Abstract
While lung ultrasonography (LUS) proved to be a useful diagnostic and prognostic tool in acute phase of COVID 19 pneumonia, its role in detecting long-term pulmonary sequelae has yet to be explored. In our prospective observational study we assessed the potential of LUS in detecting the presence of computed tomography (CT) fibrotic-like changes after 6 months from COVID-19 pneumonia. Patients who were discharged with a diagnosis of severe COVID-19 pneumonia were enrolled. After 6 months from hospital discharge they underwent LUS, chest CT scan and pulmonary function tests. A logistic regression analysis was performed to assess the association between presence of symptoms, LUS score and diffusing capacity for carbon monoxide (DLCO) at 6-month after hospital discharge and CT scan fibrotic-like changes. A second logistic model was performed to assess the value of some predefined baseline factors (age, sex, worst PaO2/FiO2, ventilator support, worst CRP value, worst D-dimer value and worst LUS score during hospitalization) to predict fibrotic-like changes on 6-month CT scan. Seventy-four patients were enrolled in the study. Twenty-four (32%) showed lung abnormalities suitable for fibrotic-like changes. At multivariate logistic regression analysis LUS score after 6 months from acute disease was significantly associated with fibrotic-like pattern on CT scan. The second logistic model showed that D-dimer value was the only baseline predictive variable of fibrotic-like changes at multivariate analysis. LUS performed after 6 months from severe COVID-19 pneumonia may be a promising tool for detection and follow-up of pulmonary fibrotic sequelae.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11739-022-03084-9.
Keywords: COVID-19 pneumonia, Ultrasonography, Pulmonary fibrosis, Lung ultrasound, Computed tomography
Introduction
Much of the knowledge about COronaVIrus Disease 2019 (COVID-19) comes from studies focusing on the acute phase of the disease. On the other hand, few data are available regarding the respiratory sequelae of COVID 19 infection. Referred dyspnoea/breathing difficulties is one of the most frequently reported symptoms in long-COVID syndrome [1–3] but the correlation of symptoms with functional and/or morphologic sequelae remains to be assessed. Considering the high proportion of patients experiencing long COVID [4] the impact of a clinical/diagnostic assessment in symptomatic patients could be very high, both at the single patient level and for the health care systems. Thus, evidence regarding long-term effect on lung function and structure is highly relevant.
An abnormal lung function with impaired diffusing capacity for carbon monoxide (DLCO) has been observed in a significant percentage of patients several months after hospital discharge [5–7]. A higher incidence of DLCO impairment was shown in those who experienced a severe course of the acute disease [8]. In addition, recent studies showed that many patients still have residual lung CT abnormalities 6 months after discharge, with peripherally-distributed ground-glass opacities (GGOs) being the most common findings [6, 7]. Residual radiological changes were significantly associated with the severity of acute disease [6–8].
In the last decades several investigations aimed to detect the long-term fibrotic burden after severe acute respiratory syndrome coronavirus (SARS-CoV) [9, 10]. Since the early phase of the pandemic, the similarities between COVID-19 and SARS have brought to hypothesize a similar risk of progression to lung fibrosis. In addition, the advancing age and multiple comorbidities in COVID-19 hospitalized patients may further increase the severity of long-term pulmonary sequelae. Moreover, the dramatic diffusion of SARS-CoV-2 worldwide makes the possible fibrotic evolution highly impacting in terms of morbidity and mortality [11]. To date several studies aimed to quantify the burden of long-term pulmonary fibrosis in hospitalized patients with COVID-19 pneumonia and identified risk factors predicting persisting lung abnormalities [12–14]. Han et al. showed lung ‘fibrotic-like’ changes in more than one-third of patients after 6 months from severe COVID-19 pneumonia [12]. These changes were associated with older age, acute respiratory distress syndrome, longer in-hospital stays, non-invasive mechanical ventilation and chest CT involvement during acute disease [12]. Lung ultrasonography (LUS) is a safe, versatile and cost-effective imaging modality for the diagnosis and monitoring of many pulmonary diseases in acute care setting [15–17]. Its use has spread up during SARS-CoV-2 pandemic, both for diagnostic and prognostic purposes [18, 19]. In the last decades it has emerged as a promising technique for the screening and burden assessment of fibrosis in interstitial lung disease (ILD), particularly in connective tissue disease (CTD) [20]. In the presence of lung fibrosis, the pulmonary sonographic pattern is characterized by an irregular pleural line with reduced sliding and the presence of vertical artifacts (B-lines) representing the pulmonary interstitial involvement [21, 22]. In particular, B-line burden has shown a high sensitivity for the detection of ILD when compared to CT [23]. Thus, lung ultrasound could theoretically be useful in detecting long-term pulmonary sequelae of COVID-19 pneumonia. To the best of our knowledge most studies focused on the role of LUS in acute COVID-19 infection while limited data are available regarding LUS findings after hospital discharge [24–26].
