Abstract
Background
Fluvoxamine is a selective serotonin reuptake inhibitor (SSRI) that has been approved for the treatment of depression, obsessive–compulsive disorder, and a variety of anxiety disorders; it is available as an oral preparation. Fluvoxamine has not been approved for the treatment of infections, but has been used in the early treatment of people with mild to moderate COVID‐19. As there are only a few effective therapies for people with COVID‐19 in the community, a thorough understanding of the current evidence regarding the efficacy and safety of fluvoxamine as an anti‐inflammatory and possible anti‐viral treatment for COVID‐19, based on randomised controlled trials (RCTs), is needed.
Objectives
To assess the efficacy and safety of fluvoxamine in addition to standard care, compared to standard care (alone or with placebo), or any other active pharmacological comparator with proven efficacy for the treatment of COVID‐19 outpatients and inpatients.
Search methods
We searched the Cochrane COVID‐19 Study Register (including Cochrane Central Register of Controlled Trials, MEDLINE, Embase, ClinicalTrials.gov, WHO ICTRP, medRxiv), Web of Science and WHO COVID‐19 Global literature on COVID‐19 to identify completed and ongoing studies up to 1 February 2022.
Selection criteria
We included RCTs that compared fluvoxamine in addition to standard care (also including no intervention), with standard care (alone or with placebo), or any other active pharmacological comparator with proven efficacy in clinical trials for the treatment of people with confirmed COVID‐19, irrespective of disease severity, in both inpatients and outpatients. Co‐interventions needed to be the same in both study arms. We excluded studies comparing fluvoxamine to other pharmacological interventions with unproven efficacy.
Data collection and analysis
We assessed risk of bias of primary outcomes using the Cochrane Risk of Bias 2 tool for RCTs. We used GRADE to rate the certainty of evidence to treat people with asymptomatic to severe COVID‐19 for the primary outcomes including mortality, clinical deterioration, clinical improvement, quality of life, serious adverse events, adverse events of any grade, and suicide or suicide attempt.
Main results
We identified two completed studies with a total of 1649 symptomatic participants. One study was conducted in the USA (study with 152 participants, 80 and 72 participants per study arm) and the other study in Brazil (study with 1497 high‐risk participants for progression to severe disease, 741 and 756 participants per study arm) among outpatients with mild COVID‐19. Both studies were double‐blind, placebo‐controlled trials in which participants were prescribed 100 mg fluvoxamine two or three times daily for a maximum of 15 days.
We identified five ongoing studies and two studies awaiting classification (due to translation issues, and due to missing published data). We found no published studies comparing fluvoxamine to other pharmacological interventions of proven efficacy.
We assessed both included studies to have an overall high risk of bias.
Fluvoxamine for the treatment of COVID‐19 in inpatients
We did not identify any completed studies of inpatients.
Fluvoxamine for the treatment of COVID‐19 in outpatients
Fluvoxamine in addition to standard care may slightly reduce all‐cause mortality at day 28 (RR 0.69, 95% CI 0.38 to 1.27; risk difference (RD) 9 per 1000; 2 studies, 1649 participants; low‐certainty evidence), and may reduce clinical deterioration defined as all‐cause hospital admission or death before hospital admission (RR 0.55, 95% CI 0.16 to 1.89; RD 57 per 1000; 2 studies, 1649 participants; low‐certainty evidence). We are very uncertain regarding the effect of fluvoxamine on serious adverse events (RR 0.56, 95% CI 0.15 to 2.03; RD 54 per 1000; 2 studies, 1649 participants; very low‐certainty evidence) or adverse events of any grade (RR 1.06, 95% CI 0.82 to 1.37; RD 7 per 1000; 2 studies, 1649 participants; very low‐certainty evidence).
Neither of the studies reported on symptom resolution (clinical improvement), quality of life or suicide/suicide attempt.
Authors' conclusions
Based on a low‐certainty evidence, fluvoxamine may slightly reduce all‐cause mortality at day 28, and may reduce the risk of admission to hospital or death in outpatients with mild COVID‐19. However, we are very uncertain regarding the effect of fluvoxamine on serious adverse events, or any adverse events.
In accordance with the living approach of this review, we will continually update our search and include eligible trials as they arise, to complete any gaps in the evidence.
Keywords: Humans, Clinical Deterioration, COVID-19 Drug Treatment, Fluvoxamine, Fluvoxamine/pharmacology, Fluvoxamine/therapeutic use, Randomized Controlled Trials as Topic, Selective Serotonin Reuptake Inhibitors, Selective Serotonin Reuptake Inhibitors/therapeutic use
Plain language summary
Fluvoxamine for treating COVID‐19
Review question
Is fluvoxamine an effective treatment for people with COVID‐19 and does it cause any unwanted effects?
Key messages
It is unclear whether fluvoxamine is an effective treatment for COVID‐19 in people with mild to moderate COVID‐19. This is because there is currently not enough research available to make a definite decision. We found five ongoing studies that are currently investigating fluvoxamine as a possible treatment for COVID‐19, and two studies for which we need more information. We will update this review if their results change our conclusions.
What is fluvoxamine?
Fluvoxamine is a type of medication known as a selective serotonin reuptake inhibitor (SSRI), available in tablet form. Recent research has found that fluvoxamine may have an effect on COVID‐19. When the immune system fights the virus, the lungs and airways can become inflamed, causing breathing difficulties. Fluvoxamine could help reduce this inflammation, potentially reducing the risk of developing severe COVID‐19 and its associated lung symptoms through its possible anti‐inflammatory and anti‐viral effects. We know that most people do not experience any serious side effects with fluvoxamine when it is taken as an antidepressant. Some people can, however, experience the following common side effects, especially when starting the medication: nausea, anxiety or restlessness, insomnia, or diarrhoea, and in rare cases, suicidal ideation.
What did we want to find out?
We wanted to know if fluvoxamine reduces death, severity of disease, and length of infection in people with COVID‐19, if it has an effect on quality of life, or causes any unwanted effects. We included studies that compared fluvoxamine to placebo (dummy treatment), no treatment, usual care, or any other treatment for COVID‐19 that is known to work to some extent, such as remdesivir or dexamethasone. We excluded treatments that we know do not work for COVID‐19, such as hydroxychloroquine, or have an unknown effect on the disease.
We evaluated the effects of fluvoxamine in adults with COVID‐19 on:
• people dying;
• whether people needed to be treated in a hospital;
• whether people's COVID‐19 symptoms got better or worse;
• unwanted effects;
• quality of life;
• and whether there is a risk of suicide or suicide attempt when taking this medication.
What did we do?
We searched for studies that investigated fluvoxamine as a treatment for adults with COVID‐19 in hospital or as outpatients. We compared and summarised the results of the studies and rated our confidence in the evidence, based on common criteria such as study methods and study sizes.
What did we find?
We found two studies that investigated fluvoxamine as an early treatment for mild COVID‐19 in 1649 self‐isolated people at home (outpatients). All studies compared fluvoxamine to placebo together with standard care. The studies used different durations of treatment (10 or 15 days).
We found five ongoing studies and two studies that are awaiting classification. We did not find any studies that investigated the effect of fluvoxamine on people in hospital with COVID‐19.
Main results
• Compared to placebo, fluvoxamine may slightly reduce the number of people who die in the 28 days after starting treatment (2 studies, 1649 people).
• Compared to placebo, fluvoxamine may reduce number of people who are admitted to a hospital or who die before hospital admission (2 studies, 1649 people).
•The number of unwanted (serious) events did not clearly differ between fluvoxamine and placebo treatment (2 studies, 1649 people).
•Neither of the studies reported on quality of life, the time needed until all initial symptoms resolved, or suicide attempts.
What are the limitations of the evidence?
We cannot be confident in the current evidence for fluvoxamine in treating people with COVID‐19, mainly due to the lack of studies that are currently available and some flaws in study design. We will continue to search for new studies to complete the current evidence gap.
It would also be important to find out the effects of a medication such as fluvoxamine on long‐Covid. We are currently waiting for research on this to become available in the near future.
Unfortunately, the studies available did not focus on children and young adults, women who are planning or trying to conceive, women who are pregnant or breastfeeding, older adults, or those people who have a weakened immune system (immunocompromised people). Likewise, no information was available on whether women or men were more likely to benefit from fluvoxamine.
Search date
The evidence is current to February 2022.
Summary of findings
Summary of findings 1. Fluvoxamine plus standard care compared to placebo plus standard care for outpatients with mild COVID‐19.
|
Patient or population: symptomatic people with COVID‐19 Setting: outpatients Intervention: fluvoxamine plus standard care Comparison: placebo plus standard care | |||||||
| Outcomes | Anticipated absolute effects (95% CI)* |
Relative effect (95% CI) |
N of participants (studies) | Certainty in the evidence (GRADE) | Comment | ||
| Risk with placebo plus standard care | Risk with fluvoxamine plus standard care | ||||||
|
All‐cause mortality (at day 28) |
30 per 1000 (95% CI 11 to 38) |
21 per 1000 (95% CI 2 to 29) |
RR 0.69 (0.38 to 1.27) |
1649 (2 RCTs) |
Lowa | Fluvoxamine may slightly reduce all‐cause mortality at day 28. | |
| All‐cause admission to hospital or death (before hospital admission) | 126 per 1000 (95% CI 105 to 150) |
94 per 1000 (95% CI 76 to 116) |
RR 0.55 (0.16 to 1.89) |
1649 (2 RCT) |
Lowb | Fluvoxamine may reduce admission to hospital or death (before hospital admission). | |
| Symptom resolution | All initial symptoms resolved | Not reported | |||||
| Time to symptom resolution | Numerical data not derivable, outcome was illustrated in a figure (graph) indicating an overlap of confidence intervals (TOGETHER 2021). | ||||||
|
Quality of life (at longest follow‐up) |
Not reported | ||||||
|
Serious adverse events (during study period) |
122 per 1000 (95% CI 18 to 248) | 95 per 1000 (95% CI 41 to 221) |
RR 0.56 (0.15 to 2.03) |
1649 (2 RCTs) |
Very lowc | The evidence is very uncertain about the effects of fluvoxamine on serious adverse events. | |
|
Any adverse events (during study period) |
118 per 1000 (95% CI 97 to 162) |
125 per 1000 (95% CI 104 to 169) |
RR 1.06 (0.82 to 1.37) |
1649 (2 RCTs) |
Very lowd | The evidence is very uncertain about the effects of fluvoxamine on any adverse events. | |
|
Suicide or suicide attempt (at longest follow‐up) |
Not reported | ||||||
| *The risk in the intervention group and its 95% CI is based on the assumed risk in the comparison group and the relative effect of the intervention and its 95% CI. CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | |||||||
aRisk difference 9 per 1000 (19 fewer to 8 more), small important effect (since mortality is the most critical outcomes for patients and clinicians). Downgraded by 2 levels for very serious imprecision since CI suggests both potential benefit and no effect/potential harm, and number of events is small. bRisk difference 57 per 1000 (106 fewer to 112 more), moderate effect. Downgraded by 2 levels for very serious imprecision since CI suggests both potential benefit and no effect/potential harm, and number of events is small. cRisk difference 54 per 1000 (50 fewer to 4 more), moderate effect. Downgraded by 1 level for serious risk of bias and by 2 levels for very serious imprecision since CI suggests both potential benefit and potential harm. dRisk difference 7 per 1000 (21 fewer to 44 more), small unimportant effect. Downgraded by 1 level for serious risk of bias and by 2 levels for very serious imprecision since CI suggests both potential benefit and potential harm.
Background
This work constitutes part of a series of Cochrane Reviews investigating the use of potential pharmacotherapies for coronavirus disease 2019 (COVID‐19) in both inpatients and outpatients. This particular review evaluates the efficacy and safety of fluvoxamine, specifically evaluating its possible use in ambulatory‐managed patients, from here on referred to as outpatients, but also considers inpatients. Reviews in this series carry a degree of overlap with the background and methodology sections of other published reviews from the German research project 'CEOsys' (COVID‐19 Evidence Ecosystem) on antibiotics (Popp 2021a), monoclonal antibodies (Kreuzberger 2021), convalescent plasma (Piechotta 2022), ivermectin (Popp 2021a), vitamin D supplementation (Stroehlein 2021), systemic corticosteroids (Wagner 2021), colchicine (Mikolajewska 2021) and remdesivir (Ansems 2021).
Description of the condition
COVID‐19 is a rapidly spreading infectious disease caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) (WHO 2020a). COVID‐19 is unprecedented in comparison to previous coronavirus outbreaks such as SARS and Middle East Respiratory Syndrome (MERS), which caused 813 and 858 deaths, respectively (WHO 2003; WHO 2019a). Despite international efforts to contain its spread, as of February 2022, COVID‐19 had resulted in more than 420 million confirmed cases and over 5.9 million deaths worldwide (WHO2022a). The emergence of novel SARS‐CoV‐2 variants is also of great concern, with the potential for augmented transmission of the disease, a shortened incubation period and a negative impact on established and proven disease control methods (Grubaugh 2020; WHO 2021a).
Vaccination has been shown to be highly effective in reducing severe illness and death from COVID‐19. More than 10.4 billion doses of vaccines had been administered globally as of February 2022, with additional vaccines in continuous development (WHO2022a). The majority of vaccines have been administered in high‐income countries, leaving populations including health care workers and older people in other countries vulnerable.
The mean incubation period is estimated at five to six days, with 97.5% of cases developing symptoms within 11.5 days of exposure (Lauer 2020). Sore throat, cough, fever, headache, fatigue, myalgia (muscle pain) and arthralgia (joint pain) are the most commonly reported symptoms (Struyf 2020). Other symptoms may include dyspnoea, rigors, nausea and vomiting, diarrhoea, and nasal congestion (WHO 2020a). The majority of infected individuals develop mild symptoms not requiring hospitalisation, or remain completely asymptomatic (80% to 90%) depending on the timing of the investigation, the cohort investigated, and the virus variant (Chen 2020; Danza 2022; Funk 2021; Pan 2020; Wu 2020). The reported frequency of asymptomatic cases also varies greatly and ranges from 6% to 96% (Buitrago‐Garcia 2020; Funk 2021; Oran 2020).
In the first two years of the pandemic (2020 to 2021), there were estimates that approximately 11% to 20% of infected individuals went on to develop severe disease, with 1% to 5% developing critical illness with respiratory failure, septic shock or multi‐organ dysfunction syndrome requiring intensive care unit (ICU) treatment (Funk 2021; Huang 2020; Wu 2020). Furthermore, COVID‐19 case fatality rates varied widely between countries and reporting periods, from 0% to over 25% (Johns Hopkins University of Medicine 2022; Williamson 2020). These numbers may have been misleading, as they depended on testing frequency, delays in reporting dates and incomplete capture of case data at the time (Johns Hopkins University of Medicine 2022; Williamson 2020). Case definitions have been adjusted and modified during the course of the pandemic (WHO 2020b), whilst contemporary considerations such as the levels of immunity in the population and type of viral strains present at the time of data collection should also be taken into account (WHO 2022).
Description of the intervention
Fluvoxamine is a selective serotonin reuptake inhibitor (SSRI) and a σ‐1 receptor (S1R) agonist that has been approved for the treatment of depression, obsessive–compulsive disorder, and a variety of anxiety disorders. Available as an oral preparation, it is widely prescribed in the primary care setting worldwide for major depressive and obsessive‐compulsive disorders; in treating anxiety disorders such as panic disorder, social anxiety disorder, and post‐traumatic stress (Figgit 2000); as well as for menopausal symptoms and functional gut disorders (off‐label use). When fluvoxamine is used to treat psychiatric conditions, the most common adverse effect is nausea (particularly at the beginning of the treatment), but adverse effects can include other gastrointestinal effects (e.g. diarrhoea, indigestion), neurological effects (e.g. asthaenia, insomnia, somnolence, anxiety, headache), and suicidal ideation. There has been much discussion over the years about increased suicide rates in people taking serotonin reuptake inhibitors. Increased suicide rates are particularly evident in younger people (Friedman 2014).
Fluvoxamine can enhance the serotonergic effects of other SSRIs or monoamine oxidase inhibitors (MAOIs), resulting in serotonin syndrome; therefore, it should not be used within two weeks of administration of other SSRIs or MAOIs. Fluvoxamine may enhance the effects of antiplatelets and anticoagulants. Hence, people receiving these drugs should be closely monitored (Kam 1997).
How the intervention might work
There are many important mechanisms of action of fluvoxamine and other SSRIs that could play a role in COVID‐19 treatment. These effects include: reduction in platelet aggregation, decreased mast cell degranulation, interference with endolysosomal viral trafficking, regulation of inositol‐requiring enzyme 1α‐driven inflammation and increased melatonin levels, collectively having a direct antiviral effect, as well as regulating coagulopathy or mitigating the cytokine storm, which are known hallmarks of severe COVID‐19 (Sukhatme 2021).
Anti‐inflammatory effects of certain pharmacological interventions are thought to be effective during phases of COVID‐19 with high inflammatory activity. S1R is an endoplasmic reticulum (ER) chaperone membrane protein involved in many cellular functions, including regulation of ER stress response–unfolded protein response and regulation of cytokine production in response to inflammatory triggers. In a preclinical model of septic shock, fluvoxamine was found to bind to S1R on immune cells, resulting in a reduced inflammatory response with inhibited cytokine production (Rosen 2019). In the presence of fluvoxamine, S1R might prevent the ER stress sensor inositol‐requiring enzyme 1α from splicing and activating the mRNA of X‐box protein 1, a key regulator of cytokine production including interleukins IL‐6, IL‐8, IL‐1β, and IL‐12.
