Abstract
OBJECTIVE
Gliomas invading the anterior corpus callosum are commonly deemed unresectable due to an unacceptable risk/benefit ratio, including the risk of abulia. In this study, the authors investigated the anatomy of the cingulum and its connectivity within the default mode network (DMN). A technique is described involving awake subcortical mapping with higher attention tasks to preserve the cingulum and reduce the incidence of postoperative abulia for patients with so-called butterfly gliomas.
METHODS
The authors reviewed clinical data on all patients undergoing glioma surgery performed by the senior author during a 4-year period at the University of Oklahoma Health Sciences Center. Forty patients were identified who underwent surgery for butterfly gliomas. Each patient was designated as having undergone surgery either with or without the use of awake subcortical mapping and preservation of the cingulum. Data recorded on these patients included the incidence of abulia/akinetic mutism. In the context of the study findings, the authors conducted a detailed anatomical study of the cingulum and its role within the DMN using postmortem fiber tract dissections of 10 cerebral hemispheres and in vivo diffusion tractography of 10 healthy subjects.
RESULTS
Forty patients with butterfly gliomas were treated, 25 (62%) with standard surgical methods and 15 (38%) with awake subcortical mapping and preservation of the cingulum. One patient (1/15, 7%) experienced postoperative abulia following surgery with the cingulum-sparing technique. Greater than 90% resection was achieved in 13/15 (87%) of these patients.
CONCLUSIONS
This study presents evidence that anterior butterfly gliomas can be safely removed using a novel, attention-task based, awake brain surgery technique that focuses on preserving the anatomical connectivity of the cingulum and relevant aspects of the cingulate gyrus.
Keywords: default mode network, connectivity, tractography, DTI, anatomy, cingulate gyrus, butterfly glioma, glioblastoma, corpus callosum, cingulum, oncology
Neurosurgeons are often taught during training that gliomas invading the corpus callosum (so-called butterfly gliomas) are “inoperable.” Yet, any brain tumor can be operated on, and what tumor surgeons actually mean by “inoperable” is that the potential benefits of trying to remove a tumor are outweighed by the risks incurred by doing so. Inquiry into why butterfly gliomas have earned this distinction yields a host of explanations from experienced surgeons: corpus callosum involvement often indicates a more aggressive phenotype; early efforts to remove these tumors often left patients with severe abulia and/or akinetic mutism; and these tumors, especially glioblastomas, are incurable. Conventional wisdom has been that it is best to biopsy to establish the diagnosis and palliate these patients with noninvasive therapies to allow them the best quality of life permitted by their disease. Consequently, the practice pattern at most institutions is divided between biopsy and surgical decompression.18
However, our experience illuminated gaps in arguments for this treatment philosophy. We became interested in resecting anterior butterfly gliomas because aspects of this conservative approach appeared inconsistent with our observations. First, the idea that we were offering these patients a good quality of life by not removing the tumor from the involved corpus callosum was contradicted by our consistent experience with these patients, who rarely had a good quality of life for any meaningful length of time. Bifrontal tumor growth and edema typically caused the patient to become abulic and akinetic shortly after diagnosis, and extensive bifrontal radiation also did not help. Thus, we believed it was hard to argue we could do worse by these patients in treating them surgically, given that, when possible, resection of low-grade gliomas (LGGs) and high-grade gliomas (HGGs) has been shown to improve tumor control and overall survival.1,11,49,62 We hypothesized that avoidance of collateral injury to critical neighboring structures—namely the default mode network (DMN)—is achievable through careful technique refinement, changing the overall risk/benefit ratio.
Avoiding the connections of the DMN may be a critical step in resecting bifrontal tumors. The DMN was originally described by Shulman and colleagues,52 who in 1997 observed (using PET) a constellation of cortical areas with reduced activity during goal-directed tasks that increased during periods of wakeful rest. Since that time, a body of work describing the DMN has grown due to widespread interest in the neuroscience community. Extensive research has led to the acceptance of the DMN as a switch between restfulness and attention,57 active during periods of repose and introspection.3 In our experience, previously described regions of the DMN44 and their connections are often involved by frontal gliomas crossing the midline through the corpus callosum.
Our present report summarizes efforts to successfully remove gliomas crossing the anterior corpus callosum over the past 4 years. We provide a description of our technique for removing these tumors, as well as describe the rationale of how and why it has evolved to its present form. We believe that this technique, which involves awake subcortical resection with higher attention tasks to preserve the anatomical connectivity of the DMN, provides insight into why earlier efforts to remove these tumors were met with high morbidity, and highlights the importance of preserving this network, especially the cingulum, which joins these structures. The importance of the latter finding has implications for numerous other procedures involving this brain region, and we provide a detailed study of the surgical anatomy of connections between the previously well-described cortices of this network to serve as a guide for complication avoidance in future endeavors.
Methods
Part 1: Operative Technique and Clinical Outcome Assessment
Patient Selection
We performed a retrospective review of prospectively collected data on all patients undergoing glioma surgery performed by the senior author (M.E.S.) during a 4-year period from 2012 to 2015 at the University of Oklahoma Health Sciences Center. For purposes of this analysis, we defined an anterior butterfly glioma as any WHO Grade II or III astrocytoma or oligodendroglioma, or a glioblastoma that had imaging evidence of tumor invading the rostrum, genu, or anterior one-fourth of the body of the corpus callosum and crossing the midpoint of the callosum. Thus, our definition includes both primarily frontal and anterior cingulate tumors that cross the corpus callosum and primarily callosal tumors with only secondary frontal involvement. All patients included in this study were diagnosed by histopathological analysis. Tumors that herniated across the midline under the falx, but did not cross the midpoint of the anterior callosum, were not defined as butterfly tumors and are not included in our study. Patients with tumors involving the expected location of the supplementary motor area (SMA; i.e., the medial surface of the posterior superior frontal gyrus) also are not included in this analysis, because the symptoms of SMA syndrome overlap with akinetic medial frontal lobe syndromes.6
This study was performed with approval of our institutional review board. Patients were counseled in frank detail during the informed consent process about the natural history and prognosis of butterfly gliomas, as well as the community view that most neurosurgeons deem these tumors inoperable, or at least high risk, and that our early surgical efforts represent preliminary efforts to improve the treatment of a devastating disease. This study includes a consecutive series of all patients with anterior butterfly gliomas on whom we have operated. Forty-four patients were prospectively identified as potential surgical candidates. Surgery was not offered to patients with more than mild akinetic mutism or abulia, or with other symptoms resulting in a Karnofsky Performance Scale score of less than 70, as we did not think a reasonable outcome was possible in these patients. Two (4%) of 44 patients were not offered surgery and 40 (95%) of 42 patients agreed to surgery after appropriate counseling.
Preoperative Evaluation
Magnetic resonance imaging with and without Gd contrast was performed preoperatively in all patients, with diffusion tensor imaging (DTI) tractography for the corticospinal tract, superior longitudinal fasciculus, arcuate fasciculus, occipitofrontal fasciculus, and optic pathways. Patients underwent preoperative clinical evaluation by physical and speech therapists, including spatiotemporal and attention testing.
Surgical Technique and Its Evolution
As with any new surgical technique, our first efforts were refined over time as we worked toward improving on our initial results. The most significant changes from our initial technique over time were the introduction of awake surgery in all patients and the use of subcortical cingulate gyrus–sparing techniques. The initial approach as described here is the standard technique, which represents the foundation upon which the cingulum-sparing technique (CST) was developed. Our data regarding the importance of cingulum preservation are based on comparison of outcomes with and without using the CST. Both techniques are described below.
Standard Technique
The standard technique was used for the first 2 years of this series, and was performed while the patient was asleep given the conventional thinking that there were no specific eloquent cortical structures to study or preserve with awake surgical techniques. The resection strategy was based on our initial hypothesis that severe frontal syndromes in medial frontal lobe resections result from inadvertent injury to the head of the caudate nucleus or the septal nuclei (which look very similar to tumor to the naked or untrained eye) or to the branches of the anterior cerebral artery (ACA). Thus, our initial method for resecting the corpus callosum involved a stepwise method of finding and avoiding damage to these structures based on anatomical landmarks.
A common perception among neurosurgeons is that the correct approach to removing these tumors is an interhemispheric approach similar to a transcallosal approach. We do not believe this is the appropriate method for removing a butterfly tumor, as the majority of the tumor is in the frontal lobe, not within the visible part of the corpus callosum. A transcallosal approach does not allow resection along the long axis of the tumor, and we have found that going through the frontal lobe is more effective and consistent with the anatomy of these tumors. This approach addresses the bulk of the tumor by removing it down the long axis of the corpus callosum.
Using image guidance, we plan a posterior and lateral cut in the superomedial frontal lobe that separates the invaded frontal lobe from eloquent structures such as the superior longitudinal fasciculus and SMA. Our goal with these cuts is to intentionally enter the frontal horn of the lateral ventricle and to identify the head of the caudate nucleus. This entry into the frontal horn is widened until the frontal portion of the tumor is circumferentially separated from the posterior brain and caudate head. Caudate tissue can look deceivingly like glioma tissue as it is gray and tends to bleed like tumor. In addition to identifying the caudate by anatomical location as it indents into the frontal horn, recognition of its gross appearance is critical. Yaşargil described it as “cut nutmeg,” which is to say, redgray tissue dotted with bright white spots.64 This feature is subtle, so entering the frontal horn early is also important in preserving the caudate head.
We then continue the posterior cut until we see the falx at the midline. The superior frontal gyrus is subpially debulked until the anterior cerebral branches are clearly delineated and the pericallosal and callosomarginal arteries are visualized. The internal frontal branches supplying the disconnected ipsilateral frontal lobe are sacrificed and the frontal portion of the tumor is removed in front of the caudate head. This step is critical. Failure to aggressively address the portion of the tumor in the frontal lobe will lead to disappointing results as there will be a great deal of tumor left, and the edema caused by leaving a large amount of this tumor will thwart efforts to improve the patient’s quality of life.