In our study, we aimed to evaluate the potential of LUS performed at six months in detecting fibrotic-like changes revealed by High-resolution chest tomography (HRCT). Furthermore, we analysed the possible role of clinical, biochemical and echographic parameters recorded during hospitalization for COVID-related pneumonia in predicting long-term evolution to fibrotic-like changes.
Methods
Study population
The study is part of a single centre, prospective, observational study including all adult patients affected by COVID-19 pneumonia and admitted to Luigi Sacco Hospital, Milan, Italy, since February 21st 2020. The study was approved by the local Ethics committee (Protocol number 16088/2020).
Patients discharged from two COVID-19 medium-intensity care units during the first pandemic wave (February–end of May 2020) have been scheduled for a comprehensive follow-up evaluation in our outpatient clinic at 6 months, including clinical assessment, LUS and pulmonary function tests. In the study we included all patients with a diagnosis of severe pneumonia [27] who needed a chest CT scan according to clinical judgement after 6 months from hospitalization. Pre-existing conditions that may mislead the evaluation of lung ultrasound (i.e. congestive heart failure, lung neoplasms, pre-existing lung interstitial diseases) were considered as exclusion criteria.
Clinical, biochemical and LUS data collected during the hospitalization were recorded; moreover, symptoms, oxygen saturation (SO2), heart rate, LUS, pulmonary function tests and CT scan data obtained at 6 months were collected for all the patients.
Pulmonary function tests were performed using a constant-volume plethysmograph (MasterScreen Body; Erich Jaeger GmbH, Würzburg, Germany) following current recommendations [28]. The following items were analysed: forced vital capacity (FVC), forced expiratory volume in one second (FEV1), diffusing capacity for carbon monoxide (DLCO). Diffusion lung capacity for carbon monoxide (DLCO) was measured with the single breath manoeuvre (MasterScreen Body; Erich Jaeger GmbH, Würzburg, Germany), assessing the VA with the inert gas dilution technique [29]. Measured DLCO < 80% of the predicted value indicated pulmonary diffusion impairment. Lung function tests were performed following the latest national and international safety standards for lung function testing during the COVID-19 pandemic [30, 31].
Lung ultrasound
LUS was conducted by trained ultrasonographers who were blinded to CT data and performed with Epiq 7 or CX50 (Philips) ultrasound devices, using a convex probe. US machines setting were optimized following the subsequent modalities: low mechanical index (0.7 or less); a single focus, positioned on the pleural line; no harmonic modality; no persistence.
The examinations were conducted through bilateral scans, dividing the thoracic surface into 12 zones, 6 for lung: two anterior (upper and lower), two lateral and two posterior regions [32]. The anterior axillary, the posterior axillary line and the internipple line were used to divide the regions.
Each lung area was scored as follows:
Score 0: presence of horizontal artifacts (A-line pattern); < 3 B lines can be present.
Score 1: presence of at least 3 B lines in at least one scan of the region; the B lines are well separated and do not merge one in the other. Small subpleural consolidations ≤ 1 cm diameter and irregular pleural line may be present.
Score 2: multiple, converging B-lines, usually determining a so-called “white lung” in at least one scan of the region. Small subpleural consolidations ≤ 1 cm diameter and irregular pleural line may be present.
Score 3: at least one consolidation with major axis > 1 cm in at least one scan of the region.
The presence of pleural effusion was recorded on the record form. Each zone was accurately scanned, with both longitudinal and transversal scanning sections, and scored according to the highest score obtained in that area. Total LUS score was obtained by summing up the scores obtained for each area. As previously reported, the left anterior inferior area was not considered due to likely issues about scoring reliability in that area related to the presence of the heart. Thus, total score was derived from 11 areas (range 0–33) [33].
For each LUS we evaluated total score, bilateral involvement, evidence of pleural line abnormalities, subpleural nodules, consolidations (i.e. with major axis > 1 cm).