The anti‐inflammatory effects of fluvoxamine through activation of S1R observed in preclinical studies suggest fluvoxamine could be evaluated as a treatment option for COVID‐19 in clinical settings (Rafiee 2016). The anti‐inflammatory effects of fluvoxamine were also shown by a significantly decreased expression of some inflammatory genes, such as intracellular adhesion molecule (ICAM1), vascular cell adhesion molecule (VCAM1), cyclooxygenase 2 (COX2), and inducible nitric oxide synthase (iNOS) in human endothelial cells and macrophages (van Harten 1995).
In a murine sepsis model, fluvoxamine was found to bind to the S1R on immune cells, resulting in reduced production of inflammatory cytokines (Rosen 2019). Furthermore, in‐vitro studies of human endothelial cells and macrophages showed that fluvoxamine reduced the expression of inflammatory genes (Sukhatme 2021). Ongoing studies are currently looking to establish whether the anti‐inflammatory effects of fluvoxamine observed in non‐clinical studies are relevant to the clinical setting of COVID‐19 (Takenaka 2022).
Why it is important to do this review
The COVID‐19 pandemic places healthcare systems under tremendous pressure to provide adequate care. The emergence of variants of concern (WHO 2021b; WHO 2022), with the potential for increased transmissibility and altered disease characteristics, combined with the ongoing scarcity of effective and established drug treatments, in addition to low global vaccination coverage (WHO 2020c), highlights the obvious and urgent need for effective and safe pharmacotherapies. The repurposing of existing medications that are also widely available, inexpensive, and with well‐understood safety profiles, such as fluvoxamine, is of great importance. Evidence‐based reviews are therefore needed to guide clinical decision‐making for people with COVID‐19.
Current treatment consists of supportive care with oxygen therapy in cases with moderate disease, and with respiratory support, such as mechanical ventilation, and extracorporeal membrane oxygenation in cases of severe disease (CDC 2020; WHO 2020b). Overall, data from randomised controlled trials (RCTs) do not demonstrate a clear, major clinical benefit with most of the drugs evaluated thus far. Data from RCTs at this stage support the role of corticosteroids for severe COVID‐19 and clinical guidelines recommend their use (Agarwal 2020; National COVID‐19 Clinical Evidence Taskforce 2021). Further, tocilizumab and janus kinase inhibitor baricitinib are recommended for certain patient groups, while other drugs, such as hydroxychloroquine, azithromycin and ivermectin, are not recommended for the treatment of COVID‐19 (Agarwal 2020; National COVID‐19 Clinical Evidence Taskforce 2021).
Effective therapy regimens are particularly necessary for the early viral phase of the disease in order to prevent a severe course of COVID‐19. Evidence is emerging about the efficacy of anti‐COVID‐19 specific neutralising monoclonal antibodies as early treatments for COVID‐19 (Kreuzberger 2021). If used in the early phase of the disease (up to day five to seven after the onset of symptoms), the neutralising monoclonal antibodies significantly reduce the risk of a severe course of the disease (Gupta 2021; Weinreich 2021). However, a parenteral form of administration and currently limited availability represent an important challenge that makes widespread use in the non‐hospitalised setting difficult. Furthermore, the effectiveness of many of the neutralising monoclonal antibodies against the new virus variants may be reduced (Hoffmann 2021; Planas 2022). Other antiviral drugs are promising, such as the ribonucleoside analogue molnupiravir or the protease inhibitor nirmatrelvir in combination with ritonavir, but are still under investigation and not yet widely available (Jayk Bernal 2021).
Systematic reviews for interventions to treat COVID‐19 have already been undertaken, including treatment with fluvoxamine (Kacimi 2021; Lee 2021; Murchu 2022; Wen 2022). However, they did not fulfil all the methodological standards for evidence synthesis. For example, they did not apply the GRADE approach for rating the certainty of the evidence or assess the risk of bias (Kacimi 2021; Wen 2022). Furthermore, Murchu 2022 considered only preliminary data of a single study on fluvoxamine and Lee 2021 focused on unpublished data. Therefore, we aim to provide a complete evidence profile for oral fluvoxamine as a treatment for COVID‐19, in both inpatients and outpatients.
Objectives
To assess the efficacy and safety of fluvoxamine in addition to standard care, compared to standard care (alone or with placebo), or any other active pharmacological comparator with proven efficacy for the treatment of COVID‐19 outpatients and inpatients.
Methods
Criteria for considering studies for this review
Types of studies
The main outline of the methods section is based on the standard template of the Cochrane Haematology review group and is in line with a series of Cochrane Reviews investigating treatments and therapies for COVID‐19 (e.g. Kreuzberger 2021; Popp 2021a; Stroehlein 2021). The original review protocol for this review was registered with PROSPERO (CRD42022299758) on 4 January 2022. As this review and the other reviews of the Cochrane Review series are living systematic reviews during the COVID‐19 pandemic, specific adaptations relating to the research question, including participants, interventions, comparators, outcomes, and methods may be necessary in further updates.
To assess the efficacy and safety of fluvoxamine for the treatment of people with COVID‐19 in outpatient and inpatient settings, we included randomised controlled trials (RCTs), as this study design, if performed appropriately, provides the best evidence for experimental therapies in highly controlled therapeutic settings. We used the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020b). In addition to observational studies, non‐standard RCT designs, such as cluster‐randomised and cross‐over trials, were not eligible for the review. The latter are not appropriate in this context, since the underlying cause of COVID‐19 is an infection with the SARS‐CoV‐2 virus and the medical condition evolves over time. Furthermore, we excluded controlled non‐randomised studies of interventions, animal studies, pharmacokinetic studies, and in vitro studies.
We included the following formats, if sufficient information was available on study design, characteristics of participants, interventions, and outcomes.
Full‐text journal publications
Abstract publications
Preprint articles
Results published in trial registries
Additional personal communication with investigators, if results were available in any of the above‐listed formats
We included preprints and conference abstracts to have a complete overview of the ongoing research activity, especially for tracking newly emerging studies on treatments for COVID‐19. We did not apply any limitations with respect to the length of follow‐up or language of the publication.
Types of participants
We included studies investigating adults with a confirmed diagnosis of COVID‐19 (with reverse transcription‐polymerase chain reaction (RT‐PCR) or antigen testing) irrespective of age, sex, ethnicity and disease severity. If studies included participants with a confirmed or suspected COVID‐19 diagnosis, we only used data for patient populations with a confirmed COVID‐19 diagnosis. In cases where data were not reported separately for participants with confirmed or suspected COVID‐19 diagnosis, we included the mixed population. The status of participants in the included studies, as well as the type of COVID‐19 diagnosis, is reported in the section Included studies. If mixed population studies contributed data to the meta‐analyses, we excluded these studies in Sensitivity analysis to test the robustness of the results.
We excluded studies that evaluated fluvoxamine for other coronavirus diseases such as SARS or MERS, or other viral diseases, such as influenza. If studies enrolled populations with, or exposed to, mixed viral diseases, we only planned to include studies if the trial authors provided subgroup data for COVID‐19.
Types of interventions
The intervention was defined as treatment with fluvoxamine. All doses and therapeutic regimes were eligible.
We compared fluvoxamine in addition to standard care (including no intervention), to standard care (alone or with placebo), or any other active pharmacological comparator with proven efficacy (within clinical trials with a high weight of evidence) for the treatment of COVID‐19.
For example, dexamethasone has been shown to reduce mortality from COVID‐19 amongst participants who were randomised to receive dexamethasone compared to those who received standard care (Agarwal 2020; RECOVERY 2021), in people who were oxygenated or received respiratory support. Remdesivir showed some benefit for people hospitalised with COVID‐19, though to a lesser extent (Beigel 2020). For people who qualify for dexamethasone therapy, for instance, or for any other intervention that is proven to be beneficial in the future, it would be unethical to further conduct trials that compare an intervention to placebo only. On the contrary, studies using comparators without proven efficacy, such as hydroxychloroquine, may confound the assessment of the efficacy or safety of fluvoxamine, so we excluded these. Although these types of intervention were possibly used at certain time points during the pandemic with the best intentions, their use was never supported through the evidence, and they may also be associated with adverse effects (Singh 2021). From those comparisons, no reliable evidence could be obtained; therefore, active comparators without proven efficacy were not eligible for this review. For the current review, we did not find any studies using an active comparator with proven efficacy.
We excluded studies evaluating fluvoxamine in combination with other active treatments, if the same treatment was not used in the control group. We also excluded studies investigating its efficacy and safety in preventing COVID‐19. We created the following comparisons.
Fluvoxamine in addition to standard care (including no intervention) versus standard care (alone or with placebo)
Fluvoxamine in addition to standard care (including no intervention) versus active pharmacological intervention for the treatment of COVID‐19 with proven efficacy (no studies were available for the current review version)
Types of outcome measures
We evaluated core outcomes in accordance with the Core Outcome Measures in Effectiveness Trials (COMET) Initiative for people with COVID‐19 (COMET 2021; Marshall 2020), and additional outcomes that have been prioritised by consumer representatives and the German guideline panel for inpatient therapy of people with COVID‐19, as well as the German Primary Care Association Guidelines (DEGAM) for the treatment of acute COVID‐19 in the outpatient setting (DEGAM 2022). The current outcome set is in alignment with the previous review in this series of Cochrane Reviews investigating the use of potential pharmacotherapies for COVID‐19 in both inpatients and outpatients (Popp 2021a).
We defined outcome sets with primary and secondary outcomes for two populations.
Inpatients (hospitalised individuals, secondary care) with moderate to severe (World Health Organization (WHO) severity score > 4) (WHO 2020e ) COVID‐19
Outpatients (ambulatory‐managed individuals, primary care) with asymptomatic or mild COVID‐19 (WHO severity score ≤ 4) (WHO 2020e)
Primary outcomes were used to inform the Table 1.
Timing of outcome measurement
We collected information on outcomes from all time points reported in the publications. If only a few studies contributed data to an outcome, we pooled different time points, provided the studies had produced valid data and pooling was clinically reasonable.
In the case of time‐to‐event analysis, for instance as with 'time to death', we included the outcome measure based on the longest follow‐up time and measured from randomisation.
We included serious adverse events and adverse events occurring during the study period, including both adverse events during active treatment and long‐term adverse events. If sufficient data were available, we grouped the measurement time points of eligible outcomes (e.g. adverse events and serious adverse events) into those measured directly after treatment (up to seven days after treatment), medium‐term outcomes (up to 14 days after treatment) and longer‐term outcomes (more than 28 days after treatment).
Primary outcomes
Inpatients with moderate to severe COVID‐19
All‐cause mortality at day 28, day 60, time‐to‐event, and at hospital discharge
-
Clinical status at day 28, day 60, and up to the longest follow‐up, including
-
worsening of clinical status
participants with clinical deterioration (new need for invasive mechanical ventilation) or death
-
improvement of clinical status
participants discharged alive (participants should be discharged without clinical deterioration or death)
-
Quality of life, including fatigue and neurological status, assessed with standardised scales (e.g. with the WHO Quality of Life‐100 (WHOQoL‐100) scale) at up to seven days; up to 28 days, and longest follow‐up available
Serious adverse events during the study period
Adverse events (any grade) during the study period, defined as the number of participants with any event
Outpatients with asymptomatic or mild COVID‐19 (WHO < 4)
All‐cause mortality at day 28, day 60, time‐to‐event, and up to the longest follow‐up
All‐cause admission to hospital or death (before hospital admission)
-
Symptom resolution
all initial symptoms resolved (asymptomatic) at day 14, day 28, and up to the longest follow‐up
duration to symptom resolution
Quality of life, including fatigue and neurological status, assessed with standardised scales (e.g. WHOQOL‐100) at day seven, up to day 28, and the longest follow‐up available
Serious adverse events during the study period
Adverse events (any grade) during the study period, defined as number of participants with any event
Suicide or suicide attempt
Secondary outcomes
Inpatients with moderate to severe COVID‐19
Additional outcomes
-
Clinical status at day 15, day 28 and up to the longest follow‐up
-
worsening of clinical status
need for invasive mechanical ventilation
need for non‐invasive mechanical ventilation or high flow
need for oxygen by mask or nasal prongs
need for hospitalisation without oxygen therapy
-
improvement of clinical status
weaning or liberation from invasive mechanical ventilation in surviving patients
ventilator‐free days
duration to liberation from invasive mechanical ventilation
liberation from supplemental oxygen in surviving patients
duration to liberation from supplemental oxygen
-
Need for dialysis at up to day 28
Admission to the intensive care unit (ICU) at day 28
Duration of hospitalisation
Viral clearance, assessed with RT‐PCR test for SARS‐CoV‐2 at baseline, up to day three, day seven, and day 14
Hospital‐acquired infections up to day 28
Outpatients with asymptomatic or mild COVID‐19 (WHO < 4)
Additional outcomes
-
Clinical status at day 15, day 28 and up to the longest follow‐up
-
worsening of clinical status (moderate to severe COVID‐19 symptoms)
need for invasive mechanical ventilation
need for non‐invasive mechanical ventilation or high flow
need for hospitalisation (with need for oxygen by mask or nasal prongs)
need for hospitalisation (without oxygen therapy)
-
Viral clearance, assessed with RT‐PCR for SARS‐CoV‐2 at baseline, up to day three, day seven, and day 14
Search methods for identification of studies
Electronic searches
Our Information Specialist (IM) designed systematic search strategies and a second Information Specialist peer reviewed them. We searched in the following sources from the inception of each database up to 1 February 2022 and did not place restrictions on the language of the publication.
-
Cochrane COVID‐19 Study Register (CCSR) (www.covid-19.cochrane.org)
Cochrane Central Register of Controlled Trials (CENTRAL) (monthly updates)
MEDLINE (PubMed) (weekly updates)
Embase.com (weekly updates)
ClinicalTrials.gov (www.clinicaltrials.gov) (daily updates)
WHO International Clinical Trials Registry Platform (ICTRP) (trialsearch.who.int/) (monthly updates)
medRxiv (www.medrxiv.org) (weekly updates)
-
Web of Science Core Collection
Science Citation Index Expanded (1945 to present)
Emerging Sources Citation Index (2015 to present)
WHO COVID‐19 Global literature on coronavirus disease (search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/)
For detailed search strategies, see Appendix 1.
Searching other resources
We searched for other potentially eligible studies or ancillary publications by searching the reference lists of included studies, systematic reviews and meta‐analyses. In addition, we contacted the investigators of included studies to obtain additional information on the retrieved studies when needed.
Data collection and analysis
Selection of studies
In accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2021), two review authors (JN, IT, AAT or CS) independently screened the results of the search strategies for eligibility of this review by reading the titles and abstracts using Covidence. We then retrieved full‐text articles and assessed eligibility of the remaining records against predefined eligibility criteria in duplicate. We resolved discrepancies by discussion within the group of review authors. We included studies in the review irrespective of whether the measured outcome data were reported in a ‘usable’ way. We collated multiple reports of the same study, so that the study, rather than the report, was the unit of interest in the review.
We documented the study selection process in a flow diagram, as recommended in the PRISMA statement (Page 2021), and show the total number of retrieved references and the numbers of included, ongoing, excluded studies, as well as those awaiting classification in Figure 1. We listed all studies that we excluded after full‐text assessment and the reasons for their exclusion in the Characteristics of excluded studies, and used the same procedure for the Studies awaiting classification.
1.
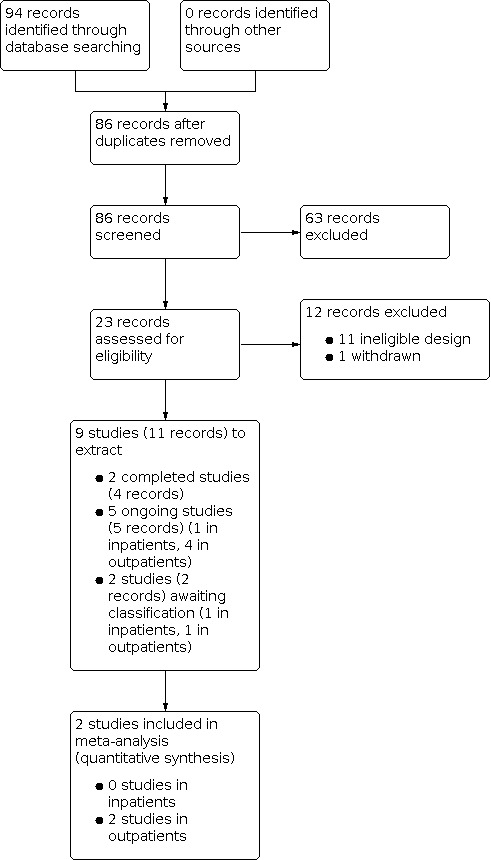
Figure 1: PRISMA flow diagram of study selection
Data extraction and management
We conducted data extraction according to the guidelines proposed by Cochrane (Li 2020). Two review authors (JN, IT or CS) extracted data independently and in duplicate, using a customised data extraction form developed in Microsoft Excel (Microsoft 2018). We solved disagreements by discussion. If no agreement was reached, we involved a third review author to resolve the disagreement.
We extracted the following information, if reported.
General information: author, title, source, country, language, type of publication, publication date
Study characteristics: setting and dates, inclusion/exclusion criteria, number of study arms, comparability of groups, treatment cross‐overs, treatment tailoring, intervention modification, length of follow‐up, funding
Participant characteristics: number of participants randomised/received intervention/analysed, COVID‐19 diagnostics, severity of disease, age, gender, comorbidities (e.g. diabetes, immunosuppression), concurrent interventions, time since symptom onset
Intervention: dose, frequency, duration, and route of administration
Control intervention: type of control, frequency, duration, and route of administration
Outcomes: as specified under Types of outcome measures
Assessment of risk of bias in included studies
We used the Risk of Bias 2 (RoB 2) tool to analyse the risk of bias of study results contributing information to our primary outcomes (risk of bias 2.0; Sterne 2019). Of interest for this review was the effect of the assignment to the intervention (the intention‐to‐treat (ITT) effect), so we performed all assessments with RoB 2 on this effect. The outcomes that we assessed are the primary outcomes specified for inclusion in the summary of findings tables.