Once the involved frontal lobe has been removed and the frontal horn is exposed, we orient ourselves to the anatomical landmarks, including the frontal horn and the foramen of Monro, which may or may not be visible. The corpus callosum is identifiable as all tissue below the pericallosal artery and above or in front of the frontal horn. Thus, the strategy for resecting this is to remove all of this tissue until the opening is widely biventricular, and the other caudate head is visualized. We do not resect white matter lateral to the contralateral caudate head to avoid coming into contact with the occipitofrontal fasciculus, although we have seldom encountered it there.
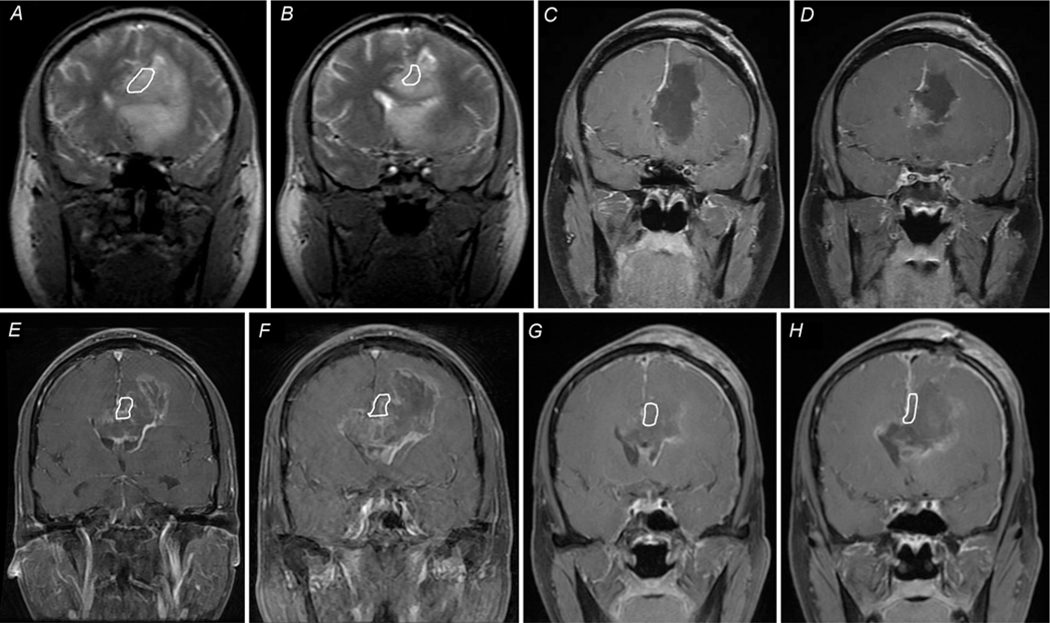
Avoidance of injury to the septal nuclei is similarly important. We accomplish this by stopping the callosal resection once we observe the A2 portion of the ACAs skeletonized on the undersurface. Here, the inferior forceps minor fans outward, and proceeding further inferiorly would lead into the subcallosal gyri where the septal nuclei are located (Fig. 1). Imaging of a patient who underwent resection using the standard technique is shown in Fig. 2A–D.
FIG. 1.
Coronal MRI demonstrating the relationship of the cingulum to other anatomical structures of the frontal region. Left: Tractography with GQI shows the laterally directed fibers of the corpus callosum (purple) cradling the bilateral fiber bundles of the cingulum (light green) crossing perpendicularly. Right: Overlays show the cingulum (light green), corpus callosum (purple), cortex of the cingulate gyrus (dark green), frontal horns of the lateral ventricles (yellow), and caudate nuclei (red). The septal nuclei (gray) lie below the rostrum of the corpus callosum. Familiarity with the “pyramid” (white lines) formed by these structures can allow for safe resection of tumors crossing in the corpus callosum.
FIG. 2.
Coronal MRI showing the standard technique (A–D) versus the CST (E–H). Key differences between the standard technique and the CST are identification and preservation of the cingulum with awake surgery and continuous speech and task monitoring, which are part of the CST but not the standard technique. A: Preoperative T2-weighted image showing a butterfly glioblastoma involving the frontal lobe of both hemispheres. The tumor crosses over the midline via the corpus callosum and involves both ventricles. B: Slice obtained posterior to panel A, demonstrating extensive involvement of the frontal lobe and corpus callosum. C and D: Postoperative T1-weighted images with Gd contrast demonstrate the EOR and surgical trajectory using the standard technique. The frontal horn of the ventricle is entered to identify the caudate nucleus, and involved superior frontal gyrus is removed. Tumor removal is continued along the corpus callosum, but not beyond the contralateral caudate head. Resection is carried inferiorly to the A2 portion of the ACAs on the underside of the callosum resection. The cingulate gyrus is within the area outlined in white in A and B, and is absent in C and D. E and F: Preoperative T1-weighted image with Gd enhancement in another patient demonstrating a butterfly glioblastoma involving the frontal lobes of both hemispheres, crossing the midline in the corpus callosum and involving both ventricles. G and H: Postoperative imaging after surgery with the CST demonstrates an intact cingulate gyrus (outlined in white) and minimal residual enhancing tumor.
Cingulum-Sparing Technique
Our initial experience with removing anterior butterfly gliomas was sufficiently promising to convince us that these tumors could be resected without severe neurological sequelae. However, our results were inconsistent, as some patients still developed the symptoms typical of the natural progression of butterfly gliomas. We began performing these cases with the patient awake throughout the subcortical and callosum portions of the resection, with the goal of avoiding abulia and akinetic medial frontal lobe syndromes. We have consistently found that patients lose attention to task as we approach or stimulate this region. We have grown increasingly confident through awake monitoring that preserving the cingulate gyrus with its connections to attention networks is the critical step in achieving good functional outcomes in these cases.
We design attention tasks for each patient by choosing a task that requires praxis, bimanual coordination, and attention. We have employed various tasks such as knitting, playing a musical instrument, and assembling auto parts. When the patient is able, we also have him or her name objects simultaneously to further strain the attention task, thus increasing its sensitivity. The inability to complete the selected tasks under stimulation indicates that we are approaching the attention network, alerting us to protect it. This often manifests as halted naming during task performance. Consequently, if under repeated stimulation, a patient is unable to perform the task, or unable to execute naming while performing the task, the area is considered to map positively. Repetitive subcortical stimulation with the Ojemann stimulator at 5 mA is performed during the entire resection to confirm that any white matter is safe to resect prior to proceeding further.
Our present technique follows the same fundamental concepts as the standard approach: a transcortical approach with an emphasis on removing the frontal portion of the tumor thoroughly, early entry into the frontal horn, identification of the caudate, skeletonization of the midline to identify the ACA branches, and removal of the corpus callosum until stopping above the septal nuclei. The key addition of awake mapping techniques allows for monitoring the lateral, posterior, and medial cuts to ensure that the attention networks of the medial frontal lobe are not damaged. The limits of resection are decided entirely by functional anatomy as determined by awake monitoring with attention tasks.
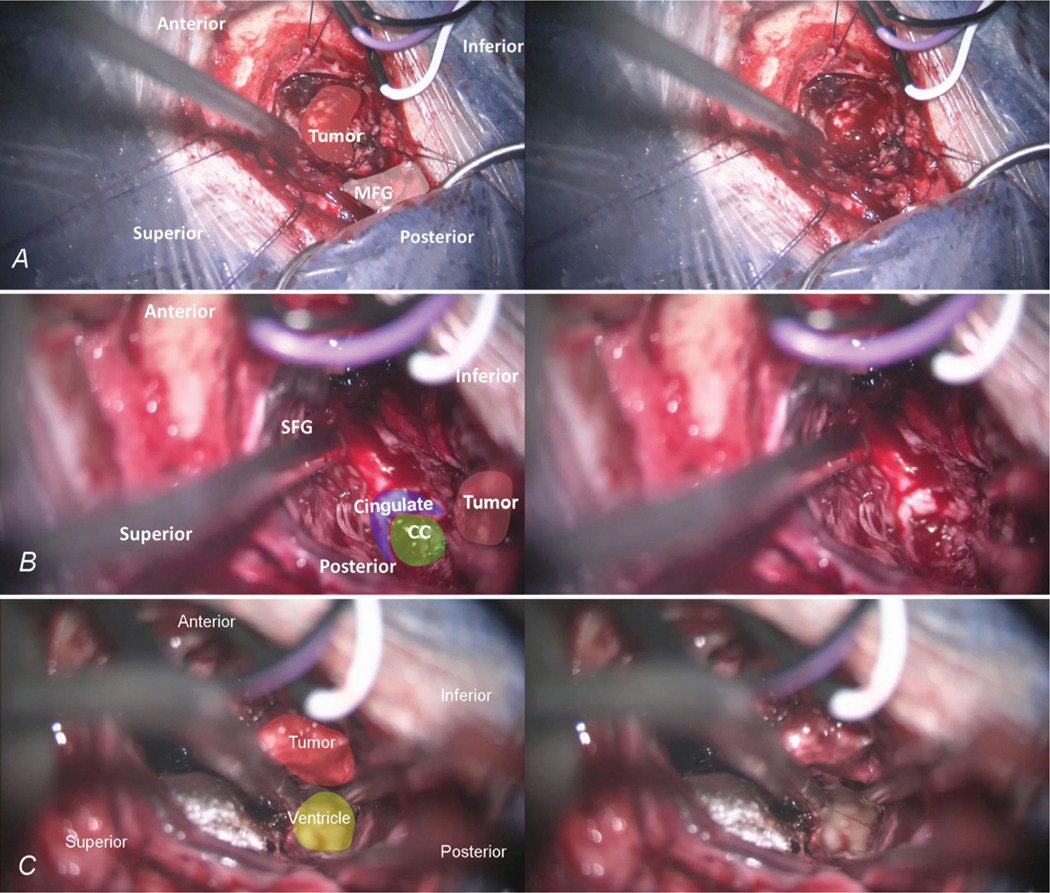
After performing the craniotomy, the exposed cortex is tested for motor, speech, and somatosensory function using standard stimulation mapping techniques. These cortices usually do not map positive as we are operating anterior to expected cortical locations of these functions. We then bring in the operating microscope and begin subcortical mapping, dividing the posterior and lateral portions of the tumor from the surrounding brain. We start by dividing the lateral border with an emphasis on continuous speech monitoring using the double task (object naming while moving the contralateral arm) described by Fernández Coello and colleagues.21
Once this lateral cut extends down to the ventricle and is free of the caudate head, we begin making the posterior cut, which is monitored using an attention task. To spare the cingulate gyrus, we first work along the medial surface of the superior frontal gyrus as the patient tolerates. Reaching subcortical areas where the patient shows decreased attention with stimulation eventually pushes us to move laterally (Fig. 3). Our cut continues until we have cut the superior frontal gyrus in half in the coronal plane. The goal is to identify the cingulate sulcus, which tells us the approximate depth of the cingulate gyrus. Once we have identified the cingulate gyrus, we continue our subcortical dissection laterally to avoid severing the cingulum tract until we are lateral to the pial border of the depth of the cingulate sulcus. We then connect this cut into the ventricle to join the lateral cut made earlier, which disconnects the tumor from the medial attention networks. If the cingulate gyrus is involved with tumor, we resect it from anterior to posterior as tolerated by the attention task.