Lung CT imaging-CT scanning protocol
High resolution chest CT (HRCT) was performed using a 64-MDCT Brilliance scanner (Philips Medical System, Netherlands). The parameters used for the scanning protocols were as follows: patients in the supine position; end-inspiratory acquisition; tube voltage, 120–140 kVp; automatic tube current modulation: 100–300 mAs; pitch, 0.5; and section thickness after reconstruction, 1.25 mm. Unenhanced CT scans were obtained for all patients.
CT scans were independently reviewed by two expert radiologists, blinded to clinical data. They firstly assessed the presence of lung residual abnormalities due to COVID-19 pneumonia (ground-glass opacities, consolidation, thickening of pleural septa, parenchymal bands) and in particular of fibrotic-like changes, defined as traction bronchiectasis, parenchymal bands and/or honeycombing, according to a previous study by Han et al. [12]. Secondly, to quantify the involvement of the lung, we used a semi-quantitative CT score system, currently used in COVID-19 literature [34], assigning a score from 0 to 5 to each pulmonary lobe, depending on the extension of pulmonary abnormalities (regardless the type of alteration): 0, no lesion; 1, < 5%; 2, 5–25%; 3, 26–49%; 4, 50–75%; 5, > 75%.
Statistical analysis
Categorical variables were reported as counts (percentages) whereas continuous variables were reported as mean (standard deviation) or median (interquartile range, IQR), as appropriate. Univariate and multivariate logistic regression analysis was performed to assess the association between LUS score, presence of symptoms and DLCO 6 months after hospital discharge and CT scan fibrotic-like changes. Only those variables statistically significant at univariate analyses were considered in multivariate models. A second univariate and multivariate logistic regression analysis was performed to assess the value of some predefined baseline factors (age, sex, worst PaO2/FiO2, ventilator support, worst C reactive protein (CRP) value, worst D-dimer value and worst LUS score during hospitalization) to predict fibrotic-like changes on 6-month CT scan. Results were expressed as odds ratio (OR) with their 95% confidence intervals (CI). P values lower than 0.05, two sided, were considered statistically significant. All the statistical analyses were conducted with the statistical software SAS (release 9.4, SAS Institute, Inc., Cary, North Carolina).
Results
Seventy-four patients were enrolled in the study. Median time point for follow-up evaluation was 195 days for clinical visit and LUS (IQR 184–210), 198 days for CT scan (IQR 183–211) and 202 days for pulmonary function tests (IQR 187–215).
Mean age was 65 (IQR 56–73). Male sex was more represented in the study population (54 patients, 73%), and the most common comorbidity was arterial hypertension (42%). Characteristics of study population are summarized in Table 1.
Table 1.
Population characteristics
| Overall population (n. 74) | |
|---|---|
| Age–median (IQR)–years | 65.5 (56.25–73) |
| Sex: female–no. (%) | 20 (27) |
| Coexisting conditions–no. (%) | |
| Hypertension | 31 (22.9) |
| CAD | 9 (6.7) |
| Diabetes | 16 (11.8) |
| Obesity BMI > 30 kg/m2 | 6 (4.4) |
| BMI > 25; ≤ 30 30 kg/m2 | 24 (17.8) |
| Charlson Comorbidity index—median (IQR) | 2 (0–4) |
| Laboratory findings during hospital stay–median (IQR) | |
| Worst CRP value (mg/L) | 107 (99–133) |
| Worst D-dimer value (ng/L) | 1157.5 (615.5–2011.5) |
| Worst P/F ratio | 215 (131–271) |
| LUS during hospital stay* | |
| Worst LUS score–median (IQR) | 17 (12–20) |
| Patients with positive LUS–no. (%) | 68 (98.5) |
| Patients with bilateral involvement–no. (%) | 68 (98.5) |
| Patients with consolidations- no. (%) | 33 (47.8) |
| Transfer to ICU | 2 (2.7) |
| Need for CPAP | 35 (47.3) |
IQR interquartile range, CAD coronary artery disease, BMI body mass index, CRP C reactive protein, P/F ratio PaO2/FiO2, LUS lung ultrasound, ICU intensive care unit, CPAP continuous positive airway pressure
*69 patients underwent LUS during hospital stay
LUS was performed in all patients, showing abnormalities in 50 of them (69.4%), with a median LUS score of 2 (IQR 0–5.25). When compared to LUS during hospitalization a decrease in total score greater than 50% was observed in 76% of patients after 6 months from COVID-19 pneumonia. Thirty-one patients presented bilateral involvement and the posterior inferior areas resulted the most frequently affected. In our population, all large consolidation had reabsorbed and only small subpleural nodules were found, often associated with fragmented and thickened pleural line. All LUS characteristics are summarized in Table 2. Examples of LUS abnormalities are presented in Fig. 1.
