19 | 124.96±61.86 | .022 * | |
| Preoperative | Serum total bilirubin (mg dL–1) | 0.915±0.379 | 0.88±0.37 | .312 |
| POD0 | 1.17±0.256 | 1.47±0.34 | .136 | |
| POD1 | 1.23±0.317 | 1.67±0.410 | .12 | |
| POD2 | 1.41±0.37 | 2.11±0.69 | .034 * | |
| POD3 | 1.29±0.53 | 2.51±1.01 | .026 * | |
| Preoperative | Serum direct bilirubin (mg dL–1) | 0.251±0.192 | 0.234±0.155 | .212 |
| POD0 | 0.292±0.109 | 0.523±0.225 | .024 * | |
| POD1 | 0.464±0.184 | 0.602±0.195 | .033 * | |
| POD2 | 0.581±0.189 | 0.837±0.32 | .011 * | |
| POD3 | 0.543±0.33 | 1.02±0.61 | .013 * |
SD, standard deviation.
Statistically significant.
Table 4 shows the comparison of coagulation parameters (INR, activated partial thromboplastin time (APTT), and platelet count) and serum electrolytes (serum sodium and potassium) between the two groups. INR and APTT were comparable preoperatively; however, INR was significantly higher in control group from first to third postoperative day, whereas APTT was raised from the day of surgery through third postoperative day. There was no significant difference between the groups in platelet count, serum sodium, and potassium levels at all time points.
Table 4.
Comparison of Coagulation Parameters and Serum Electrolytes among Two Groups
| NAC Group (Mean±SD) | Control Group (Mean±SD) | P | ||
|---|---|---|---|---|
| Preoperative | INR | 1.15±0.152 | 1.14±0.13 | .161 |
| POD0 | 1.28±0.138 | 1.33±0.118 | .214 | |
| POD1 | 1.31±0.128 | 1.4±0.148 | .041 * | |
| POD2 | 1.35±0.112 | 1.45±0.215 | .034 * | |
| POD3 | 1.32±0.101 | 1.43±0.177 | .001 * | |
| Preoperative | APTT (seconds) | 28.46±1.45 | 26.66±1.604 | .053 |
| POD0 | 31.75±6.73 | 29.73±2.62 | .011 * | |
| POD1 | 32.96±6.35 | 31.83±5.50 | .034 * | |
| POD2 | 28.83±3.24 | 29.26±3.81 | .013 * | |
| POD3 | 27.7±2.15 | 29.3±3.82 | .016 * | |
| Preoperative | Platelet count | 1.26±0.42 | 1.40±0.34 | .12 |
| POD0 | 1.20±0.215 | 1.38±0.308 | .147 | |
| POD1 | 1.18±0.28 | 1.23±0.209 | .138 | |
| POD2 | 1.13±0.27 | 1.12±0.22 | .168 | |
| POD3 | 1.16±0.28 | 1.027±0.22 | .127 | |
| Preoperative | Serum sodium (mequiv. L−1) | 134.87±3.24 | 134.97±3.66 | .412 |
| POD0 | 1.37±3.39 | 1.37±3.49 | .341 | |
| POD1 | 1.37±4.12 | 1.37±3.85 | .261 | |
| POD2 | 1.37±3.47 | 1.37±4.65 | .212 | |
| POD3 | 1.36±3.71 | 1.36±4.13 | .314 | |
| Preoperative | Serum potassium (mequiv. L−1) | 3.97±0.32 | 3.93±0.29 | .416 |
| POD0 | 3.92±0.34 | 4.02±0.414 | .054 | |
| POD1 | 4.98±5.57 | 4.09±0.59 | .124 | |
| POD2 | 3.93±0.465 | 3.9±0.398 | .113 | |
| POD3 | 3.90±0.418 | 4.01±0.213 | .067 |
SD, standard deviation.
Statistically significant.
Table 5 shows the comparison of kidney function test (serum creatinine and blood urea) and 24 hours urine output. Serum creatinine values were comparable at all times. Blood urea levels were comparable at preoperative day but were significantly raised in the control group from the day of surgery till third postoperative day. 24 hours urine output was comparable between the groups till first postoperative day but was significantly lower in the control group on the second and third postoperative days.
