Abstract
Introduction
Long term care facilities for elderly (LTCFs) in Europe encountered a high disease burden at the start of the COVID-19 pandemic. Therefore, these facilities were the first to receive COVID-19 vaccines in many European countries. A limited COVID-19 vaccine supply early 2021 resulted in a majority of residents and healthcare workers (HCWs) in LTCFs being vaccinated compared to a minority in the general population. This study exploits this imbalance to assess the efficiency of COVID-19 vaccination in containing outbreaks in LTCFs.
Methods
Exploratory statistics were performed using data from a COVID-19 surveillance system covering all 842 LTCFs in Flanders (the northern region of Belgium). The number and size of COVID-19 outbreaks in LTCFs were compared (1) before and after introducing vaccines and (2) with the status of the pandemic in the general population. Based on individual data from 15 LTCFs, the infection rate and symptoms of vaccinated and unvaccinated residents and HCWs were compared during a COVID-19 outbreak.
Results
95.8% of the residents and 90.9% of the HCWs in Flemish LTCFs were vaccinated before May 30, 2021. Before vaccine introduction, residents in LTCFs were 10 times more likely to test positive for COVID-19 than the general population of Flanders. This ratio reversed after vaccination. Furthermore, after vaccination fewer and shorter outbreaks were observed involving fewer residents. During these outbreaks, vaccinated and unvaccinated residents were equally likely to test positive, but positive vaccinated residents were less likely to develop severe symptoms. In contrast, unvaccinated HCWs were more likely to test positive.
Conclusion
In the first half of 2021, two-dose vaccination was highly efficient in preventing and containing outbreaks in LTCFs, reducing COVID-19 hospitalizations and deaths. The high likelihood of unvaccinated HCWs to be involved in COVID-19 outbreaks in vaccinated LTCFs emphasizes the importance of vaccinating HCWs.
Keywords: Healthcare workers, COVID-19, Long term care facilities, Vaccination
1. Introduction
Across the globe long-term care facilities for elderly (LTCFs) suffered high attack rates and disease burden during the COVID-19 pandemic [1]. Residents of these facilities are vulnerable due to a combination of COVID-19 severe disease risk factors, more specifically high age and frequent comorbidities [2]. In Belgium 51% of all reported deaths during the first wave (March 2020 – May 2020) occurred in LTCFs [3]. The same LTCFs were once again heavily involved during the second wave (October 2020 – January 2021) [4]. Therefore, when the European Medicines Agency approved the first COVID-19 vaccines (Comirnaty on December 21, 2020 and Spikevax on January 6, 2021), most European countries prioritized the vaccination of residents and staff in LTCFs [5]. In Belgium, the majority of LTCF residents and staff received their first two vaccine doses between January 2021 and February 2021 during large vaccination events organized in the LTCFs [4].
The effect of the first two doses of mRNA vaccination against COVID-19 in LTCFs has been extensively studied and many countries reported high vaccine effectiveness (VE) in preventing severe disease (hospitalisation and death) in elderly [6]. Several papers reported a decrease in COVID-19 incidence (positive PCR test) in LTCFs in the 28 days after mass vaccination events in these LTCFs [7], [8]. Using individual patient data White et al. (2021) found a similar decrease in case incidence for vaccinated and unvaccinated residents [9]. Prior to vaccination COVID-19 cases in LTCFs were strongly linked to infection rates in the general population and especially in the neighbourhood of the facility [10]. However, more evidence is needed on the impact of vaccination on this link between LTCFs and the general population.
This paper contributes to this open question by investigating the effectiveness of vaccinating HCWs and residents in LTCFs in preventing the spread of infection from the community to LTCFs. In Belgium, elderly in LTFC were the first to receive COVID19 mRNA vaccines in a two-dose schedule (4 weeks interval). Vaccination of HCW in LTFC was initiated shortly afterwards. We compare the number and extent of outbreaks in LTCFs in the entire region of Flanders during pandemic waves before and after the start of the vaccination campaign in LTFCs and compare these outbreaks to the observed infection rate of the pandemic in the general population. In addition, we quantify the associated burden of symptomatic disease in a subset of LTCFs who experienced an outbreak shortly after their first vaccinations. Our observations relate to the period June 2020 to May 2021 in which the Wuhan and alpha strain of the COVID-19 virus were dominant in Belgium.