Table 2.
LUS findings at six months
| LUS SCORE–median (IQR) | 2 (0–5.25) |
| Patients with positive LUS–no. (%) | 50 (69.4) |
| Patients with bilateral involvement–no. (%) | 31 (41.9) |
| Patients with small subpleural nodules no. (%) | 31 (43.1) |
| Patients with thickened and fragmented pleural line no. (%) | 38 (52.8) |
| Number of involved regions–median (IQR) | 2 (0–4) |
| Negative regions (i.e. score B0)–% | 76.8 |
| Positive regions (i.e. score B1;B2;B3)–% | 23.2 |
| Localization of positive REGIONS–% | |
| Anterior superior | (11.7) |
| Anterior inferior | (8.9) |
| Lateral superior | (9.6) |
| Lateral inferior | (23.5) |
| Posterior superior | (14.5) |
| Posterior inferior | (31.8) |
LUS lung ultrasound
Fig. 1.
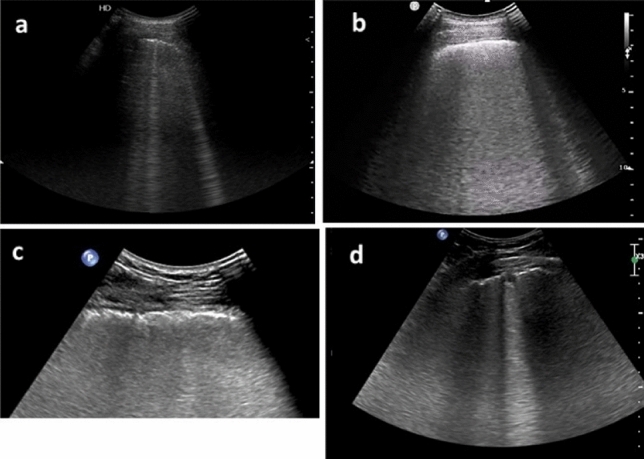
Examples of LUS findings at 6 months after Covid-19 pneumonia. a Interstitial pattern with separated B-lines, LUS score 1. b Interstitial pattern with confluent B-lines, LUS score 2. c Image blow-up for better appreciation of the irregular/fragmented pleural line. d Sub-centimetric pleural nodule, irregular pleural line
All the 74 patients underwent HRCT. Twenty-four out of 74 patients (32%) showed lung abnormalities suitable for fibrotic-like changes, while the remaining patients showed either complete resolution (33/74; 44%) or non-fibrotic-like abnormalities (17/74; 23%) (complete data are provided with Online resource 1, Table e1).
Almost two thirds of patients complained about long-lasting symptoms six months after discharge, with 49% reporting fatigue and/or dyspnoea. The analysis of lung function test, showed as the most significant finding a reduction in DLCO value < 80% of predicted in 27 patients (41.5%) (complete data are provided with Online resource 2, Table e2).
The results of univariate and multivariate analyses assessing the value of LUS to predict fibrotic-like changes on 6-month CT scan are summarized in Table 3. LUS score was significantly associated with fibrotic-like pattern on CT scan at univariate analysis. LUS score maintained the statistically significant association with fibrotic-like changes on CT scan in the multivariate analysis when performed with age as the confounding variable. Based on these findings, we assessed the accuracy of 6-month LUS score in identifying patients with fibrotic-like changes on CT scan by building a ROC curve model. With an AUC of 0.85 (95% CI 0.76–0.93), ROC curve showed that 6-month LUS score is moderately accurate when used as an indicator of 6-month fibrotic-like CT scan pattern. In particular, a LUS score lower than 2 can rule out fibrotic-like changes with a sensitivity of 0.92 (95% CI 0.73–0.99) and a specificity of 0.60 (95% CI 0.45–0.74). The ROC curve analysis for 6-months LUS score is shown in Fig. 2.
Table 3.
Univariate and multivariate analysis
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Six months LUS Score | 1.354 (1.165–1.574) | < 0.0001 | 1.35 (1.14—1.59) | 0.0004 |
| Fatigue and/or dyspnea at six months (presence vs absence) | 0.659 (0.247–1.762) | 0.4063 | − | − |
| Six months DLCO (%)° | 0.998 (0.973–1.024) | 0.9018 | − | − |
Univariate and multivariate logistic regression analysis for the association between 6-month LUS score, symptoms and DLCO and fibrotic-like changes on 6-month CT scan. LUS lung ultrasound, DLCO diffusion lung CO. ° Values are expressed as % of predicted value
Bold values indicate statistical significance at the p < 0.05 level
Fig. 2.