Table 5.
Comparison of Parameters of Kidney Function Test and 24 Hours Urine Put between the Two Groups
| NAC Group (Mean±SD) | Control Group (Mean±SD) | P | |
|---|---|---|---|
| Serum Creatinine | |||
| Preoperative | 0.915±0.813 | 1.002±0.206 | .061 |
| POD0 | 0.819±0.410 | 1.32±0.56 | .002* |
| POD1 | 1.035±0.69 | 1.505±0.76 | .127 |
| POD2 | 1.23±1.09 | 1.87±1.25 | .134 |
| POD3 | 1.10±0.62 | 1.78±1.23 | .368 |
| Serum Urea | |||
| Preoperative | 29.96±5.50 | 33.33±6.92 | .053 |
| POD0 | 30.86±12.21 | 48.03±18.71 | .013* |
| POD1 | 39.5±26.5 | 56.63±32.35 | .034* |
| POD2 | 45.03±35.78 | 68.9±42.58 | .022* |
| POD3 | 37.06±19.04 | 68.46±40.45 | .003* |
| 24 Hours Urine Output | |||
| POD0 | 1.808±0.319 | 1.45±0.330 | .146 |
| POD1 | 1.58±0.437 | 1.11±0.317 | .113 |
| POD2 | 1.6±0.402 | 0.846±0.343 | .0021* |
| POD3 | 1.63±0.36 | 0.86±0.403 | .0032* |
Statistically significant.
Table 6 shows the comparison of postoperative parameters (duration of mechanical ventilation, total chest tube drainage, duration of stay in ICU and hospital, use of RRT, need for re-exploration, and incidence of POAF).
Table 6.
Comparison of Postoperative Parameters between the Two Groups
| Postoperative Parameters | NAC Group (Mean±SD) | Control Group (Mean±SD) | P |
|---|---|---|---|
| Duration of mechanical ventilation (hours) | 8.83±2.03 | 17.2±9.056 | .002 * |
| Total chest tube drainage (mL) | 558.5±117.07 | 630.67±172.90 | .034 * |
| Duration of stay in hospital (days) | 5.93±0.78 | 7.90±1.82 | .022 * |
| Duration of stay in ICU (hours) | 9.4±0.89 | 13.33±2.03 | .034 * |
| Use of RRT, n (%) | 1 (3.3) | 2 (6.6) | .052 |
| Need for reoperation, n (%) | 0 | 1 (3.3) | .12 |
| Incidence of postoperative AF, n (%) | 10 (33.3) | 17 (56.6) | .011 * |
SD, standard deviation.
Statistically significant.
Discussion
This is the first study that evaluated the role of any specific medication in preventing hepatic dysfunction following cardiac surgery conducted using CPB. Although there is abundant literature to support that hepatic dysfunction is a common occurrence following CPB1–7 and associated with significant morbidity and mortality,2,4,5,7–11 till now no drug had been evaluated to specifically prevent such hepatic dysfunction.
