Abstract
The SCF (Skp1–Cul1–F-box protein) ubiquitin ligase complex plays a pivotal role in various biological processes, including host-pathogen interactions. Many pathogens exploit the host SCF machinery to promote efficient infection by translocating pathogen-encoded F-box proteins into the host cell. How pathogens ensure sufficient amounts of the F-box effectors in the host cell despite the intrinsically unstable nature of F-box proteins, however, remains unclear. We found that the Agrobacterium F-box protein VirF, an important virulence factor, undergoes rapid degradation through the host proteasome pathway. This destabilization of VirF was counteracted by VirD5, another bacterial effector that physically associated with VirF. These observations reveal a previously unknown counterdefense strategy used by pathogens against potential host antimicrobial responses.
One-sentence summary:
A plant pathogen prevents degradation of its key virulence factor in infected host cells.
Editor’s Summary:
Maintaining Virulence
Agrobacterium tumefaciens is a bacterial pathogen that genetically transforms its hosts, which include several commercially important plants such as grapevines and roses, and induces neoplastic growths termed crown galls (tumors). Agrobacterium-induced genetic transformation and tumorigenesis require the integration of a bacterial DNA molecule into the host genome, a process that involves the virulence factor VirF, an F-box protein that is incorporated into the host cell machinery that targets proteins for degradation. Many F-box proteins, including VirF, are themselves unstable and become degraded, leading Magori and Citovsky to investigate how Agrobacterium stabilizes VirF. They found that VirD5, another effector protein produced by Agrobacterium, associated with VirF and prevented its degradation. Indeed, a strain of Agrobacterium lacking VirD5 formed fewer tumors on tomato plants than did a wild-type strain with VirD5. These findings suggest a general strategy by which other pathogens may stabilize their F-box effector proteins in infected cells.
INTRODUCTION
The pathogen Agrobacterium genetically transforms plants, causing crown gall disease, and, under laboratory conditions, can transform virtually any eukaryotic species, from fungal to human cells (1). During transformation, a single-stranded copy of the bacterial transferred DNA (T-DNA) is exported into the host cell and ultimately integrated into the host genome (2). Within the host cell, the T-DNA is packaged into a nucleoprotein complex (T-complex) in which it is coated with the bacterial virulence (Vir) protein VirE2 (3). In addition, the plant factor VIP1 (VirE2-interacting protein 1) directly binds to VirE2, facilitating nuclear import of the T-complex and its subsequent targeting to the host chromatin (4-6). Upon Agrobacterium infection, host plants activate the mitogen-activated protein kinase (MAPK) defense signaling, leading to phosphorylation of VIP1 (7). This phosphorylation event enables the translocation of VIP1 into the nucleus, where it induces expression of several stress-responsive genes, including the pathogenesis-related PR1 (7, 8). This finding suggests that Agrobacterium co-opts the host defense response to efficiently transport the T-complex into the nucleus.
Once the T-complex reaches the host nucleus, it needs to be uncoated before T-DNA integration or its expression. This is mediated by the Agrobacterium F-box protein VirF, one of the bacterial effectors that are translocated into the host cell (9). As a subunit of the SCF (Skp1–Cul1–F-box protein) ubiquitin E3 ligase complex, VirF targets VIP1 as well as its associated protein VirE2 for ubiquitin-mediated, proteasome-dependent degradation (9). This VirF-mediated targeted proteolysis seems to be indispensable for Agrobacterium infection because deletion of the virF locus or mutations in the F-box domain of VirF substantially attenuate virulence (10-12).
An infection strategy that uses pathogen-encoded F-box proteins is employed not only by Agrobacterium but also by other viral (13-15) and bacterial pathogens (16), including the human pathogen Legionella pneumophila (17, 18). The apparent paradox of this strategy is that F-box proteins are inherently short-lived because of their own proteolysis, which is mediated by autoubiquitination activity (19, 20) or other E3 ubiquitin ligases (21-23). Assuming that only a limited amount of virulence proteins is translocated into the host cell, it would be critical for pathogens to protect their F-box effectors against degradation. Invading pathogens do not export the gene that encodes the F-box effector to the host cell and therefore must stabilize their exported F-box proteins. How this challenge is met by the pathogens remains unknown. Here, we demonstrate that Agrobacterium uses another exported virulence protein, VirD5, to stabilize VirF, which otherwise undergoes rapid degradation through the host ubiquitin-proteasome pathway. The role of VirD5 in the infection process was confirmed by the observations that an Agrobacterium strain lacking VirD5 exhibited significantly attenuated virulence on tomato plants. Thus, we propose that Agrobacterium has evolved VirD5 to counteract the host-induced degradation of VirF, thereby maximizing infection efficiency.
RESULTS
VirD5 is localized to the cell nucleus
VirD5 is a bacterial host range factor (24) that shows no apparent homology to other proteins, making it difficult to determine its biological function. Although early genetic studies had indicated that VirD5 is not essential for Agrobacterium pathogenesis (25-28), additional work demonstrated that VirD5 is translocated into the host cell (24), suggesting that it may play a role within the host cell during Agrobacterium infection. Consistent with this notion, VirD5 (accession number, AAF77175) contains four potential eukaryotic nuclear localization signals (NLSs): 170KRKR173, 324RRLGAPERTAYERWSKR340, 766KKDLEAKSVGVRQKKKE782, and 817RRVYDPRDRAQDKAFKR833. Indeed, the cyan fluorescent protein (CFP)–tagged VirD5 (CFP-VirD5) protein was observed exclusively in the cell nucleus when transiently expressed in Nicotiana benthamiana leaves (Fig. 1A). Thus, our data further suggest that Agrobacterium VirD5 has evolved to function in plant cells and, more specifically, in the plant nucleus.
