Abstract
Background:
Salmonella infections continue to be of global concern to poultry health, productivity, and public health. About 44% of the poultry in Nigeria are indigenous and kept in close interaction with farmers who are mostly rural dwellers and have limited access to veterinary and extension services.
Aim:
The perceptions and practices of farmers of indigenous poultry toward Salmonella infections were assessed to obtain and document baseline data that can be used to create awareness among farmers about these infections and their attendant public health implications.
Methods:
A cross-sectional approach using a multistage sampling method was used in this survey. A total of 419 farmers keeping indigenous poultry were interviewed using a pre-tested electronic questionnaire in three randomly selected states within North-Central Nigeria. Data were analyzed using descriptive and regression analysis.
Results:
Out of the 419 respondents, 138 (32.9%), 141 (33.7%), and 140 (33.4%) were from Benue, Kwara, and Plateau States, respectively. Of the 419, 55.4% were females, 40.8% were above 40 years, and 35.8% have over 10 years of farming experience. The majority of the poultry are not housed (58.5%) and farmers predominantly rear chickens (51.8%). Also, 49.9% of the birds were 1–6 months with 41.5% of the flock sizes being 11–20. Respondents had a poor level of perception toward Salmonella infection as the majority did not know that Salmonella affects poultry (89.3%) and that Salmonella infections are zoonotic (94.5%). Significant (p = 0.000) associations existed between categorized perception score and age, educational status, family size, and farming experience of farmers. There were significant (p = 0.000) associations of categorized practice scores with gender, age, education status, family size, and farming experience of farmers.
Conclusion:
This study has revealed the poor perception of farmers on Salmonella infections and has highlighted their practices. There is a need to raise awareness about these infections to improve indigenous poultry health and productivity as well as public health.
Keywords: Indigenous poultry farmers, Perceptions, Practices, Nigeria, Salmonella infections
Introduction
Indigenous poultry is also known as traditional, scavenging, backyard, village, local, family, rural, free-range, or native poultry (Padhi, 2016). These poultry categories are often kept in an extensive setting with minimal or no housing or feeding since birds are left to scavenge for food and water (Magothe et al., 2012; Desta et al., 2013; FAO, 2018). Also, they are hardy, can withstand harsh weather and environmental conditions (Ajayi, 2010), and are found primarily in rural community households (Kryger et al., 2010).
The rearing of indigenous poultry has been reported globally, but they are more abundant in developing countries than in developed ones (Manyelo et al., 2020). Approximately 80% of rural households are engaged in smallholder poultry production in sub-Saharan Africa (Kryger et al., 2010). Ethiopia has about 59.5 million poultry of which 91% are indigenous chickens (CSA, 2017). Similarly, Kenya has an estimated 32 million poultry, 81% are indigenous chickens that support the livelihood of over 21 million people in rural areas (Nyaga, 2007).
Nigeria is a lower-middle-income country with a population of 190 million people and a gross domestic product per capita of $ 1,968. The country has about 102 million people living below the poverty line and over 70% of Nigerians are involved in poultry production directly or indirectly (FAO, 2019). Indigenous poultry constitutes about 80 million of the total 180 million poultry in Nigeria (FMARD, 2017; FAO, 2018) and is being kept by about 6.6 million households (FAO, 2018) who are primarily rural dwellers (Ajayi, 2010; Heise, 2015).
There are several reports of Salmonella infections in commercial poultry (Muhammad et al., 2010; Agbaje et al., 2010; Fagbamila et al, 2017; Mshelbwala et al, 2017; Jibril et al, 2020) but the Salmonella status of indigenous poultry in Nigeria has not been fully elucidated. This may partly be due to the little or no veterinary attention received by indigenous poultry (Adene and Oguntade, 2006). The implication is that human and poultry health becomes compromised leading to illnesses, decreased productivity, malnutrition, and loss of income among rural dwellers. If properly harnessed, there is enormous potential for the poultry industry in Nigeria to enhance food and nutritional security while contributing to household and economic growth (Heise, 2015). Despite the high population of indigenous poultry in Nigeria, there is still a dearth of information on the demographics of these farmers, their perceptions about salmonellosis, farming practices, its significant public health importance, and its impact on poultry productivity. Hence, in this study, we evaluated the perceptions and practices of farmers of indigenous poultry towards Salmonella infections which will help increase awareness of the disease among farmers, enhance poultry productivity and improve poultry and public health.
Materials and Methods
Study area and design
This study was conducted in the North Central geopolitical zone of Nigeria, one of the country’s administrative divisions. The zone comprises six States, namely, Benue, Kogi, Kwara, Nasarawa, Niger, and Plateau.