FIG. 3.
Identifying the site of inattention in awake surgery. This artistic scheme demonstrates how we use intraoperative testing to guide resection. A coronal view shows our descent (black arrow) as the patient performs an attention task during dissection of subcortical white matter of the frontal lobe. When the patient struggles with the task, this tells us to redirect laterally (white arrow) to avoid the cingulum bundle. Stimulation is continued until the tumor is dissected off the lateral portion of the cingulate gyrus down to the corpus callosum. X = callosal sulcus. Figure is available in color online only.
Anatomical study shows that the fibers of the corpus callosum course under and around the cingulate gyrus. Thus, once the lateral portion of the cingulate has been divided from the brain along its lateral surface, we are able to remove the corpus callosum without disconnecting the cingulum tract from the rest of its network. The resection of the corpus callosum proceeds as described in the standard technique detailed above. Imaging from a patient treated with the CST is shown in Fig. 2E–H for comparison with the traditional method, and a step-by-step illustration of the operation is given in Figs. 4 and 5. A detailed operative video (Video 1) further demonstrates the CST surgery.
FIG. 4.
Intraoperative photographs of the first 3 key steps of the CST. A: The CST is performed via a transcortical approach while the patient is awake, using subcortical mapping to safely separate the frontal aspect involved by tumor from the surrounding frontal lobe. B: We focus mainly on the medial frontal lobe, working lateral to the cingulate gyrus. Following the tumor inferiorly with subcortical mapping, we dissect and preserve the cingulate gyrus, with the callosal sulcus coming into view where the corpus callosum (CC) crosses underneath the cingulate gyrus. C: We next look posterolaterally for the lateral ventricle and use this to define the lateral boundary of the corpus callosum as well as separate the tumor from the caudate nucleus. MFG = middle frontal gyrus; SFG = superior frontal gyrus.
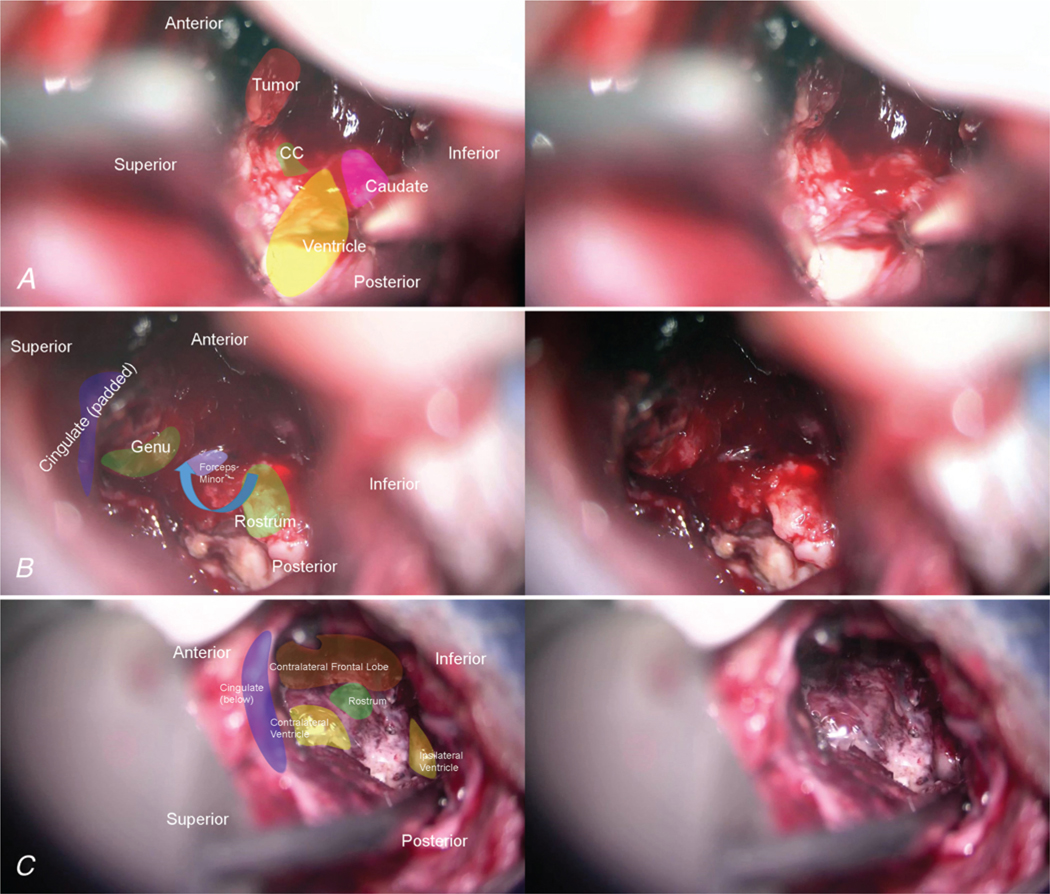
FIG. 5.
Intraoperative photographs of the last 3 key steps of the CST. A: The tumor is completely separated from the caudate nucleus and the frontal horn is widely open and defined. This gives us a clear area of the corpus callosum to be removed between the cingulate gyrus and the ventricle. B: After removing the genu of the corpus callosum, we have a clear path to the other side. Entering the contralateral ventricle, we follow the tumor into the contralateral frontal lobe, anterior to the cingulate gyrus. C: The final result is a wide, biventricular opening, with the triangular-shaped appearance of the forceps minor and rostrum clearly identified at the bottom.
VIDEO 1. Clip showing the CST surgery in detail, narrated by the senior author (M.E.S.). Copyright Michael E. Sughrue. Published with permission. CC = corpus callosum, FAT = frontal aslant tract, IFOF = inferior frontooccipital fasciculus, MFG = middle frontal gyrus, SFG = superior frontal gyrus, SLF = superior longitudinal fasciculus. Click here to view.
Outcome Assessment and Definition of Terms
Patients were assessed immediately following surgery in the hospital and at 6 weeks postoperatively by physical and speech therapists. Assessment included basic neuropsychological testing. For the purposes of our study, significant abulia and akinetic mutism were both considered undesirable outcomes and analyzed as the same thing. A large proportion of these patients present with some degree of abulia on initial diagnosis, so for the purpose of determining what constitutes a poor outcome we define significant abulia as reduced emotional responsiveness, lack of spontaneous movement, or marked passivity that leaves the patient unable to participate meaningfully in neuropsychological testing or to perform activities of daily living without assistance or prompting. Akinetic mutism is more severe and leaves a patient unable to partake in nearly all activities around him or herself. To ensure we were not ignoring marginal outcomes in this difficult-to-assess ability, we adopted a stringent definition. To achieve a desired outcome, a patient could not have more than mild personality blunting noticeable only to close contacts. Notably, of those with desirable outcomes, few had personality blunting noticeable even to close friends or family members. Additionally, abulia and akinetic mutism were defined as resolved if present initially after surgery but not at the 6-week follow-up.
All patients underwent postoperative MRI with and without contrast to evaluate the extent of resection (EOR) using volumetric analysis of pre- and postoperative images. EOR was graded as gross-total resection (GTR), near-total resection (NTR), or subtotal resection (STR),33 corresponding to 100%, 90%–99%, and 70%–89% of tumor volume resected, respectively. Resection of tumor-involved corpus callosum was also assessed with MR contrast and T2-weighted imaging, and recorded as a percentage of the total volume of corpus callosum involved by tumor.
Statistical Analysis
Potential differences in each surgical category were assessed to identify factors associated with developing both temporary and persistent abulia/akinetic mutism. Categorical variables were compared using the Pearson chi-square test. The Fisher exact test was used if more than 80% of values were less than 5. A paired-samples t-test was used for continuous variables. For the sake of survival analysis, time to death was determined from the time of surgery, and between-group differences were analyzed using the log-rank test. A p value < 0.05 was considered statistically significant. All data analysis was conducted using SPSS (version 22, IBM Inc.).