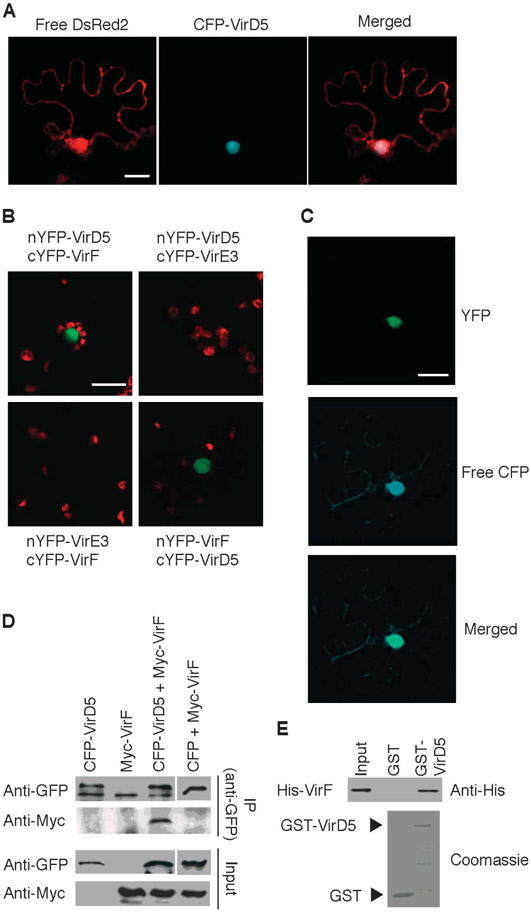
Fig. 1.
VirD5 interacts with VirF in the nucleus. (A) CFP-tagged VirD5 was transiently coexpressed with free DsRed2 in N. benthamiana leaf epidermal cells by microbombardment. CFP-VirD5 colocalized with the nuclear DsRed2. Scale bar, 20 μm. Images are single confocal sections. (B) In vivo BiFC assay for the VirD5-VirF interaction in N. benthamiana leaves. VirE3 was used as negative control. Scale bar, 20 μm. YFP signal is in green, and plastid autofluorescence is in red. (C) Nuclear localization of the VirD5-VirF complexes. The YFP signal is derived from the interacting nYFP-VirD5 and cYFP-VirF. Free CFP is a reference protein that visualizes the cell cytoplasm and the nucleus. YFP and CFP signals are in green and blue, respectively; merged image represents overlay of the BiFC and CFP signals. Scale bar, 20 μm. All images are single confocal sections. (D) Coimmunoprecipitation assay for the VirD5-VirF interaction. CFP-VirD5 and Myc-VirF expressed in N. benthamiana leaves were immunoprecipitated (IP) with anti-GFP antibody, followed by immunoblotting with anti-GFP or anti-Myc antibody. (E) GST pull-down assay for the VirD5-VirF interaction. His-VirF was detected by anti-His antibody (top). GST and GST-VirD5 proteins were detected by Coomassie blue staining (bottom). Each experiment was performed at least twice with similar results.
VirD5 interacts with VirF
To elucidate the role of VirD5 in Agrobacterium infection, we next examined possible interactions between VirD5 and other known exported Vir proteins (VirD2, VirE2, VirE3, and VirF) (24, 29) in plants, using bimolecular fluorescence complementation (BiFC) (30). Only VirF interacted with VirD5 (Fig. 1B). When coexpressed in leaf epidermal cells of N. benthamiana, the N-terminal half of yellow fluorescent protein (YFP) fused to VirD5 (nYFP-VirD5) and the C-terminal half of YFP fused to VirF (cYFP-VirF) led to reconstitution of the YFP fluorescence (Fig. 1B), indicating interaction between the proteins. Similarly, the reciprocal combination (nYFP-VirF and cYFP-VirD5) also restored YFP fluorescence (Fig. 1B). The interacting complexes were observed exclusively in the cell nucleus and colocalized with the nuclear portion of coexpressed CFP (Fig. 1C), potentially reflecting the nuclear nature of both VirD5 (Fig. 1A) and VirF (9). No YFP signal was detected when cYFP-VirF was coexpressed with nYFP-VirE3, another Agrobacterium effector that is translocated into the host cell (24), or when nYFP-VirD5 was coexpressed with cYFP-VirE3 (Fig. 1B). In addition, nYFP-VirD5 did not interact with cYFP-VBF (VIP1-binding F-box protein) (fig. S1), a plant nuclear F-box protein that mimics the molecular function of VirF (31). We next confirmed the VirD5-VirF interaction in plant cells by an independent approach. CFP-VirD5 and Myc-tagged VirF (Myc-VirF) were transiently expressed in N. benthamiana leaves. Myc-VirF coimmunoprecipitated with CFP-VirD5 but not with free CFP (Fig. 1D). In an in vitro interaction assay, glutathione S-transferase (GST)–tagged VirD5 (GST-VirD5) specifically pulled down His-tagged VirF (His-VirF) (Fig. 1E). Together, these observations demonstrate that VirD5 interacts with VirF.