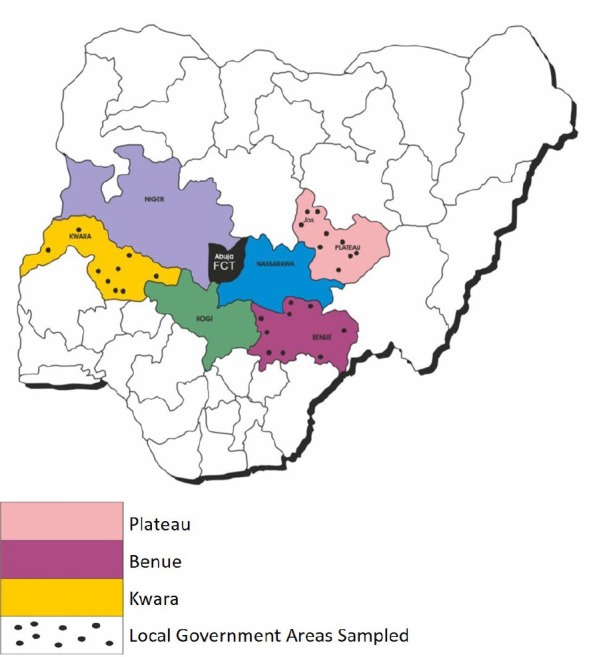
A cross-sectional approach using a multistage sampling method was used in this survey. In the first stage, the North Central zone was purposely selected because it forms the hub of indigenous poultry keeping. Three States (Benue, Kwara, and Plateau States) out of the six states in the North Central zone were selected by balloting without replacement in the second stage. Each State has three senatorial districts from which three Local Government Areas (LGAs) were further selected in the third stage by balloting. In the fourth stage, two villages were randomly selected as sampling sites in each selected LGA. Hence, six villages were sampled per senatorial district to give a total of 18 villages in each State. The Local Government Areas sampled in the three States in North Central Nigeria are shown in Figure 1.
Fig. 1. Map of Nigeria showing the North Central States and the local government areas sampled in the three selected States.
Data collection tool
A pre-tested, structured questionnaire was administered to farmers of indigenous poultry to assess their perceptions of Salmonella between November 2019 and December 2020. Households with indigenous poultry were considered eligible for the survey and were selected by the snowballing method. Questionnaire administration was carried out using the KoBo Toolbox to collect data from farmers across the three selected States. KoBo Toolbox is an open-source suite of tools for data collection and analysis developed by the Harvard Humanitarian Initiative and the International Rescue Committee. This tool offers a quick and reliable means of gathering information about humanitarian crises and research. Briefly, farmers were asked questions on demographics, their perceptions of salmonellosis, and their farming practices. These questions had been inputted on smartphones, and their responses were sent online to a designated site for analysis.
Sample size determination
The prevalence rate for Salmonella infections in indigenous chickens in Nigeria is not known, so an assumed prevalence of 50% was used for sample size determination. The sample size was calculated using the formula for cross-sectional studies (Thrusfield, 2007).
where N is the sample size
Z = 1.96 (constant)
p (prevalence) = 0.5
q (1−p) = 0.5
d (allowable error of 5%) = 0.05
Four hundred and nineteen (N = 419) farmers from different households were interviewed in the three States, with each State having at least 138 respondents across its three senatorial districts.
Questionnaire data collection
In each of the states sampled, the Veterinary Departments were contacted and approval was sought for farmers of indigenous poultry to be interviewed. Livestock extension workers were identified in each of the senatorial districts who further liaised with community leaders in the villages where farmers were interviewed. In the selected villages, farmers keeping indigenous poultry were considered eligible for the study. The farmers were informed of the purpose of the study and verbal and written consent was obtained before the interviews. Participation was voluntary and for the reliability of the research instrument and data, Livestock Extension Officers conducted the interviews which were done in the farmers’ local languages.
Data analyses
The data generated was imported into an Excel spreadsheet (Microsoft Inc.) and exported to SPSS version 25.0 (PASW Inc.) for statistical analysis. Percentages were calculated as the ratio between the responses of farmers to key questions and the number of farmers interviewed. The percentage of each response was calculated separately and presented in Tables. Perception and practice variables were scored, and associations of categorized perception and practice scores with demographic variables were tested using Chi-square statistic. Values of p ≤ 0.05 were considered to be significant.
Ethical approval
Ethical approval for this work was obtained from the National Veterinary Research Institute Vom Animal Use and Care Committee (AEC/02/70/19).
