Abstract
The logopenic variant of primary progressive aphasia is characterized by early deficits in language production and phonological short-term memory, attributed to left-lateralized temporoparietal, inferior parietal and posterior temporal neurodegeneration. Despite patients primarily complaining of language difficulties, emerging evidence points to performance deficits in non-linguistic domains. Temporoparietal cortex, and functional brain networks anchored to this region, are implicated as putative neural substrates of non-linguistic cognitive deficits in logopenic variant primary progressive aphasia, suggesting that degeneration of a shared set of brain regions may result in co-occurring linguistic and non-linguistic dysfunction early in the disease course. Here, we provide a Review aimed at broadening the understanding of logopenic variant primary progressive aphasia beyond the lens of an exclusive language disorder. By considering behavioural and neuroimaging research on non-linguistic dysfunction in logopenic variant primary progressive aphasia, we propose that a significant portion of multidimensional cognitive features can be explained by degeneration of temporal/inferior parietal cortices and connected regions. Drawing on insights from normative cognitive neuroscience, we propose that these regions underpin a combination of domain-general and domain-selective cognitive processes, whose disruption results in multifaceted cognitive deficits including aphasia. This account explains the common emergence of linguistic and non-linguistic cognitive difficulties in logopenic variant primary progressive aphasia, and predicts phenotypic diversification associated with progression of pathology in posterior neocortex.
Keywords: primary progressive aphasia, Alzheimer’s disease, temporoparietal junction, inferior parietal lobe, non-linguistic functions, language
In a new clinico-anatomical model, Ramanan et al. integrate advances from cognitive neuroscience and neuropsychology to propose a role for left temporoparietal degeneration in underpinning emergent linguistic and non-linguistic cognitive deficits in logopenic variant primary progressive aphasia.
Introduction
Primary progressive aphasia (PPA) refers to a group of neurodegenerative disorders affecting language functions in early stages, due to degeneration of a distributed, largely left-lateralized language network.1,2 Historical descriptions of PPA-like syndromes can be found in late 19th and early 20th centuries in reports by Pick, Sérieux, Dejerine, Rosenfeld and others.1 Contemporary clinical, anatomical and pathological understanding of PPAs has evolved greatly in the last 40 years. Key to this resurgence was a seminal case series by Mesulam3 describing progressive aphasia with left perisylvian atrophy and slow rates of disease progression. Marked general cognitive decline emerged only later in the disease course.4 Subsequent classifications of PPA have expanded the phenotype to include patients with motor-speech planning, grammatical and semantic memory deficits, identifying three main clinical variants: a ‘non-fluent/agrammatic’ variant (nfvPPA) characterized by motor-speech dysfunction and/or agrammatism stemming from primary fronto-insular degeneration;5 a ‘semantic’ variant (svPPA, also called semantic dementia) presenting with degradation of conceptual knowledge due to anterior temporal degeneration;6 and a third ‘logopenic’ variant (lvPPA)7 that forms the focus of the current Review. Clinically, lvPPA patients display slowed, disrupted spontaneous language production marked by word-finding pauses, anomia, phonological errors and poor length-dependent repetition of sentences, amid relatively preserved grammatical processing, motor speech (unlike nfvPPA) and semantic knowledge (unlike svPPA).2,8 Comprehension of long sentences is also affected and, in part, could arise from poor phonological short-term memory.8–10 This constellation of language difficulties emerges from primary degeneration of the left-lateralized posterior temporal gyri, temporoparietal junction and inferior parietal lobes (TPJ/IPL) and their interstices.8,11 Recent advances in neuroimaging of disease progression in PPA syndromes conceptualize them as network-level disorders, in which neurodegeneration starts in a syndrome-specific susceptible epicentre and then spreads in a predictable, constrained manner along structurally/functionally connected brain regions.12,13 Accordingly, with disease progression, atrophy in lvPPA encroaches into regions in the left hemisphere that are functionally coupled with TPJ/IPL (via language and Default Mode networks), followed by regions in the right hemisphere.14–19
To date, the lens of lvPPA clinical investigations has largely focused on language profiles, mainly because, by definition, the main presenting clinical complaint is that of language disturbances. However, a growing number of studies pose an important diagnostic challenge: that numerous lvPPA patients display concurrent, non-linguistic cognitive deficits on neuropsychological testing. Of note, even in Mesulam’s early PPA cases who probably had the logopenic variant, calculation difficulties were noted during clinical presentation.3 When non-linguistic cognitive functions are tested in depth, most lvPPA cases appear to exhibit moderate-to-marked levels of non-linguistic cognitive dysfunction.20 Others find that the severity of non-linguistic difficulties in lvPPA may parallel the severity of aphasia and emerge relatively independent of disease severity.21 This issue is complicated by overlaps in both language and non-linguistic cognitive performance between lvPPA, amnestic Alzheimer’s disease and posterior cortical atrophy: three clinical entities often sharing common pathology.16 Piecing together the diagnostic and mechanistic puzzle of why lvPPA presents with linguistic and non-linguistic cognitive difficulties is vital to ensure accurate diagnosis, characterization and prognostication.
In this paper, we propose a new clinico-anatomical framework that encompasses the ‘multidimensional’ deficits in lvPPA. The combination of core language and variable non-linguistic deficits may arise from shared neurophysiological mechanisms, and the nature and localization of neurocognitive processes supported by the left TPJ/IPL region—the epicentre of atrophy in the syndrome. The contribution of this region and functionally connected areas to language disruption in lvPPA has received significant attention, but its role in cognitive processes beyond language in this syndrome has been relatively overlooked. By integrating insights from contemporary cognitive neuroscience and neuropsychology, we review the evidence of domain-general and domain-selective mechanistic roles for these regions. By then reversing these inferences, we can consider the phenotypic outcome of left TPJ/IPL degeneration to lvPPA, updating our understanding of why some patients present with a relatively pure aphasic profile while others display additional non-linguistic dysfunction.
To be clear from the outset, our goal of this Review is not to rewrite the current diagnostic criteria for lvPPA, but rather to explain phenotypic diversification in the syndrome with implications to its accurate diagnosis and timely introduction of multidomain symptomatic treatments.
Insights from contemporary cognitive neuroscience
We propose that patterns of verbal and nonverbal cognitive difficulties observed early in the lvPPA disease course are a direct reflection of (i) the cognitive complexity of TPJ/IPL; and (ii) the amount and distribution of neuropathology, atrophy and/or hypometabolism in TPJ/IPL and structurally/functionally connected regions and networks.10,22–25 The impact of TPJ/IPL pathology in lvPPA may further cause disruptions in cognitive processing related to up/downstream regions, such as the hippocampus and right IPL, and connected functional networks, compounding cognitive dysfunction in the syndrome. The current proposal does not exclude contributions of other brain regions to cognitive dysfunction in lvPPA; but in this Review, we focus on TPJ/IPL due to its complex cognitive roles and early involvement in this syndrome. A detailed review of left TPJ/IPL functionality is beyond the scope of our Review26–29; however, by considering key themes regarding its neurocognitive organization, we can provide testable neural hypotheses explaining the multifaceted cognitive presentation of lvPPA.
Historical cognitive neuroscience and neuropsychology evidence has implicated left TPJ/IPL in multiple, apparently distinct, cognitive tasks. For instance, Gerstmann’s syndrome, emerging from left IPL damage, encompasses both linguistic (dysgraphia and occasionally, aphasia) and non-linguistic (finger agnosia, dyscalculia, left-right disorientation) complaints.30 Functional neuroimaging suggests that the multidomain involvement of TPJ/IPL can be traced to its role in deploying key neurocomputational resources to meet demands of different cognitive tasks.28,31 Three principal features of this region underpin its domain-general and domain-selective functions:
Neuroanatomically, TPJ/IPL are segregated from primary sensory cortices. This absolves them from processing dynamic fluctuations in the immediate sensory environment, and facilitates the processing of multimodal information and diverse cognitive domains.28,32,33,29,34
Computational models suggest that repeat processing, integration and buffering of information can promote extraction of time-, space- and context-invariant representations that can be repurposed to different task demands.28,35,36 TPJ/IPL harbour this functional capacity, whereas TPJ/IPL lesions characteristic of semantic aphasia impair the use of time- and context-appropriate information across semantic and non-semantic domains.37,38
Functional specialization within TPJ/IPL is not sharply fractionated, but varies between subregions in a graded manner and is tied closely to structural/functional connectivity patterns of each subregion. This characteristic is seen in the temporal granularity of information being processed here. For example, cognitive tasks processed over shorter time periods (e.g. phonology, sound) preferentially recruit anterior TPJ/IPL regions (e.g. supramarginal gyrus) whereas posterior IPL (e.g. angular gyrus) is preferentially recruited in tasks reliant on longer temporal windows, where processing of accumulating information is required (e.g. sentence and narrative processing, episodic memory retrieval).28,34,39
On the basis of these principles, we evaluate evidence for TPJ/IPL in multidomain cognition, with relevance to lvPPA (Fig. 1A–C).