The F-box protein effector VirF is stabilized by VirD5
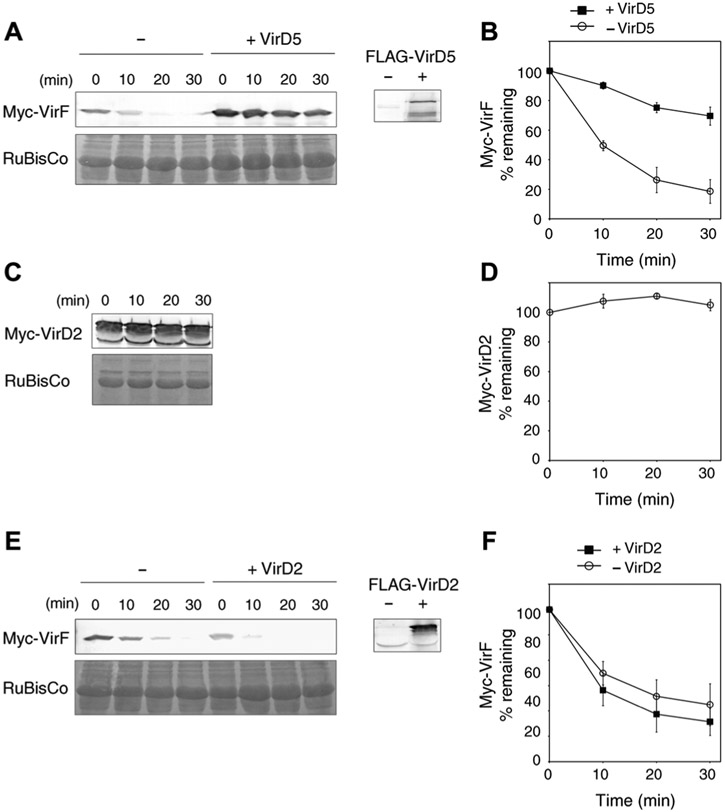
F-box proteins are intrinsically unstable (19, 20). Therefore, the direct binding of VirD5 to VirF may function to stabilize the latter, ensuring that a sufficient amount of the bacterial F-box protein effector is available in the host cell to promote efficient infection. To test this hypothesis, we first examined whether coexpression of VirF and VirD5 in N. benthamiana alters the cellular amounts of VirF. Indeed, VirF accumulated at higher amounts in the presence of VirD5 in plant cells (fig. S2). Then, we analyzed the stability of VirF in the presence or absence of VirD5 by a cell-free degradation assay. Protein extracts were prepared from N. benthamiana plants transiently expressing Myc-VirF with or without FLAG-tagged VirD5 (FLAG-VirD5). In plant extracts containing Myc-VirF alone, VirF protein amounts rapidly declined over 30 min (Fig. 2, A and B). However, this degradation of VirF was delayed in the presence of FLAG-VirD5 (Fig. 2, A and B). In contrast, Myc-VirD2 was stable over 30 min, indicating that VirF destabilization in plant extracts is specific (Fig. 2, C and D). The stabilization effect of VirD5 on VirF was also specific because FLAG-VirD2 did not affect the degradation of VirF (Fig. 2, E and F).
Fig. 2.
VirF degradation and its stabilization by VirD5. (A) Myc-VirF is destabilized in a cell-free degradation assay. Myc-VirF was expressed alone (−) or coexpressed with FLAG-VirD5 (+VirD5) in N. benthamiana leaves. The resulting protein extracts were incubated for the indicated time periods. The putative RuBisCO large chain was used as loading control. The presence of FLAG-VirD5 in the plant extract was confirmed with anti-FLAG antibody (right). (B) Quantification of Myc-VirF degradation described in (A). (C) Myc-VirD2 is stable in the cell-free degradation assay. (D) Quantification of Myc-VirD2 degradation described in (C). (E) FLAG-VirD2 has no effect on VirF degradation. Myc-VirF was expressed alone (−) or coexpressed with FLAG-VirD2 (+VirD2). The presence of FLAG-VirD2 in the plant extract was confirmed with anti-FLAG antibody (right). (F) Quantification of Myc-VirF degradation described in (E). In all quantifications, relative protein amounts were normalized to the RuBisCO loading control. All quantified data are means ± SEM (n = 3 experiments).
VirF is targeted for degradation through the host ubiquitin-proteasome pathway
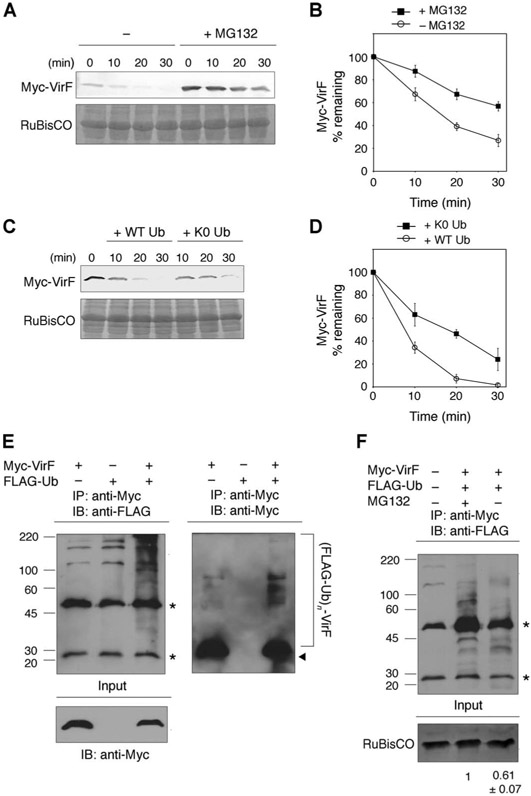
To shed light on the mechanism underlying the VirF degradation, we first tested the effect of the proteasome inhibitors MG132 and lactacystin on the stability of VirF. Pretreatment of N. benthamiana leaves with either proteasome inhibitor prolonged the half-life of VirF (Fig. 3, A and B, and fig. S3, A and B), suggesting that VirF is degraded through the host ubiquitin-proteasome pathway. Consistent with this notion, exogenous addition of the K0 ubiquitin mutant, which lacks all lysine residues required for ubiquitin polymerization, delayed the degradation of VirF (Fig. 3, C and D). These results prompted us to investigate whether VirF undergoes ubiquitination in vivo. For this purpose, Myc-VirF and FLAG-tagged ubiquitin (FLAG-Ub) were coexpressed in N. benthamiana leaves. After pretreatment of the leaves with MG132 to enrich the polyubiquitinated VirF species, total protein was extracted and immunoprecipitation was carried out with anti-Myc antibody. Subsequent immunoblot analysis using anti-FLAG or anti-Myc antibody revealed high–molecular weight protein species, an indication of polyubiquitination (Fig. 3E). The signal intensity of the high–molecular weight species was reduced when the leaves were pretreated with dimethyl sulfoxide (DMSO) instead of MG132 before harvesting (Fig. 3F). Together, these results indicate that VirF is polyubiquitinated and targeted for the proteasome-dependent degradation in the host cells.
Fig. 3.