Results
Demographic characteristics of farmers of indigenous poultry in North-Central Nigeria
A total of 419 respondents were interviewed across the three selected States of which 138 (32.9%), 141 (33.7%), and 140 (33.4%) respondents were from Benue, Kwara, and Plateau States, respectively (Table 1). Out of the total 419 respondents interviewed, 232 (55.4%) were females, and 187 (44.6%) were males. Based on the age distribution, 27 (6.4%) respondents were 15–25 years, 108 (25.8%) were 26–35 years, 113 (27.0%) were 36–45 years, and 171 (40.8%) were above 40 years. Thirty (7.2%) respondents had adult education, 72 (17.2%) had no education, 58 (13.8%) had primary education, 165 (39.4%) high school education, and 94 (22.45) had tertiary education. One hundred and sixty-eight (40.1) had a family size of 1–5, 201 (48.0%) had 6–10, 35 (8.4%) had 11–15, and 15 (3.6%) had family size of more than 16. Based on years of farming experience, 11 (2.6%) respondents had less than 1 year farming experience, 138 (32.9%) had 1–5 years, 120 (28.6%) had 6–10 years, and 150 (35.8%) respondents had over 10 years farming experience (Table 1).
Table 1. Demographic characteristics of farmers of indigenous poultry in North Central Nigeria.
| Characteristic | Frequency (N = 419) | Percent (%) |
|---|---|---|
| State of origin | ||
| Benue | 138 | 32.9 |
| Kwara | 141 | 33.7 |
| Plateau | 140 | 33.4 |
| Gender | ||
| Female | 232 | 55.4 |
| Male | 187 | 44.6 |
| Age range (years) | ||
| 15–25 | 27 | 6.4 |
| 26–35 | 108 | 25.8 |
| 36–45 | 113 | 27.0 |
| Above 45 | 171 | 40.8 |
| Educational status | ||
| Adult education | 30 | 7.2 |
| None | 72 | 17.2 |
| Primary | 58 | 13.8 |
| High school | 165 | 39.4 |
| Tertiary | 94 | 22.4 |
| Family size (number) | ||
| 1–5 | 168 | 40.1 |
| 6–10 | 201 | 48 |
| 11–15 | 35 | 8.4 |
| More than16 | 15 | 3.6 |
| Farming experience (years) | ||
| Less than 1 | 11 | 2.6 |
| 1–5 | 138 | 32.9 |
| 6–10 | 120 | 28.6 |
| Over 10 | 150 | 35.8 |
Management practices of farmers of indigenous poultry in North-Central Nigeria
Based on the type of poultry, 217 (51.8%) respondents reared chickens, 37 (8.8%) turkeys, 24 (5.7%) ducks, 9 (2.2%) guinea fowls, and 132 (33.5%) kept mixed species. More respondents, 174 (41.5%) had poultry flock size of 11–20, 60 (14.3%) had 1–10, 107 (25.5%) had 21–30, and 78 (18.6%) had flock size of over 30 birds. Seventy-one (16.9%) respondents reared birds for the purpose of having assets, 113 (27.1%) for consumption, 231 (55.1%) for income, and 4 (0.9%) reared birds for other purposes. Based on the ages of birds in the flock, 177 (42.2%) respondents had birds less than 1 month old, 209 (49.9%) had birds 1–6 months old, and 33 (7.9%) had birds aged 7 months and above. Two hundred and forty-one (57.5%) respondents bought their birds from neighbours, 50 (11.9%) obtained them as gifts, 99 (23.6%) from inheritance, and 29 (6.9%) from other sources. Only 174 (41.5%) respondents housed their birds, while 245 (58.5%) do not.
The sources of poultry feed were commercial in 92 (22.0%), scavenging in 133 (31.7%), grains in 181 (43.2%), and others in 13 (3.1%) respondents. Sixty-three (15.0%) respondents sourced water from boreholes, 21 (5.0%) from streams, lakes and rivers, 46 (11.0%) from pipe borne water, and 289 (69.0%) from wells. A total of 333 (79.5%) respondents had separate drinking points for poultry. Other animals kept were pets (dogs and cats) by 47 (11.2%), ruminants by 165 (39.4%), swine by 20 (4.8%) and mixed by 55 (13.1%) respondents; 132 (31.5%) respondents kept no other animals (Table 2).