2. Methods
2.1. Data sources
Aggregated LTCF data. An online COVID-19 surveillance system in LTCFs was organized in Flanders by the Flemish Health Authority. Between June 2nd, 2020 and May 30th, 2020 daily data regarding the past week was collected once a week through an online application from all 842 Flemish LTCFs. The data contains the number of residents and HCWs per LTCF, daily vaccine coverage (partial/full) and COVID-19 infections (positive PCR tests) for both HCWs and residents and daily hospitalizations and deaths for residents with COVID-19 within these LTCFs. This data was pseudonymized and aggregated per LTCF by the Flemish health authority for this study.
Individual LTCF data. A second surveillance data set monitored 15 selected LTCFs that experienced a severe COVID-19 outbreak (>5 cases) between February 1st, 2021 and April 30th, 2021. During this period also the second dose of the COVID-19 vaccine was administered in LTCFs. All LTCFs experiencing a severe outbreak in this period were contacted and the majority participated. The data is recorded at the level of individual residents and HCWs and includes date of vaccination and whether the resident/HCW tested positive during the outbreak. For infected residents and HCWs, the data registers the date of the first positive PCR test, symptom severity, hospitalization and death. The data were pseudonymized.
Population COVID-19 data. Open data on the number of confirmed SARS-CoV-2 cases in Flanders were collected and made available by the Belgian institute for Health (Sciensano, https://epistat.wiv-isp.be/covid/). The number of inhabitants of Flanders was obtained from the Belgian statistical office (StatBel, https://statbel.fgov.be/nl/open-data/bevolking-naar-woonplaats-nationaliteit-burgerlijke-staat-leeftijd-en-geslacht-10). These open data sources allow a comparison between the evolution of the COVID-19 pandemic in LCTFs and the general population.
2.2. Data analysis
Although daily reporting of the aggregated LTCF data was mandatory and monitored by the Flemish health agency, most LTCFs miss information for some dates. On average 80% of the LTCFs reported data on weekdays (Monday – Friday), whereas during the weekend only 20% of the LTCFs complied with the required reporting. Missing residential and HCW population sizes were imputed based on neighboring dates and missing incidences, hospitalizations and deaths are set to zero, assuming no events had occurred. This assumption was inspired by the fact that LTCFs should have been highly motivated to enter data when new cases emerged as this would result in outbreak management support by the health authorities.
A compliant LTCF was defined as an LTCF with less than 50% missing data, more than 10 patients and for which the registered partial and full vaccination coverage of both residents and HCWs exceeded 80% at the end of the study period (May 30th, 2021). These criteria were matched by 545 out of the 842 LTCFs in Flanders.
The daily partial and full vaccination coverage of residents (respectively HCWs) per LTCF was computed by dividing the number of vaccinated residents (respectively HCWs) by the total number of residents (respectively HCWs). Since there are substantial fluctuations in the reported number of HCWs and, to a minor extent, the number of residents, the vaccination coverage fluctuates heavily from day to day. An increasing partial and full vaccine coverage was obtained by using the cumulative maximum of the partial and full vaccine coverage over all previous dates in the analysis. The total partial and full vaccination coverage of an LTCF was computed as the vaccination coverage reached in the LTCF by the end of the study period (May 30th, 2021).
A COVID-19 outbreak was defined as one or more reported cases in residents occurring in the same LTCF with a delay of at most five days between subsequent cases and no reported cases in the five days preceding the first case as well as the five days following the last case. The start and end date of the outbreak were defined as the date of the first and last case in the outbreak, respectively. The size of the outbreak was defined as the total number of infected residents in the LTCF during the outbreak.