The liver receives about 25% of the cardiac output. The hepatic artery contributes 20-25%, while the portal system supplies 75-80% of the blood flow to the liver. This dual blood supply and the fact that liver can extract as much as 95% of oxygen from the blood both impart it endurance to necrosis following hypoperfusion.18 But, when the insult becomes overwhelming, in the presence of precipitating factors or reduced pre-existing reserve, CPB might unmask liver dysfunction. Preoperative risk factors are right-sided heart failure, moderate to severe TR, pulmonary hypertension, chronic heart failure, New York heart association functional status class II to IV, and low ejection fraction.19 Postoperative liver dysfunction can be because of either a proinflammatory syndrome (with the release of hepatotoxic cytokines) or the haemodynamic changes related to surgery and CPB (i.e., hypotension, low cardiac output syndrome, hypoxia, and RV dysfunction). These may lead to ischemic hepatitis or passive liver congestion or both.19
Prolonged CPB time had been shown in previous studies to be an independent predictor of postoperative hepatic dysfunction.6,10 So, we evaluated the effects of NAC in patients with expected CPB time of more than 120 minutes. The criteria for the diagnosis of hepatitis based on levels of serum enzymes differ with the underlying cause (ischemic, drug induced, etc.). Although previous studies agreed that the aetiology of hepatic dysfunction following CPB could be multifactorial, hepatic ischemia definitely has a role. We took the cutoff of serum level of enzymes for labelling hepatic failure as elucidated for ischemic hepatitis. A serum aminotransferase level at more than equal to 20 times the normal level was considered to be minimum requirement for the diagnosis of hypoxic liver injury,20,21 whereas in a recent review, it was mentioned that 2.5-10 times the upper limit of normal (ULN) value of liver enzymes was sufficient to diagnose hypoxic liver injury.22 We took 10 times the upper limit of normal for serum aminotransferases and alkaline phosphatase as the cut-off level. Regarding serum bilirubin, there seems to be unanimity among authors to label hepatic dysfunction when it exceeds 3 mg dL−1. It needs to be appreciated that criteria for the diagnosis of liver injury/failure do not include raised serum bilirubin level.20,22 This could be because serum bilirubin may be raised in several conditions with normal liver enzymes.23 Furthermore, had raised serum aminotransferase been taken (10 times of ULN) as marker of hepatic dysfunction, the reported incidence could have been different. We measured the serum bilirubin, serum aminotransferases, and serum alkaline phosphatase sequentially from day of surgery till third postoperative day. With cutoff value of serum bilirubin >3 mg dL– 1, the incidence of hepatic dysfunction on POD1, POD2, and POD3 was 33%, 26.35%, and 33.3%, respectively, which were comparable to the figures reported in previous studies.3–6 But, if we label hepatic dysfunction based on serum AST (cut-off value 400 units), the incidences on POD1, POD2, and POD3 were 46.6%, 50%, and 52%, respectively, or based on serum ALT (500 U L−1), the incidences on first 3 days postoperatively were 33%, 43.3%, and 46.6%, respectively. On all these days, by any of the cited criteria, there was no incidence of hepatic dysfunction in the NAC group (Table 3).
We found that serum aminotransferase levels were significantly higher in control group compared to the NAC group from the day of surgery till the third postoperative day11 and also found that there was a significant correlation between prolonged pump time (>100 minutes) and magnitude of rise in AST on POD2. Serum bilirubin (total and direct) levels were comparable between the groups till first postoperative day but became significantly higher in control group from second postoperative day onward (Table 3). Serum bilirubin levels after hepatic dysfunction follows rise in serum transferases.
The INR was significantly higher in the control group from first postoperative day till third postoperative day (Table 4). This difference may be ascribed to the hepatic dysfunction in the control group. The APTT was also prolonged from the day of surgery till third postoperative day (Table 4). APTT may be deranged as a consequence of hepatic dysfunction but, in our case, could also be the result of continuing heparin administration because of hepatic dysfunction. Since the treating physician was free to continue medical management in the postoperative period as per existing protocols, this aspect was beyond the control of the authors.
The duration of mechanical ventilation and the duration of hospital and ICU stays were all statistically longer, and the total chest tube drainage statistically higher in control group compared to NAC group (Table 6). Previous studies also reported that patients with early POJ required prolonged mechanical ventilation, longer stay in ICU, and higher mortality.5,8
Comorbidities associated with the occurrence of postoperative hepatic dysfunction were preoperative deranged liver function (hyperbilirubinemia and raised PT), valvular dysfunction,24 right sided heart failure, and pulmonary hypertension and low EF.19 We had excluded patients with preoperative deranged liver function tests. All patients were optimised prior to surgery with decongestant therapy, so that none of the patients had right-sided heart failure. Many of the patients posted for valve replacement had mild to moderate pulmonary hypertension, but their numbers were comparable among the two groups (Table 1).