Table 2. Management practices among farmers of indigenous poultry in North Central Nigeria.
| Management practice | Frequency (N = 419) | Percent (%) |
|---|---|---|
| Types of Poultry reared | ||
| Chickens | 217 | 51.8 |
| Turkeys | 37 | 8.8 |
| Ducks | 24 | 5.7 |
| Guinea fowls | 9 | 2.2 |
| Mixed | 132 | 33.5 |
| Flock size | ||
| 1–10 | 60 | 14.3 |
| 11–20 | 174 | 41.5 |
| 21–30 | 107 | 25.5 |
| over 30 | 78 | 18.6 |
| Purpose of rearing birds | ||
| Asset | 71 | 16.9 |
| Consumption | 113 | 27.1 |
| Income | 231 | 55.1 |
| Others | 4 | 0.9 |
| Age of birds in the flock | ||
| Less than 1month | 177 | 42.2 |
| 1–6 months | 209 | 49.9 |
| 7 months and above | 33 | 7.9 |
| Source of birds | ||
| Purchased from a neighbour | 241 | 57.5 |
| Gift | 50 | 11.9 |
| Inheritance | 99 | 23.6 |
| Others | 29 | 6.9 |
| Housing of birds | ||
| Yes | 174 | 41.5 |
| No | 245 | 58.5 |
| Poultry feed source | ||
| Commercial | 92 | 22.0 |
| Scavenge | 133 | 31.7 |
| Grains | 181 | 43.2 |
| Others | 13 | 3.1 |
| Feeding pattern | ||
| Once daily | 192 | 45.8 |
| Twice daily | 159 | 37.9 |
| Thrice daily | 19 | 4.5 |
| Others (Specify) | 49 | 11.7 |
| Source of drinking water for poultry | ||
| Boreholes | 63 | 15.0 |
| Streams, lakes, rivers etc. | 21 | 5.0 |
| Pipe borne water | 46 | 11.0 |
| Wells | 289 | 69.0 |
| Separate drinking point for poultry | ||
| Yes | 333 | 79.5 |
| No | 86 | 20.5 |
| Other Animals kept by farmers | ||
| None | 132 | 31.5 |
| Pets (dogs/cats) | 47 | 11.2 |
| Ruminants | 165 | 39.4 |
| Swine | 20 | 4.8 |
| Mixed | 55 | 13.1 |
Perceptions of farmers of indigenous poultry towards Salmonella infection
Among the respondents, 373 (89.0%) knew that Salmonella affects humans, 45 (10.7%) knew that Salmonella affects poultry. Additionally, 19 (4.5%) respondents had recorded Salmonella infection in their flocks out of which 11 (57.9%) had digestive signs, 2 (10.5%) respiratory, 5 (26.3%) mixed signs, and 1 (5.3%) recorded death. Only 23 (5.5%) respondents knew that Salmonella infections in humans can affect poultry and vice versa (Table 3).
Table 3. Perception of farmers of indigenous poultry towards Salmonella infections in North Central Nigeria.
| Variables | Frequency (%) |
|---|---|
| Salmonella affect humans | |
| Yes | 373 (89.0) |
| No | 46 (11.0) |
| Salmonella affects poultry | |
| Yes | 45 (10.7) |
| No | 374 (89.3) |
| Salmonella infection recorded in flock | |
| Yes | 19 (4.5) |
| No | 400 (95.5) |
| Signs of Salmonella infection | |
| Digestive | 11 (57.9) |
| Respiratory | 2 (10.5) |
| Mixed | 5 (26.3) |
| Death | 1 (5.3) |
| Salmonella infections in humans can affect poultry and vice versa | |
| Yes | 23 (5.5) |
| No | 396 (94.5) |
The associations of demographic characteristics of farmers of indigenous poultry with categorized perception score of Salmonella infection in North Central Nigeria were assessed (Table 4). There were statistically significant associations with age (χ2 = 23.230; df = 3; p = 0.000), education status (χ2 = 21.656; df = 4; p = 0.000), family size (χ2 = 20.731; df = 3; p = 0.000) and farming experience (χ2 = 19.260; df = 3; p = 0.000) of farmers with the poor level of perception.
Table 4. Association of demographic characteristics of farmers of indigenous poultry with categorized perception score of Salmonella infection in North Central Nigeria.