3. Results
3.1. COVID-19 vaccination in LTFC in Flanders
Registered full vaccination coverage at the end of the study period (May 30th) was 95.8% for residents and 90.9% for HCWs. In most Belgian LTCFs residents and HCWs were vaccinated on the same date. When this was not possible, vaccination of residents was prioritized. Therefore, the vaccination period for residents and HCWs largely coincides, although on average residents were vaccinated slightly earlier. Of the doses administered for LTCF residents, 80% of the first doses was administered between January 11th and January 25th, 2021 and 80% of the second doses between February 2nd and February 17th, 2021. Of the doses administered for HCWs in LTCFs, 80% of the first doses was administered between January 14th and January 29th, 2021 and 80% of the second doses between February 4th and February 19th, 2021.
3.2. Evolution of COVID-19 cases in Flanders
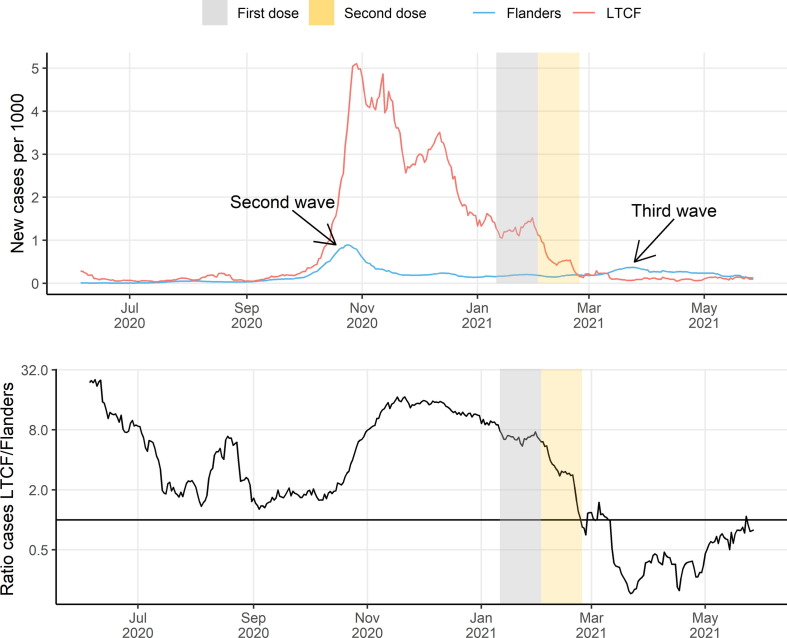
To appreciate the effect of vaccination, we compared incidence rates between LTCF residents and the general public. Fig. 1 (a) shows the evolution of the number of COVID-19 cases per 1000 LTCF residents and per 1000 inhabitants in Flanders. Before March 2021, LTCFs reported similar or higher infection rates as were seen in the general population. LTCFs reported considerably more cases during the second Belgian COVID-19 wave, which started around October 2020. At the peak of the second wave (November and December 2020) weekly incidence ranged from 1.58 to 4.86 cases per 1000 LTCF residents compared to 0.14 to 0.60 cases per 1000 adults in the general population. Between March 2021 and June 2021, incidence rates in LTCFs dropped sharply and fell well below those in the general population. In April 2021, weekly case numbers dropped to 0.05 to 0.14 cases per 1000 LTCF residents compared to 0.24 to 0.32 cases per 1000 adults in the general population. This drop in cases within LTCFs compared to the general population coincided with the vaccination campaign in LTCFs. This shift is better seen on Fig. 1(b) which shows the ratio between the number of cases per 1000 LTCF residents and per 1000 inhabitants in Flanders. The solid line indicates an equal infection rate in LTCFs to that in the general population. At the peak of the second wave, residents of LTCFs were ten times more likely to report an infection than other inhabitants of Flanders. Once most LTCF residents were fully vaccinated (Fig. 1(b), gold area) this ratio flipped over and they became four times less likely to test positive for COVID-19 relative to the general population.
Fig. 1.
Comparison of evolution of confirmed COVID-19 cases in LTCF residents and in the general population. (a) Number of registered, new COVID-19 cases per 1000 inhabitants in LTCFs and in the general population of Flanders. (b) Ratio number of new COVID-19 cases in LTCFs and Flanders per 1000 inhabitants. The silver (resp. gold) areas indicate the time window in which most residents and HCWs received their first (resp. second) vaccination dose.
