Abstract
Rhodobacter capsulatus synthesizes two homologous protein complexes capable of activating molecular H2, a membrane-bound [NiFe] hydrogenase (HupSL) linked to the respiratory chain, and an H2 sensor encoded by the hupUV genes. The activities of hydrogen-deuterium (H-D) exchange catalyzed by the hupSL-encoded and the hupUV-encoded enzymes in the presence of D2 and H2O were studied comparatively. Whereas HupSL is in the membranes, HupUV activity was localized in the soluble cytoplasmic fraction. Since the hydrogenase gene cluster of R. capsulatus contains a gene homologous to hoxH, which encodes the large subunit of NAD-linked tetrameric soluble hydrogenases, the chromosomal hoxH gene was inactivated and hoxH mutants were used to demonstrate the H-D exchange activity of the cytoplasmic HupUV protein complex. The H-D exchange reaction catalyzed by HupSL hydrogenase was maximal at pH 4.5 and inhibited by acetylene and oxygen, whereas the H-D exchange catalyzed by the HupUV protein complex was insensitive to acetylene and oxygen and did not vary significantly between pH 4 and pH 11. Based on these properties, the product of the accessory hypD gene was shown to be necessary for the synthesis of active HupUV enzyme. The kinetics of HD and H2 formed in exchange with D2 by HupUV point to a restricted access of protons and gasses to the active site. Measurement of concentration changes in D2, HD, and H2 by mass spectrometry showed that, besides the H-D exchange reaction, HupUV oxidized H2 with benzyl viologen, produced H2 with reduced methyl viologen, and demonstrated true hydrogenase activity. Therefore, not only with respect to its H2 signaling function in the cell, but also to its catalytic properties, the HupUV enzyme represents a distinct class of hydrogenases.
In the photosynthetic bacterium Rhodobacter capsulatus, the ability to use H2 as an electron donor is conferred by an H2-uptake hydrogenase, a membrane-bound [NiFe] hydrogenase linked to the respiratory chain (31) and encoded by the hupSL genes (21).
The hupSL genes are part of a cluster of hup (for hydrogen uptake) and hyp (for hydrogenase pleiotropic) genes necessary for the biosynthesis of the hupSL-encoded hydrogenase (7). The hup and hyp gene products bear significant structural identity to hydrogenase gene products from Escherichia coli, Ralstonia eutropha (formerly Alcaligenes eutrophus), Rhizobium leguminosarum, Bradyrhizobium japonicum, and Azotobacter vinelandii. Some of these products are necessary for maturation of the enzyme, some for Ni insertion at the active site, and some for regulation of hupSL gene expression (reviewed in references 17 and 42).
The hup-hyp cluster comprises the hupTUV operon, the products of which exert a negative control on hupSL gene expression. The hupU gene product shares 20% amino acid sequence similarity with the small subunit (HupS) of the hupSL-encoded hydrogenase, and that of hupV shares 29% similarity with the large subunit (HupL) (12, 15). It is thought that HupU and HupV proteins function as a complex, since mutants with inactivated hupU or hupV or deleted hupUV genes have the same phenotype (12). The HupUV protein complex can catalyze the hydrogen-deuterium (H-D) exchange reaction in the presence of D2 gas and was suggested to function as a cellular H2 sensor (40). The hupT gene product is a protein histidine kinase (13, 15). With the response regulator HupR, it forms the two-component HupT-HupR system, which regulates the synthesis of HupSL hydrogenase in R. capsulatus (16). In the absence of H2, HupT represses the transcription of hydrogenase (hupSL) genes by phosphorylating HupR (16).
We demonstrate in this study that the H-D exchange reaction catalyzed by the HupUV protein complex can be differentiated from that of the HupSL hydrogenase by different relative rates of H2 and HD formation in exchange with D2, a different sensitivity to acetylene, and a different in situ response to oxygen. Thus, this report defines specific features of a new type of hydrogenase, the H2-signaling HupUV hydrogenase.
MATERIALS AND METHODS
Bacterial strains and cultures.
The strains and plasmids used in this work are listed in Table 1. R. capsulatus strains were grown heterotrophically either anaerobically in the light or aerobically in darkness at 30°C as described previously (7) in minimal salts RCV medium (43) supplemented with 30 mM Na dl-malate as a carbon source and either 7 mM ammonium sulfate (MN medium) or 7 mM glutamate (MG medium) as a nitrogen source. The concentrations of the antibiotics used were as follows: kanamycin, 10 μg ml−1, tetracycline, 1 μg ml−1; and streptomycin, 25 μg ml−1.
TABLE 1.
Bacterial strains and plasmids used in this study
| R. capsulatus strain or plasmid | Relevant genotype or phenotype | Source or reference |
|---|---|---|
| Strains | ||
| B10 | Wild type, Hup+ Nif+ | 26 |
| BSE16 | ΔhupUV | 12 |
| JP91 | hupSL | 6 |
| RCC12 | hypD | 7 |
| RCC44 | Δ(hoxH, ORF2 hupTUV hypF) | This work |
| JBC12 | SmrhoxH | This work |
| JBC13 | SmrhoxH hupSL | This work |
| Plasmids | ||
| pAC142 | Tcr; pPHU234 with 730-bp HindIII-XhoII insert containing a phupS::lacZ fusion | 8 |
| pAC206 | Tcr; pPHU231 with 4.8-kb SalI-HindIII insert containing the hupTUV operon | 12 |
| pAC63 | Tcr; pRK292 with 9.6-kb HindIII-HindIII insert containing the hypAB hupR hypCDE genes | 7 |
| pAC171 | Apr; pUC18 with a 2.2-kb HindIII-HindIII insert containing the hoxH gene | This work |
| pAC229 | Tcr, pPHU281 with a 6.0-kb BamHI-PstI deletion replaced by an omega cassette | This work |
DNA manipulations and bacterial mating.
DNA preparation and cloning were carried out according to reference 34. Restriction endonucleases and DNA-modifying enzymes were used by following the instructions of the manufacturers. Plasmids were introduced in R. capsulatus by the helper plasmid pRK2013 (10), as described earlier (5). The hoxH gene carried on plasmid pAC171 was inactivated by insertion of an omega cassette at the HindII site, and the mutated gene was exchanged with wild-type hoxH in the chromosome by double recombination. Two mutant strains having the chromosomal hoxH gene inactivated were isolated. The first, termed JBC12, was obtained from the wild-type strain B10, and the second, termed JBC13, was obtained from the hupSL mutant JP91. Strain JBC13 is therefore an Hup(SL)− HoxH− double mutant. The RCC44 mutant was obtained from B10 by exchange with the insert of plasmid pAC229, which has the 6.0-kb BamHI-PstI fragment carrying the hoxH, open reading frame 2 (ORF2), hupTUV, and hypF genes replaced by an omega cassette.