| Characteristic | Categorized score (N = 419) | χ2 | df | p-value | |
|---|---|---|---|---|---|
| Poor (%) | Good (%) | ||||
| State of origin | |||||
| Benue | 128 (30.5) | 10 (2.4) | 0.082 | 2 | 0.960 |
| Kwara | 131(31.3) | 10 (2.4) | |||
| Plateau | 131(31.3) | 9 (2.1) | |||
| Gender | |||||
| Female | 231 (55.1) | 20 (4.8) | 1.065 | 1 | 0.333 |
| Male | 159 (37.9) | 9 (2.1) | |||
| Age range (years) | |||||
| 15–25 | 23 (5.5) | 4 (1.0) | 23.230 | 3 | 0.000* |
| 26–35 | 91 (21.7) | 17 (4.1) | |||
| 36–45 | 109 (26.0) | 4 (1.0) | |||
| Above 45 | 167 (39.9) | 4 (1.0) | |||
| Educational status | |||||
| Adult education | 24 (5.7) | 6 (1.4) | 21.656 | 4 | 0.000* |
| None | 62 (14.8) | 10 (2.4) | |||
| Primary | 52 (12.4) | 6 (1.4) | |||
| Secondary | 161 (38.4) | 4 (1.0) | |||
| Tertiary | 91 (21.7) | 3 (0.7) | |||
| Family size (number) | |||||
| 1–5 | 145 (34.6) | 23 (5.5) | 20.731 | 3 | 0.000* |
| 6–10 | 197 (47.0) | 4 (1.0) | |||
| 11–15 | 33 (7.9) | 2 (0.5) | |||
| More than16 | 15 (3.6) | 0 (0.0) | |||
| Farming experience (years) | |||||
| Less than 1 | 9 (2.1) | 2 (0.5) | 19.260 | 3 | 0.000* |
| 1–5 | 119 (28.4) | 19 (4.5) | |||
| 6–10 | 115 (27.4) | 5 (1.2) | |||
| Over 10 | 147 (35.1) | 3(0.7) | |||
Practices of farmers of indigenous poultry towards Salmonella infection
Out of the 419 respondents, 261 (62.3%) administer medication to poultry flock and this medication included solely antimicrobials [191 (73.0%)], antimicrobials and others [21 (8.0%)], and vaccines [13 (5.0%)]. Out of the 261 that give medication, 204 (78.2%) administered for 1–3 days, 49 (18.8%) for 4–7 days, and 8 (13.0%) gave for over 1 week (Table 5). Fifty-one (19.5%) respondents provided self-veterinary care, 176 (67.4%) sought veterinary care from veterinary assistants, and 34 (13.1%) sought veterinary care from veterinary doctors (Table 5).
Table 5. Practices of farmers of indigenous poultry regarding Salmonella infections in North Central Nigeria.
| Variables | Frequency (%) |
|---|---|
| Medication of poultry flock | |
| Yes | 261 (62.3) |
| No | 158 (37.7) |
| Type of intervention | |
| Antimicrobials alone | 191 (73.0) |
| Antimicrobials and others | 21 (8.0) |
| Vaccines | 13 (5.0) |
| Do not know | 36 (14.0) |
| Duration of medication for poultry | |
| 1–3 days | 204 (78.2) |
| 4–7 days | 49 (18.8) |
| Over 1 week | 8 (13.0) |
| Provision of veterinary care | |
| Self | 51 (19.5) |
| Veterinary assistant | 176 (67.4) |
| Veterinary doctor | 34 (13.1) |
The associations of demographic characteristics of farmers of indigenous poultry with categorized practice score towards Salmonella infection in North Central Nigeria revealed statistically significant associations with gender (χ2 = 14.245; df = 1; p = 0.000), age (χ2 = 21.545; df = 3; p = 0.000), educational status (χ2 = 41.498; df = 4; p = 0.000), family size (χ2 = 22.295; df = 3; p = 0.000) and farming experience (χ2 = 25.155; df = 3; p = 0.000) of farmers with good level of practice (Table 6).
Table 6. Association of demographic characteristics of farmers of indigenous poultry with categorized practice score of Salmonella infection in North Central Nigeria.
| Characteristic | Categorized score (N = 419) | χ2 | df | p-value | |
|---|---|---|---|---|---|
| Poor (%) | Good (%) | ||||
| State of origin | |||||
| Benue | 53 (12.6) | 85 (20.3) | 0.048 | 2 | 0.976 |
| Kwara | 53 (12.6) | 88 (21.0) | |||
| Plateau | 52 (12.4) | 88 (21.0) | |||
| Gender | |||||
| Female | 113 (27.0) | 138 (32.9) | 14.245 | 1 | 0.000* |
| Male | 45 (10.7) | 123 (29.4) | |||
| Age range (years) | |||||
| 15–25 | 7 (1.7) | 20 (4.8) | 21.545 | 3 | 0.000* |
| 26–35 | 49 (11.7) | 59 (14.1) | |||
| 36–45 | 57 (13.6) | 56 (13.4) | |||
| Above 45 | 45 (10.7) | 126 (30.1) | |||
| Educational status | |||||
| Adult education | 9 (2.1) | 21(5.0) | 41.498 | 4 | 0.000* |
| None | 32 (7.6) | 40 (9.5) | |||
| Primary | 28 (6.7) | 30 (7.2) | |||
| Secondary | 79 (18.9) | 86 (20.5) | |||
| Tertiary | 10 (2.4) | 84 (20.0) | |||
| Family size (number) | |||||
| 1–5 | 74 (17.7) | 94 (22.4) | 22.295 | 3 | 0.000* |
| 6–10 | 80 (19.1) | 121 (28.9) | |||
| 11–15 | 2 (0.5) | 33 (7.9) | |||
| More than16 | 2 (0.5) | 13 (3.1) | |||
| Farming experience (years) | |||||
| Less than 1 | 3 (0.7) | 8 (1.9) | 25.155 | 3 | 0.000* |
| 1–5 | 61 (14.6) | 77 (18.4) | |||
| 6–10 | 60 (14.3) | 60 (14.3) | |||
| Over 10 | 34 (8.1) | 116 (27.7) | |||
Discussion
Poultry farming generally thrives in Nigeria due to its acceptability across cultural and religious lines. Demand for poultry is also higher because of population growth, economic growth, and lifestyle changes. Indigenous poultry keeping will readily provide protein sources for consumers both at the rural and urban settings at lower rates than what is obtainable for commercial poultry due to the lower cost of production.