3.3. Outbreak occurrence and propagation in LTCFs in relation to the general population
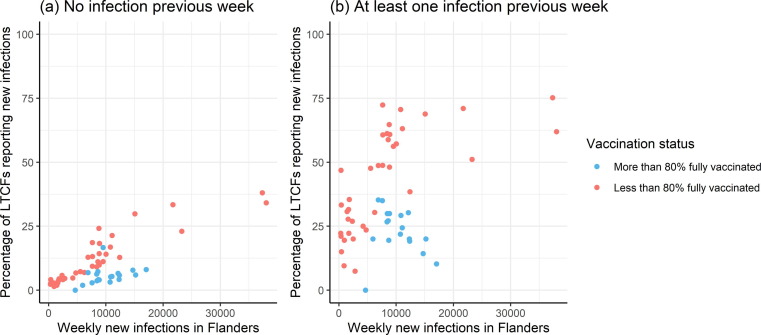
Next to overall incidence, the fraction of LTCF affected by COVID-19 outbreaks is of important from a public health management point of view. Fig. 2 plots the percentage of compliant reporting LTCFs with at least one new infection in a given week against the total number of new COVID-19 cases in Flanders during the same week. Percentages are shown by vaccination status at LTCF level and whether a new case was detected in the previous week. The figure excludes observations from weeks in which there were less than five LTCFs with the selected combination of vaccination status and past infection status. For a given infection rate in the general population, new outbreaks were 2.37 (95% CI 2.13–2.64) times more likely in LTCFs which reported a case during the previous week. In both LTCFs with and without cases in the previous week the probability of new infections increased steadily with the infection rate in the general population. After vaccination, fewer LTCFs report new infections given the same case numbers in the general population. Given an infection rate of 7,500–12,500 weekly cases in the general population, the average probability of a new outbreak dropped from 58.6% before vaccination to 26.1% after fully vaccinating 80% of the residents. Remarkably, after vaccination, the probability that an LTCF with an infection in the previous week also reports an infection in the current week was lower in weeks with a high infection rate in the general population.
Fig. 2.
Percentage of LTCFs reporting at least one new COVID-19 infection per week. Percentage of LTCFs reporting at least one new COVID-19 infection in a given week versus the number of new COVID-19 cases in Flanders during the same week. Results are stratified by vaccination status and whether the LTCF reported at least one COVID-19 case in the previous week.
3.4. Outbreak characteristics
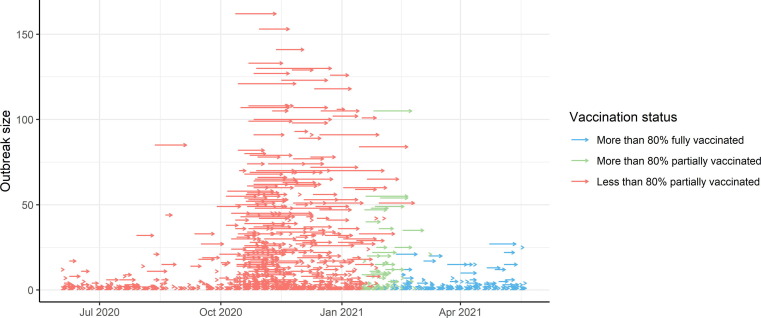
The magnitude of an outbreak has an important impact on local management in LTCF and implies measures to limit additional morbidity and mortality. The data records 1588 outbreaks in LTCFs in Flanders during the study period. Fig. 3 visualizes each outbreak with an arrow, where the start and end of the arrow mark the duration of the outbreak and the position on the y-axes denotes its size. Both the frequency and magnitude of outbreaks drastically increased between October 2020 and January 2021, which corresponds to the second wave of the COVID-19 pandemic. After vaccination, the size of outbreaks reduced to the low levels observed in July-August 2020. The third COVID-19 wave in Belgium (March-April 2021) was not reflected in the size and duration of outbreaks in LTCFs.
Fig. 3.