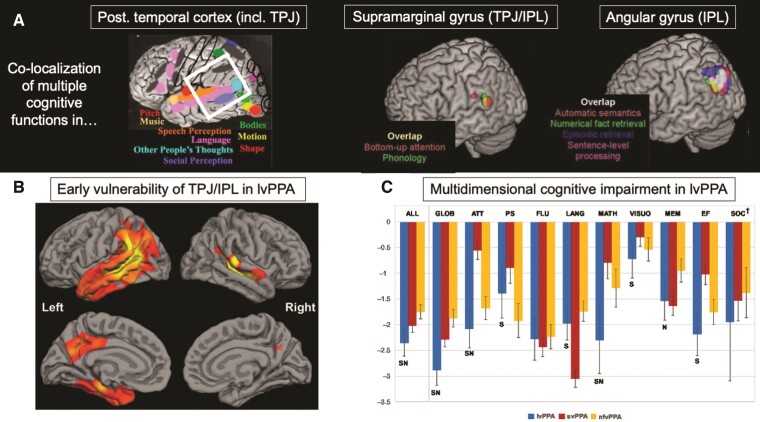
Figure 1.
Graphical summary of the main premise of this Review. (A) Insights from cognitive neuroscience indicate that the left posterior temporal cortices (including superior/middle/inferior temporal gyrus and TPJ; together, denoted within the white square) (reprinted from Kanwisher40) and left supramarginal and angular gyri (components of the IPL) (reprinted from Humphreys and Lambon Ralph28) support multiple cognitive functions, possibly through deployment of domain-general and domain-selective computations. These computations support phonology and working memory, episodic and semantic memory, social and numerical cognition, visuospatial and executive abilities, as well as attention and praxis functions. (B) lvPPA targets left posterior temporal and TPJ/IPL regions (depicted here as reduced cortical thickness, i.e. warmer colours) suggesting the aforementioned cognitive functions dependent on TPJ/IPL functionality should be affected (reprinted from Leyton et al.11 with permission from IOS Press). (C) A recent meta-analysis of neuropsychological performance in 663 lvPPA patients (across 51 studies) found significant performance deficits across standardized neuropsychological measures of episodic memory, social and numerical cognition, executive functions, and attention in lvPPA relative to nfvPPA (‘N’ in figure) and svPPA (‘S’ in figure) groups (reprinted from Kamath et al.21 with permission from Cambridge University Press), showcasing the multidimensional cognitive profile of this syndrome.
Considering language functions, posterior superior temporal and inferior supramarginal gyri are involved in extraction of spectro-temporal features of sound41 and extraction and maintenance of phonological representations.42 These specific functions, in turn, may be supported by shared neurophysiological mechanisms within TPJ/IPL. For example, the planum temporale acts as an auditory ‘computational hub’, disambiguating incoming auditory information, matching these representations to existing auditory templates (derived from experience), in turn, supporting its transformation into subsequent motor programmes for different cognitive/behavioural domains (e.g. object recognition, emotional vocalization).43–45 Ventral and posterior portions, including posterior middle temporal regions, are associated with multimodal semantic-executive related processing,46–48 while angular gyrus participates in constructive elements of verbal episodic recollection,49–51 sentential and combinatorial processing52 and buffering sequential information in coherent contexts.53,54 These functional specializations are corroborated by extant neuropsychological evidence. For example, conduction aphasia, emerging from damage to the inferior supramarginal gyrus and underlying arcuate fasciculus, specifically disrupts speech repetition but spares semantic-executive control.55,56 Conversely, transcortical sensory aphasia and semantic aphasia, occurring from lesions in the middle/posterior cerebral artery watershed territory affecting posterior middle temporal regions (outside the supramarginal gyrus), display poor semantic-executive processing but relatively intact phonological functions.38,57 Finally, combined damage to the entire left TPJ/IPL, such as in Wernicke’s aphasia, disrupts both phonological and semantic-executive control functions, irrespective of tested modality.58
For non-linguistic domains, left IPL emerges at the nexus of multiple non-linguistic cognitive capacities. As per our third principle (i.e. functional gradation within TPJ/IPL for processing information varying in temporal granularity), the anterior IPL (supramarginal gyrus) shows involvement in tasks requiring relatively shorter temporal processing windows, such as directing bottom-up attention to external stimuli and internally generated thought (e.g. episodic recollection).59 Longer temporal windows in posterior IPL (angular gyrus) facilitate its involvement in transformation and integration of accumulating information, irrespective of its nature or modality.28,54 For example, during memory recollection and future-oriented thinking: (i) angular gyrus and medial temporal regions show functional coupling; and (ii) angular gyrus activity scales with the vividness, strength and accuracy of retrieved memories, and shows demonstrable sensitivity to integration of multimodal information.49,50,60,61 Accordingly, primary IPL damage, irrespective of aetiology, impairs performance on cognitive endeavours requiring mental simulations, including episodic retrieval and scene construction,62–64 theory of mind and social cognition65 and spatial navigation.66 Angular gyrus is further integral to transformation and retrieval of numerical knowledge,67 explaining dyscalculia following IPL damage in Gerstmann’s syndrome. The integration role of the IPL further extends to motor behaviour, where supramarginal gyrus directs attention to motor sequences68 while supramarginal and angular gyri together aid integration of individual motor goals and their transformation into external motor actions.69 This particular finding implicates the IPL as a neural substrate of ideational apraxia as well as volitional apathy.69,70 Taken together, the evidence points to a role for the TPJ/IPL in supporting multiple cognitive processes spanning language and beyond.
lvPPA clinical observations mirror cognitive neuroscience
Reverse inference from the cognitive neuroscience and neuropsychology literature on the nature of multidomain cognitive processes of the TPJ/IPL region (reviewed previously, see also Fig. 1) provides a potential foundation for explaining the core language and co-occurring variable cognitive deficits in lvPPA in terms of stochastic spreading of pathology and atrophy from the TPJ/IPL epicentre.
A prototypical language deficit
The core language deficit of lvPPA is in keeping with the role of the posterior superior temporal gyrus/inferior supramarginal gyrus in phonological processing and working memory. In lvPPA, errors of phonology and lexical retrieval during word formation emerge from dysfunction of these specific regions.11,71,72 Difficulties in decoding phonemic structure in lvPPA patients further relate to attenuated activation of superior temporal regions as noted on functional neuroimaging.73 With disrupted phonological short-term memory, length-dependent repetition deficits are a canonical feature of lvPPA, manifesting in a profound inability to retain, manipulate and reproduce long segments of verbal information (e.g. repeating lengthy words, sentences and letter/digit strings in forward/reverse orders).74,75 On this view, the language and anatomical profile of lvPPA most closely resembles conduction aphasia post-stroke,74,76,77 although it must be acknowledged that the major PPA syndromes have either little or only partial analogues in vascular aphasiology, in terms of overall linguistic and cognitive performance.78 If and when the underlying pathology encroaches into anterior and ventral temporal cortices, semantic comprehension impairments, such as those in svPPA, are expected.79,80 Similarly, if the disease spreads into fronto-insular regions and connecting arcuate/superior longitudinal fasciculus, then motor-speech difficulties (resembling those observed in nfvPPA) may become part of the clinical profile.81
The multidimensional non-linguistic deficits
Relative to healthy controls and other PPA variants, neuropsychological investigations of well-characterized lvPPA patients identify deficits in processing speed, executive function, sustained attention, working memory and visuospatial function.82–85 Dyscalculia as early as 1 year post disease onset, and emergence of apraxia within 2 years are also reported.7,86 A recent case report of an lvPPA patient with canonical TPJ/IPL atrophy noted attentional and visuospatial complaints (manifesting as hemispatial neglect)87—a constellation of complaints typically seen in posterior cortical atrophy following superior/inferior parieto-occipital neurodegeneration. Another case report of a patient meeting lvPPA clinico-radiological criteria evidenced deregulated semantic-executive control when using electrical appliances, wherein the patient ‘clearly understood what these appliances are and what they are used for, but was not sure how to use them’.86(p2). This profile appears to fulfil standard definitions of ideational apraxia, and is highly reminiscent of semantic control deficits in semantic aphasia (who present with concurrent ideational/dysexecutive apraxia),88–90 where damage to inferior/middle posterior temporal and IPL (angular gyrus) regions results in context-invariant executive deregulation of verbal and nonverbal semantic information.38,89,90 In this regard, probing the status of semantic control functions in lvPPA forms an important line of inquiry as it may inform whether deregulated semantic control partly explains the co-occurrence of some non-linguistic symptoms, such as apraxia, in this syndrome.