Our findings revealed that more women in rural areas keep poultry compared to men. This finding agrees with Siyaya and Masuku (2013), who reported more female farmers of indigenous poultry in Swaziland than males. This might be because poultry are mostly raised around the home where women take care of the children and perform other chores. Women are known to use their income, mostly generated from the sale of poultry products (OECD, 2009) to provide for family needs such as food or medical care (Wong et al., 2017). Men are more likely to be involved in rearing cattle and crop farming, leaving the rearing of poultry to the women and children. In contrast, Mose et al. (2018) and Moussaa et al. (2018) reported more males involved in indigenous poultry in Kenya (76.3%) and Niger Republic (56.9%), respectively. Our findings revealed older persons compared to younger ones are involved in raising indigenous poultry. This could either be as a result of urban migration by the younger generation seeking a better life or the hierarchical nature of rural settings where animals owned by family members are all under the direct care of the head of the family. A high proportion of our respondents (39.4%) had high school education which is relevant for understanding and translating knowledge to improve livelihoods. In most instances, highly educated individuals prefer to stay in townships, leaving the not-so-educated in the villages. It is, therefore, worthy of note that even with high school education, most of the farmers (94.5%) were not aware of the zoonotic nature of Salmonella. The study has revealed that the average family size of indigenous farmers in North-Central Nigeria is large (between 6 and 10 members). This might be due to the value placed on children as assets of labour in agrarian settings, as a social symbol, or for physical or security purposes (Owumi et al., 2016; Alaba et al., 2017).
Chickens are accepted across different cultures, which could explain why they are the most commonly kept indigenous poultry, as observed in this study (51.8%). Similar finding (59.2%) was obtained in Niger Republic (Moussaa et al., 2018). The practice of farmers keeping more than one species of animal has been brought to the fore. Farmers do this to increase the income-generating power of their families. Flock sizes are generally small (averagely 11–20 birds as seen in this study). These poultry were kept as a regular source of marginal income or sold to buy food or medical supplies (Wong et al., 2017). Families may treat visitors to sumptuous dishes made from the poultry they own.
Interestingly, most farmers/respondents reported not housing the indigenous poultry (58.5%). This is in agreement with Moussaa et al. (2018) who reported a similar finding (60.6%) in Niger Republic. Poultry could perch on trees, fences, and any other such places (Adene and Oguntade, 2006). Most of the respondents (43.2%) feed their poultry on grains. This practice is most seen where farmers pour grains for poultry in the mornings before the go out to scavenge. This practice is quite good as it boosts their nutrition in combination with scavenging/free-ranging and has the potential to increase flock sizes. It is, however, intriguing why the flock sizes are quite small in the households.
Water can be a significant source of contamination and disease transmission in rural areas. Findings from this study revealed that most farmers use well water (69%) as drinking water for their poultry, with the majority (79.5%) having separate drinking points for poultry. This practice is very important in preventing food-borne diseases infections such as Salmonella infections.
Significant differences between poultry-keeping duration were also observed. This observation implies that more farmers had been rearing poultry for a long period in the villages as a “way of life.” Poultry keeping duration suggests that farmers who kept poultry for longer periods were more at risk of getting Salmonella infections than those who do not. This is true because birds that harbor Salmonella shed the bacteria intermittently into the environment serving as a source of infection to newer additions to the flock and also the farmers. Salmonella infections tend to occur in older birds. Shedding happens when birds are exposed to stressors such as extreme weather changes (Gole et al., 2017).