The secondary objective of our study was to evaluate the effect of prophylactic NAC on the incidence of AKI and need for RRT in postoperative period following cardiac surgery with prolonged CPB. Although the mean serum creatinine was significantly higher in control group compared to NAC group, only two patients in control group and one patient in NAC group required RRT (P = .053) (Table 6). Urine output, though, significantly, higher in NAC group compared to control group, cannot be relied upon, as most of these patients received diuretic on the advice of treating intensivist whenever urine out fell below 0.5 mL kg−1 h−1. An earlier study reported that NAC was not associated with a decrease in mortality, AKI needing RRT, length of ICU stay in adult patients who underwent major cardiac surgery.25 This was further validated in a recent meta-analysis that evaluated the effects of perioperative NAC administration on reducing the risk of cardiac surgery-induced AKI and found that NAC-treated placebo group had similar rates of occurrence of AKI, change in creatinine levels and in hospital mortality.26 Risk factors strongly associated with the development of postoperative AKI after cardiac surgery include valve replacement surgery, age, redo surgeries, postoperative use of norepinephrine, and dobutamine.27 Among the patients studied, the number of valve replacement surgeries (29 in NAC group vs 28 in control group) and age were comparable. As per the protocol, we used epinephrine infusion for weaning from CPB, so no patient received either adrenaline or dobutamine infusion.
The findings of our study reveal that the incidence of POAF was much significantly lower among NAC-treated group (33.3%) compared to control group (56.6%) (Table 6). This finding had been corroborated by several studies and metanalyses.16,17 POAF had been demonstrated to be caused by oxidative stress induced by CPB. NAC, being a free radical scavenger, effectively reduced the incidence of POAF (as shown in the meta-analysis).28
Possible mechanism of action of NAC
As far as the protective effect of NAC is considered, it was proposed that NAC in nonacetaminophen ALF was shown to increase oxygen utilisation in microcirculation. NAC may act by enhancing the effect of nitric oxide on guanylate cyclase, increasing the formation of 3’,5’ guanosine monophosphate, and thereby resulting in vasodilation.15 Perioperative NAC was used as it is a free radical scavenger antioxidant that may attenuate this physiologic response and reduce post-CPB complications.16,17 It is also claimed to reduce ischemia/reperfusion injury.16
Safety of NAC
We found that NAC administration was safe, and we did not encounter any adverse effects. Preoperative exposure to NAC did not increase postoperative blood loss in the first 72 postoperative hours in the cardiac surgery patient. Furthermore, NAC did not negatively affect PT time, haemoglobin, haematocrit, platelets, or number of transfusion requirements.29 Adverse drug reaction to NAC in patients with acetaminophen overdose was reported to be common, but mostly minor and all reported adverse effects were easily managed.30
Limitations of the study: our study had the following limitations:
Follow-up of the patients for studied parameters except length of ICU stay and hospital stay, and mortality was done only till third postoperative day.
Correlation of risk factors of postoperative dysfunction with the possible risk factors was not evaluated to find out the possibility of which subset of patients is likely to benefit more from prophylactic NAC administration.
Absence of criteria for hepatic dysfunction and failure hampered the calculation of the incidence of each of these entities separately.
Small sample size.
Conclusion
From the findings of our study, we conclude that prophylactic intravenous N-acetylcysteine has a protective role in preventing hepatic dysfunction in patients undergoing cardiac surgery with prolonged CPB. Studies with larger sample sizes are warranted to further validate the findings of our study.
Figure 1.
CONSORT diagram.
Footnotes
Ethics Committee Approval: Ethical committee approval was received from the Institutional Review Board of Dr Ram Manohar Lohia Institute of Medical Sciences, Lucknow, India (IEC No. 10/17).
Informed Consent: Written informed consent was obtained from all participants who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - R.K., S.S.N.; Design - R.K., S.S.N.; Supervision - S.S.N., D.M.; Materials - D.S.; Data Collection and/or Processing - R.K.; Analysis and/or Interpretation - M.B.; Literature Search - S.S.N., V.K.; Writing Manuscript - S.S.N.; Critical Review - V.K., D.M., D.S.
Acknowledgement: We acknowledge the assistance of Dr. Ruchi Mitra in statistical analysis.
Conflict of Interest: The authors have no conflicts of interest to declare.
Financial Disclosure: The study was funded by the authors’ institute as an intramural project. The funding agent had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication (Dr Ram Manohar Lohia Institute of Medical Sciences, Lucknow, INDIA).