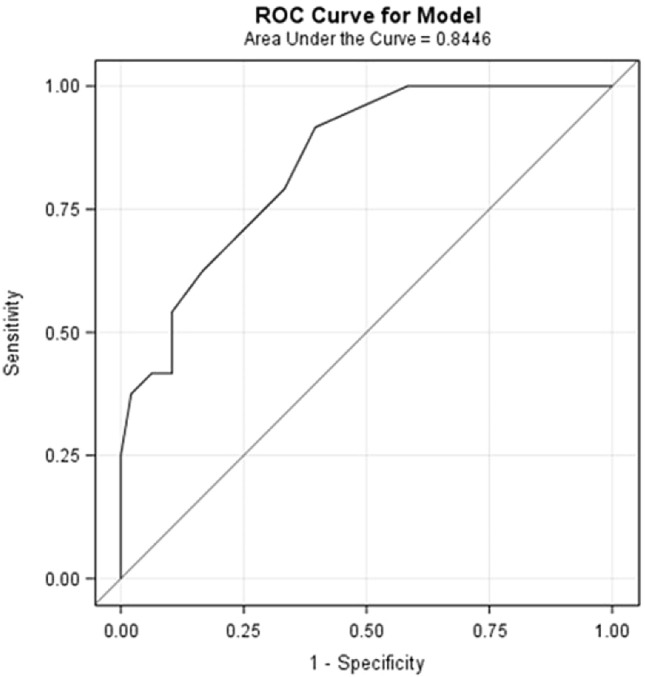
ROC curve model: LUS score accuracy for detecting patients with 6-month fibrotic-like changes on CT scan
The results of univariate and multivariate analyses assessing the value of intra-hospitalization factors (age, sex, worst PaO2/FiO2, ventilator support, worst CRP value, worst D-dimer value and worst LUS score during hospitalization) to predict fibrotic-like changes on 6-month CT scan are summarized in Table 4.
Table 4.
Univariate and multivariate analysis
| Univariate | Multivariate | |||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age (years) | 1.05 (1.002–1.1) | 0.04 | – | ns |
| Sex (male vs female) | 1.63 (0.51–5.17) | 0.41 | – | – |
| Need for MV or CPAP support | 1.78 (0.66–4.77) | 0.25 | ||
| Worst P/F | 0.99 (0.987–1.00) | 0.05 | – | – |
| Worst CPR value (mg/L) | 1.002 (0.997–1.01) | 0.51 | – | – |
|
Worst D-Dimer (ng/L) (> 1157,5 vs ≤ 1157,5) |
11.62 (2.97–45.43) | 0.0004 | 10.32 (2.48- 42.91) | 0.013 |
| Worst LUS score | 1.04 (0.95–1.14) | 0.4 | – | – |
Univariate and multivariate logistic regression for age, sex, ventilator support, respiratory impairment (expressed as P/F), biochemical variables and LUS score during hospitalization as predictors of fibrotic-like changes on 6-month CT scan. MV mechanical ventilation, CPAP continuous positive airway pressure, P/F ratio PaO2/FiO2, CRP C reactive protein, LUS lung ultrasound
Bold values indicate statistical significance at the p < 0.05 level
Univariate analysis showed that only age and worst D-dimer were significantly associated with the presence of six-month fibrotic-like changes. After a multivariate analysis D-dimer value remained the only independent predictive variable of fibrotic-like changes on 6-month lung CT scan.
Discussion
The main finding of our study is that LUS performed after 6 months from a severe COVID-19 pneumonia is a reliable tool in detecting pulmonary fibrotic sequelae. Moreover, D-dimer level during the acute disease was a strong predictor of subsequent development of fibrotic abnormalities.