Part 2: Surgical Anatomy of the Attention Network
Our initial experiences with awake mapping for anterior butterfly glioma surgery focused our interest on the DMN. Specifically, we selected cortices shown to co-activate in resting-state functional MRI (fMRI) studies,15,34,50,56,58 and believed to be involved in switching from undirected thinking to attention-driven, goal-directed thinking.44,45 We found that our intraoperative dissections performed with attention tasks were repeatedly directed away from these areas to preserve attention function. This section thus focuses on outlining the surgical anatomy of this network and its connections in normal patients to guide surgeons in these cases.
Tractography
Diffusion imaging from 10 healthy adult controls from the publicly available Human Connectome Project were obtained for this study from the Human Connectome Project database (http://humanconnectome.org, release Q3). A multishell diffusion scheme was used, and the b-values were 990, 1985, and 1980 sec/mm2. Each b-value was sampled in 90 directions. The in-plane resolution was 1.25 mm. The slice thickness was 1.25 mm. The diffusion data were reconstructed using generalized q-sampling imaging (GQI)66 with a diffusion sampling length ratio of 1.25.
Following registration to Montreal Neurological Institute space, virtual dissections were performed in DSI Studio (Carnegie-Mellon) using 2 regions of interest (ROIs) to isolate single tracts.10 Dissecting in Montreal Neurological Institute space allowed for ready assessment of variability among patients. Voxels within each ROI were automatically traced with randomized seeding of the voxel and/or tract with a maximum angular threshold of 45°. When a voxel was approached with no tract direction or a direction greater than 45°, the tract was halted. Tractography was stopped after reaching a length of 450 mm. In some instances, exclusion ROIs were placed to exclude spurious tracts or tracts not involved in the network of interest. Dissections proceeded systematically anteriorly to posteriorly along the cingulate gyrus. All cingulate tracts were dissected in both hemispheres.
The tractography analysis was driven by functional imaging data demonstrating the locations of cortices associated with the DMN.50,56 The functional data were used to determine appropriate placement of ROIs, and connectivity analysis with randomized seeding in these regions was used to create the final maps published in this study.
Postmortem Dissections
Our goal with postmortem dissections was to demonstrate the location of the DMN and its connections using gross surgical anatomy familiar to neurosurgeons. Postmortem dissections were performed using a modified Klingler technique.33 Ten specimens were used for this study, obtained from our institution’s Willed Body Program with approval of the state’s anatomical board. The cadaveric brains were fixed in 10% formalin for at least 3 months after removal from the cranium. Up until the time of dissection, the pia-arachnoid membrane was left attached.
After fixation with formalin, specimens were rinsed with water for 2 days, and then frozen at −10°C for 8 hours causing disruption of the white matter. After thawing, dissection of the “freeze-fractured” specimens began with removal of meninges and identification of cortical anatomy, including gyri and sulci. Cortical areas were peeled back to reveal white matter areas of interest, and care was taken to leave intact those cortical areas corresponding to white tracts of interest to preserve their relationship to one another. Tracts were dissected with blunt instruments to avoid disrupting the natural tract anatomy. Photographs were taken at each stage in the dissection.
Results
Patient Population
A total of 40 patients were treated surgically by the senior author between 2012 and 2015 for WHO Grade II–IV glioma tumors crossing the anterior corpus callosum. Characteristics of these patients are given in Table 1. We treated 25 patients with the standard technique before we developed the CST, after which 15 patients were treated with the CST. Patients treated with the standard technique had a median age of 52 years (range 29–77 years), and patients treated with the CST had a median age of 45 years (range 24–68 years; p = 0.95). Eleven (44%) of 25 patients treated with the standard technique and 2 (13%) of 15 treated with the CST had WHO Grade II tumors. Four (16%) of 25 patients treated with the standard technique and 2 (13%) of 15 treated with the CST had Grade III tumors. Ten (40%) of 25 patients treated with the standard technique and 11 (74%) of 15 treated with the CST had Grade IV tumors (p = 0.09). There was no significant difference in patient sex between the 2 treatment groups (p = 0.41).
TABLE 1.
Patient characteristics
| Technique | |||
|---|---|---|---|
|
|
|||
| Variable | Standard | CST | p Value |
|
| |||
| No. of patients (%) | 25 (62) | 15 (38) | — |
|
| |||
| Age (yrs) | 0.95 | ||
|
| |||
| Median | 52 | 45 | |
|
| |||
| Range | 29–77 | 24–68 | |
|
| |||
| Sex (%) | 0.41 | ||
|
| |||
| Male | 17 (68) | 12 (80) | |
|
| |||
| Female | 8 (32) | 3 (20) | |
|
| |||
| WHO grade (%) | 0.09 | ||
|
| |||
| II | 11 (44) | 2 (13) | |
|
| |||
| III | 4 (16) | 2 (13) | |
|
| |||
| IV | 10 (40) | 11 (73) | |
|
| |||
| EOR | 0.83 | ||
|
| |||
| 100% (GTR) | 21 (84) | 12 (80) | |
|
| |||
| 90%—99% (NTR) | 2 (8) | 1 (7) | |
|
| |||
| 70%—89% (STR) | 2 (8) | 2 (13) | |
|
| |||
| Involved corpus callosum resected | 0.38 | ||
|
| |||
| 100% (GTR) | 23 (92) | 15 (100) | |
|
| |||
| 90%—99% (NTR) | 0 (0) | 0 (0) | |
|
| |||
| 70%—89% (STR) | 2 (8) | 0 (0) | |
Of patients treated with the standard technique, 21/25 (84%) had GTR, 2/25 (8%) had NTR, and 2/25 (8%) had STR. Of patients treated with the CST, 12/15 (80%) had GTR, 1/15 (7%) had NTR, and 2/15 (13%) had STR. There was no significant difference in EOR between the 2 surgical groups (p = 0.83). In patients treated with the standard technique, tumor-involved corpus callosum was 100% resected in 23/25 (92%), 90%–99% resected in 0/25 (0%), and 70%–89% resected in 2/25 (8%). All 15 patients (100%) treated with CST experienced 100% resection of tumor-involved corpus callosum (p = 0.38).
Clinical Outcome Comparison
Eleven (44%) of 25 patients had significant abulia/akinesis on postoperative Day 1 with the standard technique and 1/15 (7%) had abulia/akinesis on postoperative Day 1 with the CST (Table 2). Seven (28%) of 25 had abulia/akinesis at the 6-week follow-up using the standard technique, and no patients had abulia/akinesis at the 6-week follow-up with the CST (p = 0.01 and 0.03, respectively). Resolution of abulia did not differ significantly between surgical groups (p = 0.41; Table 3). Rates of other surgical complications did not significantly differ between groups (Table 4).
TABLE 2.
Interventions
| Method | Abulic/Akinetic | p Value |
|---|---|---|
| Postop Day 1 (%) | 0.01 | |
| Non-cingulum sparing | 11/25 (44) | |
| CST | 1/15 (7) | |
| Postop 6 wks (%) | 0.03 | |
| Non-cingulum sparing | 7/25 (28) | |
| CST | 0/15 (0) |
TABLE 3.
Resolution of abulia in each group
| Method | Abulia Resolved | p Value |
|---|---|---|
| CST | 1/1 (100%) | 0.41 |
| Non-cingulum sparing | 4/11 (36%) |
TABLE 4.
Postoperative complications*
| Technique | |||
|---|---|---|---|
|
|
|||
| Variable | Standard (%) | CST (%) | p Value |
|
| |||
| Overall complication rate† | 7/25 (28) | 2/15 (13) | 0.28 |
|
| |||
| Postop stroke | 0 (0) | 0 (0) | — |
|
| |||
| Postop deep vein thrombosis | 4/25 (16) | 0/15 (0) | 0.10 |
|
| |||
| Postop hemorrhage | 1/25 (4) | 1/15 (7) | 0.71 |
|
| |||
| Infection | 5/25 (20) | 1/15 (7) | 0.25 |
Some patients experienced more than 1 complication. Values are expressed as the number of patients.
All complications examined in this study, including postoperative stroke, intracerebral hemorrhage, postoperative deep vein thrombosis/pulmonary embolism, and infection.
As noted in Table 5, none of 13 patients (0%) with LGGs died during the study period, while 10/27 (37%) with HGGs died during the study. Survival analysis found a median survival for patients with HGG of 15.0 months compared with 100% survival in patients with LGG (p < 0.01). Of patients with HGG only, 4/13 (31%) treated with CST died during the study, while 6/14 (43%) treated with traditional surgery died during this period. Survival analysis found median survival for patients with HGGs treated with CST was 15.0 months compared with 12.0 months for patients treated with traditional surgery (p = 0.63). Survival curves are shown in Fig. 6.
TABLE 5.
Overall survival following surgery
| Variable | Deaths (%) | Median Survival (mos) | P Value |
|---|---|---|---|
| Tumor type | <0.01 | ||
| LGG | 0/13 (0) | — | |
| HGG | 10/27 (37) | 15.0 | |
| Op technique (HGG only) | 0.63 | ||
| CST | 4/13 (31) | 15.0 | |
| Traditional | 6/14 (43) | 12.0 |
FIG. 6.
Charting survival outcomes after surgery. A: Survival in patients with LGG compared with patients with HGG. B: Survival in patients after surgery with the CST compared with surgery using the standard technique.