References
- 1. Lockey E, McIntyre N, Ross DN, Brookes E, Sturridge MF. Early jaundice after open-heart surgery. Thorax . 1967;22:(2):165–169.. 10.1136/thx.22.2.165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Collins JD, Bassendine MF, Ferner R.et al. Incidence and prognostic importance of jaundice after cardiopulmonary bypass surgery. Lancet . 1983;21: (8334):1119–1123.. 10.1016/S0140-6736(83)92863-5) [DOI] [PubMed] [Google Scholar]
- 3. Chu CM, Chang CH, Liaw YF, Hsieh MJ. Jaundice after open heart surgery: A prospective study. Thorax . 1984;39:(1):52–56.. 10.1136/thx.39.1.52) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang MJ, Chao A, Huang CH.et al. Hyperbilirubinemia after cardiac operation. Incidence, risk factors, and clinical significance. J Thorac Cardiovasc Surg . 1994;108:(3):429–436.. 10.1016/S0022-5223(94)70252-7) [DOI] [PubMed] [Google Scholar]
- 5. An Y, Xiao YB, Zhong QJ. Hyperbilirubinemia after extracorporeal circulation surgery: A recent and prospective study. World J Gastroenterol . 2006;12:(41):6722–6726.. 10.3748/wjg.v12.i41.6722) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mastoraki A, Karatzis E, Mastoraki S, Kriaras I, Sfirakis P, Geroulanos S. Postoperative jaundice after cardiac surgery. Hepatobiliary Pancreat Dis Int . 2007;6:(4):383–387.. [PubMed] [Google Scholar]
- 7. Farag M, Veres G, Szabó G, Ruhparwar A, Karck M, Arif R. Hyperbilirubinaemia after cardiac surgery: The point of no return. ESC Heart Fail . 2019;6:(4):694–700.. 10.1002/ehf2.12447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Michalopoulos A, Alivizatos P, Geroulanos S. Hepatic dysfunction following cardiac surgery: Determinants and consequences. Hepatogastroenterology . 1997;44:(15):779–783.. [PubMed] [Google Scholar]
- 9. Kraev AI, Torosoff MT, Fabian T, Clement CM, Perez-Tamayo RA. Postoperative hyperbilirubinemia is an independent predictor of long term outcomes after cardiopulmonary bypass. J Am Coll Surg . 2008;206:(4):645–653.. 10.1016/j.jamcollsurg.2007.11.021) [DOI] [PubMed] [Google Scholar]
- 10. Nishi H, Sakaguchi T, Miyagawa S, Yoshikawa Y, Sawa Y. Cardiac surgery in patients with Gilbert's syndrome. J Card Surg . 2012;27:(1):60–61.. 10.1111/j.1540-8191.2011.01353.x) [DOI] [PubMed] [Google Scholar]
- 11. Sabzi F, Faraji R. Liver function tests following open cardiac surgery. J Cardiovasc Thorac Res . 2015;7:(2):49–54.. 10.15171/jcvtr.2015.11) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yamada T, Ochiai R, Takeda J, Kikuchi H, Ishibashi M, Watanabe K. Off-pump coronary artery bypass attenuates transient hepatocellular damage after myocardial revascularization. J Cardiothorac Vasc Anesth . 2005;19:(5):603–607.. 10.1053/j.jvca.2005.02.004) [DOI] [PubMed] [Google Scholar]
- 13. Paparella D, Yau TM, Young E. Cardiopulmonary bypass induced inflammation: Pathophysiology and treatment. An update. Eur J Cardiothorac Surg . 2002;21:(2):232–244.. 10.1016/S1010-7940(01)01099-5) [DOI] [PubMed] [Google Scholar]
- 14. Prescott LF, Illingworth RN, Critchley JA, Stewart MJ, Adam RD, Proudfoot AT. Intravenous N-acetylcystine: The treatment of choice for paracetamol poisoning. Br Med J . 1979;2:1097–1100.. 10.1136/bmj.2.6198.1097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mumtaz K, Azam Z, Hamid S.et al. Role of N-acetylcysteine in adults with non-acetaminophen-induced acute liver failure in a center without the facility of liver transplantation. Hepatol Int . 2009;3:(4):563–570.. 