Given the role of the angular gyrus in episodic memory processing, atrophy/pathological deposition to this region can impair episodic retrieval.34,49,60,91 Accordingly, an additional under-appreciated cognitive complaint in lvPPA is episodic amnesia, closely linked to IPL involvement in the syndrome. By the time of their first clinic appointment, a third of patients report misplacing objects, missing appointments, getting lost and facing difficulty in learning new tasks.92,93 Both carer- and clinician-indexed reports further attest to daily memory difficulties.94 On objective standardized neuropsychological memory assessment, verbal and nonverbal episodic memory deficits in lvPPA can be prominent to a magnitude comparable to amnesic Alzheimer’s disease and are associated with changes to integrity of the bilateral IPL (angular gyri) and their structural connections to memory processing regions in the medial temporal lobe (e.g. hippocampus).94–96 Episodic amnesia in lvPPA can also extend to remote autobiographical memories encoded decades before the onset of clinical symptoms (e.g. from teenage and early adulthood years), with poor autobiographical recall, irrespective of the temporal epoch of the memory, correlating with angular gyrus involvement in the syndrome.97
Beyond episodic memory, lvPPA patients show difficulties on tasks of nonverbal auditory object processing (e.g. phoneme discrimination, prosody perception, global pitch and timbre processing), in the absence of peripheral hearing difficulties.98–101 These impairments have been found to be closely associated with dysfunctional working memory,98,100 further correlating with integrity of the left IPL and parietal cortices.100,101 Spatial working memory deficits are also prominent in lvPPA, again associated with degeneration of superior parietal cortices and their functional connections with prefrontal brain regions.102–104 Across clinical variants of Alzheimer’s disease (including lvPPA), increased pathological accumulation in TPJ/IPL and parietal cortex strongly correlates with emergent impairments of episodic and semantic memory, plus executive and visuospatial functions.105 In lvPPA particularly, patients displaying greater visuospatial, executive and general cognitive disturbances typically show intensified atrophy to left IPL/superior parietal regions.20,106 Corroborative evidence also comes from studies adopting the inverse ‘imaging-first’ classification approach, where lvPPA patients with greater left IPL/parietal atrophy/hypometabolism display poorer nonverbal memory, visuospatial and executive performance, in contrast to lvPPA patients with temporal-dominant atrophy/hypometabolism.107 Finally, there is some evidence for behavioural dysfunction in lvPPA, such as increased apathy, anxiety, irritability, agitation and difficulties in emotion detection.108–112 Ultimately, it is unsurprising that a combination of aphasia, poor memory and general cognition, increased apathy and agitation significantly compounds carer burden and caring-related challenges in lvPPA.110,113,114 Additional investigations of the inter-dependence between behavioural and mood symptoms, the neural bases of non-linguistic cognitive and behavioural difficulties, and how these changes relate to underlying pathological and genetic changes in lvPPA are required (see Box 1). Nevertheless, the extant evidence consistently implicates TPJ/IPL dysfunction as one of the brain regions central to multidimensional cognitive decline in the syndrome. This anatomical framework also potentially explains the development of incipient lvPPA-like features in other Alzheimer’s disease clinical variants, especially in early onset atypical presentations such as posterior cortical atrophy,92,131,132 as the underlying pathology encroaches into TPJ/IPL.
Box 1 Links between multidimensional cognitive impairment, underlying Alzheimer’s disease pathology and progranulin gene mutations in lvPPA. Clinicopathological associations are key to achieving accurate prognosis and treatment decisions. Over 80% of lvPPA patients have underlying Alzheimer pathology, mostly noted as amyloid-positive PET scans.16,115–118 More recently, reports have emerged of cases showing an lvPPA-like clinical picture amid amyloid-negative profiles. In these cases, a common finding is the mutation of the progranulin gene, which is typically associated with frontotemporal lobar degeneration syndromes.119–122 In addition to the classic linguistic profile of lvPPA, many such cases display concurrent difficulties in reading, episodic memory, executive functions and calculation,123,124 suggesting links between cognitive multidimensionality and underlying neurobiological and genetic factors. In current neurodegenerative dementia clinico-pathological conceptualizations, lvPPA is considered as an atypical clinical variant of Alzheimer’s disease.16 Therefore, an lvPPA patient with an amyloid-positive scan, a prototypical aphasic profile, poor memory, visuospatial and/or executive dysfunction may rule in several different diagnostic labels including mixed PPA, advanced amnesic Alzheimer’s disease and an Alzheimer’s disease clinical variant with visuospatial and/or language features. As the syndrome straddles clinicopathological boundaries of both PPA and Alzheimer’s disease taxonomies, it is sensible to ask whether multidimensional cognitive difficulties in lvPPA are parsimoniously explained by underlying pathological and/or genetic mechanisms.
Given the paucity of evidence on correspondence between clinicopathological and cognitive performance data in lvPPA, a direct answer to this question is currently hard to establish. For example, one study noted that PPA with Alzheimer’s disease pathology (most of whom met a clinical diagnosis of lvPPA) is associated with episodic memory preservation, despite these patients presenting significant TPJ/IPL and medial temporal atrophy.125 On the other hand, accumulating evidence suggests that lvPPA patients with and without amyloid-positive profiles perform comparably on standardized measures of lexical retrieval, speech and language, executive function, processing speed and verbal memory.126–129 Comparable profiles between high- and low-amyloid burden lvPPA patients are also evident on visuospatial and non-verbal memory tasks, even when disease severity and overall language status are accounted for.127,130 Moreover, data-driven clustering solutions of lvPPA cognitive performance find no significant differences in amyloid burden, demographic and disease duration indices between lvPPA with mild, marked or no co-occurring general cognitive deficits.20 Likewise, when contrasting lvPPA cases with and without progranulin gene mutations, comparable language, cognitive, and neuroanatomical profiles (on structural MRI) are notable.119,120 Due to the rarity of such reports, more work is required on this front. However, most findings suggest that the pathological or genetic profile, by itself, may not be sufficient to explain variations in non-linguistic cognitive difficulties in lvPPA. Rather, as we propose, the key factor in variable cognitive profiles early in the syndrome may be the extent to which the distribution and loading of pathology and atrophy/hypometabolism are localized in the TPJ/IPL and neighbouring regions.
Other contributory mechanisms
Non-linguistic deficits and their relationship with primary aphasia
Primary verbal degradation in lvPPA greatly influences performance on tasks taxing working memory and phonological abilities, such as reading, verbal fluency and verbal episodic memory.133–135 These deficits often interfere with patients’ ability to understand complex verbal instructions. However, there is compelling evidence that many nonverbal cognitive deficits observed in lvPPA are not simply a reflection/by-product of the patients’ progressive aphasia. Patients with lvPPA can show marked deficits on cognitive functions that circumvent language demands, such as nonverbal episodic memory, nonverbal auditory object processing, spatial orientation, spatial working memory and visuospatial functioning.83,96,98,99,136,137 Likewise, lvPPA patients exhibit impaired performance on verbal memory, and visuospatial and executive processing tasks, even after statistically controlling effects of aphasia severity.94,97,106,109 In this regard, detailed lvPPA case reports are particularly insightful. In one report, the earliest recorded complaints involved calculation and sustained attention difficulties occurring a few months prior to the emergence of a florid lvPPA aphasic profile.87 In another study, Pozzebon et al.138 interviewed spouses of five lvPPA patients and found all carers to report incipient apathy and social withdrawal, plus deficits of episodic memory and sustained attention emerging almost 1–3 years before spousal recognition of frank expressive language deficits. These deficits were not core of the patients’ presentation, which was of language impairment, thus meeting the primary criteria for progressive aphasia. These findings indicate that non-linguistic deficits in lvPPA can emerge independent of and in parallel to primary aphasia, even where the patient’s core complaint is that of language difficulty.
Non-linguistic deficits emerge with disease progression
As with other progressive disorders, relatively ‘pure’ cases with circumscribed clinico-anatomical presentation evolve to show clear and increasing multidomain dysfunction as the underlying pathology propagates. Although longitudinal profiles of some lvPPA patients neatly fit this pattern,139 this hypothesis is not a sufficient explanation the early non-linguistic deficits in many other people with lvPPA. Even at the mildest clinician-indexed disease stages (mean Clinical Dementia Rating score of 0.5–0.7), difficulties in calculation and spatial working memory can be evident in lvPPA.7,8 Likewise, poor nonverbal episodic memory performance remains prominent in lvPPA, even after statistically controlling for disease severity.94 The confounding issue of disease severity has been addressed by recent data-driven studies parcellating its effects. For example, when overall language performance is employed as a categorical proxy for disease severity, the magnitude of general cognitive impairment scales with aphasia severity.79,140 A key limitation of this approach is that categorizing lvPPA patients first on aphasia severity and then on non-linguistic cognitive performance overlooks linguistic and non-linguistic difficulties occurring in parallel. Using step-wise classifications, therefore, may bias our determination of the absence/magnitude of non-linguistic complaints.
A recent study overcame this limitation by using principal component analysis in 43 well-characterized lvPPA patients, at varying disease stages, who had undergone comprehensive multidomain neuropsychological testing.106 The principal component analysis revealed multiple, concurrent sources of variance across neuropsychological performance in lvPPA. Extraction of orthogonal dimensions of performance variation allowed (i) exploration of confounds associated with disease and aphasia severity that otherwise pervade across measures; and (ii) situation of patients along independent dimensions of verbal and nonverbal cognitive performance thereby capturing their graded, inter-individual variations in cognitive impairment. The results indicated that the cognitive profile of lvPPA is best characterized along two orthogonal dimensions: (i) speech production and verbal memory deficits; and (ii) visuospatial and executive changes (Fig. 2B). Supporting our proposed framework, reduced integrity of left IPL and right fronto-parietal grey matter was characteristic of lvPPA cases with poorer visuo-executive performance. Three results from this study argue against the hypothesis that disease severity is the primary modulator of general cognitive deficits in lvPPA: (i) language and non-linguistic performance did not align with a single ‘disease severity’ factor but lay on orthogonal dimensions; (ii) the visuo-executive factor correlated weakly with independent clinical measures of disease severity and disease duration; and (iii) visuo-executive deficits were present systematically across the lvPPA cohort, irrespective of the magnitude of speech production difficulties.106 These findings are consistent with the case studies reporting concurrent verbal and non-verbal dysfunction early in the disease course; and formally show that multidimensional nonverbal cognitive difficulties are systematically present and form an early and integral part of the symptom complex of lvPPA.