Two review authors (JN, IT) independently assessed the risk of bias for each outcome. In case of discrepancies among their judgements and inability to reach consensus, we consulted the third review author to reach a final decision. We assessed the following types of bias as outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
Bias arising from the randomisation process
Bias due to deviations from the intended interventions
Bias due to missing outcome data
Bias in measurement of the outcome
Bias in selection of the reported result
To address these types of bias we used the signalling questions recommended in RoB 2 and make a judgement using the following options.
'Yes': if there is firm evidence that the question is fulfilled in the study (i.e. the study is at low or high risk of bias for the given the direction of the question)
'Probably yes': a judgement has been made that the question is fulfilled in the study (i.e. the study is at low or high risk of bias given the direction of the question)
'No': if there is firm evidence that the question is unfilled in the study (i.e. the study is at low or high risk of bias for the given the direction of the question)
'Probably no': a judgement has been made that the question is unfilled in the study (i.e. the study is at low or high risk of bias given the direction of the question)
'No information': if the study report does not provide sufficient information to allow any judgement
We used the algorithms proposed by RoB 2 to assign each domain one of the following levels of bias.
Low risk of bias
Some concerns
High risk of bias
Subsequently, we derived an overall risk of bias rating for each prespecified outcome in each study in accordance with the following suggestions.
'Low risk of bias': we judged the trial to be at low risk of bias for all domains for this result
'Some concerns': we judged the trial to raise some concerns in at least one domain for this result, but not to be at high risk of bias for any domain
'High risk of bias': we judged the trial to be at high risk of bias in at least one domain for the result, or we judged the trial to have some concerns for multiple domains in a way that substantially lowers confidence in the results
To implement RoB 2, we used the RoB 2 Excel tool (available on the website www.riskofbias.info/welcome/rob-2-0-tool/current-version-of-rob-2), and stored and presented our detailed RoB 2 assessments in the analyses section.
Measures of treatment effect
For dichotomous outcomes, we recorded the number of events and total number of participants in both treatment and control groups and reported the pooled risk ratio (RR) with 95% confidence intervals (CIs) and the risk difference (RD) (Deeks 2020).
For continuous outcomes, we recorded the mean, standard deviation and total number of participants in both treatment and control groups. Where continuous outcomes used the same scale, we performed analyses using the mean difference (MD) with 95% CIs (Deeks 2020). For continuous outcomes measured with different scales, we planned to perform analyses using the standardised mean difference (SMD) (Deeks 2020). For interpreting SMDs, we planned to re‐express SMDs in the original units of a particular scale with the most clinical relevance and impact. For the current review, all outcomes were measured on comparable scales.
If available, we extracted and reported hazard ratios (HRs) for time‐to‐event outcomes (e.g. time to hospital discharge). If HRs were not available, we would have made every effort to estimate the HR as accurately as possible from available data using the methods proposed by Parmar and Tierney (Parmar 1998; Tierney 2007). If sufficient studies provided HRs, we would have used HRs rather than RRs or MDs in a meta‐analysis, as HRs provide more information.
Unit of analysis issues
The unit of analysis for this review is the individually‐randomised participant. In studies with multiple intervention groups, we followed the recommendations in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020a). For studies with multiple treatment groups of the same intervention (i.e. dose, route of administration), we combined the study arms if they were sufficiently homogeneous. If arms could not be pooled, which was not the case for the current review, we had planned to compare each arm with the common comparator separately. For pairwise meta‐analysis, we had planned to split the ‘shared’ group into two or more groups with smaller sample size, and include two or more (independent) comparisons. For this purpose, in the case of dichotomous outcomes, we would have divided both the number of events and the total number of participants. For continuous outcomes, we would have divided the total number of participants and retained unchanged means and standard deviations (SDs).
Dealing with missing data
There are many potential sources of missing data in a systematic review or meta‐analysis, which can affect the level of studies, outcomes, summary data, individuals, or study‐level characteristics (Deeks 2020). Incomplete data can introduce bias into the meta‐analysis if they are not missing at random, which is addressed in the section Assessment of risk of bias in included studies.
First, when data were missing at outcome and study level, we checked for any evidence for the data being missing at random. When we could not retrieve information about data being missing at random, we contacted principal investigators and requested these data (Table 2). If, after this, data were still missing, we would have assumed that data were not missing at random. On the other hand, if there were indications that data were not missing at random, we would have conducted complete case analysis for the primary analysis and would have discussed its potential impact in the discussion section. If we were concerned regarding missing data across studies, we would not have performed meta‐analyses, but would have provided subtotals per study.
1. Author communication.
| Study ID | Date of request and question of the authors' of this review | Date when feedback was received and author response provided |
| Lenze 2020 | 9 June 2022: Question regarding the outcome 'all‐cause hospitalisation', which is mentioned in the primary study, but definite numerical data were missing. | 9 June 2022: Numbers for all‐cause hospitalisation: 1 participant in the fluvoxamine and 5 participants in the placebo group. |
| TOGETHER 2021 | 27 January 2022: Question regarding the outcome 'symptom resolution' which is mentioned in the primary study, but definite numerical data are not reported. | Feedback was not provided. |
Communications are listed after considering communication date
Assessment of heterogeneity
We used the descriptive statistics reported in the Characteristics of included studies to assess whether the studies within each pairwise comparison were homogenous enough, with respect to study and intervention details and population baseline characteristics, that the assumption of homogeneity might be plausible. In case of excessive clinical heterogeneity, we did not pool the findings of included studies. We measured statistical heterogeneity using the Chi2 test and the I2 statistic (Deeks 2020), and the 95% prediction interval (PI) for random‐effects meta‐analysis (IntHout 2016). The prediction interval helps in the clinical interpretation of heterogeneity by estimating what true treatment effects can be expected in future settings (IntHout 2016).
We restricted the calculation of a 95% PI to meta‐analyses with four or more studies (≥ 200 participants), since the interval would be imprecise if a summary estimate were based on only a few small studies. We planned to use the open‐source statistical software R package Meta to calculate 95% PIs, and declare statistical heterogeneity if the P value was less than 0.1 for the Chi2 statistic, or the I2 statistic was 40% or more (40% to 60%: moderate heterogeneity; 50% to 90%: substantial heterogeneity; 75% to 100%: considerable heterogeneity) (Deeks 2020); or the range of the 95% PI revealed a different clinical interpretation of the effect estimate compared to the 95% CI.
Assessment of reporting biases
We sought to identify all research that meets our predefined eligibility criteria. Missing studies can introduce bias to the analysis. We searched for completed non‐published trials in trials registers, contacted authors to seek assurance that the results will be made available, and classified them as 'awaiting classification' until the results are reported. We reported the number of completed non‐published trials. We planned to investigate the risk of reporting bias (publication bias) in pairwise meta‐analyses using contour‐enhanced funnel plots, when there were 10 or more relevant studies pooled in a meta‐analysis. In the current review, there are no meta‐analyses including 10 or more studies. For future review updates, if funnel plot asymmetry is suggested by a visual assessment, we plan to perform exploratory analyses (e.g. Ruecker’s arcsine test for dichotomous data and Egger’s linear regression test for continuous data) to further investigate funnel plot asymmetry (Egger 1997). A P value of less than 0.1 will be considered as the level of statistical significance. In future review updates, we will analyse reporting bias using the open‐source statistical software R package Meta.
Data synthesis
We analysed trials including different severities of disease separately, grouping them into asymptomatic to mild, and moderate to severely ill, as these are different populations in different settings, resulting in different outcome sets (see Types of outcome measures). We analysed trials with the following participant populations separately.
Inpatients with moderate to severe COVID‐19
Outpatients with asymptomatic or mild COVID‐19
For these two distinct populations, we created the following comparisons.
Fluvoxamine in addition to standard care versus standard care (alone or with placebo)
Fluvoxamine in addition to standard care versus active pharmacological intervention for the treatment of COVID‐19 with proven efficacy in clinical trials with a high weight of evidence (no studies were available for the current review version) in addition to standard care
Placebo and standard care alone (including no intervention) were treated as the same intervention, as well as standard care at different institutions and time points during the pandemic.
We performed meta‐analyses according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2020). If clinical and methodological characteristics of individual studies were sufficiently homogeneous, we pooled the data into meta‐analyses. We used the RevMan Web software for meta‐analyses (RevMan Web 2022). One review author entered the data in the software, and a second review author checked the data for accuracy. When meta‐analysis was feasible, we used the random‐effects model as we assumed that the intervention effects were related but were not the same for the included studies. For dichotomous outcomes, we performed meta‐analyses using the Mantel‐Haenszel method under a random‐effects model to calculate the summary (combined) intervention effect estimate as a weighted average of the intervention effects estimated in the individual studies. For continuous outcomes, we used the inverse‐variance method.
We planned to present descriptive statistics only if we deemed meta‐analysis inappropriate for a certain outcome because of heterogeneity or because of serious study limitations leading to a considerably high risk of bias (e.g competing risk of death not taken into account in outcome measurements). This was not the case for the current review version.
If meta‐analysis was possible, we assessed the effects of potential biases in sensitivity analyses (see Sensitivity analysis) and considered investigating heterogeneity in subgroup analyses (see Subgroup analysis and investigation of heterogeneity). Subgroup analyses were not possible due to the low number of studies per outcome. For future review updates, if we cannot find a cause for the heterogeneity, we will not undertake a meta‐analysis but will comment instead on the results as a narrative and present the results from all studies in tables.
We used forest plots to visualise meta‐analyses of primary outcomes only, including risk of bias assessment. We reported secondary outcomes without risk of bias assessments in additional tables.
Subgroup analysis and investigation of heterogeneity
We planned subgroup analyses to investigate clinical heterogeneity for the following characteristics (considering primary outcomes):
Disease severity at baseline as defined by the WHO Clinical Progression Scale (mild, moderate, severe)
Dose of fluvoxamine (usual dose versus low dose versus high dose)
In the current review version, none of these analyses were possible due to a lack of data and trials (only two included studies).
If enough studies are identified during future review updates, we will also perform subgroup analyses, if statistical heterogeneity is present (P < 0.1 for the Chi2 test of heterogeneity, I2 ≥ 50%), or a different clinical conclusion (when comparing 95% CI with 95% PI). We also plan to undertake tests for interaction to test for differences between subgroup results.
Sensitivity analysis
We planned sensitivity analyses of the following characteristics (considering primary outcomes).
Risk of bias assessment (only trials with a low risk of bias or some concerns)
Comparison of preprint articles versus peer‐reviewed publications (only trials published as journal articles)
Confirmed versus mixed (suspected and confirmed) COVID‐19 diagnosis (only trials/participants with confirmed COVID‐19 diagnosis)
Similar to the subgroup analyses, such analyses were not possible in the current review version (due to the low number of trials).
Summary of findings and assessment of the certainty of the evidence
Summary of findings
We evaluated the certainty of the evidence using the GRADE approach for the interventions evaluated in RCTs.
We planned to create a separate summary of findings tables for the different patient populations (outpatients, inpatients) and for the different comparisons. For the current review version, there were no studies addressing inpatients or an active comparator.
We used the GRADEpro GDT to create a summary of findings table. For time‐to‐event outcomes, we planned to calculate absolute effects at specific time points, as recommended in the GRADE guidance 27 (Skoetz 2020). For the current review, there were no time‐to‐event data available. According to Chapter 14 of the updated Cochrane Handbook for Systematic Reviews of Interventions, the “most critical and/or important health outcomes, both desirable and undesirable, limited to seven or fewer outcomes” should be included in the summary of findings table (Schünemann 2020). We included the following outcomes prioritised according to the Core Outcome Set for intervention studies (COMET 2021, Marshall 2020) and patient relevance.
Inpatients with moderate to severe COVID‐19
All‐cause mortality (most favourable time point: at hospital discharge, if not reported for this time point we considered day 60, followed by day 28, or time‐to‐event estimate)
-
Worsening of clinical status at day 28
participants with clinical deterioration (new need for invasive mechanical ventilation) or death
-
Improvement of clinical status at day 28
participants discharged alive
Quality of life at longest follow‐up available
Serious adverse events during the study period
Any adverse events during the study period
Suicide or suicide attempt
Outpatients with asymptomatic or mild COVID‐19
All‐cause mortality (most favourable at longest follow‐up (> 60 days), if not reported at longest follow‐up we considered day 60, followed by day 28, or time‐to‐event estimate)
Admission to hospital (all cause) or death (combined outcome, within 28 days)
-
Symptom resolution
all initial symptoms resolved (asymptomatic) at day 14
duration to symptom resolution
Quality of life at longest follow‐up available
Serious adverse events during the study period
Any adverse events during the study period
Suicide or suicide attempt
Assessment of certainty in the evidence
We used the GRADE approach to assess the certainty in the evidence for the outcomes listed above. The GRADE approach uses five domains (risk of bias, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty in the body of evidence for each prioritised outcome.
We downgraded our certainty of evidence as follows.
Serious (‐1) or very serious (‐ 2) risk of bias
Serious (‐1) or very serious (‐ 2) inconsistency
Serious (‐1) or very serious (‐ 2) uncertainty about directness
Serious (‐1) or very serious (‐ 2) imprecise or sparse data
Serious (‐1) or very serious (‐ 2) probability of reporting bias
The GRADE system used the following criteria for assigning grade of evidence.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
We followed the current GRADE guidance for these assessments in its entirety as recommended in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 14 (Schünemann 2020). We used the overall risk of bias judgement, derived from the RoB 2 Excel tool, to inform our decision on downgrading for risk of bias. We phrased the findings and certainty in the evidence as suggested in the informative statement guidance (Santesso 2020).
Methods for future updates (living systematic review considerations)
Our information specialists (IM, KG) will provide us with new search records each week, which two review authors will screen, extract, evaluate, and integrate following the guidance for Cochrane living systematic reviews (Cochrane LSR). We will also manually check platform trials that were previously identified and listed as Studies awaiting classification for additional relevant treatment arms.
We will wait until the accumulating evidence changes our conclusions of the implications of research and practice before republishing the review. We will consider one or more of the following components to inform this decision.
The findings of one or more prioritised outcomes
The credibility (e.g. GRADE rating) of one or more prioritised outcomes
New settings, populations, interventions, comparisons or outcomes studied
In case of emerging policy relevance because of global controversies on the intervention, we will consider republishing an update the review even though our conclusions will remain unchanged. We will review the review scope and methods approximately monthly, or more frequently if appropriate, in light of potential changes in COVID‐19 research (for example, when additional comparisons, interventions, subgroups or outcomes, or new review methods become available).
Results
Description of studies
Results of the search
The literature search resulted in 94 records. No records were identified via additional searches of reference lists. After removing duplicates, 86 records remained. During title and abstract screening, we judged 63 records to be irrelevant.
We proceeded to full‐text screening with 23 records, considering published full texts or, if these were not available, trial register entries. From these 23 records, we excluded 12 records: one record (one study) was cancelled before starting patient recruitment and 11 records (11 studies) were not RCTs. From the 11 records (nine studies) that we considered to be relevant (included), five records referred to ongoing studies and two are awaiting classification. Finally, we included two studies (four records) in our quantitative synthesis, contributing data to the primary and secondary outcomes of this review. The flow of records is illustrated in Figure 1.
Inpatients
We did not include any clearly‐eligible, completed studies that investigated fluvoxamine in the inpatient setting.
Studies awaiting classification
We considered one study conducted in the inpatient setting to be relevant (Safa 2020), and listed this study in the section Studies awaiting classification. This study was only available in the Persian language, and we will re‐evaluate it once the study authors have clarified some translation issues.
Ongoing studies
The characteristics of ongoing studies are available in the section Ongoing studies. In the inpatient setting, we identified one ongoing study (NCT04718480). This trial is still active and compares fluvoxamine with placebo treatment (both in addition to standard care) in people hospitalised with COVID‐19. The expected completion date is December 2022. The trial records refer to a planned sample size of 100.
Outpatients
Study design and publication status
Details of the included studies are available in the section Characteristics of included studies.
We included two randomised controlled trials in this review with a total of 1649 allocated participants, of whom 821 were allocated to fluvoxamine in addition to standard care and 828 to placebo and standard care (Lenze 2020; TOGETHER 2021). TOGETHER 2021 was a randomised, adaptive platform trial to investigate the efficacy of different repurposed treatments for non‐hospitalised people with COVID‐19, allocating 1497 participants to the comparison of interest. Lenze 2020 allocated 152 participants in total.
The study by Lenze 2020 was performed as a remote outpatient trial and the TOGETHER 2021 study used both in‐person and remote follow‐up assessments (i.e. relying on physical examination or self‐assessments via phone contact or video calls). Both studies were multicentric: Lenze 2020 was performed in two centres in the greater St. Louis metropolitan area, USA, while the TOGETHER 2021 study was conducted at 11 clinical sites in Brazil. With regard to equity, applicability, and resources, we must highlight that one of the included studies was performed in a high‐income country while the other was performed in an upper‐middle‐income country. Both studies reported information about the responsible ethics committee and financial support. TOGETHER 2021 was supported by FastGrants and the Rainwater Foundation, while Lenze 2020 was supported by the Taylor Family Institute for Innovative Psychiatric Treatment at Washington University and the COVID‐19 Early Treatment Fund, the Center for Brain Research in Mood Disorders at Washington University, the Bantly Foundation, and a grant from the National Institutes of Health. In both studies, a number of co‐authors reported conflicts of interests.
Both trials were peer‐reviewed publications in indexed journals and were prospectively registered.