10.1007/s12072-009-9151-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu XH, Xu CY, Fan GH. Efficacy of N-acetylcysteine in preventing atrial fibrillation after cardiac surgery: A meta-analysis of published randomized controlled trials. BMC Cardiovasc Disord . 2014;14:(1):52. 10.1186/1471-2261-14-52) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baker WL, Anglade MW, Baker EL, White CM, Kluger J, Coleman CI. Use of N-acetylcysteine to reduce post-cardiothoracic surgery complications: A meta-analysis. Eur J Cardiothorac Surg . 2009;35:(3):521–527.. 10.1016/j.ejcts.2008.11.027) [DOI] [PubMed] [Google Scholar]
- 18. Chacon MM, Schulte TE. Liver dysfunction in cardiac surgery-what causes it and is there anything we can do?. J Cardiothorac Vasc Anesth . 2018;32:(4):1719–1721.. 10.1053/j.jvca.2018.02.037) [DOI] [PubMed] [Google Scholar]
- 19. Di Tomasso N, Monaco F, Landoni G. Hepatic and renal effects of cardiopulmonary bypass. Best Pract Res Clin Anaesthesiol . 2015;29:(2):151–161.. 10.1016/j.bpa.2015.04.001) [DOI] [PubMed] [Google Scholar]
- 20. Ebert EC. Hypoxic liver injury. Mayo Clin Proc . 2006;81:(9):1232–1236.. 10.4065/81.9.1232) [DOI] [PubMed] [Google Scholar]
- 21. Henrion J, Schapira M, Luwaert R, Colin L, Delannoy A, Heller FR. Hypoxic hepatitis: Clinical and hemodynamic study in 142 consecutive cases. Medicine (Baltimore) . 2003;82:(6):392–406.. 10.1097/01.md.0000101573.54295.bd) [DOI] [PubMed] [Google Scholar]
- 22. Waseem N, Chen PH. Hypoxic hepatitis: A review and clinical update. J Clin Transl Hepatol . 2016;4:(3):263–268.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. VanWagner LB, Green RM. Evaluating elevated bilirubin levels in asymptomatic adults. JAMA . 2015;313:(5):516–517.. 10.1001/jama.2014.12835) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sharma P, Ananthanarayanan C, Vaidhya N, Malhotra A, Shah K, Sharma R. Hyperbilirubinemia after cardiac surgery: An observational study. Asian Cardiovasc Thorac Ann . 2015;23:(9):1039–1043.. 10.1177/0218492315607149) [DOI] [PubMed] [Google Scholar]
- 25. Ho KM, Morgan DJ. Meta-analysis of N-acetylcysteine to prevent acute renal failure after major surgery. Am J Kidney Dis . 2009;53:(1):33–40.. 10.1053/j.ajkd.2008.05.019) [DOI] [PubMed] [Google Scholar]
- 26. Mei M, Zhao HW, Pan QG, Pu YM, Tang MZ, Shen BB. Efficacy of N-acetylcysteine in preventing acute kidney injury after cardiac surgery: A meta-analysis study. J Invest Surg . 2018;31:(1):14–23.. 10.1080/08941939.2016.1269853) [DOI] [PubMed] [Google Scholar]
- 27. Ramos KA, Dias CB. Acute kidney injury after cardiac surgery in patients without chronic kidney disease. Braz J Cardiovasc Surg . 2018;33:(5):454–461.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gu WJ, Wu ZJ, Wang PF, Aung LH, Yin RX. N-Acetylcysteine supplementation for the prevention of atrial fibrillation after cardiac surgery: A meta-analysis of eight randomized controlled trials. BMC Cardiovasc Disord . 2012;12:10. 10.1186/1471-2261-12-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wesner AR, Brackbill ML, Systma CS. Effect of preoperative N-acetylcysteine on postoperative blood loss parameters in cardiac surgery patients. Int J Vasc Med . 2011;2011:859020. 10.1155/2011/859020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zyoud SH, Awang R, Syed Sulaiman SA, Sweileh WM, Al-Jabi SW. Incidence of adverse drug reactions induced by N-acetylcysteine in patients with acetaminophen overdose. Hum Exp Toxicol . 2010;29:(3):153–160.. 10.1177/0960327109359642) [DOI] [PubMed] [Google Scholar]



 Content of this journal is licensed under a
Content of this journal is licensed under a