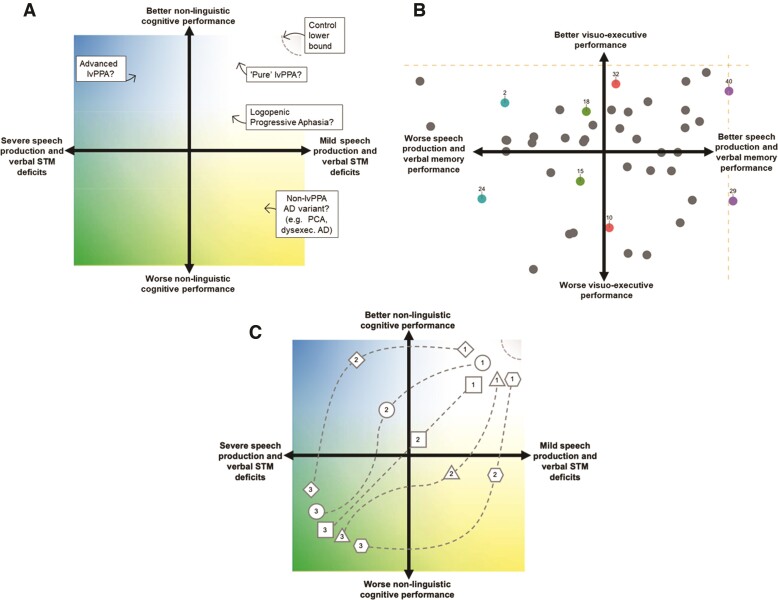
Figure 2.
Capturing phenotypic variations in lvPPA within a graded, multidimensional space. (A) Capturing cognitive changes to language and non-linguistic domains within a multidimensional space allows an examination of the graded, individual-level variations in patients’ profiles, as well as the underlying neural machinery that is affected in each case. STM = short-term memory; PCA = posterior cortical atrophy; AD = Alzheimer’s disease. (B) The nature of such a multidimensional graded space including the lvPPA phenotype receives support from the empirical findings of Ramanan et al.106 Here, principal component analysis on comprehensive neuropsychological performance data (n = 43 lvPPA) revealed multiple, independent sources of variation, captured best by two orthogonal dimensions of performance labelled ‘speech production and verbal memory’ and ‘visuospatial and executive’ (visuo-executive) performances. The finding that lvPPA patients, irrespective of the extent of their speech production and verbal memory performance, display some level of visuo-executive changes (c.f. coloured pairs) suggests individual-level variation in cognitive phenotype that necessitates the use of a multidimensional space. Here, dashed gold lines indicate lower bound of performance in healthy Controls (adapted from Ramanan et al.106). (C) The adoption of a multidimensional space to account for linguistic and non-linguistic changes in lvPPA can further afford mapping of heterogeneous disease trajectories dynamically varying at the individual-level. This includes individuals showing generalized cognitive impairment that are (i) linear with disease progression (square); (ii) emerge quickly into disease onset (triangle and pentagon); or (iii) emerge slowly with disease advancement (diamond and circle). Here, numbers correspond to the clinical assessment round (1 = 1st assessment etc.). STM = short term memory.
Accommodating lvPPA cognitive variations within a graded multidimensional phenotypic space
Given the evidence for multidomain cognitive dysfunction, it has been argued whether the term ‘lvPPA’ be reserved for ‘pure’ cases (impaired single-word retrieval and sentence/phrase repetition without concurrent non-linguistic impairment), while ‘Logopenic Progressive Aphasia’ might better capture individuals displaying a dynamic, multifaceted phenotype beyond the linguistic constraints of a pure PPA.125 Terminological distinctions are non-trivial; terminologies themselves are carefully derived and require universal consensus. Although language difficulties remain the primary presenting complaint for these patients, the evidence reviewed here might bring into question (i) whether additional non-linguistic cognitive dysfunction is representative of the syndromic designation of lvPPA; (ii) whether ‘logopenic progressive aphasia’ might better reflect the cognitive profile of such patients; or (iii) whether such cases may represent a new subtype/subcategory of lvPPA. How do we then best capture these terminological differences and inter-individual differences in cognitive performance?
As per recent developments in post-stroke aphasia and frontotemporal dementia studies,78,141–143 we propose that cognitive changes in individual lvPPA patients are best conceptualized as graded, individual-level differences within a multidimensional space (Fig. 2A). By this view, differences between two lvPPA patients in non-linguistic cognition do not necessarily reflect a sharp categorical distinction in their clinical conceptualization. Instead, these differences reflect graded, individual-level variations directly tied to disease encroachment into different brain regions. Importantly, embracing a graded, multidimensional approach does not require discarding current categorical labels. Instead, categorical labels serve as direct pointers to a specific region within this multidimensional space (Fig. 2A).
To simplify, we can use a colour analogy to represent this multidimensional space, with each axis representing systematic variation on verbal and nonverbal cognitive performance (Fig. 2A) as has been indicated by previous evidence (Fig. 2B).106 In this space, some cases may be ‘yellow’, others ‘blue’ and many others various intersections of these colours. In this hue space, ‘pure’ lvPPA patients (with prototypical aphasia and atrophy centred in posterior superior temporal gyrus/supramarginal gyrus) may represent a subsample of individuals within this larger space. What is important to recognize is that ‘pure’ lvPPA as a clinical entity does not occupy the entire multidimensional space, but rather locates in an area of a larger space spanning variable clinical presentations of the syndrome. Variations in atrophy/functional disruptions within the TPJ/IPL cortices, and connected regions, will generate graded variations in the type and severity of the individual verbal and nonverbal profile, determining locations of individuals within this space. In short, phenotypic deviations from prototypical lvPPA may be graded rather than absolute, and reflect the variations in local pathology (Fig. 2A).
The adoption of such a multidimensional space may further afford the mapping of dynamic cognitive devolution of individual lvPPA patients, tied closely to patterns of disease encroachment (Fig. 2C). It is proposed that, if phenotype can be multidimensional, disease progression can be too and the adoption of such a space can accommodate fluid and heterogeneous cognitive degradation patterns. This includes individuals showing generalized cognitive impairment that emerge (i) linearly with disease and aphasia progression (Fig. 2C, square); (ii) quickly into disease onset (Fig. 2C, triangle and pentagon); or (iii) slowly with disease advancement (Fig. 2C, diamond and circle). This space further enables comparison of different clinical syndromes (lvPPA, amnestic Alzheimer’s disease, posterior cortical atrophy) to derive transdiagnostic understandings of symptom progression. Ultimately, we propose that clinical subtypes of Alzheimer’s disease can be coherently conceptualized as displaying graded patterns of cognitive variation, mainly dependent on the neuroanatomical regions of impact.23,106,144 This proposal holds important clinical implications, especially when patients present with co-occurring language, memory and visuospatial difficulties that each require recognition and management. It holds importance for how individuals are sampled for research and clinical studies as ‘representative’ of a clinical condition, as the subspace from where we sample directly determines emergent results. This approach can also accommodate discrepancies regarding usage of the terminologies in published literature, such as ‘lvPPA’ versus ‘Logopenic Progressive Aphasia’ to refer to patients with prototypical lvPPA clinico-anatomical profiles, but with varying, graded levels of additional non-linguistic difficulties.
Conclusions
The evidence for non-linguistic cognitive deficits in lvPPA does not necessitate a change in current diagnostic criteria. Rather, the combination of linguistic and non-linguistic deficits indicates a need to re-evaluate the phenotype and pathophysiology, and consider the use of non-linguistic deficits in the diagnostic and management plan of lvPPA. We foresee three direct clinical advantages to this change.
First is the need to assess both language and non-linguistic features in PPA diagnosis. Naming, spontaneous speech and paraphasic/lexical error patterns, as individual measures, are increasingly recognized as providing poor specification of PPA type.78,126,145,146 On language performance alone, specific expertise is necessary to distinguish lvPPA from other PPA types78,147 and the clinical accuracy of language measures in differentiating PPA variants may not always improve upon the incorporation of additional pathological information.126,127 This complicates identification of the syndrome and, to the untrained clinical eye, risks conferring an lvPPA diagnosis through eliminating nfvPPA and svPPA labels. In contrast, recent work suggests that non-linguistic measures, such as nonverbal memory, may hold >80% accuracy in distinguishing lvPPA from other non-fluent variants (if not from amnestic Alzheimer’s disease).94 While the discriminative use of other non-linguistic tests remains to be explored, co-reliance on linguistic and non-linguistic measures uniquely stressing functions supported by TPJ/IPL may significantly improve accurate diagnosis of lvPPA. For practising clinicians, detailed testing of verbal and nonverbal cognition in the syndrome can improve understanding of the structure of this multidimensional space and help identify measures that are highly representative of each axis of space. In turn, this information can be used to cull lengthy test batteries and retain those efficient and effective measures that capture the most important cognitive-behavioural variation in lvPPA. The success of this method has recently been demonstrated in post-stroke aphasia148; how informative it is in PPA, where aetiology, cognitive performance and progression patterns are complex and highly variable, remains to be better understood. At the point of diagnosis, specific quadrants/locations within this space may also inform selection, titration and timing of management approaches, therapies and treatments, as well as inclusion and stratification of specific individuals into trials.