Evolution of outbreak dynamics in LTCFs in Flanders. Every arrow represents a single outbreak in an LTCF, where the horizontal position marks the start and end of the outbreak and the value on the y-axes denotes the total number of residents infected during the outbreak. Colors of arrows represent vaccination status of the LTCF at the start of the outbreak.
Table 1 shows the average duration, size, number of hospitalizations and deaths per outbreak, stratified by vaccination status of the LTCF at the start of the outbreak. The unvaccinated period is further split into the month June – Sept 2020 when the overall intensity of the pandemic was low and the period Oct 2002 until vaccination which includes the second wave of the COVID-19 pandemic in Belgium. Both periods were similar in terms of testing guidelines, outbreak response protocols and the availability of personal protective equipment. Despite this similarity, outbreaks in the later period lasted longer, infected more residents and resulted in more hospitalizations and deaths compared to both the preceding period when COVID-19 transmission in general was low and the succeeding period after vaccination. After vaccination, outbreak dynamics returned to levels similar to those observed in July-August 2020 despite that COVID-19 incidences remain high in the general population.
Table 1.
Average duration, number of cases, number of hospitalizations and number of deaths per outbreak along with 95% Poisson confidence intervals, in 15 LTFC. Because vaccination dates varied considerably between LTCFs, general periods reflecting ‘partial vaccination > 80%’ and ‘full vaccination > 80%’ were not defined. Vaccination abbreviated as vacc..
| Vaccination status | Number of Outbreaks | Average duration in days | Average number of cases | Average number of hospitalizations | Average number of deaths |
|---|---|---|---|---|---|
| Partial vacc. < 80% (June – Sept 2020) |
264 | 2.2 (2.1–2.4) |
3.5 (3.3–3.8) |
0.34 (0.27–0.41) |
0.14 (0.10–0.19) |
| Partial vacc. < 80% (after Sept 2020) |
956 | 6.5 (6.3–6.6) |
15.8 (15.6–16.1) |
0.93 (0.87–1.00) |
0.97 (0.91–1.04) |
| Partial vacc. > 80% | 109 | 4.4 (4.1–4.9) |
8.9 (8.3–9.4) |
0.42 (0.31–0.56) |
0.40 (0.29–0.54) |
| Full vacc. > 80% | 259 | 2.1 (1.9–2.2) |
2.6 (2.5–2.9) |
0.19 (0.14–0.25) |
0.03 (0.02–0.07) |
3.5. Comparing COVID-19 in vaccinated and unvaccinated residents and HCWs
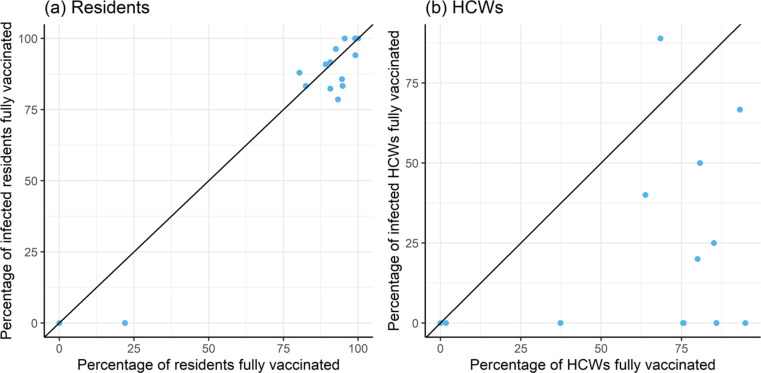
To further assess effect of vaccination, we investigated the relative risk of infection according to vaccination status. Fig. 4 compares, per outbreak, the vaccination coverage in the LTCF at the start of the outbreak and the vaccination coverage within infected cases. The unit line represents the situation where infections are independent of vaccination status. Points below the unit line indicate relatively more infections in unvaccinated residents and HCWs, whereas points above the line indicate more infections in vaccinated residents and HCWs. Applied to residents (panel a) the observed points were close to the unit line. Hence, during outbreaks the probability of testing positive was similar for vaccinated and unvaccinated residents. Applied to HCWs (panel b), almost all points were located below the unit line. This shows that unvaccinated HCWs were much more likely to get infected during a COVID-19 outbreak.