VirF is degraded through the host ubiquitin-proteasome pathway. (A) Effect of the proteasome inhibitor MG132 on VirF degradation. N. benthamiana leaves transiently expressing Myc-VirF were treated with either DMSO (−) or 10 μM MG132 (+MG132) for 4 hours and analyzed by the cell-free degradation assay. (B) Quantification of Myc-VirF degradation described in (A). (C) VirF degradation is delayed by the K0 ubiquitin mutant. Recombinant wild-type (WT) or K0 ubiquitin (K0 Ub) (1 μg/μl) was added to protein extracts, and the stability of Myc-VirF was analyzed by the cell-free degradation assay. (D) Quantification of Myc-VirF degradation described in (C). (E) Ubiquitination of VirF. Myc-VirF and FLAG-ubiquitin were transiently coexpressed in N. benthamiana leaves. After pretreatment of the leaves with 10 μM MG132 for 4 hours, total proteins were immunoprecipitated (IP) with anti-Myc antibody, followed by immunoblotting (IB) with anti-FLAG (left) or anti-Myc antibody (right). Polyubiquitinated VirF species are indicated by a bracket [(FLAG-Ub)n–VirF]. Arrowhead indicates nonubiquitinated VirF. (F) Omission of pretreatment of plants with MG132 reduced the amount of polyubiquitinated VirF. Asterisks indicate immunoglobulin G (IgG) heavy and light chains. Molecular mass markers (in kilodaltons) are indicated on the left. Relative polyubiquitinated VirF amounts are shown below the blot. All quantified data are means ± SEM (n = 3 experiments).
The autocatalytic mechanism of F-box proteins is not involved in the degradation of VirF
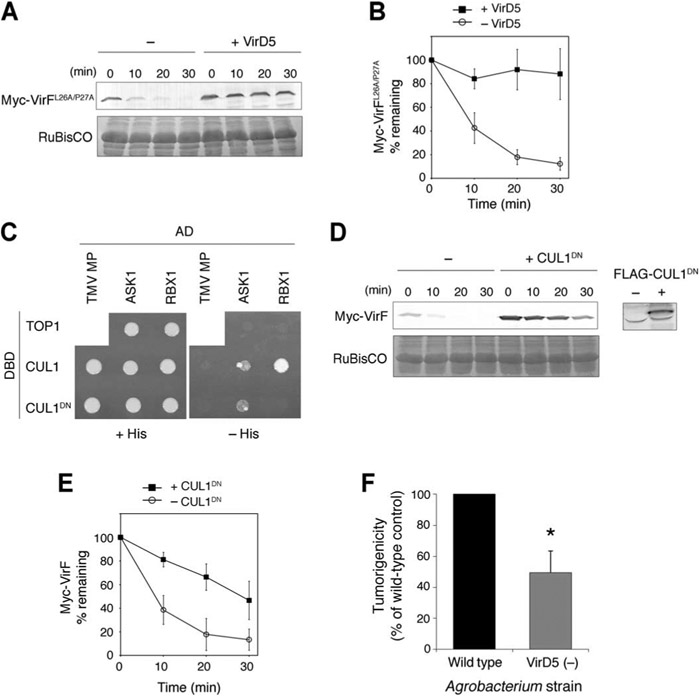
Several F-box proteins promote their own degradation through auto-ubiquitination, which requires an intact F-box domain and its association with the core SCF (19, 20). However, this is not likely for VirF because mutations in its F-box domain (Leu26→Ala and Pro27→Ala), which abolish the interaction of VirF with ASK1 (Arabidopsis SKP1-like 1) (10), did not substantially affect the degradation of VirF (Fig. 4, A and B). Moreover, VirD5 could still stabilize the mutant form of VirF (Fig. 4, A and B). These results suggest that VirF degradation may be mediated primarily by other E3 ubiquitin ligases rather than the auto-catalytic mechanism.
Fig. 4.
Mechanism of VirF degradation and the requirement of VirD5 for Agrobacterium infection of tomato. (A) Cell-free degradation assay with the mutant VirF (VirFL26A/P27A). Myc-VirFL26A/P27A was expressed alone (−) or coexpressed with FLAG-VirD5 (+VirD5) in N. benthamiana leaves. The resulting protein extracts were incubated for the indicated time periods. The putative RuBisCO large chain was used as loading control. (B) Quantification of Myc-VirFL26A/P27A degradation described in (A). (C) CUL1DN interacts with ASK1, but not RBX1, in a yeast two-hybrid system. Yeast cells expressing the indicated LexA DNA binding domain (DBD) fusions and GAL4 activation domain (AD) fusions were grown in the presence (+His) or absence of histidine (−His). Tobacco mosaic virus movement protein (TMV MP) and Saccharomyces cerevisiae DNA topoisomerase I (TOP1) were used as negative controls. (D) Effect of CUL1DN on VirF degradation. Myc-VirF was expressed alone (−) or coexpressed with FLAG-CUL1DN (+CUL1DN) in N. benthamiana leaves. The resulting protein extracts were incubated for the indicated time periods. The presence of CUL1DN in the plant extract was confirmed with anti-FLAG antibody (right). (E) Quantification of Myc-VirF degradation described in (D). In all quantifications, relative protein amounts were normalized to the RuBisCO loading control. All quantified data are means ± SEM (n = 3 experiments). (F) VirD5 is necessary for efficient infection of tomato by Agrobacterium. Tomato seedlings were inoculated with either a wild-type Agrobacterium strain or a mutant strain that does not express VirD5 [VirD5 (−)]. Tumorigenicity was quantified as percentage of inoculation sites (50 to 70 per experiment) that developed a tumor and expressed as means ± SD (n = 3 experiments). The value of the wild-type control was set at 100%. *P < 0.01.