In this study, we also observed that majority of the farmers (89%) were aware of Salmonella infections in humans known as typhoid fever but unaware of the same in poultry as revealed by most of the respondents (89.3%). A high proportion of farmers (95.5 %) also reported never having recorded Salmonella infection in their flocks. These knowledge gaps among farmers may be associated with poor disease surveillance and weak veterinary extension services in the villages/rural areas. More worrisome is that these farmers do not know the inherent risks related to zoonotic transmission of Salmonella infections commonly associated with poultry as seen in this study. The close interaction between poultry, farmers, and the environment in which Salmonella could be shed will enhance its persistence in the environment and horizontal transmission in the ecosystem. However, strengthening the veterinary and extension services to rural areas will improve farmer knowledge and public health of the communities.
Additionally, 62.3% of the respondents revealed that they give medication to their poultry out of which 78.2% admitted that they only give these medications for between 1 and 3 days. This may be connected to the high cost of medication and drug availability in rural areas. The practice can lead to the development of antimicrobial resistance in poultry and subsequently in humans. Most of the prescriptions are done by veterinary attendants who are mostly working without equipment/ reagents for diagnosis.
Our findings showed that 58% of the 19 farmers that had experienced Salmonella infections in their flocks noticed digestive signs while 26% reported digestive and respiratory signs. Taken together, these data highlight the potential importance of Salmonella infections as causes of ill health in the indigenous poultry and also a possible source of salmonellosis in humans in North-Central Nigeria.
Conclusion
This study has provided some baseline data on the demographics of farmers that raise indigenous poultry in the North-Central part of Nigeria. Management practices and other vital information about rearing indigenous poultry have also been bought to the fore. Knowledge gaps among farmers of indigenous poultry towards Salmonella infections, their potential significance in causing ill health among poultry and its attendant public health implications have been highlighted.
There is a need to conduct in-depth studies to underpin the salmonellosis burden and its significance in this poultry sub-sector. There is also a need for further studies on the interactions between indigenous poultry, humans, and the environment as regards the occurrence, control, and prevention of Salmonella infections. This will help improve public health, poultry productivity, and enhance food security.
Acknowledgments
The authors would like to thank farmers of indigenous poultry in the states sampled for taking time to openly share their experiences. The authors also would like to thank the Drs Dayo Akinsola, Kingsley Idahor, Adeyinka Adedeji, Ochuko Orakpoghenor, Ndadilnasiya Endie Waziri, and Asabe Dzikwi-Emennaa for the statistical analyses and valuable inputs. The extension staff in the states sampled are also being appreciated.
Conflict of interest
The authors declare that there is no conflict of interest.
Authors’ contributions
NMS and IOF: designed the study, analyzed the data, and contributed to the writing of the manuscript. PDL, FNM, RPW, MM and JE: interpreted and analyzed the data and also involved in the writing of the manuscript.
References
- Adene D.F, Oguntade A.E. Rome, Italy: FAO; 2006. The structure and importance of the commercial and village-based poultry industry in Nigeria. [Google Scholar]
- Agbaje M, Davies R, Oyekunle M.A, Ojo O.E, Fasina F.O, Akinduti P.A. Observation on the occurrence and transmission of Salmonella gallinarum in commercial poultry farms in Ogun State, South Western Nigeria. Afr. J. Microbiol. Res. 2010;4(9):796–800. [Google Scholar]
- Ajayi F.O. Nigerian indigenous chicken: a valuable genetic resource for meat and egg production. Asian J. Poult. Sci. 2010;4(4):164–172. [Google Scholar]
- Alaba O.O, Olubusoye O.E, Olaomi J.O. Spatial patterns and determinants of fertility levels among women of childbearing age in Nigeria. S. Afr. Fam. Pract. 2017;59(4):143–147. [Google Scholar]
- Central Statistics Agency (CSA) Report on livestock and livestock characteristics, Agricultural Sample Survey 2016/17 (2009 E.C) Statistical Bulletin No 585, Vol. II. Addis Ababa. 2017 [Google Scholar]
- Desta T.T, Dessie T, Bettridge J, Lynch S.E, Melese K, Collins M, Christley R.M, Wigley P, Kaiser P, Terfa Z, Mwacharo J.M, Hanotte O. Signature of artificial selection and ecological landscape on morphological structures of Ethiopian village chickens. Anim. Genet. Resour. 2013;52:17–29. [Google Scholar]