Fig. 4.
Percentage of infected cases in fully vaccinated (a) residents or (b) HCWs against the overall percentage of vaccinated (a) residents or (b) HCWs within 15LTCF. Each dot represents one LTFC.The diagonal line indicates the situation where the distribution of vaccination status is the same among the infected and non-infected (a) residents or (b) HCWs.
Across the 15 outbreaks for which individual data were available, 272 of the 1567 residents tested positive for SARS-CoV-2. Table 2 lists the vaccination status of the residents who tested positive at the time of infection as well as the number of residents per vaccination status and the fraction of these residents developing symptoms, severe symptoms, becoming hospitalized or dying. As a result of the high vaccination coverage in Flemish LTCFs only few infected patients were not vaccinated, which results in broad confidence intervals for the other ratios in the table. Despite these broad confidence intervals, COVID-19 vaccination resulted in significant reductions of the probability of developing severe symptoms or dying following a COVID-19 infection.
Table 2.
Number of infected residents per vaccination status along with symptomatic rate, severe symptomatic rate, hospitalization rate and mortality rate along with 95% Bernoulli confidence intervals, within 15 LTFC.
| Vaccination status | Infected | Fraction symptomatic | Fraction with severe symptoms | Fraction hospitalized | Fraction death |
|---|---|---|---|---|---|
| Not fully vaccinated | 37 | 65 (47–79) | 32 (19–50) | 8 (2–23) | 27 (14–44) |
| Fully vaccinated | 235 | 46 (39–52) | 13 (9–18) | 11 (7–16) | 9 (5–14) |
4. Discussion and conclusion
In Flanders, Belgium, LTCFs for elderly were heavily affected by the second wave (autumn 2020) of the SARS-CoV-2 pandemic, but they largely escaped from the third wave (April 2021). Seroprevalence data indicated higher attack rates in LTCFs (both residents and staff) than in the general population before vaccination started [11]. The current analysis of aggregated data comparing the outbreak dynamics in LTFC to overall population dynamics show how the outbreak pattern in LTFC changed after the vast majority of the residents and HCWs were vaccinated in January-March 2021. As a result, the incidence in LTFC residents was lower during the third wave than in the general population, where vaccination coverage was still low around that time, and the outbreak pattern mimicked the one seen during the low-endemic period July-August 2020, preceding the second wave. Similarly, lower numbers of LTFC outbreaks and lower numbers of cases per outbreak than in previous waves were reported in the third pandemic wave in Germany, shortly after vaccination in LTFC [12].
When more people in the general adult population were vaccinated, the gap in COVID-19 incidence between the general population and LTCFs narrowed. Vaccination of the general adult population started mid-March 2021, with prioritization based on age (elderly) and health status (comorbidity), and by end of May 2021 54% and 24% of adults(18+) in Flanders had received one or two doses, respectively (Sciensano.be). Over this period infections in LTCFs remained low. This is consistent with waning of VE against infection with the variants circulating during the study period which was shown to become apparent only about 6 months after vaccination [13]. Due to the simultaneous vaccination of both HCWs and residents it was not possible in our study to disentangle this joint benefit into the benefits of only vaccinating HCWs or residents.
New outbreaks in LTCFs were more often detected when the COVID-19 infection rate in the general population was high. Vaccination within LTFC thus reduces the risk of new outbreaks, but retains positive association with the status of the pandemic in the general population. This finding corroborates analysis from the US [14] and stresses the need to obtain and keep high population immunity for maximal protection of vulnerable populations.
Once an outbreak was detected, the risk of finding additional cases was considerably lower in LTCFs in which a majority of the residents and HCWs was already vaccinated. Interestingly, after vaccination this risk did not depend on the status of the pandemic in the general population. This independence suggests that after vaccination, additional cases mainly arise via internal transmission. It is unclear how disease propagation within LTCFs compares to other risk groups or to the general public, since, after an initial case detection, a more elaborated screening of residents and HCWs was conducted according to standard practice, which increased the probability to detect additional asymptomatic cases.