VirF degradation occurs through the host SCF pathway
Plants encode an unusually large number of F-box subunits (32), making the SCF complexes the largest class of E3 ubiquitin ligases for plants. Therefore, VirF degradation may be induced by a plant SCF complex. To explore this possibility, we generated a plant dominant-negative CULLIN1 (CUL1DN). In a yeast two-hybrid assay, this C-terminal deletion mutant of Arabidopsis CULLIN1 (amino acid residues 1 to 420) interacted with ASK1, but not with RBX1, the catalytic core of the SCF complex (Fig. 4C). Similar dominant-negative forms of CUL1 have been used to identify substrates of mammalian SCF E3 ligases (33). We took advantage of this molecular tool to test potential involvement of a host SCF complex in VirF degradation. FLAG-tagged CUL1DN (FLAG-CUL1DN) and Myc-VirF were coexpressed in N. benthamiana leaves, and the protein extracts were analyzed by a cell-free degradation assay. VirF proteins were stabilized in the presence of FLAG-CUL1DN (Fig. 4, D and E), suggesting that a plant SCF complex is at least partly responsible for the degradation of VirF in the host cell.
VirD5 is necessary for efficient infection of Agrobacterium
Although our data indicate an important role for VirD5 in the Agrobacterium infection, several previous genetic studies suggested that VirD5 is not essential for this process (25-28). However, most of these experiments were performed in plant species that do not require VirF for infection. If the main function of VirD5 is to stabilize VirF, its effect on infection should be tested in a host, such as tomato, in which VirF enhances infection (12). We thus inoculated the stems of tomato seedlings with either wild-type Agrobacterium or a mutant bacterial strain lacking virD5 (28) and scored the resulting crown gall tumors. The number of the inoculation sites that developed a tumor was significantly decreased in the VirD5 (−) mutant compared to the wild-type bacterium (Fig. 4F), supporting the requirement of VirD5 for efficient Agrobacterium infection.
DISCUSSION
Host-pathogen interactions represent a never-ending arms race. Most organisms have evolved elaborate defense systems to fight against invading pathogens, whereas pathogens exploit or interfere with the host cellular functions to maximize their own infection efficiency. For example, RNA silencing serves as a well-conserved antiviral defense mechanism in both plants and animals (34). Conversely, many viral pathogens evade this defense by using their silencing suppressors that disrupt the host RNA silencing pathway (34). Such biotic interaction exemplifies a coevolution of defense and counterdefense between the host and the pathogens (35). Here, we reveal a previously unknown type of counterdefense strategy used by pathogens against the potential host antimicrobial response.
The Agrobacterium F-box protein VirF plays an essential role in uncoating of the T-complex in the host cells (9). Using a cell-free degradation assay, we demonstrated that VirF is unstable in protein extracts prepared from N. benthamiana, one of the natural host plants of Agrobacterium. This destabilization of VirF was reduced by the proteasome inhibitors MG132 and lactacystin, suggesting that the host ubiquitin-proteasome pathway is responsible for VirF degradation. Consistent with this notion, we observed that VirF undergoes polyubiquitination in the plant cells. Together, our data suggest the presence of a host defense mechanism that targets the Agrobacterium virulence factor for proteasome-dependent degradation. Because VirF effector function is critical for the Agrobacterium infection (10-12), a plant defense strategy that targets VirF makes biological sense. On the other hand, the destabilization of VirF might also represent the “unstable when active” phenomenon observed in other F-box proteins (36). Assembly of some SCF complexes leads to destabilization not only of their target proteins but also of the F-box adaptor proteins (19, 20). However, this autocatalytic mechanism is not likely to be the case for VirF because the mutations in its F-box domain, which is required for the assembly of the SCF complex (10), did not stabilize VirF. Thus, VirF is likely destabilized through a host E3 ubiquitin ligase.
The fact that plants encode an unusually large number of F-box proteins (32) may reflect an evolutionary adaptation of the sessile organism that necessitates broad protection against pathogens. Indeed, increasing evidence suggests that many plant F-box proteins play substantial roles in defense response (31, 37, 38). Thus, we suggest that during Agrobacterium infection, the host plant activates a cellular SCF complex containing an as-yet-unidentified F-box protein to destabilize VirF. This idea is further corroborated by the observation that disruption of the plant SCF function by a dominant-negative CUL1 led to the increased stability of VirF.
As a counterdefense strategy, Agrobacterium has evolved another effector, VirD5, which is exported into the host cell and acts to bind and stabilize VirF. Thus, VirD5 functions as a positive regulator of the VirF protein and, by implication, Agrobacterium infection. Indeed, an Agrobacterium mutant that does not produce VirD5 significantly attenuated virulence in a host known to require VirF for efficient infection, further substantiating the role of VirD5 in the infection process. One limitation of our data, however, is that we were unable to identify mutations that specifically abolish the VirD5-VirF interaction and test the effects of such mutations on the bacterial virulence and VirF stability.
That VirD5, like VirF and VirE3, is a host range factor (24) not absolutely essential for infection in some hosts (25-28) suggests that in these hosts Agrobacterium may also exploit cellular factors that are functionally redundant with VirD5. Such functional redundancy between Agrobacterium effectors and host proteins is seen for VirE3 and VIP1 (39) and for VirF and VBF (31). Alternatively, in some hosts, the cellular F-box proteins, such as VBF (31), may substitute for VirF when the VirF protein amounts are reduced in the absence of VirD5.
Our observations reveal a previously unknown type of the host-pathogen molecular arms race that takes place over control of the stability of pathogen-encoded F-box protein effectors. Considering that diverse pathogens use their F-box proteins for infection, the host defense targeting such F-box proteins and the counterdefense strategy exploiting protective bacterial effectors could be potentially widespread.
MATERIALS AND METHODS
Sequence analysis
The WoLF PSORT (http://wolfpsort.org/) Web server was used to predict the NLSs of VirD5. The amino acid sequence of VirD5 (AAF77175) was submitted as the query.
Bimolecular fluorescence complementation
Microprojectiles were prepared with 200 ng of nYFP- and cYFP-fused constructs per shot and bombarded into N. benthamiana leaves with the Helios Gene Gun System (Bio-Rad). For studies of the subcellular localization of the VirD5-VirF interaction, free CFP was coexpressed from pSAT5-ECFP-C1 (40). Reconstitution of YFP fluorescence and free CFP fluorescence was detected with a Zeiss LSM 5 Pascal confocal microscope.