Fibers of the Cingulum to the Superior Frontal Gyrus
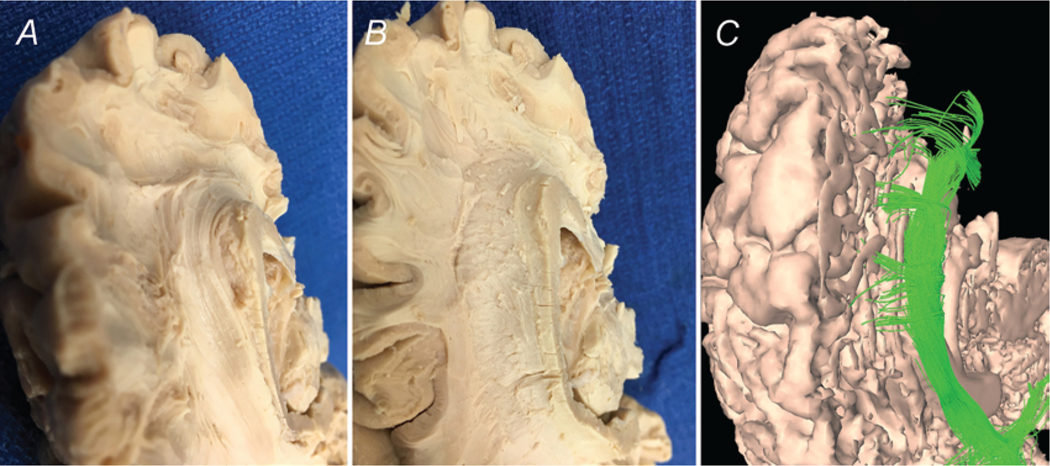
After dividing the cerebral hemispheres of our freeze-fractured specimens, we began dissecting from the midsagittal aspect. The corpus callosum is readily identified by its distinctive “C” shape, and the unique texture created by the cut ends of its many crossing fibers. It is enveloped by the cingulate gyrus, which rests superiorly and wraps anteriorly around the bulb formed by the genu of the corpus callosum. Posterior to the genu is the septum pellucidum separating the frontal horns of the lateral ventricles. The cingulate sulcus forms the junction of the cingulate gyrus and the superior frontal gyrus; this is the boundary of the initial dissection. With blunt instrumentation, the gray matter of the anterior cingulate gyrus can be teased away to reveal fibers of the cingulum beneath. We found it was easiest to begin at the cingulate sulcus just above the body of the corpus callosum, working inferiorly and laterally. Once the cingulum bundle was identified, the dissection was carried forward and inferiorly to reveal the bundles branching off the cingulum to the inferomedial part of the superior frontal gyrus. Continuing the dissection down and around the genu reveals a continuation of the cingulum around the posterior inferior boundary of the genu. Carefully removing the gray matter of the medial portion of the superior frontal gyrus reveals fiber bundles originating from the cingulum both inferior and superior to the genu. Deeper dissection above the body of the corpus callosum reveals connections to the more posterior regions of the medial superior frontal gyrus and precentral gyrus. These anatomical findings are consistent with the anterior portion of the DMN from past studies of fMRI data. Anatomical dissection findings were consistent with tractography (Fig. 7).
FIG. 7.
Anterior connections of the cingulum. Green and black arrows represent the directionality of the fiber bundles of the cingulum. A: Medial view of dissected left hemisphere demonstrates fibers projecting off the cingulum from above the genu of the corpus callosum (CC) into the medial SFG. B: Deeper dissection shows fibers projecting from below the genu (G) as well. C: Diffusion tractography re-demonstrates the connections described. CR = corona radiata; GO = gyrus orbitalis; GR = gyrus rectus; LV = lateral ventricle; S = splenium of the corpus callosum.
Fibers of the Cingulum to the Cuneus
After removal of the anterior cingulate gyrus, fibers of the cingulum can be traced posteriorly, coursing above the body of the corpus callosum to the splenium. Postcentral fibers can be observed branching off of the cingulum as it courses posteriorly over the body of the corpus callosum. Complete removal of the superior frontal gyrus and paracentral lobule superiorly reveals the fibers of the corona radiata, with no visible connections into the cingulum. These fibers instead course under the cingulum as part of the corpus callosum, which connects the two hemispheres of the cerebrum. We also note there are no visible fibers joining the cingulum from any part of the corpus callosum. Instead, fibers of the corpus callosum cradle the cingulum bundle coursing perpendicularly (Figs. 1 and 8). Fibers of the cingulum continue around the posterior portion of the corpus callosum, turning inferiorly. Here the cingulum divides, with some fibers continuing inferiorly around the splenium and others continuing posteriorly to the retrosplenial cortex of the cingulate gyrus (Fig. 9). Some fibers appear to terminate rather than continuing further inferiorly. Removal of retrosplenial cortex reveals a fiber bundle connecting to the precuneal area of the superior parietal lobe medially near the midline. This tract represents contributions of the parietal aspect of the DMN. Notably, fiber tracts coming off the cingulum in this region were deeper and not as readily identifiable. We found that cortical anatomy in this area was particularly helpful in identifying these bundles, as gyral shape and, particularly, sulci course often informed the anatomy beneath. These bundles were clearly represented in tractography.
FIG. 8.
Anatomy of the cingulum. A: Medial view of an intact right hemisphere. Note the relationship of the cingulate gyrus to the corpus callosum inferiorly, and the medial superior frontal gyrus superiorly. B: Partial removal of medial cortical structures reveals the white matter tracts beneath. Fibers of the cingulum connect into the cingulate gyrus above the body of the corpus callosum. C: Further removal of cortical structures allows for visualization of projections of the cingulum into the superior frontal gyrus, precentral gyrus, paracentral lobule, and precuneus. Red overlays indicate cortical areas of the DMN. D: Complete removal of the cingulum reveals the anatomy of the corpus callosum, with its connections into the superior frontal gyrus, pre- and postcentral gyrii, and precuneus, lateral to the projections of the cingulum. E: Diffusion imaging with surface reconstruction and tractography re-demonstrates the anatomy and projections of the cingulum (light green). F: Tractography showing fibers of the corpus callosum passing beneath and lateral to the cingulum. 1 = genu of the corpus callosum; 2 = splenium of the corpus callosum; 3 = cingulate gyrus; 4 = medial superior frontal gyrus; 5 = precentral gyrus; 6 = paracentral lobule; 7 = precuneus; 8 = isthmus of the cingulate gyrus.
FIG. 9.
Posterior connections of the cingulum. Left: Medial view of left hemisphere fibers of the cingulum coursing posteriorly around the genu of the corpus callosum, giving off projections into the precuneus (PC). Black arrows represent the directionality of the fiber bundles of the cingulum. Right: Diffusion tractography shows fibers projecting from the posterior cingulum to terminate in the PC. C = cuneus; POS = parietooccipital sulcus (pink).
Fibers of the Cingulum to the Medial Temporal Lobe
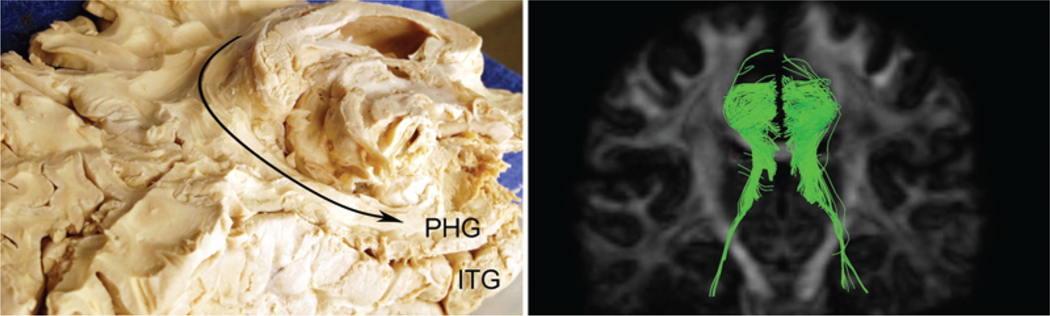
Again from the medial aspect, the isthmus of the cingulate gyrus wraps around just below the most inferior part of the splenium. Posterior to the pineal gland, it bulges slightly medially giving the inferior aspect the shape of a conch shell. It continues anteriorly below the splenium to connect with the parahippocampal gyrus. The isthmus is formed inferiorly by the parietooccipital sulcus and superiorly by the splenium. In some specimens, the lingual gyrus continues anteriorly to cover the connection of the isthmus to the parahippocampal gyrus. Additionally, in our specimens, this most inferior portion of the cingulate gyrus was particularly friable, and required finesse to avoid severing the tracts below. This area is best approached inferomedially from the parietooccipital sulcus. Careful removal of the isthmus demonstrated a continuation of the tracts that wrap around the splenium above. At this point, we removed the brainstem at the level of the pons to allow for better visualization of the medial temporal lobe. Dissecting along the direction of the tracts, they appeared to terminate in the parahippocampal gyrus well before reaching the temporal pole (Fig. 10). These tracts were apparent on diffusion tractography, although their appearance was more variable.
FIG. 10.
Temporal connections of the cingulum. Left: Posteromedial view of right hemisphere fiber tracts continuing around the splenium of the corpus callosum to terminate in the parahippocampal gyrus (PHG). Right: Posterior view demonstrated with diffusion tractography. ITG = inferior temporal gyrus.
Discussion
In this study we provide what is, to our knowledge, the first technical report providing evidence that infiltrating gliomas crossing the anterior corpus callosum can be resected with good results, implying that these tumors are not actually “inoperable.” We emphasize that this is possible with a novel approach involving attention monitoring, preservation of the anterior cingulate gyrus and its connections, and caution near the caudate and septal nuclei. All of these structures are at risk when the anterior corpus callosum is removed. We propose that the anterior corpus callosum is not “sacred” tissue in and of itself, as decades of epilepsy literature support sectioning it. Similar to the insula,48 this structure can be removed if careful attention is given to preserving critical surrounding structures by use of microsurgical technique, understanding anatomical relationships, and defining resection limits with functional brain mapping.