Participants
Both studies recruited participants from the outpatient setting. Lenze 2020 used an electronic health record system, physician referrers, doctors' hotlines, COVID‐19 Test Centres, Emergency Rooms (microbiology lab) as per the Healthy Mind Lab website (www.healthymind.wustl.edu), flyers, and email notifications. TOGETHER 2021 recruited participants at community health facilities (emergency settings, influenza‐symptom referral centres, or primary care community centres), with the help of notices through physical and social media as per local public health authorities.
In Lenze 2020, all participants had a polymerase chain reaction (PCR)‐confirmed SARS‐CoV‐2 infection, while in TOGETHER 2021 PCR for SARS‐CoV‐2 was not mandatory and participants were also included in the study if they had a positive rapid test for SARS‐CoV‐2 antigen done after obtaining informed consent.
Lenze 2020 performed randomisation and start of study medication within seven days of symptom onset, without further specification. In TOGETHER 2021, all patients had symptoms beginning within seven days of the screening date: 42.6% within the first three days of symptom onset and 34.5% between days four and seven. For the remaining participants, information on the duration of symptoms within the first seven days was missing.
In both studies, all participants were symptomatic adults. In Lenze 2020 the median age of participants was 46 years in the intervention group and 45 years in the control group (interquartile range (IQR) 35 to 58 and 36 to 54 years, respectively). In TOGETHER 2021 the median age of participants was 50 years (IQR 39 to 56) in the intervention group and 49 years (IQR 38 to 56) in the control group. In total, 50.9% of all participants in this study were less than 50 years old. The majority of participants were female (Lenze 2020: 71.7%; TOGETHER 2021: 58.7%).
The most common comorbidities in Lenze 2020 were asthma (21%) and hypertension (19%) in the intervention group and hypertension (21%) in the control group. In TOGETHER 2021 the most common risk factors were uncontrolled hypertension (in 14% of participants in the intervention group and 12% of participants in the control group) and type 2 diabetes mellitus (in 14% of participants in the intervention group and 12% of participants in the control group).
The most common symptoms of COVID‐19 in Lenze 2020 were loss of sense of smell (in 33% of participants in the intervention group and 25% of participants in the control group) and fatigue (in 21% of participants in the intervention group and 25% of participants in the control group). TOGETHER 2021 did not report the details of baseline symptoms.
Interventions and comparators
Both studies compared fluvoxamine in addition to standard care with placebo and standard care (Lenze 2020; TOGETHER 2021). In Lenze 2020 the dose of fluvoxamine (capsules manufactured by Apotex) was titrated from 50 mg on the day of randomisation to 100 mg twice daily on days two and three and finally 100 mg three times daily on the remaining days up to day 15. After the completion of 15 days of fluvoxamine or placebo, participants were given the option to receive a six‐day open‐label course of fluvoxamine. This was a change from the original study protocol, but no data collection was conducted for this phase. Matching placebo gelatin capsules contained microcrystalline cellulose and silica gel, micronised. All active drug and placebo preparations were performed by the same pharmacy. In TOGETHER 2021 participants were randomly assigned to fluvoxamine (manufactured by Abbott) at a dose of 100 mg twice daily for 10 days or a corresponding placebo starting directly after randomisation. Neither study specified the placebo.
In the TOGETHER 2021 study, standard care typically focused on the management of symptoms and provision of antipyretics, while antibiotics were provided when clinicians suspected bacterial pneumonia. Lenze 2020 did not report standard care, and the participants received either fluvoxamine or placebo during quarantine. However, taking immunosuppressant biological drugs or high‐dose corticosteroids (> 20 mg/d of prednisone) were exclusion criteria in the Lenze 2020 study. TOGETHER 2021 did not allow current use of an SSRI.
Outcome measures
The primary outcome in Lenze 2020 was clinical deterioration, defined by meeting both criteria of (1) shortness of breath or hospitalisation for shortness of breath or pneumonia and (2) oxygen saturation < 92% on room air or need for supplemental oxygen to achieve oxygen saturation of 92% or greater (within 15 days). The primary outcome in TOGETHER 2021 was admission to hospital, defined as COVID‐19 emergency setting visits (participants remaining under observation for over six hours) or admission to hospitalisation due to progression of COVID‐19 (within 28 days).
Secondary outcomes included in Lenze 2020 were 30 days of post‐trial observation events (emergency department visit, hospitalisation, or both) and ventilator support. All outcomes were measured using participants’ self‐reported responses on twice‐daily surveys during the 15 days after randomisation and assessed remotely (via Zoom videoconference, phone, text, email, as well as REDCap surveys pushed out to participants via their smartphones or other devices). In order to standardise the procedure, criteria were formulated for which an emergency department visit was indicated (a decrease in oxygen saturation < 90% on room air on more than two readings, persistent increase in respiratory rate to > 30 breaths per minute, persistent increase in heart rate to > 120 beats per minute, alteration in mentation, or severe worsening in shortness of breath). At 30 days after the conclusion of the 15‐day trial, a follow‐up survey was performed by phone, email, or electronic medical record review. In the follow‐up, the participants were asked if they were hospitalised or had visited a hospital or emergency department since the last study survey.
In TOGETHER 2021, secondary outcomes included time to clinical improvement, number of days with respiratory symptoms, time to hospitalisation (for any cause or due to COVID‐19 progression), length of hospitalisation (days), proportion of participants with mechanical ventilation, time on mechanical ventilator (days), proportion of participants who were non‐adherent with the study drugs, and adverse reactions to the study medications. Study personnel collected outcome data on days one, two, three, four, five, seven, ten, 14, and 28 in‐person or via telephone or social media using video‐teleconferencing. At the baseline visit, a six‐lead electrocardiogram (Kardiamobile, Mountain View, CA, USA) was performed for all participants and transferred to a central facility (Cardresearch, Belo Horizonte, Brazil) for reading. Vital signs included oxygen status assessed by means of a pulse oximeter for non‐invasive arterial oxygen saturation and pulse (Jumper Medical Equipment, Shenzhen, China), and temperature by a standard digital oral thermometer.
Studies awaiting classification
We considered one study conducted in the outpatient setting to be relevant (NCT04668950), and listed it in the section Studies awaiting classification. Although the study is related to the included and published study of Lenze 2020 (but conducted across the United States and two provinces of Canada), we have not yet identified any published reports.
Ongoing Studies
The characteristics of ongoing studies in outpatients are available in the section Ongoing studies. We classified four trials as ongoing: three are still active and are currently recruiting (NCT04510194; NCT04885530; NCT05087381), while the other trial (TCTR20210615002) is pending (not yet recruiting). In all trials, fluvoxamine is being compared to placebo or standard care or with other pharmacological interventions in the outpatient setting. Ongoing studies are evaluating participants aged 18 years and older. The original completion date for the TCTR20210615002 trial was December 2021, and the expected completion date for NCT05087381 was March 2022, while those for NCT04885530 and NCT04510194 are in early 2023. These four trial records refer to planned sample sizes of 296 (TCTR20210615002), 1350 (NCT04510194), 1800 (NCT05087381), and 15000 (NCT04885530), respectively.
Excluded studies
We excluded 12 records after full‐text assessment, and these are listed in the section Excluded studies. One record (one study) was cancelled before starting participant recruitment and 11 records (11 studies) were not RCTs.
Risk of bias in included studies
We assessed methodological quality and risk of bias for two RCTs contributing results to our primary outcomes using the RoB 2 tool. The RoB 2 judgements for all study results per outcome and for all domains are available in an interactive risk of bias table (Table 11; Table 12; Table 13; Table 14) and are briefly summarised below.
Risk of bias for analysis 1.1 All‐cause mortality (at day 28).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Lenze 2020 | Low risk of bias | Quote: "Patients were randomized 1:1 to fluvoxamine or matching placebo capsules. Randomization schedules were generated that stratified by age (18‐44, 45‐54, 55‐64, and ≥65 years)16 and sex. Treatments were randomly allocated using alternating block sizes of 2 and 4. Randomization allocation was conducted via REDCap, which displayed randomization assignment to the laboratory manager (J.S.), who prepared the study materials, including the study drug or placebo. All outcome assessors, investigators, and research staff who were in contact with participants were blinded to participant treatment assignment." "Participants were well‐matched in demographic and clinical characteristics. The baseline oxygen saturation level did not differ between the groups." | Low risk of bias | Placebo controlled trial. All outcome assessors, investigators, and research staff who were in
contact with participants were blinded to participant treatment assignment. There is a substantial number of participants who did not complete the study. There were 18 out of 80 (22.5%) in intervention group and 19 out of 72 (26.4%) in the placebo group. Patients were analysed according to randomization group. |
High risk of bias | 37 study participants did not finish the study. 18 patients in the intervention group and 19 patients in the placebo group did not complete the study. No evidence to support that the results were not bias by missing outcome data. The missing patients might have different outcomes compared to the patients that remained in the trial. High proportion of withdrawals without explanation | Low risk of bias | No detailed information on outcome measurement reported. Medical records were used to measure outcomes that were not self‐reported. No patients died during the trial. Participants and personnel were blinded to the treatment, so differences in the ascertainment of the outcome are unlikely. Ascertainment of mortality considered objective enough. | Low risk of bias | The primary analysis reported agrees with that of the protocol. Unlikely that multiple outcome measures or analyses were used since 0 patients died during the trial. | High risk of bias | Quote: "Patients were randomized 1:1 to fluvoxamine or matching
placebo capsules. Randomization schedules were generated
that stratified by age (18‐44, 45‐54, 55‐64, and ≥65 years)16
and sex. Treatments were randomly allocated using alternating
block sizes of 2 and 4. Randomization allocation was conducted
via REDCap, which displayed randomization assignment
to the laboratory manager (J.S.), who prepared the
study materials, including the study drug or placebo. All outcome
assessors, investigators, and research staff who were in
contact with participants were blinded to participant treatment
assignment." "Participants were well‐matched in demographic and clinical characteristics. The baseline oxygen saturation level did not differ between the groups." No, placebo controlled trial. All outcome assessors, investigators, and research staff who were in contact with participants were blinded to participant treatment assignment. There is a substantial number of participants who did not complete the study. There were 18 in intervention group and 19 in the placebo group. Patients were analysed according to randomization group. 37 study participants did not finish the study. 18 patients in the intervention group and 19 patients in the placebo group did not complete the study. No evidence to support that the results were not bias by missing outcome data. The missing patients might have different outcomes compared to the patients that remained in the trial. High proportion of the withdrawals had no reason explaining it. No detailed information on outcome measurement reported. Medical records were used to measure outcomes that were not self‐reported. No patients died during the trial. Participants and personnel were blinded to the treatment, so differences in the ascertainment of the outcome are unlikely. Ascertainment of mortality considered subjective enough. Participants and personnel were blinded to the treatment, so differences in the ascertainment of the outcome are unlikely. The primary analysis reported in the paper agrees with that of the protocol. Unlikely that multiple outcome measures or analyses were used since 0 patients died during the trial. |
| TOGETHER 2021 | Low risk of bias | Quote: "Participants were randomly assigned by means of a centralised core randomisation process handled by an independent unmasked pharmacist who was not aware of any protocol‐related procedures and contracted specifically for this process. Patients were randomly assigned (1:1) by means of a block randomisation procedure for each participating site, stratified by age (<50 years or ≥50 years)." Quote: "With respect to covariates of age, body‐mass index, and comorbidities, the groups were generally well balanced." | Low risk of bias | Quote: "The trial team, site staff, and patients were masked to treatment allocation. The active drugs and the placebo pills were packaged in identically shaped bottles and labelled with alphabet letters corresponding to the active group or placebo group." Comment: Patients were analysed according to randomization group with intention‐to treat analyses. |
Low risk of bias | Quote: "84 participants stopped fluvoxamine and 64 participants stopped placebo owing to issues of tolerability." Comment: Proportions of drop‐out are comparable between groups and < 12%. This proportion seems tolerable and is not considered to introduce bias to the results. | Low risk of bias | Quote: "Study personnel collected outcome data on days 1, 2, 3, 4, 5, 7, 10, 14, and 28 in person or via telephone contact or social media applications using video‐teleconferencing. We collected outcome data irrespective of whether participants took study medication." Comment: All outcomes were reported to be self‐reported. No information about measuring mortality are reported. Outcome measurement or ascertainment of the outcome are unlikely to differ between groups for mortality. | Low risk of bias | Comment: The primary analysis reported in the paper agrees with that of the protocol. Numerical results are unlikely to be selected from multiple outcome measures or analyses. | Low risk of bias | Quote: "Participants were randomly assigned by means of a centralised core randomisation process handled by an independent unmasked pharmacist who was not aware of any protocol‐related procedures and contracted specifically for this process. Patients were randomly assigned (1:1) by means of a block randomisation procedure for each participating site, stratified by age (<50 years or ≥50 years)."
Quote: "With respect to covariates of age,
body‐mass index, and comorbidities, the groups were generally well balanced." Quote: "The trial team, site staff, and patients were masked to treatment allocation. The active drugs and the placebo pills were packaged in identically shaped bottles and labelled with alphabet letters corresponding to the active group or placebo group." Comment: Patients were analysed according to randomization group with intention‐to treat analyses. Quote: "84 participants stopped fluvoxamine and 64 participants stopped placebo owing to issues of tolerability." Comment: Proportions of drop‐out are comparable between groups and < 12%. This proportion seems tolerable and is not considered to introduce bias to the results. Quote: "Study personnel collected outcome data on days 1, 2, 3, 4, 5, 7, 10, 14, and 28 in person or via telephone contact or social media applications using video‐teleconferencing. We collected outcome data irrespective of whether participants took study medication." Comment: All outcomes were reported to be self‐reported. No information about measuring mortality are reported. Comment: Outcome measurement or ascertainment of the outcome are unlikely to differ for mortality. Comment: The primary analysis reported in the paper agrees with that of the protocol. Comment: Numerical results are unlikely to be selected from multiple outcome measures or analyses. |
Risk of bias for analysis 1.2 All‐cause hospital admission or death (before hospital admission).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Lenze 2020 | Low risk of bias | Quote: "Patients were randomized 1:1 to fluvoxamine or matching placebo capsules. Randomization schedules were generated that stratified by age (18‐44, 45‐54, 55‐64, and ≥65 years)16 and sex. Treatments were randomly allocated using alternating block sizes of 2 and 4. Randomization allocation was conducted via REDCap, which displayed randomization assignment to the laboratory manager (J.S.), who prepared the study materials, including the study drug or placebo. All outcome assessors, investigators, and research staff who were in contact with participants were blinded to participant treatment assignment." "Participants were well‐matched in demographic and clinical characteristics. The baseline oxygen saturation level did not differ between the groups." | Low risk of bias | All outcome assessors, investigators, and research staff who were in contact with participants were blinded to participant treatment assignment. Patients were analyzed according to randomization group. | Low risk of bias | Although 37 study participants did not finish the study. 18 patients in the intervention group and 19 patients in the placebo group did not complete the study, the authors performed a senstivity analysis to assess the missing data. | Low risk of bias | In this case we consider hospitalisation as obejctive enough to allow for an unbiased assessment. Study personnel and participants were blinded to the intervention. | Low risk of bias | The primary analysis reported in the paper agrees with that of the protocol. Unlikely that multiple outcome measures or analyses were used since 0 patients died during the trial. | Low risk of bias | No concerns. |
| TOGETHER 2021 | Low risk of bias | Quote: "Participants were randomly assigned by means of a centralised core randomisation process handled by an independent unmasked pharmacist who was not aware of any protocol‐related procedures and contracted specifically for this process. Patients were randomly assigned (1:1) by means of a block randomisation procedure for each participating site, stratified by age (<50 years or ≥50 years)." Quote: "With respect to covariates of age, body‐mass index, and comorbidities, the groups were generally well balanced." | Low risk of bias | Quote: "The trial team, site staff, and patients were masked to treatment allocation. The active drugs and the placebo pills were packaged in identically shaped bottles and labelled with alphabet letters corresponding to the active group or placebo group." Comment: Patients were analysed according to randomization group with intention‐to treat analyses. |
Low risk of bias | Quote: "84 participants stopped fluvoxamine and 64 participants stopped placebo owing to issues of tolerability." Comment: Proportions of drop‐out are comparable between groups and < 12%. This proportion seems tolerable and is not considered to introduce bias to the results. | Low risk of bias | Quote: "Study personnel collected outcome data on days 1, 2, 3, 4, 5, 7, 10, 14, and 28 in person or via telephone contact or social media applications using video‐teleconferencing. We collected outcome data irrespective of whether participants took study medication." Comment: All outcomes were reported to be self‐reported. Hospital admission is judged to be objective enough to allow for an unbiased assessment. Outcome measurement or ascertainment of the outcome are unlikely to differ between both groups. | Low risk of bias | Comment: The primary analysis reported in the publication agrees with that of the protocol. Numerical results are unlikely to be selected from multiple outcome measures or analyses. Outcomes are sufficiently different and details are reported for each measurement separately. |
Low risk of bias | Quote: "Participants were randomly assigned by means of a centralised core randomisation process handled by an independent unmasked pharmacist who was not aware of any protocol‐related procedures and contracted specifically for this process. Patients were randomly assigned (1:1) by means of a block randomisation procedure for each participating site, stratified by age (<50 years or ≥50 years)."