Transient expression by agroinfiltration
Agrobacterium EHA105 strain harboring an appropriate pPZP-RCS1 binary vector (41) was grown in LB medium supplemented with spectinomycin (100 μg/ml) overnight at 28°C. Cells were harvested by centrifugation and resuspended to OD600 (optical density at 600 nm) of 0.5 in infiltration buffer [10 mM MgCl2, 10 mM MES (pH 5.5), 100 μM acetosyringone]. Bacterial suspension was incubated for 2 hours at room temperature and then infiltrated into the abaxial sides of 3- to 4-week-old N. benthamiana leaves with a 1-ml needleless syringe. Plants were grown for 48 hours under regular growth conditions before being harvested.
Coimmunoprecipitation assays from plant cells
Fusion proteins were transiently expressed in N. benthamiana leaves by agroinfiltration. Infiltrated leaves were harvested and ground into fine powder in liquid nitrogen. Total proteins were extracted from ground tissues in immunoprecipitation buffer [50 mM tris-HCl (pH 7.5), 150 mM NaCl, 0.1% NP-40, 1 mM EDTA, 3 mM dithiothreitol (DTT), 1× plant protease inhibitor cocktail (Sigma-Aldrich), and 50 μM MG132 (Calbiochem)]. Protein extracts were incubated with anti–green fluorescent protein (GFP) antibody (Clontech) for 3 hours at 4°C, followed by incubation with Protein G–Sepharose 4B (Invitrogen) for an additional 3 hours at 4°C to capture the immunocomplexes. After three washes with washing buffer [50 mM tris-HCl (pH 7.5), 150 mM NaCl, 0.1% NP-40, and 1 mM EDTA], immunoprecipitates were eluted in SDS sample buffer. CFP-VirD5 and Myc-VirF proteins were detected by immunoblotting with anti-GFP anti-body (Clontech) and anti–c-Myc antibody conjugated to horseradish peroxidase (Roche), respectively.
GST pull-down assays
GST and GST-VirD5 were expressed in Escherichia coli BL21(DE3) and immobilized on Glutathione Sepharose 4B (GE Healthcare). GST- and GST-VirD5–bound glutathione beads were incubated with purified His-VirF (4) in phosphate-buffered saline buffer containing 0.1% NP-40 for 2 hours at room temperature. After four washes with the same buffer, pulled-down proteins were resuspended in SDS sample buffer. His-VirF was detected by immunoblotting with anti-His antibody (Bethyl Laboratories) followed by anti-rabbit antibody conjugated to horseradish peroxidase (Pierce).
Cell-free degradation assay
Myc or 3×FLAG fusion protein alone or together was transiently expressed in N. benthamiana leaves by agroinfiltration. Infiltrated leaves were harvested and ground into fine powder in liquid nitrogen. For studies of effects of proteasome inhibitor MG132, leaves were infiltrated with 10 μM MG132 (Calbiochem) or mock-treated with 0.1% DMSO and incubated for an additional 4 hours before harvesting. For studies of effects of the proteasome inhibitor lactacystin, leaves were infiltrated with 10 μM lactacystin (Sigma-Aldrich) or mock-treated with distilled water and incubated for an additional 4 hours before harvesting. Cell-free degradation assay was performed as described with slight modifications (42). Total proteins were extracted from ground tissues in degradation buffer [50 mM tris-HCl (pH 7.5), 100 mM NaCl, 10 mM MgCl2, 5 mM DTT, 5 mM adenosine 5′-triphosphate, and 1× plant protease inhibitor cocktail (Sigma-Aldrich)]. Equal volumes of extracts were transferred to microfuge tubes and incubated at room temperature for the indicated periods of time. For studies of effects of the lysineless ubiquitin mutant, purified wild-type ubiquitin or K0 ubiquitin (Boston Biochem) was added to protein extracts at 1 μg/μl. Reactions were terminated by addition of SDS sample buffer, followed by boiling for 3 min. Myc- and 3×FLAG-tagged proteins were detected by anti–c-Myc 9E10 antibody (Covance) and anti-FLAG M5 antibody (Sigma-Aldrich), respectively, followed by anti-mouse antibodies conjugated to alkaline phosphatase (Sigma-Aldrich). For loading controls, a major band at about 50 kD [putative RuBisCO (ribulose-1,5-bisphosphate carboxylase oxygenase) large chains] on Coomassie-stained gels was used. All immunoblots were quantified with ImageJ software (National Institutes of Health).
Ubiquitination assay
Myc-VirF or FLAG-Ub alone or together was transiently expressed in N. benthamiana leaves by agroinfiltration. After 48 hours, the infiltrated leaves were treated with 10 μM MG132 and incubated for an additional 4 hours unless otherwise noted. Leaves were harvested and ground into fine powder in liquid nitrogen. Total proteins were extracted from ground tissues in immunoprecipitation buffer (see above). Protein extracts were incubated with anti–c-Myc 9E10 antibody (Covance) for 3 hours at 4°C, followed by incubation with Protein G–Sepharose 4B (Invitrogen) for an additional 3 hours at 4°C to capture the immunocomplexes. After four washes with washing buffer [50 mM tris-HCl (pH 7.5), 150 mM NaCl, 0.1% NP-40, 1 mM EDTA, and 3 mM DTT], immunoprecipitates were eluted in SDS sample buffer. The presence of (FLAG-Ub)n–VirF was analyzed by immunoblotting with anti–c-Myc antibody conjugated to horseradish peroxidase (Roche) or anti-FLAG M5 antibody (Sigma-Aldrich) followed by anti-mouse antibody conjugated to horseradish peroxidase (Sigma-Aldrich).
Yeast two-hybrid analysis
Plasmids were transformed into the S. cerevisiae strain L40 by means of the lithium acetate method and selected on leucine- and tryptophan-deficient medium. Protein interactions were assayed by growing cells for 3 days at 30°C on leucine-, tryptophan-, and histidine-deficient medium.