- Fagbamila I.O, Barco L, Mancin M, Kwaga J, Ngulukun S.S, Zavagnin P, Lettini A.A, Lorenzetto M, Abdu P.A, Kabir J, Umoh J, Ricci A, Muhammad M. Salmonella serovars and their distribution in Nigerian commercial chicken layer farms. PLoS One. 2017;12(3):e0173097. doi: 10.1371/journal.pone.0173097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal ministry of Agriculture and Rural Development. Animal population data. FMARD. 2017 [Google Scholar]
- Food and Agriculture Organization (FAO) Livestock production systems spotlight Nigeria. Rome, Italy: FAO; 2018. Africa Sustainable Livestock 2050. [Google Scholar]
- Food and Agriculture Organization (FAO) Rome, Italy: FAO; 2019. The future of livestock in Nigeria. Opportunities and challenges in the face of uncertainty. [Google Scholar]
- Gole V.C, Woodhouse R, Caraguel C, Moyle T, Rault J.L, Sexton M, Chousalkar K. Dynamics of Salmonella shedding and welfare of hens in free-range egg production systems. Appl. Environ. Microbiol. 2017;83(5):e03313–16. doi: 10.1128/AEM.03313-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise H, Crisan A, Theuvsen L. The poultry market in Nigeria: market structures and potential for investment in the market. Int. Food Agribus. Manag. Rev. 2015;18(Special Issue A):197–222. [Google Scholar]
- Jibril A.H, Okeke I.N, Dalsgaard A, Kudirkiene E, Akinlabi O.C, Bello M.B, Olsen J.E. Prevalence and risk factors of Salmonella in commercial poultry farms in Nigeria. PLoS One. 2020;15(9):e0238190. doi: 10.1371/journal.pone.0238190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryger K.N, Thomsen K.A, Whyte M.A, Dissing M. Small holder Poultry Production. Rome, Italy: FAO; 2010. [22 August 2021]. Small holder poultry production–Livelihoods, food security and sociocultural significance. Available via http://www.fao.org/docrep/013/al674e/al674e00.pdf . [Google Scholar]
- Magothe T.M, Okeno T.O, Muhuyi W.B, Kahi A.K. Indigenous chicken production in Kenya: I. Current status. World’s Poult. Sci. J. 2012;68:119–132. [Google Scholar]
- Manyelo T.G, Selaledi L, Hassan Z.M, Mabelebele M. Local chicken breeds of Africa: their description, uses and conservation methods. Animals. 2020;10(12):2257. doi: 10.3390/ani10122257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mose P.B, Wasike C.B., Ombok B.O, Kipsat M.J. Farmers’ attitude towards risk on indigenous chicken in Nyanza region. J. Agric. Econ. Rural. Dev. 2018;4(2):469–476. [Google Scholar]
- Moussaa H.O, Keambouc T.C, Himaa K, Issab S, Motsa’ad S.J, Bakassoa Y. Indigenous chicken production in Niger. Vet. Anim. Sci. 2018;7C:40. doi: 10.1016/j.vas.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mshelbwala F.M, Ibrahim N.D, Saidu S.N, Azeez A.A, Akinduti P.A, Kwanashie C.N, Fakilahyel Kadiri A.K, Muhammed M, Fagbamila I.O, Luka P.D. Motile Salmonella serotypes causing high mortality in poultry farms in three South-Western States of Nigeria. Vet. Rec. Open. 2017;4(1):e000247. doi: 10.1136/vetreco-2017-000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad M, Muhammad L.U, Ambali A.G, Mani A.U, Azard S, Barco L. Prevalence of Salmonella associated with chick mortality at hatching and their susceptibility to antimicrobial agents. Vet. Microbiol. 2010;140(1–2):131–135. doi: 10.1016/j.vetmic.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Nyaga P. Food and Agriculture organization of the United Nations, Animal Production and Health Division. Rome, Italy: FAO; 2007. [July 2007]. Poultry sector country review. [Google Scholar]
- Organization for Economic Cooperation and Development (OECD) DAC Guiding Principles for Aid, Effectiveness, Gender Equality and Women’s Empowerment. 2009. [16 August 2021]. Available via https://www.oecd.org/social/gender-development/42310124.pdf .
- Owumi B.E, Eboh A, Owoyemi J.O, Akpata G.O. Perceived causes of childhood illnesses and herbal medicine utilization among mothers and patients in Lokoja, Kogi State, North-Central Nigeria. Res. Humanit. Soc. Sci. 2016;6(23):14–28. [Google Scholar]
- Padhi M.K. Importance of indigenous breeds of chicken for rural economy and their improvements for higher production performance. Scientifica. 2016;2016:9. doi: 10.1155/2016/2604685. Article ID 2604685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siyaya B, Masuku M. Determinants of profitability of indigenous chickens in Swaziland. Bus. Econ. Res. 2013;3(2):205. [Google Scholar]
- Thrusfield M. 3rd. Oxford, UK: Blackwell Science Ltd, A Blackwell Publishing Company; 2007. Veterinary epidemiology. [Google Scholar]
- Wong J.T, de Bruyn J, Bagnol B, Grieve H, Li M, Pym R, Alders R.G. Small-scale poultry and food security in resource-poor settings: a review. Glob. Food Sec. 2017;15:43–52. [Google Scholar]