Enzyme assays and protein determination.
Hydrogenase activity was assayed by the rate of H2 (or D2) uptake, H2 production, or H2 and HD formed in exchange with D2 (H-D exchange). Hydrogen uptake was determined spectrophotometrically by using methylene blue (MB) (0.15 mM) (9) or by mass spectrometry with oxidized benzyl viologen (BV2+) or MB (4 mM) as an electron acceptor. One unit of hydrogenase activity is 1 nmol of H2 (D2) consumed (produced)/min/mg of protein. The rates of H2 uptake with BV2+, of H2 production by reduction of protons in the presence of Zn-reduced methyl viologen (MV+), and of H2 and HD formed in exchange with D2, measured at 30°C, were monitored continuously in the aqueous phase of cell suspensions (either whole cells, membranes, or soluble cytoplasmic fraction) by the mass spectrometric method described in detail previously (19, 41).
β-Galactosidase activity was assessed from the rate of o-nitrophenol (ONP) released from o-nitrophenyl-β-d-galactopyranoside (ONPG) at 30°C, according to Miller (29) as described previously (8). One unit of β-galactosidase activity is 1 μmol of ONP formed min/mg of protein.
The protein concentration of whole cells was estimated by the empirical relationship optical density at 660 nm (OD660)/5 = mg of protein ml−1 (27), and that of membranes and cell free extracts (obtained by cell breakage in a French pressure cell followed by two successive centrifugations at 20,000 × g for 30 min and then 100,000 × g for 70 min) was estimated by the bicinchoninic acid protein assay (Bio-Rad Laboratories, Hercules, Calif.) with bovine serum albumin as a standard.
RESULTS
The hydrogenase hup-hyp gene cluster of R. capsulatus can encode more than one hydrogenase.
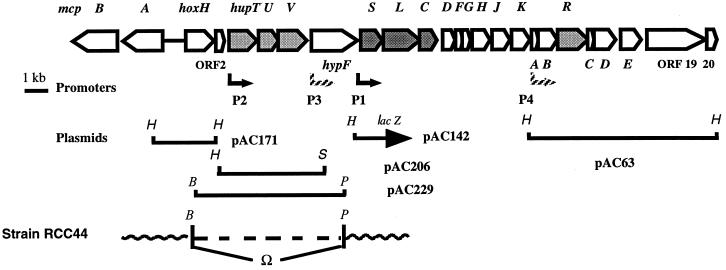
The gene organization at the hup locus of the chromosome of R. capsulatus (strain B10) is shown in Fig. 1. The hup-hyp gene cluster comprises the structural hydrogenase genes for H2 uptake (hup) and accessory genes for the synthesis of active hydrogenase(s) (hup and hyp genes). The hupSLC operon encodes the membrane-bound H2-uptake [NiFe] hydrogenase (HupSL) (21) and HupC, a cytochrome b, which links HupSL to the respiratory chain (3). It is expressed from the hupS promoter (phupS) (8), which depends on ς70 factor (16). The hupTUV operon encodes proteins that negatively control hupSL gene expression (12). The hupU gene product, homologous to the small hydrogenase subunit HupS, lacks the long twin-Arg signal peptide present at the N terminus of HupS. This type of signal peptide has been shown in E. coli to lead to the export of dimeric hydrogenase to the periplasm by the Tat system (33, 35). Indeed, evidence is given below that, in contrast to the membrane-bound, periplasmically oriented HupSL hydrogenase, the HupUV protein complex is localized in the cytoplasm. Upstream from the hupTUV operon lies an ORF, termed hoxH, whose predicted product shares significant similarity with the large subunit of [NiFe] hydrogenases, in particular with HoxH, the β-subunit of the tetrameric soluble NAD-linked hydrogenase (39). The genes encoding the other three subunits of the tetrameric NAD-linked hydrogenases were not found in the hydrogenase gene cluster. Downstream from hoxH, ORF2 can encode a protein of 181 amino acids, which shares no significant similarity with known proteins. Upstream from hoxH, separated by approximately 500 nucleotides (nt) and transcribed in the opposite direction, are the mcpA and mcpB genes capable of encoding methyl-accepting-type chemoreceptors (28).
FIG. 1.
Gene organization at the hup locus of the chromosome of R. capsulatus. The coding region at the hup locus of R. capsulatus chromosome comprises 21 ORFs, all contiguous and transcribed from the same strand. At the 5′ end, it is separated by around 500 nt from the mcpA and mcpB genes transcribed in the opposite direction (28). The positions of known promoters and plasmid inserts are shown. B, BamHI; H, HindIII; P, PstI; S, SalI.
It is not known whether hoxH is expressed and, if so, under what conditions. The genes encoding a tetrameric, reversible, NAD-linked hydrogenase have not yet been identified in the (nearly completely sequenced) genome of R. capsulatus (B. Billoud, A. Colbeau, and P. M. Vignais, unpublished data). However, it is possible that the product of hoxH can function with some subunits of complex I, as suggested by Appel and Schulz (1). To eliminate any interference with the putative HoxH protein, the hoxH gene was inactivated in the chromosome of R. capsulatus, and the mutants were used to study HupUV activity in the cell.
The H-D exchange activity catalyzed by the HupUV protein complex is not sensitive to oxygen.