Tumorigenesis in tomato
The assay was performed as described (31) with the wild-type R10 strain or the virD5 mutant VIK36 strain (28). Briefly, 11-day-old Solanum lycopersicum cv. Micro-Tom seedlings grown on Murashige and Skoog (MS) medium were punctured by a 27-gauge needle, inoculated with Agrobacterium suspension (OD600 = 0.02), grown for an additional 2 days on MS, and then further grown on MS supplemented with timentin (100 μg/ml). Tumors were scored 28 days after inoculation. All quantitative data were analyzed by the Student’s t test; P values <0.01, corresponding to the statistical probability of greater than 99%, were considered statistically significant.
Supplementary Material
Fig. S1. VirD5 does not interact with VBF.
Fig. S2. Increased amounts of VirF in the presence of VirD5 in plant cells.
Fig. S3. Lactacystin, a specific inhibitor of the 26S proteasome, stabilizes VirF.
Acknowledgments:
We thank S. C. Winans (Cornell University) for the gift of the Agrobacterium strain VIK36. We also thank A. Zaltsman for technical assistance with tomato tumor assay.
Funding:
Supported by grants from the United States Department of Agriculture National Institute of Food and Agriculture, NIH, NSF, and United States–Israel Binational Agricultural Research and Development Fund to V.C. and by Grant-in-Aid for Japan Society for the Promotion of Science Fellows to S.M.
Footnotes
SUPPLEMENTARY MATERIALS
Competing interests: The authors declare that they have no competing financial interests.
REFERENCES AND NOTES
- 1.Lacroix B, Tzfira T, Vainstein A, Citovsky V, A case of promiscuity: Agrobacterium’s endless hunt for new partners. Trends Genet. 22, 29–37 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Gelvin SB, Agrobacterium and plant genes involved in T-DNA transfer and integration. Annu. Rev. Plant Physiol. Plant Mol. Biol 51, 223–256 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Citovsky V, Kozlovsky SV, Lacroix B, Zaltsman A, Dafny-Yelin M, Vyas S, Tovkach A, Tzfira T, Biological systems of the host cell involved in Agrobacterium infection. Cell. Microbiol 9, 9–20 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Lacroix B, Loyter A, Citovsky V, Association of the Agrobacterium T-DNA–protein complex with plant nucleosomes. Proc. Natl. Acad. Sci. U.S.A 105, 15429–15434 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tzfira T, Vaidya M, Citovsky V, VIP1, an Arabidopsis protein that interacts with Agrobacterium VirE2, is involved in VirE2 nuclear import and Agrobacterium infectivity. EMBO J. 20, 3596–3607 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Krichevsky A, Vaidya M, Tzfira T, Citovsky V, Uncoupling of the functions of the Arabidopsis VIP1 protein in transient and stable plant genetic transformation by Agrobacterium. Proc. Natl. Acad. Sci. U.S.A 102, 5733–5738 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Djamei A, Pitzschke A, Nakagami H, Rajh I, Hirt H, Trojan horse strategy in Agrobacterium transformation: Abusing MAPK defense signaling. Science 318, 453–456 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Pitzschke A, Djamei A, Teige M, Hirt H, VIP1 response elements mediate mitogen-activated protein kinase 3-induced stress gene expression. Proc. Natl. Acad. Sci. U.S.A 106, 18414–18419 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzfira T, Vaidya M, Citovsky V, Involvement of targeted proteolysis in plant genetic transformation by Agrobacterium. Nature 431, 87–92 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Schrammeijer B, Risseeuw E, Pansegrau W, Regensburg-Tuïnk TJ, Crosby WL, Hooykaas PJ, Interaction of the virulence protein VirF of Agrobacterium tumefaciens with plant homologs of the yeast Skp1 protein. Curr. Biol 11, 258–262 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Melchers LS, Maroney MJ, den Dulk-Ras A, Thompson DV, van Vuuren HA, Schilperoort RA, Hooykaas PJ, Octopine and nopaline strains of Agrobacterium tumefaciens differ in virulence; molecular characterization of the virF locus. Plant Mol. Biol 14, 249–259 (1990). [DOI] [PubMed] [Google Scholar]
- 12.Regensburg-Tuïnk AJ, Hooykaas PJ, Transgenic N. glauca plants expressing bacterial virulence gene virF are converted into hosts for nopaline strains of A. tumefaciens. Nature 363, 69–71 (1993). [DOI] [PubMed] [Google Scholar]
- 13.Aronson MN, Meyer AD, Gyorgyey J, Katul L, Vetten HJ, Gronenborn B, Timchenko T, Clink, a nanovirus-encoded protein, binds both pRB and SKP1. J. Virol 74, 2967–2972 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baumberger N, Tsai CH, Lie M, Havecker E, Baulcombe DC, The Polerovirus silencing suppressor P0 targets ARGONAUTE proteins for degradation. Curr. Biol 17, 1609–1614 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Bortolamiol D, Pazhouhandeh M, Marrocco K, Genschik P, Ziegler-Graff V, The Polerovirus F box protein P0 targets ARGONAUTE1 to suppress RNA silencing. Curr. Biol 17, 1615–1621 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Angot A, Peeters N, Lechner E, Vailleau F, Baud C, Gentzbittel L, Sartorel E, Genschik P, Boucher C, Genin S, Ralstonia solanacearum requires F-box-like domain-containing type III effectors to promote disease on several host plants. Proc. Natl. Acad. Sci. U.S.A 103, 14620–14625 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price CT, Al-Khodor S, Al-Quadan T, Santic M, Habyarimana F, Kalia A, Kwaik YA, Molecular mimicry by an F-box effector of Legionella pneumophila hijacks a conserved polyubiquitination machinery within macrophages and protozoa. PLoS Pathog. 5, e1000704 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lomma M, Dervins-Ravault D, Rolando M, Nora T, Newton HJ, Samson FM, Sahr T, Gomez-Valero L, Jules M, Hartland EL, Buchrieser C, The Legionella pneumophila F-box protein Lpp2082 (AnkB) modulates ubiquitination of the host protein parvin B and promotes intracellular replication. Cell. Microbiol 12, 1272–1291 (2010). [DOI] [PubMed] [Google Scholar]