Quote: "With respect to covariates of age,
body‐mass index, and comorbidities, the groups were generally well balanced." Quote: "The trial team, site staff, and patients were masked to treatment allocation. The active drugs and the placebo pills were packaged in identically shaped bottles and labelled with alphabet letters corresponding to the active group or placebo group." Comment: Patients were analysed according to randomisation group with intention‐to treat analyses. Quote: "We assessed time‐to‐event outcomes using Cox proportional hazard models and binary outcomes using logistic regression." Quote: "84 participants stopped fluvoxamine and 64 participants stopped placebo owing to issues of tolerability." Comment: Proportions of drop‐out are comparable between groups and < 12%. This proportion seems tolerable and is not considered to introduce bias to the results. Quote: "Study personnel collected outcome data on days 1, 2, 3, 4, 5, 7, 10, 14, and 28 in person or via telephone contact or social media applications using video‐teleconferencing. We collected outcome data irrespective of whether participants took study medication." Comment: All outcomes were reported to be self‐reported. Hospital admission is judged to be objective enough to allow for an unbiased assessment. Comment: Outcome measurement or ascertainment of the outcome are unlikely to differ between both groups. Comment: The primary analysis reported in the paper agrees with that of the protocol. Comment: Numerical results are unlikely to be selected from multiple outcome measures or analyses. Outcomes re sufficiently different and details are reported for each measurement separately. |
Risk of bias for analysis 1.3 Serious adverse events.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Lenze 2020 | Low risk of bias | Quote: "Patients were randomized 1:1 to fluvoxamine or matching placebo capsules. Randomization schedules were generated that stratified by age (18‐44, 45‐54, 55‐64, and ≥65 years)16 and sex. Treatments were randomly allocated using alternating block sizes of 2 and 4. Randomization allocation was conducted via REDCap, which displayed randomization assignment to the laboratory manager (J.S.), who prepared the study materials, including the study drug or placebo. All outcome assessors, investigators, and research staff who were in contact with participants were blinded to participant treatment assignment." "Participants were well‐matched in demographic and clinical characteristics. The baseline oxygen saturation level did not differ between the groups." | Low risk of bias | Placebo controlled trial. All outcome assessors, investigators, and research staff who were in contact with participants were blinded to participant treatment assignment. There is a substantial number of participants who did not complete the study. There were 18 out of 80 (22.5%) in the intervention group and 19 out of 72 (26.4%) in the placebo group. Quote: "the data appeared to be missing at random and no data imputation was conducted" Comment: For missing data, the researchers compared available scores with missing scores to check if the missing scores were non‐random. |
High risk of bias | 37 study participants did not complete the study. 18 patients in the intervention group and 19 patients in the placebo group did not complete the study.
No evidence to support that the results were not bias by missing outcome data. Outcome data could potentially be missing when participants experienced serious adverse events. |
High risk of bias | Quote: "Adverse events and serious adverse events were recorded each day via participant self‐report for 15 days after randomization." Comment: This was a remote trial with minimal patient‐practitioner contact. (Serious) adverse events were self‐reported and not verified by practitioner or medical record. Differences in measurement and ascertainment of the outcome unlikely between groups. | Low risk of bias | The protocol states: "we will assess for adverse events (including serious adverse events) daily via participant self‐report during the first 15 days, and then again at the end of the study." | High risk of bias | Quote: "Patients were randomized 1:1 to fluvoxamine or matching placebo capsules. Randomization schedules were generated
that stratified by age (18‐44, 45‐54, 55‐64, and ≥65 years)16 and sex. Treatments were randomly allocated using alternating block sizes of 2 and 4. Randomization allocation was conducted
via REDCap, which displayed randomization assignment to the laboratory manager (J.S.), who prepared the study materials, including the study drug or placebo. All outcome assessors, investigators, and research staff who were in
contact with participants were blinded to participant treatment assignment." "Participants were well matched in demographic and clinical characteristics. The baseline oxygen saturation level did not differ between the groups." No, placebo controlled trial. All outcome assessors, investigators, and research staff who were in contact with participants were blinded to participant treatment assignment. There is a substantial number of participants who did not complete the study. There were 18 in intervention group and 19 in the placebo group. Quote: "the data appeared to be missing at random and no data imputation was conducted" Comment: For missing data, the researchers compared available scores with missing scores to check if the missing scores were non‐random. 37 study participants did not finish the study. 18 patients in the intervention group and 19 patients in the placebo group did not complete the study. No evidence to support that the results were not bias by missing outcome data. Outcome data could potentially be missing when participants experienced serious adverse events. The outcomes for the missing observations would have been significantly different from the final participants analysed. Quote: Adverse events and serious adverse events were recorded each day via participant self‐report for 15 days after randomization. Comment: This was a remote trial with minimal patient‐practitioner contact. (Serious) adverse events were self‐reported and not verified by practitioner or medical record. Comment: Differences in measurement and ascertainment of the outcome unlikely. The protocol states: "we will assess for adverse events (including serious adverse events) daily via participant self‐report during the first 15 days, and then again at the end of the study." |
| TOGETHER 2021 | Low risk of bias | Quote: "Participants were randomly assigned by means of a centralised core randomisation process handled by an independent unmasked pharmacist who was not aware of any protocol‐related procedures and contracted specifically for this process. Patients were randomly assigned (1:1) by means of a block randomisation procedure for each participating site, stratified by age (<50 years or ≥50 years)." Quote: "With respect to covariates of age, body‐mass index, and comorbidities, the groups were generally well balanced." | Low risk of bias | Quote: "The trial team, site staff, and patients were masked to treatment allocation. The active drugs and the placebo pills were packaged in identically shaped bottles and labelled with alphabet letters corresponding to the active group or placebo group." Comment: Patients were analysed according to randomization group with intention‐to treat analyses. |
Low risk of bias | Quote: "84 participants stopped fluvoxamine and 64 participants stopped placebo owing to issues of tolerability." Comment: Proportions of drop‐out are comparable between groups and < 12%. This proportion seems tolerable and is not considered to introduce bias to the results. | High risk of bias | Quote: "Study personnel collected outcome data on days 1, 2, 3, 4, 5, 7, 10, 14, and 28 in person or via telephone contact or social media applications using video‐teleconferencing. We collected outcome data irrespective of whether participants took study medication."; "All serious and non‐serious adverse events were reported to study personnel as per local regulatory requirements." Comment: The outcomes appears to also be self‐reported. Outcome measurement or ascertainment of the outcome are unlikely to differ in both groups. | Low risk of bias | Comment: The primary analysis reported in the paper agrees with that of the protocol. Numerical results are unlikely to be selected from multiple outcome measures or analyses. |
High risk of bias | Quote: "Participants were randomly assigned by means of a centralised core randomisation process handled by an independent unmasked pharmacist who was not aware of any protocol‐related procedures and contracted specifically for this process. Patients were randomly assigned (1:1) by means of a block randomisation procedure for each participating site, stratified by age (<50 years or ≥50 years)."
Quote: "With respect to covariates of age,
body‐mass index, and comorbidities, the groups were generally well balanced." Quote: "The trial team, site staff, and patients were masked to treatment allocation. The active drugs and the placebo pills were packaged in identically shaped bottles and labelled with alphabet letters corresponding to the active group or placebo group." Comment: Patients were analysed according to randomization group with intention‐to treat analyses. Quote: "84 participants stopped fluvoxamine and 64 participants stopped placebo owing to issues of tolerability." Comment: Proportions of drop‐out are comparable between groups and < 12%. This proportion seems tolerable and is not considered to introduce bias to the results. Quote: "Study personnel collected outcome data on days 1, 2, 3, 4, 5, 7, 10, 14, and 28 in person or via telephone contact or social media applications using video‐teleconferencing. We collected outcome data irrespective of whether participants took study medication." Comment: All outcomes were reported to be self‐reported. Comment: Outcome measurement or ascertainment of the outcome are unlikely to differ in both groups. Comment: The primary analysis reported in the paper agrees with that of the protocol. Comment: Numerical results are unlikely to be selected from multiple outcome measures or analyses. |
Risk of bias for analysis 1.4 Adverse events (any grade).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Lenze 2020 | Low risk of bias | Quote: "Patients were randomized 1:1 to fluvoxamine or matching placebo capsules. Randomization schedules were generated that stratified by age (18‐44, 45‐54, 55‐64, and ≥65 years)16 and sex. Treatments were randomly allocated using alternating block sizes of 2 and 4. Randomization allocation was conducted via REDCap, which displayed randomization assignment to the laboratory manager (J.S.), who prepared the study materials, including the study drug or placebo. All outcome assessors, investigators, and research staff who were in contact with participants were blinded to participant treatment assignment." "Participants were well‐matched in demographic and clinical characteristics. The baseline oxygen saturation level did not differ between the groups." | Low risk of bias | Placebo controlled trial. All outcome assessors, investigators, and research staff who were in
contact with participants were blinded to participant treatment assignment. Comment: For missing data, the researchers compared available scores with missing scores to check if the missing scores were non‐random. |
High risk of bias | 37 study participants did not finish the study. There were 18 out of 80 (22.5%) in the intervention group and 19 out of 72 (26.4%) in the placebo group. No evidence to support that the results were not bias by missing outcome data. The outcomes for the missing observations would have been significantly different from the final participants analysed. | High risk of bias | Quote: "Adverse events and serious adverse events were recorded each day via participant self‐report for 15 days after randomization." Comment: This was a remote trial with minimal patient‐practitioner contact. (Serious) adverse events were self‐reported and not verified by practitioner or medical record. Differences in measurement and ascertainment of outcome unlikely. | Low risk of bias | The protocol states: "we will assess for adverse events (including serious adverse events) daily via participant self‐report during the first 15 days, and then again at the end of the study." | High risk of bias | Quote: "Patients were randomized 1:1 to fluvoxamine or matching placebo capsules. Randomization schedules were generated
that stratified by age (18‐44, 45‐54, 55‐64, and ≥65 years)16 and sex. Treatments were randomly allocated using alternating block sizes of 2 and 4. Randomization allocation was conducted
via REDCap, which displayed randomization assignment to the laboratory manager (J.S.), who prepared the study materials, including the study drug or placebo. All outcome assessors, investigators, and research staff who were in
contact with participants were blinded to participant treatment assignment." "Participants were well matched in demographic and clinical characteristics. The baseline oxygen saturation level did not differ between the groups." No, placebo controlled trial. All outcome assessors, investigators, and research staff who were in contact with participants were blinded to participant treatment assignment. There is a substantial number of participants who did not complete the study. There were 18 in intervention group and 19 in the placebo group. Quote: " the data appeared to be missing at random and no data imputation was conducted" 37 study participants did not finish the study. 18 patients in the intervention group and 19 patients in the placebo group did not complete the study. No evidence to support that the results were not bias by missing outcome data. The outcomes for the missing observations would have been significantly different from the final participants analysed. Quote: Adverse events and serious adverse events were recorded each day via participant self‐report for 15 days after randomization. Comment: This was a remote trial with minimal patient‐practitioner contact. (Serious) adverse events were self‐reported and not verified by practitioner or medical record. Comment: Differences in measurement and ascertainment of outcome unlikely. In the protocol it states: "we will assess for adverse events (including serious adverse events) daily via participant self‐report during the first 15 days, and then again at the end of the study." |
| TOGETHER 2021 | Low risk of bias | Quote: "Participants were randomly assigned by means of a centralised core randomisation process handled by an independent unmasked pharmacist who was not aware of any protocol‐related procedures and contracted specifically for this process. Patients were randomly assigned (1:1) by means of a block randomisation procedure for each participating site, stratified by age (<50 years or ≥50 years)." Quote: "With respect to covariates of age, body‐mass index, and comorbidities, the groups were generally well balanced." | Low risk of bias | No, The trial team, site staff, and patients were masked to treatment allocation. The active drugs and the placebo pills were packaged in identically shaped bottles and labelled with alphabet letters corresponding to the active group or placebo group. Comment: Patients were analysed according to randomization group with intention‐to treat analysis. Quote: "We assessed time‐to‐event outcomes using Cox proportional hazard models and binary outcomes using logistic regression." |
Low risk of bias | Quote: "84 participants stopped fluvoxamine and 64 participants stopped placebo owing to issues of tolerability." Comment: Proportions of drop‐out are comparable between groups and < 12%. This proportion seems tolerable and is not considered to introduce bias to the results. | High risk of bias | Quote: "Study personnel collected outcome data on days 1, 2, 3, 4, 5, 7, 10, 14, and 28 in person or via telephone contact or social media applications using video‐teleconferencing. We collected outcome data irrespective of whether participants took study medication." Comment: All outcomes were reported to be self‐reported. Outcome measurement or ascertainment of the outcome are unlikely to differ between both groups. | Low risk of bias | Comment: The primary analysis reported in the paper agrees with that of the protocol. Numerical results are unlikely to be selected from multiple outcome measures or analyses. |
High risk of bias | Quote: "Participants were randomly assigned by means of a centralised core randomisation process handled by an independent unmasked pharmacist who was not aware of any protocol‐related procedures and contracted specifically for this process. Sites requested randomisation by text message to the pharmacist at the coordinating centre.
This maintained concealment of allocation. Patients were randomly assigned (1:1) by means of a block randomisation procedure for each participating site, stratified by age (<50 years or ≥50 years)." "Participants were well matched in demographic and clinical characteristics." The trial team, site staff, and patients were masked to treatment allocation. The active drugs and the placebo pills were packaged in identically shaped bottles and labelled with alphabet letters corresponding to the active group or placebo group. Comment: Patients were analysed according to randomization group with intention‐to treat analyses. Quote: "We assessed time‐to‐event outcomes using Cox proportional hazard models and binary outcomes using logistic regression." Quote: "84 participants stopped fluvoxamine and 64 participants stopped placebo owing to issues of tolerability." Comment: Proportions of drop‐out are comparable between groups and < 12%. This proportion seems tolerable and is not considered to introduce bias to the results. Quote: "Study personnel collected outcome data on days 1, 2, 3, 4, 5, 7, 10, 14, and 28 in person or via telephone contact or social media applications using video‐teleconferencing. We collected outcome data irrespective of whether participants took study medication." Comment: All outcomes were reported to be self‐reported. Outcome measurement or ascertainment of the outcome are unlikely to differ both groups. The primary analysis reported in the paper agrees with that of the protocol. Numerical results are unlikely to be selected from multiple outcome measures or analyses. |
Overall risk of bias by study
We assessed both studies to have an overall high risk of bias (Lenze 2020; TOGETHER 2021), mainly due to concerns with the outcome measurement (mostly self‐reported outcomes collected remotely), and due to missing participant data. In Lenze 2020, 20% of study participants stopped responding to surveys during the 15‐day trial. The possibility that some of these participants (n = 6) received care at an urgent care centre outside the trial could not be excluded. For 31 participants, it was confirmed that they did not receive any medical care for worsening of COVID‐19.
Overall risk of bias by outcome
The following section summarises the risk of bias per outcome for all primary outcomes included in the summary of findings table (Table 1.)
We have no concerns regarding the risk of bias across studies for the outcome 'all‐cause mortality at day 28'. We identified a high risk of bias due to missing outcome data for one trial that contributed data to this outcome (18 out of 80 (22.5%) in the intervention group and 19 out of 72 (26.4%) in the placebo group did not complete the study and reasons for missing data were not provided) (Lenze 2020). However, this study did not impact the result of the analysis (zero event study, Analysis 1.1), so we based the bias summary on TOGETHER 2021, where the dropout rate was less than 12%.
1.1. Analysis.

Comparison 1: Fluvoxamine plus standard care compared to placebo plus standard care for outpatients with mild COVID‐19, Outcome 1: All‐cause mortality (at day 28)
We also have no concerns regarding risk of bias for the outcome 'admission to hospital and death' reported by one study (TOGETHER 2021). Although hospital admission rates may be more frequent in trials with a remote patient assessment, particularly when patients are diagnosed with an infectious disease, and direct (face‐to‐face) contact with a clinician is lacking, the randomised allocation of participants should balance this issue between study arms.
For 'serious adverse events' and 'adverse events', we also identified a high risk of bias due to the measurement of the outcome. All (serious and non‐serious) adverse events appear to be self‐reported, and it is unclear whether they were verified by medical records or practitioner reports, which may be associated with an under‐ or overestimation of such events. Furthermore, the results could be impacted due to missing participant data, particularly in the study of Lenze 2020.
Effects of interventions
See: Table 1
on time to ssssAs we did not include any studies investigating efficacy and safety of fluvoxamine in the inpatient setting, this section effectively only addresses the outpatient setting.
Primary outcomes are displayed in the summary of findings table (Table 1 'Fluvoxamine plus standard care compared to placebo plus standard care for outpatients with mild COVID‐19').
We included two placebo‐controlled studies in both qualitative synthesis and quantitative synthesis (meta‐analysis) of this review (Lenze 2020; TOGETHER 2021). Both studies investigated outpatients with confirmed mild COVID‐19.
We did not conduct sensitivity analysis regarding the risk of bias, since only one study had a low risk of bias for the outcome 'all‐cause mortality' (TOGETHER 2021). Lenze 2020 did not report any events reported for this outcome. So the study with a low risk of bias contributed 100% to the weight of the meta‐analysis.
There were no indications of data not missing at random, so predefined analyses for such cases were not applicable.
In the current review, there are not enough studies available to perform the planned subgroup analyses to investigate heterogeneity for study characteristics (such as differing doses and severity of the disease).
Primary outcomes
All‐cause mortality at day 28
Both studies reported on all‐cause mortality either at day 15 (Lenze 2020) or day 28 (TOGETHER 2021). Fluvoxamine may slightly reduce all‐cause mortality (RR 0.69, 95% CI 0.38 to 1.27; 1649 participants; RD 9 per 1000; Analysis 1.1). While no deaths were recorded in either the treatment group or placebo group at day 15 (Lenze 2020), at day 28 the risk with fluvoxamine and standard care was 21 per 1000 and the risk in the placebo group was 30 per 1000 (TOGETHER 2021). Certainty of the evidence for this outcome was low. While we did not downgrade for risk of bias (as the study with a high risk of bias had a weight of 0% (Lenze 2020), we did downgrade for very serious imprecision because the 95% CI suggests both potential benefit and no effect or potential harm, and the number of events was small.