To our knowledge, this is the first study where LUS was performed after a follow-up of 6 months from COVID-19 pneumonia. When comparing baseline and 6-month LUS findings in our study, a significant improvement of total score was evident in most patients; however, a relevant percentage of patients still had a bilateral involvement after 6 months. These data suggest that COVID-19-associated sonographic abnormalities may take several months to resolve thus adding evidence to what recently reported at a 3-months control by Hernandez-Piriz et al. [35]. Our study observed a significant association between LUS score and the presence of fibrotic-like changes on chest HRCT after a follow-up of 6 months from the acute disease. Our results are consistent with two previous studies, in which patients were evaluated 3 [24] and 2–5 [26] months after COVID-19 hospitalization. In particular, the study by Clofent et al. reported a strong correlation between LUS and CT scores in a patient cohort characterized by a wide range of clinical severity in the acute phase, including a relevant amount of patients who did not require oxygen supplementation [26]. In contrast with our study, a high variability in the timing of follow-up evaluation (median 90 days, IQR, 64–114) was described.
In our study, all patients were evaluated at 6 months; in our population, the percentage of patients with fibrotic-like changes at follow-up chest CT was similar to previous investigations [12, 13]. To note, in our population all patients had a severe COVID-19 pneumonia in acute stage; mean age and need of ventilatory support were higher as compared to Han et al. [12]. Our findings differ from Caruso et al. who reported fibrotic-like changes in 72% of patients at 6-month follow-up [14] probably reflecting a population with increased number of critical patients during acute disease and a different definition of lung fibrosis. Han et al. and Caruso et al. observed that age and the severity of COVID-19 pneumonia were significantly associated to the presence of fibrotic-like changes at chest CT scan after 6 months from acute disease [12, 14]. Although age was associated with the development of pulmonary fibrotic sequelae, D-dimer level during acute disease was the only independent predictive factor in our study. This finding suggests the potential value of this test as a prognostic marker even in the long-term setting of COVID-19 disease.
Although fibrotic-like changes were present at chest CT scan after 6 months from COVID-19 pneumonia in a significant percentage of individuals, their clinical meaning remains debatable. In our study there was not association between symptoms and pulmonary function tests and fibrotic-like changes on chest CT, suggesting the need for further research in order to define the clinical relevance of these radiologic abnormalities. Furthermore, recent studies showed a partial reversibility of fibrotic lesions, thus suggesting that–at least in some cases–they may not reflect a permanent damage to lung parenchyma [36–39]. In this context, it does not seem appropriate to perform a follow-up chest CT for detecting the presence of fibrotic-like changes in all patients with COVID-19 pneumonia. On the contrary, LUS may be the first-line ideal tool in the long-term follow-up of COVID-19 patients due to low cost, absence of radiation and repeatability. Our data suggest that LUS can potentially be used to follow-up lung lesions after hospital discharge. In particular, LUS could be used for ruling-out the presence of fibrotic lesions in patients who have multiple risk factors for evolution to lung fibrosis (older age, severe pneumonia, ventilatory support, elevated D-dimer levels) thus avoiding a CT examination; moreover, the possibility to follow-up lung alterations could allow a watch and wait strategy, particularly in patients without functional abnormalities. Overall, LUS may reduce the need for chest CT scan thus sparing radiation related damage for patients and healthcare economical resources.
Limits
Our study has several limitations.
Firstly, sample size was small and follow-up was too short to determine whether fibrotic-like changes are permanent, progressive or reversible.
Secondly, only a small percentage of patients performed a chest CT scan during hospitalization so we could not assess dynamic CT changes.
Finally, inter-reader agreement was not performed for LUS.
Conclusions
Our data suggest that LUS performed after 6 months from severe COVID-19 pneumonia may be a promising tool for detection and follow-up of pulmonary fibrotic sequelae.
Further studies on greater populations and new evidences on the long-term evolution and clinical impact of fibrotic-like changes at chest-CT will be necessary to better define the role of LUS in long-term management of patients with COVID-19 pneumonia.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank all the health professionals of the Internal Medicine Department of Hospital Luigi Sacco who contributed to the follow-up clinical evaluations and ultrasound examinations.
Author contribution
GR, FC, NF, SI and CC conceived the idea and designed the project; GR and FL performed LUS examinations; NF and SI analysed CT examinations; DR performed lung function tests; GR, FL and FV analysed the data and prepared the figures; GR, FL, FC and CC drafted the manuscript; GC conducted the statistical analyses. All authors read and approved the final manuscript.
Funding
No funding was received for conducting this study.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
All authors declare that they have no conflicts of interest. Conflict of interest disclosure forms for each author are submitted separately as a single file.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the local Ethics Committee (Protocol Number 16088/2020).