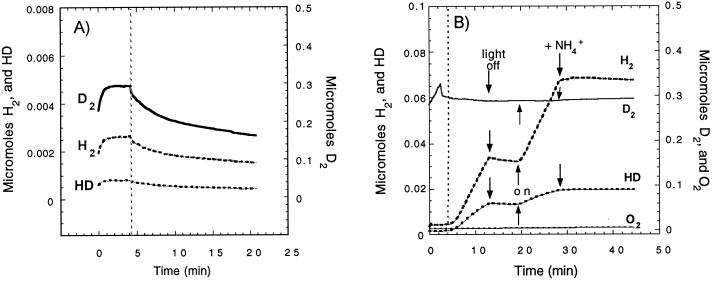
After the discovery of the presence of hoxH in the hydrogenase gene cluster, and because HupUV has a low level of activity (40), special care was taken to assess the cellular H-D exchange activity. A mutant strain, RCC44, with the DNA encompassing hoxH, orf2, hupTUV, and hypF deleted (Fig. 1), was constructed and used in control experiments. In the absence of the hypF gene product (4), there was no active membrane-bound (HupSL) hydrogenase. When grown under nitrogenase-repressing conditions (in MN medium), there was no H-D exchange reaction at all and no formation of HD and H2 in exchange of D2, and the curves displayed in Fig. 2A show only the consumption of D2, H2, and HD by the mass spectrometer (in this experiment, H2 and HD were brought as contaminants of D2—hence the difference in scales). On the other hand, RCC44 cells grown photoheterotrophically in MG medium (nitrogenase-inducing conditions) catalyzed an H-D exchange reaction due to nitrogenase activity (19). Figure 2B shows the pattern of H2 and HD formation in exchange with D2, and also some proton reduction, catalyzed by the nitrogenase complex. The typical features of the nitrogenase-catalyzed H-D exchange are that it requires light (for the regeneration by photophosphorylation of the ATP needed for nitrogenase activity) and it is completely inhibited by ammonia. These two simple tests (insensitivity to darkness and to ammonia) were used to check that the H-D exchange activities measured in our experiments represented hydrogenase and not nitrogenase activity. Moreover, for in vivo experiments, the cells were systematically grown in MN medium, although the synthesis of the HupSL hydrogenase is strongly repressed under such conditions.
FIG. 2.
Nitrogenase-mediated H2 and HD production, in the presence of D2 and H2O, by the RCC44 mutant. The RCC44 mutant (lacking the hoxH, hupTUV, and the hypF genes) was grown photoheterotrophically in either MN medium (A) or MG medium (B). (A) The MN culture (1.5 ml, 0.7 mg of protein) was sparged with D2, and the reaction vessel was closed at the time indicated by the vertical dotted line. The curves (not corrected for the consumption of gasses of the apparatus) exhibit the real changes with time in D2 (▄), H2 (–––), and HD (•••) concentrations in the reaction chamber. (B) The MG culture (1.5 ml, 0.6 mg of protein) in the reaction chamber was sparged with D2. At the time indicated by the vertical dotted line, the cell was closed and the H-D exchange reaction in whole cells was measured under light (light on) or in darkness (light off) and after addition of 10 mM ammonium sulfate, as indicated by arrows. The curves have been corrected for gas consumption by the mass spectrometer.
The H-D exchange activity of HupUV was also determined in strain JP91 (hupSL mutant) and in its derivative, in which the hoxH gene has been inactivated, JBC13 (hupSL hoxH double mutant). Figure 3 shows that, although feeble, the rates of HD and H2 formed in the course of the H-D exchange reaction catalyzed by HupUV in JBC13 cells could be determined. The initial rates of H2 and HD formation were determined for three time intervals following gassing by D2 and then closing of the reaction chamber: between 5 and 6 min (interval 1), between 22 and 23 min (interval 2), and between 35.3 and 36.5 min (interval 3). Light was off for intervals 2 and 3, and O2 was present in interval 3 when the rates of HD and H2 formation were determined. The initial rates were 0.8, 0.9, and 0.8 nmol of H2 formed/min and 1.4, 1.5, and 1.4 nmol of HD formed/min for intervals 1, 2, and 3, respectively. This experiment shows that (i) the H-D exchange proceeded in the absence of light; (ii) the initial rates were reproducible and unchanged in the presence of O2; and (iii) the H-D exchange reaction was due to HupUV, since JBC13 cells have no hoxH or hupSL genes. There were no significant differences between the cellular activities of JBC13 (hoxH hupSL mutant) and JP91 (hupSL mutant). Thus, either hoxH was not expressed in cells grown anaerobically in the light in MN medium or, if it was expressed, the level of expression was even lower than that of the hupUV genes and was not detectable in our tests.
FIG. 3.
Time course of H2 and HD production in the D2-H2O system by the HupUV protein complex in JBC13 cells. Cells were grown anaerobically in the light in MN medium. The H-D exchange reaction in whole cells of JBC13 (hupSL hoxH double mutant) (3.7 mg of protein) was measured in 1.5 ml of 50 mM Tris-HCl buffer (pH 8.0). After gassing the cell suspension with D2, the reaction chamber was closed (vertical grey lines), and the H-D exchange reaction was allowed to proceed. At 18 min, the reaction chamber was regassed with D2; at 22 min, the light was turned off and the vessel was closed; at 27 min, H2O2 (5 μl, 0.3%) was added and O2 was liberated by decomposition (the lower trace shows O2 concentrations measured at a mass of 32 Da); at 30 min, the chamber was regassed with D2; at 35 min, the light was turned off, the vessel was closed, and there was new addition of H2O2 (10 μl, 0.3%). The figure shows the real gas concentrations of H2 (▄), HD (••••), and O2 (▄ ▄ ▄) in the reaction chamber.
Unlike the membrane-bound HupSL hydrogenase, the HupUV H2 sensor is a soluble cytoplasmic enzyme.
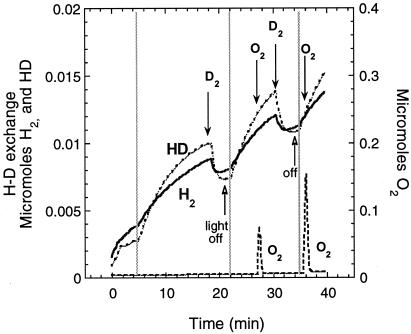
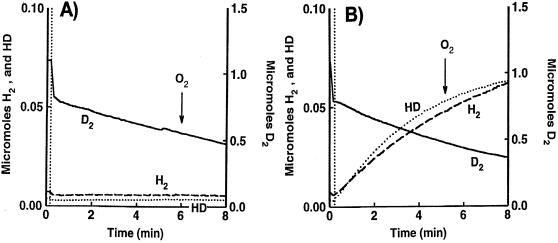
The lack of a signal peptide at the N terminus of HupU predicted that the hupUV gene products are cytoplasmically located. The experiments shown in Fig. 4 demonstrate that the HupUV protein complex in cells of the hupSL mutant JP91 cannot transfer H2 electrons to O2 or to the impermeant electron acceptor ferricyanide, in contrast to HupSL in the hupUV mutant, BSE16. Figure 4A shows that the initial rate of H2 formation (14.6 nmol/min/mg of protein) was higher than that of HD (7.5 nmol/min/mg of protein) in the HupSL-catalyzed H-D exchange reaction (in BSE16 cells). On the other hand, for the HupUV-catalyzed H-D exchange reaction [in JP91(pAC206) cells], the reverse was observed (initial HD production rate, 5.3 nmol/min/mg of protein > initial H2 rate, 3.7 nmol/min/mg of protein) (Fig. 4B). Upon addition of a small amount of O2, there was immediate and rapid uptake by HupSL of the three isotopic forms of hydrogen gas, D2, HD, and H2. The formation of H2 and HD resumed when all O2 had been consumed, and a further addition of ferricyanide immediately reoxidized the three hydrogen species (Fig. 4A). Neither the addition of O2 nor that of ferricyanide affected significantly the H-D exchange reaction catalyzed by the HupUV protein complex (Fig. 4B). (On the figure, the decrease in HD concentration is due to the further exchange of HD with protons and not to an inhibition by O2 or ferricyanide.) The same types of results were observed with JP91 cells without pAC206 (not shown) and with JBC13 (hupSL hoxH double mutant) (Fig. 3). This is further evidence that HupSL transmits H2 electrons to O2 through the respiratory chain and that HupUV is not directly connected to the cytoplasmic membrane.