All‐cause admission to a hospital or death (before hospital admission)
Both studies reported data on admission to hospital or death within 28 days for participants with symptomatic COVID‐19. Fluvoxamine may reduce admission to hospital or death (RR 0.55, 95% CI 0.16 to 1.89; 1649 participants; RD 57 per 1000; Analysis 1.2). Certainty of the evidence for this outcome was low. We downgraded this by two levels due to concerns of very serious imprecision because the 95% CI suggests both potential benefit and no effect or potential harm, and the number of events was small.
1.2. Analysis.

Comparison 1: Fluvoxamine plus standard care compared to placebo plus standard care for outpatients with mild COVID‐19, Outcome 2: All‐cause hospital admission or death (before hospital admission)
Symptom resolution
Neither of the studies reported numerical data for this outcome. One study illustrated data in a graph that indicated an overlap of confidence intervals (TOGETHER 2021).
Quality of life
Neither of the studies reported this outcome.
Serious adverse events
Both studies reported data for this outcome (Lenze 2020; TOGETHER 2021). There is very low‐certainty evidence on fluvoxamine regarding the number of participants with serious adverse events during the study period (RR 0.56, 95% CI 0.15 to 2.03; 1649 participants; RD 54 per 1000; Analysis 1.3). We downgraded the certainty of the evidence by three levels: by one level for serious concerns due to high risk of bias (measurement of the outcome), and by two levels for very serious concerns due to imprecision. The 95% CI includes a potential benefit and harm.
1.3. Analysis.

Comparison 1: Fluvoxamine plus standard care compared to placebo plus standard care for outpatients with mild COVID‐19, Outcome 3: Serious adverse events
Any adverse events (any grade)
The studies of Lenze 2020 and TOGETHER 2021 also reported data for any adverse events. There is very low‐certainty evidence on fluvoxamine regarding adverse events of any grade during the short‐term follow‐up (RR 1.06, 95% CI 0.82 to 1.37; 1649 participants; RD 7 per 1000; Analysis 1.4). We downgraded the certainty of the evidence by three levels: by one level for serious concerns due to high risk of bias (measurement of the outcome), and by two levels for very serious concerns due to imprecision. The 95% CI includes a potential benefit and harm.
1.4. Analysis.

Comparison 1: Fluvoxamine plus standard care compared to placebo plus standard care for outpatients with mild COVID‐19, Outcome 4: Adverse events (any grade)
Suicide or suicide attempt
None of the included studies reported this outcome.
Secondary outcomes
The secondary outcomes reported included 'clinical status' at day 15, 28 or the longest follow‐up and 'viral clearance'. Clinical status was measured in one study each as 'need for invasive mechanical ventilation', 'need for non‐invasive mechanical ventilation or high flow' and 'need for hospitalisation with oxygen therapy'. Quantitative data for the secondary outcomes are listed in Table 3.
2. Fluvoxamine plus standard care compared to placebo plus standard care for outpatients with mild COVID‐19.
| Outcomes | Effect estimate (95% CI) | N of participants (studies) |
Fluvoxamine n/N |
Placebo n/N |
Measures of heterogeneity | Comment |
| Need for invasive mechanical ventilation | RR: 0.77 (0.45 to 1.30) |
1497a(1) | 26/741 (3,5%) |
34/756 (4,5%) |
NA |
TOGETHER 2021 no significant difference |
| Need for non‐invasive mechanical ventilation or high flow (ventilator support) | AD: 0.00 (‐8.41 to 0.49) |
152 (1) | 0/80 (0%) |
0/72 (0%) |
NA |
Lenze 2020 no significant difference |
| Need for hospitalisation (with oxygen therapy by masks or nasal prongs) | AD: ‐4.21 (‐13.22 to 2.04) |
152 (1) | 0/80 (0%) |
3/72 (4.2%) |
NA |
Lenze 2020 no significant difference |
| Viral clearance (assessed with RT‐PCR) at day 7 | OR: 0.67 (0.42 to 1.06) |
428b (1) | 40/207 (19%) |
58/221 (26%) |
NA |
TOGETHER 2021 no significant difference |
AD: absolute difference; n: number of events; N: number of participants; NA: not applicable; OR: odds ratio; RR: relative risk; RT‐PCR: reverse transcription‐polymerase chain reaction
a Number of participants hospitalised. b Number of participants available for outcome assessment.
Need for invasive mechanical ventilation
TOGETHER 2021 reported that 26 of 741 fluvoxamine treated patients versus 34 of 756 participants in the placebo group needed mechanical ventilation (RR 0.77, 95% CI 0.45 to 1.30; 1497 participants; RD 10 per 1000; Table 3).
Need for non‐invasive mechanical ventilation or high flow
Lenze 2020 reported that none of the 80 fluvoxamine treated participants versus three of 72 participants in the placebo group required supplemental oxygen plus ventilator support for three days or more (absolute difference 0.00, 95% CI ‐8.41 to 0.49; 152 participants; RD 0 per 1000; Table 3). This outcome was assessed with a 7‐point scale.
Need for hospitalisation with oxygen therapy by masks or nasal prongs
Lenze 2020 also reported that none of the 80 participants treated with fluvoxamine versus three of 72 participants in the placebo group needed hospitalisation and required supplemental oxygen therapy by masks or nasal prongs (absolute difference ‐4.21, 95% CI ‐13.22 to 2.04; 152 participants; RD 42 per 1000; Table 3). Again, this outcome was assessed with a 7‐point scale.
Viral clearance (assessed with PCR) at day 7
TOGETHER 2021 reported that 40 of 207 fluvoxamine treated patients versus 58 of 221 participants in the placebo group showed successful viral clearance at day seven (OR 0.67, 95% CI 0.42 to 1.06; 428 participants; RD 70 per 1000; Table 3).
Discussion
Summary of main results
The aim of this review was to investigate the efficacy and safety of fluvoxamine in people with COVID‐19. We identified two RCTs including more than 1500 non‐hospitalised people (outpatients) with symptomatic COVID‐19 (Lenze 2020; TOGETHER 2021). We did not find any RCTs that investigated asymptomatic people or those hospitalised with COVID‐19 who were being treated with fluvoxamine.
Both included studies evaluated composite endpoints including clinical worsening according to the definition of the respective study and retention in a COVID‐19 emergency setting or inpatient setting. From the prioritised primary endpoints of this review, the studies reported all‐cause mortality at day 28 (Lenze 2020; TOGETHER 2021), admission to hospital (all cause) or death (before hospital admission) (TOGETHER 2021), serious adverse events (Lenze 2020; TOGETHER 2021) and adverse events (any grade) (Lenze 2020; TOGETHER 2021). Furthermore, the studies also reported data for the secondary outcomes: need for mechanical ventilation (TOGETHER 2021), need for non‐invasive mechanical ventilation or high flow oxygen supplementation (Lenze 2020), need for hospitalisation with need for oxygen therapy (by masks or nasal prongs) (Lenze 2020), and viral clearance at day 7 (TOGETHER 2021).
Our analyses show that fluvoxamine compared to placebo (both in addition to standard care) may slightly reduce all‐cause mortality at day 28 (RR 0.69, 95% CI 0.38 to 1.27; 2 studies, 1649 participants; low‐certainty evidence) and may reduce the number of people admitted to a hospital or who died beforehand (RR 0.55, 95% CI 0.16 to 1.89; 2 studies, 57 participants; low‐certainty evidence). The number of serious adverse events did not clearly differ between fluvoxamine and placebo, and we are very uncertain about the effect of fluvoxamine on serious adverse events during the study period (RR 0.56, 95% CI 0.15 to 2.03; 2 studies, 1649 participants; very low‐certainty evidence). Likewise, we are very uncertain about the effect of fluvoxamine on adverse events of any grade (RR 1.06, 95% CI 0.82 to 1.37; 2 studies, 1649 participants; very low‐certainty evidence). Although TOGETHER 2021 mentioned that there was no difference in time to symptom resolution between the groups, we could not derive any numerical data from this study and contacting the authors was unsuccessful. Neither study reported on quality of life or suicide and suicide attempt.
Overall completeness and applicability of evidence
The evidence summarised in this review applies to the use of fluvoxamine in symptomatic COVID‐19 outpatients. It is mainly restricted to unvaccinated people at high‐risk and the virus variants of concern that were present at the time of the conducted trials (up to August 2021). We did not identify any randomised trials for inpatients with moderate, severe and critical COVID‐19, or any studies reporting on fluvoxamine versus other active pharmacological comparators with proven effectiveness.
One small study was conducted in a single geographical area of the USA, with recruitment between April and August 2020 (the first phase of the pandemic) (Lenze 2020). The other, much larger, study was conducted in high‐risk people in Brazil and recruitment took place between January and August 2021 (TOGETHER 2021). The recruitment period in Lenze 2020 was before the start of the vaccination campaign, and in TOGETHER 2021 only 6% of recruited participants reported one dose of a COVID‐19 vaccine at the end of the trial. Taking into account that widespread vaccination programmes have now become established in some areas, it is very unclear whether the results of the studies can be transferred to a predominantly immunised population in case of a breakthrough infection.
Particularly for immunocompromised people, who have a relevant risk of an inadequate immune response to vaccination, an effective treatment reducing disease progression would be clinically important. However, immunosuppression was an exclusion criterion in Lenze 2020, and in TOGETHER 2021 the number of immunosuppressed participants was unclear. Older people were also underrepresented in both studies: the median age of participants was 46 years (intervention arm) and 45 years (control arm) in Lenze 2020, and 50% of participants in the TOGETHER 2021 trial were younger than 50 years old. Because the focus of the study of TOGETHER 2021 was people at high‐risk, the applicability of our findings for subgroups including the immunocompromised or older people must be applied with caution.
Since recruitment in TOGETHER 2021 was based on the results of rapid SARS‐CoV‐2 antigen testing, diagnostic uncertainty may be another limitation in the interpretation of the results of this study. However, the concordance of positive COVID‐19 rapid antigen tests with RT‐PCR was evaluated in a group of participants who underwent PCR testing. A concordance rate of greater than 99% for both tests collected at baseline was found.
Furthermore, the changing dynamics in the development of virus variants, with possible limitations on the effectiveness of antiviral therapies, needs to be considered when interpreting these results. There are no studies to date that evaluate the efficacy of fluvoxamine in the presence of virus variants of concern. Furthermore, we currently we do not know how immunomodulators will be affected by viral variants.
Certainty of the evidence
We assessed the certainty of evidence for primary outcomes presented in the Table 1 according to Schünemann 2020, and this ranged from low to very low. We found all‐cause mortality and admission to hospital, or death prior to hospital admission, to have low‐certainty evidence. The certainty of evidence for the outcomes ‘serious adverse events’ and ‘adverse events of any grade’ was very low. Downgrading was due to very serious imprecision (because the 95% CI suggests both potential benefit and no effect or potential harm) and a serious risk of bias (because of concerns in measurement of the outcome 'adverse events' which were self‐reported in both studies). Although our findings are mainly restricted to unvaccinated persons and virus variants of concern that were present up to August 2021, we did not downgrade due to indirectness. The reason for not downgrading was due to the ongoing and still dynamic pandemic. Judging the effectiveness of current anti‐COVID‐19 therapies against emerging variants poses an enormous challenge, as has been seen with the assessment of vaccine effectiveness over the last year (Altmann 2021).
In the current phase of the pandemic, it is also impossible to reliably assess the risk of publication bias. There are registered studies that are ongoing. We will follow the publication and trial history of each ongoing study, including those awaiting classification. We do not suspect any current publication bias to be present, for any of the outcomes. However, this may change in future updates of this review.
Potential biases in the review process
To avoid potential bias in the review process, we committed ourselves to conducting this systematic review according to published guidance provided by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020b).
Furthermore, this review is part of a Cochrane Living Systematic Reviews Series on different interventions for treatment of COVID‐19 (Ansems 2021; Kreuzberger 2021; Popp 2021a; Popp 2021b; Stroehlein 2021; Wagner 2021). Initially, a common core outcome set was established for all reviews in accordance with the Core Outcome Measures in Effectiveness Trials (COMET) Initiative for people with COVID‐19 (COMET 2021; Marshall 2020). These outcomes are continuously discussed and modified as necessary, taking into account the dynamics of the pandemic.
Although we did not exclude preprint studies, this review contains data from peer‐reviewed journal publications only. We contacted study authors if the publication included unclear or missing information. Details of communication with authors are provided in Table 2.
Two trials are classified as awaiting classification either due to inconclusive information regarding outcome measures related to the language of the study (trial in Persian language in an inpatient setting), or because the study was stopped for futility by a data safety monitoring board and results have not been published (trial in an inpatient setting). We will closely monitor these trials for further publications in the near future.
None of the authors has an affiliation with a stakeholder group that favours or disapproves of any selective serotonin reuptake inhibitors, or any of the comparators used in relevant studies.
Agreements and disagreements with other studies or reviews
We have identified two published reviews dedicated solely to the efficacy and safety of fluvoxamine in the treatment of COVID‐19 (Kacimi 2021; Lee 2021). Only one review, published as a preprint, analysed randomised trials (Lee 2021), while Kacimi 2021 also considered observational studies.
Lee 2021 came to similar conclusions as our review. However, that review included three randomised trials. Besides the study of TOGETHER 2021 and Lenze 2020, the review considered a third completed, but not published, trial (NCT04668950). The authors stated that they obtained outcome data directly from the principal investigators (who were co‐authors of the cited review).
Kacimi 2021 (a preprint labelled as a living systematic review) also focused on fluvoxamine and included two randomised trials and one observational study. The authors concluded that repurposing of fluvoxamine for the treatment of people with COVID‐19 had not shown any efficacy in reducing the mortality rate or the rate of progress to mechanical ventilation. Although the authors described a reduced risk of hospitalisation amongst people with COVID‐19, they stressed that there was still an urgent need for more clinical studies to determine the extent of the effectiveness of fluvoxamine, and to know more about its mechanism of action in people with COVID‐19.
There are several reviews analysing fluvoxamine as one of the therapeutics for COVID‐19.
A systematic review by Murchu 2022 focused on differing oral interventions (including fluvoxamine, bamlanivimab as monotherapy and combined with etesevimab, casirivimab plus imdevimab, ivermectin, nitazoxanide, and peginterferon lambda) in the outpatient setting to prevent progression to severe disease in people with COVID‐19. Although this review did not include the large TOGETHER trial on fluvoxamine (TOGETHER 2021), and most findings of the evaluated medications were based on single study results, the authors concluded that promising results in relation to clinical deterioration (defined by dyspnoea or hospitalisation), oxygen saturation (on room air) or need for supplemental oxygen (in the ambulatory setting) are available.
Wen 2022 conducted a meta‐analysis to investigate the effectiveness of fluvoxamine and other oral antivirals (molnupiravir and nirmatrelvir/ritonavir). The authors included both randomised and non‐randomised trials and concluded that each oral medication assessed was effective in reducing mortality and hospitalisation rates in people with COVID‐19. In addition, they found that the risk of adverse events did not increase whilst taking medication compared to placebo or standard care. Taking into account that no bias assessment had been conducted, and the study results were combined without differentiating between inpatient and outpatient settings, these findings needed to be interpreted with caution.
Based on their review of trials, including two RCTs which had the greatest impact on the Panel's recommendation (Lenze 2020; TOGETHER 2021), the U.S. National Institutes of Health COVID‐19 Treatment Guidelines Panel stated that there is insufficient evidence to recommend either for or against the use of fluvoxamine in the treatment of COVID‐19 (National Institutes of Health 2022).
Although there are some published reviews on fluvoxamine as early treatment for COVID‐19, none fulfilled all the methodological standards for evidence syntheses, and they did not apply the GRADE approach for rating the certainty of the evidence. But, overall, the findings of these reviews are in alignment with our review, indicating that the current evidence for fluvoxamine is limited, especially considering that only two randomised controlled trials currently exist.
Authors' conclusions
Implications for practice.
Based on the current evidence, the use of fluvoxamine plus standard care in comparison to standard care plus placebo in outpatients with mild COVID‐19 may reduce slightly all cause mortality (at day 28) and may reduce admission to hospital or death. However, we are very uncertain regarding the effect on serious adverse events. These findings are restricted to unvaccinated symptomatic outpatients and virus variants of concern that were present at the time that the trials were conducted. There were no numerical data currently available for other prioritised outcomes, including symptom resolution, quality of life, and suicide. No completed studies were identified that focused on inpatients, hence no statements could be made on this aspect of fluvoxamine use.
Implications for research.
Effective pharmacological therapies to treat COVID‐19 in the outpatient setting are important to reduce the risk of progression to severe disease in people with COVID‐19, and reduce the need for hospital admission. Trials considering both prioritised outcomes, such as quality of life, and current vaccination status, as well as older populations, women who are pregnant or breastfeeding, or people who have a weakened immune system are needed to further investigate the role of fluvoxamine in the treatment of COVID‐19. Furthermore, it would be important to establish the efficacy and safety of fluvoxamine as compared to other active pharmacotherapies targeting novel COVID‐19 variants, and whether closely related selective serotonin reuptake inhibitors could be used interchangeably.
Risk of bias
Acknowledgements
This work is part of a series of reviews investigating treatments and therapies for COVID‐19 as part of the project CEOsys. We thank the authors of the first published reviews of this series for providing and sharing some information in the background (particularly in the section 'Why it is important to do this review') and thank the Cochrane Haematology working group for using the template for the description of methods.
The research was part of a project supported by the German Federal Ministry of Education and Research (NaFoUniMedCovid19, funding number: 01KX2021; part of the project ‘CEOSys’). The contents of this document reflect only the authors' views, and the German Ministry is not responsible for any use that may be made of the information it contains.