We do not know for certain if removing butterfly gliomas in fact leads to meaningful long-term survival compared with avoiding removal of involved corpus callosum. Certainly, a study of mixed-grade tumors will not satisfy questions about survival; those will require numerous, more focused inquiries from different groups to demonstrate that CST is not only plausible, but also beneficial. In this context, we offer survival curves to be cautiously interpreted. These tentative results suggest that patients may do as well following resection of butterfly gliomas as following resection of gliomas in other locations, but again we note that we do not have enough data to make a definitive conclusion. And while some of the existing literature fails to show that surgery can help these patients in any meaningful way,18 we would note numerous studies that show a stepwise improvement in survival with increased EOR of both HGGs and LGGs1,7,49,62 to hypothesize that resection of these tumors will ultimately be helpful. As with any new surgical technique, however, a review of ongoing results will be essential in determining if resection by this operative approach should be pursued further.
Development of the CST and the Ethics of Operating on “Inoperable” Brain Tumors
Although we are not the first surgeons to remove gliomas from the corpus callosum,40 the tumor neurosurgical community has discouraged the practice, given realistic fears of devastating neurological consequences to the patient. Causing profound abulia and akinetic mutism would make any additional treatment pointless, and rob the patient of any meaningful time he or she has left. Many of our patents were not offered surgery at other credible institutions for these reasons. Interestingly, these patients show a general willingness to undergo surgery, even after being informed that the risks are high, the benefit uncertain, and the treatment unproven. We suspect that a primary motivation of patients facing dismal prognoses such as butterfly gliomas is to try to live, yet we as physicians often misjudge our patients’ willingness to assume risk in these situations.
A reluctance to resect tumors involving the corpus callosum may be misplaced, as noted by previous authors.17 The corpus callosum is a white matter tract that has been sectioned for decades without leaving patients neurologically devastated.36 Thus, the idea that removing it somehow carries greater risk than sectioning it appears inconsistent with its anatomical organization as a collection of axons without internal cell bodies. In other words, cutting the tract should be anatomically equivalent to removing it. Consequently, we believed that poor outcomes following removal of these tracts24,25,54 were a result of damage to other surrounding structures involved in attention, language production, and motivation.
Additionally, there are limited data supporting the conclusion that the process of these tumors crossing over the midline is biologically driven (i.e., that these tumors are more aggressive),11 especially when surgeons’ unwillingness to remove this part of the tumor makes the grim prognosis a self-fulfilling prophecy. An alternative theory is that these tumors happen to originate near the callosal fibers, following the neighboring white matter tracts in a fashion typical of infiltrating tumors. In other words, there is no obvious reason to view a tumor following the corpus callosum as fundamentally different from one following the optic radiations, local subcortical u-fibers, or any other white matter tract. It follows that the cytoreductive benefits of operating on a butterfly glioma are not necessarily less than for a glioma in any other brain region with a similar histopathological grade and molecular profile, assuming it can be performed with a reasonable outcome.
Many of the problems feared in removing butterfly tumors occurred in our initial experience, and at times we considered that our approach was not the answer for these patients. This was especially easy to do after a bad outcome. We were surprised, however, at how often this did not happen, which led us to believe there was probably a way to make the surgery work. Additionally, our view was that, especially in glioblastoma, the idea that these patients go home after a biopsy and chemoradiotherapy to have meaningful remaining time with a good quality of life is largely mythology. Our experience has instead been that the condition of many of these patients rapidly deteriorates to a neurological state similar to that occurring after a bad outcome in our efforts to remove the tumor. In other words, we could only improve on a grim natural history by trying. We persisted to work out our technique in its current form, inspired that even our initial efforts (i.e., the standard technique described above) focused merely on preserving the caudate head, anterior cerebral artery, and septal nuclei resulted in good outcomes in many patients. What remained was a refinement of our technique to account for variable patient anatomy.
Based on what is known about the variability in anatomical organization of other neocortical brain functions in patients with glioma (as in speech areas47), we hypothesized the presence of cortical variability due to functional reorganization occurring in these patients as well. Infiltrating gliomas can change the functional organization from the Platonic ideal noted in classic anatomical diagrams, and this is well reported in the literature.16,29,53 Shifting cortical functions result in changes to the network in these patients, allowing the patient to compensate from the loss of function in this cortex. This likely contributed to our finding that the anterior cingulate cortex was not uniformly unresectable either, as it was partially removed in patients undergoing surgery with both techniques. Most patients tolerated this removal without problems. However, we have not performed fMRI in these patients, and cannot say for certain how the DMN changes in the presence of infiltrative tumors. As many of these cases are HGGs, blood oxygen level–dependent (BOLD) signal in the area of the tumor may be misleading on fMRI, as BOLD signal has been shown to be strongly related to tumor grade.61 This is possibly due to abnormal autoregulation of blood flow around the tumor.32
Our opinion is not that we cannot resect any portion of the DMN, including the anterior cingulate gyrus, but rather that awake monitoring techniques aimed at avoiding disruption of the network improve outcomes by preventing removal of an essential part of the DMN. By keeping patients awake, we quickly realized that attention function was variably dependent on the anterior cingulate gyrus and the cingulum, with occasional contribution from parts of the neighboring superior frontal gyri. We also found that we could consistently achieve good outcomes in these patients, all while aggressively removing the involved corpus callosum.
Notably, we did not perform formal frontal lobe tests such as Stroop35 or Trails A,8 but we did conduct limited neuropsychological testing on all of our patients. To ensure we captured any perceptible problem with attention, we adopted a stringent definition of an undesirable outcome based on our experience that formal testing is of limited utility in these patients. Testing by an independent observer further supports our belief that we are not hurting these patients, as we would expect to see an inability to focus on our basic neuropsychological tests. Additionally, while most patients with butterfly tumors already have subtle cognitive changes upon presentation, we argue that in treating them we provide a chance for meaningful survival.
Attention Mapping: The Evolution of Awake Brain Mapping From Cortical Mapping to Subcortical Monitoring
The observation that electrical stimulation of motor cortex could help define the functional organization of the cerebrum has been known for more than 150 years,23 and its role in brain surgery was established by surgeons such as Penfield41 and refined for decades until the present state, where cortical mapping is the standard approach to eloquent cortex brain tumors.14 However, it is increasingly clear to us and others19,63 that the common approach of mapping the cortex and then putting the patient to sleep to remove the tumor ignores the fact that brain cortices do not work when disconnected from their targets. Ignoring the subcortical white matter can lead to destruction of functional networks after the patient has been put to sleep.19 Furthermore, not all potentially relevant brain regions are easily stimulated without removing or manipulating other brain areas first, most notably the insula and cingulate gyrus.
A view of brain mapping as a single stage performed prior to cutting the cortex is one reason we initially did not consider awake surgery in avoiding inattention syndromes with butterfly gliomas. The fact that our initial results were not worse is likely attributable to variable anatomy between patients as discussed above; that we did not disrupt the attention network in many patients was therefore attributable to reorganization. In our experience, the role of the cingulate gyrus in the attention network can be variable, and it is preservation of the network that is paramount. As such, we can be confident that we are probably not neurologically devastating a patient who is performing a complex task while we are removing their tumor.
Understanding that mapping and functional preservation should continue throughout the white matter dissection allowed us to quickly identify what we were doing wrong. It also gave us insights that have wider implications in complication avoidance in other surgeries in this area. We feel a continuous monitoring paradigm is critical to reducing errors in butterfly glioma surgery by permitting technique refinement, and improving the EOR. Awake, continuous, multimodality monitoring during the subcortical phase of tumor removal should form the foundation for increasing the number of patients who can safely undergo aggressive glioma resections in areas previously believed to be inoperable.
Is the Disruption of the DMN the Cause of Abulia in Medial Frontal Lobe Surgery?
Obviously, a single study of comparative clinical outcomes based on a change in technique cannot definitively answer this question beyond all doubt. At least 3 networks have been linked to the transition from inattention to focused thought based on functional connectivity analysis: the DMN, the Salience Network,27 and the Central Executive Network.9,22 The Salience Network is located in the frontoinsular cortex and dorsal anterior cingulate cortex, and the Central Executive Network is largely located in the dorsolateral prefrontal cortex.37 Consequently, studying which of these networks is damaged in medial frontal lobe surgery, such as resecting an anterior butterfly glioma, presents a challenge. There is also a possibility that other networks exist that have yet to be identified. Additionally, studying detailed connections of these networks in patients with glioma has not yet been possible given the known difficulties with performing DTI tractography in edematous brain tissue.68
We first considered the role of the DMN in clinical outcomes based on our experience operating on splenium butterfly gliomas. Following resection of a tumor crossing in the splenium of the corpus callosum we unexpectedly observed a clinical picture oddly consistent with frontal lobe–type inattention. We subsequently altered our resection through the splenium to leave bundles of the cingulum intact, resulting in consistently favorable outcomes. We chose not to include those data in this study as these cases are less common, and are a separate problem. We subsequently employed this technique in resecting bifrontal tumors crossing in the genu of the corpus callosum, and noticed a significantly reduced incidence of abulia/akinetic mutism between the two surgical groups. Some time after observing these differences in clinical outcomes, we recognized the correlation to the anatomy of the DMN, and noted that they resulted when key connections of the DMN were left intact. We believe the narrative that drove our hypothesis adds to its strength.
We also believe this hypothesis best explains our observations because the primary difference between the two techniques described in this paper is the identification and sparing of portions of the cingulate gyrus involved in attention, and its connections with the rest of the DMN via the cingulum. Thus, while other connections necessary for attention, motivation, and additional medial frontal functions may play a role in our findings, we suggest that because the DMN areas connect directly through an anterior-to-posterior running tract within the cingulate gyrus—and as we are essentially removing all white matter, inferior, superior, and lateral to the DMN—that this is the best explanation based on our present understanding. Therefore, we based our explanation of our findings in a detailed study of the anatomy of the DMN and its connections in the following sections.