FIG. 4.
Time course of H2 and HD production and D2 consumption in the D2-H2O system by the HupSL (A) and HupUV (B) protein complexes. Cells were grown anaerobically in the light in MN medium. The H-D exchange reaction in whole cells of BSE16, a ΔhupUV mutant (2.5 mg of protein) (A), and in whole cells of JP91(pAC206), a hupSL mutant, with the hupTUV operon-containing plasmid pAC206 (5.2 mg of protein) (B), was measured in 50 mM citrate-phosphate buffer (pH 7.0). At the time indicated by the vertical dotted line, the reaction vessel was closed, and the concentrations of D2 (▄), H2 (----), and HD (····) were recorded. The arrows indicate the time of O2 appearance in the medium after H2O2 addition (2 μl of 0.3% H2O2) and the time of ferricyanide addition (10 mM). The changes in O2 concentration were monitored at a mass of 32 Da (data not shown). The figure shows the real concentrations of the hydrogen species present in the vessel.
The use of detergents brought another piece of evidence of the cytoplasmic localization of HupUV. Whereas treatment of B10 or BSE16 cells by sodium dodecyl sulfate plus chloroform (the same treatment used for β-galactosidase assays) abolished the H-D exchange activity of HupSL hydrogenase, the activity of the HupUV protein complex only dropped from 3.9 to 3.3 nmol of HD produced/min/mg of protein after that treatment. Finally, it is demonstrated below that the HupUV H-D exchange activity was found in the soluble cytoplasmic fraction obtained after breakage of the cells and removal of the membranes by centrifugation.
The H-D exchange reaction catalyzed by HupUV is distinguishable from that of HupSL by its insensitivity to acetylene.
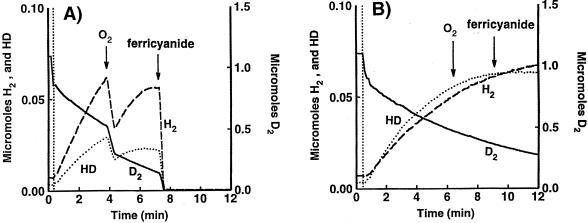
Typically, as shown in Fig. 3 and 4, in the H-D exchange catalyzed by HupUV, the initial rate of HD formation was higher than that of H2. Then, as the D2 concentration decreased, HD further exchanged with protons and H2 became predominant. We show now (Fig. 5) that acetylene inhibited the H-D exchange reaction catalyzed by HupSL, but not that catalyzed by HupUV. The hupSL-encoded hydrogenase of BSE16 (ΔhupUV) cells was 95% inhibited after a 1-h 40-min incubation under a gas phase containing a 1:1 mixture of acetylene and argon (Fig. 5A), while under the same conditions, the H-D exchange activity of the HupUV protein was practically not inhibited by acetylene (Fig. 5B). The lack of an acetylene effect on HupUV was not due to a lack of acetylene penetration in the cytoplasmic compartment, since acetylene is the substrate commonly used to measure the activity of nitrogenase, which is also cytoplasmically located (19). Acetylene, which inhibits the H-D exchange activity of Thiocapsa roseopersicina hydrogenase, had been shown earlier to interact with the Ni atom of the hydrogenase active site (abolishing the electron paramagnetic resonance signal due to Ni-C and stabilizing it in an electron paramagnetic resonance-silent state) (44). Thus, apparently acetylene cannot reach the active site of HupUV as it can do for T. roseopersicina hydrogenase.
FIG. 5.
Effect of acetylene on the H-D exchange reaction catalyzed by the hupSL-encoded hydrogenase (A) and by the hupUV-encoded hydrogenase (B). The conditions were the same as those in Fig. 4, with cells grown overnight anaerobically in the light in MN medium. H2 (▄ ▄ ▄) and HD (····) production in exchange with D2 (––––) uptake catalyzed by whole cells of BSE16 (2.5 mg of protein) (A) and JP91(pAC206) (5.2 mg of protein) (B) was measured at pH 7 after the cells had been incubated for 1 h at room temperature under a gas phase of C2H2-Ar (1:1). The figure shows the real concentrations of the hydrogen species in the vessel.
This ability of acetylene to specifically inhibit the hupSL-encoded hydrogenase was then used to demonstrate that the synthesis of the hupUV-encoded enzyme necessitates the product of the accessory hypD gene (24). The RCC12 mutant contains an in-frame deletion of 54 bp within the hypD gene. It is complemented by plasmid pAC63, which provides in trans the wild-type form of HypD. In the mutant, the hupSL genes are transcribed, but the hydrogenase is not processed and thus not active (7). It is shown here that the hypD RCC12 mutant also lacks the active HupUV protein complex and that complementation of the mutant restored HupUV activity. Table 2 provides the rates of H2 and HD formation in cells of the wild-type strain B10, the hupSL mutant JP91, and the hypD mutant RCC12. The RCC12 cells have no H-D exchange activity; the experimental values given in Table 2 (experiment 4) indicate the sensitivity of the measurements. The presence of plasmid pAC63 in the hypD mutant restored H-D exchange activity to a level even higher than that in wild-type B10 cells, for the plasmid also provided additional copies of the transcriptional activator HupR (Fig. 1 and Table 2) (16, 38). The residual activity measured in RCC12(pAC63) cells incubated with C2H2-Ar (1:1), which was not significantly diminished by addition of O2 (not shown), was attributed to HupUV (insensitive to acetylene; Fig. 5). The data demonstrate that the hypD gene product was required for the synthesis of mature and active HupSL and HupUV enzymes.
TABLE 2.