Cochrane Haematology supported the authors in the development of this review. Ina Monsef is a member of Cochrane Haematology but was not involved in the editorial process or decision‐making for this review.
The following people conducted the editorial process for this article:
Sign‐off Editor (final editorial decision): Robert Boyle, Imperial College London, UK; Co‐ordinating Editor of the Cochrane Skin Group
Managing Editor (selected peer reviewers, provided comments, collated peer‐reviewer comments, provided editorial guidance to authors, edited the article): Lara Kahale; Cochrane Central Editorial Service
Editorial Assistant (conducted editorial policy checks and supported editorial team): Leticia Rodrigues, Cochrane Central Editorial Service
Copy Editor (copy‐editing and production): Andrea Takeda (Central Production Service)
Peer‐reviewers (provided comments and recommended an editorial decision): Kenji Hashimoto, Chiba University, Japan (clinical); Eileen Doyle, PharmD (clinical); Robert Walton, Senior Fellow in General Practice, Cochrane UK (clinical); Liz Doney, Cochrane Skin University of Nottingham (search); Robin Featherstone, Cochrane Central Editorial Service (search); Stella Maria O'Brien (consumer); and Nuala Livingstone, Associate editor, Cochrane Evidence Production and Methods Directorate (methods).
One additional peer reviewer provided clinical peer review, but chose not to be publicly acknowledged.
Appendices
Appendix 1. Search strategies
Cochrane COVID‐19 Study Register
fluvoxamin* or luvox* or floxyfral* or fevarin* or faverin* or fluvoxadura* or dumirox* or desiflu*
Web of Science (Core Collection) – Science Citation Index and Emerging Sources Citation Index
#1 TI=((COVID OR COVID19 OR "SARS‐CoV‐2" OR "SARS‐CoV2" OR SARSCoV2 OR "SARSCoV‐2" OR "SARS coronavirus 2" OR "2019 nCoV" OR "2019nCoV" OR "2019‐novel CoV" OR "nCov 2019" OR "nCov 19" OR "severe acute respiratory syndrome coronavirus 2" OR "novel coronavirus disease" OR "novel corona virus disease" OR "corona virus disease 2019" OR "coronavirus disease 2019" OR "novel coronavirus pneumonia" OR "novel corona virus pneumonia" OR "severe acute respiratory syndrome coronavirus 2"))) OR AB=((COVID OR COVID19 OR "SARS‐CoV‐2" OR "SARS‐CoV2" OR SARSCoV2 OR "SARSCoV‐2" OR "SARS coronavirus 2" OR "2019 nCoV" OR "2019nCoV" OR "2019‐novel CoV" OR "nCov 2019" OR "nCov 19" OR "severe acute respiratory syndrome coronavirus 2" OR "novel coronavirus disease" OR "novel corona virus disease" OR "corona virus disease 2019" OR "coronavirus disease 2019" OR "novel coronavirus pneumonia" OR "novel corona virus pneumonia" OR "severe acute respiratory syndrome coronavirus 2")
#2 (TI=((fluvoxamin* or luvox* or floxyfral* or fevarin* or faverin* or fluvoxadura* or dumirox* or desiflu*))) OR AB=((fluvoxamin* or luvox* or floxyfral* or fevarin* or faverin* or fluvoxadura* or dumirox* or desiflu*))
#3 #1 AND #2
WHO COVID‐19 Global literature on coronavirus disease
(fluvoxamin* or luvox* or floxyfral* or fevarin* or faverin* or fluvoxadura or dumirox* or desiflu*)
Data and analyses
Comparison 1. Fluvoxamine plus standard care compared to placebo plus standard care for outpatients with mild COVID‐19.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause mortality (at day 28) | 2 | 1649 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.38, 1.27] |
| 1.2 All‐cause hospital admission or death (before hospital admission) | 2 | 1649 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.16, 1.89] |
| 1.3 Serious adverse events | 2 | 1649 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.15, 2.03] |
| 1.4 Adverse events (any grade) | 2 | 1649 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.82, 1.37] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Lenze 2020.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
TOGETHER 2021.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
COVID‐19: coronavirus disease 2019; RT‐PCR: reverse transcription‐polymerase chain reaction; SARS‐CoV‐2: Severe Acute Respiratory Syndrome Coronavirus 2
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Assanovich 2021 | Ineligible study design: a description of properties of fluvoxamine |
| Brown University 2021 | Ineligible study design: not original study publication, rather a commentary on how fluvoxamine may limit deterioration from COVID‐19 |
| Glebov 2021 | Ineligible study design: a highlight of protective effect of fluvoxamine antidepressant against COVID‐19, highlighting therapeutic and prophylactic potential |
| Hashimoto 2021 | Ineligible study design: a commentary on mechanisms of action of fluvoxamine for COVID‐19 |
| Khosravi 2022 | Ineligible study design: a narrative review |
| Marcec 2021 | Ineligible study design: description of fluvoxamine mechanisms of action and potential use in COVID‐19 treatment |
| McCarthy 2021 | Ineligible study design: paper reviewed the pleiotropic properties of fluvoxamine and explored how the drug may be used to treat the inflammatory sequelae of COVID‐19 in the future |
| Medical Letter 2021 | Ineligible study design: a commentary on properties of fluvoxamine |
| Murchu 2021 | Ineligible study design: not an original publication |
| NCT04711863 | Suspended Trial: trial was suspended due to closure of the main treatment centre |
| Seftel 2021 | Ineligible study design: a prospective cohort study in real‐world experience using fluvoxamine |
| Sukhatme 2021 | Ineligible study design: description of fluvoxamine mechanisms of action and potential use in COVID‐19 treatment |
Characteristics of studies awaiting classification [ordered by study ID]
NCT04668950.
| Methods |
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g. type, dose, route of administration)
|
| Outcomes | Primary study outcomes
Relevant study outcomes planned
Secondary outcomes
|
| Notes |
|
Safa 2020.
| Methods |
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention of relevant arms
Treatment details of control group (e.g. type, dose, route of administration)
|
| Outcomes | Primary study outcomes
|
| Notes |
|
COVID‐19: coronavirus disease 2019; SARS‐CoV‐2: Severe Acute Respiratory Syndrome Coronavirus 2; SSRIs: selective serotonin reuptake inhibitors
Characteristics of ongoing studies [ordered by study ID]
NCT04510194.
| Study name | COVID‐OUT: Early Outpatient Treatment for SARS‐CoV‐2 Infection (COVID‐19) |
| Methods |
|
| Participants | Inclusion Criteria
Exclusion Criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g. dose, route of administration)
|
| Outcomes |
|
| Starting date | 1 January 2021 |
| Contact information | covidout@umn.edu |
| Notes |
|
NCT04718480.
| Study name | Fluvoxamine Administration in Moderate SARS‐CoV‐2 (COVID‐19) Infected Patients |
| Methods |
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g. dose, route of administration)
|
| Outcomes |
|
| Starting date | 18 January 2021 |
| Contact information | andrea.fekete@sigmadrugs.com |
| Notes |
|
NCT04885530.
| Study name | ACTIV‐6: COVID‐19 Outpatient Randomized Trial to Evaluate Efficacy of Repurposed Medications |
| Methods |
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g. dose, route of administration)
|
| Outcomes |
|
| Starting date | 13 May 2021 |
| Contact information | sybil.wilson@duke.edu, and april.ray@duke.edu |
| Notes |
|
NCT05087381.
| Study name | Randomized‐controlled Trial of the Effectiveness of COVID‐19 Early Treatment in Community |
| Methods |
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g. dose, route of administration)
|
| Outcomes |
|
| Starting date | 21 October 2021 |
| Contact information | Dhammika.L@chula.ac.th, Phatthranit.pha@mahidol.edu |
| Notes |
|
TCTR20210615002.
| Study name | Effect of Combined Fluvoxamine with Favipiravir versus Favipiravir Monotherapy in Prevention of Clinical Deterioration among mild to moderate COVID‐19 patients Monitoring by Telemedicine in Virtual Clinic |
| Methods |
|
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Details of intervention
Treatment details of control group (e.g. dose, route of administration)
|
| Outcomes |
|
| Starting date | 16 June 2021 |
| Contact information | taweegrit.sir@pccms.ac.th. |
| Notes |
|
COVID‐19: Coronavirus Disease 2019; JAK: Janus Kinase; PCR: Polymerase Chain Reaction; SARS‐CoV‐2: Severe Acute Respiratory Syndrome Coronavirus 2
Differences between protocol and review
There were no changes between the initial review protocol and the final review.
Contributions of authors
John Nyirenda (JN)*: search and selection of studies for inclusion in the review; collection of data for the review; assessment of the risk of bias in the included studies; analysis of data; assessment of the certainty in the body of evidence; writing and proofreading of the review.
Mario Sofroniou (MSo)*: conception of the review; interpretation of data; writing and proofreading of the review.
Ingrid Toews (IT): conception of the review; search and selection of studies for inclusion in the review; collection of data for the review; assessment of the risk of bias in the included studies; assessment of the certainty in the body of evidence, writing and proofreading the review.
Agata Mikolajewska (AMi): conception of the review; interpretation of data; writing and proofreading of the review.
Cornelius Lehane (CL): interpretation of data and proofreading the review.
Ina Monsef (IM): search strategy design; running the searches and writing the review.
Aesha Abu‐taha (AA): search and selection of studies for inclusion in the review.
Andy Maun (AMa): conception of the review; interpretation of data; writing and proofreading of the review.
Miriam Stegemann (MSt): conception of the review; interpretation of data; writing and proofreading of the review.
Christine Schmucker (CS): conception of the review; design of the review; co‐ordination of the review; collection of data for the review; assessment of the risk of bias in the included studies; analysis of data; assessment of the certainty in the body of evidence; interpretation of data; writing of the review.
*Shared first authorship.
Sources of support
Internal sources
-
Charité –University Berlin, Germany, Germany
Department of Infectious Diseases and Respiratory Medicine, corporate member of Freie Universität Berlin and Humboldt‐Universität zu Berlin.
-
Medical Center ‐ University of Freiburg, Germany
Institute for Evidence in Medicine (for Cochrane Germany Foundation)
External sources
-
Federal Ministry of Education and Research, Germany
Development, synthesis and translation of evidence in the COVID‐19 pandemic (01KA2022).
Declarations of interest
John Nyirenda (JN): has no known conflicts of interest to declare.
Mario Sofroniou (MSo): has no known conflicts of interest to declare.
Ingrid Toews (IT): has no known conflicts of interest to declare.
Agata Mikolajewska (AMi): is affiliated with not‐for‐profit organisation: Coordination of Section COVRIIN and Work in Office of STAKOB (Competence and Treatment Centres for high consequence infectious diseases) at Robert Koch Institute Centre for Biological Threats and Special Pathogens (ZBS), Section Clinical Management and Infection Control.
Cornelius Lehane (CL): has no known conflicts of interest to declare.
Ina Monsef (IM): has no known conflicts of interest to declare. She is part of the Cochrane Haematology editorial team, but was not involved in the editorial process of this review.
Aesha Abu‐taha (AA): has no known conflicts of interest to declare.
Andy Maun (AMa) is a member of the guideline group 'Covid 19, Ambulatory Treatment', member of the German College of General Practitioners and Family Physicians, and affiliated with the Practice Kreusel/Höltner, Titisee‐Neustadt, Germany (employed as a General Practitioner).
Miriam Stegemann (MSt): has no known conflicts of interest to declare.
Christine Schmucker (CS): has no known conflicts of interest to declare.
Shared first authorship
New
References
References to studies included in this review
Lenze 2020 {published data only}
- Lenze EJ, Mattar C, Zorumski CF, Stevens A, Schweiger J, Nicol GE, et al. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. Journal of the American Medical Association 2020;324(22):2292-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04342663. A double-blind, placebo-controlled clinical trial of fluvoxamine for symptomatic individuals with COVID-19 infection. clinicaltrials.gov/ct2/show/NCT04342663 (first received 13 April 2020).
TOGETHER 2021 {published data only}
- NCT04727424. Repurposed approved and under development therapies for patients with early-onset COVID-19 and mild symptoms. www.clinicaltrials.gov/ct2/show/NCT04727424 (first received 27 January 2021).
- Reis G, Dos Santos Moreira-Silva EA, Silva DC, Thabane L, Milagres AC, Ferreira TS, et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalisation among patients with COVID-19: the TOGETHER randomised, platform clinical trial. Lancet Global Health 2021;10(1):e42-e51. [DOI: 10.1016/S2214-109X(21)00448-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Assanovich 2021 {published data only}
- Assanovich M. Fluvoxamine in the treatment of patients with COVID-19. Psychiatry, Psychotherapy and Clinical Psychology 2021;12(2):260-8. [Google Scholar]
Brown University 2021 {published data only}
- Anonymous. Study suggests fluvoxamine may limit deterioration from COVID‐19. Brown University Psychopharmacology Update 2021;32(4):6-7. [Google Scholar]
Glebov 2021 {published data only}
- Glebov OO. Low-dose fluvoxamine modulates endocytic trafficking of SARS-CoV-2 spike protein: a potential mechanism for anti-COVID-19 protection by antidepressants. Frontiers in Pharmacology 2021;12:787261. [DOI: 10.3389/fphar.2021.787261] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hashimoto 2021 {unpublished data only}
- Hashimoto Y, Suzuki T, Hashimoto K. Old drug fluvoxamine, new hope for COVID-19. European archives of psychiatry and clinical neuroscience 2021;2:1-3. [DOI: 10.1007/s00406-021-01326-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khosravi 2022 {published data only}
- Khosravi M. Candidate Psychotropics against SARS - CoV-2: a narrative review. Pharmacopsychiatry 2022;55(1):16-23. [DOI: 10.1055/a-1551-3756] [DOI] [PubMed] [Google Scholar]
Marcec 2021 {published data only}
- Marcec R, Likic R. Could fluvoxamine keep COVID-19 patients out of hospitals and intensive care units? Croatian Medical Journal 2021;62(1):95-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarthy 2021 {published data only}
- McCarthy MW. Repurposing approach of fluvoxamine for COVID-19. Drug Future 2021;46(10):813-8. [Google Scholar]
Medical Letter 2021 {published data only}
- Anonymous. Fluvoxamine for COVID-19? Medical Letter on Drugs and Therapeutics 2021;63(1623):69-70. [PubMed] [Google Scholar]
Murchu 2021 {published data only}
- Murchu EO, Spillane S, Byrne P, O'Neill M, Harrington P, Ryan M. Interventions in an ambulatory setting to prevent progression to severe disease in patients with COVID-19: a systematic review. Annals of Pharmacotherapy 2022;56(3):309-318. [DOI: 10.1177/10600280211028242] [DOI] [PubMed] [Google Scholar]
NCT04711863 {unpublished data only}
- NCT04711863. Fluvoxamine for adults with mild to moderate COVID-19 [Fluvoxamine for adults with mild to moderate COVID-19: a single-blind, randomized, placebo-controlled trial]. clinicaltrials.gov/ct2/show/record/NCT04711863 (first received 15 January 2021).
Seftel 2021 {published data only}
- Seftel D, Boulware DR. Prospective cohort of fluvoxamine for early treatment of coronavirus disease 19. Open Forum Infectious Diseases 2021;8(2):ofab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sukhatme 2021 {published data only}
- Sukhatme VP, Reiersen AM, Vayttaden SJ, Sukhatme VV. Fluvoxamine: a review of its mechanism of action and its role in COVID-19. Frontiers in Pharmacology 2021;12:ofab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT04668950 {unpublished data only}
- NCT04668950. Fluvoxamine for early treatment of Covid-19 (Stop Covid 2). clinicaltrials.gov/ct2/show/NCT04668950 (first received 16 December 2020).
Safa 2020 {published and unpublished data}
- Safa M, Hashemian MR, Abedi M, Malek MM. Effect of fluvoxamine medicine on cytokine level of COVID-19 patients, hospitalized in ICU ward [أثیر داروی فلووکسامین بر سطح سیتوکین بیماران مبتال به9-COVID بستری در بخش ICU]. Journal of Medical Council of Iran 2020;38(3):135-8. [URL: jmciri.ir/browse.php?a_code=A-10-1-2643&sid=1&slc_lang=en] [Google Scholar]
References to ongoing studies
NCT04510194 {unpublished data only}
- NCT04510194. COVID-OUT: early outpatient treatment for SARS-CoV-2 infection (COVID-19). clinicaltrials.gov/ct2/show/NCT04510194. (first received 12 August 2020).
NCT04718480 {unpublished data only}
- NCT04718480. Fluvoxamine administration in moderate SARS-CoV-2 (COVID-19) infected patients. clinicaltrials.gov/ct2/show/record/NCT04718480 (first received 22 January 2021).
NCT04885530 {unpublished data only}
- NCT04885530. ACTIV-6: COVID-19 study of repurposed medications. clinicaltrials.gov/ct2/show/NCT04885530 (first received on 13 May 2021).
NCT05087381 {unpublished data only}
- NCT05087381. Randomized-controlled trial of the effectiveness of COVID-19 early treatment in community. clinicaltrials.gov/ct2/show/record/NCT05087381 (first received 21 October 2021).
TCTR20210615002 {unpublished data only}
- TCTR20210615002. Effect of combined fluvoxamine with favipiravir versus favipiravir monotherapy in prevention of clinical deterioration among mild to moderate COVID-19 patients monitoring by telemedicine in virtual clinic: open-label randomized controlled trial. www.thaiclinicaltrials.org/show/TCTR20210615002 (first received 15 June 2021).