Second, given their importance in the multifaceted clinical profile of lvPPA, TPJ/IPL and parietal cortex seem likely to become neuroanatomical targets of symptomatic intervention in this syndrome. In this regard, recent work has revealed links between excitatory neurostimulation of prefrontal regions and facilitation of oral and written language improvement in lvPPA.149,150 As for the IPL, emerging evidence in PPA indicates that a combination of IPL neurostimulation and speech and language training can induce (i) improved naming and verbal fluency performance; (ii) transfer of gains to unlearnt items and select cognitive domains (e.g. overall digit span performance); and (iii) benefits sustaining for up to 2 weeks.151,152 These findings indicate the need for more studies exploring interventions targeting the parietal cortex for linguistic, non-linguistic cognitive and functional improvement in lvPPA. Moreover, TPJ/IPL and general parietal dysfunction are largely considered to form the common neuroanatomical denominator associated with phenotypic diversification across several posterior cortical neurodegenerative syndromes, many of which share Alzheimer’s disease pathology.23,92,153,154 Shifting the focus to reveal the importance of parietal integrity in Alzheimer’s disease will move the field a step closer to achieving a full understanding of the interplay between neurodegeneration and cognitive dysfunction in multiple clinical variants of Alzheimer’s disease.
As we make progress on the aforementioned steps, it seems likely that we will uncover deeper layers to the lvPPA cognitive and behavioural profile. Therefore, a final, important step forward is to build integrative frameworks of cognitive and brain mechanisms that underpin the broader symptom complex of lvPPA, with relevance to other neurodegenerative dementia syndromes affecting the posterior neocortex. This larger programme requires a two-pronged approach, with each step informing the other. What we first need is a better understanding of how psychological and pathophysiological changes in lvPPA relate to changes in local neural populations and their neurochemistry, genetics and neuropathology, and neuroimaging in the syndrome. This will allow integration of new knowledge regarding brain, behaviour and biological changes in lvPPA with current neurocognitive models. Next, testing the stability and interpretability of these renewed models requires international, multi-centre studies to establish large lvPPA cohorts with deep neuropsychological and neuroimaging phenotyping, and identify candidate neurophysiological biomarkers in the diagnostic process. We acknowledge that this future direction is ambitious and requires navigating recruitment challenges and nosological disagreements. However, we hope that our framework can aid this process by accommodating terminological discrepancies and diverse clinical-cognitive presentations within a multidimensional space, and potentially offer a common research framework to springboard future efforts towards a complete understanding of the lvPPA phenotype.
Abbreviations
- IPL =
inferior parietal lobe
- lvPPA =
logopenic variant primary progressive aphasia
- nfvPPA =
non-fluent/agrammatic variant PPA
- PPA =
primary progressive aphasia
- svPPA =
semantic variant PPA
- TPJ =
temporoparietal junction
Contributor Information
Siddharth Ramanan, Medical Research Council Cognition and Brain Sciences Unit, University of Cambridge, Cambridge, UK.
Muireann Irish, The University of Sydney, Brain and Mind Centre and School of Psychology, Sydney, Australia.
Karalyn Patterson, Medical Research Council Cognition and Brain Sciences Unit, University of Cambridge, Cambridge, UK.
James B Rowe, Medical Research Council Cognition and Brain Sciences Unit, University of Cambridge, Cambridge, UK; Department of Clinical Neurosciences, Cambridge University Centre for Frontotemporal Dementia, Cambridge, UK; Cambridge University Hospitals NHS Trust, Cambridge, UK.
Maria Luisa Gorno-Tempini, Memory and Aging Center, University of California San Francisco, San Francisco, USA.
Matthew A Lambon Ralph, Medical Research Council Cognition and Brain Sciences Unit, University of Cambridge, Cambridge, UK.
Funding
M.I. is supported by an Australian Research Council Future Fellowship (FT160100096). J.B.R. is supported by the Medical Research Council (SUAG/051 G101400) and the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014; the views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care); and the Cambridge Centre for Parkinson-Plus. M.L.G.T. is supported by the National Institutes of Health (NINDS R01NS050915, K24DC015544). M.A.L.R. is supported by an advanced ERC award (GAP: 670428-30 BRAIN2MIND_NEUROCOMP), MRC programme grant (MR/R023883/1) and intramural funding (MC_UU_00005/18).
Competing interests
The authors report no competing interests.
References
- 1. Mesulam MM. Primary progressive aphasia. Ann Neurol. 2001;49:425–432. [PubMed] [Google Scholar]
- 2. Gorno-Tempini ML, Hillis AE, Weintraub S, et al. . Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mesulam MM. Slowly progressive aphasia without generalized dementia. Ann Neurol. 1982;11:592–598. [DOI] [PubMed] [Google Scholar]
- 4. Weintraub S, Rubin NP, Mesulam MM. Primary progressive aphasia. Longitudinal course, neuropsychological profile, and language features. Arch Neurol. 1990;47:1329–1335. [DOI] [PubMed] [Google Scholar]
- 5. Grossman M. The non-fluent/agrammatic variant of primary progressive aphasia. Lancet Neurol. 2012;11:545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hodges JR, Patterson K. Semantic dementia: A unique clinicopathological syndrome. Lancet Neurol. 2007;6:1004–1014. [DOI] [PubMed] [Google Scholar]
- 7. Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. . Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gorno-Tempini ML, Brambati SM, Ginex V, et al. . The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71:1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wilson SM, Galantucci S, Tartaglia MC, Gorno-Tempini ML. The neural basis of syntactic deficits in primary progressive aphasia. Brain Lang. 2012;122:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tee BL, Gorno-Tempini ML. Primary progressive aphasia: A model for neurodegenerative disease. Curr Opin Neurol. 2019;32:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leyton CE, Piguet O, Savage S, Burrell J, Hodges JR. The neural basis of logopenic progressive aphasia. J Alzheimers Dis. 2012;32:1051–1059. [DOI] [PubMed] [Google Scholar]
- 12. Brown JA, Deng J, Neuhaus J, et al. . Patient–tailored, connectivity-based forecasts of spreading brain atrophy. Neuron. 2019;104:856–868.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mandelli ML, Vilaplana E, Brown JA, et al. . Healthy brain connectivity predicts atrophy progression in non-fluent variant of primary progressive aphasia. Brain. 2016;139(Pt 10):2778–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Battistella G, Borghesani V, Henry M, et al. . Task-free functional language networks: Reproducibility and clinical application. J Neurosci. 2020;40:1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sintini I, Graff-Radford J, Senjem ML, et al. . Longitudinal neuroimaging biomarkers differ across Alzheimer’s disease phenotypes. Brain. 2020;143:2281–2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Graff-Radford J, Yong KXX, Apostolova LG, et al. . New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet Neurol. 2021;20:222–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rohrer JD, Caso F, Mahoney C, et al. . Patterns of longitudinal brain atrophy in the logopenic variant of primary progressive aphasia. Brain Lang. 2013;127:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brambati SM, Amici S, Racine CA, et al. . Longitudinal gray matter contraction in three variants of primary progressive aphasia: A tenser-based morphometry study. Neuroimage Clin. 2015;8:345–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rogalski EJ, Sridhar J, Martersteck A, et al. . Clinical and cortical decline in the aphasic variant of Alzheimer’s disease. Alzheimers Dement. 2019;15:543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Owens TE, Machulda MM, Duffy JR, et al. . Patterns of neuropsychological dysfunction and cortical volume changes in logopenic aphasia. J Alzheimers Dis. 2018;66:1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kamath V, Sutherland ER, Chaney GA. A meta-analysis of neuropsychological functioning in the logopenic variant of primary progressive aphasia: Comparison with the semantic and non-fluent variants. J Int Neuropsychol Soc. 2020;26:322–330. [DOI] [PubMed] [Google Scholar]
- 22. Ruksenaite J, Volkmer A, Jiang J, et al. . Primary progressive aphasia: Toward a pathophysiological synthesis. Curr Neurol Neurosci Rep. 2021;21:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Warren JD, Fletcher PD, Golden HL. The paradox of syndromic diversity in Alzheimer disease. Nat Rev Neurol. 2012;8:451–464. [DOI] [PubMed] [Google Scholar]