Restoration of HupSL and HupUV activities in the complemented hypD mutant RCC12 as seen by the H2 and HD production in exchange with D2 uptake and the effect of acetylenea
| Expt | Strain | Conditions | Formation (nmol · min−1 · mg of protein−1) of:
|
|
|---|---|---|---|---|
| H2 | HD | |||
| pH 4 | ||||
| 1 | B10 (wild type) | Zero time | 20.3 | 11.3 |
| B10 | 2 h under C2H2-Ar | 0.9 | 1.7 | |
| 2 | JP91 (hupSL) | 0.8 | 1.7 | |
| 3 | JP91(pAC206) | Zero time | 2.5 | 5.6 |
| JP91(pAC206) | 2 h under C2H2-Ar | 2.6 | 5.7 | |
| 4 | RCC12 (hypD) | 0.03 | 0.03 | |
| 5 | RCC12(pAC63) | Zero time | 65.5 | 30.5 |
| RCC12(pAC63) | 2 h under C2H2-Ar | 4.9 | 4.2 | |
| pH 7 | ||||
| 6 | B10 (wild type) | Zero time | 3.1 | 2.8 |
| B10 | 1 h 30 min under C2H2-Ar | 1.5 | 2.0 | |
| 7 | JP91(pAC206) | Zero time | 3.7 | 5.2 |
| JP91(pAC206) | 1 h 30 min under C2H2-Ar | 3.9 | 5.1 | |
| 8 | RCC12(pAC63) | Zero time | 11.1 | 6.4 |
| RCC12(pAC63) | 1 h 30 min under C2H2-Ar | 1.6 | 2.1 | |
| 9 | BSE16 (ΔhupUV) | Zero time | 13.9 | 6.0 |
| BSE16 | 1 h 30 min under C2H2-Ar | 0.3 | 0.2 | |
Cells were grown photoheterotrophically in MN medium to an OD660 of ca. 2.4 and collected by centrifugation. The initial rates of H2 or HD formation were determined in whole cells, in 50 mM citrate-phosphate buffer, at time zero, and after incubation under C2H2-Ar (1:1) as indicated.
The catalytic properties of the HupUV protein complex are those of hydrogenase enzymes.
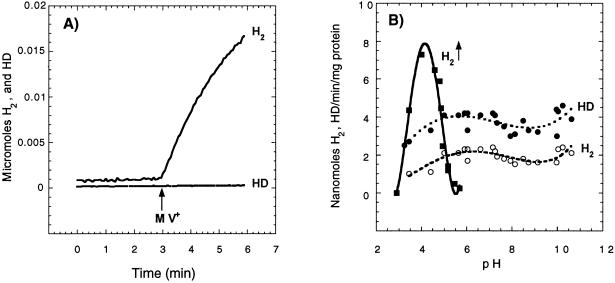
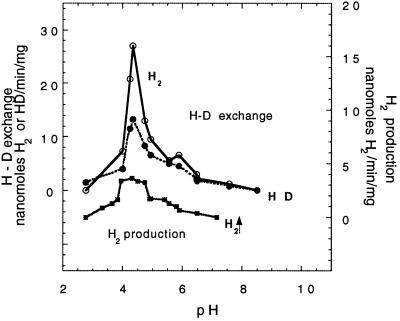
Not only can HupUV activate the H2 molecule, as indicated by the H-D exchange reaction, but it can also catalyze the other hydrogenase partial reactions, namely H2 production and H2 oxidation. Cell extracts were used to overcome the permeability barrier to electron acceptors or donors and to measure HupUV activity as a function of pH. To ascertain whether the activity was due to HupUV, it was systematically checked whether the presence of plasmid pAC206 containing the hupTUV operon that produces active HupUV protein complex (12) resulted in an increase (three- to fourfold) in hydrogenase activity of JP91(pAC206) cells compared to JP91 cells. H2 production by HupUV upon addition of MV+ (Fig. 6A) was observed at acidic pH, even at pH 4, where proteins precipitated. It occurred within a narrow range of acidic pH, in contrast to the H-D exchange activity, which did not vary significantly between pH 5 and 11 (Fig. 6B). The lack of pH dependence of the H-D exchange reaction catalyzed by HupUV is in contrast with the sharp pH dependence of the H-D exchange reaction catalyzed by the HupSL hydrogenase (Fig. 7) and other membrane-bound hydrogenases, e.g., in T. roseopersicina (44) and in Paracoccus denitrificans (41). The rates of HupSL-catalyzed HD and H2 formation peaked at around pH 4.5 and were close to zero at pH 9 with BSE16 membranes. The apparent maximal rate of H2 production by HupSL was also around pH 4 to 5, in agreement with what was observed for other [NiFe] hydrogenases, such as the hydrogenase 1 of T. roseopersicina (44), but was sevenfold lower than the H-D exchange reaction (Fig. 7).
FIG. 6.
HupUV hydrogenase activities in the soluble cytosolic fraction of JP91(pAC206) cells as a function of pH. JP91(pAC206) cells grown photoheterotrophically in MN medium were broken by passage through a French pressure cell. The soluble cytoplasmic fraction obtained by centrifugation at 100,000 × g for 70 min was used to determine HupUV hydrogenase activities. (A) H2 production linked to MV oxidation at pH 4.0. The phosphate-citrate buffer (1.25 ml, final concentration of 100 mM) in the reaction chamber was first sparged with argon to remove O2, and then the reaction vessel was closed, and 0.25 ml of soluble cytosolic fraction (0.9 mg of protein) was added. Two minutes later, MV+ (50 μl, final concentration of 120 mM) was injected into the reaction vessel. (B) pH dependence of MV+-mediated H2 production and H2 and HD formation in exchange with D2. Initial rates determined for the first minute of H2 (○) and HD (●) production (in 1.5 ml, 0.8 mg of protein) are plotted versus pH. To measure the H-D exchange, the reaction vessel was sparged first with D2. H2 (■) was formed by proton reduction with MV+. The buffers used (final concentration of 100 mM) were phosphate-citrate (pH 2.9 to 7.0), phosphate-Tris (pH 6.6 to 8.5), phosphate-glycine-NaOH (pH 7.5 to 10), and glycine-NaOH (pH 9.0 to 12.8).
FIG. 7.
pH dependence of the H-D exchange reaction and H2 production catalyzed by the hupSL-encoded hydrogenase in BSE16 membranes. The experimental conditions were the same as those in Fig. 6. H2 (○) and HD (●) were produced by the H-D exchange reaction and 0.4 mg of protein. Production of H2 (■) was measured with MV+ and 0.8 mg of protein.