Nature of Our Analysis of the DMN
Consistent with earlier reports,10,13,59 we found good concordance between the two methods implemented for fiber-tract dissection and provide illustrations of the connections. Tractography was especially useful in our quantitative analysis of tract volume. Furthermore, GQI allowed us to perform tractography in voxels that contained crossing fibers,66 which was particularly useful in visualizing tracts to the temporal lobe. Other connections of the cingulate gyrus not linked to the DMN, such as the thalamus,30 were not included in our analysis as this was beyond our scope. Thus, it is possible that this network is more complex than we are depicting in this study.
Past studies have mapped the DMN using resting-state functional connectivity MRI.4,22,26,67 The DMN has accordingly been defined as regions of neurons that fire synchronously during wakeful rest. Past anatomical studies of the cingulum65 have used derivations of the technique originally described by Klingler.31 However, comprehensive studies of cerebral white matter tracts now use diffusion MRI tractography in combination with the technique attributed to Klingler.10,13,38,59,60 Advanced imaging techniques that make use of the diffusion of water along white matter tracts makes visualization of these tracts possible, and these findings can then be verified with careful, gross anatomical dissection. This method of studying white matter tracts has been used to characterize a number of important fiber bundles,10,13,59 and can be integrated with previous studies to show connections between regions identified on fMRI. An overview of the anatomy of the cingulum with respect to the DMN is given in Fig. 8.
Anterior Connections of the Cingulum
Anterior connections of the cingulate gyrus are important to decision making that takes place during the engagement of the DMN. This region, including the inferior superior frontal gyrus, has been implicated in retrieval of personal knowledge, considering future goals, and simulating future personal interactions in fMRI studies.2,39,55 Our study shows fibers from the superior frontal gyrus joining the cingulum, which connects with the cingulate gyrus, precuneus, and medial temporal lobe. Anterior connections are shown in Fig. 7, and shown from the surgical aspect in Fig. 11.
FIG. 11.
Dissection of fiber tracts of the left hemisphere shown from the surgical angle. The cingulum bundle can be observed coursing around the genu of the corpus callosum anteriorly, with radiations to the superior frontal gyrus (A). Removal of the cingulum bundle (B) reveals the fibers of the corpus callosum, which connects cortices of both hemispheres. Tractography with GQI demonstrates the cingulum and its radiations (C).
The medial temporal lobe is believed to play a role in planning by contributing to episodic retrieval for imagining the future.4 This is evidenced by studies that have shown that damage to the hippocampus leads to deficits in imagining,46 and damage to the parahippocampus leads to deficits in scene and spatial recognition.42 Additionally, the medial temporal region has been reliably activated in subjects engaging in autobiographical thought and in those imagining the future.51 Episodic recall and future planning in the medial temporal lobe are important components of the DMN,43 and we have shown its anatomical connections to the cingulate gyrus and superior frontal gyrus.
Posterior Connections of the Cingulum
The posterior cingulate gyrus and precuneus are involved in responses to external stimuli.45 This region is likely responsible for gathering information from the outside world while the DMN is active. One author has suggested this is an evolutionary mechanism, so that one automatically focuses his or her attention in the presence of danger.45 Notably, visual information travels through this region en route to visual cortex.5 The parietal lobe is particularly affected in Alzheimer’s dementia, and may help explain impaired awareness in these patients.12,53 Our anatomical findings confirm connections of the precuneus to other parts of the DMN, and demonstrate tracts connecting the precuneus to both the inferior superior frontal gyrus and the hippocampus by way of the cingulum (Figs. 8 and 9).
Connectivity of the DMN
Global connectivity of the DMN can be altered by intracranial pathology. Two studies using fMRI have demonstrated decreased connectivity of the DMN in patients with diffuse gliomas.20,28 Both found that higher WHO grade corresponded to decreased connectivity. One group additionally found that DMN connectivity was more affected by parietal tumors than frontal tumors,28 which could be explained by the extensive connections in this region noted in our dissections. Accordingly, these studies concluded that cognitive changes in these patients are most likely due to disruptions of the DMN.20,28
What Cannot Be Concluded From This Work
We note again that this study aims to report the plausibility of removing anterior butterfly gliomas with good results, and does not provide evidence that this is the correct treatment for these patients or that this treatment improves meaningful or overall survival; a definitive study would entail a limited spectrum of glioma grade and histopathology, and a longer range of follow-up in a larger cohort.
Additionally, our work does not claim that abulia can be universally avoided in all patients by monitoring them with an attention task that aims to preserve the DMN and cingulum. So far this strategy appears to be the most significant improvement in technique, suggesting that this approach represents a major step forward. However, we acknowledge that preservation of other frontal networks and other connections may be needed to continue to improve the consistency of our outcomes. Additional studies that implement fMRI in patients with tumors may also be helpful in understanding DMN reorganization.
Finally, we are not claiming that all these patients are without any neurological problems or that the anterior corpus callosum and frontal lobes are unessential and can be expended without consequence. These patients often present with significant frontal lobe–based cognitive problems, and although many of the patients in this series live a normal life—with some even returning to high-functioning employment—sophisticated neuropsychological assessment would likely reveal mild-to-moderate cognitive dysfunction referable to some aspect of frontal lobe function in a significant portion of this population. However, in most cases, leaving a butterfly tumor growing on both sides of the brain into both frontal lobes is not a realistic strategy for avoiding this problem. In many cases further damage can be prevented, or at least delayed, by preserving critical functional networks while treating the patient with a strategy better able to control the tumor. Thus, we believe our approach is a promising avenue toward improving upon the dour natural history of anterior butterfly gliomas.
Conclusions
In this study we present evidence that anterior butterfly gliomas can be safely removed using a novel attention-task-based awake brain surgery technique that focuses on preserving the anatomical connectivity of the cingulum and relevant aspects of the cingulate gyrus. We believe that the findings may be helpful for a broad range of brain surgeries involving the anteromedial frontal lobe.
ABBREVIATIONS
- ACA
anterior cerebral artery
- BOLD
blood oxygen level–dependent
- CST
cingulum-sparing technique
- DMN
default mode network
- DTI
diffusion tensor imaging
- EOR
extent of resection
- fMRI
functional MRI
- GQI
generalized q-sampling imaging
- GTR
gross-total resection
- HGG
high-grade glioma
- LGG
low-grade glioma
- NTR
near-total resection
- ROI
region of interest
- SMA
supplementary motor area
- STR
subtotal resection
Footnotes
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Aghi MK, Nahed BV, Sloan AE, Ryken TC, Kalkanis SN, Olson JJ: The role of surgery in the management of patients with diffuse low grade glioma: A systematic review and evidence-based clinical practice guideline. J Neurooncol 125:503–530, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Andrews-Hanna JR: The brain’s default network and its adaptive role in internal mentation. Neuroscientist 18:251–270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL: Evidence for the default network’s role in spontaneous cognition. J Neurophysiol 104:322–335, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews-Hanna JR, Smallwood J, Spreng RN: The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci 1316:29–52, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker JF, Petersen SE, Newsome WT, Allman JM: Visual response properties of neurons in four extrastriate visual areas of the owl monkey (Aotus trivirgatus): a quantitative comparison of medial, dorsomedial, dorsolateral, and middle temporal areas. J Neurophysiol 45:397–416, 1981 [DOI] [PubMed] [Google Scholar]
- 6.Bannur U, Rajshekhar V: Post operative supplementary motor area syndrome: clinical features and outcome. Br J Neurosurg 14:204–210, 2000 [DOI] [PubMed] [Google Scholar]
- 7.Berger MS, Deliganis AV, Dobbins J, Keles GE: The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer 74:1784–1791, 1994 [DOI] [PubMed] [Google Scholar]