Case-based data from a subset of these outbreaks show that mainly unvaccinated HCW were at risk to be involved and thus they may have played a part in the internal transmission. The protective effect of HCW vaccination to prevent outbreaks in LTFC was demonstrated in Japan, where HCW were prioritized to be vaccinated before elderly [15]. Despite high vaccination coverage in LTFCs there was remaining burden among residents, especially the unvaccinated ones, through severe symptomatic infection. These findings emphasize the importance of HCW vaccination and continued non-pharmaceutical interventions to complement vaccine-induced protection in the vulnerable LTFC population.
An important strength of this study is the huge amount of available data. The data were not specifically collected for this analysis and likely suffer from registration errors. However, as registration of both vaccination status and infection status was necessary to receive outbreak management (e.g. testing materials) from health authorities, we presume registration to be rather complete. It is possible that small outbreaks of asymptomatic infections have stayed unnoticed, which may have happened more frequently after vaccination. However, this would not change our major findings.
The results in this paper were obtained over a period in which the Wuhan and alpha strain of SARS-CoV-2 were dominant, and where an epidemic wave arrived immediately after vaccination of LTFC residents. Further investigation is required to see how these results change in the presence of new SARS-CoV-2 strains, or when more time has elapsed after vaccine administration. A European study reported increasing breakthrough outbreaks with high attack rates in LTFC in July-October 2021, during the epidemic wave caused by the delta variant, and stressed the importance of early detection and rapid containment [16]. In Israel a similar rise in LTFC cases during the delta variant, which occurred several months after vaccination, could be counteracted with a booster dose in the LTFC population [17].
The data sources and methods used in the current study allow for a clear illustration of the preventive impact of vaccination within LTFCs not only on hospitalization and death among residents, but also on outbreak risk in LTFCs, even during a period with low immunity in the general population. Since outbreak management puts considerable pressure on HCW within LTFC and on mental health of the residents, the added value of avoiding outbreaks cannot be underestimated.
5. Disclosures
HT, HM and NH initiated the study. JC and TN performed statistical analyses. HT retrieved relevant background literature. JC, HT and TN wrote the first draft. All authors interpreted the results and revised the manuscript for important intellectual content. All authors attest they meet the ICMJE criteria for authorship and have approved the final article.
Funding
TN gratefully acknowledges funding by the Internal Funds KU Leuven (project number 3M190682). NH acknowledges support from the European Union’s Horizon 2020 research and innovation program - project EpiPose (No 101003688) and from the Flemish Research Fund (FWO 1150017N), BV acknowledges support from Ghent University Special Research Fund (COV021-20 BOF) and from the Flemish Research Fund (G.0H44.20N). The funders had no role in study design, data collection, data analysis, data interpretation, writing or submitting of the report.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The universities of Hasselt and Antwerp received funding for grants from GSK Biologicals, Pfizer, Merck and J&J, outside the submitted work.
References
- 1.ECDC Public Health Emergency Team, Kostas D, Laure F, Scarlett G, Côme D, Sibylle B, et al. High impact of COVID-19 in long-term care facilities, suggestion for monitoring in the EU/EEA, May 2020. Euro Surveillance. 2020;25(22). https://doi.org/10.2807/1560-7917.ES.2020.25.22.2000956. [DOI] [PMC free article] [PubMed]
- 2.Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J., et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J Infect. 2020;81(2) doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vandael E., Latour K., Islamaj E., Panis L.I., Callies M., Haarhuis F., et al. COVID-19 cases, hospitalizations and deaths in Belgian nursing homes: results of a surveillance conducted between April and December 2020. Arch Public Health. 2022;80(1) doi: 10.1186/s13690-022-00794-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catteau L, Haarhuis F, Dequeker S, Vandael E, Stouten V, Litzroth A, Wyndham C. Surveillance van de COVID-19 vaccinatie in Belgische woonzorgcentra. Resultaten tot 24 maart 2021. 2021. Dutch. https://www.sciensano.be/nl/pershoek/thematisch-rapport-surveillance-van-de-covid-19-vaccinatie-belgische-woonzorgcentra [accessed 11/03/2022].