The H2-uptake activities of HupSL and HupUV, as a function of pH, were determined by using BV2+ or MB as an electron acceptor. Maximal uptake activity of HupSL was in the range of pH 8.5 to 9.0, while the uptake activity of HupUV paralleled that of the HupUV H-D exchange activity (not shown). At pH 8.7, the initial rate of hydrogen uptake with MB as an electron acceptor was 260 nmol of D2/min/mg of protein for HupSL in BSE16 (ΔhupUV) cells and 21 nanomoles of D2/min/mg of protein for HupUV in the soluble fraction of JP91(pAC206) cells with BV2+ as an electron acceptor. If we take into account an estimated 3-fold increase in HupUV protein complex brought by plasmid pAC206, there would be a 30-fold difference in activity between HupSL and HupUV.
In short, the HupUV protein complex exhibited all of the typical hydrogenase reactions, and thus HupU and HupV form an active hydrogenase. However, the measured H2 uptake and H2-activating activities are very low, near the limit of detection, and more than 10 times lower than those measured for HupSL. Differences in the expression levels of the hupTUV operon and the hupSLC operon, monitored by the lacZ reporter gene, have also been observed. The ratio of phupT::lacZ to phupS::lacZ expression was between 1/50 and 1/100 (8, 12). Therefore the low HupUV activity found in situ may be due to a low level of protein, although it is also quite possible that HupUV has a low specific activity. The main feature of HupUV hydrogenase is the pH insensitivity of the H-D exchange reaction, while the H-D exchange catalyzed by HupSL showed a strong pH dependence with a sharp peak at around pH 4.5.
DISCUSSION
This work deals with the H-D exchange properties of the energy-transducing hydrogenase, HupSL, and the H2 sensor, HupUV. Since a gene homologous to hoxH of R. eutropha (39) was found in the hydrogenase gene cluster of R. capsulatus, new mutants with an inactivated hoxH gene were constructed and studied comparatively with the hupUV and hupSL mutants. No detectable change in H-D exchange was found in the hoxH mutants. Thus, either the hoxH gene was not expressed under the growth conditions used (repressing conditions for HupSL synthesis), or the activity of the hoxH gene product was too low to be detected.
HupUV has a low hydrogenase activity, but its activity is detectable with a mass spectrometer by the measurement of changes in the concentrations of D2, H2, and HD in cell suspensions. Production of H2 by extracts of cells lacking HupSL [JP91, JP91(pAC206), and JBC13] could be observed at acidic pH values after addition of MV+. The rate of H2 production by HupUV at pH 4 was twice as high as the rate of H-D exchange (Fig. 6), unlike with HupSL, whose specific activity for H2 production was sevenfold lower than the H-D exchange reaction at pH 4 (Fig. 7). The insensitivity of HupUV to acetylene and oxygen is probably due to limited gas access to the HupUV active site (30). The lack of pH effect on the H-D exchange reaction may also reflect the poor physical connection of the active site to the surface of the protein or a narrow putative proton channel. Thus, besides its specific cellular function in signal transduction, the HupUV hydrogenase displays catalytic (and probably structural) features specific to this new class of hydrogenases.
The product of the accessory hypD gene is necessary for the synthesis of active HupSL and HupUV hydrogenases (Table 2). This implies that HupUV requires the same posttranslation activation as other [NiFe] hydrogenases (24, 25) and thus has a Ni atom at its active site. Indeed, the presence of Ni and of the ligands to the Fe atom of the active site, CN and CO (18), has been demonstrated in the homologous HoxBC protein of R. eutropha (32). In a very recent report, Kleihues et al. (20) have proposed that these H2 sensors form a new subclass of so-called “regulatory hydrogenases.”
The demonstrated hydrogenase activity of HupUV is a first step for understanding how the HupUV H2 sensor and the protein histidine kinase HupT communicate and interact to control the transcriptional induction of hydrogenase (hupSL) genes in response to H2 (12, 15). H2 sensing systems homologous to HupUV have been found in bacteria other than R. capsulatus. They include HupUV in B. japonicum (2), HoxBC in Alcaligenes hydrogenophilus (23), and HoxBC in R. eutropha (22), shown to be necessary, as in R. capsulatus, for hydrogenase gene expression in response to H2.
In cells lacking hupT, maximal hupSL derepression was observed in the presence of O2. A region of the phupS regulatory region similar or very close to the binding site of HupR appears to be involved in O2 derepression of hupSL gene expression (38). Either the transcriptional factor RegA, which responds to redox and binds to the same phupS region (14), HupR, or both control hupSL expression in response to oxygen. It is not known whether the HupUV protein complex could also be involved. The sensor kinase HupT has been shown recently to belong to the PAS domain-containing superfamily of proteins (37). PAS domains are signaling modules that detect changes in light, redox potential, oxygen, small ligands, and overall energy level of a cell. According to Taylor and Zhulin (37), most PAS domains in prokaryotes are in histidine kinase sensor proteins, and their primary role is sensing oxygen, redox potential, and light. The sensing function of PAS proteins is commonly associated with the binding of specific cofactors. The flavoprotein NifL of A. vinelandii, a PAS protein, is a redox sensor which regulates nitrogen fixation by modulating the activity of the transcriptional factor NifA in response to oxygen and to fixed nitrogen (11). It is believed that the oxidation state of the prosthetic group flavin adenine dinucleotide (FAD) acts as a switch to control transcriptional activation by NifA. FAD binds to the N-terminal region of NifL in the PAS core region (36), alignable to the PAS core of HupT (37). Does HupT contain a redox-sensitive chromophore reducible by the HupUV hydrogenase, such as FAD or NAD, or is the PAS core of HupT a domain involved in protein-protein interaction between the histidine kinase HupT and the sensory HupUV protein complex?
The three-dimensional structure of the H2-signaling HupUV hydrogenase should reveal the features able to account for the inaccessibility of acetylene to the active site and for the restricted proton access reflected by the lack of pH sensitivity of HupUV activity; hopefully, this structure will also provide molecular basis for the specific function of HupUV hydrogenase in signal transduction.
ACKNOWLEDGMENTS
This work was supported by the Commissariat à l'Energie Atomique and the Centre National de la Recherche Scientifique (CEA/CNRS/UJF UMR 5092). N. Zorin received financial support from the International Association for the Promotion of Cooperation with Scientists from the Independent States of the Former Soviet Union (INTAS) (grants 94-882 and 98-862) and partial support by a travel grant through the COST Action 8.18. M. Tomiyama (Tsukuba, Japan) was supported by the Special Coordination Funds for Promoting Science and Technology based on Japan-French Bilateral International Joint Research Programme.