Additional references
Agarwal 2020
- Agarwal A, Rochwerg B, Lamontagne F, Siemieniuk RA, Agoritsas T, Askie L, et al. A living WHO guideline on drugs for Covid-19. BMJ 2020;m3379:1-14. [DOI: 10.1136/bmj.m3379] [DOI] [PubMed] [Google Scholar]
Altmann 2021
- Altmann DM, Boyton RJ, Beale R. Immunity to SARS-CoV-2 variants of concern. Science 2021;371(6534):1103-4. [DOI: 10.1126/science.abg7404] [DOI] [PubMed] [Google Scholar]
Ansems 2021
- Ansems K, Grundeis F, Dahms K, Mikolajewska A, Thieme V, Piechotta V, et al. Remdesivir for the treatment of COVID-19. Cochrane Database of Systematic Reviews 2021, Issue 8. Art. No: CD014962. [DOI: 10.1002/14651858.CD014962] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beigel 2020
- Beigel JK, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19. New England Journal of Medicine 2020;383(19):1813-26. [PMID: DOI: 10.1056/NEJMoa2007764] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buitrago‐Garcia 2020
- Buitrago-Garcia D, Egli-Gany D, Counotte MJ, Hossmann S, Imeri H, Ipekci Aziz M, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2infections: a living systematic review and meta-analysis. PloS Medicine 2020;17(9):e1003346. [DOI: 10.1371/ journal.pmed.1003346] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2020
- Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020 March 26 [Epub ahead of print]:1-12. [DOI: 10.1136/bmj.m1091] [DOI] [PMC free article] [PubMed]
COMET 2021
- COMET Initiative. Core outcome set developers’ response to COVID-19; April 2021. www.comet-initiative.org/Studies/Details/1538.
Danza 2022
- Danza P, Koo TH, Haddix M, Fisher R, Traub E, Yong K, et al. SARS-CoV-2 infection and hospitalization among adults aged≥ 18 Years, by vaccination status, before and during SARS-CoV-2 B. 1.1. 529 (omicron) variant predominance—Los Angeles County, California, November 7, 2021 to January 8, 2022. Morbidity and Mortality Weekly Report 2022;71(5):177. [DOI: 10.15585/mmwr.mm7105e1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2020
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
DEGAM 2022
- Blankenfeld H, Kaduszkiewicz H, Kochen MM, Pömsl J, Scherer M. SARS-CoV-2/Covid-19information and practical aids for established general practitioners and general practitioners (Version 22) [SARS-CoV-2/ Covid-19-Informationen & Praxishilfen für niedergelassene Hausärztinnen und Hausärzte (Version 22)]. www.degam-leitlinien.de/ (accessed 16 June 2022).
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Figgit 2000
- Figgitt DP, McClellan KJ. Fluvoxamine. An updated review of its use in the management of adults with anxiety disorders. SpringerLink 2000;60(4):925-54. [PMID: ] [DOI] [PubMed] [Google Scholar]
Friedman 2014
- Friedman R A. Antidepressants' black-box warning — 10 years later. New England Journal of Medicine 2014;371(18):1666-8. [DOI: 10.1056/nejmp1408480] [DOI] [PubMed] [Google Scholar]
Funk 2021
- Funk T, Pharris A, Spiteri G, Bundle N, Melidou A, Carr M, et al. Characteristics of SARS-CoV-2 variants of concern B.1.1.7,B.1.351 or P.1: data from seven EU/EEA countries, weeks 38/2020 to 10/2021. Europe's Journal on Infectious Disease Surveillance, Epidemiology, Prevention and Control 2021;26(16):pii=2100348. [DOI: 10.2807/1560-7917.ES.2021.26.16.2100348] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 15 August 2022. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Grubaugh 2020
- Grubaugh ND, Hanage WP, Rasmussen AL. Making sense of mutation: what D614G means for the COVID-19 pandemic remains unclear. Cell 2020;182(4):794-5. [DOI: 10.1016/j.cell.2020.06.040] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2021
- Gupta A, Gonzalez-Rojas Y, Juarez E, Crespo Casal M, Moya J, Falci DR, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. New England Journal of Medicine 2021;385(21):1941-50. [DOI: 10.1056/NEJMoa2107934] [DOI] [PubMed] [Google Scholar]
Higgins 2020a
- Higgins JP, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020. Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Higgins 2020b
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Higgins 2021
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Hoffmann 2021
- Hoffmann M, Krüger N, Schulz S, Cossmann A, Rocha C, Kempf A, et al. The omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell 2021;185(3):447-56. [DOI: 10.1016/j.cell.2021.12.032] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2020
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395(10223):497-506. [DOI: 10.1016/S0140-6736(20)30183-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
IntHout 2016
- IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 2016;6(7):e010247. [DOI: 10.1136/bmjopen-2015-010247] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jayk Bernal 2021
- Jayk Bernal A, Gomes da Silva MM, Musungaie DB, Kovalchuk E, Gonzalez A, Delos Reyes V, et al MOVe-OUT Study Group. Molnupiravir for oral treatment of COVID19 in nonhospitalized patients. New England Journal of Medicine 2021:1-12. [DOI: 10.1056/NEJMoa2116044] [DOI] [PMC free article] [PubMed]
Johns Hopkins University of Medicine 2022
- Johns Hopkins University & Medicine Coronavirus Resource Center. Mortality analyses: How does mortality differ across countries? coronavirus.jhu.edu/data/mortality (accessed 10 February 2022).
Kacimi 2021
- Kacimi SE, Greca E, Haireche MA, ElHawary SA, Setti MO, Caruana R, et al. The place of fluvoxamine in the treatment of non-critically ill patients with COVID-19: a living systematic review and meta-analysis. medRxiv 2021. [DOI: 10.1101/2021.12.19.21268044] [DOI]
Kam 1997
- Kam PCA, Chang GWM. Selective serotonin reuptake inhibitors Pharmacology and clinical implications in anaesthesia and critical care medicine. Anaesthesia 1997;52(10):982-8. [DOI: 10.1111/j.1365-2044.1997.162-az0176.x] [DOI] [PubMed] [Google Scholar]
Kreuzberger 2021
- Kreuzberger N, Hirsch C, Chai KL, Tomlinson E, Khosravi Z, Popp M, et al. SARS‐CoV‐2‐neutralising monoclonal antibodies for treatment of COVID‐19. Cochrane Database of Systematic Reviews 2021, Issue 9. Art. No: CD013825. [DOI: 10.1002/14651858.CD013825.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lauer 2020
- Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, et al. The incubation period of coronavirus disease 2019(COVID-19) from publicly reported confirmed cases: estimation and application. Annals of Internal Medicine 2020;172(9):577–82. [DOI: 10.7326/M20-0504] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2021
- Lee TC, Vigod S, Bortolussi-Courval E, Hanula R, Boulware DR, Lenze EJ, et al. Fluvoxamine for outpatient COVID-19 to prevent hospitalization: a systematic review and meta-analysis. medRxiv. [DOI: 10.1101/2021.12.17.21268008] [DOI] [PMC free article] [PubMed]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf MI, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook.
Marshall 2020
- Marshall JC, Murthy S, Diaz J, Adhikari NK, Angus DC, Arabi YM, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infectious Diseases 2020;20(8):e192-7. [DOI: 10.1016/S1473-3099(20)30483-7]] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mikolajewska 2021
- Mikolajewska A, Fischer AL, Piechotta V, Mueller A, Metzendorf MI, et al. Colchicine for the treatment of COVID-19. Cochrane Database of Systematic Reviews 2021, Issue 10. Art. No: CD015045. [DOI: 10.1002/14651858.CD015045] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murchu 2022
- Murchu OE, Spillane S, Byrne P, O’Neill M, Harrington P, Ryan M. Interventions in an ambulatory setting to prevent progression to severe disease in patients with COVID-19: a systematic review. Annals of Pharmacotherapy 2022;56(3):309-18. [DOI: 10.1177/10600280211028242] [DOI] [PubMed] [Google Scholar]
National Institutes of Health 2022
- National Institutes of Health. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. www.covid19treatmentguidelines.nih.gov/ (accessed 17 February 2022).
NCT04510194
- NCT04510194. COVID-OUT: early outpatient treatment for SARS-CoV-2 infection (COVID-19). clinicaltrials.gov/ct2/show/NCT04510194 (first received 12 August 2020).
NCT04668950
- NCT04668950. Fluvoxamine for early treatment of Covid-19 (Stop Covid 2). clinicaltrials.gov/ct2/show/NCT04668950 (first received 16 December 2020).
NCT04718480
- NCT04718480. Fluvoxamine administration in moderate SARS-CoV-2 (COVID-19) infected patients. clinicaltrials.gov/ct2/show/NCT04718480 (first received 22 January 2021).
NCT04885530
- NCT04885530. ACTIV-6: COVID-19 outpatient randomized trial to evaluate efficacy of repurposed medications. clinicaltrials.gov/ct2/show/NCT04885530 (first received on 13 May 2021).
NCT05087381
- NCT05087381. Randomized-controlled trial of the effectiveness of COVID-19 early treatment in community. clinicaltrials.gov/ct2/show/record/NCT05087381 (first received 21 October 2021).
Oran 2020
- Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2infection: a narrative review. Annals of Internal Medicine 2020;173(5):362-7. [DOI: 10.7326/M20-3012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Page 2021
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372(71):1-9. [DOI: 10.1136/bmj.n71] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pan 2020
- Pan A, Liu L, Wang C, Guo H, Hao X, Wang Q, et al. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. Journal of the American Medical Association 2020;323(19):1915-23. [DOI: 10.1001/jama.2020.6130] [DOI] [PMC free article] [PubMed] [Google Scholar]
Piechotta 2022
- Piechotta V, Iannizzi C, Chai KL, Valk SJ, Kimber C, Dorando E, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database of Systematic Reviews 2022, Issue 2. Art. No: CD013600. [DOI: 10.1002/14651858.CD013600.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Planas 2022
Popp 2021a
- Popp M, Stegemann M, Riemer M, Metzendorf M-I, Romero CS, Mikolajewska A, et al. Antibiotics for the treatment of COVID‐19. Cochrane Database of Systematic Reviews 2021, Issue 10. Art. No: CD015025. [DOI: 10.1002/14651858.CD015025] [DOI] [PMC free article] [PubMed] [Google Scholar]
Popp 2021b
- Popp M, Stegemann M, Metzendorf MI, Gould S, Kranke P, Meybohm P, et al. Ivermectin for preventing and treating COVID-19. Cochrane Database of Systematic Reviews 2021, Issue 7. Art. No: CD015017. [DOI: 10.1002/14651858.CD015017.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rafiee 2016
- Rafiee L, Hajhashemi V, Javanmard SH. Fluvoxamine inhibits some inflammatory genes expression in LPS/stimulated human endothelial cells, U937 macrophages, and carrageenan-induced paw edema in rat. Iranian Journal of Basic Medical Sciences 2016;19(9):977-84. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
RECOVERY 2021
- RECOVERY Collaborative Group. Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. Respiratory medicine 2021;9(12):1419-26. [DOI: 10.1016/S2213-2600(21)00435-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.12.0. The Cochrane Collaboration, 2022. Available from revman.cochrane.org.
Rosen 2019
- Rosen DA, Seki SM, Fernández-Castañeda A, Beiter RM, Eccles JD, Woodfolk JA, et al. Modulation of the sigma-1 receptor–IRE1 pathway is beneficial in preclinical models of inflammation and sepsis. Science Translational Medicine 2019;11(478):5266. [DOI: 10.1126/scitranslmed.aau5266] [DOI] [PMC free article] [PubMed] [Google Scholar]
Safa 2020
- Safa M, Hashemian MR, Abedi M, Malek MM. Effect of fluvoxamine medicine on cytokine level of COVID-19 patients, hospitalized in ICU ward [تأثیر داروی فلووکسامین بر سطح سیتوکین بیماران مبتال به9-COVID بستری در بخش ICU]. Journal of Medical Council of Iran 2020;30(3):135-8. [URL: jmciri.ir/browse.php?a_code=A-10-1-2643&sid=1&slc_lang=en] [Google Scholar]
Santesso 2020
- Santesso N, Glenton C, Dahm P, Garner P, Akl A, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;119:126-35. [DOI: 10.1016/j.jclinepi.2019.10.014] [DOI] [PubMed] [Google Scholar]
Schünemann 2020
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing 'Summary of findings' tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from training.cochrane.org/handbook.
Singh 2021
- Singh B, Ryan H, Kredo T, Chaplin M, Fletcher T. Chloroquine or hydroxychloroquine for prevention and treatment of COVID-19. Cochrane Database of Systematic Reviews 2021, Issue 2. Art. No: CD013587. [DOI: 10.1002/14651858.CD013587.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Skoetz 2020
- Skoetz N, Goldkuhle M, Van Dalen EC, Akl EA, Trivella M, Mustafa RA, et al. GRADE guidelines 27: how to calculate absolute effects for time-to-event outcomes in summary of findings tables and Evidence Profiles. Journal of Clinical Epidemiology 2020;118:124-31. [DOI: 10.1016/j.jclinepi.2019.10.015] [DOI] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:1-8. [DOI: 10.1136/bmj.l4898] [DOI] [PubMed] [Google Scholar]
Stroehlein 2021
- Stroehlein JK, Wallqvist J, Iannizzi C, Mikolajewska A, Metzendorf MI, Benstoem C, et al. Vitamin D supplementation for the treatment of COVID-19: a living systematic review. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD015043. [DOI: 10.1002/14651858.CD015043] [DOI] [PMC free article] [PubMed] [Google Scholar]
Struyf 2020
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013665. [DOI: 10.1002/14651858.CD013665] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sukhatme 2021
- Sukhatme VP, Reiersen AM, Vayttaden SJ, Sukhatme VV. Fluvoxamine: a review of its mechanism of action and its role in COVID-19. Frontiers in Pharmacology 2021;12:652688. [DOI: 10.3389/fphar.2021.652688] [DOI] [PMC free article] [PubMed] [Google Scholar]
Takenaka 2022
- Takenaka Y, Tanaka R, Kitabatake K, Kuramochi K, Aoki S, Tsukimoto M. Profiling differential effects of 5 selective serotonin reuptake inhibitors on TLRs-dependent and -independent IL-6 production in immune cells identifies fluoxetine as preferred anti-inflammatory drug candidate. Frontiers in Pharmacology 2022;13:874375. [DOI: 10.3389/fphar.2022.874375] [DOI] [PMC free article] [PubMed] [Google Scholar]
TCTR20210615002
- TCTR20210615002. Effect of combined fluvoxamine with favipiravir versus favipiravir monotherapy in prevention of clinical deterioration among mild to moderate COVID-19 patients monitoring by telemedicine in virtual clinic: open-label randomized controlled trial. trialsearch.who.int/Trial2.aspx?TrialID=TCTR20210615002 (first received 14 June 2021). [CENTRAL: CN-02281273]
van Harten 1995
- Harten J. Overview of the pharmacokinetics of fluvoxamine. Clinical Pharmacokinetics 1995;29(1):1-9. [DOI: 10.2165/00003088-199500291-00003] [DOI] [PubMed] [Google Scholar]
Wagner 2021
- Wagner C, Griesel M, Mikolajewska A, Mueller A, Nothacker M, Kley K, et al. Systemic corticosteroids for the treatment of COVID-19. Cochrane Database of Systematic Reviews 2021, Issue 8. Art. No: CD014963. [DOI: 10.1002/14651858.CD014963] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinreich 2021
- Weinreich DM, Sivapalasingam S, Norton T, Ali S, Gao H, et al. REGEN-COV antibody combination and outcomes in outpatients with Covid-19. New England Journal of Medicine 2021;385(23):1-12. [DOI: 10.1056/NEJMoa2108163] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wen 2022
- Wen W, Chen C, Tang J, Wang C, Zhou M, Cheng Y, et al. Efficacy and safety of three new oral antiviral treatment (molnupiravir, fluvoxamine and Paxlovid) for COVID-19: a meta-analysis. Annals of Medicine 2022;54(1):516-23. [DOI: 10.1080/07853890.2022.2034936] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2003
- World Health Organization. Cumulative number of reported probable cases of SARS; 2003. www.who.int/csr/sars/country/2003_07_11/en (accessed 16 December 2021).
WHO 2019a
- World Health Organization. Middle East respiratory syndrome coronavirus (MERS-CoV): 2019. www.who.int/emergencies/mers-cov/en (accessed 6 December 2021).
WHO 2020a
- World Health Organization. Report of the WHO-China JointMission on coronavirus disease 2019 (COVID-19). www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-oncovid-19-final-report (accessed 6 December 2021).
WHO 2020b
- World Health Organization. Estimating mortality from COVID-19 - scientific brief. www.who.int/publications/i/item/WHO-2019-nCoV-Sci-Brief-Mortality-2020 (accessed 6 December 2021).
WHO 2020c
- World Health Organization. DraR landscape of COVID-19 candidate vaccines. www.who.int/emergencies/diseases/novel-coronavirus-2019/covid-19-vaccines (accessed 6 December 2021).
WHO 2020e
- World Health Organization. COVID-19 Therapeutic Trial Synopsis. www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis (accessed 14 February 2022).
WHO 2021a
- World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard. covid19.who.int (accessed 25 February 2022).
WHO 2021b
- World Health Organization. SARS-CoV-2 Variants. www.who.int/csr/don/31-december-2020-sars-cov2-variants/en/ (accessed 6 December 2021).
WHO 2022
- World Health Organization. Tracking SARS-CoV-2 variants. www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed 18 February 2022). [PubMed]
WHO2022a
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. covid19.who.int/ (accessed 25 February 2022).
Williamson 2020
- Williamson E, Walker AJ, Bhaskaran KJ, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584(7821):430-6. [DOI: 10.1038/s41586-020-2521-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2020
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323(13):1239-42. [DOI: 10.1001/jama.2020.2648] [DOI] [PubMed] [Google Scholar]