- 24. Mesulam MM, Rogalski EJ, Wieneke C, et al. . Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10:554–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Marshall CR, Hardy CJD, Volkmer A, et al. . Primary progressive aphasia: A clinical approach. J Neurol. 2018;265:1474–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Igelstrom KM, Graziano MSA. The inferior parietal lobule and temporoparietal junction: A network perspective. Neuropsychologia. 2017;105:70–83. [DOI] [PubMed] [Google Scholar]
- 27. Seghier ML. The angular gyrus: Multiple functions and multiple subdivisions. Neuroscientist. 2013;19:43–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Humphreys GF, Lambon Ralph MA. Fusion and fission of cognitive functions in the human parietal cortex. Cereb Cortex. 2015;25:3547–3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patel GH, Sestieri C, Corbetta M. The evolution of the temporoparietal junction and posterior superior temporal sulcus. Cortex. 2019;118:38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vallar G. Spatial neglect, Balint-Homes’ and Gerstmann’s syndrome, and other spatial disorders. CNS Spectr. 2007;12:527–536. [DOI] [PubMed] [Google Scholar]
- 31. Humphreys GF, Jackson RL, Lambon Ralph MA. Overarching principles and dimensions of the functional organization in the inferior parietal cortex. Cereb Cortex. 2020;30:5639–5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Margulies DS, Ghosh SS, Goulas A, et al. . Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc Natl Acad Sci U S A. 2016;113:12574–12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mesulam MM. From sensation to cognition. Brain. 1998;121(Pt 6):1013–1052. [DOI] [PubMed] [Google Scholar]
- 34. Ramanan S, Bellana B. A domain-general role for the angular gyrus in retrieving internal representations of the external world. J Neurosci. 2019;39:2978–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McClelland JL, St John M, Taraban R. Sentence comprehension: A parallel distributed processing approach. Lang Cogn Process. 1989;4:SI287–SI335. [Google Scholar]
- 36. Botvinick M, Plaut DC. Doing without schema hierarchies: A recurrent connectionist approach to normal and impaired routine sequential action. Psychol Rev. 2004;111:395–429. [DOI] [PubMed] [Google Scholar]
- 37. Head H. Aphasia and kindred disorders of speech. Cambridge Univ. Press; 1926. [DOI] [PubMed] [Google Scholar]
- 38. Jefferies E, Lambon Ralph MA. Semantic impairment in stroke aphasia versus semantic dementia: a case-series comparison. Brain. 2006;129(Pt 8):2132–2147. [DOI] [PubMed] [Google Scholar]
- 39. Lerner Y, Honey CJ, Silbert LJ, Hasson U. Topographic mapping of a hierarchy of temporal receptive windows using a narrated story. J Neurosci. 2011;31:2906–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kanwisher N. The quest for the FFA and where it led. J Neurosci. 2017;37:1056–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Warren JD, Zielinski BA, Green GG, Rauschecker JP, Griffiths TD. Perception of sound-source motion by the human brain. Neuron. 2002;34:139–148. [DOI] [PubMed] [Google Scholar]
- 42. Hartwigsen G, Baumgaertner A, Price CJ, Koehnke M, Ulmer S, Siebner HR. Phonological decisions require both the left and right supramarginal gyri. Proc Natl Acad Sci U S A. 2010;107:16494–16499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Warren JE, Wise RJ, Warren JD. Sounds do-able: auditory-motor transformations and the posterior temporal plane. Trends Neurosci. 2005;28:636–643. [DOI] [PubMed] [Google Scholar]
- 44. Griffiths TD, Warren JD. The planum temporale as a computational hub. Trends Neurosci. 2002;25:348–353. [DOI] [PubMed] [Google Scholar]
- 45. Hickok G, Okada K, Serences JT. Area SPT in the human planum temporale supports sensory-motor integration for speech processing. J Neurophysiol. 2009;101:2725–2732. [DOI] [PubMed] [Google Scholar]
- 46. Noonan KA, Jefferies E, Visser M, Lambon Ralph MA. Going beyond inferior prefrontal involvement in semantic control: Evidence for the additional contribution of dorsal angular gyrus and posterior middle temporal cortex. J Cogn Neurosci. 2013;25:1824–1850. [DOI] [PubMed] [Google Scholar]
- 47. Jackson RL. The neural correlates of semantic control revisited. Neuroimage. 2021;224:117444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ralph MA, Jefferies E, Patterson K, Rogers TT. The neural and computational bases of semantic cognition. Nat Rev Neurosci. 2017;18:42–55. [DOI] [PubMed] [Google Scholar]
- 49. Rugg MD, King DR. Ventral lateral parietal cortex and episodic memory retrieval. Cortex. 2018;107:238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. [DOI] [PubMed] [Google Scholar]
- 51. Humphreys GF, Jung J, Lambon Ralph MA. The convergence and divergence of episodic and semantic functions across lateral parietal cortex. Cereb Cortex. Published online 23 Feb 2022. doi:10.1093/cercor/bhac044. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Price AR, Bonner MF, Peelle JE, Grossman M. Converging evidence for the neuroanatomic basis of combinatorial semantics in the angular gyrus. J Neurosci. 2015;35:3276–3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Branzi FM, Pobric G, Jung J, Lambon Ralph MA. The left angular gyrus is causally involved in context-dependent integration and associative encoding during narrative reading. J Cogn Neurosci. 2021;33:1082–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Humphreys GF, Lambon Ralph MA. Mapping domain-selective and counterpointed domain-general higher cognitive functions in the lateral parietal cortex: Evidence from fMRI comparisons of difficulty-varying semantic versus visuo-spatial tasks, and functional connectivity analyses. Cereb Cortex. 2017;27:4199–4212. [DOI] [PubMed] [Google Scholar]
- 55. Dronkers NF, Wilkins DP, Van Valin RD Jr, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. [DOI] [PubMed] [Google Scholar]
- 56. Fridriksson J, Kjartansson O, Morgan PS, et al. . Impaired speech repetition and left parietal lobe damage. J Neurosci. 2010;30:11057–11061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Berthier ML. Transcortical aphasias. Psychology Press; 1999. [Google Scholar]
- 58. Robson H, Sage K, Ralph MA. Wernicke’s aphasia reflects a combination of acoustic-phonological and semantic control deficits: A case-series comparison of Wernicke’s aphasia, semantic dementia and semantic aphasia. Neuropsychologia. 2012;50:266–275. [DOI] [PubMed] [Google Scholar]
- 59. Hutchinson JB, Uncapher MR, Wagner AD. Posterior parietal cortex and episodic retrieval: convergent and divergent effects of attention and memory. Learn Mem. 2009;16:343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ramanan S, Piguet O, Irish M. Rethinking the role of the angular gyrus in remembering the past and imagining the future: The contextual integration model. Neuroscientist. 2018;24:342–352. [DOI] [PubMed] [Google Scholar]
- 61. Sestieri C, Corbetta M, Romani GL, Shulman GL. Episodic memory retrieval, parietal cortex, and the default mode network: Functional and topographic analyses. J Neurosci. 2011;31:4407–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ben-Zvi S, Soroker N, Levy DA. Parietal lesion effects on cued recall following pair associate learning. Neuropsychologia. 2015;73:176–194. [DOI] [PubMed] [Google Scholar]
- 63. Ramanan S, Strikwerda-Brown C, Mothakunnel A, Hodges JR, Piguet O, Irish M. Fronto-parietal contributions to episodic retrieval—Evidence from neurodegenerative disorders. Learn Mem. 2019;26:262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ramanan S, Alaeddin S, Goldberg ZL, Strikwerda-Brown C, Hodges JR, Irish M. Exploring the contribution of visual imagery to scene construction—Evidence from posterior cortical atrophy. Cortex. 2018;106:261–274. [DOI] [PubMed] [Google Scholar]
- 65. Samson D, Apperly IA, Chiavarino C, Humphreys GW. Left temporoparietal junction is necessary for representing someone else’s belief. Nat Neurosci. 2004;7:499–500. [DOI] [PubMed] [Google Scholar]
- 66. Ciaramelli E, Rosenbaum RS, Solcz S, Levine B, Moscovitch M. Mental space travel: Damage to posterior parietal cortex prevents egocentric navigation and reexperiencing of remote spatial memories. J Exp Psychol Learn Mem Cogn. 2010;36:619–634. [DOI] [PubMed] [Google Scholar]
- 67. Grabner RH, Ansari D, Koschutnig K, Reishofer G, Ebner F, Neuper C. To retrieve or to calculate? Left angular gyrus mediates the retrieval of arithmetic facts during problem solving. Neuropsychologia. 2009;47:604–608. [DOI] [PubMed] [Google Scholar]
- 68. Rushworth MF, Krams M, Passingham RE. The attentional role of the left parietal cortex: The distinct lateralization and localization of motor attention in the human brain. J Cogn Neurosci. 2001;13:698–710. [DOI] [PubMed] [Google Scholar]
- 69. Tumati S, Martens S, de Jong BM, Aleman A. Lateral parietal cortex in the generation of behavior: Implications for apathy. Prog Neurobiol. 2019;175:20–34. [DOI] [PubMed] [Google Scholar]
- 70. Mesulam M. Principles of behavioral and cognitive neurology. Oxford University Press; 2000. [Google Scholar]