We gratefully acknowledge Jacqueline Chabert for participating in the construction and verification by sequencing of the JBC12 and JBC13 mutants, Patrick Carrier for the mass spectrometric measurements, and J. C. Willison (UMR 5092) for critical reading of the manuscript.
REFERENCES
- 1.Appel J, Schulz R. Hydrogen metabolism in organisms with oxygenic photosynthesis: hydrogenases as important regulatory devices for a proper redox poising? J Photochem Photobiol. 1998;47:1–11. [Google Scholar]
- 2.Black L K, Fu C, Maier R J. Sequences and characterization of hupU and hupV genes of Bradyrhizobium japonicum encoding a possible nickel-sensing complex involved in hydrogenase expression. J Bacteriol. 1994;176:7102–7106. doi: 10.1128/jb.176.22.7102-7106.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cauvin B, Colbeau A, Vignais P M. The hydrogenase structural operon in Rhodobacter capsulatus contains a third gene, hupM, necessary for the formation of a physiologically competent hydrogenase. Mol Microbiol. 1991;5:2519–2527. doi: 10.1111/j.1365-2958.1991.tb02098.x. [DOI] [PubMed] [Google Scholar]
- 4.Colbeau A, Elsen S, Tomiyama M, Zorin N A, Dimon B, Vignais P M. Rhodobacter capsulatus HypF is involved in regulation of hydrogenase synthesis through the HupUV proteins. Eur J Biochem. 1998;251:65–71. doi: 10.1046/j.1432-1327.1998.2510065.x. [DOI] [PubMed] [Google Scholar]
- 5.Colbeau A, Godfroy A, Vignais P M. Cloning of DNA fragments carrying hydrogenase genes of Rhodopseudomonas capsulata. Biochimie. 1986;68:147–155. doi: 10.1016/s0300-9084(86)81079-3. [DOI] [PubMed] [Google Scholar]
- 6.Colbeau A, Magnin J P, Cauvin B, Champion T, Vignais P M. Genetic and physical mapping of a hydrogenase gene cluster from Rhodobacter capsulatus. Mol Gen Genet. 1990;220:393–399. [Google Scholar]
- 7.Colbeau A, Richaud P, Toussaint B, Caballero J, Elster C, Delphin C, Smith R L, Chabert J, Vignais P M. Organization of the genes necessary for hydrogenase expression in Rhodobacter capsulatus. Sequence analysis and identification of two hyp regulatory mutants. Mol Microbiol. 1993;8:15–29. doi: 10.1111/j.1365-2958.1993.tb01199.x. [DOI] [PubMed] [Google Scholar]
- 8.Colbeau A, Vignais P M. Use of hupS::lacZ gene fusion to study regulation of hydrogenase expression in Rhodobacter capsulatus: stimulation by H2. J Bacteriol. 1992;174:4258–4264. doi: 10.1128/jb.174.13.4258-4264.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colbeau A, Vignais P M. The membrane-bound hydrogenase of Rhodopseudomonas capsulata is inducible and contains nickel. Biochim Biophys Acta. 1983;748:128–138. [Google Scholar]
- 10.Ditta G, Schmidhauser T, Yakobson E, Lu P, Liang X-W, Finlay D R, Guiney D, Helinski D R. Plasmids related to the broad host range vector, pRK290, useful for gene cloning and for monitoring gene expression. Plasmids. 1985;13:149–153. doi: 10.1016/0147-619x(85)90068-x. [DOI] [PubMed] [Google Scholar]
- 11.Dixon R. The oxygen-responsive NIFL-NIFA complex: a novel two-component regulatory system controlling nitrogenase synthesis in γ-Proteobacteria. Arch Microbiol. 1998;169:371–380. doi: 10.1007/s002030050585. [DOI] [PubMed] [Google Scholar]
- 12.Elsen S, Colbeau A, Chabert J, Vignais P M. The hupTUV operon is involved in negative control of hydrogenase synthesis in Rhodobacter capsulatus. J Bacteriol. 1996;178:5174–5181. doi: 10.1128/jb.178.17.5174-5181.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elsen S, Colbeau A, Vignais P M. Purification and in vitro phosphorylation of HupT, a regulatory protein controlling hydrogenase gene expression in Rhodobacter capsulatus. J Bacteriol. 1997;179:968–971. doi: 10.1128/jb.179.3.968-971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elsen S, Dischert W, Colbeau A, Bauer C E. Expression of uptake hydrogenase and molybdenum nitrogenase in Rhodobacter capsulatus is coregulated by the RegB-RegA two-component regulatory system. J Bacteriol. 2000;182:2831–2837. doi: 10.1128/jb.182.10.2831-2837.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elsen S, Richaud P, Colbeau A, Vignais P M. Sequence analysis and interposon mutagenesis of the hupT gene, which encodes a sensor protein involved in repression of hydrogenase synthesis in Rhodobacter capsulatus. J Bacteriol. 1993;175:7404–7412. doi: 10.1128/jb.175.22.7404-7412.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dischert W, Vignais P M, Colbeau A. The synthesis of Rhodobacter capsulatus HupSL hydrogenase is regulated by the two-component HupT/HupR system. Mol Microbiol. 1999;34:995–1006. doi: 10.1046/j.1365-2958.1999.01660.x. [DOI] [PubMed] [Google Scholar]
- 17.Friedrich B, Schwartz E. Molecular biology of hydrogen utilization in aerobic chemolithotrophs. Annu Rev Microbiol. 1993;47:351–383. doi: 10.1146/annurev.mi.47.100193.002031. [DOI] [PubMed] [Google Scholar]
- 18.Happe R P, Roseboom W, Pierik A J, Albracht S P J. Biological activation of hydrogen. Nature. 1997;385:126. doi: 10.1038/385126a0. [DOI] [PubMed] [Google Scholar]
- 19.Jouanneau Y, Kelley B C, Berlier Y, Lespinat P A, Vignais P M. Continuous monitoring, by mass spectrometry, of H2 production and recycling in Rhodopseudomonas capsulata. J Bacteriol. 1980;143:628–636. doi: 10.1128/jb.143.2.628-636.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleihues L, Lenz O, Bernhard M, Buhrke T, Friedrich B. The H2 sensor of Ralstonia eutropha is a member of the subclass of regulatory [NiFe] hydrogenases. J Bacteriol. 2000;182:2716–2724. doi: 10.1128/jb.182.10.2716-2724.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leclerc M, Colbeau A, Cauvin B, Vignais P M. Cloning and sequencing of the genes encoding the large and the small subunits of the H2 uptake hydrogenase (hup) of Rhodobacter capsulatus. Mol Gen Genet. 1988;214:97–107. doi: 10.1007/BF00340186. . (Erratum, 215:368, 1989.) [DOI] [PubMed] [Google Scholar]