- 5.European Centre for Disease Prevention and Control. COVID-19 outbreaks in long-term care facilities in the EU/EEA in the context of current vaccination coverage, 26 July 2021. 2021. https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-in-LTCFs-in-the-EU-EEA-in-the-context-of-current-vaccination-coverage.pdf [accessed 11/03/2022].
- 6.Lopez Bernal J., Andrews N., Gower C., Robertson C., Stowe J., Tessier E., et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ (Clinical research ed.), 2021;373. https://doi.org/10.1136/bmj.n1088. [DOI] [PMC free article] [PubMed]
- 7.Mor V., Gutman R., Yang X., White E.M., McConeghy K.W., Feifer R.A., et al. Short-term impact of nursing home SARS-CoV-2 vaccinations on new infections, hospitalizations, and deaths. J Am Geriatr Soc. 2021;69(8) doi: 10.1111/jgs.17176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Domi M., Leitson M., Gifford D., Nicolaou A., Sreenivas K., Bishnoi C. The BNT162b2 vaccine is associated with lower new COVID-19 cases in nursing home residents and staff. J Am Geriatr Soc. 2021;69(8) doi: 10.1111/jgs.17224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.White E.M., Yang X., Blackman C., Feifer R.A., Gravenstein S., Mor V. Incident SARS-CoV-2 Infection among mRNA-Vaccinated and Unvaccinated Nursing Home Residents. N Engl J Med. 2021;385(5):474–476. doi: 10.1056/NEJMc2104849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabilloud M, Riche B, Etard JF, Elsensohn M-H, Voirin N, Bénet T, et al. COVID-19 outbreaks in nursing homes: A strong link with the coronavirus spread in the surrounding population, France, March to July 2020. PLoS ONE. 2020;17(1). https://doi.org/10.1371/journal.pone.0261756. [DOI] [PMC free article] [PubMed]
- 11.Janssens H., Heytens S., Meyers E., De Schepper E., De Sutter A.n., Devleesschauwer B., et al. Pre-vaccination SARS-CoV-2 seroprevalence among staff and residents of nursing homes in Flanders (Belgium) in fall 2020. Epidemiol Infect. 2020;150 doi: 10.1017/S095026882200036X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suwono B., Steffen A., Schweickert B., Schönfeld V., Brandl M., Sandfort M., et al. SARS-CoV-2 outbreaks in hospitals and long-term care facilities in Germany: a national observational study. The Lancet Regional Health Europe. 2022;14:100303. doi: 10.1016/j.lanepe.2021.100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldberg Y., Mandel M., Bar-On Y.M., Bodenheimer O., Freedman L., Haas E.J., et al. Waning Immunity after the BNT162b2 Vaccine in Israel. The New England J Med. 2021;385(24):e85. doi: 10.1056/NEJMoa2114228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaffney K.K., Jana Broadhurst M., Brett-Major D.M. Mumps to COVID-19: Vaccinated persons remain vulnerable when community uptake is low. Vaccine. 2022;40(12):1691–1694. doi: 10.1016/j.vaccine.2022.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sasanami M., Kayano T., Nishiura H. The number of COVID-19 clusters in healthcare and elderly care facilities averted by vaccination of healthcare workers in Japan, February–June 2021. Mathematical Biosciences and Engineering, 2022;19(3). https://doi.org/10.3934/mbe.2022126. [DOI] [PubMed]
- 16.Suetens C, Kinross P, Gallego BP, Arroyo NV, Hassan E, Calba C, et al. Increasing risk of breakthrough COVID-19 in outbreaks with high attack rates in European long-term care facilities, July to October 2021. Euro Surveillance. 2021;26(49). https://doi.org/10.2807/1560-7917.ES.2021.26.49.2101070. [DOI] [PMC free article] [PubMed]
- 17.Muhsen K., Maimon N., Mizrahi A., Varticovschi B., Bodenheimer O., Gelbshtein U., et al. Effects of BNT162b2 Covid-19 Vaccine Booster in Long-Term Care Facilities in Israel. The New England J Med. 2022;386(4):399–401. doi: 10.1056/NEJMc2117385. [DOI] [PMC free article] [PubMed] [Google Scholar]