- 71. Leyton CE, Hodges JR. Towards a clearer definition of logopenic progressive aphasia. Curr Neurol Neurosci Rep. 2013;13:396. [DOI] [PubMed] [Google Scholar]
- 72. Teichmann M, Kas A, Boutet C, et al. . Deciphering logopenic primary progressive aphasia: A clinical, imaging and biomarker investigation. Brain. 2013;136(Pt 11):3474–3488. [DOI] [PubMed] [Google Scholar]
- 73. Hardy CJD, Agustus JL, Marshall CR, et al. . Functional neuroanatomy of speech signal decoding in primary progressive aphasias. Neurobiol Aging. 2017;56:190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lukic S, Mandelli ML, Welch A, et al. . Neurocognitive basis of repetition deficits in primary progressive aphasia. Brain Lang. 2019;194:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Henry ML, Gorno-Tempini ML. The logopenic variant of primary progressive aphasia. Curr Opin Neurol. 2010;23:633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Baldo JV, Dronkers NF. The role of inferior parietal and inferior frontal cortex in working memory. Neuropsychology. 2006;20:529–538. [DOI] [PubMed] [Google Scholar]
- 77. Hickok G, Poeppel D. Dorsal and ventral streams: A framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. [DOI] [PubMed] [Google Scholar]
- 78. Ingram RU, Halai AD, Pobric G, Sajjadi S, Patterson K, Lambon Ralph MA. Graded, multidimensional intra- and intergroup variations in primary progressive aphasia and post-stroke aphasia. Brain. 2020;143:3121–3135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Leyton CE, Hodges JR, McLean CA, Kril JJ, Piguet O, Ballard KJ. Is the logopenic-variant of primary progressive aphasia a unitary disorder? Cortex. 2015;67:122–133. [DOI] [PubMed] [Google Scholar]
- 80. Louwersheimer E, Keulen MA, Steenwijk MD, et al. . Heterogeneous language profiles in patients with primary progressive aphasia due to Alzheimer’s disease. J Alzheimers Dis. 2016;51:581–590. [DOI] [PubMed] [Google Scholar]
- 81. Mandelli ML, Caverzasi E, Binney RJ, et al. . Frontal white matter tracts sustaining speech production in primary progressive aphasia. J Neurosci. 2014;34:9754–9767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Harris JM, Saxon JA, Jones M, Snowden JS, Thompson JC. Neuropsychological differentiation of progressive aphasic disorders. J Neuropsychol. 2019;13:214–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Magnin E, Chopard G, Ferreira S, et al. . Initial neuropsychological profile of a series of 20 patients with logopenic variant of primary progressive aphasia. J Alzheimers Dis. 2013;36:799–808. [DOI] [PubMed] [Google Scholar]
- 84. Macoir J, Lavoie M, Laforce R Jr, Brambati SM, Wilson MA. Dysexecutive symptoms in primary progressive aphasia: Beyond diagnostic criteria. J Geriatr Psychiatry Neurol. 2017;30:151–161. [DOI] [PubMed] [Google Scholar]
- 85. Mendez MF, Monserratt LH, Liang LJ, et al. . Neuropsychological similarities and differences between amnestic Alzheimer’s disease and its non-amnestic variants. J Alzheimers Dis. 2019;69:849–855. [DOI] [PubMed] [Google Scholar]
- 86. Funayama M, Nakagawa Y, Yamaya Y, Yoshino F, Mimura M, Kato M. Progression of logopenic variant primary progressive aphasia to apraxia and semantic memory deficits. BMC Neurol. 2013;13:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zilli EM, Heilman KM. Spatial neglect in a patient with logopenic progressive aphasia. Neurocase. 2016;22:30–39. [DOI] [PubMed] [Google Scholar]
- 88. Corbett F, Jefferies E, Burns A, Lambon Ralph MA. Deregulated semantic cognition contributes to object-use deficits in Alzheimer’s disease: A comparison with semantic aphasia and semantic dementia. J Neuropsychol. 2015;9:219–241. [DOI] [PubMed] [Google Scholar]
- 89. Corbett F, Jefferies E, Ehsan S, Lambon Ralph MA. Different impairments of semantic cognition in semantic dementia and semantic aphasia: Evidence from the non-verbal domain. Brain. 2009;132(Pt 9):2593–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Corbett F, Jefferies E, Ralph MA. Exploring multimodal semantic control impairments in semantic aphasia: Evidence from naturalistic object use. Neuropsychologia. 2009;47:2721–2731. [DOI] [PubMed] [Google Scholar]
- 91. Berryhill ME. Insights from neuropsychology: pinpointing the role of the posterior parietal cortex in episodic and working memory. Front Integr Neurosci. 2012;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Migliaccio R, Agosta F, Rascovsky K, et al. . Clinical syndromes associated with posterior atrophy: early age at onset AD spectrum. Neurology. 2009;73:1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kakinuma K, Baba T, Ezura M, et al. . Logopenic aphasia due to Lewy body disease dramatically improved with donepezil. eNeurologicalSci. 2020;19:100241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Ramanan S, Flanagan E, Leyton CE, et al. . Non-verbal episodic memory deficits in primary progressive aphasias are highly predictive of underlying amyloid pathology. J Alzheimers Dis. 2016;51:367–376. [DOI] [PubMed] [Google Scholar]
- 95. Casaletto KB, Marx G, Dutt S, et al. . Is ‘learning’ episodic memory? Distinct cognitive and neuroanatomic correlates of immediate recall during learning trials in neurologically normal aging and neurodegenerative cohorts. Neuropsychologia. 2017;102:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ramanan S, Marstaller L, Hodges JR, Piguet O, Irish M. Understanding the neural basis of episodic amnesia in logopenic progressive aphasia: A multimodal neuroimaging study. Cortex. 2020;125:272–287. [DOI] [PubMed] [Google Scholar]
- 97. Ramanan S, Foxe D, El-Omar H, et al. . Evidence for a pervasive autobiographical memory impairment in logopenic progressive aphasia. Neurobiol Aging. 2021;108:168–178. [DOI] [PubMed] [Google Scholar]
- 98. Goll JC, Kim LG, Hailstone JC, et al. . Auditory object cognition in dementia. Neuropsychologia. 2011;49:2755–2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Golden HL, Clark CN, Nicholas JM, et al. . Music perception in dementia. J Alzheimers Dis. 2017;55:933–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Johnson JCS, Jiang J, Bond RL, et al. . Impaired phonemic discrimination in logopenic variant primary progressive aphasia. Ann Clin Transl Neurol. 2020;7:1252–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Johnson JCS, Marshall CR, Weil RS, Bamiou DE, Hardy CJD, Warren JD. Hearing and dementia: From ears to brain. Brain. 2021;144:391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Foxe D, Leyton CE, Hodges JR, Burrell JR, Irish M, Piguet O. The neural correlates of auditory and visuospatial span in logopenic progressive aphasia and Alzheimer’s disease. Cortex. 2016;83:39–50. [DOI] [PubMed] [Google Scholar]
- 103. Foxe D, Irish M, Roquet D, et al. . Visuospatial short-term and working memory disturbance in the primary progressive aphasias: Neuroanatomical and clinical implications. Cortex. 2020;132:223–237. [DOI] [PubMed] [Google Scholar]
- 104. Whitwell JL, Jones DT, Duffy JR, et al. . Working memory and language network dysfunctions in logopenic aphasia: A task-free fMRI comparison with Alzheimer’s dementia. Neurobiol Aging. 2015;36:1245–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Bejanin A, Schonhaut DR, La Joie R, et al. . Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer’s disease. Brain. 2017;140:3286–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ramanan S, Roquet D, Goldberg ZL, et al. . Establishing two principal dimensions of cognitive variation in logopenic progressive aphasia. Brain Commun. 2020;2:fcaa125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Krishnan K, Machulda MM, Whitwell JL, et al. . Varying degrees of temporoparietal hypometabolism on FDG-PET reveal amyloid-positive logopenic primary progressive aphasia is not a homogeneous clinical entity. J Alzheimers Dis. 2017;55:1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Fittipaldi S, Ibanez A, Baez S, Manes F, Sedeno L, Garcia AM. More than words: Social cognition across variants of primary progressive aphasia. Neurosci Biobehav Rev. 2019;100:263–284. [DOI] [PubMed] [Google Scholar]
- 109. Multani N, Galantucci S, Wilson SM, et al. . Emotion detection deficits and changes in personality traits linked to loss of white matter integrity in primary progressive aphasia. Neuroimage Clin. 2017;16:447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wong S, Irish M, Husain M, Hodges JR, Piguet O, Kumfor F. Apathy and its impact on carer burden and psychological wellbeing in primary progressive aphasia. J Neurol Sci. 2020;416:117007. [DOI] [PubMed] [Google Scholar]
- 111. Foxe D, Irish M, Ramanan S, et al. . Longitudinal changes in behaviour, mood and functional capacity in the primary progressive aphasia variants. Eur J Neurosci. doi: 10.1111/ejn.15557. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 112. Rohrer JD, Warren JD. Phenomenology and anatomy of abnormal behaviours in primary progressive aphasia. J Neurol Sci. 2010;293:35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hazelton JL, Irish M, Hodges JR, Piguet O, Kumfor F. Cognitive and affective empathy disruption in non-fluent primary progressive aphasia syndromes. Brain Impair. 2017;18:117–129. [Google Scholar]