Standard of reporting
The items included in the STROBE checklist were addressed during the preparation of the manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Garrigues E, Janvier P, Kherabi Y, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020 doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daher A, Balfanz P, Cornelissen C, et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): pulmonary and extrapulmonary disease sequelae. Respir Med. 2020;174:106197. doi: 10.1016/j.rmed.2020.106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davis HE, Assaf GS, McCorkell L, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38:101019. doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayoubkhani D, Gaughan C. Technical article : Updated estimates of the prevalence of post-acute symptoms among people with coronavirus (COVID-19) in the UK : 26 April 2020 to 1 August 2021. 2021; (April 2020):1–19. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/technicalarticleupdatedestimatesoftheprevalenceofpostacutesymptomsamongpeoplewithcoronaviruscovid19intheuk/26april2020to1august2021#:~:text=1.- Main%20points,data%20to%201%20August%202021
- 5.Huang Y, Tan C, Wu J, et al. Impact of coronavirus disease 2019 on pulmonary function in early convalescence phase. Respir Res. 2020;21(1):1–16. doi: 10.1186/s12931-020-01429-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Q, Zhong L, Li H, et al. A follow-up study of lung function and chest computed tomography at 6 months after discharge in patients with coronavirus disease 2019. Can Respir J. 2021;2021:6692409. doi: 10.1155/2021/6692409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu X, Liu X, Zhou Y, et al. 3-month, 6-month, 9-month, and 12-month respiratory outcomes in patients following COVID-19-related hospitalisation: a prospective study. Lancet Respir Med. 2021;9(7):747–754. doi: 10.1016/S2213-2600(21)00174-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet (London, England) 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu X, Dong D, Ma D. Thin-section computed tomography manifestations during convalescence and long-term follow-up of patients with severe acute respiratory syndrome (SARS) Med Sci Monit Int Med J Exp Clin Res. 2016;22:2793–2799. doi: 10.12659/msm.896985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang P, Li J, Liu H, et al. Long-term bone and lung consequences associated with hospital-acquired severe acute respiratory syndrome: a 15-year follow-up from a prospective cohort study. Bone Res. 2020 doi: 10.1038/s41413-020-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spagnolo P, Balestro E, Aliberti S, et al. Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir Med. 2020;8(8):750–752. doi: 10.1016/S2213-2600(20)30222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han X, Fan Y, Alwalid O, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. 2021;299(1):E177–E186. doi: 10.1148/RADIOL.2021203153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu M, Lv F, Huang Y, Xiao K. Follow-up study of the chest CT characteristics of COVID-19 survivors seven months after recovery. Front Med. 2021;8:636298. doi: 10.3389/fmed.2021.636298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caruso D, Guido G, Zerunian M, et al. Post-acute sequelae of COVID-19 pneumonia: six-month chest CT follow-up. Radiology. 2021;301(2):E396–E405. doi: 10.1148/radiol.2021210834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure the BLUE protocol. Chest. 2008;134(1):117–125. doi: 10.1378/chest.07-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayo PH, Copetti R, Feller-Kopman D, et al. Thoracic ultrasonography: a narrative review. Intensive Care Med. 2019;45(9):1200–1211. doi: 10.1007/s00134-019-05725-8. [DOI] [PubMed] [Google Scholar]
- 17.Chavez MA, Shams N, Ellington LE, et al. Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Respir Res. 2014;15(1):1–9. doi: 10.1186/1465-9921-15-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosso G, Allegorico E, Pagano A, et al. Lung ultrasound as diagnostic tool for SARS-CoV-2 infection. Intern Emerg Med. 2021;16(2):471–476. doi: 10.1007/s11739-020-02512-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji L, Cao C, Gao Y, et al. Prognostic value of bedside lung ultrasound score in patients with COVID-19. Crit Care. 2020;24(1):700. doi: 10.1186/s13054-020-03416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y, Gargani L, Barskova T, Furst DE, Cerinic MM. Usefulness of lung ultrasound B-lines in connective tissue disease-associated interstitial lung disease: a literature review. Arthritis Res Ther. 2017;19(1):206. doi: 10.1186/s13075-017-1409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cogliati C, Antivalle M, Torzillo D, et al. Standard and pocket-size lung ultrasound devices can detect interstitial lung disease in rheumatoid arthritis patients. Rheumatology (Oxford) 2014;53(8):1497–1503. doi: 10.1093/rheumatology/keu033. [DOI] [PubMed] [Google Scholar]