- 8.Bowie CR, Harvey PD: Administration and interpretation of the Trail Making Test. Nat Protoc 1:2277–2281, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Bressler SL, Menon V: Large-scale brain networks in cognition: emerging methods and principles. Trends Cogn Sci 14:277–290, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Catani M, Dell’acqua F, Vergani F, Malik F, Hodge H, Roy P, et al. : Short frontal lobe connections of the human brain. Cortex 48:273–291, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Chaichana KL, Jusue-Torres I, Lemos AM, Gokaslan A, Cabrera-Aldana EE, Ashary A, et al. : The butterfly effect on glioblastoma: is volumetric extent of resection more effective than biopsy for these tumors? J Neurooncol 120:625–634, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chhatwal JP, Schultz AP, Johnson K, Benzinger TL, Jack C Jr, Ances BM, et al. : Impaired default network functional connectivity in autosomal dominant Alzheimer disease. Neurology 81:736–744, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Benedictis A, Duffau H, Paradiso B, Grandi E, Balbi S, Granieri E, et al. : Anatomo-functional study of the temporoparieto-occipital region: dissection, tractographic and brain mapping evidence from a neurosurgical perspective. J Anat 225:132–151, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS: Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol 30:2559–2565, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Di X, Biswal BB: Identifying the default mode network structure using dynamic causal modeling on resting-state functional magnetic resonance imaging. Neuroimage 86:53–59, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duffau H: The huge plastic potential of adult brain and the role of connectomics: new insights provided by serial mappings in glioma surgery. Cortex 58:325–337, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Duffau H, Khalil I, Gatignol P, Denvil D, Capelle L: Surgical removal of corpus callosum infiltrated by low-grade glioma: functional outcome and oncological considerations. J Neurosurg 100:431–437, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Dziurzynski K, Blas-Boria D, Suki D, Cahill DP, Prabhu SS, Puduvalli V, et al. : Butterfly glioblastomas: a retrospective review and qualitative assessment of outcomes. J Neurooncol 109:555–563, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enatsu R, Kanno A, Ohtaki S, Akiyama Y, Ochi S, Mikuni N: Intraoperative subcortical fiber mapping with subcorticocortical evoked potentials. World Neurosurg 86:478–483, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Esposito R, Mattei PA, Briganti C, Romani GL, Tartaro A, Caulo M: Modifications of default-mode network connectivity in patients with cerebral glioma. PLoS One 7:e40231, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernández Coello A, Moritz-Gasser S, Martino J, Martinoni M, Matsuda R, Duffau H: Selection of intraoperative tasks for awake mapping based on relationships between tumor location and functional networks. J Neurosurg 119:1380–1394, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME: The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102:9673–9678, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fritsch G, Hitzig E: Ueber die elektrische Erregbarkeit des Grossbirns. Arch Anat Physiol Wissen 37:300–332, 1870 [Google Scholar]
- 24.Geschwind N: Disconnexion syndromes in animals and man. I. Brain 88:237–294, 1965 [DOI] [PubMed] [Google Scholar]
- 25.Geschwind N: Disconnexion syndromes in animals and man. II. Brain 88:585–644, 1965 [DOI] [PubMed] [Google Scholar]
- 26.Greicius MD, Krasnow B, Reiss AL, Menon V: Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A 100:253–258, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ham T, Leff A, de Boissezon X, Joffe A, Sharp DJ: Cognitive control and the salience network: an investigation of error processing and effective connectivity. J Neurosci 33:7091–7098, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris RJ, Bookheimer SY, Cloughesy TF, Kim HJ, Pope WB, Lai A, et al. : Altered functional connectivity of the default mode network in diffuse gliomas measured with pseudo-resting state fMRI. J Neurooncol 116:373–379, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ius T, Angelini E, Thiebaut de Schotten M, Mandonnet E, Duffau H: Evidence for potentials and limitations of brain plasticity using an atlas of functional resectability of WHO grade II gliomas: towards a “minimal common brain”. Neuroimage 56:992–1000, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Jang SH, Yeo SS: Thalamocortical tract between anterior thalamic nuclei and cingulate gyrus in the human brain: diffusion tensor tractography study. Brain Imaging Behav 7:236–241, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Klingler J: Erleichterung der makroskopischen Präparation des Gehirns durch den Gefrierprozess. Schweiz Arch Neurol Psychiatr 36:247–256, 1935 [Google Scholar]
- 32.Korvenoja A, Kirveskari E, Aronen HJ, Avikainen S, Brander A, Huttunen J, et al. : Sensorimotor cortex localization: comparison of magnetoencephalography, functional MR imaging, and intraoperative cortical mapping. Radiology 241:213–222, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Koutsarnakis C, Liakos F, Kalyvas AV, Sakas DE, Stranjalis G: A laboratory manual for stepwise cerebral white matter fiber dissection. World Neurosurg 84:483–493, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Lee RF: Emergence of the default-mode network from resting-state to activation-state in reciprocal social interaction via eye contact. Conf Proc IEEE Eng Med Biol Soc 2015:1821–1824, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Lezak MD, Howieson DB, Bigler ED, Tranel D: Neuropsychological Assessment, ed 5. New York: Oxford University Press, 2012 [Google Scholar]
- 36.Malmgren K, Rydenhag B, Hallböök T: Reappraisal of corpus callosotomy. Curr Opin Neurol 28:175–181, 2015 [DOI] [PubMed] [Google Scholar]
- 37.Menon V: Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 15:483–506, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Middlebrooks EH, Yagmurlu K, Bennett JA, Bidari S: Normal relationship of the cervicomedullary junction with the obex and olivary bodies: a comparison of cadaveric dissection and in vivo diffusion tensor imaging. Surg Radiol Anat 37:493–497, 2015 [DOI] [PubMed] [Google Scholar]
- 39.Moran JM, Kelley WM, Heatherton TF: What can the organization of the brain’s default mode network tell us about self-knowledge? Front Hum Neurosci 7:391, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oszvald Á, Quick J, Franz K, Güresir E, Szelényi A, Vatter H, et al. : Resection of gliomas in the cingulate gyrus: functional outcome and survival. J Neurooncol 109:341–348, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Penfield W, Boldrey E: Somatic motor and sensory representations in the cerebral cortex of man as studied by electrical stimulation. Brain 60:389–443, 1937 [Google Scholar]
- 42.Ploner CJ, Gaymard BM, Rivaud-Péchoux S, Baulac M, Clémenceau S, Samson S, et al. : Lesions affecting the parahippocampal cortex yield spatial memory deficits in humans. Cereb Cortex 10:1211–1216, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Race E, Keane MM, Verfaellie M: Medial temporal lobe damage causes deficits in episodic memory and episodic future thinking not attributable to deficits in narrative construction. J Neurosci 31:10262–10269, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raichle ME: The brain’s default mode network. Annu Rev Neurosci 38:433–447, 2015 [DOI] [PubMed] [Google Scholar]
- 45.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL: A default mode of brain function. Proc Natl Acad Sci U S A 98:676–682, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosenbaum RS, Gilboa A, Levine B, Winocur G, Moscovitch M: Amnesia as an impairment of detail generation and binding: evidence from personal, fictional, and semantic narratives in K.C. Neuropsychologia 47:2181–2187, 2009 [DOI] [PubMed] [Google Scholar]
- 47.Sanai N, Mirzadeh Z, Berger MS: Functional outcome after language mapping for glioma resection. N Engl J Med 358:18–27, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Sanai N, Polley MY, Berger MS: Insular glioma resection: assessment of patient morbidity, survival, and tumor progression. J Neurosurg 112:1–9, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS: An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg 115:3–8, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Sandrone S, Catani M: Journal Club. Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology 81:e172–e175, 2013 [DOI] [PubMed] [Google Scholar]
- 51.Schacter DL, Addis DR, Buckner RL: Remembering the past to imagine the future: the prospective brain. Nat Rev Neurosci 8:657–661, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, et al. : Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J Cogn Neurosci 9:648–663, 1997 [DOI] [PubMed] [Google Scholar]
- 53.Southwell DG, Hervey-Jumper SL, Perry DW, Berger MS: Intraoperative mapping during repeat awake craniotomy reveals the functional plasticity of adult cortex. J Neurosurg 124:1460–1469, 2016 [DOI] [PubMed] [Google Scholar]
- 54.Sperry RW, Gazzaniga MS, Bogen JE: Interhemispheric relationships: the neocortical commissures; syndromes of hemisphere disconnection, in Vinken PJ, Bruyn GW (eds): Handbook of Clinical Neurology. Amsterdam: Elsevier, 1969, Vol 4, pp 273–290 [Google Scholar]
- 55.Spreng RN, Mar RA, Kim AS: The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J Cogn Neurosci 21:489–510, 2009 [DOI] [PubMed] [Google Scholar]
- 56.Tessitore A, Esposito F, Vitale C, Santangelo G, Amboni M, Russo A, et al. : Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology 79:2226–2232, 2012 [DOI] [PubMed] [Google Scholar]
- 57.Teves D, Videen TO, Cryer PE, Powers WJ: Activation of human medial prefrontal cortex during autonomic responses to hypoglycemia. Proc Natl Acad Sci U S A 101:6217–6221, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vatansever D, Menon DK, Manktelow AE, Sahakian BJ, Stamatakis EA: Default mode network connectivity during task execution. Neuroimage 122:96–104, 2015 [DOI] [PubMed] [Google Scholar]
- 59.Vergani F, Lacerda L, Martino J, Attems J, Morris C, Mitchell P, et al. : White matter connections of the supplementary motor area in humans. J Neurol Neurosurg Psychiatry 85:1377–1385, 2014 [DOI] [PubMed] [Google Scholar]
- 60.Wang F, Sun T, Li X, Xia H, Li Z: Microsurgical and tractographic anatomical study of insular and transsylvian transinsular approach. Neurol Sci 32:865–874, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang L, Chen D, Olson J, Ali S, Fan T, Mao H: Re-examine tumor-induced alterations in hemodynamic responses of BOLD fMRI: implications in presurgical brain mapping. Acta Radiol 53:802–811, 2012 [DOI] [PubMed] [Google Scholar]
- 62.Woernle CM, Péus D, Hofer S, Rushing EJ, Held U, Bozinov O, et al. : Efficacy of surgery and further treatment of progressive glioblastoma. World Neurosurg 84:301–307, 2015 [DOI] [PubMed] [Google Scholar]
- 63.Yamao Y, Matsumoto R, Kunieda T, Arakawa Y, Kobayashi K, Usami K, et al. : Intraoperative dorsal language network mapping by using single-pulse electrical stimulation. Hum Brain Mapp 35:4345–4361, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yaşargil GM, Krisht AF, Türe U, Al-Mefty O, Yaşargil DCH: Microsurgery of insular gliomas: Part IV: Surgical treatment and outcome. Contemp Neurosurg 24:1–8, 2002 [Google Scholar]
- 65.Yaşargil MG, Türe U, Yaşargil DC: Surgical anatomy of supratentorial midline lesions. Neurosurg Focus 18(6B):E1, 2005 [DOI] [PubMed] [Google Scholar]
- 66.Yeh FC, Wedeen VJ, Tseng WY: Generalized q-sampling imaging. IEEE Trans Med Imaging 29:1626–1635, 2010 [DOI] [PubMed] [Google Scholar]
- 67.Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. : The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol 106:1125–1165, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang H, Wang Y, Lu T, Qiu B, Tang Y, Ou S, et al. : Differences between generalized q-sampling imaging and diffusion tensor imaging in the preoperative visualization of the nerve fiber tracts within peritumoral edema in brain. Neurosurgery 73:1044–1053, 2013 [DOI] [PubMed] [Google Scholar]