- 114. Goldberg ZL, El-Omar H, Foxe D, et al. . Cognitive and neural mechanisms of social communication dysfunction in primary progressive aphasia. Brain Sci. 2021;11:1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Chare L, Hodges JR, Leyton CE, et al. . New criteria for frontotemporal dementia syndromes: Clinical and pathological diagnostic implications. J Neurol Neurosurg Psychiatry. 2014;85:865–870. [DOI] [PubMed] [Google Scholar]
- 116. Leyton CE, Villemagne VL, Savage S, et al. . Subtypes of progressive aphasia: application of the international consensus criteria and validation using beta-amyloid imaging. Brain. 2011;134(Pt 10):3030–3043. [DOI] [PubMed] [Google Scholar]
- 117. Rabinovici GD, Jagust WJ, Furst AJ, et al. . Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol. 2008;64:388–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Santos-Santos MA, Rabinovici GD, Iaccarino L, et al. . Rates of amyloid imaging positivity in patients with primary progressive aphasia. JAMA Neurol. 2018;75:342–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Saracino D, Ferrieux S, Nogues-Lassiaille M, et al. . Primary progressive aphasia associated with GRN mutations: New insights into the nonamyloid logopenic variant. Neurology. 2021;97:e88–e102. [DOI] [PubMed] [Google Scholar]
- 120. Josephs KA, Duffy JR, Strand EA, et al. . Progranulin-associated PiB-negative logopenic primary progressive aphasia. J Neurol. 2014;261:604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Snowden JS, Pickering-Brown SM, Mackenzie IR, et al. . Progranulin gene mutations associated with frontotemporal dementia and progressive non-fluent aphasia. Brain. 2006;129(Pt 11):3091–3102. [DOI] [PubMed] [Google Scholar]
- 122. Mesulam M, Johnson N, Krefft TA, et al. . Progranulin mutations in primary progressive aphasia: The PPA1 and PPA3 families. Arch Neurol. 2007;64:43–47. [DOI] [PubMed] [Google Scholar]
- 123. Rohrer JD, Ridgway GR, Crutch SJ, et al. . Progressive logopenic/phonological aphasia: Erosion of the language network. Neuroimage. 2010;49:984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Rohrer JD, Rossor MN, Warren JD. Syndromes of nonfluent primary progressive aphasia: A clinical and neurolinguistic analysis. Neurology. 2010;75:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Mesulam MM, Coventry C, Kuang A, et al. . Memory resilience in Alzheimer’s disease with primary progressive aphasia. Neurology. 2021;96:e916–e925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Giannini LAA, Irwin DJ, McMillan CT, et al. . Clinical marker for Alzheimer disease pathology in logopenic primary progressive aphasia. Neurology. 2017;88:2276–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Tetzloff KA, Whitwell JL, Utianski RL, et al. . Quantitative assessment of grammar in amyloid-negative logopenic aphasia. Brain Lang. 2018;186:26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Whitwell JL, Duffy JR, Strand EA, et al. . Clinical and neuroimaging biomarkers of amyloid-negative logopenic primary progressive aphasia. Brain Lang. 2015;142:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Matias-Guiu JA, Cabrera-Martin MN, Moreno-Ramos T, et al. . Amyloid and FDG-PET study of logopenic primary progressive aphasia: Evidence for the existence of two subtypes. J Neurol. 2015;262:1463–1472. [DOI] [PubMed] [Google Scholar]
- 130. Whitwell JL, Lowe VJ, Duffy JR, et al. . Elevated occipital beta-amyloid deposition is associated with widespread cognitive impairment in logopenic progressive aphasia. J Neurol Neurosurg Psychiatry. 2013;84:1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Magnin E, Sylvestre G, Lenoir F, et al. . Logopenic syndrome in posterior cortical atrophy. J Neurol. 2013;260:528–533. [DOI] [PubMed] [Google Scholar]
- 132. Crutch SJ, Lehmann M, Warren JD, Rohrer JD. The language profile of posterior cortical atrophy. J Neurol Neurosurg Psychiatry. 2013;84:460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Putcha D, Dickerson BC, Brickhouse M, Johnson KA, Sperling RA, Papp KV. Word retrieval across the biomarker-confirmed Alzheimer’s disease syndromic spectrum. Neuropsychologia. 2020;140:107391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Brambati SM, Ogar J, Neuhaus J, Miller BL, Gorno-Tempini ML. Reading disorders in primary progressive aphasia: A behavioral and neuroimaging study. Neuropsychologia. 2009;47:1893–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Win KT, Pluta J, Yushkevich P, et al. . Neural correlates of verbal episodic memory and lexical retrieval in logopenic variant primary progressive aphasia. Front Neurosci. 2017;11:330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Watson CL, Possin K, Allen IE, et al. . Visuospatial functioning in the primary progressive aphasias. J Int Neuropsychol Soc. 2018;24:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Foxe D, Irish M, Hodges JR, Piguet O. Verbal and visuospatial span in logopenic progressive aphasia and Alzheimer’s disease. J Int Neuropsychol Soc. 2013;19:247–253. [DOI] [PubMed] [Google Scholar]
- 138. Pozzebon M, Douglas J, Ames D. Spousal recollections of early signs of primary progressive aphasia. Int J Lang Commun Disord. 2018;53:282–293. [DOI] [PubMed] [Google Scholar]
- 139. Etcheverry L, Seidel B, Grande M, et al. . The time course of neurolinguistic and neuropsychological symptoms in three cases of logopenic primary progressive aphasia. Neuropsychologia. 2012;50:1708–1718. [DOI] [PubMed] [Google Scholar]
- 140. Machulda MM, Whitwell JL, Duffy JR, et al. . Identification of an atypical variant of logopenic progressive aphasia. Brain Lang. 2013;127:139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Butler RA, Lambon Ralph MA, Woollams AM. Capturing multidimensionality in stroke aphasia: mapping principal behavioural components to neural structures. Brain. 2014;137(Pt 12):3248–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Murley AG, Coyle-Gilchrist I, Rouse MA, et al. . Redefining the multidimensional clinical phenotypes of frontotemporal lobar degeneration syndromes. Brain. 2020;143:1555–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Ramanan S, El-Omar H, Roquet D, et al. . Mapping behavioural, cognitive and affective transdiagnostic dimensions in frontotemporal dementia, medRxiv, https://www.medrxiv.org/content/10.1101/2021.10.29.21265655v1, 2021 preprint: not peer reviewed. [DOI] [PMC free article] [PubMed]
- 144. Lambon Ralph MA, Patterson K, Graham N, Dawson K, Hodges JR. Homogeneity and heterogeneity in mild cognitive impairment and Alzheimer’s disease: A cross-sectional and longitudinal study of 55 cases. Brain. 2003;126(Pt 11):2350–2362. [DOI] [PubMed] [Google Scholar]
- 145. Tippett DC. Classification of primary progressive aphasia: challenges and complexities. F1000Res. 2020;9:F1000 Faculty Rev-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Budd MA, Kortte K, Cloutman L, et al. . The nature of naming errors in primary progressive aphasia versus acute post-stroke aphasia. Neuropsychology. 2010;24:581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Sajjadi SA, Patterson K, Arnold RJ, Watson PC, Nestor PJ. Primary progressive aphasia: A tale of two syndromes and the rest. Neurology. 2012;78:1670–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Halai AD, Perez BDD, Stefaniak JD, Lambon Ralph MA. Comparing short and long batteries to assess deficits and their neural bases in stroke aphasia, biorxiv,https://www.biorxiv.org/content/10.1101/2020.11.24.395590v1, 2020 preprint: not peer reviewed.
- 149. Trebbastoni A, Raccah R, de Lena C, Zangen A, Inghilleri M. Repetitive deep transcranial magnetic stimulation improves verbal fluency and written language in a patient with primary progressive aphasia-logopenic variant (LPPA). Brain Stimul. 2013;6:545–553. [DOI] [PubMed] [Google Scholar]
- 150. Bereau M, Magnin E, Nicolier M, et al. . Left prefrontal repetitive transcranial magnetic stimulation in a logopenic variant of primary progressive aphasia: A case report. Eur Neurol. 2016;76:12–18. [DOI] [PubMed] [Google Scholar]
- 151. Roncero C, Kniefel H, Service E, Thiel A, Probst S, Chertkow H. Inferior parietal transcranial direct current stimulation with training improves cognition in anomic Alzheimer’s disease and frontotemporal dementia. Alzheimers Dement (N Y). 2017;3:247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. de Aguiar V, Rofes A, Wendt H, Ficek BN, Webster K, Tsapkini K. Treating lexical retrieval using letter fluency and tDCS in primary progressive aphasia: a single-case study. Aphasiology. 2022;36:353–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Ossenkoppele R, Cohn-Sheehy BI, La Joie R, et al. . Atrophy patterns in early clinical stages across distinct phenotypes of Alzheimer’s disease. Hum Brain Mapp. 2015;36:4421–4437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Jacobs HI, Van Boxtel MP, Jolles J, Verhey FR, Uylings HB. Parietal cortex matters in Alzheimer’s disease: An overview of structural, functional and metabolic findings. Neurosci Biobehav Rev. 2012;36:297–309. [DOI] [PubMed] [Google Scholar]