- 19.Zhou P, Howley PM, Ubiquitination and degradation of the substrate recognition subunits of SCF ubiquitin-protein ligases. Mol. Cell 2, 571–580 (1998). [DOI] [PubMed] [Google Scholar]
- 20.Galan JM, Peter M, Ubiquitin-dependent degradation of multiple F-box proteins by an autocatalytic mechanism. Proc. Natl. Acad. Sci. U.S.A 96, 9124–9129 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ayad NG, Rankin S, Murakami M, Jebanathirajah J, Gygi S, Kirschner MW, Tome-1, a trigger of mitotic entry, is degraded during G1 via the APC. Cell 113, 101–113 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Margottin-Goguet F, Hsu JY, Loktev A, Hsieh HM, Reimann JD, Jackson PK, Prophase destruction of Emi1 by the SCFβTrCP/Slimb ubiquitin ligase activates the anaphase promoting complex to allow progression beyond prometaphase. Dev. Cell 4, 813–826 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Guardavaccaro D, Kudo Y, Boulaire J, Barchi M, Busino L, Donzelli M, Margottin-Goguet F, Jackson PK, Yamasaki L, Pagano M, Control of meiotic and mitotic progression by the F box protein β-Trcp1 in vivo. Dev. Cell 4, 799–812 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Vergunst AC, van Lier MC, den Dulk-Ras A, Stuve TA, Ouwehand A, Hooykaas PJ, Positive charge is an important feature of the C-terminal transport signal of the VirB/D4-translocated proteins of Agrobacterium. Proc. Natl. Acad. Sci. U.S.A 102, 832–837 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Porter SG, Yanofsky MF, Nester EW, Molecular characterization of the virD operon from Agrobacterium tumefaciens. Nucleic Acids Res. 15, 7503–7517 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koukolíkova-Nicola Z, Raineri D, Stephens K, Ramos C, Tinland B, Nester EW, Hohn B, Genetic analysis of the virD operon of Agrobacterium tumefaciens: A search for functions involved in transport of T-DNA into the plant cell nucleus and in T-DNA integration. J. Bacteriol 175, 723–731 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin TS, Kado CI, The virD4 gene is required for virulence while virD3 and orf5 are not required for virulence of Agrobacterium tumefaciens. Mol. Microbiol 9, 803–812 (1993). [DOI] [PubMed] [Google Scholar]
- 28.Kalogeraki VS, Zhu J, Stryker JL, Winans SC, The right end of the vir region of an octopine-type Ti plasmid contains four new members of the vir regulon that are not essential for pathogenesis. J. Bacteriol 182, 1774–1778 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vergunst AC, Schrammeijer B, den Dulk-Ras A, de Vlaam CM, Regensburg-Tuïnk TJ, Hooykaas PJ, VirB/D4-dependent protein translocation from Agrobacterium into plant cells. Science 290, 979–982 (2000). [DOI] [PubMed] [Google Scholar]
- 30.Citovsky V, Gafni Y, Tzfira T, Localizing protein–protein interactions by bimolecular fluorescence complementation in planta. Methods 45, 196–206 (2008). [DOI] [PubMed] [Google Scholar]
- 31.Zaltsman A, Krichevsky A, Loyter A, Citovsky V, Agrobacterium induces expression of a host F-box protein required for tumorigenicity. Cell Host Microbe 7, 197–209 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gagne JM, Downes BP, Shiu SH, Durski AM, Vierstra RD, The F-box subunit of the SCF E3 complex is encoded by a diverse superfamily of genes in Arabidopsis. Proc. Natl. Acad. Sci. U.S.A 99, 11519–11524 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yen HC, Elledge SJ, Identification of SCF ubiquitin ligase substrates by global protein stability profiling. Science 322, 923–929 (2008). [DOI] [PubMed] [Google Scholar]
- 34.Díaz-Pendón JA, Ding SW, Direct and indirect roles of viral suppressors of RNA silencing in pathogenesis. Annu. Rev. Phytopathol 46, 303–326 (2008). [DOI] [PubMed] [Google Scholar]
- 35.Vance V, Vaucheret H, RNA silencing in plants—Defense and counterdefense. Science 292, 2277–2280 (2001). [DOI] [PubMed] [Google Scholar]
- 36.Hermand D, F-box proteins: More than baits for the SCF? Cell Div. 1, 30 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ditt RF, Kerr KF, de Figueiredo P, Delrow J, Comai L, Nester EW, The Arabidopsis thaliana transcriptome in response to Agrobacterium tumefaciens. Mol. Plant Microbe Interact 19, 665–681 (2006). [DOI] [PubMed] [Google Scholar]
- 38.Citovsky V, Zaltsman A, Kozlovsky SV, Gafni Y, Krichevsky A, Proteasomal degradation in plant-pathogen interactions. Semin. Cell Dev. Biol 20, 1048–1054 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Lacroix B, Vaidya M, Tzfira T, Citovsky V, The VirE3 protein of Agrobacterium mimics a host cell function required for plant genetic transformation. EMBO J. 24, 428–437 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tzfira T, Tian GW, Lacroix B, Vyas S, Li J, Leitner-Dagan Y, Krichevsky A, Taylor T, Vainstein A, Citovsky V, pSAT vectors: A modular series of plasmids for autofluorescent protein tagging and expression of multiple genes in plants. Plant Mol. Biol 57, 503–516 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Goderis IJ, De Bolle MF, FranÇois IE, Wouters PF, Broekaert WF, Cammue BP, A set of modular plant transformation vectors allowing flexible insertion of up to six expression units. Plant Mol. Biol 50, 17–27 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Más P, Kim WY, Somers DE, Kay SA, Targeted degradation of TOC1 by ZTL modulates circadian function in Arabidopsis thaliana. Nature 426, 567–570 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. VirD5 does not interact with VBF.
Fig. S2. Increased amounts of VirF in the presence of VirD5 in plant cells.
Fig. S3. Lactacystin, a specific inhibitor of the 26S proteasome, stabilizes VirF.