- 22.Lenz O, Friedrich B. A novel multicomponent regulatory system mediates H2 sensing in Alcaligenes eutrophus. Proc Natl Acad Sci USA. 1998;95:12474–12479. doi: 10.1073/pnas.95.21.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenz O, Strack A, Tran-Betcke A, Friedrich B. A hydrogen-sensing system in transcriptional regulation of hydrogenase gene expression in Alcaligenes species. J Bacteriol. 1997;179:1655–1663. doi: 10.1128/jb.179.5.1655-1663.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lutz S, Jacobi A, Schlensog V, Böhm R, Sawers G, Böck A. Molecular characterisation of an operon (hyp) necessary for the activity of the three hydrogenase isoenzymes in Escherichia coli. Mol Microbiol. 1991;5:123–135. doi: 10.1111/j.1365-2958.1991.tb01833.x. [DOI] [PubMed] [Google Scholar]
- 25.Maier T, Böck A. Nickel incorporation into hydrogenases. In: Hausinger R P, Eichhorn G L, Marzilli L G, editors. Advances in inorganic biochemistry: mechanisms of metallocenter assembly. New York, N.Y: VCH Publishers, Inc.; 1996. pp. 173–192. [Google Scholar]
- 26.Marrs B L. Genetic recombination in Rhodopseudomonas capsulata. Proc Natl Acad Sci USA. 1974;71:971–973. doi: 10.1073/pnas.71.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer J, Kelley B C, Vignais P M. Effect of light on nitrogenase function and synthesis in Rhodopseudomonas capsulata. J Bacteriol. 1978;136:201–208. doi: 10.1128/jb.136.1.201-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michotey V, Toussaint B, Richaud P, Vignais P M. Characterization of the mcpA and mcpB genes capable of encoding methyl-accepting type chemoreceptors in Rhodobacter capsulatus. Gene. 1996;170:73–76. doi: 10.1016/0378-1119(95)00844-6. [DOI] [PubMed] [Google Scholar]
- 29.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 30.Montet Y, Amara P, Volbeda A, Vernede X, Hatchikian E C, Field M J, Frey M, Fontecilla-Camps J C. Gas access to the active site of Ni-Fe hydrogenases probed by X-ray crystallography and molecular dynamics. Nat Struct Biol. 1997;4:523–526. doi: 10.1038/nsb0797-523. [DOI] [PubMed] [Google Scholar]
- 31.Paul F, Colbeau A, Vignais P M. Phosphorylation coupled to H2 oxidation by chromatophores from Rhodopseudomonas capsulata. FEBS Lett. 1979;106:29–33. doi: 10.1016/0014-5793(79)80688-2. [DOI] [PubMed] [Google Scholar]
- 32.Pierik A J, Schmelz M, Lenz O, Friedrich B, Albracht S P J. Characterization of the active site of a hydrogen sensor from Alcaligenes eutrophus. FEBS Lett. 1998;438:231–235. doi: 10.1016/s0014-5793(98)01306-4. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigue A, Chanal A, Beck K, Müller M, Wu L-F. Co-translocation of a periplasmic enzyme complex by a hitchhiker mechanism through the bacterial Tat pathway. J Biol Chem. 1999;274:13223–13228. doi: 10.1074/jbc.274.19.13223. [DOI] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 35.Sargent F, Bogsch E G, Stanley N R, Wexler M, Robinson C, Berks B C, Palmer T. Overlapping functions of components of a bacterial Sec-independent protein export pathway. EMBO J. 1998;17:3640–3650. doi: 10.1093/emboj/17.13.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Söderbäck E, Reyes-Ramirez F, Eydmann T, Austin S, Hill S, Dixon R. The redox- and fixed nitrogen-responsive regulatory protein NIFL from Azotobacter vinelandii comprises discrete flavin and nucleotide-binding domains. Mol Microbiol. 1998;28:179–192. doi: 10.1046/j.1365-2958.1998.00788.x. [DOI] [PubMed] [Google Scholar]
- 37.Taylor B L, Zhulin I B. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol Mol Biol Rev. 1999;63:479–506. doi: 10.1128/mmbr.63.2.479-506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Toussaint B, de Sury d'Aspremont R, Delic-Attree I, Berchet V, Elsen S, Colbeau A, Dischert W, Lazzaroni Y, Vignais P M. The Rhodobacter capsulatus hupSLC promoter: identification of cis-regulatory elements and of trans-activating factors involved in H2 activation of hupSLC transcription. Mol Microbiol. 1997;26:927–937. doi: 10.1046/j.1365-2958.1997.6291996.x. [DOI] [PubMed] [Google Scholar]
- 39.Tran-Betcke A, Warnecke U, Böcker C, Zaborosch C, Friedrich B. Cloning and nucleotide sequences of the genes for the subunits of NAD-reducing hydrogenase of Alcaligenes eutrophus H16. J Bacteriol. 1990;172:2920–2929. doi: 10.1128/jb.172.6.2920-2929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vignais P M, Dimon B, Zorin N A, Colbeau A, Elsen S. HupUV proteins of Rhodobacter capsulatus can bind H2: evidence from the H-D exchange reaction. J Bacteriol. 1997;179:290–292. doi: 10.1128/jb.179.1.290-292.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vignais P M, Henry M-F, Berlier Y, Lespinat P A. Effect of pH on the H-2H exchange, H2 production and H2 uptake catalysed by the membrane-bound hydrogenase of Paracoccus denitrificans. Biochim Biophys Acta. 1982;681:519–529. [Google Scholar]
- 42.Vignais P M, Toussaint B. Molecular biology of membrane- bound H2 uptake hydrogenases. Arch Microbiol. 1994;161:1–10. doi: 10.1007/BF00248887. [DOI] [PubMed] [Google Scholar]
- 43.Weaver P F, Wall J D, Gest H. Characterization of Rhodopseudomonas capsulata. Arch Microbiol. 1975;105:207–216. doi: 10.1007/BF00447139. [DOI] [PubMed] [Google Scholar]
- 44.Zorin N A, Dimon B, Gagnon J, Gaillard J, Carrier P, Vignais P M. Inhibition by iodoacetamide and acetylene of the H-D-exchange reaction catalyzed by Thiocapsa roseopersicina hydrogenase. Eur J Biochem. 1996;241:675–681. doi: 10.1111/j.1432-1033.1996.00675.x. [DOI] [PubMed] [Google Scholar]