- 22.Barskova T, Gargani L, Guiducci S, et al. Lung ultrasound for the screening of interstitial lung disease in very early systemic sclerosis. Ann Rheum Dis. 2013;72(3):390–395. doi: 10.1136/annrheumdis-2011-201072. [DOI] [PubMed] [Google Scholar]
- 23.Vassalou EE, Raissaki M, Magkanas E, Antoniou KM, Karantanas AH. Lung ultrasonography in patients with idiopathic pulmonary fibrosis: evaluation of a simplified protocol with high-resolution computed tomographic correlation. J ultrasound Med Off J Am Inst Ultrasound Med. 2018;37(3):689–696. doi: 10.1002/jum.14406. [DOI] [PubMed] [Google Scholar]
- 24.Giovannetti G, De Michele L, De Ceglie M, et al. Lung ultrasonography for long-term follow-up of COVID-19 survivors compared to chest CT scan. Respir Med. 2021;181:106384. doi: 10.1016/j.rmed.2021.106384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alharthy A, Abuhamdah M, Balhamar A, et al. Residual lung injury in patients recovering from COVID-19 critical illness: a prospective longitudinal point-of-care lung ultrasound study. J ultrasound Med Off J Am Inst Ultrasound Med. 2021;40(9):1823–1838. doi: 10.1002/jum.15563. [DOI] [PubMed] [Google Scholar]
- 26.Clofent D, Polverino E, Felipe A, et al. Lung Ultrasound as a first-line test in the evaluation of post-COVID-19 pulmonary sequelae. Front Med (Lausanne) 2022;13(8):815732. doi: 10.3389/fmed.2021.815732.PMID:35096906;PMCID:PMC8794580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. (2020). Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance, 13 March 2020. World Health Organization. https://apps.who.int/iris/handle/10665/331446. License: CC BY-NC-SA 3.0 IGO
- 28.Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 29.Graham BL, Brusasco V, Burgos F, et al. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur Respir J. 2017;49(1):1600016. doi: 10.1183/13993003.00016-2016. [DOI] [PubMed] [Google Scholar]
- 30.Milanese M, Corsico AG, Bellofiore S, et al. SIP-IRS. Esami di funzionalità respiratoria nel contesto COVID-19. Published online 2020:1–14. https://irn.sipirs.it/storage/61/Documento-EsamiFunzionalitàRes-Covid_Vers.1_12.05.2020.pdf
- 31.McGowan A, Sylvester K, Burgos F, et al. Recommendation from ERS Group 9.1 (Respiratory function technologists /Scientists) Lung function testing during COVID-19 pandemic and beyond. Ers. Published 2020. https://ers.app.box.com/s/zs1uu88wy51monr0ew- d990itoz4tsn2h
- 32.Bouhemad B, Mongodi S, Via G, Rouquette I. Ultrasound for “lung monitoring” of ventilated patients. Anesthesiology. 2015;122(2):437–447. doi: 10.1097/ALN.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 33.Casella F, Barchiesi M, Leidi F, et al. Lung ultrasonography: a prognostic tool in non-ICU hospitalized patients with COVID-19 pneumonia. Eur J Intern Med. 2021;85(January):34–40. doi: 10.1016/j.ejim.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan F, Ye T, Sun P, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020 doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernández-Píriz A, Tung-Chen Y, Jiménez-Virumbrales D, et al. Importance of lung ultrasound follow-up in patients who had recovered from coronavirus disease 2019: results from a prospective study. J Clin Med. 2021 doi: 10.3390/jcm10143196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu M, Lv F, Zheng Y, Xiao K. A prospective cohort study on radiological and physiological outcomes of recovered COVID-19 patients 6 months after discharge. Quant Imaging Med Surg. 2021;11(9):4181–4192. doi: 10.21037/qims-20-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X, Shen C, Wang L, et al. Pulmonary fibrosis and its related factors in discharged patients with new coronavirus pneumonia: a cohort study. Respir Res. 2021;22(1):203. doi: 10.1186/s12931-021-01798-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Ding C, Yu L, et al. One-year follow-up of chest CT findings in patients after SARS-CoV-2 infection. BMC Med. 2021;19(1):191. doi: 10.1186/s12916-021-02056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martini K, Larici AR, Revel MP, Ghaye B, Sverzellati N, et al. European society of thoracic imaging (ESTI), the European society of radiology (ESR) COVID-19 pneumonia imaging follow-up: when and how? A proposition from ESTI and ESR. Eur Radiol. 2022;32(4):2639–2649. doi: 10.1007/s00330-021-08317-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.


